Abstract
Aim
To determine the most effective DNA extraction method for bacteria in faecal samples.
Materials and Results
This study assessed five commercial methods, that is, NucliSens easyMag, QIAamp DNA Stool Mini kit, PureLink Microbiome DNA purification kit, QIAamp PowerFecal DNA kit and RNeasy PowerMicrobiome kit, of which the latter has been optimized for DNA extraction. The DNA quantity and quality were determined using Nanodrop, Qubit and qPCR. The PowerMicrobiome kit recovered the highest DNA concentration, whereby this kit also recovered the highest gene copy number of Gram positives, Gram negatives and total bacteria. Furthermore, the PowerMicrobiome kit in combination with mechanical pre‐treatment (bead beating) and with combined enzymatic and mechanical pre‐treatment (proteinase K+mutanolysin+bead beating) was more effective than without pre‐treatment.
Conclusion
From the five DNA extraction methods that were compared, the PowerMicrobiome kit, preceded by bead beating, which is standard included, was found to be the most effective DNA extraction method for bacteria in faecal samples.
Significance and Impact of the Study
The quantity and quality of DNA extracted from human faecal samples is a first important step to optimize molecular methods. Here we have shown that the PowerMicrobiome kit is an effective DNA extraction method for bacterial cells in faecal samples for downstream qPCR purpose.
Keywords: bacteria, DNA extraction, faeces, Nanodrop, qPCR, Qubit
Introduction
During the last decades, nucleic acid‐based methods (e.g. quantitative PCR and high‐throughput sequencing) have revolutionized our knowledge of the gut microbiome and its role in human health and disease. The gastrointestinal microbial community protects against invading pathogens, guides the development of the mammalian immune system and contributes to various metabolic functions (Flint et al. 2012). Alterations of the gastrointestinal microbial composition have been associated with various conditions such as inflammatory bowel diseases (Frank et al. 2007), cancer (Francescone et al. 2014), obesity (Ley et al. 2006), diabetes (Qin et al. 2012; Dunne et al. 2014), cardiovascular disease (Rajendhran et al. 2013), autism and depression (Vuong et al. 2017) and kidney disease (Ramezani and Raj 2014). Conventional cultivation‐based methods to identify the gastrointestinal microbes depend upon bacterial growth on several different selective media. However, cultivation‐based methods have several limitations to efficiently assess the bacterial complexity of the gut, such as the underrepresentation of fastidious and uncultivable bacterial species, the need for special culture conditions and nutrients, and the need for identification of isolates. Although conventional culture methods remain important to gain knowledge regarding physiological properties of the bacterial species (Vartoukian et al. 2010), molecular non‐culture‐based techniques have revealed the large diversity of the gastrointestinal microbial composition, usually through amplification and sequencing of universally present but variable genes, such as the 16S ribosomal RNA gene (Eckburg et al. 2005; Bragg and Tyson 2014). The quantity and quality of the nucleic acids that can be extracted from microbes in faecal samples is of utmost importance to optimize the information that can be obtained from these molecular methods. In addition, different extraction methods may have different efficiency for microbes with strongly differing cells walls, that is, Gram‐positive and Gram‐negative cell walls. The outcome may even differ between Gram positives for different DNA extraction methods (Maukonen et al. 2012). Although numerous studies have addressed this issue in detail (Holland et al. 2000; McOrist et al. 2002; Anderson and Lebepe‐Mazur 2003; Li et al. 2003; Yu and Morrison 2004; Nechvatal et al. 2008; Ariefdjohan et al. 2010; Nylund et al. 2010; Salonen et al. 2010; Persson et al. 2011; Smith et al. 2011; Yuan et al. 2012; Andersen et al. 2013; Henderson et al. 2013; Mirsepasi et al. 2014; Wesolowska‐Andersen et al. 2014; Kumar et al. 2016; Costea et al. 2017), only few of them included the QIAamp PowerFecal DNA kit (Nechvatal et al. 2008; Kumar et al. 2016), and none included the commonly used PureLink Microbiome DNA Purification kit or the RNeasy PowerMicrobiome kit. It remains to be established to what extent these procedures are efficient to extract DNA from endospores.
In this study, we compared five commercial DNA extraction assays. We analysed the DNA quality and quantity by means of Nanodrop and Qubit, and with qPCR assays specific for one Gram‐positive bacterial genus (i.e. Bifidobacterium spp.) and for one Gram‐negative bacterial species (i.e. Escherichia coli), and one qPCR assay to assess total bacterial 16S rRNA gene copies.
Materials and methods
Study population and faecal sample collection
One faecal sample was collected from three volunteers, all women between 20 and 23 years of age, two African and one European descent ancestry, without signs of disease and not taking any antibiotics during 3 months before sampling. Samples were stored at −80°C within an hour after sampling. To aliquot the samples, the faecal samples were thawed, divided into 0·2 g aliquots and stored again at −80°C until processing. The study was approved by, and performed according to the guidelines of the Medical Ethics Committee of the Ghent University Hospital (Ghent, Belgium) (B670201214999‐2012/063). Before inclusion, all volunteers provided written informed consent.
DNA extraction assays for faecal samples
We included the following five DNA extraction methods: (i) Semi‐automated NucliSens easyMag (EM; BioMérieux, Marcy‐l’Etoile, France), which is the generally used method to extract DNA in the Ghent University laboratory (El Aila et al. 2011); (ii) RNeasy PowerMicrobiome kit (PM; Qiagen, Hilden, Germany), which was already used in the KU Leuven laboratory to extract DNA from faecal samples (Falony et al. 2016; Joossens et al. 2019); (iii) The QIAamp DNA Stool Mini kit (QIA; Qiagen) was selected as an appropriate DNA extraction method, found to be most efficient in several studies (Holland et al. 2000; McOrist et al. 2002; Salonen et al. 2010); (iv) the QIAamp PowerFecal DNA kit (PF; Qiagen) and (v) the PureLink Microbiome DNA purification kit (PL; Invitrogen, Carlsbad, CA) were included as kits that had become available recently, were affordable and were based on silica‐based DNA separation techniques, as was the case for the kits already included. In case of the EM, which is the only method that is semi‐automated due to the use of the NucliSens easyMag instrument, the silica is coated onto magnetic particles, whereas in the other assays the silica is bound to a column.
In this study, the term pre‐treatment indicates the treatment of the faecal sample to optimize bacterial cell lysis before using the assay, whereas a combined pre‐treatment and assay is designated as a procedure. For each pre‐treatment and each assay, two faecal aliquots per volunteer were used to check the intra‐sample variability. Furthermore, each procedure was performed at two different time points to check the inter‐run variability of each procedure. As such, a total of 40 faecal samples of 0·2 g were used for every volunteer (Fig. 1).
Figure 1.
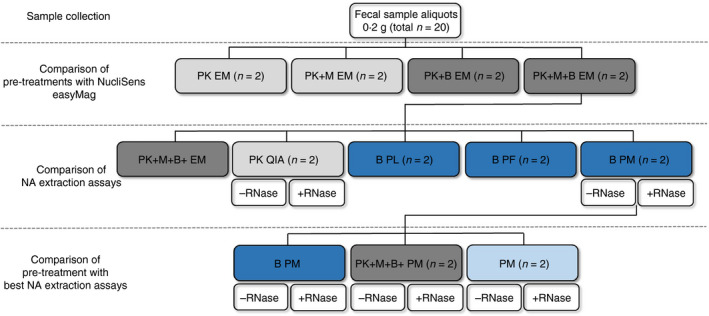
Experimental set‐up for one volunteer for one time point measurement. This set‐up was performed for all three volunteers, each at two different time points. Selection of best assay each round was based on Nanodrop and Qubit quantification and quality assessment and quantification by means of qPCR for Escherichia coli, Bifidobacterium spp. and all bacterial species. Procedure was carried out twice on two different days in duplicate. n: Number of faecal samples used; PK: proteinase K pre‐treatment; M: mutanolysin pre‐treatment; B: bead beating pre‐treatment; RNase: RNase treatment followed by a DNA purification step; EM: semi‐automated NucliSens easyMag; QIA: QIAamp DNA Stool Mini kit; PL: PureLink Microbiome DNA Purification kit; PF: QIAamp PowerFecal DNA kit; PM: RNeasy PowerMicrobiome kit; light grey: mechanical lysis; dark grey: combination of mechanical and enzymatic lysis; light blue: no enzymatic or mechanical lysis; dark blue: enzymatic lysis. [Colour figure can be viewed at wileyonlinelibrary.com]
Semi‐automated NucliSens easyMag DNA extraction (EM)
The programme ‘Specific A’, specifically developed by BioMérieux for the extraction of DNA from faecal samples, was used to extract DNA from bacterial cells in faecal samples. To extract high DNA yields with the NucliSens easyMag, four pre‐treatment modifications (steps 2–5 in ‘Specific A’ protocol) were evaluated: (i) proteinase K (PK), (ii) proteinase K and mutanolysin (PK+M), (iii) proteinase K and bead beating (zirconium beads, 0·5 mm; Sigma Aldrich, St‐Louis, MO) (PK+B) and (iv) proteinase K, mutanolysin and bead beating (PK+M+B). The protocols for the four pre‐treatments combined with the NucliSens easyMag are illustrated in Fig. S1.
QIAamp DNA Stool Mini kit
DNA extraction with the QIAamp DNA Stool Mini kit (QIA) was performed according to the manufacturer’s instructions (Qiagen) without modifications. The lysis step in this assay includes ASL buffer as well as PK for the degradation and digestion of proteins. InhibitEX matrix tablets (provided in the kit) are used to adsorb DNA‐damaging substances and PCR inhibitors (Fig. S2).
PureLink Microbiome DNA Purification kit
The PureLink Microbiome DNA Purification kit (PL) was performed according to the manufacturer’s instructions (Invitrogen) without modifications. S1 (lysis buffer) and S2 (lysis enhancer) are used to lyse the cells and beads (0·070–0·125 mm; provided in the kit) were used for mechanical lysis (Fig. S2).
QIAamp PowerFecal DNA kit
The QIAamp PowerFecal DNA kit (PF) (previously PowerFecal DNA Isolation kit, commercialized by MO BIO Laboratories, Carlsbad, CA) was performed according to the manufacturer’s instructions (Qiagen) without modifications. Chemical lysis is performed by adding C1 solution to the sample, and mechanical lysis is obtained by garnet beads (0·7 mm; provided in the kit). An inhibitor removal solution (C2; provided in the kit) is added to remove contaminating organic and inorganic matter (Fig. S2).
RNeasy PowerMicrobiome kit
The RNeasy PowerMicrobiome kit (PM) (previously PowerMicrobiome RNA Isolation kit, commercialized by MO BIO Laboratories) has been optimized for DNA extraction (Falony et al. 2016) and thus performed according to the manufacturer’s instruction (Qiagen), with the following modifications: the DNase steps (steps 12–16) were not performed, to extract DNA and RNA. Furthermore, an additional heating step of 95°C for 10 min after step 4 was added because this was shown previously to increase the DNA yield (Falony et al. 2016) (Fig. S2). PM1+1% β‐mercaptoethanol (βME) solution is, respectively, used to lyse the cells and to denature ribonucleases to protect RNA. Glass beads (0·1 mm; provided in the kit) are used for mechanical lysis. PM2 solution is an inhibitor removal solution to remove contaminants. A volume of 100 μl of nucleic acids (NA, i.e., both DNA and RNA) is eluted by PM8 solution. In half of the extracts, after NA elution, RNase treatment was performed, followed by DNA purification (QIAquick PCR purification kit; Qiagen) to remove the RNA from the DNA–RNA mix. The pre‐treatment described in the protocol according to the manufacturer’s instructions uses only a bead beating (B) step. This pre‐treatment was compared with (i) proteinase K, mutanolysin and bead beating (PK+M+B) and (ii) without any pre‐treatment (none). The PM protocol with the different pre‐treatments is illustrated in Fig. S3.
DNA and RNA yield and quality
DNA and RNA concentrations of the extracts were measured with the Nanodrop ND‐1000 spectrophotometer (Isogen Life Science, Utrecht, the Netherlands). In case of the NA extracts obtained with the EM, 10‐fold dilutions in sterile water were used, to avoid interference from the elution buffer. To determine DNA and RNA purity, the A 260 nm/A 280 nm ratio of each sample was determined. In addition, the DNA concentration of the extracts was measured with the Qubit 2·0 Fluorimeter (ds DNA high‐sensitivity assays kit; Invitrogen). For both methods, every sample was measured three times and the average was normalized to an equal elution volume of 100 µl for all DNA extraction assays and used for further analysis. In addition, a serial dilution of a deoxy nucleotide triphosphate (dNTP) mix (10 mmol l−1 dNTP mix PCR grade; Invitrogen) was determined with the Nanodrop and the Qubit.
Quantitative PCR assays
The extent to which the different methods provided amplifiable DNA was determined by means of species‐specific qPCRs for two gut bacterial taxa, a Bifidobacterium spp. (Gram‐positive bacterial genus) and E. coli (Gram‐negative bacterial species), and a general bacterial 16S rRNA gene qPCR. We used primers for E. coli and total bacteria as described, respectively, by Chern et al. (2011) and Vaneechoutte et al. (2000), and the primers for Bifidobacterium spp. were designed on the basis of those described by Hauther et al. (2015) (Table 1). Also the E. coli probe was designed de novo in this study (Table 1). Specificity of primers and of the probe was evaluated in silico using nucleotide BLAST (basic local alignment search tool). Primer sequences and the amplicon sequence were further evaluated with the programs ‘OligoAnalyzer 3.1’, ‘mfold’ and ‘DINAMelt’ for homo‐ and hetero‐dimers, sequence length, GC% and melting temperature. All primers and the probe were purchased from Eurogentec (Seraing, Belgium). To validate the specificity of the amplification of the primers and probe, qPCR amplification was performed using template DNA from different bacterial species (Table S1), with the LightCycler480 (LC480; Roche Life Science, Vilvoorde, Belgium). Table S2 summarizes the qPCR mixes and thermal cycling conditions for each qPCR. The specificity of the amplification of the Bifidobacterium spp. was verified by the presence of a melting peak between 84 and 86°C. For all qPCR reactions, every sample was amplified in duplicate, standard dilution series were used as positive controls, and qPCR mixes without DNA were included as negative controls. The concentrations obtained with qPCR were, for all DNA extraction assays, normalized to an equal elution volume of 100 µl. Standard 10‐fold dilution series were prepared from DNA extracts of the following strains: Bifidobacterium pseudocatenulatum LBR 0715206 and E. coli LMG 2092T.
Table 1.
qPCR primers and probe sequences
| Target species (gene) | Sequence 5′–3′ | Amplicon length (bp) | Reference |
|---|---|---|---|
| Bifidobacterium spp. (16S rRNA gene) |
F: GAATAGCTCCTGGAAACG R: ATAGGACGCGACCCCA |
99 |
This paper This paper |
| Escherichia coli (uidA gene) |
F: CAACGAACTGAACTGGCAGA R: CATTACGCTGCGATGGAT P: FAM‐TATCCCGCCGGGAATGGTGA‐TAMRA |
121 |
Chern et al. (2011) Chern et al. (2011) This paper |
| Total bacteria (16S rRNA gene) |
F: CTCCTACGGGAGGCAGCAGT R: GTATTACCGCGGCTGCTG |
170–200 |
Vaneechoutte et al. (2000) Vaneechoutte et al. (2000) |
F: forward primer; R: reverse primer; P: probe; bp: base pair; FAM: 6‐carboxyfluorescein, fluorescence reporter dye; TAMRA: 5(6)‐carboxy‐tetramethylrhodamine, fluorescence quencher dye.
qPCR inhibition
Possible qPCR inhibition due to the presence of PCR inhibitors in the DNA extracts was assessed by comparing the calculated bacterial cell counts with the cell counts as assessed based on the 10‐fold diluted DNA extracts, as obtained by the Bifidobacterium spp., E. coli and total bacteria qPCRs.
Statistics
Prior to statistical analysis of the qPCR concentration results of the bacterial species, the data were log10 transformed. All statistical analyses were performed by IBM SPSS Statistics for Windows, ver. 25 (IBM, Armonk, NY) and graphs were made with R (2018, ver. 3.5.2.). To correct for possible intra‐sample variability, the mean of two faecal samples (0·2 g) per volunteer was used for further analysis. Inter‐run variability was also checked by the coefficient of variation (Cv%), and values below 20% were considered as indicative for limited inter‐run variability. For each volunteer, faecal samples were considered as dependent samples. Thus, the Friedman test (not normally distributed data) or linear mixed models (normally distributed data) were performed to compare the different pre‐treatments and the different DNA extraction assays. Moreover, a paired sample t test or a Wilcoxon signed‐rank test was performed to analyse the concentrations of the PowerMicrobiome assay before and after the RNase treatment and also for the assessment of possible PCR inhibition. Depending on the distribution of the data, mean values (normally distributed) or median values (not normally distributed) are given representing the three volunteers together. For correlation between the total yield of DNA extracted and the total bacterial 16S rRNA gene copies, a Spearman rank (rs) correlation was performed. Differences were considered as statistically significant when the P value was below 0·05.
Results
Comparison of five commercial DNA extraction assays
Yield and quality of the DNA extracted with five different assays
The following five different NA extraction procedures were compared: (i) PK QIA; (ii) B PL; (iii) B PF; (iv) B PM and (v) PK+M+B EM (Fig. 1). The latter procedure, that is, PK+M+B EM, showed the highest yield of NA and highest amount of bacterial gene copies compared to three other pre‐treatments in combination with EM (Fig. S4). After performing the extraction protocols, two assays had high A 260 nm/A 280 nm median ratios according to the Nanodrop, that is, 2·02 for the RNeasy PowerMicrobiome kit including a bead beating pre‐treatment (B PM), and 2·03 for the QIAamp DNA Stool Mini kit (PK QIA). These high A 260 nm/A 280 nm median ratios are an indication for the presence of high levels of RNA. Therefore, an RNase step followed by a DNA purification step was performed to measure the quantity of DNA present in the extracts. The initial abundance of RNA is confirmed by median ratios of 1·75 for B PM and 1·81 for PK QIA after the RNase purification step, corresponding with almost pure DNA, according to the Nanodrop guidelines (Table S3).
Significant differences were found between the Nanodrop concentrations of PK QIA (median 1·9 µg g−1 faeces) and the B PM (median 60·0 µg g−1 faeces) (Fig. 2a, Table S3). Only for PK+M+B EM, PK QIA and B PL, the inter‐run variability of the DNA yield for every extraction was below 20% (Table S4). B PL, B PM and PK QIA were found to yield almost pure DNA (median A 260 nm/A 280 nm ratios of, respectively, 1·74, 1·75 and 1·81). The observed median A 260 nm/A 280 nm ratios of 1·61 and 1·02 for, respectively, B PF and PK+M+B EM indicate low DNA purity (Table S3). In addition, the DNA levels obtained with the five different DNA extraction assays were also measured with the Qubit, which measures only dsDNA. The same results as the Nanodrop were found, a significant difference between PK QIA (median 0·8 µg g−1 faeces) and B PM (median 16·0 µg g−1 faeces) (Fig. 2c, Table S3). For none of the assays was the inter‐run variability below 20% for all volunteers (Table S4). Compared to the Qubit DNA kit, which measures only dsDNA, the higher levels observed with the Nanodrop (Fig. 2) might be due to fact that this method also measures RNA, single‐stranded oligonucleotides and single dNTPs (Table S5) for PK+M+B EM, B PL and B PF (Fig. 2), and loss of DNA due to column purification after RNase treatment for PK QIA and B PM.
Figure 2.
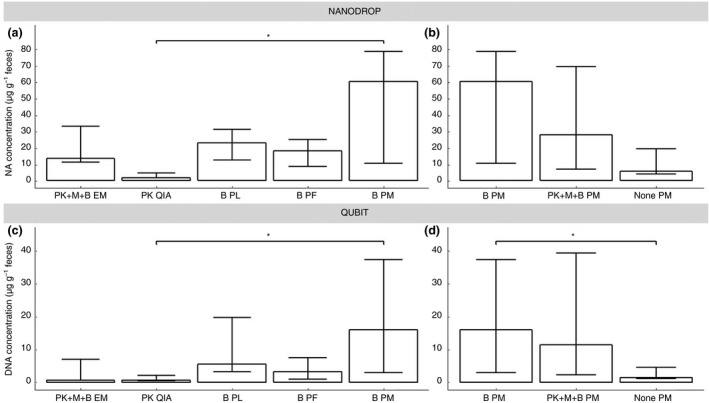
Nucleic acid concentrations (µg per gram faeces), as determined by Nanodrop and Qubit. (a and c) the five DNA extraction assays and (b and d) the three different pre‐treatment methods for the PowerMicrobiome assay. Data presented as median with 95% CI; EM: semi‐automated NucliSens easyMag; QIA: QIAamp DNA Stool Mini kit; PL: PureLink Microbiome DNA Purification kit; PF: QIAamp PowerFecal DNA kit; PM: RNeasy PowerMicrobiome kit; PK: proteinase K pre‐treatment; M: mutanolysin pre‐treatment; B: bead beating pre‐treatment; *P < 0·050.
qPCR results of bacterial DNA
qPCR performed on the DNA extracts obtained with the five different assays yielded a substantially higher estimate of the number of E. coli uidA gene copies for B PM compared to PK QIA (Fig. 3a). The highest E. coli concentration was obtained with B PM (median 108·4 uidA gene copies per gram faeces), followed by B PL (median 108·2 uidA gene copies per gram faeces) and PK+M+B EM (median 108·1 uidA gene copies per gram faeces). The number of Bifidobacterium spp. 16S rRNA gene copies was considerably higher when extracting DNA using a bead beating pre‐treatment with the highest yields recovered with B PL (median 109·5 16S rRNA gene copies per gram faeces), B PM (median 109·4 16S rRNA gene copies per gram faeces) and PF (median 108·9 16S rRNA gene copies per gram faeces). B PM also recovered substantially more Bifidobacterium spp. 16S rRNA gene copies compared to PK+M+B EM (median 107·8 16S rRNA gene copies per gram faeces, P = 0·001) (Fig. 3c, Table S3). For the total bacterial 16S rRNA gene copies of the DNA extracts, a significantly higher quantity was observed using B PM (median 1011·9 16S rRNA gene copies per gram faeces) when compared to PK+M+B EM (median 109·8 16S rRNA gene copies per gram faeces; P < 0·001) and PK QIA (median 1010·3 16S rRNA gene copies per gram faeces; P < 0·050). PK QIA also had lower total bacterial 16S rRNA gene copy number than B PL (median 1011·4 16S rRNA gene copies per gram faeces) (Fig. 3e, Table S3). For all assays, the inter‐run variability of all three qPCRs was lower than 20% (Table S6).
Figure 3.
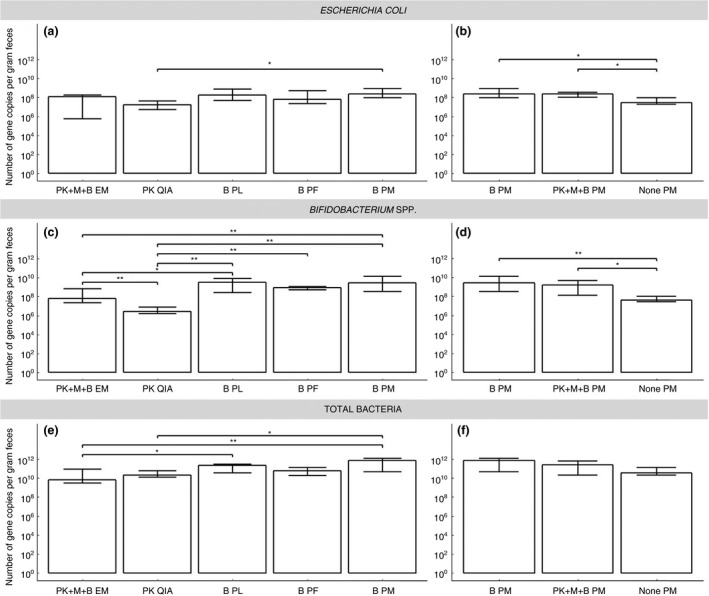
Escherichia coli, Bifidobacterium spp. and total bacterial gene copies per gram faeces. (a, c and e) the five DNA extraction assays, and (b, d and f) the three different pre‐treatment methods for the PowerMicrobiome assay. Data presented as median with 95% CI; EM: Semi‐automated NucliSens easyMag; QIA: QIAamp DNA Stool mini kit; PL: PureLink Microbiome DNA Purification kit; PF: QIAamp PowerFecal DNA kit; PM: RNeasy PowerMicrobiome kit; PK: Proteinase K pre‐treatment; M: mutanolysin pre‐treatment; B: bead beating pre‐treatment; *P < 0·050; **P ≤ 0·001.
B PM was used for the further comparisons because of the high DNA yield and the strong qPCR signal for E. coli, Bifidobacterium spp. and for total bacterial gene copies.
Comparison of three pre‐treatments in combination with the RNeasy PowerMicrobiome kit
To assess what part of the B PM procedure was most essential to explain the efficiency of this approach, we compared the yield and quality of DNA obtained from faecal samples by means of this procedure, with that of PM without pre‐treatment (None PM) and with that of PM preceded by proteinase K, mutanolysin and bead beating (PK+M+B PM).
Yield and quality of the DNA extracted with the RNeasy PowerMicrobiome kit
Also on these extracts, due to a DNA ratio of about 2·00 indicating the presence of RNA, an RNase treatment was performed. For the DNA concentrations obtained with the Nanodrop, no significant differences were found between the three PM procedures. Although the DNA concentration of B PM (median 60·0 µg g−1 faeces) and PK+M+B PM (median 28·0 µg g−1 faeces) were observably higher than PM without pre‐treatment (median 5·5 µg g−1 faeces) (Fig. 2b, Table S3). However, for the Qubit DNA concentrations, a significant higher DNA concentration was found for B PM (median 16·0 µg g−1 faeces) compared to PM without pre‐treatment (median 1·4 µg g−1 faeces) (Fig. 2d, Table S3). No pre‐treatments showed an inter‐run variability less than 20%, for all three volunteers, based on Nanodrop or Qubit DNA levels (Table S4).
These results indicate that the bead beating pre‐treatment results in a higher yield of DNA than PM without pre‐treatment, and that the treatment with proteinase K and mutanolysin do not further increase the additional yield that is obtained by bead beating alone.
qPCR results of bacterial DNA
For E. coli as well as for Bifidobacterium spp., higher values were observed for B PM (median 108·4 uidA gene copies per gram faeces and 109·4 16S rRNA gene copies per gram faeces, respectively) and PK+M+B PM (median 108·4 uidA gene copies per gram faeces and 109·2 16S rRNA gene copies per gram faeces, respectively) using qPCR, suggesting significantly better DNA extraction for the pre‐treatments with mechanical lysis (B) and the combination of mechanical and enzymatic lysis (PK+M+B), compared to no lysis pre‐treatment (median 107·5 uidA gene copies per gram faeces and 107·6 16S rRNA gene copies per gram faeces, respectively) (all P < 0·050) (Fig. 3b,d, Table S3). The pre‐treatments including mechanical lysis (B and PK+M+B) were equally efficient for lysis of Gram‐positive and Gram‐negative bacterial species. For the total number of bacterial 16S rRNA gene copies, no differences were found between the three PM procedures (none PM: median 1010·6 16S rRNA gene copies per gram faeces; B PM: median 1011·9 16S rRNA gene copies per gram faeces; PK+M+B PM: median 1011·4 16S rRNA gene copies per gram faeces) (Fig. 3f, Table S3). For none of the three pre‐treatments, the inter‐run variability for all three qPCRs was higher than 20% (Table S6).
When comparing the qPCR results for the three pre‐treatments with PM, used for the analyses, with the initial DNA extractions without RNase treatment, significantly lower levels of E. coli and Bifidobacterium spp. were found for all three pre‐treatments with RNase treatment. In addition, the total number of bacterial 16S rRNA gene copies was significantly lower for B PM and PK+M+B compared to the samples without RNase treatment. Same results were found for the E. coli and Bifidobacterium spp. levels for QIA with and without RNase treatment (Fig. S5). Using an RNase treatment followed by a DNA purification step significantly decreased the gene copy number of Gram‐positive as well as Gram‐negative cells that could be detected by qPCR.
Based on all the obtained results, PM with the bead beating pre‐treatment (B PM) was selected as the most appropriate procedure to extract DNA from bacterial cells in faecal samples for downstream qPCR.
qPCR inhibition
For the different pre‐treatments and assays, no significant differences in calculated numbers of gene copies were observed between the undiluted and 10‐fold‐diluted DNA extracts (P > 0·050). Thus, the qPCR values of the undiluted extracts were used for further analyses (data not shown).
Correlation between total DNA and 16S rRNA gene copy number
The total bacterial 16S rRNA gene copies as determined by qPCR correlated well with the total yield of DNA extracted, as determined by Nanodrop and Qubit, for the five DNA extraction assays (Nanodrop: n = 30, rs = 0·774; Qubit: n = 29, rs = 0·884; all P < 0·001), the three PM procedures (Nanodrop: n = 17, rs = 0·949; Qubit: n = 17, rs = 0·895; all P < 0·001), and all the DNA extraction assays (Nanodrop: n = 41, rs = 0·817; Qubit: n = 40, rs = 0·897; all P < 0·001) (Fig. S6). This positive correlation indicates that the variation in efficiency of DNA extraction between the procedures, as observed in this study, is rather due to differences in DNA quantity than in DNA quality.
Discussion
This study evaluated to what extent different assays for the extraction of DNA from faecal samples yielded high‐quality bacterial DNA for downstream qPCR purposes. The DNA quality and quantity as determined by Nanodrop and Qubit, and the abundance of two gut bacterial taxa and overall bacteria as determined by qPCR were used as screening criteria to select the method with the highest yield. To evaluate the effect of the different pre‐treatments and assays on the lysis of different bacterial cell walls, qPCRs for the Gram‐positive Bifidobacterium spp. as well as for the Gram‐negative Escherichia coli were performed, next to qPCR with universal bacterial primers, enumerating the total bacterial 16S rRNA gene copies.
To our knowledge, this is the first study to assess the DNA extraction efficacy of the PureLink Microbiome DNA Purification kit (PL) and the RNeasy PowerMicrobiome kit (PM). These two assays were compared with the QIAamp PowerFecal DNA kit (PF) (Nechvatal et al. 2008; Kumar et al. 2016), the QIAamp DNA Stool Mini kit (QIA) (Holland et al. 2000; McOrist et al. 2002; Li et al. 2003; Yu and Morrison 2004; Nechvatal et al. 2008; Ariefdjohan et al. 2010; Nylund et al. 2010; Salonen et al. 2010; Persson et al. 2011; Smith et al. 2011; Yuan et al. 2012; Henderson et al. 2013; Mirsepasi et al. 2014; Kumar et al. 2016; Costea et al. 2017) and the semi‐automated NucliSens easyMag (EM) (Nylund et al. 2010; Persson et al. 2011; Andersen et al. 2013; Mirsepasi et al. 2014; Costea et al. 2017). The latter extraction assay was combined with four different pre‐treatments of which the use of bead beating substantially increased the DNA yield and also the lysis of Gram‐positive bacterial cells. Also the PL, the PF and the PM assays, which use bead beating combined with a lysis buffer as a lysis method, resulted in substantially stronger qPCR results for the Gram positives compared to the QIA assay, which uses only chemical lysis (McOrist et al. 2002). Several other studies also showed the importance of bead beating to enhance the lysis of Gram positives and which subsequently obtained higher concentrations of DNA (Ariefdjohan et al. 2010; Smith et al. 2011; Yuan et al. 2012; Costea et al. 2017). The combination PK+M+B EM did not result in a higher concentration of DNA or higher gene copy number for Gram‐negative E. coli, when compared with the other assays. However, a lower 16S rRNA gene copy number of Gram‐positive Bifidobacterium spp. and total bacteria was observed compared with the PL and PM assays. This indicates that EM is a less appropriate method to obtain high yields of (bacterial) DNA from human faecal samples compared to the PL and PM assays. However, Nylund et al. (2010) used repetitive bead beating prior to EM and found better A 260 nm/A 280 nm ratio values. In addition Mirsepasi et al. (2014) recovered with the Nanodrop a fourfold higher mean DNA concentration in comparison with our results, without taking into account for the interpatient variability of the bacterial composition in human faecal samples, while a threefold higher DNA concentration was found in our study for EM combined with mechanical pre‐treatment compared to Mirsepasi et al. (2014). On the other hand, Nylund et al. (2010), using the EM assay, obtained similar total bacterial 16S rRNA gene copies, based on universal qPCR, when compared to our EM results, whatever pre‐treatment. It is important to notice that the DNA levels obtained with the Nanodrop are overestimated, which is assumed by the comparison with the Qubit DNA levels, which only measures dsDNA. This overestimation of DNA with the Nanodrop can be explained by the determination of also RNA, single‐stranded oligonucleotides and dNTPs for PK+M+B EM, B PL and B PF, and loss of DNA due to column purification after RNase treatment for PK QIA and B PM (as was also observed with qPCR).
Several studies reported QIA as the best assay to extract DNA for further PCR or qPCR applications, with the highest sensitivity for Gram‐negative bacterial species (Holland et al. 2000; McOrist et al. 2002; Salonen et al. 2010). In our study, we found only a significant difference for Gram‐negatives between the QIA assay and the PM assay. This indicates that mechanical lysis can disrupt more Gram‐negative bacteria, even with the absence of a thick peptidoglycan cell wall in these bacteria.
The highest DNA yield, determined with Nanodrop as well as with Qubit, was obtained by PM using the bead beating pre‐treatment. For all three PM procedures (B; PK+M+B and no pre‐treatment), considerably lower gene copy numbers of E. coli and Bifidobacterium spp. were found compared to the same procedures without RNase treatment. This was also the case for the QIA assay, for which we performed an additional RNase treatment. This result might be explained by the loss of a certain amount of DNA because the RNase treatment is followed by a DNA purification step. This indicates that RNase treatment in combination with DNA purification is not necessary and even deleterious to obtain better qPCR results. The B PM and the PK+M+B PM procedures resulted in the most efficient amplification of bacterial DNA, according to both species‐specific qPCR assays, even compared with other studies (Nylund et al. 2010; Salonen et al. 2010). This also applies for the DNA yield, determined with Nanodrop as well as with Qubit (Nechvatal et al. 2008; Mirsepasi et al. 2014; Kumar et al. 2016). Yuan et al. (2012) also established that 16S rRNA gene sequencing on DNA, obtained with extraction methods using bead beating and/or mutanolysin, resulted in a better representation of bacterial community structure. Thus, B PM and PK+M+B PM are the most appropriate procedures to extract DNA for downstream qPCR, with the highest qPCR values obtained by the B PM procedure.
Previously, studies noted the presence of PCR inhibitors in faecal extracts, which can interfere with the qPCR reaction, decreasing its efficacy, as such leading to an underestimation of the quantity of the target species (Nechvatal et al. 2008; Mirsepasi et al. 2014). These inhibitory compounds are derived from the complex composition of faecal material, for example, bile salts, bilirubins and complex polysaccharides (Monteiro et al. 1997; McOrist et al. 2002; Nechvatal et al. 2008; Persson et al. 2011). Although dilution of at least 10 times is recommended for molecular diagnostics (Mirsepasi et al. 2014), no inhibitory effects were found in our experiment because the qPCR results of the non‐diluted and 10‐fold diluted extracts were not significantly different and were in the same order of magnitude. These findings correspond with the studies of Holland et al. (2000) and Salonen et al. (2010).
Our study had some possible shortcomings. First, we did not homogenize the faecal samples before dividing into aliquots, which might have caused inter‐aliquot, intra‐sample variation for each volunteer. However, our data indicate that this is not a problem, since only 20·0 and 38·9% of the samples show an intra‐sample variability of more than 25% for the Nanodrop and the Qubit, respectively. And for the E. coli, Bifidobacterium spp. and total number of 16S rRNA gene copies qPCRs, respectively, only 0, 0 and 2·6%. Moreover, data on the importance of homogenization of samples are conflicting (Swidsinski et al. 2008; Salonen et al. 2010; Wesolowska‐Andersen et al. 2014). We therefore used the mean values of the aliquots per individual and per extraction assay for further analysis, to correct for possible intra‐sample variability. Second, the mechanical lysis was performed by vortexing with beat beads, as recommended by the manufacturer, although repetitive bead beating or the use of a tissue lyser has been shown to result in higher DNA yields (Ariefdjohan et al. 2010; Salonen et al. 2010; Smith et al. 2011; Yuan et al. 2012).
In conclusion, this study confirms the importance of comparing DNA extraction methods for the purpose of quantification by means of qPCR of bacteria in faecal samples. To obtain high‐quality DNA for downstream qPCR purposes, the PowerMicrobiome assay, that is, including bead beating, was found to yield high‐quality DNA and the highest numbers of Gram‐positive, Gram‐negative and total bacterial cells compared to the other assays examined. Bead beating was shown to be necessary to increase yield as well as to be sufficient as no further improvement was obtained when adding protease and mutanolysin treatment.
Conflict of Interest
The authors declare no competing interests.
Supporting information
Figure S1. Protocol overview of four different pre‐treatments, followed by DNA extraction with the NucliSens easyMag.
Figure S2. Protocol overview of the five DNA extraction assays.
Figure S3. Protocol overview of three different pre‐treatments, followed by DNA extraction with the RNeasy PowerMicrobiome kit.
Figure S4. Comparison of four pre‐treatments combined with NucliSens easyMag.
Figure S5. qPCR results on DNA extracts with and without RNase treatment.
Figure S6. The spearman correlation between total bacterial cell 16S rRNA gene copies and total DNA, as determined by Nanodrop and Qubit.
Table S1. Specificity of the Escherichia coli, Bifidobacterium spp. and total bacterial gene copies qPCR.
Table S2. qPCR mixes and thermal cycling conditions.
Table S3. DNA quality and yield for all extraction assays, as determined by Nanodrop, Qubit and qPCR for E. coli, Bifidobacterium spp. and total bacterial gene copies.
Table S4. Nucleic acid extraction yield and quality of the different extraction assays for three volunteers, as determined by Nanodrop and Qubit.
Table S5. Measurement of dNTP mix with Nanodrop and Qubit.
Table S6. qPCR for E. coli, Bifidobacterium spp. and total bacterial gene copies.
Acknowledgements
T.G., the first author, is supported by The Research Foundation Flanders (FWO Vlaanderen) grant no. G017815N. The authors also thank Pascaline‐Marie Bonne Maholo for the assistance in the lab.
References
- Andersen, L.O. , Roser, D. , Nejsum, P. , Nielsen, H.V. and Stensvold, C.R. (2013) Is supplementary bead beating for DNA extraction from nematode eggs by use of the NucliSENS easyMag protocol necessary? J Clin Microbiol 51, 1345–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, K.L. and Lebepe‐Mazur, S. (2003) Comparison of rapid methods for the extraction of bacterial DNA from colonic and caecal lumen contents of the pig. J Appl Microbiol 94, 988–993. [DOI] [PubMed] [Google Scholar]
- Ariefdjohan, M.W. , Savaiano, D.A. and Nakatsu, C.H. (2010) Comparison of DNA extraction kits for PCR‐DGGE analysis of human intestinal microbial communities from fecal specimens. Nutr J 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg, L. and Tyson, G.W. (2014) Metagenomics using next‐generation sequencing. Methods Mol Biol 1096, 183–201. [DOI] [PubMed] [Google Scholar]
- Chern, E.C. , Siefring, S. , Paar, J. , Doolittle, M. and Haugland, R.A. (2011) Comparison of quantitative PCR assays for Escherichia coli targeting ribosomal RNA and single copy genes. Lett Appl Microbiol 52, 298–306. [DOI] [PubMed] [Google Scholar]
- Costea, P.I. , Zeller, G. , Sunagawa, S. , Pelletier, E. , Alberti, A. , Levenez, F. , Tramontano, M. , Driessen, M. et al (2017) Towards standards for human fecal sample processing in metagenomic studies. Nat Biotechnol 35, 1069–1076. [DOI] [PubMed] [Google Scholar]
- Dunne, J.L. , Triplett, E.W. , Gevers, D. , Xavier, R. , Insel, R. , Danska, J. and Atkinson, M.A. (2014) The intestinal microbiome in type 1 diabetes. Clin Exp Immunol 177, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg, P.B. , Bik, E.M. , Bernstein, C.N. , Purdom, E. , Dethlefsen, L. , Sargent, M. , Gill, S.R. , Nelson, K.E. et al (2005) Diversity of the human intestinal microbial flora. Science 308, 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aila, N.A. , Tency, I. , Saerens, B. , De Backer, E. , Cools, P. , dos Santos Santiago, G.L. , Verstraelen, H. , Verhelst, R. et al (2011) Strong correspondence in bacterial loads between the vagina and rectum of pregnant women. Res Microbiol 162, 506–513. [DOI] [PubMed] [Google Scholar]
- Falony, G. , Joossens, M. , Vieira‐Silva, S. , Wang, J. , Darzi, Y. , Faust, K. , Kurilshikov, A. , Bonder, M.J. et al (2016) Population‐level analysis of gut microbiome variation. Science 352, 560–564. [DOI] [PubMed] [Google Scholar]
- Flint, H.J. , Scott, K.P. , Louis, P. and Duncan, S.H. (2012) The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 9, 577–589. [DOI] [PubMed] [Google Scholar]
- Francescone, R. , Hou, V. and Grivennikov, S.I. (2014) Microbiome, inflammation, and cancer. Cancer J 20, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, D.N. , St Amand, A.L. , Feldman, R.A. , Boedeker, E.C. , Harpaz, N. and Pace, N.R. (2007) Molecular‐phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104, 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauther, K.A. , Cobaugh, K.L. , Jantz, L.M. , Sparer, T.E. and DeBruyn, J.M. (2015) Estimating time since death from postmortem human gut microbial communities. J Forensic Sci 60, 1234–1240. [DOI] [PubMed] [Google Scholar]
- Henderson, G. , Cox, F. , Kittelmann, S. , Miri, V.H. , Zethof, M. , Noel, S.J. , Waghorn, G.C. and Janssen, P.H. (2013) Effect of DNA extraction methods and sampling techniques on the apparent structure of cow and sheep rumen microbial communities. PLoS ONE 8, e74787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, J.L. , Louie, L. , Simor, A.E. and Louie, M. (2000) PCR detection of Escherichia coli O157:H7 directly from stools: evaluation of commercial extraction methods for purifying fecal DNA. J Clin Microbiol 38, 4108–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joossens, M. , Faust, K. , Gryp, T. , Nguyen, A.T.L. , Wang, J. , Eloot, S. , Schepers, E. , Dhondt, A. et al (2019) Gut microbiota dynamics and uraemic toxins: one size does not fit all. Gut 68, 2257–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, J. , Kumar, M. , Gupta, S. , Ahmed, V. , Bhambi, M. , Pandey, R. and Chauhan, N.S. (2016) An improved methodology to overcome key issues in human fecal metagenomic DNA extraction. Genom Proteom Bioinform 14, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley, R.E. , Turnbaugh, P.J. , Klein, S. and Gordon, J.I. (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023. [DOI] [PubMed] [Google Scholar]
- Li, M. , Gong, J. , Cottrill, M. , Yu, H. , de Lange, C. , Burton, J. and Topp, E. (2003) Evaluation of QIAamp DNA Stool Mini Kit for ecological studies of gut microbiota. J Microbiol Methods 54, 13–20. [DOI] [PubMed] [Google Scholar]
- Maukonen, J. , Simoes, C. and Saarela, M. (2012) The currently used commercial DNA‐extraction methods give different results of clostridial and actinobacterial populations derived from human fecal samples. FEMS Microbiol Ecol 79, 697–708. [DOI] [PubMed] [Google Scholar]
- McOrist, A.L. , Jackson, M. and Bird, A.R. (2002) A comparison of five methods for extraction of bacterial DNA from human faecal samples. J Microbiol Methods 50, 131–139. [DOI] [PubMed] [Google Scholar]
- Mirsepasi, H. , Persson, S. , Struve, C. , Andersen, L.O. , Petersen, A.M. and Krogfelt, K.A. (2014) Microbial diversity in fecal samples depends on DNA extraction method: easyMag DNA extraction compared to QIAamp DNA stool mini kit extraction. BMC Res Notes 7, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro, L. , Bonnemaison, D. , Vekris, A. , Petry, K.G. , Bonnet, J. , Vidal, R. , Cabrita, J. and Megraud, F. (1997) Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J Clin Microbiol 35, 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechvatal, J.M. , Ram, J.L. , Basson, M.D. , Namprachan, P. , Niec, S.R. , Badsha, K.Z. , Matherly, L.H. , Majumdar, A.P. et al (2008) Fecal collection, ambient preservation, and DNA extraction for PCR amplification of bacterial and human markers from human feces. J Microbiol Methods 72, 124–132. [DOI] [PubMed] [Google Scholar]
- Nylund, L. , Heilig, H.G. , Salminen, S. , de Vos, W.M. and Satokari, R. (2010) Semi‐automated extraction of microbial DNA from feces for qPCR and phylogenetic microarray analysis. J Microbiol Methods 83, 231–235. [DOI] [PubMed] [Google Scholar]
- Persson, S. , de Boer, R.F. , Kooistra‐Smid, A.M. and Olsen, K.E. (2011) Five commercial DNA extraction systems tested and compared on a stool sample collection. Diagn Microbiol Infect Dis 69, 240–244. [DOI] [PubMed] [Google Scholar]
- Qin, J. , Li, Y. , Cai, Z. , Li, S. , Zhu, J. , Zhang, F. , Liang, S. , Zhang, W. et al (2012) A metagenome‐wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60. [DOI] [PubMed] [Google Scholar]
- Rajendhran, J. , Shankar, M. , Dinakaran, V. , Rathinavel, A. and Gunasekaran, P. (2013) Contrasting circulating microbiome in cardiovascular disease patients and healthy individuals. Int J Cardiol 168, 5118–5120. [DOI] [PubMed] [Google Scholar]
- Ramezani, A. and Raj, D.S. (2014) The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25, 657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen, A. , Nikkila, J. , Jalanka‐Tuovinen, J. , Immonen, O. , Rajilic‐Stojanovic, M. , Kekkonen, R.A. , Palva, A. and de Vos, W.M. (2010) Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J Microbiol Methods 81, 127–134. [DOI] [PubMed] [Google Scholar]
- Smith, B. , Li, N. , Andersen, A.S. , Slotved, H.C. and Krogfelt, K.A. (2011) Optimising bacterial DNA extraction from faecal samples: comparison of three methods. Open Microbiol J 5, 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski, A. , Loening‐Baucke, V. , Verstraelen, H. , Osowska, S. and Doerffel, Y. (2008) Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology 135, 568–579. [DOI] [PubMed] [Google Scholar]
- Vaneechoutte, M. , Claeys, G. , Steyaert, S. , De Baere, T. , Peleman, R. and Verschraegen, G. (2000) Isolation of Moraxella canis from an ulcerated metastatic lymph node. J Clin Microbiol 38, 3870–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartoukian, S.R. , Palmer, R.M. and Wade, W.G. (2010) Strategies for culture of 'unculturable' bacteria. FEMS Microbiol Lett 309, 1–7. [DOI] [PubMed] [Google Scholar]
- Vuong, H.E. , Yano, J.M. , Fung, T.C. and Hsiao, E.Y. (2017) The microbiome and host behavior. Annu Rev Neurosci 40, 21–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowska‐Andersen, A. , Bahl, M.I. , Carvalho, V. , Kristiansen, K. , Sicheritz‐Ponten, T. , Gupta, R. and Licht, T.R. (2014) Choice of bacterial DNA extraction method from fecal material influences community structure as evaluated by metagenomic analysis. Microbiome 2, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Z. and Morrison, M. (2004) Improved extraction of PCR‐quality community DNA from digesta and fecal samples. Biotechniques 36, 808–812. [DOI] [PubMed] [Google Scholar]
- Yuan, S. , Cohen, D.B. , Ravel, J. , Abdo, Z. and Forney, L.J. (2012) Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS ONE 7, e33865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Protocol overview of four different pre‐treatments, followed by DNA extraction with the NucliSens easyMag.
Figure S2. Protocol overview of the five DNA extraction assays.
Figure S3. Protocol overview of three different pre‐treatments, followed by DNA extraction with the RNeasy PowerMicrobiome kit.
Figure S4. Comparison of four pre‐treatments combined with NucliSens easyMag.
Figure S5. qPCR results on DNA extracts with and without RNase treatment.
Figure S6. The spearman correlation between total bacterial cell 16S rRNA gene copies and total DNA, as determined by Nanodrop and Qubit.
Table S1. Specificity of the Escherichia coli, Bifidobacterium spp. and total bacterial gene copies qPCR.
Table S2. qPCR mixes and thermal cycling conditions.
Table S3. DNA quality and yield for all extraction assays, as determined by Nanodrop, Qubit and qPCR for E. coli, Bifidobacterium spp. and total bacterial gene copies.
Table S4. Nucleic acid extraction yield and quality of the different extraction assays for three volunteers, as determined by Nanodrop and Qubit.
Table S5. Measurement of dNTP mix with Nanodrop and Qubit.
Table S6. qPCR for E. coli, Bifidobacterium spp. and total bacterial gene copies.


