Abstract
Aims
To characterize the epidemiology and treatment patterns of adult men (≥40 years) diagnosed with, or treated for, overactive bladder (OAB) and/or benign prostatic hyperplasia (BPH).
Methods
This retrospective observational study used data extracted from the IBM MarketScan Commercial Claims and Encounters database and the Medicare Supplemental Coordination of Benefits database. Men with BPH and/or OAB were identified and observed to assess treatment and diagnostic patterns.
Results
Within the entire study sample (N = 462 400), BPH diagnosis (61.5%) and BPH treatment (73.7%) were more common than the corresponding values for OAB (25.8% and 7.0%, respectively). Notably, among diagnosed individuals, the dispensation of a corresponding treatment was more likely in individuals diagnosed with BPH (183 672 out of 284 416 = 64.6%) compared with OAB (16 468 out of 119 236 = 13.8%). Among newly diagnosed and/or treated patients (n = 196 576), only 60.3% received treatment. Among treated patients, most experienced only a single type of treatment (93.4%), 6.6% went on to receive a secondary treatment and 3.5% a tertiary. The most common primary treatment was alpha‐blocker monotherapy (76.9%) followed by tadalafil monotherapy (16.4%). Among those untreated at first diagnosis, the median time between diagnosis and treatment initiation was 128 days.
Conclusions
Diagnosis and management of OAB among males are challenging given the inherent overlap in symptoms observed with BPH. Unsurprisingly, we found that BPH is diagnosed and treated more frequently than OAB; but the differences between diagnosis and treatment patterns for the two conditions highlight the potential undertreatment of OAB and misdirection of therapy for men with a combination of voiding and storage symptoms.
Keywords: benign prostatic hyperplasia, epidemiology, lower urinary tract symptoms, overactive bladder
1. INTRODUCTION
Lower urinary tract symptoms (LUTS), an umbrella term for a constellation of urinary storage and voiding problems, is prevalent among men, increases in frequency with age, and is associated with a decrease in quality of life. 1 , 2 , 3 Previously, LUTS in men was thought to be primarily a result of prostate obstruction, given that the age‐related increase in the prevalence of both LUTS and benign prostatic hyperplasia (BPH) occurs in tandem. 4 Historically, both irritative and obstructive symptoms observed in men were attributed to BPH; however, it is now recognized that the irritative symptoms are due to overactive bladder (OAB), a condition that is defined as urinary urgency that occurs with or without incontinence. 5 Furthermore, while OAB was previously thought to predominately affect females, epidemiologic studies indicate that the overall prevalence is equal between both sexes (16.0% and 16.9% among adult men and women, respectively). 6 , 7 , 8 Despite this, participants in clinical studies of OAB are disproportionately female, 9 which may limit the generalizability of findings. 9
The diagnosis of LUTS etiology in men is complicated by the frequent concurrence of storage and voiding problems, which makes it difficult to discern BPH from OAB. 3 , 10 While BPH is predominately associated with voiding problems, including urinary hesitancy and a poor and/or intermittent stream, storage problems that are the hallmark of OAB, including frequent urination, urgency, nocturia, and the sensation of incomplete bladder emptying, may also occur. 4 Therefore, it is likely that some cases of LUTS due to OAB may be attributed incorrectly to BPH and vice versa. 11 However, the etiology is often not distinguished in the clinical setting and a more broad diagnosis of LUTS is given.
Failure to determine to what degree prostate pathology, bladder dysfunction, or both contribute to LUTS in men can have important implications for treatment outcomes. 10 When BPH is suspected, alpha‐blockers such as tamsulosin hydrochloride, often are used as a primary therapy for LUTS. 12 However, these therapies may fail to alleviate OAB‐induced storage symptoms. 1 Furthermore, treatment uptake for LUTS is low, particularly when OAB is suspected; only an estimated 19% of men with OAB are prescribed medications compared with 60% of men with BPH. 11 This could be due in part to an underappreciation of OAB in men, particularly given the higher prevalence of BPH (>50% of men aged 60 and older are affected), 13 as well as the disproportionate use of BPH therapies when storage symptoms are predominant.
Previous studies have assessed the real‐world diagnosis and treatment patterns associated with BPH and OAB in men. 6 , 11 , 14 , 15 However, no attempts have been made to characterize a population of males with LUTS secondary to BPH and/or OAB, both in terms of observed diagnoses and subsequent treatment sequencing. This perspective would allow for a better understanding of how men with LUTS are diagnosed and treated in a real‐world setting, particularly given that most men present with both storage and voiding symptoms. Therefore, the overarching objective of this study was to characterize the epidemiology and treatment patterns of adult men (aged 40 years and older) diagnosed with, or treated for, BPH and/or OAB (collectively referred to as LUTS). The specific aims included: (a) to characterize patterns of treatment and diagnoses among men with LUTS and (b) to summarize baseline clinical and demographic characteristics of men with LUTS.
2. METHODS
2.1. Databases
This retrospective observational study used data extracted from the IBM MarketScan Commercial Claims and Encounters database (Commercial) and the Medicare Supplemental Coordination of Benefits (Medicare supplemental) database from 2012 until the end of 2017. The Commercial database contains longitudinal medical and drug information, including paid amounts, for several million individuals (including spouses and dependents) across multiple employer‐sponsored private health insurance plans. The Medicare supplemental database contains similar information for seniors with Medicare supplemental insurance through employers and includes approximately three million individuals annually.
2.2. Study design
Eligibility was determined during the first 24 months of the 2013 to 2017 study period. To meet the inclusion criteria, individuals were required to have at least one inpatient code, and/or two outpatient codes and/or one medication claim(s) specific to OAB and/or BPH (Supporting Information Tables A1 and A2). Individuals were excluded if they had a record of any of the following during the study period: neurogenic bladder/neurogenic detrusor overactivity, Parkinson's disease, multiple sclerosis, spinal cord injury, malignant neoplasm, renal impairment, hepatic insufficiency, trauma, or organ transplantation based on diagnosis codes. The date of the first OAB‐ or BPH‐related International Classification of Disease Version 9 diagnosis codes (ICD‐9) and/or fill for an OAB‐ or BPH‐specific medication was defined as the index date.
The overall LUTS cohort of men was identified using previously used ICD‐9 and/or medication claims for OAB and BPH. ICD‐9 codes for OAB included 788.3, 788.31, 788.33, 788.37, 788.41, 788.43, 788.63, and 788.91 16 , 17 , 18 , 19 ; medications for OAB included darifenacin, fesoterodine, oxybutynin, solifenacin, tolterodine, trospium, and mirabegron. ICD‐9 codes for BPH included 596.0, 600, 600.0×, 600.1, 600.1×, 600.2, 600.2×, 600.3, 600.9, and 600.9× 11 , 20 ; medications for BPH included terazosin, doxazosin, tamsulosin, alfuzosin, silodosin, dutasteride. and daily tadalafil. Given that ICD‐10 codes were introduced into the data set in 2015, and the identification period was between 2013 and 2014, they were not used to identifying individuals with these conditions.
While the overall sample included men from all stages of LUTS management, two subcohorts were identified to assess the study objectives from the perspective of individuals who were newly treated and/or diagnosed. These cohorts included individuals who were treatment naïve (“treatment‐patterns cohort”) and/or newly diagnosed (“new‐LUTS cohort”). Individuals who received pharmacotherapy during the identification or follow‐up period but had no record of therapy during the 12‐month preindex (baseline) period were assigned to the treatment‐patterns cohort; likewise, patients who had no OAB‐ or BPH‐related diagnosis or treatment codes in the baseline period and at least 12 months of post‐index follow‐up available, were assigned to the new‐LUTS cohort. Although these two cohorts were not mutually exclusive and could, therefore, have overlapping membership, they each enabled a distinct assessment of the data that addresses: (a) treatment patterns following initial treatment (treatment‐patterns cohort) and (b) treatment patterns following initial diagnosis (new‐LUTS cohort).
2.3. Outcomes and analyses
All cohorts (overall LUTS, LUTS with ≥12 months follow‐up post‐index, treatment‐patterns, and new‐LUTS) were characterized based on the demographic data, including age and race, and clinical data, such as the Elixhauser comorbidity score, 21 as well as individual comorbidities. To estimate disease prevalence, the overall LUTS cohort was divided by the total number of men 40 years and older on 1 January 2013 in MarketScan who were observed at any point in time during the identification period (2013‐2014). The observed prevalence was subsequently applied to US Census data (2010) to obtain an age‐standardized estimate. 22
Treatment patterns were characterized by pharmacotherapies at the class level, so that within‐class treatment switches were considered part of the same primary, secondary, or tertiary therapy. Treatments examined included antimuscarinics, alpha‐blockers, tadalafil, the beta‐3 receptor agonist mirabegron, and 5‐alpha reductase inhibitors. Dose changes were not assessed. The following procedures were also included in the characterization of treatment patterns: onabotulinumtoxinA injection, sacral nerve stimulation, percutaneous tibial nerve stimulation, and BPH surgery. In addition to tabulated data, Sankey charts were used to visualize treatment sequencing. Originally developed as a means to visualize the flow of energy in various networks, Sankey charts are beginning to be used as a tool to graphically represent the complexity of treatment patterns, particularly within oncology. 23
All outcome variables were summarized by means, SDs, medians, and interquartile ranges (IQRs) for continuous variables and by numbers and percentages for categorical variables.
3. RESULTS
The overall LUTS cohort included 462 400 individuals. Of these, 326 994 had at least 12 months of available follow‐up post‐index. The new‐LUTS cohort included 196 576 individuals, while the treatment‐patterns cohort included 128 951 individuals (118 591 individuals were in both cohorts). Table 1 shows the demographic and clinical characteristics of the four cohorts. Incident patient cohorts (treatment‐patterns and new‐LUTS) were slightly younger than the overall LUTS cohort (58.0 vs 61.3 years, respectively) and had lower frequencies of almost all comorbidities evaluated, with the exception of depression (7.2% in both the treatment‐patterns and overall LUTS cohorts) and obesity (5.5%: treatment‐patterns vs 5.4%: overall LUTS). The age‐standardized prevalence of LUTS was estimated at 12.2% and is generalizable to a population of commercially insured men aged 40 and older.
Table 1.
Demographic and clinical characteristics
| Characteristic | LUTS (N = 462 400), n (%) | LUTS with 12‐mo post‐index (N = 326 994), n (%) | Treatment‐patterns (N = 128 951), n (%) | New‐LUTS (N = 196 576), n (%) |
|---|---|---|---|---|
| Age | ||||
| Median (Q1‐Q3) | 61 (54‐67) | 60 (54‐66) | 57 (51‐63) | 58 (52‐63) |
| Mean (95% CI) | 61.3 (61.3‐61.4) | 60.6 (60.6‐60.7) | 58.0 (57.9‐58.0) | 58.5 (58.5‐58.5) |
| 40‐49 | 60 118 (13.0) | 44 632 (13.6) | 25 890 (20.1) | 34 955 (17.8) |
| 50‐59 | 149 027 (32.2) | 113 667 (34.8) | 50 795 (39.4) | 76 768 (39.1) |
| 60‐69 | 157 590 (34.1) | 107 245 (32.8) | 36 484 (28.3) | 59 541 (30.3) |
| 70‐79 | 64 913 (14.0) | 43 026 (13.2) | 11 380 (8.8) | 18 687 (9.5) |
| 80+ | 30 752 (6.7) | 18 424 (5.6) | 4402 (3.4) | 6625 (3.4) |
| Region of residence | ||||
| Northeast | 93 827 (20.3) | 69 889 (21.4) | 24 130 (18.7) | 43 756 (22.3) |
| North Central | 110 006 (23.8) | 84 850 (25.9) | 32 347 (25.1) | 48 293 (24.6) |
| South | 158 848 (34.4) | 121 871 (37.3) | 52 056 (40.4) | 74 574 (37.9) |
| West | 93 291 (20.2) | 46 811 (14.3) | 19 219 (14.9) | 27 932 (14.2) |
| Unknown | 6428 (1.4) | 3573 (1.1) | 1199 (0.9) | 2021 (1.0) |
| Type of health care plan | ||||
| Commercial | 312 526 (67.6) | 228 586 (69.9) | 101 628 (78.8) | 152 543 (77.6) |
| Medicare (supplemental) | 149 874 (32.4) | 98 408 (30.1) | 27 323 (21.2) | 44 033 (22.4) |
| Elixhauser index score | ||||
| Median (Q1‐Q3) | 2.00 (1.00‐3.00) | 2.00 (1.00‐3.00) | 2.00 (1.00‐3.00) | 2.00 (1.00‐2.00) |
| Mean (95% CI) | 2.10 (2.10‐2.11) | 2.04 (2.03‐2.04) | 2.00 (1.99‐2.01) | 1.97 (1.97‐1.98) |
| Most prevalent Elixhauser index comorbidities | ||||
| Hypertension, uncomplicated | 219 508 (47.5) | 151 935 (46.5) | 55 253 (42.8) | 84 113 (42.8) |
| Diabetes, uncomplicated | 88 038 (19.0) | 60 909 (18.6) | 22 532 (17.5) | 32 639 (16.6) |
| Chronic pulmonary disease | 47 110 (10.2) | 31 368 (9.6) | 11 330 (8.8) | 16 895 (8.6) |
| Cardiac arrhythmias | 45 703 (9.9) | 30 057 (9.2) | 9949 (7.7) | 15 368 (7.8) |
| Depression | 33 191 (7.2) | 21 892 (6.7) | 9343 (7.2) | 12 945 (6.6) |
| Hypothyroidism | 29 970 (6.5) | 20 576 (6.3) | 7506 (5.8) | 11 763 (6.0) |
| Obesity | 25 167 (5.4) | 16 475 (5.0) | 7131 (5.5) | 10 165 (5.2) |
| Peripheral vascular disorders | 24 139 (5.2) | 15 386 (4.7) | 5054 (3.9) | 7837 (4.0) |
| Valvular disease | 24 035 (5.2) | 16 309 (5.0) | 5216 (4.0) | 8630 (4.4) |
| Diabetes, complicated | 20 349 (4.4) | 12 778 (3.9) | 4598 (3.6) | 6652 (3.4) |
| Diagnostic sequencing | ||||
| OAB Dx only | 30 589 (6.6) | 21 847 (6.7) | 8995 (7.0) | 20 093 (10.2) |
| BPH Dx only | 195 769 (42.3) | 145 043 (44.4) | 42 964 (33.3) | 90 657 (46.1) |
| OAB Dx then BPH Dx | 22 450 (4.9) | 18 349 (5.6) | 8203 (6.4) | 7753 (3.9) |
| BPH Dx then OAB Dx | 43 793 (9.5) | 37 986 (11.6) | 12 850 (10.0) | 8495 (4.3) |
| BPH Dx and OAB Dx (on the same day) | 22 404 (4.8) | 16 540 (5.1) | 5926 (4.6) | 8909 (4.5) |
| Never received OAB or BPH diagnosis | 147 395 (31.9) | 87 229 (26.7) | 50 013 (38.8) | 60 669 (30.9) |
| Treatment sequencing | ||||
| OAB Rx only | 12 943 (2.8) | 8272 (2.5) | 4259 (3.3) | 3909 (2.0) |
| BPH Rx only | 321 342 (69.5) | 221 415 (67.7) | 117 887 (91.4) | 102 120 (51.9) |
| OAB Rx then BPH Rx | 4759 (1.0) | 3721 (1.1) | 1396 (1.1) | 535 (0.3) |
| BPH Rx then OAB Rx | 13 050 (2.8) | 10 852 (3.3) | 4998 (3.9) | 2185 (1.1) |
| BPH Rx and OAB Rx (on the same day) | 1722 (0.4) | 1144 (0.3) | 411 (0.3) | 328 (0.2) |
| Never treated for OAB or BPH with Rx | 108 584 (23.5) | 81 590 (25.0) | 0 (0.0) | 87 499 (44.5) |
Abbreviations: BPH, benign prostate hyperplasia; Dx: diagnosis; LUTS, lower urinary tract symptoms; OAB, overactive bladder; Rx: treatment.
3.1. Treatment patterns
Table 1 shows that among the overall LUTS cohort, BPH diagnosis (61.5%) and BPH treatment (ie, medication) (73.7%) were more frequent than the corresponding values for OAB (25.8% and 7.0%, respectively). In Table 2, the co‐occurrence of diagnosis and treatment in the overall LUTS cohort is presented. Overall, a higher percentage of individuals received treatment with BPH medication (73.7% of all LUTS patients [340 873 out of 462 400] and 64.6% of patients with a BPH diagnosis [183 672 out of 284 416]), while a lower percentage of individuals received an OAB medication (7.0% of all LUTS patients [32 474 out of 462 400] and 13.8% of patients with an OAB diagnosis [16 468 out of 119 236]).
Table 2.
Co‐occurrence of OAB and BPH diagnosis and treatment, respectively, in the overall LUTS cohort
| Diagnosis | Prescription | ||||
|---|---|---|---|---|---|
| BPH | OAB | BPH | OAB | N | % |
| ✓ | X | ✓ | X | 120 931 | 26.2 |
| ✓ | X | ✓ | ✓ | 4711 | 1.0 |
| ✓ | X | X | ✓ | 1833 | 0.4 |
| ✓ | X | X | X | 68 294 | 14.8 |
| ✓ | ✓ | ✓ | X | 48 626 | 10.5 |
| ✓ | ✓ | ✓ | ✓ | 9404 | 2.0 |
| ✓ | ✓ | X | ✓ | 2805 | 0.6 |
| ✓ | ✓ | X | X | 27 812 | 6.0 |
| X | ✓ | ✓ | X | 13 852 | 3.0 |
| X | ✓ | ✓ | ✓ | 1640 | 0.4 |
| X | ✓ | X | ✓ | 2619 | 0.6 |
| X | ✓ | X | X | 12 478 | 2.7 |
| X | X | ✓ | X | 137 933 | 29.8 |
| X | X | ✓ | ✓ | 3776 | 0.8 |
| X | X | X | ✓ | 5686 | 1.2 |
| X | X | X | X | … | 0.0 |
Abbreviations: BPH, benign prostate hyperplasia; LUTS, lower urinary tract symptoms; OAB, overactive bladder.
With regard to treatment sequences following incident treatment, the majority of individuals in the treatment‐patterns cohort experienced only one type of treatment (OAB medication or BPH medication, or OAB + BPH medication) ([128 951‐8568]/128 951, or 93.4%) (Figure 1). Among those who received two or more types of treatment, the majority received OAB medication as their secondary treatment (48%), followed by a BPH procedure (24%), BPH medication (17%), and a small proportion moved on to an OAB + BPH combination therapy (10%) (Figure 1). Regarding discontinuations, a higher proportion of men who received alpha‐blockers as their primary treatment for LUTS discontinued the treatment altogether (62.4%), compared with men who received either antimuscarinics (55.5%) or mirabegron (47.2%) (data not shown). Among men who received an OAB medication as primary treatment, the proportion either discontinuing (any treatment for LUTS) or moving onto BPH procedure was less for mirabegron compared with antimuscarinics. It is notable that almost half (46.6%) of the patients who discontinued LUTS treatment after primary treatment with alpha‐blockers never received a BPH or OAB diagnosis. Among those with a diagnosis, 56.3% only had a BPH diagnosis, 13.8% only had an OAB diagnosis, and 29.9% had both a BPH and an OAB diagnosis (data not shown).
Figure 1.
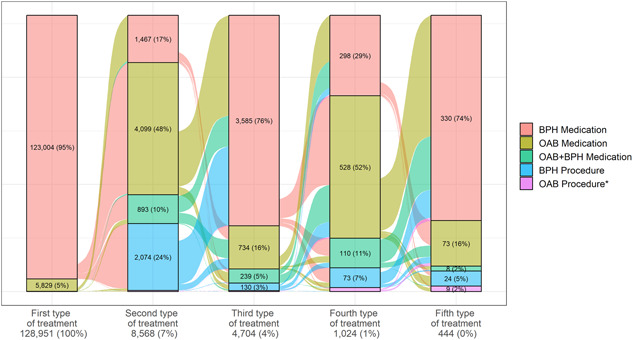
Sankey chart of treatment sequencing among (a) BPH Medications, (b) OAB Medications and (c) OAB+BPH Medications in the Treatment‐Patterns. BPH, benign prostate hyperplasia; OAB, overactive bladder. *OAB procedures include onabotulinumtoxinA injection, sacral nerve stimulation, and percutaneous tibial nerve stimulation
With regard to the assessment of treatment patterns among individuals who did not receive treatment at first diagnosis, the median (IQR: 21‐466) time to initiating treatment among individuals in the new‐LUTS cohort was 128 days (Table 3). The most common primary treatment received in this cohort was alpha‐blocker monotherapy (76.9%) followed by tadalafil monotherapy (16.4%). Among those who initiated a primary therapy, 12.8% went on to receive a secondary therapy and 6.6% a tertiary. Among the new‐LUTS patients who went on to receive a secondary therapy, alpha‐blocker monotherapy was most frequently (26.0%) observed as secondary therapy, followed closely by antimuscarinics (21.0%).
Table 3.
Treatments received by treatment sequence in the new‐LUTS cohort
| Sequence of treatment received | |||
|---|---|---|---|
| Primary (N = 118 591) | Secondary (N = 15 237) | Tertiary (N = 7859) | |
| Time from index until treatment initiation, among those untreated at index (days) a (N = 107 671) | |||
| Median (Q1‐Q3) | 128 (21‐466) | ||
| Mean (95% CI) | 310 (306‐315) | ||
| Therapy | |||
| Antimuscarinics (monotherapy) | 4765 (4.0) | 3204 (21.0) | 709 (9.0) |
| Mirabegron (monotherapy) | 364 (0.3) | 459 (3.0) | 178 (2.3) |
| Multiple OAB | 0 (0) | 5 (0.0) | 26 (0.3) |
| OAB procedures (onabotulinumtoxinA, SNS, PTNS) | 0 (0) | 25 (0.2) | 12 (0.2) |
| Alpha‐blockers (monotherapy) | 91 167 (76.9) | 3967 (26.0) | 4960 (63.1) |
| 5‐Alpha reductase inhibitors (monotherapy) | 2530 (2.1) | 1253 (8.2) | 355 (4.5) |
| Tadalafil 2.5 mg or 5 mg (monotherapy) | 19 424 (16.4) | 2753 (18.1) | 854 (10.9) |
| BPH surgery | 0 (0) | 1552 (10.2) | 211 (2.7) |
| Multiple BPH | 239 (0.2) | 1297 (8.5) | 330 (4.2) |
| OAB + BPH | 102 (0.1) | 722 (4.7) | 224 (2.9) |
Abbreviations: BPH, benign prostate hyperplasia; CI, confidence interval; LUTS, lower urinary tract symptoms; OAB, overactive bladder; PTNS, percutaneous tibial nerve stimulation; Q, quartile; SNS, sacral neuromodulation.
Patients without corresponding line of therapies are excluded.
4. DISCUSSION
To the authors' knowledge, this is the first US study that has characterized a commercially insured population of males with LUTS secondary to OAB and BPH, where individuals were not compartmentalized into either OAB or BPH, but rather described according to observed diagnoses and treatment sequencing. This approach allowed for a better understanding of how males with LUTS are diagnosed and treated according to their suspected underlying condition. Another strength of this study is the use of data from the IBM MarketScan database, which is a large, generalizable US claims data set, well‐suited for addressing the study objectives.
In this study, LUTS was of relatively high prevalence among commercially insured men aged 40 and older. 24 Diagnoses for BPH were more frequent than for OAB, which was also reflected in treatment patterns. While the frequency of OAB diagnoses was notably higher than the frequency of OAB treatment, the reverse was true for BPH diagnoses and treatment. Thus, these data indicate that OAB symptoms in men are potentially undertreated. Additionally, when treatments following initial treatment were examined, a large uptake of OAB‐specific medications/procedures was observed, potentially indicating that symptoms were originally misdiagnosed and treated as BPH rather than OAB. While it is possible that the frequency of OAB treatment was low due to the use of behavior/physical therapies (consistent with current guidelines), OAB has previously been recognized as an underdiagnosed and undertreated condition. 11,25 Furthermore, these findings may highlight a need for physicians to provide more clarification on determining the best treatments for patients presenting with different LUTS symptoms. With respect to treatment patterns, alpha‐blockers were identified as the most frequent primary treatment prescribed in the new‐LUTS cohort, which is also aligned with current guidelines. Also, as alpha‐blockers are better tolerated than anticholinergics, 26 their frequency was not unexpected.
The age‐standardized prevalence estimate reported here (12.2%) falls in the range of other published estimates, which have ranged from 3.5% to 19.0%. 7 , 13 , 24 , 27 , 28 The variation in estimates may be due to a number of reasons. Within studies conducted using administrative and claims data sets, variation in specific LUTS definition and/or study population, and inclusion criteria may result in variability of epidemiological estimates. Secondly, prevalence estimates of OAB and LUTS generated from database studies (which describe populations of patients with treatment‐seeking behavior) have been historically lower than cross‐sectional studies where LUTS are self‐reported. 7 , 14 , 27 , 28 , 29 , 30 , 31 There is evidence that a high proportion of males with LUTS symptoms do not seek medical help 30 , 32 ; these individuals would be captured in a study where the condition is defined based on self‐reported symptoms, but not in a study relying on administrative data. Overall, the prevalence estimated here can be regarded as a more accurate representation of the prevalence of LUTS among commercially insured, treatment‐seeking males in the United States.
An important limitation of the present study is that treatment persistence and adherence were not investigated, which limited the ability to assess treatment switching. In this study, a substantially higher proportion of patients who received an OAB therapy as their primary therapy subsequently switched to a BPH therapy compared with patients who first received a BPH therapy and subsequently switched to an OAB therapy. This is consistent with the trends observed in other studies. 15 Our data may be reflective, in part, of current American Urological Association BPH treatment guidelines that lack clarity when providing recommendations on sequencing or combination therapies for men with mixed symptoms. Furthermore, a recent US study found that treatment persistence was higher among those with BPH relative to those with OAB. 11 Therefore, to better characterize the appropriateness and tolerability of OAB and BPH therapies received, it would be of interest to further consider adherence and persistence to therapies, in addition to overall treatment sequencing.
There are limitations inherent to any retrospective analysis using administrative claims data, which include errors that may influence key outcomes, exposures, and control variables. Administrative claims data are collected for billing rather than research purposes, which therefore introduces the potential for misclassification as coding may be driven by reimbursement (rather than clinical) factors. For example, it is possible that patients who presented for erectile dysfunction were misclassified at LUTS, which may have increased the LUTS cohort. A further limitation of administrative claims data is that given that individuals with intermittent health care coverage may have been included, transitions to subsequent types of therapy in the analysis of treatment patterns may have been missed, although this limitation was not expected to have a relevant impact on the study findings. Administrative claims data are also unable to capture the use of behavioral therapies to manage symptoms. Finally, the study findings are reflective of commercially insured individuals and therefore may not be generalizable to noncommercially insured individuals.
In conclusion, diagnosis and management of LUTS among males is challenging, particularly given the inherent overlap in symptoms of BPH and OAB. The analysis conducted here found that, not surprisingly, BPH is diagnosed and treated more frequently than OAB. However, the differential between diagnosis and treatments for the two conditions highlight the potential undertreatment of OAB in this population and warrants further investigation, particularly as experts have begun to acknowledge the etiological complexity of LUTS in men.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was funded by Astellas Pharma Global Development Inc., medical writing/editorial support was provided by Meagan Harwood, MPH from Broadstreet Health Economics & Outcomes Research and funded by the study sponsor.
Burnett AL, Walker DR, Feng Q, et al. Undertreatment of overactive bladder among men with lower urinary tract symptoms in the United States: A retrospective observational study. Neurourology and Urodynamics. 2020;39:1378–1386. 10.1002/nau.24348
DATA AVAILABILITY STATEMENT
Researchers may request access to anonymized participant‐level data, trial‐level data, and protocols from Astellas‐sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study‐Sponsors/Study‐Sponsors‐Astellas.aspx.
REFERENCES
- 1. Lee SH, Byun SS, Lee SJ, Kim KH, Lee JY. Effects of initial combined tamsulosin and solifenacin therapy for overactive bladder and bladder outlet obstruction secondary to benign prostatic hyperplasia: a prospective, randomized, multicenter study. Int Urol Nephrol. 2014;46(3):523‐529. [DOI] [PubMed] [Google Scholar]
- 2. Maserejian NN, Chen S, Chiu GR, et al. Incidence of lower urinary tract symptoms in a population‐based study of men and women. Urology. 2013;82(3):560‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woo HH, Gillman MP, Gardiner R, Marshall V, Lynch WJ. A practical approach to the management of lower urinary tract symptoms among men. Med J Aust. 2011;195(1):34‐39. [DOI] [PubMed] [Google Scholar]
- 4. Lepor H. Pathophysiology of lower urinary tract symptoms in the aging male population. Rev Urol. 2005;7(suppl 7):S3‐S11. [PMC free article] [PubMed] [Google Scholar]
- 5. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub‐committee of the International Continence Society. Urology. 2003;61(1):37‐49. [DOI] [PubMed] [Google Scholar]
- 6. Irwin DE, Milsom I, Hunskaar S, et al. Population‐based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306‐1314. [DOI] [PubMed] [Google Scholar]
- 7. Stewart W, Van Rooyen J, Cundiff G, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327‐336. [DOI] [PubMed] [Google Scholar]
- 8. Liu AB, Liu Q, Yang CC, et al. Patient characteristics associated with more bother from lower urinary tract symptoms. J Urol. 2019;202(3):585‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clinicaltrial.gov . 2019. https://clinicaltrials.gov/. Accessed 30 April 2019.
- 10. Gacci M, Sebastianelli A, Spatafora P, et al. Best practice in the management of storage symptoms in male lower urinary tract symptoms: a review of the evidence base. Ther Adv Urol. 2017;10(2):79‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anger JT, Goldman HB, Luo X, et al. Patterns of medical management of overactive bladder (OAB) and benign prostatic hyperplasia (BPH) in the United States. Neurourol Urodyn. 2018;37(1):213‐222. [DOI] [PubMed] [Google Scholar]
- 12. McVary L, Roehrborn C, Avins A, et al. Management of Benign Prostatic Hyperplasia. American Urological Association. 2010.
- 13. Bechis SK, Otsetov AG, Ge R, Olumi AF. Personalized medicine for the management of benign prostatic hyperplasia. J Urol. 2014;192(1):16‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cisternas MG, Foreman AJ, Marshall TS, Runken MC, Kobashi KC, Seifeldin R. Estimating the prevalence and economic burden of overactive bladder among Medicare beneficiaries prior to Medicare Part D coverage. Curr Med Res Opin. 2009;25(4):911‐919. [DOI] [PubMed] [Google Scholar]
- 15. Hakimi Z, Johnson M, Nazir J, Blak B, Odeyemi IA. Drug treatment patterns for the management of men with lower urinary tract symptoms associated with benign prostatic hyperplasia who have both storage and voiding symptoms: a study using the health improvement network UK primary care data. Curr Med Res Opin. 2015;31(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 16. Vonesh E, Gooch KL, Khangulov V, et al. Cardiovascular risk profile in individuals initiating treatment for overactive bladder—Challenges and learnings for comparative analysis using linked claims and electronic medical record databases. PLOS One. 2018;13(10):e0205640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chancellor MB, Migliaccio‐Walle K, Bramley TJ, Chaudhari SL, Corbell C, Globe D. Long‐term patterns of use and treatment failure with anticholinergic agents for overactive bladder. Clin Ther. 2013;35(11):1744‐1751. [DOI] [PubMed] [Google Scholar]
- 18. Pelletier EM, Vats V, Clemens JQ. Pharmacotherapy adherence and costs versus nonpharmacologic management in overactive bladder. Am J Manag Care. 2009;15(4 suppl):S108‐S114. [PubMed] [Google Scholar]
- 19. Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei J, Calhoun E, Jacobson S. Benign prostatic hyperplasia In: Litwin M, Saigal C, eds. Urologic Diseases in America: US Department of Health and Human Services, Public Health Services, National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2007:48‐53. [Google Scholar]
- 21. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626‐633. [DOI] [PubMed] [Google Scholar]
- 22. Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes. 2001;(20):1‐10. https://www.cdc.gov/nchs/data/statnt/statnt20.pdf [PubMed] [Google Scholar]
- 23. Tarhini A, Atzinger C, Gupte‐Singh K, Johnson C, Macahilig C, Rao S. Treatment patterns and outcomes for patients with unresectable stage III and metastatic melanoma in the USA. J Comp Eff Res. 2019;8:461‐473. [DOI] [PubMed] [Google Scholar]
- 24. Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am. 2016;43(3):289‐297. [DOI] [PubMed] [Google Scholar]
- 25. Helfand BT, Evans RM, McVary KT. A comparison of the frequencies of medical therapies for overactive bladder in men and women: analysis of more than 7.2 million aging patients. Eur Urol. 2010;57(4):586‐591. [DOI] [PubMed] [Google Scholar]
- 26. Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2007;9(4):181‐190. [PMC free article] [PubMed] [Google Scholar]
- 27. Coyne KS, Sexton CC, Bell JA, et al. The prevalence of lower urinary tract symptoms (LUTS) and overactive bladder (OAB) by racial/ethnic group and age: results from OAB‐POLL. Neurourol Urodyn. 2013;32(3):230‐237. [DOI] [PubMed] [Google Scholar]
- 28. Logie J, Clifford GM, Farmer RD. Incidence, prevalence and management of lower urinary tract symptoms in men in the UK. BJU Int. 2005;95(4):557‐562. [DOI] [PubMed] [Google Scholar]
- 29. Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology. 2011;77(5):1081‐1087. [DOI] [PubMed] [Google Scholar]
- 30. Sexton CC, Coyne KS, Thompson C, Bavendam T, Chen CI, Markland A. Prevalence and effect on health‐related quality of life of overactive bladder in older Americans: results from the epidemiology of lower urinary tract symptoms study. J Am Geriatr Soc. 2011;59(8):1465‐1470. [DOI] [PubMed] [Google Scholar]
- 31. Ganz ML, Liu J, Zou KH, Bhagnani T, Luo X. Real‐world characteristics of elderly patients with overactive bladder in the United States. Curr Med Res Opin. 2016;32(12):1997‐2005. [DOI] [PubMed] [Google Scholar]
- 32. Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM‐7). Eur Urol. 2003;44(6):637‐649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Data Availability Statement
Researchers may request access to anonymized participant‐level data, trial‐level data, and protocols from Astellas‐sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study‐Sponsors/Study‐Sponsors‐Astellas.aspx.


