Abstract
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) are recommended for glycaemic management in patients with type 2 diabetes (T2D). Oral semaglutide, the first oral GLP‐1RA, has recently been approved for clinical use, based on the results of the randomized, Phase 3a Peptide InnOvatioN for Early diabEtes tReatment (PIONEER) clinical trials. The PIONEER programme tested oral semaglutide in patients with T2D of duration ranging from 3.5 to 15 years, from monotherapy through to insulin add‐on, in global populations and two trials dedicated to Japanese patients. Outcomes (glycated haemoglobin [HbA1c] and body weight reduction, plus other relevant efficacy and safety endpoints) were tested against both placebo and active standard‐of‐care medications. A separate trial evaluated the cardiovascular safety of oral semaglutide in patients with T2D at high cardiovascular risk. Over periods of treatment up to 78 weeks, oral semaglutide 7 and 14 mg once daily reduced HbA1c and body weight across the spectrum of T2D, and improved other diabetes‐related endpoints, such as fasting plasma glucose. Oral semaglutide provided significantly better efficacy than placebo and commonly used glucose‐lowering medications from the dipeptidyl peptidase‐4 inhibitor (sitagliptin) and sodium‐glucose co‐transporter‐2 inhibitor (empagliflozin) classes, as well as the subcutaneous GLP‐1RAs liraglutide and dulaglutide. Oral semaglutide was well tolerated in line with the known safety profile of GLP‐1RAs, with transient gastrointestinal events being the most common side effects reported. Cardiovascular safety was demonstrated for oral semaglutide in patients with cardiovascular disease or high cardiovascular risk. The results of the PIONEER programme suggest that oral semaglutide is efficacious and well tolerated for glycaemic control of T2D. The availability of oral semaglutide may help to broaden treatment choice and facilitate adoption of earlier GLP‐1RA treatment in the paradigm of T2D management.
Keywords: cardiovascular disease, clinical trial, GLP‐1 analogue, Phase 3 study, type 2 diabetes
1. INTRODUCTION
Incretin‐based therapies such as glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) and dipeptidyl peptidase‐4 (DPP4) inhibitors act on multiple pathophysiological pathways to help normalize insulin and glucagon secretion, and lower blood glucose levels. GLP‐1RAs also reduce appetite and facilitate weight loss. 1 , 2 , 3 The American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) guidelines recommend a GLP‐1RA or DPP4 inhibitor, or a sodium‐glucose co‐transporter‐2 inhibitor (SGLT2i), sulfonylurea or thiazolidinedione, as second‐line treatment in people with type 2 diabetes (T2D) uncontrolled on metformin. 4 , 5 Regardless of glycated haemoglobin (HbA1c), patients with or at risk of cardiovascular disease (CVD) should preferentially receive GLP‐1RA that has shown CV benefit, or an SGLT2i with CV benefit if a GLP‐1RA is not appropriate; SGLT2is are preferred over GLP‐1RAs for those with heart failure or chronic kidney disease (CKD). 4 , 5
Semaglutide is a GLP‐1 analogue approved as a once‐weekly subcutaneous injection for glycaemic control in patients with T2D inadequately controlled on at least one oral glucose‐lowering medication. 6 , 7 , 8 In the Phase 3 SUSTAIN clinical trials, once‐weekly subcutaneous semaglutide provided consistently significantly better reductions in HbA1c and body weight against placebo and a variety of active comparator drugs in multiple patient groups with T2D. 9 Additionally, semaglutide, like several other GLP‐1RAs, 10 , 11 , 12 showed a cardioprotective effect in patients with T2D and CVD or CV risk factors. 13
2. ORAL SEMAGLUTIDE
Until recently, all available GLP‐1RAs were given subcutaneously. An oral GLP‐1RA could help to encourage earlier and wider usage of this class, particularly in patients hesitant to take an injectable formulation. However, formulating a peptide for oral administration brings considerable challenges in terms of protecting the active molecule during its passage through the gastrointestinal (GI) tract and facilitating absorption across the GI epithelium. 14 Indeed, these issues have prevented the production of oral insulin for many years. 15
Oral semaglutide, a co‐formulation of semaglutide with the absorption enhancer sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate (SNAC), is the first oral GLP‐1RA that has been approved for clinical use for improving glycaemic control in patients with T2D in the United States, 16 and has received a positive opinion from European regulators. 17 SNAC has previously been co‐formulated with heparin and ibandronate to increase drug absorption, 18 , 19 and is currently available in a vitamin B12 supplement. 20 It helps to protect semaglutide from proteolytic degradation in the stomach and facilitates its absorption across the gastric mucosa (Figure 1). 21 SNAC itself is mostly eliminated after approximately 4‐6 hours. 21
FIGURE 1.
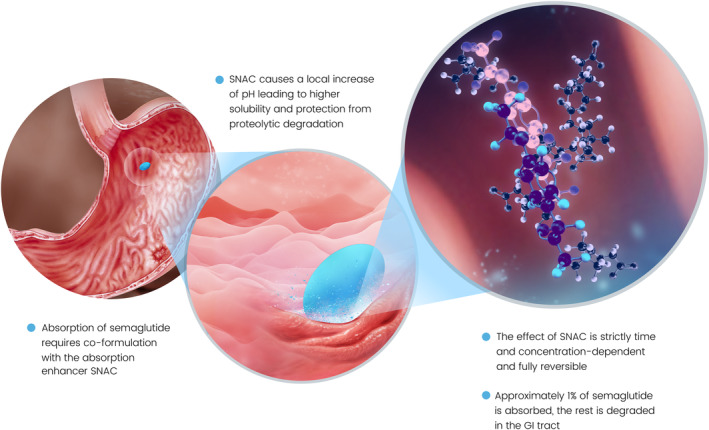
Mechanism of absorption of oral semaglutide. 21 GI, gastrointestinal; SNAC, sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate
The presence of food and/or substantial volumes of water in the stomach reduces the gastric absorption of oral semaglutide. 22 , 23 , 24 Therefore, in clinical trials, patients were required to take oral semaglutide in the morning in a fasted state with no more than 120 mL of water and waiting at least 30 minutes before taking any food, drink or other oral medication. Dosing under these conditions led to clinically relevant semaglutide plasma concentrations. 25 At equivalent levels of exposure, similar glycaemic and weight responses were seen with both oral and subcutaneous semaglutide. 26
In Phase I studies, the pharmacokinetics of oral semaglutide were not affected by any level of renal impairment (estimated glomerular filtration rate [eGFR] 15‐89 mL/min/1.73 m2), including end‐stage renal disease, 27 any level of hepatic impairment (Child‐Pugh A [mild]‐C [severe]) 28 or presence of upper GI disease (chronic gastritis, GI reflux disease, or both). 29 There are no studies of the use of oral semaglutide after bariatric surgery. There were no clinically relevant interactions between oral semaglutide and medications commonly prescribed to patients with T2D, including lisinopril, metformin, warfarin and statins, as well as the combined oral contraceptive pill; there was also no interaction with omeprazole. 30 , 31 , 32 Exposure to levothyroxine was increased in the presence of oral semaglutide, suggesting that thyroid parameters should be monitored when these two medications are co‐administered, in line with clinical practice. 33
In a Phase II dose‐finding trial, oral semaglutide at doses of 2.5‐40 mg once daily demonstrated significant dose‐dependent reductions in glucose and body weight in patients with early T2D. 34 Oral semaglutide was well tolerated, and the main adverse events were GI‐related, which (in common with other GLP‐1RAs) mainly consisted of dose‐dependent, mild‐to‐moderate and transient nausea when initiating or titrating therapy. Fewer GI events were reported when patients started on a lower dose, suggesting that a gradual escalation could be a helpful mitigation strategy. 34 Based on the results of the Phase II trial, 34 oral semaglutide doses of 3, 7 and 14 mg were taken forward to the Phase 3 Peptide InnOvatioN for the Early diabEtes tReatment (PIONEER) programme, a series of 10 multicentre, randomized clinical trials.
3. OVERVIEW OF THE PIONEER TRIALS
3.1. Design
The PIONEER programme was designed to test oral semaglutide across the spectrum of disease and background therapies, from patients with early T2D managed by diet and exercise through to those requiring daily insulin, and in patients with comorbidities such as CVD and CKD (Table 1). 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Each Phase 3a study was a randomized, controlled trial with the aim of comparing oral semaglutide with an active comparator and/or placebo. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
TABLE 1.
| Trial | Treatment arms a | Key inclusion criteria | Trial duration; blinded or open‐label | Primary endpoint/outcome | Key baseline characteristics (mean values) | Trial product discontinuation/rescue medication use by end of treatment period (proportion of patients) |
|---|---|---|---|---|---|---|
| Placebo‐controlled trials | ||||||
| PIONEER 1 (N = 703) | Oral semaglutide 3 mg, Oral semaglutide 7 mg, Oral semaglutide 14 mg, Placebo | Treated with diet and exercise, HbA1c 7.0%‐9.5% | 26‐week; blinded | Change in HbA1c from baseline to week 26 | Age: 55 years, HbA1c: 8.0% (63 mmol/mol), Duration of T2D: 3.5 years | Oral semaglutide 3 mg: 3%/7%, Oral semaglutide 7 mg: 8%/2%, Oral semaglutide 14 mg: 7%/1%, Placebo: 5%/15% |
| PIONEER 5 (N = 324) | Oral semaglutide 14 mg, Placebo | Moderate renal impairment, treated with metformin ± sulfonylurea; or basal insulin ± metformin, HbA1c 7.0%‐9.5% | 26‐week; blinded | Change in HbA1c from baseline to week 26 | Age: 70 years, HbA1c: 8.0% (64 mmol/mol), Duration of T2D: 14.0 years | Oral semaglutide: 18%/4%, Placebo: 12%/10% |
| PIONEER 6 (N = 3183) | Oral semaglutide 14 mg, Placebo | Age ≥50 years with CVD/CKD or age ≥60 years with CV risk factors | Event‐driven; blinded | 3‐point composite MACE b | Age: 66 years, HbA1c: 8.2% (66 mmol/mol), Duration of T2D: 14.9 years | Oral semaglutide: 15%/NR, Placebo: 10%/NR |
| PIONEER 8 (N = 731) | Oral semaglutide 3 mg, Oral semaglutide 7 mg, Oral semaglutide 14 mg, Placebo | Treated with insulin c ± metformin, HbA1c 7.0%‐9.5% | 52‐week; blinded | Change in HbA1c from baseline to week 26 | Age: 61 years, HbA1c: 8.2% (66 mmol/mol), Duration of T2D: 15.0 years | Oral semaglutide 3 mg: 13%/29%, Oral semaglutide 7 mg: 19%/18%, Oral semaglutide 14 mg: 20%/17%, Placebo: 12%/36% |
| Active‐controlled trials | ||||||
| PIONEER 2 (N = 822 d ) | Oral semaglutide 14 mg, Empagliflozin 25 mg | Treated with metformin, HbA1c 7.0%‐10.5% | 52‐week; open‐label | Change in HbA1c from baseline to week 26 | Age: 58 years, HbA1c: 8.1% (65 mmol/mol), Duration of T2D: 7.4 years | Oral semaglutide: 18%/8%, Empagliflozin: 11%/11% |
| PIONEER 3 (N = 1864) | Oral semaglutide 3 mg, Oral semaglutide 7 mg, Oral semaglutide 14 mg, Sitagliptin 100 mg | Treated with metformin ± sulfonylurea, HbA1c 7.0%‐10.5% | 78‐week; blinded | Change in HbA1c from baseline to week 26 | Age: 58 years, HbA1c: 8.3% (67 mmol/mol), Duration of T2D: 8.6 years | Oral semaglutide 3 mg: 17%/34%, Oral semaglutide 7 mg: 15%/22%, Oral semaglutide 14 mg: 19%/10%, Sitagliptin: 13%/28% |
| PIONEER 4 (N = 711) | Oral semaglutide 14 mg, Liraglutide 1.8 mg s.c., Placebo | Treated with metformin ± SGLT2i, HbA1c 7.0%‐9.5% | 52‐week; blinded | Change in HbA1c from baseline to week 26 | Age: 56 years, HbA1c: 8.0% (64 mmol/mol), Duration of T2D: 7.6 years | Oral semaglutide: 15%/7%, Liraglutide: 13%/6%, Placebo: 12%/30% |
| PIONEER 7 (N = 504) | Oral semaglutide (flexible dose adjustment: 3, 7 or 14 mg), Sitagliptin 100 mg | Treated with 1‐2 OADs e , HbA1c 7.5%‐9.5% | 52‐week; open‐label f | Proportion of patients with HbA1c <7.0% at week 52 | Age: 57 years, HbA1c: 8.3% (67 mmol/mol), Duration of T2D: 8.8 years | Oral semaglutide: 17%/3%, Sitagliptin: 9%/16% |
| PIONEER 9 (N = 243) | Oral semaglutide 3 mg, Oral semaglutide 7 mg, Oral semaglutide 14 mg, Liraglutide 0.9 mg s.c., Placebo | Treated with diet and exercise or stable dose of 1 OAD g , HbA1c 7.0%‐10.0% if on diet and exercise or HbA1c 6.5%‐9.5% if on 1 OAD | 52‐week; open‐label | Change from baseline to week 26 in HbA1c | Age: 59 years, HbA1c: 8.2% (66 mmol/mol), Duration of T2D: 7.6 years | Oral semaglutide 3 mg: 8%/14%,Oral semaglutide 7 mg: 2%/10%, Oral semaglutide 14 mg: 6%/8%, Liraglutide: 8%/6%,Placebo: 0%/31% |
| PIONEER 10 (N = 458) | Oral semaglutide 3 mg, Oral semaglutide 7 mg, Oral semaglutide 14 mg, Dulaglutide 0.75 mg s.c. | Treated with stable doses of 1 OAD h , HbA1c 7.0%‐10.5% | 52‐week; open‐label | Number of treatment‐emergent adverse events at week 57 | Age: 58 years, HbA1c: 8.3% (67 mmol/mol), Duration of T2D: 9.4 years | Oral semaglutide 3 mg: 5%/17%,Oral semaglutide 7 mg: 7%/6%,Oral semaglutide 14 mg: 12%/2%, Dulaglutide: 6%/9% |
Abbreviations: CKD, chronic kidney disease; CVD, established cardiovascular disease; HbA1c, glycated haemoglobin; MACE, major adverse cardiovascular events; NR, not reported; OAD, oral antidiabetic drug; s.c., subcutaneous; SGLT2i, sodium‐glucose co‐transporter‐2 inhibitor; T2D, type 2 diabetes.
All agents were administered once daily, except for dulaglutide 0.75 mg (PIONEER 10), which was administered once weekly;
Non‐fatal myocardial infarction, non‐fatal stroke or cardiovascular death;
Basal, basal‐bolus or premixed;
One patient enrolled at two sites so analyses were based on 821 patients;
Metformin, sulfonylurea, SGLT2i or thiazolidinedione;
52‐week extension study is ongoing;
Metformin, sulfonylurea, glinide, α‐glucosidase inhibitor, dipeptidyl peptidase‐4 inhibitor or SGLT2i;
Sulfonylurea, glinide, thiazolidinedione, α‐glucosidase inhibitor or SGLT2i.
In the PIONEER programme, two scientific questions related to the efficacy objectives were addressed through the definition of two estimands. 45 The treatment policy estimand evaluated the treatment effect for all randomized patients regardless of trial product discontinuation or use of rescue medication. This estimand reflects the population‐level effect of oral semaglutide compared with a comparator. The trial product estimand evaluated the treatment effect, assuming that all patients remained on the trial product for the entire planned trial duration and did not use rescue medication. This estimand aimed to show the efficacy of oral semaglutide without the confounding effects of rescue medication and trial product discontinuation. Data were analysed for both estimands by logistic regression for binary endpoints, including data irrespective of discontinuation of trial product or initiation of rescue medication in the case of the treatment policy estimand, and excluding data obtained after these intercurrent events for the trial product estimand. For continuous endpoints, the treatment policy estimand was estimated by a pattern‐mixture model with multiple imputation for missing data, whereas a mixed model for repeated measurements was employed for the trial product estimand. 45
Here, we will focus on efficacy outcomes with the treatment policy estimand, which was the primary estimand in most of the PIONEER trials and the only estimand employed in the PIONEER 6 CV outcomes trial. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
3.2. Patients
In most PIONEER trials, patients were aged ≥18 years, had been diagnosed with T2D at least 3 months before screening, and had baseline HbA1c in the range of 7.0%‐9.5% (Table 1). 35 , 36 , 37 , 38 , 39 , 41 , 42 In the PIONEER 6 CV outcomes trial, patients were aged ≥50 years and had clinical evidence of CVD or CKD, or were ≥60 years with CV risk factors. 40
3.3. Comparators, doses and treatment duration
Doses of oral semaglutide at 3, 7 and 14 mg were tested in the PIONEER programme (Table 1). To mitigate potential GI side effects, all patients randomized to oral semaglutide started treatment at the 3 mg dose, after which the dose was escalated in 4‐week increments to 7 mg and then 14 mg, until the randomized dose was achieved.
In PIONEER 1, oral semaglutide 3, 7 and 14 mg once daily were compared with placebo for 26 weeks in patients with early T2D managed by diet and exercise. 35 In PIONEER 2‐4, once‐daily oral semaglutide was compared with the SGLT2i empagliflozin (25 mg once daily), the DPP4 inhibitor sitagliptin (100 mg once daily) and the subcutaneous GLP‐1RA liraglutide (1.8 mg once daily) for either 52 or 78 weeks in patients with T2D who were already receiving one or two oral glucose‐lowering drugs. 36 , 37 , 38 In PIONEER 7, flexible dose adjustment of oral semaglutide (where each individual patient's daily dose could be increased or decreased between 3, 7 or 14 mg dependent on glycaemic efficacy and GI tolerability) was compared with 100 mg sitagliptin for 52 weeks. 41
Patients in PIONEER 5, 6 and 8 had T2D of 14‐15 years’ duration and received oral semaglutide or placebo added on to background medication. 39 , 40 , 42 In PIONEER 8, patients were all taking insulin, the dose of which was recommended to be reduced by 20% at randomization, after which it could be raised between weeks 8 and 26, without exceeding the pre‐randomization dosage, and was freely adjustable after week 26. 42 Patients in PIONEER 5 had moderate renal impairment (eGFR 30‐59 mL/min/1.73 m2). 39 PIONEER 6 was an event‐driven, placebo‐controlled CV outcomes study designed to demonstrate the CV safety of oral semaglutide 14 mg once daily in patients with established T2D and CVD, CKD or CV risk factors. 40
The two Japanese trials tested oral semaglutide monotherapy compared with placebo or liraglutide (PIONEER 9), 43 and oral semaglutide compared with dulaglutide in patients receiving stable doses of background oral antidiabetic drugs (OADs) (PIONEER 10). 44
3.4. Endpoints
The primary and confirmatory secondary endpoints in most of the trials were change from baseline in HbA1c and body weight, respectively, at week 26 (Table 1). 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 The exceptions were the flexible dose study (PIONEER 7), 38 the CV outcomes trial (PIONEER 6), 40 and PIONEER 10, which was primarily a safety study. 44 Supportive endpoints included achievement of HbA1c targets, weight‐loss thresholds and composite outcomes, and changes in parameters such as fasting plasma glucose, blood pressure and body mass index, as well as patient‐reported outcomes. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
Safety endpoints (evaluated in all randomized patients) included the number of treatment‐emergent adverse events, hypoglycaemic episodes, laboratory tests, physical examinations and outcomes of special interest, such as diabetic retinopathy, acute pancreatitis and CV events. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Hypoglycaemia was classified as severe according to the ADA definition, 46 and confirmed if blood glucose was <3.1 mmol/L (<56 mg/dL).
4. CLINICAL EVIDENCE
In total, 9543 patients were randomized to receive oral semaglutide or comparators during the PIONEER programme (including PIONEER 9 and 10). Over 80% of patients completed each trial, mostly while still receiving their randomized treatment, and the rate of rescue medication use in the oral semaglutide groups was <10% after 26 weeks’ treatment. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
4.1. Glycaemic control with oral semaglutide
Oral semaglutide was effective in reducing HbA1c across the continuum of T2D, using both the treatment policy estimand (results summarized below, and in Table 2 and Figure 2) and the trial product estimand (Figure S1). 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Monotherapy with oral semaglutide 3, 7 and 14 mg once daily dose‐dependently and significantly reduced HbA1c (baseline 8.0%) compared with placebo after 26 weeks’ treatment in patients with early T2D (mean duration 3.5 years) in PIONEER 1 (estimated treatment difference [ETD] –0.6% [3 mg] to −1.1% [14 mg] at week 26; P < .001 for all doses vs placebo). 35
TABLE 2.
Summary of main efficacy outcomes across the PIONEER trials 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
| Trial name and setting/main comparator | PIONEER 1 Monotherapy | PIONEER 2 Empagliflozin | PIONEER 3 Sitagliptin | PIONEER 4 Liraglutide | PIONEER 5 Renal | PIONEER 6 a , b CVD/CKD | PIONEER 7 a Flex | PIONEER 8 Insulin add‐on | PIONEER 9 Liraglutide Japan | PIONEER 10 Dulaglutide Japan | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparators | Sema | Pbo | Sema | Empa | Sema | Sita | Sema | Lira | Pbo | Sema | Pbo | Sema | Pbo | Sema | Sita | Sema | Pbo | Sema | Lira | Pbo | Sema | Dula | ||||||||||
| Dose, mg | 3 | 7 | 14 | 14 | 25 | 3 | 7 | 14 | 100 | 14 | 1.8 | 14 | 3 | 7 | 14 | 100 | 3 | 7 | 14 | 3 | 7 | 14 | 0.9 | 3 | 7 | 14 | 0.75 | |||||
| Estimated mean reductions from baseline (at 26 weeks except end of trial in PIONEER 6 and 52 weeks in PIONEER 7) | ||||||||||||||||||||||||||||||||
| HbA1c, % point | −0.9* | −1.2* | −1.4* | −0.3 | −1.3* | −0.9 | −0.6 | −1.0* | −1.3* | −0.8 | −1.2 | −1.1 | −0.2 | −1.0* | −0.2 | −1.0 | −0.3 | −1.3* | −0.8 | −0.6* | −0.9* | −1.3* | −0.1 | −1.1 | −1.6 | −1.8* | −1.4 | −0.4 | −1.1* | −1.7 | −2.0* | −1.5 |
| FPG, mmol/L c | −0.9* | −1.5* | −1.8* | −0.2 | −2.0 | −2.0 | −0.8 | −1.2* | −1.7* | −0.9 | −2.0 | −1.9 | −0.4 | −1.5* | −0.4 | NE | −2.2* | −1.4 | −0.2 | −1.1* | −1.3* | 0.3 | −1.7 | −1.9 | −2.5 | −2.0 | −0.7 | −1.4* | −2.2 | −2.6* | −2.0 | |
| Body weight, kg | −1.5 | −2.3 | −3.7* | −1.4 | −3.8 | −3.7 | −1.2* | −2.2* | −3.1* | −0.6 | −4.4* | −3.1 | −0.5 | −3.4* | −0.9 | −4.2 | −0.8 | −2.6* | −0.7 | −1.4* | −2.4* | −3.7* | −0.4 | −0.6 | −1.1* | −2.4* | −0.0 | −1.1 | −0.2 | −1.0* | −2.2* | 0.3 |
| Observed proportions of patients (%) achieving thresholds (at 26 weeks except 52 weeks in PIONEER 7) d | ||||||||||||||||||||||||||||||||
| HbA1c <7% | 55* | 69* | 77* | 31 | 67* | 40 | 27 | 42* | 55* | 32 | 68 | 62 | 14 | 58* | 23 | NE | 58* | 25 | 28* | 43* | 58* | 7 | 52 | 69 | 81* | 53 | 16 | 46* | 75 | 82* | 70 | |
| Weight loss ≥5% | 20 | 27* | 41* | 15 | 41 | 36 | 13 | 19* | 30* | 10 | 44* | 28 | 8 | 36* | 10 | NE | 27* | 12 | 13* | 31* | 39* | 3 | 4 | 10 | 34* | 0 | 10 | 5 | 18* | 31* | 6 | |
| HbA1c <7%, no weight gain or hypoglycaemia | 37* | 57* | 69* | 23 | 61* | 36 | 20 | 34* | 46* | 20 | 61 | 54 | 11 | 51* | 17 | NE | 45* | 15 | 18* | 27* | 44* | 2 | 33 | 53 | 70* | 33 | 8 | 31 | 49 | 66* | 39 | |
Data are for the treatment policy estimand (including data from patients who discontinued treatment or required rescue medication).
Abbreviations: CKD, chronic kidney disease; CVD, cardiovascular disease; dula, dulaglutide; empa, empagliflozin; imp, impairment; lira, liraglutide; met, metformin; NE, not evaluated; OAD, oral antidiabetic drug; pbo, placebo; sema, semaglutide; sita, sitagliptin; SU, sulfonylurea.
P < .05 in favour of oral semaglutide vs placebo or active comparator for the estimated treatment difference/odds ratio (comparison vs placebo not shown for PIONEER 4 and 9, nor are comparisons shown when the comparator was significantly better than oral semaglutide, which occurred in some cases with oral semaglutide 3 mg).
HbA1c reduction was not the primary endpoint in PIONEER 6 or 7;
Event‐driven trial: efficacy outcomes were not analysed statistically;
Converted from mg/dL when reported as such by multiplying by 0.055494;
Values rounded to whole numbers (estimated values reported for PIONEER 3).
FIGURE 2.
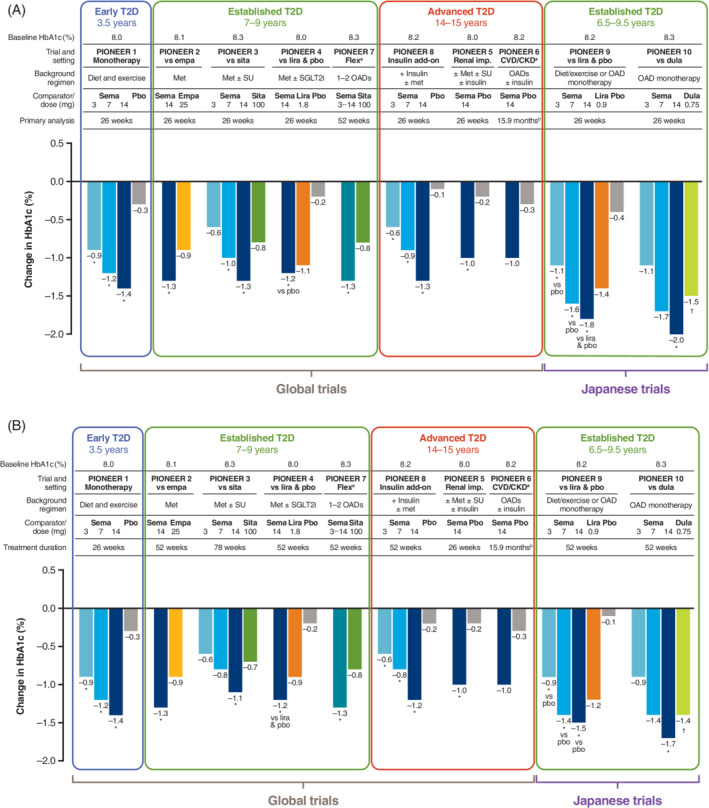
Reduction in HbA1c with oral semaglutide and comparators. A, Primary analysis time point (26 weeks except for PIONEER 6 and 7). B, End of treatment in the PIONEER trials, by the treatment policy estimand 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 (part B adapted with permission from Rasmussen. Diabetol Int. 2020;11(2):76‐86 55 ). Data are for the treatment policy estimand (including data from patients who discontinued treatment or required rescue medication). aHbA1c reduction was not the primary endpoint in PIONEER 6 or 7; bevent‐driven trial: efficacy outcomes were not analysed statistically. *P < .05 for the estimated treatment difference with oral semaglutide vs placebo and/or active comparator; † P < .05 for the estimated treatment difference with comparator vs oral semaglutide 3 mg. CKD, chronic kidney disease; CVD, cardiovascular disease; dula, dulaglutide; empa, empagliflozin; HbA1c, glycated haemoglobin; imp, impairment; lira, liraglutide; met, metformin; OAD, oral antidiabetic drug; pbo, placebo; sema, semaglutide; SGLT2i, sodium‐glucose co‐transporter‐2 inhibitor; sita, sitagliptin; SU, sulfonylurea; T2D, type 2 diabetes
In patients with established T2D (mean duration of between 7.4 and 8.6 years) who were receiving background OADs (PIONEER 2‐4), oral semaglutide 14 mg was more effective than empagliflozin 25 mg (ETD –0.4%; P < .0001), sitagliptin 100 mg (ETD –0.5%; P < .001) and similar to liraglutide 1.8 mg (ETD –0.1%; P = .0645) for HbA1c reduction (baseline 8.0%‐8.3%) at week 26 (treatment policy estimand); 36 , 37 , 38 differences remained in favour of oral semaglutide at end of treatment. Oral semaglutide 7 mg, but not 3 mg, was also more effective than sitagliptin for reducing HbA1c in PIONEER 3 (ETD −0.3% at week 26; P < .001). 37 In PIONEER 7, flexible dose adjustment of oral semaglutide was more effective than sitagliptin 100 mg for reducing HbA1c (ETD –0.5%, P < .0001) at 52 weeks (at which time 30% of patients in the oral semaglutide group were receiving the 7 mg dose and 59% the 14 mg dose). 41 In patients with advanced T2D (mean duration 15 years) receiving insulin, oral semaglutide 3, 7 and 14 mg reduced HbA1c (baseline 8.2%) significantly more than placebo at week 26 (PIONEER 8: ETD −0.5% [3 mg] to −1.2% [14 mg]; P < .0001 for all) and week 52. 42
In patients with moderate renal impairment (eGFR 30‐59 mL/min/1.73 m2) and long‐standing diabetes (mean duration 14 years) who took part in PIONEER 5, oral semaglutide 14 mg was significantly more effective than placebo in reducing HbA1c (baseline 8.0%) at week 26 (ETD –0.8%; P < .0001). 39 In patients with T2D (mean duration at baseline of 15 years) and at high CV risk in PIONEER 6, oral semaglutide reduced HbA1c by a mean of −1.0% versus −0.3% with placebo, both on top of standard of care, over a median trial duration of 15.9 months (outcome not analysed statistically). 40
Similar to the global trials, glycaemic control was improved with oral semaglutide 14 mg compared with both placebo and the GLP‐1RAs liraglutide 0.9 mg and dulaglutide 0.75 mg in the Japanese PIONEER 9 and 10 studies (efficacy for the 7 mg dose was similar to that of the active comparators). HbA1c reductions tended to be somewhat greater with oral semaglutide in the Japanese trials than in the global trials (from similar baseline levels). 43 , 44
The proportion of patients achieving the ADA‐recommended target of HbA1c <7.0% was consistently greater with oral semaglutide 7 and 14 mg (42%‐77%) than with placebo (7%‐31%) and active comparators (25%‐62%) at the primary analysis time point across the global trials (Table 2), and this advantage was generally maintained or improved upon at the end of the trial. 35 , 36 , 37 , 38 , 39 , 41 , 42 Generally, more patients also achieved the HbA1c target of ≤6.5% with oral semaglutide than with placebo or active comparators. 35 , 36 , 37 , 38 , 39 , 41 , 42 Other measures of glycaemic control, including fasting plasma glucose (Table 2), were also generally reduced in patients receiving oral semaglutide compared with those randomized to placebo or active comparators. 35 , 36 , 37 , 38 , 39 , 41 , 42
4.2. Body weight reductions with oral semaglutide
Oral semaglutide was effective in reducing body weight across the continuum of T2D using the treatment policy estimand (results summarized below and in Table 2 and Figure 3); outcomes were similar using the trial product estimand (Figure S2). 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Oral semaglutide 3, 7 and 14 mg given as monotherapy dose‐dependently and significantly reduced body weight (baseline 88.1 kg) compared with placebo in patients with early T2D managed with diet and exercise (PIONEER 1: ETD −0.1 kg [3 mg] to −2.3 kg [14 mg] at week 26; P < .001 vs placebo). 35
FIGURE 3.
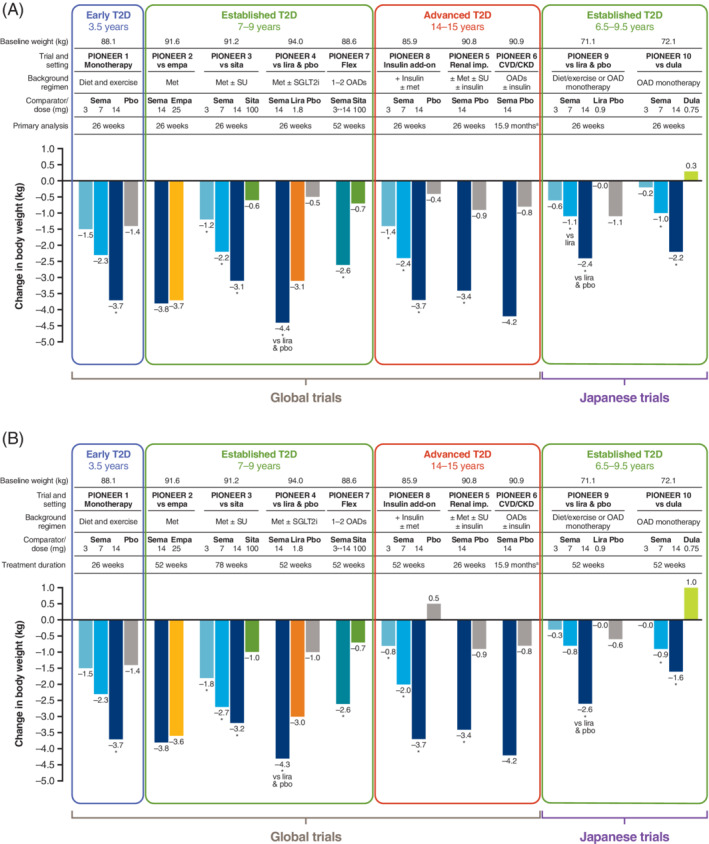
Reduction in body weight with oral semaglutide and comparators. A, Primary analysis time point (26 weeks except for PIONEER 6 and 7). B, End of treatment in the PIONEER trials, by the treatment policy estimand 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 (part B adapted with permission from Rasmussen. Diabetol Int. 2020;11(2):76‐86 55 ). Data are for the treatment policy estimand (including data from patients who discontinued treatment or required rescue medication). aEvent‐driven trial: efficacy outcomes were not analysed statistically. *P < .05 for the estimated treatment difference with oral semaglutide vs placebo and/or active comparator. CKD, chronic kidney disease; CVD, cardiovascular disease; dula, dulaglutide; empa, empagliflozin; imp, impairment; lira, liraglutide; met, metformin; OAD, oral antidiabetic drug; pbo, placebo; sema, semaglutide; SGLT2i, sodium‐glucose co‐transporter‐2 inhibitor; sita, sitagliptin; SU, sulfonylurea; T2D, type 2 diabetes
In patients with more established T2D receiving background OADs (baseline weight 88.6‐94.0 kg), oral semaglutide 14 mg had similar efficacy to empagliflozin in reducing body weight at week 26 (PIONEER 2: ETD −0.11 kg; P = .7593) and week 52 (ETD −0.2 kg; P = .6231) using the treatment policy estimand. 36 Oral semaglutide 14 mg provided a significantly greater body weight reduction compared with liraglutide at week 26 (PIONEER 4: ETD −1.2 kg; P = .0003) and week 52 (ETD −1.3 kg; P = .0019). 38 All three tested doses of oral semaglutide were also associated with significantly greater weight loss compared with sitagliptin at week 26 (PIONEER 3: ETD −0.6 kg [3 mg] to −2.5 kg [14 mg]; P ≤ .02 for all doses) and up to week 78, 37 and when oral semaglutide was dosed flexibly for 52 weeks (PIONEER 7: ETD −1.9 kg; P < .0001). 41
In patients with advanced T2D receiving background insulin, oral semaglutide 3, 7 and 14 mg were significantly better than placebo in reducing body weight (baseline 85.9 kg) at week 26 (PIONEER 8: ETD −0.9 kg [3 mg] to −3.3 kg [14 mg]; P ≤ .04 for all) and week 52 (ETD −1.3 kg to −4.3 kg; P ≤ .0101). 42 In patients with moderate renal impairment (PIONEER 5), oral semaglutide 14 mg significantly reduced body weight (baseline 90.8 kg) at week 26 (ETD −2.5 kg; P < .0001 vs placebo). 39 In PIONEER 6, in patients with CVD, CKD or CV risk factors, oral semaglutide reduced body weight (baseline 90.9 kg) by a mean of −4.2 kg over the course of the trial versus −0.8 kg with placebo (outcome not analysed statistically). 40
The proportion of patients achieving weight loss of ≥5% across the global trials was consistently greater with oral semaglutide 7 and 14 mg (13%‐44%) than with placebo (3%‐15%) and active comparators (10%‐36%) at week 26, and the difference was maintained at the end of treatment in each trial. 35 , 36 , 37 , 38 , 39 , 41 , 42 Other measures of body size (body mass index and waist circumference) were reduced with oral semaglutide compared with placebo and active comparators. 35 , 36 , 37 , 38 , 39 , 41 , 42
4.3. Composite outcomes
With the exception of PIONEER 6, 40 two composite outcomes were studied in the PIONEER trials. 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 At week 26, a greater proportion of patients achieved HbA1c <7% with no weight gain or severe/blood glucose‐confirmed hypoglycaemia with oral semaglutide 7 and 14 mg than with placebo and active comparators (Table 2). 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 This advantage was maintained over longer treatment durations. Similarly, more patients achieved HbA1c reduction ≥1% with body weight loss ≥3% at week 26 with oral semaglutide 7 and 14 mg than with placebo and active comparators. 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 The difference also remained over longer treatment durations.
4.4. Quality of life endpoints
Patient‐reported outcomes were collected in the PIONEER programme. In PIONEER 4, patients reported significantly better overall treatment satisfaction when treated with oral semaglutide compared with placebo. 38 Outcomes between oral semaglutide and active comparators were generally similar across the trials. In PIONEER 2, scores using the 36‐item short‐form survey (version 2) were significantly better for oral semaglutide than empagliflozin for the domains of general health and social functioning at week 26, but favoured empagliflozin for physical health scores at week 52; oral semaglutide was favoured for reported improvements in craving control at weeks 26 and 52 using the control of eating questionnaire. 36 Patient‐reported outcomes were similar between oral semaglutide and sitagliptin in PIONEER 3, 37 and patients reported similar convenience for oral semaglutide and sitagliptin after 52 weeks when oral semaglutide was flexibly dosed (PIONEER 7). 41 Oral semaglutide 7 and 14 mg were associated with improvements in general health compared with placebo after 52 weeks’ treatment in patients with long‐standing T2D also receiving insulin (PIONEER 8); significant improvements were also reported regarding the impact of weight on patients’ quality of life (oral semaglutide 14 mg). 42
4.5. Cardiovascular outcomes
Among 3183 patients with T2D and high CV risk enrolled in PIONEER 6, oral semaglutide was non‐inferior to placebo for the incidence of first major adverse cardiovascular events (MACE) (3.8% in the oral semaglutide group and 4.8% in the placebo group; hazard ratio [HR] = 0.79 [95% CI 0.57‐1.11], P < .001 for non‐inferiority). 40 A lower incidence of CV‐related death (HR = 0.49 [95% CI 0.27‐0.92]) and all‐cause death (1.4% vs 2.8%; HR = 0.51 [95% CI 0.31‐0.84]) was observed with oral semaglutide versus placebo. 40
In the other PIONEER trials, among patients at lower CV risk, the incidence of CV events between patients receiving oral semaglutide and those receiving placebo or other glucose‐lowering drugs was low. 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 Fasting lipid levels (very low, low and high‐density lipoprotein cholesterol, and triglycerides) remained similar or were reduced with oral semaglutide (generally at the 14 mg dose) versus comparators, and reductions were maintained over time. 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44
4.6. Safety and tolerability
The proportion of patients with any adverse event was generally similar or higher with oral semaglutide than with placebo and active comparator (Table 3). 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Adverse events in patients receiving oral semaglutide were mainly mild or moderate in severity, and generally did not result in permanent drug discontinuation (≤15% in any trial) (Table 3). 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
TABLE 3.
Summary of on‐treatment safety outcomes in the PIONEER trials 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
| Trial (number of patients enrolled) | Treatment arm | Patients with any AE (% of total patients) | Severe AEs (% of total patients) | Serious AEs (% of total patients) | AEs leading to trial product discontinuation (% of total patients) | Hypoglycaemic episodes (% of total patients) a | |
|---|---|---|---|---|---|---|---|
| Overall | GI | ||||||
| Placebo‐controlled trials | |||||||
| PIONEER 1 (N = 703) | Oral semaglutide 3 mg (n = 175) | 101 (58) | 8 (5) | 5 (3) | 4 (2) | 3 (2) | 5 (3) |
| Oral semaglutide 7 mg (n = 175) | 93 (53) | 1 (1) | 3 (2) | 7 (4) | 4 (2) | 2 (1) | |
| Oral semaglutide 14 mg (n = 175) | 99 (57) | 3 (2) | 2 (1) | 13 (7) | 9 (5) | 1 (1) | |
| Placebo (n = 178) | 99 (56) | 5 (3) | 8 (4) | 4 (2) | 1 (1) | 1 (1) | |
| PIONEER 5 (N = 324) | Oral semaglutide 14 mg (n = 163) | 120 (74) | 10 (6) | 17 (10) | 24 (15) | 19 (12) | 9 (6) |
| Placebo (n = 161) | 105 (65) | 15 (9) | 17 (11) | 8 (5) | 3 (2) | 3 (2) | |
| PIONEER 6 (N = 3183) | Oral semaglutide 14 mg (n = 1591) | NR | NR | 301 (19) | 184 (12) | 108 (7) | NR b |
| Placebo (n = 1592) | NR | NR | 358 (22) | 104 (7) | 26 (2) | NR b | |
| PIONEER 8 c (N = 731) | Oral semaglutide 3 mg (n = 184) | 137 (74) | 17 (9) | 25 (14) | 13 (7) | 9 (5) | 52 (28) |
| Oral semaglutide 7 mg (n = 181) | 142 (78) | 17 (9) | 19 (10) | 16 (9) | 12 (7) | 47 (26) | |
| Oral semaglutide 14 mg (n = 181) | 151 (83) | 13 (7) | 12 (7) | 24 (13) | 19 (10) | 48 (27) | |
| Placebo (n = 184) | 139 (76) | 9 (5) | 17 (9) | 5 (3) | 1 (1) | 54 (29) | |
| Active‐controlled trials | |||||||
| PIONEER 2 (N = 822)d | Oral semaglutide 14 mg (n = 410) | 289 (70) | 24 (6) | 27 (7) | 44 (11) | 33 (8) | 7 (2) |
| Empagliflozin 25 mg (n = 409) | 283 (69) | 23 (6) | 37 (9) | 18 (4) | 3 (1) | 8 (2) | |
| PIONEER 3 (N = 1864) | Oral semaglutide 3 mg (n = 466) | 370 (79) | 47 (10) | 64 (14) | 26 (6) | 11 (2) | 23 (5) |
| Oral semaglutide 7 mg (n = 464) | 363 (78) | 37 (8) | 47 (10) | 27 (6) | 16 (3) | 24 (5) | |
| Oral semaglutide 14 mg (n = 465) | 370 (80) | 40 (9) | 44 (9) | 54 (12) | 32 (7) | 36 (8) | |
| Sitagliptin 100 mg (n = 466) | 388 (83) | 53 (11) | 58 (12) | 24 (5) | 12 (3) | 39 (8) | |
| PIONEER 4 (N = 711) | Oral semaglutide 14 mg (n = 285) | 229 (80) | 23 (8) | 31 (11) | 31 (11) | 22 (8) | 2 (1) |
| Liraglutide 1.8 mg (n = 284) | 211 (74) | 22 (8) | 22 (8) | 26 (9) | 17 (6) | 7 (2) | |
| Placebo (n = 142) | 95 (67) | 7 (5) | 15 (11) | 5 (4) | 3 (2) | 3 (2) | |
| PIONEER 7 (N = 504) | Oral semaglutide (flexible 3, 7 or 14 mg) (n = 253) | 197 (78) | 16 (6) | 24 (9) | 22 (9) | 14 (6) | 14 (6) |
| Sitagliptin 100 mg (n = 250) | 172 (69) | 18 (7) | 24 (10) | 8 (3) | 2 (1) | 14 (6) | |
| PIONEER 9 (N = 243) | Oral semaglutide 3 mg (n = 49) | 37 (76) | 1 (2) | 2 (4) | 1 (2) | 17 (35) | 0 |
| Oral semaglutide 7 mg (n = 49) | 37 (76) | 2 (4) | 3 (6) | 1 (2) | 18 (37) | 0 | |
| Oral semaglutide 14 mg (n = 48) | 34 (71) | 0 | 0 | 2 (4) | 16 (33) | 0 | |
| Liraglutide 0.9 mg (n = 48) | 32 (67) | 0 | 0 | 0 | 18 (38) | 2 (4) | |
| Placebo (n = 49) | 39 (80) | 0 | 3 (6) | 0 | 10 (20) | 0 | |
| PIONEER 10 (N = 458) | Oral semaglutide 3 mg (n = 131) | 101 (77) | 3 (2) | 9 (7) | 4 (3) | 40 (31) | 3 (2) |
| Oral semaglutide 7 mg (n = 132) | 106 (80) | 1 (1) | 4 (3) | 8 (6) | 51 (39) | 3 (2) | |
| Oral semaglutide 14 mg (n = 130) | 111 (85) | 1 (1) | 7 (5) | 8 (6) | 70 (54) | 4 (3) | |
| Dulaglutide 0.75 mg (n = 65) | 53 (82) | 0 | 1 (2) | 2 (3) | 26 (40) | 0 | |
Data are n (%) with proportions rounded to whole numbers.
Abbreviations: AE, adverse event; GI, gastrointestinal; NR, not reported.
Hypoglycaemic episodes were either severe (defined according to the American Diabetes Association classification) or confirmed by a whole‐blood glucose value <56 mg/dL (<3.1 mmol/L), with symptoms consistent with hypoglycaemia unless otherwise stated;
Cases of severe hypoglycaemia were identified through a search of terms in the Medical Dictionary for Regulatory Activities, version 20.1 (23 and 13 patients identified with oral semaglutide and placebo, respectively);
Additional data from Zinman et al, poster presented at 79th Scientific Sessions of the American Diabetes Association Congress, San Francisco, CA, USA, 7‐11 June, 2019;
822 patients were enrolled, of whom 821 were included in the safety analysis set.
In line with the known tolerability profile of GLP‐1RAs, the most frequent class of adverse events across the PIONEER trials was GI disorders, which mainly consisted of nausea, vomiting, diarrhoea, constipation, dyspepsia and upper abdominal pain. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 GI events were more common with oral semaglutide than placebo or active comparators, but were generally mild to moderate in severity and transient, occurring predominantly during the dose escalation period. Nausea was usually the most common GI adverse event; respiratory tract infections (typically nasopharyngitis and influenza) were usually the most common non‐GI adverse events and occurred with a similar incidence to comparators. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
Patients with proliferative retinopathy or maculopathy requiring acute treatment were excluded from the PIONEER trials. The incidence of diabetic retinopathy identified during the trials was similar to that seen with placebo and active comparators. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Most cases were diagnosed during routine eye examinations and did not require active treatment. External independent adjudication committees were used to confirm occurrences of adverse events of special interest in each trial. There was a low incidence of adjudicated events, including acute pancreatitis and acute kidney injury, and rates were similar between oral semaglutide and comparators. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Malignant neoplasms, including malignant thyroid neoplasms, were few in number, and there was no clustering of events in any particular system organ or class. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
The incidence of severe or blood glucose‐confirmed symptomatic hypoglycaemia was low in patients receiving oral semaglutide (Table 3) and did not exceed 8% in any oral semaglutide treatment group, 35 , 36 , 37 , 38 , 39 , 41 , 43 , 44 with the exception of patients receiving background insulin in PIONEER 8 (among whom the greatest incidence was associated with basal/bolus insulin). 42 Across the studies, very few cases of hypoglycaemia were classed as severe (according to the ADA definition 46 ). Blood pressure remained similar or was slightly reduced over time in patients treated with oral semaglutide compared with those receiving comparator treatments. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
As expected for a GLP‐1RA, 9 mean pulse rate was 1‐4 beats/min higher with oral semaglutide than with placebo at the end of treatment. 35 , 38 , 39 , 41 Changes were similar between oral semaglutide and liraglutide in PIONEER 4. 38
Amylase and lipase levels generally increased with oral semaglutide compared with placebo, as seen with other GLP‐1RAs, 47 and changes were similar to those observed with liraglutide; no increase in acute pancreatitis was reported. 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 Renal function was unaffected during oral semaglutide treatment, including in patients with renal impairment at baseline. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 There were a low and similar number of deaths in the trials among patients receiving oral semaglutide and comparators. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
5. DISCUSSION
In the PIONEER programme, oral semaglutide was shown to be effective for glycaemic control across the spectrum of disease durations, background therapies and comorbidities (CVD/CKD) in patients with T2D. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Although most trials analysed the primary outcome after 26 weeks, treatment continued in most of the global trials for 52 weeks (78 weeks in PIONEER 3 37 ), and HbA1c remained significantly better for oral semaglutide 7 and 14 mg versus comparators at these later times. 36 , 37 , 38 , 41 , 42 , 43 , 44 At least half of the patients treated with oral semaglutide 14 mg in any given trial had HbA1c below the ADA target of 7.0% at the end of treatment. 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 When dose adjustment of oral semaglutide was permitted to account for a need to improve glycaemic control or to alleviate side effects (PIONEER 7), 41 oral semaglutide was more effective than sitagliptin after 52 weeks, suggesting that such an approach, in which the dose can be adjusted per individual patient, may be beneficial in clinical practice. Only 9% of patients were not escalated to the 7 or 14 mg dose by week 52. 41 The results of an open‐label extension phase of PIONEER 7, during which patients continued with (or switched from sitagliptin to) flexible oral semaglutide dosing for a further 52 weeks, are due to be reported soon (NCT02849080).
Compared with patients in the global trials, Japanese patients tended to have greater HbA1c reductions than global patients (from similar baselines), 43 , 44 supporting the hypothesis that T2D in East Asian patients is mainly a disease of beta‐cell dysfunction rather than of obesity and insulin resistance. 48
In the global trials, oral semaglutide was as effective as the oral SGLT2i empagliflozin in helping patients to lose weight at 26 and 52 weeks, 36 and was more effective than the DPP4 inhibitor sitagliptin 37 , 41 and the subcutaneous GLP‐1RA liraglutide at both time points. 38 In these trials, up to 4.3 kg of weight loss was achieved at 52 weeks in patients receiving oral semaglutide 14 mg once daily (treatment policy estimand), 36 , 37 , 38 , 41 and in PIONEER 3 weight loss was maintained up to 78 weeks. 37 Patients with moderate renal impairment (eGFR 15‐29 mL/min/1.73 m2) and those at high CV risk also lost weight over the course of oral semaglutide treatment. 36 , 37 Up to 69% of patients treated with oral semaglutide in the global trials achieved the composite outcome of HbA1c <7.0% with no weight gain or severe/blood glucose‐confirmed hypoglycaemia. 35 , 36 , 37 , 38 , 39 , 41 , 42
Patient‐reported outcomes were generally improved with oral semaglutide compared with placebo. 38 , 42 Such outcomes were generally similar to those of active comparators, but there were some improvements in patients’ food cravings, with oral semaglutide compared with empagliflozin. 36 Outcomes related to satisfaction and convenience of treatment suggested that patients found the dosing requirements for oral semaglutide similarly acceptable to those for sitagliptin, a commonly administered oral glucose‐lowering medication. 37 , 41
Here, we have primarily reported efficacy outcomes using the treatment policy estimand, which includes data from patients who discontinued treatment and/or required rescue medication. 45 This was the primary estimand in most of the PIONEER trials and may be of most interest to clinical decision‐makers, regulators, public health officials and payers because it gives a population‐level estimate of efficacy. Results were similar but somewhat more favourable for oral semaglutide against comparators for the trial product estimand, in which it was assumed that all patients remained on the trial product for the entire planned trial duration and did not use rescue medication. 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 This estimand shows the efficacy that oral semaglutide can achieve without these confounding effects, and clinicians may wish to take results with both estimands into account when evaluating probable efficacy across a population and in an individual patient. 45
Caution should be exercised when comparing the results of the oral and subcutaneous formulations of semaglutide in the SUSTAIN and PIONEER programmes because the trial designs and populations (including baseline characteristics and background medications), comparators and analysis time points varied. However, clinically relevant reductions in HbA1c and body weight were observed with semaglutide regardless of the route of administration. 9 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Outcomes with oral semaglutide should be considered in the context of expanding the treatment choices for patients, the potential for improvements in treatment adherence, and a new option for patients with a preference for oral versus injectable therapy.
CVD and CKD are some of the most important concomitant diseases in patients with T2D. 49 In the CV outcomes trial (PIONEER 6), oral semaglutide did not increase the risk of MACE compared with placebo in patients with CVD/CKD or CV risk factors. 40 While PIONEER 6 was not powered to show superiority for CV events with oral semaglutide, 50 subcutaneous semaglutide has been shown to reduce the risk of MACE compared with placebo in similarly high‐risk patients in the larger and longer SUSTAIN 6 trial, 13 with an HR estimate similar to that observed in PIONEER 6. 40 A cardioprotective effect has also been demonstrated in other CV outcomes trials of GLP‐1RAs. 10 , 11 , 12 This led to the ADA/EASD recommendation for the use of a GLP‐1RA with proven CV benefit in patients with T2D who are at elevated CV risk. 4 , 5 The ongoing SOUL trial (NCT03914326) will recruit nearly 10 000 patients with T2D and CVD/CKD, and monitor their CV outcomes for up to 5 years to determine whether oral semaglutide is superior to placebo in preventing MACE.
The safety profile of oral semaglutide was generally consistent with the known tolerability of the GLP‐1RAs, with the most frequent adverse events being mild‐to‐moderate and transient GI effects. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 The incidence of GI events was similar to that seen in SUSTAIN 1‐7, 9 indicating no effect of the oral route of semaglutide delivery on tolerability. There was no adverse effect on renal function in patients treated with oral semaglutide, and no additional safety concerns in patients with moderate renal impairment, 39 meaning that no dose adjustment is deemed necessary in patients with impaired renal function. 16 , 23 GLP‐1RAs have a positive effect on albuminuria, 51 , 52 which is a marker for kidney damage and CV risk, 53 , 54 but the clinical implications are yet to be fully examined. Nevertheless, oral semaglutide may be a useful option for patients with T2D and renal impairment, for whom current treatment options are limited. 39
Severe and/or blood glucose‐confirmed symptomatic hypoglycaemic episodes were uncommon in patients receiving oral semaglutide (with the exception of those receiving concomitant insulin and sulfonylurea, in whom the rate would be expected to be higher 42 ). This confirms that semaglutide‐mediated HbA1c reduction is not associated with uncontrolled lowering of blood glucose levels, in line with the glucose‐dependent mechanism of action. 1
Patients in the SUSTAIN 6 trial who received semaglutide had a higher incidence of diabetic retinopathy than those who did not, 13 which may have been related to substantial and rapid reductions in HbA1c during the first 16 weeks of treatment. 9 Therefore, gradual dose escalation is recommended with both oral and subcutaneous semaglutide. 6 , 7 , 8 , 16 Patients were thus excluded from the PIONEER trials if they had proliferative retinopathy or maculopathy requiring acute treatment, and there was a low incidence of diabetic retinopathy and related events. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
The PIONEER programme was a robustly designed series of 10 clinical trials and established the premise of oral GLP‐1RA therapy. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Given that injections represent a barrier for some patients and health care providers, the availability of an oral GLP‐1RA should increase the number of eligible patients who receive this effective means of T2D management, and encourage use at an earlier stage. Thus, oral semaglutide addresses an important unmet need in the management of T2D.
In conclusion, the PIONEER programme demonstrated that the novel formulation of oral semaglutide was efficacious across the spectrum of T2D and more effective than comparators for glycaemic control and weight loss. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Tolerability was consistent with the known profile of GLP‐1RAs. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Real‐world data will be required to confirm if the outcomes seen in the PIONEER trials are translated into clinical practice, and whether the availability of semaglutide in an innovative oral formulation will promote earlier and more frequent utilization of the GLP‐1RA class.
CONFLICT OF INTEREST
T.K.T. is on the speaker's bureau for Novo Nordisk and is one of the US national leads for the Semaglutide Cardiovascular Outcomes Trial in Patients With Type 2 Diabetes (SOUL) trial (NCT03914326). R.P. has received lecture and consulting fees from AstraZeneca; consulting fees from Boehringer Ingelheim, Eisai, GlaxoSmithKline and Mundipharma; grants and lecture/consulting fees from Glytec, Janssen, Novo Nordisk, Pfizer and Takeda; grants from Lexicon Pharmaceuticals; and grants and consulting fees from Ligand Pharmaceuticals, Lilly, Merck and Sanofi‐Aventis US. Except for consulting fees in February 2018 and June 2018 received from Sanofi US Services, all fees for services were paid directly to AdventHealth, a non‐profit organization. J.J.M. has received lecture honoraria and consulting fees from AstraZeneca, Berlin‐Chemie, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme (MSD), Novo Nordisk, Novartis and Sanofi; has received reimbursement of congress participation fees and travel expenses from MSD, Novo Nordisk and Sanofi; and has initiated projects supported by Boehringer Ingelheim, MSD, Novo Nordisk and Sanofi.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGEMENTS
We are grateful to all the patients, investigators and trial site staff who took part in the PIONEER trials. In addition, we would like to thank Stephen Purver of Spirit Group Ltd for medical writing and editorial assistance (funded by Novo Nordisk A/S). Novo Nordisk was also provided with the opportunity to perform a medical accuracy review.
Thethi TK, Pratley R, Meier JJ. Efficacy, safety and cardiovascular outcomes of once‐daily oral semaglutide in patients with type 2 diabetes: The PIONEER programme. Diabetes Obes Metab. 2020;22:1263–1277. 10.1111/dom.14054
All PIONEER trials were registered with ClinicalTrials.gov (NCT02906930 [PIONEER 1], NCT02863328 [PIONEER 2], NCT02607865 [PIONEER 3], NCT02863419 [PIONEER 4], NCT02827708 [PIONEER 5], NCT02692716 [PIONEER 6], NCT02849080 [PIONEER 7], NCT03021187 [PIONEER 8], NCT03018028 [PIONEER 9] and NCT03015220 [PIONEER 10]).
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14054.
Funding information Novo Nordisk
REFERENCES
- 1. Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors. Diabetes Obes Metab. 2016;18(3):203‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696‐1705. [DOI] [PubMed] [Google Scholar]
- 3. Dailey MJ, Moran TH. Glucagon‐like peptide 1 and appetite. Trends Endocrinol Metab. 2013;24(2):85‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221‐228. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43(Suppl 1):S98‐S110. [DOI] [PubMed] [Google Scholar]
- 6. European Medicines Agency . Ozempic summary of product characteristics. 2019; https://www.ema.europa.eu/en/documents/product-information/ozempic-epar-product-information_en.pdf. Accessed September 13, 2019.
- 7. Food and Drug Administration . Ozempic prescribing information. 2017; https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf. Accessed September 13, 2019.
- 8. Japanese Ministry of Health Labour and Welfare . Ozempic Japanese prescribing information. 2018.
- 9. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcomes with once‐weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1‐7 trials. Diabetes Metab. 2019;45(5):409‐418. [DOI] [PubMed] [Google Scholar]
- 10. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392(10157):1519‐1529. [DOI] [PubMed] [Google Scholar]
- 12. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394(10193):121‐130. [DOI] [PubMed] [Google Scholar]
- 13. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 14. Renukuntla J, Vadlapudi AD, Patel A, Boddu SH, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm. 2013;447(1–2):75‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brazil R . Binning the sharps: the quest for oral insulin. Pharm J. 2019;303(7929). [Google Scholar]
- 16. Food and Drug Administration . Rybelsus prescribing information. 2019; https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf. Accessed October 1, 2019.
- 17. European Medicines Agency . First oral GLP‐1 treatment for type 2 diabetes. https://www.ema.europa.eu/en/news/first-oral-glp-1-treatment-type-2-diabetes. Accessed March 5, 2020.
- 18. Mousa SA, Zhang F, Aljada A, et al. Pharmacokinetics and pharmacodynamics of oral heparin solid dosage form in healthy human subjects. J Clin Pharmacol. 2007;47(12):1508‐1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bittner B, McIntyre C, Tian H, et al. Phase I clinical study to select a novel oral formulation for ibandronate containing the excipient sodium N‐[8‐(2‐hydroxybenzoyl) amino] caprylate (SNAC). Pharmazie. 2012;67(3):233‐241. [PubMed] [Google Scholar]
- 20. Castelli MC, Friedman K, Sherry J, et al. Comparing the efficacy and tolerability of a new daily oral vitamin B12 formulation and intermittent intramuscular vitamin B12 in normalizing low cobalamin levels: a randomized, open‐label, parallel‐group study. Clin Ther. 2011;33(3):358‐371.e2. [DOI] [PubMed] [Google Scholar]
- 21. Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon‐like peptide‐1 receptor agonist. Sci Transl Med. 2018;10(467):eaar7047. [DOI] [PubMed] [Google Scholar]
- 22. Bækdal TA, Borregaard J, Donsmark M, Breitschaft A, Søndergaard FL. Evaluation of the effects of water volume with dosing and post‐dose fasting period on pharmacokinetics of oral semaglutide. Paper presented at American Diabetes Association 77th Annual Scientific Sessions; 9‐13 June, 2017; San Diego, USA.
- 23. Connor A, Donsmark M, Hartoft‐Nielsen M‐L, Sondergaard FL, Bækdal TA. A pharmacoscintigraphic study of the relationship between tablet erosion and pharmacokinetics of oral semaglutide. Paper presented at American Diabetes Association 77th Annual Scientific Sessions; 9‐13 June, 2017; San Diego, USA.
- 24. Maarbjerg SJ, Borregaard J, Breitschaft A, Donsmark M, Søndergaard FL. Evaluation of the effect of food on the pharmacokinetics of oral semaglutide. Abstract #148 (Study 4154). Paper presented at: European Association for the Study of Diabetes (EASD) – 53rd Annual Meeting; 11‐15 September, 2017; Lisbon, Portugal
- 25. Granhall C, Donsmark M, Blicher TM, et al. Safety and pharmacokinetics of single and multiple ascending doses of the novel oral human GLP‐1 analogue, oral semaglutide, in healthy subjects and subjects with type 2 diabetes. Clin Pharmacokinet. 2019;58(6):781‐791. [DOI] [PubMed] [Google Scholar]
- 26. Overgaard RV, Navarria A, Hertz CL, Ingwersen SH. Similar efficacy and gastrointestinal tolerability versus exposure for oral and subcutaneous semaglutide. Paper presented at 55th Annual Meeting of the European Association for the Study of Diabetes (EASD); 16‐20 September, 2019; Barcelona, Spain
- 27. Granhall C, Sondergaard FL, Thomsen M, Anderson TW. Pharmacokinetics, safety and tolerability of oral semaglutide in subjects with renal impairment. Clin Pharmacokinet. 2018;57(12):1571‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bækdal TA, Thomsen M, Kupcova V, Hansen CW, Anderson TW. Pharmacokinetics, safety, and tolerability of oral semaglutide in subjects with hepatic impairment. J Clin Pharmacol. 2018;58(10):1314‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meier JJ, Granhall C, Hoevelmann U, et al. Effect of upper gastrointestinal disease on the pharmacokinetics of oral semaglutide in subjects with type 2 diabetes. Paper presented at American Diabetes Association 79th Annual Scientific Sessions; 7‐11 June, 2019; San Francisco, USA. [DOI] [PubMed]
- 30. Jordy AB, Breitschaft A, Christiansen E, et al. Oral semaglutide does not affect the bioavailability of the combined contraceptive ethinylestradiol/levonorgestrel. Paper presented at 54th Annual Meeting of the European Association for the Study of Diabetes (EASD); 1‐5 October, 2018; Berlin, Germany
- 31. Bækdal TA, Breitschaft A, Navarria A, Hansen CW. A randomized study investigating the effect of omeprazole on the pharmacokinetics of oral semaglutide. Exp Opin Drug Metab Toxicol. 2018;14(8):869‐877. [DOI] [PubMed] [Google Scholar]
- 32. Bækdal TA, Borregaard J, Hansen CW, Thomsen M, Anderson TW. Effect of oral semaglutide on the pharmacokinetics of lisinopril, warfarin, digoxin, and metformin in healthy subjects. Clin Pharmacokinet. 2019;58(9):1193‐1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hauge C, Breitschaft A, Hartoft‐Nielsen ML, Jensen S, Bækdal TA. A drug‐drug interaction trial of oral semaglutide with levothyroxine and multiple co‐administered tablets. Paper presented at 101st Annual Meeting and Expo of the Endocrine Society (ENDO); 23‐26 March, 2019; New Orleans, USA
- 34. Davies M, Pieber TR, Hartoft‐Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318(15):1460‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial comparing the efficacy and safety of oral semaglutide monotherapy with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724‐1732. [DOI] [PubMed] [Google Scholar]
- 36. Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(9):1724‐1732. [DOI] [PubMed] [Google Scholar]
- 37. Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321(15):1466‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double‐blind, phase 3a trial. Lancet. 2019;394(10192):39‐50. [DOI] [PubMed] [Google Scholar]
- 39. Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo‐controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515‐527. [DOI] [PubMed] [Google Scholar]
- 40. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841‐851. [DOI] [PubMed] [Google Scholar]
- 41. Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open‐label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528‐539. [DOI] [PubMed] [Google Scholar]
- 42. Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42(12):2262‐2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamada Y, Katagiri H, Hamamoto Y, et al. Dose‐response, efficacy and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52‐week, phase 2/3a, randomised, multicentre trial. Lancet Diabetes Endocrinol. 2020;8(5):377‐391. [DOI] [PubMed] [Google Scholar]
- 44. Yabe D, Nakamura J, Kaneto H, et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): a multicentre, open‐label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2020;8(5):392‐406. [DOI] [PubMed] [Google Scholar]
- 45. Aroda VR, Saugstrup T, Buse JB, Donsmark M, Zacho J, Davies MJ. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: the PIONEER 1 randomized clinical trial as an example. Diabetes Obes Metab. 2019;21(10):2203‐2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frias JP. Safety of once‐weekly glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes. J Fam Pract. 2018;67(6 suppl):S25‐S34. [PubMed] [Google Scholar]
- 48. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on east Asian perspectives. J Diabetes Investig. 2016;7(Suppl 1):102‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. American Diabetes Association . Cardiovascular disease and risk management: standards of medical care in diabetes‐2020. Diabetes Care. 2019;43(Suppl 1):S111‐S134. [DOI] [PubMed] [Google Scholar]
- 50. Bain SC, Mosenzon O, Arechavaleta R, et al. Cardiovascular safety of oral semaglutide in patients with type 2 diabetes: rationale, design and patient baseline characteristics for the PIONEER 6 trial. Diabetes Obes Metab. 2019;21(3):499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of liraglutide versus placebo as add‐on to glucose‐lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA‐RENAL): a randomized clinical trial. Diabetes Care. 2016;39(2):222‐230. [DOI] [PubMed] [Google Scholar]
- 52. Bloomgarden Z. The kidney and cardiovascular outcome trials. J Diabetes. 2018;10(2):88‐89. [DOI] [PubMed] [Google Scholar]
- 53. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421‐426. [DOI] [PubMed] [Google Scholar]
- 54. Schmieder RE, Mann JF, Schumacher H, et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol. 2011;22(7):1353‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rasmussen MF. The development of oral semaglutide, an oral GLP‐1 analog, for the treatment of type 2 diabetes. Diabetol Int. 2020;11(2):76‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


