Summary
Strigolactones (SLs) represent a class of plant hormones that regulate developmental processes and play a role in the response of plants to various biotic and abiotic stresses. Both in planta hormonal roles and ex planta signalling effects of SLs are potentially interesting agricultural targets. In this review, we explore various aspects of SL function and highlight distinct areas of agriculture that may benefit from the use of synthetic SL analogues, and we identify possible bottlenecks. Our objective is to identify where the contributions of science and stakeholders are still needed to achieve harnessing the benefits of SLs for a sustainable agriculture of the near future.
Keywords: agriculture, application, microbiome, stress, strigolactones
Introduction
Strigol, the first strigolactone (SL), was isolated in 1966 from cotton root exudate (Cook et al., 1966), yet it took more than 40 years to realize that SLs represent a new class of phytohormones (Gomez‐Roldan et al., 2008; Umehara et al., 2008). Despite the time lapse between discovery, elucidation of its structure, and recognition as a hormone, the recent rise in research focus on SLs suggests a promising future for this class of signalling molecules (Cook et al., 1972; Zwanenburg & Blanco‐Ania, 2018). This review introduces the prospects of such a future by highlighting the science of SLs and their potential application in agriculture. SLs are derived from β‐carotene (Alder et al., 2012). Partial elucidation of their biosynthesis in several plant species has identified the involvement of the following genes: DWARF27 (β‐carotene isomerase), CAROTENOID CLEAVAGE DIOXYGENASE 7 and 8 (CCD7 and CCD8), and MAX1 homologues (cytochrome P450s) (Lopez‐Obando et al., 2015; Fig. 1). According to recent reviews, more than 25 SLs have been identified across the plant kingdom, categorized into canonical and noncanonical SLs based on the presence or absence, respectively, of the complete ABC‐ring system (Wang & Bouwmeester, 2018; Bürger & Chory, 2020). A conserved feature in both canonical and noncanonical SLs is the D‐ring, but otherwise many structural variations – including differences in stereochemistry and lack of the conventional ABC‐ring system (noncanonical SLs) – have been reported (Wang & Bouwmeester, 2018; Fig. 2). A butenolide structure similar to the SL D‐ring is also found in smoke‐derived signalling molecules, karrikins, which have been implicated in the germination of dormant seeds after a bush‐fire (Flematti et al., 2015). Karrikins are presumed to mimic an as yet‐unknown endogenous signalling molecule involved in early plant development, and share a paralogous signalling pathway with the SLs (Flematti et al., 2015).
Fig. 1.
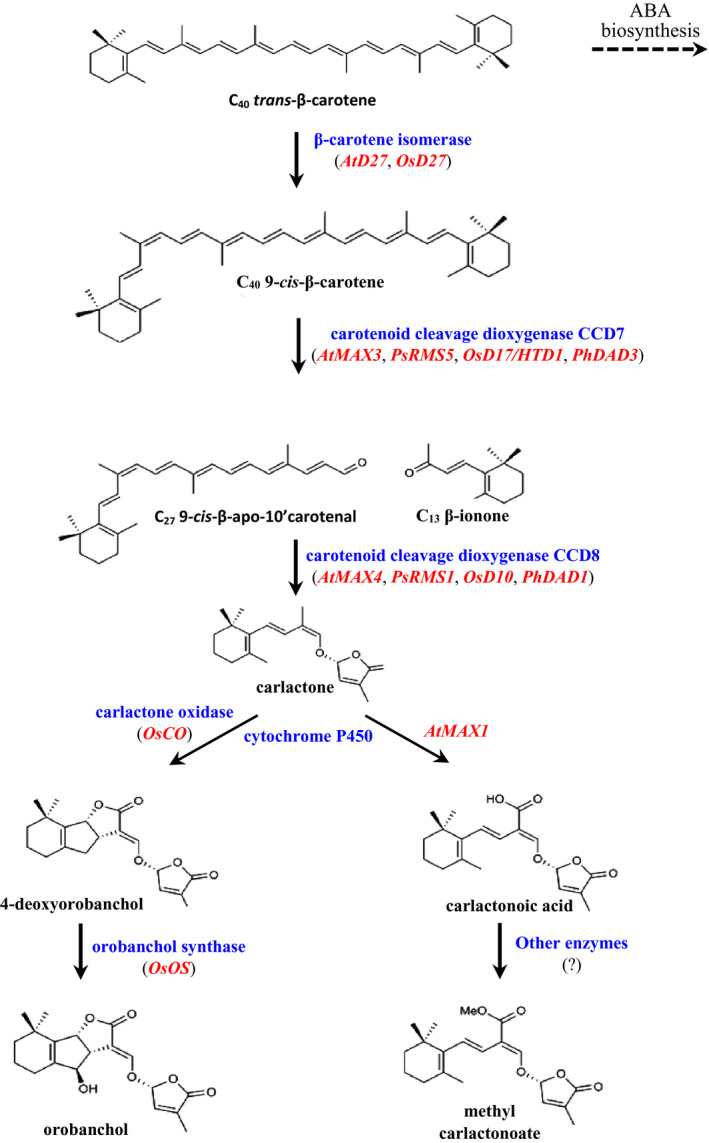
Strigolactone biosynthesis pathway showing the substrates (in black), proteins (in blue), and genes (in red) encoding the enzymes involved in Arabidopsis (At), rice (Os), pea (Ps), and petunia (Ph) (Flematti et al., 2016). Abscisic acid (ABA) biosynthesis competes with strigolactone biosynthesis for all‐trans‐β‐carotene as substrate.
Fig. 2.
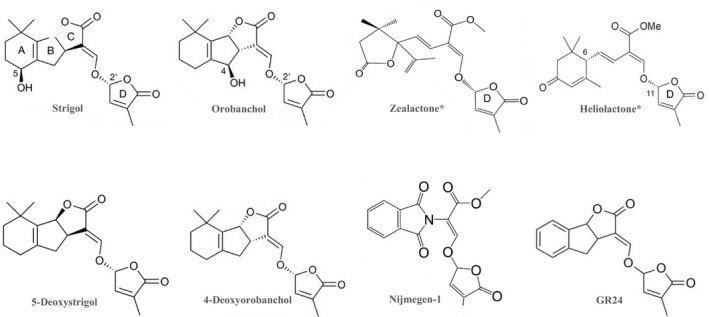
Structural variation in natural and synthetic strigolactones (SLs). All these SLs have the conserved D‐ring (see strigol). The canonical SLs have the full ABC‐ring system, as in strigol, orobanchol, 5‐deoxystrigol, and 4‐deoxyorobanchol. The noncanonical SLs have an incomplete ABC‐ring system, as illustrated with zealactone and heliolactone (marked with asterisk). 5‐deoxystrigol and 4‐deoxyorobanchol illustrate the differences in orientation of the BC ring junction. Nijmegen‐1 and GR24 are examples of synthetic SLs. Adapted from Wang & Bouwmeester (2018) and Yoshimura et al. (2019).
Synthetic SLs are an important tool in the biological research on the functions of these signalling molecules. Chemical synthesis involves either a total synthesis of the entire SL structure or synthesis of analogues with a simplified structure that retains the bio‐properties of SLs (Zwanenburg et al., 2015; Oancea et al., 2017). Total synthesis of the ABC scaffold and subsequent attachment of the functional side‐chains and D‐ring is tedious, and yield is low (Zwanenburg et al., 2015). Chemical synthesis of SL analogues is more promising and is feasible based on the identification of the bioactiphore in SLs, the D‐ring, which is required for activity. Although the contribution of the A‐ring to activity is low, it should have the required stereochemistry (molecular freedom) to get reasonably active analogues (Zwanenburg et al., 2015). A report has shown that structural modification of the D‐ring into a γ‐lactam functional group may give insight into the variations in SL binding interaction with its receptor (Lombardi et al., 2017). Another important analogue is the fluorescence turn‐on probe, Yoshimulactone Green, which can be used to track SL perception (Tsuchiya et al., 2015). All these synthetic SLs have greatly contributed to improve our understanding of the biological role of SLs. However, SL synthesis is faced with a number of challenges (see Box 1).
Box 1. Synthetic production and regulatory bottlenecks to strigolactone application.
Having highlighted the potential that lies in the application of strigolactones (SLs) in agriculture, an inevitable prerequisite for harnessing such potential is that synthetic SL products need to be developed. Some of the synthetic SLs that have been produced so far include GR24 (Besserer et al., 2008), Nijmegen‐1 (Nefkens et al., 1997), Strigolactams (De Mesmaeker et al., 2019), and the fluorescent EGO‐15 and ST‐23b (Prandi et al., 2011), sphynolactone‐7 for Striga control (Uraguchi et al., 2018), and CISA‐1 (Rasmussen et al., 2013) (Fig. 2). The core bioactivity of these synthetic SLs mainly depends on the presence of the D‐ring, although the side‐chain functional groups do modify SL function and activity (Boyer et al., 2012).
One of the challenging requirements for synthetic SLs is their stability in solvent media in which they are formulated, as this contributes to efficacy, which may be negatively affected by environmental conditions, such as temperature and pH (Zwanenburg & Pospíšil, 2013). Furthermore, these SL products must meet the relevant regulations that are in place to ensure their environmental friendliness and safety. For instance, transportability to target tissues and rapid hydrolysis after its action has been initiated.
In terms of regulatory requirements for agricultural end‐uses, SL products may be categorized either as plant protection products, plant strengtheners, or plant bio‐stimulants during product registration procedures, and such diversified categorization may make it hard for regulatory bodies to have a definitive regulation in the use of SL products (Vurro et al., 2016). Additionally, overall investment to optimize and develop a new synthetic agrochemical costs c. USD 300 million (Syngenta Crop Protection AG, 2016), and the years involved in the application procedure without a guarantee of return on investment may dissuade industrial partners from commercializing synthetic SLs (Vurro et al., 2016). Despite the aforementioned requirements and complexities, a number of industries have already been investing significant resources into SL‐based agricultural trials (Davidson, 2015; Screpanti et al., 2016). These efforts may contribute to unveiling the usefulness of SLs to all stakeholders and possibly ease their registration, for instance, as plant strengtheners or plant bio‐stimulants.
The structural variations in the SLs are reflected in their functional diversity (Scaffidi et al., 2014). For instance, differences in the effects of various SLs and their stereoisomers on the germination of parasitic weeds, such as Striga hermonthica and Striga gesnerioides, could be attributed to their structural variation (Nomura et al., 2013). Using germination assays, Nomura et al. (2013) showed that SLs that have the same configuration as 5‐deoxystrigol at their C3a, C8b and C2 positions triggered high germination of S. hermonthica but not S. gesnerioides. Furthermore, the recognition of natural SLs and nonnatural SL isomers (and karrikins), which is mediated by the receptors D14 and KAI2, respectively, is dependent on the structural variations in the chiral carbon orientations at the junction of the BC and D‐rings (Scaffidi et al., 2014). This specificity in SL recognition was demonstrated in Arabidopsis using 5‐deoxystrigol, which showed active, D14‐dependent, inhibition of hypocotyl elongation but not KAI2‐dependent seed germination (Scaffidi et al., 2014). Both of the specific receptor molecules (D14 and KAI2) use MORE AXILLARY GROWTH2 (MAX2) for downstream signalling (Fig. 3). MAX2 is an F‐box protein that forms part of a Skp‐Cullin‐F‐box (SCFMAX2) complex and targets the downstream repressors of karrikin and SLs signalling for degradation by ubiquitination (Soundappan et al., 2015). These repressors include SUPPRESSOR OF MAX2 1 (SMAX1) and SMAX1‐LIKE (SMXL)2 that repress karrikin signalling (Stanga et al., 2016), and SMXL6, SMXL7, and SMXL8 that repress SL signalling (Soundappan et al., 2015). The other SMXLs (SMXL3, SMXL4 and SMXL5) have so far not been reported as repressors of karrikin or SL signalling (Wallner et al., 2017). Research efforts are ongoing in order to gain more insight into the unique and common aspects of these two paralogous signalling pathways, karrikin and SL signalling (Hakoshima, 2018).
Fig. 3.
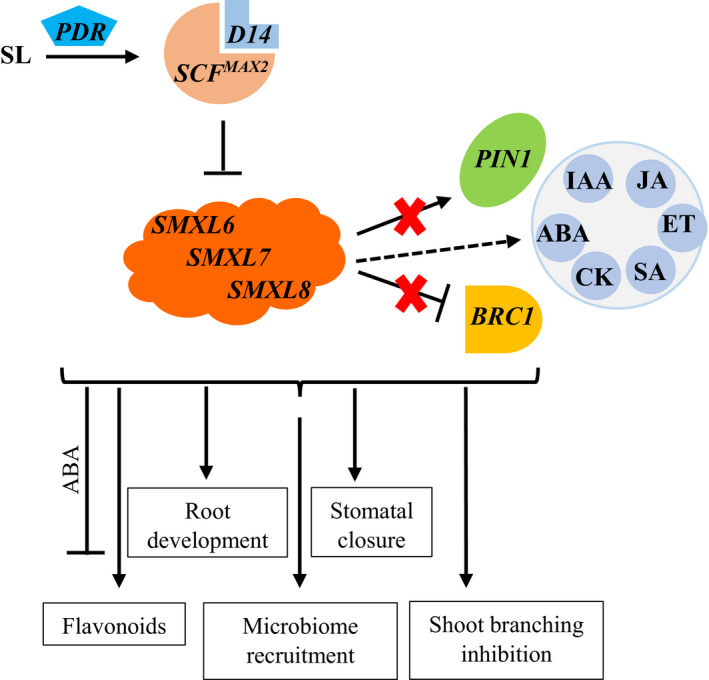
Model of strigolactone (SL) signalling and downstream physiological and phenotypic effects. The dashed arrow indicates the complex inter‐hormonal interactions of SLs. ABA, abscisic acid; CK, cytokinin; ET, ethylene; IAA, auxin; JA, jasmonic acid; SA, salicylic acid.
In addition to their effects on the germination of parasitic weeds ex planta (Nomura et al., 2013), SL signalling facilitates the interaction of plants with arbuscular mycorrhizal fungi (AMFs) by triggering hyphal branching and the formation of the fungal hyphopodia, as has been shown in rice (Oryza sativa) using SL‐biosynthesis mutants (Kobae et al., 2018). Another study that investigated the transcriptomic changes in germinated spores of the AMF Gigaspora margarita following SL (GR24) treatment suggests that SL induces expression changes in fungal genes involved in respiration, production of chitin oligosaccharides, and transcriptional reprograming in the fungus (Lanfranco et al., 2018). SL‐specific effects on AMFs have also been reported; for example, dose–response analysis showed that sorgolactone and GR24 induced hyphal branching in Gigaspora rosea at 10−13 M, whereas GR7, which lacks the A‐ring, only stimulated branching at 10−7 M (Besserer et al., 2006). Another study of SL structural specificity in AMF interactions demonstrated using the AMF G. margarita that intact AB‐ring structure is required for a high hyphal branching activity (Akiyama et al., 2010). Furthermore, SLs are involved in the interaction of plant roots with nitrogen‐fixing bacteria (Rhizobium). There are reports of increased nodulation of alfalfa inoculated with Sinorhizobium meliloti following SL (GR24) treatment (Soto et al., 2010; De Cuyper et al., 2015). In pea also, SLs have been shown to enhance the development of infection threads during the interaction with Rhizobium, and endogenous SLs influence the number of nodules that are formed (Foo & Davies, 2011; McAdam et al., 2017).
In planta, SL hormonal signals have been shown to inhibit axillary bud outgrowth (branching or tillering), in principle, independent of auxin signals (Brewer et al., 2015), presumably by regulating the downstream expression of BRANCHED 1 (BRC1). There are also reports of their involvement in moderating auxin canalization from buds to the main stem through the internalization of the auxin export protein (PIN1), thereby maintaining apical dominance (Hayward et al., 2009; Shinohara et al., 2013). Additionally, they play a role in the regulation of root architecture (Ruyter‐Spira et al., 2011; Sun et al., 2016). We hypothesize that the increasing scientific knowledge of the biological effects of SLs could translate into potential applications in agriculture (Box 2), as discussed in the following section.
Box 2. Overview of requirements for strigolactone application (Fig. 4).
There is currently little information on the field‐scale application of strigolactones (SLs), probably due to the high cost of SL synthesis and the knowledge gap on any potential off‐target environmental risks and/or side‐effects from SL degradation products. Targeted research is therefore needed to understand the specificity of various SLs, the importance of time of application, the possible dose‐dependent environmental risks above or belowground, SL effects on soil microbiota at the community level beyond the effects on a single species or genus, and so on. Importantly, SLs should be properly acknowledged and emphasized in studies in which the effects of SLs are evident, just like with other hormones. Also, collaboration among all stakeholders (government, private/industrial sector, and research institutes) is inevitable for the eventual large‐scale application of SL products. Finally, regulatory advocacy and public awareness may be necessary to provide law makers, farmers, and the general public with the correct information about SLs on which to base their judgements.
Fig. 4.
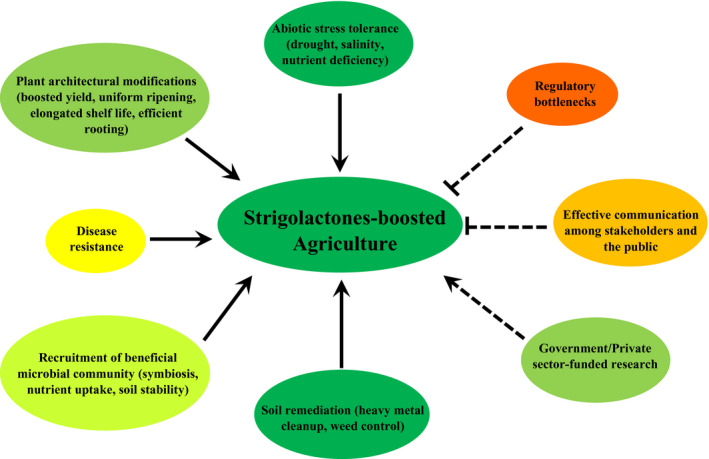
Illustration of potential application benefits (solid lines) of strigolactones in agriculture and the factors (broken lines) that are contributing to its potential realization, either positively (←) or negatively ( ). The colour hues of the panels represent the extent of knowledge and/or application on a field scale and the intensity of effects of the contributing factors (red, negative; green, positive).
). The colour hues of the panels represent the extent of knowledge and/or application on a field scale and the intensity of effects of the contributing factors (red, negative; green, positive).
Do strigolactones mediate the interaction with other soil‐dwelling organisms?
Recruitment of beneficial microbial communities
In addition to the already established involvement of SLs in the interaction of plants with AMFs, recent studies have suggested that SLs can also affect the interaction with other soil (micro)organisms at both the individual and community levels (Lareen et al., 2016; Schlemper et al., 2017). For instance, in a nonfertile soil, the Striga‐resistant cv SRN‐39, which exudes a high orobanchol to 5‐deoxystrigol (5‐DS) ratio, recruits a different bacterial community than high 5‐DS/orobanchol‐exuding genotypes (Schlemper et al., 2017). The dissimilarity in bacterial community recruitment observed by Schlemper et al. (2017) was mainly reflected in the abundance of Comamonadaceae and Burkholderiaceae families that were recruited significantly more by ‘SRN‐39’ than by other genotypes. This may suggest that these two bacterial families contribute to the Striga resistance of ‘SRN‐39’. The genetic basis for the unique SL profile of ‘SRN‐39’ and its resultant Striga resistance was further investigated using a recombinant inbred line population by Gobena et al. (2017). Interestingly, allelic deletion mutations in the LOW GERMINATION STIMULANT 1 gene were shown to be responsible for the observed SL profile and resistance phenotype (Gobena et al., 2017). Indeed, a potential ‘allelic’ control of SL biosynthesis and effects has also been demonstrated in tobacco (Nicotiana tabacum) using CRISPR/Cas9‐targeted mutation of the CCD8 SL‐biosynthesis gene (Gao et al., 2018). The two mutant alleles of the closely related CCD8 genes (NtCCD8A and NtCCD8B) showed distinctive and differential gene‐expression levels in root tissues, and in response to exogenous auxin, respectively, suggesting that SL biosynthesis and function may be altered at the allelic level (Gao et al., 2018). Also, in Arabidopsis, a mutant line of the SL‐biosynthesis enzyme CCD8 (max4) has been used to demonstrate SL effects on plant–microbial interactions at the community level (Carvalhais et al., 2018). The authors showed that SLs influence the composition of fungal communities in the rhizosphere. The fungal taxa/family/species, Epicoccum nigrum, Penicillium, Fibulochlamys chilensis, Herpotrichiellaceae, Mycosphaerella and Mycosphaerellaceae were more abundantly recruited to the root rhizosphere of the wild‐type than the max4 mutant, whereas some members of other families/taxa, including Fusarium, Alternaria and Pleosporaceae, were more abundant in the rhizosphere of max4 (Carvalhais et al., 2018), suggesting that SLs can possibly also repel harmful microbes like Fusarium. The notion that SLs may also attract pathogenic fungi like E. nigrum and Mycosphaerella should be treated with care, as the taxonomic assignment, without identification to the species level due to lack of sufficient sequence information, makes conclusions on the involvement and identity of such microbes only speculative. For instance, Mycosphaerella is a vast genus that includes various species that have not yet been ascertained as pathogenic (Crous et al., 2009), making it unreliable to conclude that the recruited Mycosphaerella in Carvalhais et al. (2018) is pathogenic. On the other hand, Epicoccum nigrum, which has a taxonomic assignment to the species level, has been studied in depth to understand its intermicrobial associations, and there is a report of how it may be utilized as a biological control agent of other phytopathogens (de Lima Fávaro et al., 2012). As a step towards understanding the mechanisms involved in these plant–microbial interactions, Belmondo et al. (2017) have used a fungal mutant screening approach to show that SL signal perception may induce the production of reactive oxygen species and other changes in the mitochondria of fungi. Also, the work of Lanfranco et al. (2018) highlighted earlier is a part of the efforts towards unravelling such mechanisms of interaction.
In view of our current understanding, we speculate that the response to SLs in microbes may vary per plant species depending on the SL profile produced. Indeed, several studies have shown that the biosynthetic pathway differences among plant species in the conversion of carlactonoic acid (CLA) influence the SL profile produced (Charnikhova et al., 2017; Iseki et al., 2018; Y. Zhang et al., 2018). According to Iseki et al. (2018), sorghum converts CLA to 5‐DS and finally into sorgomol, whereas moonseed converts CLA directly to strigol without a 5‐DS intermediate. Similarly, in tomato (Solanum lycopersicum), CLA was reportedly converted directly to orobanchol through the action of an as‐yet‐unknown enzyme(s) (Y. Zhang et al., 2018). Interestingly, a recent study has demonstrated that the previously unknown enzyme catalysing the direct conversion of CLA to orobanchol in cowpea and tomato is cytochrome P450 CYP722C (Wakabayashi et al., 2019). In maize (Zea mays), oxidation, epoxidation, ring cleavage, and lactonization steps have been postulated to result in the unique SL zealactone from CLA (Charnikhova et al., 2017).
As already described, several studies have tried to unravel the mechanisms underlying the relation between SL profile and microbial recruitment. Further research is needed to improve our understanding of these mechanisms and the SL‐based interaction of plants with the soil microbiome. This may aid the implementation of SLs in recruiting microbial communities of agronomic importance (Hartman et al., 2018; Toju et al., 2018; Bouwmeester et al., 2019). Moreover, other potential indirect benefits of SLs in the soil, like phytoremediation, may be linked to its role in plant–microbe interactions (Wu et al., 2011; Lenoir et al., 2016), although more dedicated research is needed to evaluate the mechanisms underlying this effect and its feasibility for application.
Defence against biotic agents
Though the foregoing emphasizes the potential benefits of SL‐based recruitment of (components of) the soil microbiome by plants, other beneficial agricultural implementations of SLs in the soil are the control of parasitic weeds and defence against diseases. Parasitic weeds pose a serious threat to agriculture, considering the long viability of their seeds in the soil and their spread to other agricultural fields (Rubiales et al., 2018). In rice, for instance, annual economic losses resulting from parasitic weeds in Africa is estimated at USD 200 million, and this increases by USD 30 million per annum (Rodenburg et al., 2016). As a potential measure to mitigate parasitic weeds, Screpanti et al. (2016) reviewed practical applications such as suicidal germination, which has been tested in tobacco to control Orobanche ramosa (Zwanenburg et al., 2016) and in sorghum to control S. hermonthica (Samejima et al., 2016). Suicidal germination is an SL‐based induction of the germination of parasitic weeds in the absence of a suitable host such that the weeds die and are eliminated from the soil before the cultivation of the crop. In terms of resistance to plant diseases, the role of SLs is far less understood. In tomato, SL biosynthetic mutants have been used to demonstrate the positive role of SL in plant defence against fungal pathogens (Botrytis cinerea and Alternaria alternate) and root‐knot nematode (Meloidogyne incognita), based on its cross‐talk with other hormones, like jasmonic acid, salicylic acid, and abscisic acid (ABA) (Torres‐Vera et al., 2014; Xu et al., 2019). On the other hand, results from studies on pea plant resistance to Fusarium oxysporum and Pythium irregulare show no involvement of SL in plant defence (Blake et al., 2016; Foo et al., 2016). In Arabidopsis, the beet cyst nematode, Heterodera schachtii, was used to demonstrate that the involvement of SLs in the attraction of plant‐cyst nematodes to the host may be MAX2‐dependent (Martinez et al., 2019), suggesting a possible crosstalk with the karrikin paralogous pathway. These findings suggest that the role of SLs in defence may be specific for certain plant–pathogen combinations only, requiring further research to fully unravel the underlying mechanisms.
How can strigolactones contribute towards plant response to environmental stress?
Physiological response to stress
There is increasing evidence for a role of SLs in the response of plants to osmotic stresses, such as drought and salinity (Visentin et al., 2016; Ma et al., 2017). Using SL‐biosynthesis mutants (i.e. max3 and max4), Ha et al. (2014) demonstrated in Arabidopsis that there is crosstalk between SLs and ABA in regulating abiotic stress responses. Physiologically, the SL mutant lines had a low germination rate, but also a poor stomatal regulation during stress that could be attributed to ABA insensitivity (Ha et al., 2014). Similar ABA–SL crosstalk has been demonstrated in Lotus japonica, showing the ABA insensitivity of the SL‐biosynthesis mutant Ljccd7 (Liu et al., 2015). However, in contrast to the findings of Ha et al. (2014), another recent study in Arabidopsis did not show ABA insensitivity of stomatal closure in SL‐biosynthesis mutants relative to wild‐type: the SL‐biosynthesis mutants (max3 and max4) in Kalliola et al. (2019) were as equally sensitive to ABA as the wild‐type. As an addition to this contrast, a drought experiment in rice using SL‐biosynthesis and signalling mutants also showed that both SL‐deficient (d10 and d17) and insensitive (d3) mutants had a high ABA accumulation in the shoot, resulting in drought tolerance, whereas the mutant, d27, was deficient in ABA and susceptible to drought (Haider et al., 2018). The overexpression of OsD27 (which the authors speculate is perhaps in addition to SLs also involved in ABA biosynthesis) and its high expression in the SL‐deficient and insensitive mutants suggest that D27 may be involved in SL–ABA crosstalk. A further investigation of the aforementioned contrast using Arabidopsis single and double mutants of SL signalling (max2), ABA biosynthesis (aba2), and ABA guard cell signalling (ost1) in Kalliola et al. (2019) showed that a combined impairment of SL signalling and ABA biosynthesis or signalling led to higher stomatal conductance in the double mutants (i.e. an enhanced impairment of stomatal closure) than in the respective single mutants, pointing to a possible ABA‐independent/MAX2‐mediated stress response. This ABA‐independent (MAX2‐mediated) stress response, however, may be a concerted role of the strigolactones–karrikins paralogous pathways, since both molecules signal through MAX2. Also, microarray analysis of the max2 mutant in Ha et al. (2014) revealed a downregulation of flavonoid biosynthesis‐related genes that are drought‐inducible in an ABA‐independent manner. Therefore, despite the unexpected contrasts between the findings from Ha et al. (2014) and those of Kalliola et al. (2019) and Haider et al. (2018), the results from all these studies suggest that SLs may contribute to abiotic stress responses both in concert with ABA and in parallel to it via the MAX2‐dependent signalling pathway.
Other reports re‐emphasize that the basis of the aforementioned ABA–SL crosstalk may be upstream of their respective biosynthesis pathways. Wang et al. (2018) demonstrated that the endogenous accumulation of ABA due to RNA interference silencing of HvABA 8‐hydroxylase 1 and 3 in barley (Hordeum vulgare) resulted in a transcriptional downregulation of SL biosynthesis genes. Such ABA regulation of SL biosynthesis may depend on the direction of the reversible conversion of all‐trans‐β‐carotene to 9‐cis‐β‐carotene by DWARF27, since both hormones share all‐trans‐β‐carotene as a common precursor (Haider et al., 2018; Wang et al., 2018; Fig. 1). In tobacco also, the characteristics of the CRISPR/Cas9‐mutated alleles of the closely related NtCCD8 biosynthetic genes (NtCCD8A and NtCCD8B) suggest that mutations in SL biosynthesis genes may contribute to ABA (in)sensitivity (Gao et al., 2018). The loss‐of‐function tobacco mutants in Gao et al. (2018) differed in a point insertion (NtCCD8A) and a three‐point deletion (NtCCD8B), and the exogenous ABA treatment of these mutants resulted in a three‐fold boost in the gene expression of NtCCD8B but not NtCCD8A. Interestingly, however, the gene expression of NtCCD8A under phosphate starvation (nutrient stress) was six‐fold higher than that of NtCCD8B, suggesting that allelic variations in SL biosynthesis genes may also influence response mechanisms to different stresses.
In terms of the practicality for agricultural implementation of the aforementioned SL physiological roles on stomatal regulation, other studies have suggested that foliar spray is sufficient to induce SLs effects, thus circumventing the rigours of root treatment (Visentin et al., 2016; Min et al., 2019). In tomato, Visentin et al. (2016) grafted wild‐type (SCWT) and SL‐depleted (SCSL) scions on to wild‐type (RSWT) and SL‐depleted (RSSL) root stocks to investigate drought responses (Visentin et al., 2016). Interestingly, drought stress in the wild‐type graft (SCWT/RSWT) induced the expression of SL biosynthetic genes in the shoot, which was similarly observed in the SL‐depleted root stock grafted on wild‐type scion (SCWT/RSSL) under irrigated conditions. The graft combinations with wild‐type scions were more drought tolerant than the graft with SLs‐depleted scion. This suggests that local SLs availability in the shoot may be critical for drought tolerance response. In fact, Visentin et al. (2016), using the mutant lines, showed that lack of SLs in the shoot limited the plants’ sensitivity to ABA‐induced stomatal closure, and exogenous SL application boosted this essential drought‐response phenotype. Also, foliar application of GR24 on grapevine seedlings subjected to polyethylene glycol treatment has recently been shown to alleviate drought stress through stomatal regulation (Min et al., 2019). In agriculture, different genotypes of various crop species display varied levels of ABA sensitivity during drought – including delayed stomatal regulation in sensitive genotypes. Exogenous SL application may be used in the field to synchronize stomatal closure before water limitation results in a stress on the often‐sensitive crops. In essence, there is evidence of the application of SLs in maize fields leading to more effective drought tolerance compared with nontreated fields (Davidson, 2015). Nevertheless, further research is needed to understand the possibilities of combinatorial formulations of SLs and ABA that could be used in agriculture for synchronized stomatal regulations.
Root development towards stress adaptation
Several reports have demonstrated the involvement of SLs in the regulation of plant root development, even though the specific effects may vary across species and conditions. For instance, lateral root development may be inhibited by SLs under optimal growing conditions, whereas during nutrient stress they are enhanced to facilitate nutrient uptake (Ruyter‐Spira et al., 2011; Marzec & Melzer, 2018). A common observation among these reports, however, is the interaction of SLs with auxin, in which SLs play a superimposing regulatory role in modulating phenotypic response to the hormonal interplay (Ruyter‐Spira et al., 2011; Kapulnik & Koltai, 2014; Sun et al., 2019). In addition to its effects on lateral root development, SLs also positively regulate primary root length, seminal root length, root biomass, and root hair length and density (Ruyter‐Spira et al., 2011; Kapulnik & Koltai, 2014). In a study using PLEIOTROPIC DRUG RESISTANCE1 (PDR1)‐overexpressing lines in Petunia hybrida, it was shown that an optimization of SL transport could release the feedback inhibition on SL biosynthesis and boost plant rooting properties (Liu et al., 2018). The overexpression of PDR1, an ABCG‐class transporter involved in SL transport to the shoot and exudation to the soil, resulted not only in the enhancement of root biomass and lateral root growth, but also induced root hair elongation (Liu et al., 2018). In fact, the P. hybrida PDR1‐overexpressing lines showed a weaker auxin reporter intensity when compared with wild‐type, suggesting that the boosted endogenous SL levels may have influenced auxin distribution in the root, thus driving the concerted hormonal regulation of the root development in Liu et al. (2018). In terms of root hair formation and elongation, it is known that auxin transport from the root tip to the root hair zone is essential to trigger root hair formation, whereas a synergy of both auxin and ethylene pathways is essential in root hair elongation (Rahman et al., 2002; Muday et al., 2012; D. J. Zhang et al., 2018). Interestingly, it has been reported that SLs regulate auxin‐efflux carriers like PIN1 that influence auxin levels in root cells and thus affect root hair growth, thereby introducing another layer of hormonal control of root hair development centred around SLs (Koltai et al., 2010; Kapulnik et al., 2011). Indeed, complex hormonal interactions between SL, auxin (IAA), cytokinin, ethylene, and karrikin pathways may all contribute to the eventual root architectural modifications that the plant can benefit from during environmental stress (Marzec et al., 2013; Marzec & Melzer, 2018; Fig. 3). An additional role of SLs in these complex hormonal interactions that may be harnessed in nutrient‐poor soils and in organic agriculture to enhance nutrient uptake is the initiation of root symbiosis with nutrient‐fixing microbes during nutrient stress. The potential of this may be explored in seed‐propagated crops by seed treatment with SL formulations before planting in nutrient‐poor soils. The idea is to boost root development for specific soil environments such that crops thrive amidst a stressful environment. Hence, this approach may also be exploited in drought‐prone areas, so that crops develop increased root density early in the growing season before the onset of drought.
How important is the effect of strigolactones on shoot architecture?
SL signalling via the receptor, D14, plays a significant role in the regulation of plant shoot architecture (Fig. 3). Among the degradation targets of SL signalling mediated by MAX2, SMXL7 was shown to be rapidly degraded by the synthetic SL GR24 (Soundappan et al., 2015), and the features of SMXL6 were further reported to be similar to SMXL7 (Bennett et al., 2016). A combined loss‐of‐function mutation of these SMXL genes (SMXL6, 7, and 8) suppressed shoot branching in max2 (Soundappan et al., 2015), indicating that the regulation of these SMXLs plays a pivotal role in shoot architectural modification. Furthermore, it has been shown with an smxl6,7,8 max2 quadruple mutant that these SMXLs promote auxin transport and PIN1 accumulation in the stem, suggesting that SLs modulate shoot branching through their effect on auxin via these SMXLs (Soundappan et al., 2015). The SMXLs also repress the expression of BRC1 in axillary buds, thus releasing the suppression of axillary bud outgrowth by BRC1 (Soundappan et al., 2015). BRC1 is known to act downstream of the SLs signalling pathway in some species like pea (PsBRC1) and Arabidopsis (AtBRC1) (Aguilar‐Martinez et al., 2007; Braun et al., 2012). A typical example of the agricultural implications of BRC1 is the domestication of maize, in which the selection for less shoot branching in favour of yield is associated with the TEOSINTE BRANCHED 1 (TB1) locus, a maize homologue of BRC1 (Kellogg, 1997). The interaction between SLs and the TB1 locus of maize in the regulation of shoot branching is, however, quite complex. Analysis of a maize SL‐biosynthesis (Zmccd8) and tb1 mutant and the double mutant (tb1‐Zmccd8) revealed that although SLs may not completely regulate the TB1 gene in maize, they have significant additive effects on shoot branching and plant height under limited TB1 allele dosage (Guan et al., 2012). The findings of Guan et al. (2012) also showed that, without SLs, maize ear length and diameter were significantly reduced and the tassel drooped. These architectural–phenotypic traits are essential for the productivity of the crop and may provide applications for SLs differing between species depending on the preferred trait. For instance, whereas branchless single stems are preferred in maize, the production of more tillers, as in rice and wheat (Triticum aestivum), may be of higher agronomic value, and tiller number may be associated with the production of less SLs (Wu et al., 1998; Jamil et al., 2012; Zhao et al., 2019). With this understanding, an application of SL formulations on seedlings in the field may be used to influence shoot architecture to facilitate a preferential partitioning of metabolites to the yield organ. In the case of maize, for instance, there are reports of a 20% yield increase following SL foliar application in three field trials (Davidson, 2015). Also, in Brassica napus, there is evidence from a glasshouse study in growth chambers that GR24 application significantly increased plant biomass within 7 days of application (Ma et al., 2017). Furthermore, there is emerging evidence of a potential role for exogenous SL application in late developmental stages of crop species. This was observed in grapevine both in vitro and in the field, whereby exogenously applied synthetic SLs interacted with exogenously co‐treated ABA (but not with endogenous ABA) to delay anthocyanin accumulation and ripening (Ferrero et al., 2018). The latter finding suggests that there may be more levels and effects of SL hormonal interactions that can essentially be further investigated and harnessed to plan the timing of SLs application in the field. For instance, considering the food losses due to post‐harvest challenges in the handling of crop products that are delicate, field treatment with a combination of synthetic SL + ABA may delay ripening, which could be beneficial in enhancing the shelf life of farm products.
Conclusions and perspectives
Variation in SLs (types, profiles, and concentrations) between plant species may differentially affect microbial communities. Research efforts to identify the respective SL biosynthesis pathways in distinct plant species would aid our understanding of such effects. It is essential to understand the roles that plant developmental stage, root architecture, environmental conditions, soil types, and so on play in the interactions between SLs and microbial communities. For this, the fundamental biology of SLs needs to be understood; for instance, the downstream signalling components in plants and perception in microbes, and the mechanisms of SL molecular and physiological interactions. The use of targeted mutation techniques (like CRISPR/Cas9) and population‐based studies could unlock the genetic basis of SL effects and potential for heritability. Key for this improved understanding is the use of pure SLs instead of racemic mixtures in SL studies, as different isomers may signal through different pathways (Scaffidi et al., 2014). Furthermore, including functional assays, like protein‐level interactions in SL experiments, will ensure that each experiment is exhaustively utilized to improve our understanding of SL effects. For application purposes, the translation of the SL potential into agriculture will require that field trials are also conducted as a follow‐up to findings from controlled environments. This would require stereoselective synthesis of only biologically active configurations in order to reduce chemical pollution in the field.
In conclusion, many of the beneficial effects described herein are highly aligned with the sustainability criteria; for example: the better use of natural resources, such as soil nutrients; and the increase of crop resilience, particularly in relation to climate change threats. New SL‐based technologies will represent a substantial paradigm shift in the area of crop protection by moving from a classic pest/disease control approach to more of a crop enhancement effect that harnesses the soil potential.
Author contributions
HJB, ADM and CS initiated the idea of the paper. EBA, TM and HJB reviewed the scientific literature. CS and ADM contributed on the industrial aspect of the manuscript.
References
- Aguilar‐Martinez JA, Poza‐Carrion C, Cubas P. 2007. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell Online 19: 458–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Ogasawara S, Ito S, Hayashi H. 2010. Structural requirements of strigolactones for hyphal branching in AM fungi. Plant and Cell Physiology 51: 1104–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al‐Babili S. 2012. The path from β‐carotene to carlactone, a strigolactone‐like plant hormone. Science 335: 1348–1351. [DOI] [PubMed] [Google Scholar]
- Belmondo S, Marschall R, Tudzynski P, López Ráez JA, Artuso E, Prandi C, Lanfranco L. 2017. Identification of genes involved in fungal responses to strigolactones using mutants from fungal pathogens. Current Genetics 63: 201–213. [DOI] [PubMed] [Google Scholar]
- Bennett T, Liang Y, Seale M, Ward S, Müller D, Leyser O. 2016. Strigolactone regulates shoot development through a core signalling pathway. Biology Open 5: 1806–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besserer A, Becard G, Jauneau A, Roux C, Sejalon‐Delmas N. 2008. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiology 148: 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besserer A, Puech‐Pagès V, Kiefer P, Gomez‐Roldan V, Jauneau A, Roy S, Portais JC, Roux C, Becard G, Sejalon‐Delmas N. 2006. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biology 4: e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake SN, Barry KM, Gill WM, Reid JB, Foo E. 2016. The role of strigolactones and ethylene in disease caused by Pythium irregulare . Molecular Plant Pathology 17: 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester HJ, Fonne‐Pfister R, Screpanti C, De Mesmaeker A. 2019. Strigolactones: plant hormones with promising features. Angewandte Chemie 58: 12778–12786. [DOI] [PubMed] [Google Scholar]
- Boyer FD, de Saint Germain A, Pillot JP, Pouvreau JB, Chen VX, Ramos S, Stevenin A, Simier P, Delavault P, Beau JM et al 2012. Structure–activity relationship studies of strigolactone‐related molecules for branching inhibition in garden pea: molecule design for shoot branching. Plant Physiology 159: 1524–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, de Saint Germain A, Pillot JP, Boutet‐Mercey S, Dalmais M, Antoniadi I, Li X, Maia‐Grondard A, Le Signor C, Bouteiller N et al 2012. The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiology 158: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Gui R, Mason MG, Beveridge CA. 2015. Strigolactone inhibition of branching independent of polar auxin transport. Plant Physiology 168: 1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürger M, Chory J. 2020. The many models of strigolactone signaling. Trends in Plant Science. doi: 10.1016/j.tplants.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalhais LC, Rincon‐Floreza VA, Brewer PB, Beveridge CA, Dennis PG, Schenk PM. 2018. The ability of plants to produce strigolactones affects rhizosphere community composition. Rhizosphere 9: 18–26. [Google Scholar]
- Charnikhova TV, Gaus K, Lumbroso A, Sanders M, Vincken JP, De Mesmaeker A, Ruyter‐Spira CP, Screpanti C, Bouwmeester HJ. 2017. Zealactones. Novel natural strigolactones from maize. Phytochemistry 137: 123–131. [DOI] [PubMed] [Google Scholar]
- Cook CE, Whichard LP, Turner B, Wall ME, Egley GH. 1966. Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science 154: 1189–1190. [DOI] [PubMed] [Google Scholar]
- Cook CE, Whichard LP, Wall ME, Egley GH, Coggon P, Luhan PA, McPhail AT. 1972. Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea). Journal of the American Chemical Society 94: 6198–6199. [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, Burgess TI, Andjic V, Barber PA, Groenewald JZ. 2009. Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. 2015. Enhancing drought tolerance in maize using strigolactones. USDA. URL [WWW document] https://portal.nifa.usda.gov/web/crisprojectpages/1002887‐enhancing‐drought‐tolerance‐in‐maize‐using‐strigolactones.html [accessed 10 June 2019].
- De Cuyper C, Fromentin J, Yocgo RE, De Keyser A, Guillotin B, Kunert K, Boyer FD, Goormachtig S. 2015. From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula . Journal of Experimental Botany 66: 137–146. [DOI] [PubMed] [Google Scholar]
- De Mesmaeker A, Screpanti C, Fonné‐Pfister R, Lachia M, Lumbroso A, Bouwmeester H. 2019. Design, synthesis and biological evaluation of strigolactone and strigolactam derivatives for potential crop enhancement applications in modern agriculture. Chimia 73: 549–560. [DOI] [PubMed] [Google Scholar]
- Ferrero M, Pagliarani C, Novák O, Ferrandino A, Cardinale F, Visentin I, Schubert A. 2018. Exogenous strigolactone interacts with abscisic acid‐mediated accumulation of anthocyanins in grapevine berries. Journal of Experimental Botany 69: 2391–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flematti GR, Dixon KW, Smith SM. 2015. What are karrikins and how were they ‘discovered’ by plants? BMC Biology 13: e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flematti GR, Scaffidi A, Waters MT, Smith SM. 2016. Stereospecificity in strigolactone biosynthesis and perception. Planta 243: 1361–1373. [DOI] [PubMed] [Google Scholar]
- Foo E, Blake SN, Fisher BJ, Smith JA, Reid JB. 2016. The role of strigolactones during plant interactions with the pathogenic fungus Fusarium oxysporum . Planta 243: 1387–1396. [DOI] [PubMed] [Google Scholar]
- Foo E, Davies NW. 2011. Strigolactones promote nodulation in pea. Planta 234: 1073–1081. [DOI] [PubMed] [Google Scholar]
- Gao J, Zhang T, Xu B, Jia L, Xiao B, Liu H, Liu L, Yan H, Xia Q. 2018. CRISPR/Cas9‐mediated mutagenesis of Carotenoid Cleavage Dioxygenase 8 (CCD8) in tobacco affects shoot and root architecture. International Journal of Molecular Sciences 19: e1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobena D, Shimels M, Rich PJ, Ruyter‐Spira C, Bouwmeester H, Kanuganti S, Mengiste T, Ejeta G. 2017. Mutation in sorghum LOW GERMINATION STIMULANT 1 alters strigolactones and causes Striga resistance. Proceedings of the National Academy of Sciences, USA 114: 4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Roldan V, Fermas S, Brewer PB, Puech‐Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC et al 2008. Strigolactone inhibition of shoot branching. Nature 455: 189–194. [DOI] [PubMed] [Google Scholar]
- Guan JC, Koch KE, Suzuki M, Wu S, Latshaw S, Petruff T, Goulet C, Klee HJ, McCarty DR. 2012. Diverse roles of strigolactone signaling in maize architecture and the uncoupling of a branching‐specific subnetwork. Plant Physiology 160: 1303–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CV, Leyva‐González MA, Osakabe Y, Tran UT, Nishiyama R, Watanabe Y, Tanaka M, Seki M, Yamaguchi S, Dong NV et al 2014. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proceedings of the National Academy of Sciences, USA 111: 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider I, Andreo‐Jimenez B, Bruno M, Bimbo A, Floková K, Abuauf H, Ntui VO, Guo X, Charnikhova T, Al‐Babili S et al 2018. The interaction of strigolactones with abscisic acid during the drought response in rice. Journal of Experimental Botany 69: 2403–2414. [DOI] [PubMed] [Google Scholar]
- Hakoshima T. 2018. Strigolactone and karrikin signaling proteins In: Hejátko J, Hakoshima T, eds. Plant structural biology: hormonal regulations. Cham, Switzerland: Springer, 97–112. [Google Scholar]
- Hartman K, van der Heijden MGA, Wittwer RA, Banerjee S, Walser JC, Schlaeppi K. 2018. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 6: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A, Stirnberg P, Beveridge C, Leyser O. 2009. Interactions between auxin and strigolactone in shoot branching control. Plant Physiology 151: 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki M, Shida K, Kuwabara K, Wakabayashi T, Mizutani M, Takikawa H, Sugimoto Y. 2018. Evidence for species‐dependent biosynthetic pathways for converting carlactone to strigolactones in plants. Journal of Experimental Botany 69: 2305–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil M, Charnikhova T, Houshyani B, van Ast A, Bouwmeester HJ. 2012. Genetic variation in strigolactone production and tillering in rice and its effect on Striga hermonthica infection. Planta 235: 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliola M, Jakobson L, Davidsson P, Pennanen V, Waszczak C, Yarmolinsky D, Zamora O, Palva ET, Kariola T, Kollist H et al 2019. Plant stomata encyclopedia. The role of strigolactones in regulation of stomatal conductance and plant‐pathogen interactions in Arabidopsis thaliana. [WWW document] URL https://plantstomata.wordpress.com/tag/dmitry‐yarmolinsky/ [accessed 18 December 2019].
- Kapulnik Y, Delaux PM, Resnick N, Mayzlish‐Gati E, Wininger S, Bhattacharya C, Séjalon‐Delmas N, Combier JP, Bécard G, Belausov E et al 2011. Strigolactones affect lateral root formation and root‐hair elongation in Arabidopsis . Planta 233: 209–216. [DOI] [PubMed] [Google Scholar]
- Kapulnik Y, Koltai H. 2014. Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant Physiology 166: 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EA. 1997. Plant evolution: the dominance of maize. Current Biology 7: 411–413. [DOI] [PubMed] [Google Scholar]
- Kobae Y, Kameoka H, Sugimura Y, Saito K, Ohtomo R, Fujiwara T, Kyozuka J. 2018. Strigolactone biosynthesis genes of rice are required for the punctual entry of arbuscular mycorrhizal fungi into the roots. Plant and Cell Physiology 59: 544–553. [DOI] [PubMed] [Google Scholar]
- Koltai H, Dor E, Hershenhorn J, Joel DM, Weininger S, Lekalla S, Shealtiel H, Bhattacharya C, Eliahu E, Resnick N et al 2010. Strigolactones’ effect on root growth and root‐hair elongation may be mediated by auxin‐efflux carriers. Journal of Plant Growth Regulation 29: 129–136. [Google Scholar]
- Lanfranco L, Fiorilli V, Venice F, Bonfante P. 2018. Strigolactones cross the kingdoms: plants, fungi, and bacteria in the arbuscular mycorrhizal symbiosis. Journal of Experimental Botany 69: 2175–2188. [DOI] [PubMed] [Google Scholar]
- Lareen A, Burton F, Schäfer P. 2016. Plant root–microbe communication in shaping root microbiomes. Plant Molecular Biology 90: 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir I, Lounes‐Hadj Sahraoui A, Fontaine J. 2016. Arbuscular mycorrhizal fungal‐assisted phytoremediation of soil contaminated with persistent organic pollutants: a review. European Journal of Soil Science 67: 624–640. [Google Scholar]
- de Lima Fávaro LC, de Souza Sebastianes FL, Araújo WL. 2012. Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS ONE 7: e36826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Pfeifer J, de Brito Francisco R, Emonet A, Stirnemann M, Gübeli C, Hutter O, Sasse J, Mattheyer C, Stelzer E et al 2018. Changes in the allocation of endogenous strigolactone improve plant biomass production on phosphate‐poor soils. New Phytologist 217: 784–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, He H, Vitali M, Charnikhova T, Haider I, Schubert A, Ruyter‐Spira C, Bouwmeester HJ, Lovisolo C, Cardinale F. 2015. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: exploring the interaction between strigolactones and ABA under abiotic stress. Planta 241: 1435–1451. [DOI] [PubMed] [Google Scholar]
- Lombardi C, Artuso E, Grandi E, Lolli M, Spirakys F, Priola E, Prandi C. 2017. Recent advances in the synthesis of analogues of phytohormones strigolactones with ring‐closing metathesis as a key step. Organic & Biomolecular Chemistry 15: 8218–8231. [DOI] [PubMed] [Google Scholar]
- Lopez‐Obando M, Ligerot Y, Bonhomme S, Boyer FD, Rameau C. 2015. Strigolactone biosynthesis and signaling in plant development. Development 142: 3615–3619. [DOI] [PubMed] [Google Scholar]
- Ma N, Hu C, Wan L, Hu Q, Xiong J, Zhang C. 2017. Strigolactones improve plant growth, photosynthesis, and alleviate oxidative stress under salinity in rapeseed (Brassica napus L.) by regulating gene expression. Frontiers in Plant Science 8: e1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez CME, Guarneri N, Overmars H, van Schaik C, Bouwmeester HJ, Ruyter‐Spira C, Goverse A. 2019. Distinct roles for strigolactones in cyst nematode parasitism of Arabidopsis roots. European Journal of Plant Pathology 154: 129–140. [Google Scholar]
- Marzec M, Melzer M. 2018. Regulation of root development and architecture by strigolactones under optimal and nutrient deficiency conditions. International Journal of Molecular Sciences 19: e1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Muszynska A, Gruszka D. 2013. The role of strigolactones in nutrient‐stress responses in plants. International Journal of Molecular Sciences 14: 9286–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam EL, Hugill C, Fort S, Samian E, Cottaz S, Davies NW, Reid JB, Foo E. 2017. Determining the site of action of strigolactones during nodulation. Plant Physiology 175: 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Z, Li R, Chen L, Zhang Y, Li Z, Liu M, Ju Y, Fang Y. 2019. Alleviation of drought stress in grapevine by foliar‐applied strigolactones. Plant Physiology and Biochemistry 135: 99–110. [DOI] [PubMed] [Google Scholar]
- Muday GK, Rahman A, Binder BM. 2012. Auxin and ethylene: collaborators or competitors? Trends in Plant Science 17: 181–195. [DOI] [PubMed] [Google Scholar]
- Nefkens GHL, Thuring JWJF, Beenakkers MFM, Zwanenburg B. 1997. Synthesis of a phthaloylglycine‐derived strigol analogue and its germination stimulatory activity toward seeds of the parasitic weeds Striga hermonthica and Orobanche crenata . Journal of Agriculture and Food Chemistry 45: 2273–2277. [Google Scholar]
- Nomura S, Nakashima H, Mizutani M, Takikawa H, Sugimoto Y. 2013. Structural requirements of strigolactones for germination induction and inhibition of Striga gesnerioides seeds. Plant Cell Reports 32: 829–838. [DOI] [PubMed] [Google Scholar]
- Oancea F, Georgescu E, Matusova R, Georgescu F, Nicolescu A, Raut I, Jecu ML, Vladulescu MC, Vladulescu L, Deleanu C. 2017. New strigolactone mimics as exogenous signals for rhizosphere organisms. Molecules 22: 961–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prandi C, Occhiato EG, Tabasso S, Bonfante P, Novero M, Scarpi D, Bova ME, Miletto I. 2011. New potent fluorescent analogues of strigolactones: synthesis and biological activity in parasitic weed germination and fungal branching. European Journal of Organic Chemistry 2011: 3781–3793. [Google Scholar]
- Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S. 2002. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiology 130: 1908–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A, Heugebaert T, Matthys C, Van Deun R, Boyer FD, Goormachtig S, Stevens C, Geelen D. 2013. A fluorescent alternative to the synthetic strigolactone GR24. Molecular Plant 6: 100–112. [DOI] [PubMed] [Google Scholar]
- Rodenburg J, Demont M, Zwart SJ, Bastiaans L. 2016. Parasitic weed incidence and related economic losses in rice in Africa. Agriculture, Ecosystems & Environment 235: 306–317. [Google Scholar]
- Rubiales D, Fernandez‐Aparicio M, Vurro M, Eizenberg H. 2018. Editorial: Advances in parasitic weed research. Frontiers in Plant Science 9: e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter‐Spira C, Kohlen W, Charnikhova T, van Zeijl A, van Bezouwen L, de Ruijter N, Cardoso C, Lopez‐Raez JA, Matusova R, Bours R et al 2011. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiology 155: 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima H, Babiker AG, Takikawa H, Sasaki M, Sugimoto Y. 2016. Practicality of the suicidal germination approach for controlling Striga hermonthica . Pest Management Science 72: 2035–2042. [DOI] [PubMed] [Google Scholar]
- Scaffidi A, Waters MT, Sun YK, Skelton BW, Dixon KW, Ghisalberti EL, Flematti GR, Smith SM. 2014. Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in Arabidopsis. Plant Physiology 165: 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlemper TR, Leite MFA, Lucheta AR, Shimels M, Bouwmeester HJ, van Veen JA, Kuramae EE. 2017. Rhizobacterial community structure differences among sorghum cultivars in different growth stages and soils. FEMS Microbiology Ecology 93: fix096. [DOI] [PubMed] [Google Scholar]
- Screpanti C, Yoneyama K, Bouwmeester HJ. 2016. Strigolactones and parasitic weed management 50 years after the discovery of the first natural strigolactone strigol: status and outlook. Pest Management Science 72: 2013–2015. [DOI] [PubMed] [Google Scholar]
- Shinohara N, Taylor C, Leyser O. 2013. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biology 11: e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto MJ, Fernandez‐Aparicio M, Castellanos‐Morales V, Garcia‐Garrido JM, Ocampo JA, Delgado MJ, Vierheilig H. 2010. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biology and Biochemistry 42: 383–385. [Google Scholar]
- Soundappan I, Bennett T, Morffy N, Liang Y, Stanga JP, Abbas A, Leyser O, Nelson DC. 2015. SMAX1‐LIKE/D53 family members enable distinct MAX2‐dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell 27: 3143–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanga JP, Morffy N, Nelson DC. 2016. Functional redundancy in the control of seedling growth by the karrikin signaling pathway. Planta 243: 1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Tao J, Gu P, Xu G, Zhang Y. 2016. The role of strigolactones in root development. Plant Signaling & Behavior 11: e1110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Xu F, Guo X, Wu D, Zhang X, Lou M, Luo F, Zhao Q, Xu G, Zhang Y. 2019. A strigolactone signal inhibits secondary lateral root development in rice. Frontiers in Plant Science 10: 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syngenta Crop Protection AG. 2016. Annual review 2016. [WWW document] URL https://www.syngenta.com/sites/syngenta/files/presentation‐and‐publication/updated/annual%20reports/2016/syngenta‐annual‐review‐2016.pdf [accessed 18 December 2019].
- Toju H, Peay KG, Yamamichi M, Narisawa K, Hiruma K, Naito K, Fukuda S, Ushio M, Nakaoka S, Onoda Y et al 2018. Core microbiomes for sustainable agroecosystems. Nature Plants 4: 247–257. [DOI] [PubMed] [Google Scholar]
- Torres‐Vera R, García JM, Pozo MJ, López‐Ráez JA. 2014. Do strigolactones contribute to plant defence? Molecular Plant Pathology 15: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y, Yoshimura M, Sato Y, Kuwata K, Toh S, Holbrook‐Smith D, Zhang H, McCourt P, Itami K, Kinoshita T et al 2015. Probing strigolactone receptors in Striga hermonthica with fluorescence. Science 349: 864–868. [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda‐Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K et al 2008. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200. [DOI] [PubMed] [Google Scholar]
- Uraguchi D, Kuwata K, Hijikata Y, Yamaguchi R, Imaizumi H, Sathiyanarayanan AM, Rakers C, Mori N, Akiyama K, Irle S et al 2018. A femtomolar‐range suicide germination stimulant for the parasitic plant Striga hermonthica . Science 362: 1301–1305. [DOI] [PubMed] [Google Scholar]
- Visentin I, Vitali M, Ferrero M, Zhang Y, Ruyter‐Spira C, Novak O, Strnad M, Lovisolo C, Schubert A, Cardinale F et al 2016. Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytologist 212: 954–963. [DOI] [PubMed] [Google Scholar]
- Vurro M, Prandi C, Baroccio F. 2016. Strigolactones: how far is their commercial use for agricultural purposes? Pest Management Science 72: 2026–2034. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Hamana M, Mori A, Akiyama R, Ueno K, Osakabe K, Osakabe Y, Suzuki H, Takikawa H, Mizutani M et al 2019. Direct conversion of carlactonoic acid to orobanchol by cytochrome P450 CYP722C in strigolactone biosynthesis. Science Advances 5: eaax9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner ES, López‐Salmerón V, Belevich I, Poschet G, Jung I, Grünwald K, Sevilem I, Jokitalo E, Hell R, Helariutta Y et al 2017. Strigolactone and karrikin‐independent SMXL proteins are central regulators of phloem formation. Current Biology 27: 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chen W, Eggert K, Charnikhova T, Bouwmeester H, Schweizer P, Hajirezaei MR, Seiler C, Sreenivasulu N, von Wirén N et al 2018. Abscisic acid influences tillering by modulation of strigolactones in barley. Journal of Experimental Botany 69: 3883–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bouwmeester HJ. 2018. Structural diversity in the strigolactones. Journal of Experimental Botany 69: 2219–2230. [DOI] [PubMed] [Google Scholar]
- Wu G, Wilson LT, McClung AM. 1998. Contribution of rice tillers to dry matter accumulation and yield. Agronomy Journal 90: 317–323. [Google Scholar]
- Wu SC, Wong CC, Shu WS, Khan AG, Wong MH. 2011. Mycorrhizo‐remediation of lead/zinc mine tailings using vetiver: a field study. International Journal of Phytoremediation 13: 61–74. [DOI] [PubMed] [Google Scholar]
- Xu X, Fang P, Zhang H, Chi C, Song L, Xia X, Shi K, Zhou Y, Zhou J, Yu J. 2019. Strigolactones positively regulate defense against root‐knot nematodes in tomato. Journal of Experimental Botany 70: 1325–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Fonné‐Pfister R, Screpanti C, Hermann K, Rendine S, Dieckmann M, Quinodoz P, De Mesmaeker A. 2019. Total synthesis and biological evaluation of heliolactone. Helvetica Chimica Acta 102: e1900211. [Google Scholar]
- Zhang Y, Cheng X, Wang Y, Díez‐Simón C, Flokova K, Bimbo A, Bouwmeester HJ, Ruyter‐Spira C. 2018. The tomato MAX1 homolog, SlMAX1, is involved in the biosynthesis of tomato strigolactones from carlactone. New Phytologist 219: 297–309. [DOI] [PubMed] [Google Scholar]
- Zhang DJ, Yang YJ, Liu CY, Zhang F, Hu W, Gong SB, Wu QS. 2018. Auxin modulates root‐hair growth through its signaling pathway in citrus. Scientia Horticulturae 236: 73–78. [Google Scholar]
- Zhao B, Wu TT, Ma SS, Jiang DJ, Bie XM, Sui N, Zhang XS, Wang F. 2019. TaD27‐B gene controls the tiller number in hexaploid wheat. Plant Biotechnology Journal 18: 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwanenburg B, Blanco‐Ania D. 2018. Strigolactones: new plant hormones in the spotlight. Journal of Experimental Botany 69: 2205–2218. [DOI] [PubMed] [Google Scholar]
- Zwanenburg B, Mwakaboko AS, Kannan C. 2016. Suicidal germination for parasitic weed control. Pest Management Science 72: 2016–2025. [DOI] [PubMed] [Google Scholar]
- Zwanenburg B, Pospíšil T. 2013. Structure and activity of strigolactones: new plant hormones with a rich future. Molecular Plant 6: 38–62. [DOI] [PubMed] [Google Scholar]
- Zwanenburg B, Zeljkovic CS, Pospisil T. 2015. Synthesis of strigolactones, a strategic account. Pest Management Science 72: 15–19. [DOI] [PubMed] [Google Scholar]


