Abstract
Background
Comel‐Netherton syndrome (NS) is a rare autosomal disease, characterized by severe skin disease, hair shaft defects, atopic diathesis, and increased susceptibility for skin infections. Since patients with NS suffer from recurrent infections, it has been hypothesized that an underlying immunodeficiency attributes to this. Here, we studied clinical and immunological characteristics of the cohort of NS patients in the Netherlands in order to identify whether potential immunodeficiencies result in the increased risk of infectious complications.
Methods
Phenotypes were scored for severity of skin condition, specific hair shaft defects, atopy, and recurrent infections. Patients’ blood samples were collected for quantification of serum immunoglobulin (Ig) levels, specific antibodies against Streptococcus pneumoniae, and allergen‐specific IgE, as well as detailed immunophenotyping of blood leukocyte and lymphocyte subsets by flow cytometry.
Results
A total of 14 patients were included with age range 3‐46 years and varying degrees of skin involvement. All patients presented with atopic symptoms (food allergy, n = 13; hay fever, n = 10; asthma, n = 7). Recurrent skin infections were common, particularly in childhood (n = 12). Low levels of specific antibodies against S pneumoniae were found in 10 of 11 evaluated patients. Detailed immunological analysis was performed on 9 adult patients. Absolute numbers of lymphocyte subsets and serum immunoglobulin levels were all within normal ranges.
Conclusion
Multidisciplinary evaluation of our national cohort showed no evidence for a severe, clinically relevant systemic immunodeficiency. Therefore, we conclude that in Dutch NS patients the increased risk of infections most likely results from the skin barrier disruption and that increased allergen penetration predisposes to allergic sensitization.
Keywords: allergy, Comel‐Netherton syndrome, dermatitis, immunodeficiency, SPINK5
Fourteen patients with Netherton disease with varying degrees of skin involvement were evaluated for clinical and immunological characteristics. Multidisciplinary evaluation, including blood immunophenotyping, shows no evidence for a severe, clinically relevant systemic immunodeficiency. Increased risk of infections in Netherton patients most likely results from the skin barrier disruption and increased allergen penetration predisposes to allergic sensitization.
Abbreviations
- BSA
body surface area
- ELA
elastase
- IGA‐NS
Investigator's Global Assessment for Netherton syndrome
- ILC
ichthyosis linearis circumflexa
- KLK
kallikrein‐related peptidase
- LEKTI
lymphoepithelial kazal type‐related inhibitor
- NS
Comel‐Netherton syndrome
- PAR
protease‐activated receptor
- SPINK5
serine protease inhibitor of kazal type 5
- TARC
thymus and activation‐regulated chemokine
- TSLP
thymic stromal lymphopoietin
1. INTRODUCTION
Comel‐Netherton syndrome (NS) (OMIM #256500) is a severe genodermatosis typically characterized by chronic inflammatory skin lesions (ichthyosis and scaly erythroderma), specific hair shaft defects (trichorrhexis invaginata), and atopic diathesis with elevated serum IgE levels.1, 2 The disease is caused by variants in the SPINK5 gene (serine protease inhibitor of kazal type 5) encoding LEKTI (lymphoepithelial kazal type‐related inhibitor), which is expressed in the stratified epithelium of the skin, the mucosa, and the Hassal corpuscles of the thymus.3, 4, 5, 6
The consequence of LEKTI deficiency is a loss of inhibition of serine proteinases such as plasmin, trypsin, subtilisin A, cathepsin G, and elastase.7, 8, 9, 10, 11 This particularly leads to unopposed activity of kallikrein‐related peptidase 5 (KLK5), which activates KLK7, KLK14, and elastase 2 (ELA2).12, 13, 14 In the skin, this leads to increased degradation of corneodesmosomal cadherins through increased degradation of desmoglein 1, increased desmosome cleavage, and reduced filaggrin proteolytic processing.15, 16, 17 The result is an abnormal skin homeostasis and detachment of the stratum corneum, which contributes to a defective skin barrier and thereby enabling microbe and allergen penetration.18 KLK5 also activates protease‐activated receptor 2 (PAR‐2) which is expressed on the surface of keratinocytes.19 Observations from SPINK5 knockout mouse embryos indicate a KLK5‐PAR2 cascade, leading to enhanced production of thymic stromal lymphopoietin (TSLP), a T‐helper 2 (Th2)‐related cytokine, enhancing the allergic predisposition.20 Furthermore, PAR‐2 leads to increased expression of TNF‐α, IL‐8, and ICAM‐1, thereby augmenting an inflammatory process.21
The effects of defective LEKTI expression in the thymus and its effect on T‐cell maturation are less well‐described. However, apart from dermal and allergic inflammation, the disease has also been associated with immunodeficiency and increased susceptibility to skin, respiratory tract, and systemic infections.22, 23, 24, 25 Although recurrent skin infections with Staphylococcus aureus can be related to altered skin homeostasis, some have attributed these observations to intrinsic immune defects, with improvement after intravenous immunoglobulin treatment.22 Described immune defects include decreased numbers of natural killer (NK) cells, an immature phenotype of NK cells with reduced lytic function, reduced numbers of memory B cells, and reduced responses to pneumococcal vaccinations.22, 23, 24, 25, 26 Although a premature senescent state of T cells has been proposed, there have been no reports on dysfunctional T‐cell properties. This is likely due to the fact that NS is a rare disease, and the described patient cohorts are small (mostly 2‐9 patients) and mainly consist of children.
To examine the potential of immunodeficiency in NS, we actively recruited all known patients in the Netherlands for detailed clinical and immunological examinations including skin phenotype, allergic manifestations, and blood leukocyte immunophenotyping.
2. METHODS
2.1. Study design and patient characteristics
The study was designed as a cross‐sectional study. All known 16 patients with NS in the Netherlands were invited to participate in the study. Patients were actively recruited through the Erasmus MC University Medical Centre Rotterdam, an acknowledged national expert center for patients with NS, through the patient association and by social media. Patients were diagnosed based on the presence of germline variants in SPINK5, LEKTI deficiency in skin biopsy, and/or trichorrhexis invaginata (ISRCTN12831121).27 Patients and controls were included after obtaining written informed consent and with approval of the Medical Ethical Committee of the Erasmus MC (MEC‐2013‐026), which complies with the Helsinki declaration. Control blood samples were obtained from healthy volunteers among department staff (MEC‐2016‐022).
All patients completed questionnaires about their medical well‐being, including medical history, (daily) medication use, daily skin appearance, growth rate, frequency of infections, hospital admissions, and (food) allergies. Subsequently, patients were invited for blood sampling and a multidisciplinary evaluation by a (pediatric) dermatologist and (pediatric) immunologist of our Netherton team.
Detailed immunological evaluation was performed in 9 out of 14 patients. Four of the five patients not included were children who were excluded because patient numbers in these age groups were too low for reliable statistical analysis. One adult patient was excluded who did not consent to blood collection. For specific flow cytometric analysis of the immune cells, a control group (n = 27) was included to determine assay‐specific reference values.
2.2. Skin assessment and recurrent infections
To objectively score skin lesions, the body surface area (BSA in %), the Investigator's Global Assessment for NS (IGA‐NS) and the total lesional sign score (TLSS) were used (Table S1).28, 29, 30
Definitions of recurrent infections were based on the 10 warning signs for primary immunodeficiency stated by the working party of ESID (European Society for Immunodeficiencies)31 and are defined as follows: recurrent skin infections >1 per year; recurrent respiratory tract infections >2 per year for adults, >6 per year for children; recurrent ear nose and throat (ENT) infections >4 per year; and recurrent gastrointestinal tract infections >1 per year.
2.3. Immunoglobulin serology
Specific IgE for inhalation and food allergens were determined using ImmunoCAP and/or the Immuno Solid‐phase Allergen Chip (ImmunoCAP ISAC, Phadia), according to manufacturer's instructions.
Total IgM, IgG, and IgA serum levels were measured by immunonephelometry with a Siemens BN II nephelometer according to manufacturer guidelines. Specific antibody titers against Streptococcus pneumoniae were analyzed using a Luminex assay according to a protocol adopted from Borgers et al32
2.4. Flow cytometric immunophenotyping of blood leukocytes
Absolute numbers of granulocytes, monocytes, lymphocytes, and NK cells (CD16+/CD56+), T cells (CD3+), and B cells (CD19+) were obtained with a diagnostic lyse‐no‐wash protocol using commercial Trucount tubes (BD Biosciences). For detailed 11‐color flow cytometry, red blood cells were lysed with NH4Cl prior to incubation of 1 million nucleated cells with antibody cocktails for 15 minutes at room temperature in a total volume of 100 µL.33 After preparation, cells were measured on a 4‐laser LSRFortessa flow cytometer (BD Biosciences) using standardized settings.34 Data were analyzed with FACSDiva software V8.0 (BD Biosciences).
2.5. Statistical analyses
Frequencies and absolute cell numbers were assumed a non‐Gaussian distribution. All results are expressed as median values with interquartile range if applicable. Results were analyzed using the nonparametric Mann‐Whitney U test; all tests were two‐tailed, and P values < 0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism software, version 6 (GraphPad Software).
3. RESULTS
3.1. Clinical characteristics of the patient cohort
For the present study, all known patients with NS in the Netherlands (n = 16) were invited; however, due to private circumstances two patients refused to participate. The included 14 patients (6 males and 8 females) ranged in age from 3 to 46 years (median, 24 years), and included four children, which were all female (Table 1).
Table 1.
Patient characteristics and clinical manifestations (n = 2)
| Patient | Sex | Age (y) | Diagnosis | Skin at birth | Skin present | Hair | Atopic manifestation | Serum IgE (IU/mL) | BSA | IGA | TLSS | Age of improvement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 43 | Genetic | ED | ED | TI, short, easily broken | Rhinitis, food allergy | 22 | Total | 4 | E3I2L2 | None |
| 2 | F | 46 | Genetic | Other | ILC + ED | TI | Asthma, rhinitis, atop. dermatitis, food allergy | 159 | >50% | 2 | E1I1L0 | None |
| 3 | M | 21 | Genetic | ED | ILC + ED | TI, easily broken | Atop. dermatitis, food allergy | >5000 | Total | 4 | E3I3L2 | None |
| 4 | F | 9 | Genetic | Normal | ILC + ED | TI, short | Rhinitis, food allergy | 4524 | Total | 4 | E1I1L2 | None |
| 5 | F | 8 | Genetic | Other | ED | TI, short, easily broken | Asthma, rhinitis, food allergy | >5000 | Total | 4 | E3I3L3 | 1.5 y |
| 6 | F | 24 | Genetic | ED | ILC + ED | TI, short, easily broken | Rhinitis, food allergy | 4083 | >75% | 3 | E3I3L2 | 5 y |
| 7 | F | 22 | Genetic | ED | ILC + ED | TI | Food allergy | >5000 | Total | 3 | E2I2L2 | None |
| 8 | M | 24 | Genetic | ED + ILC | ILC | TI, easily broken | Asthma, rhinitis, atop. dermatitis, food allergy | >5000 | >75% | 3 | E2I2L2 | 12 y |
| 9 | M | 36 | Genetic | Normal | ILC | TI | None | >5000 | Total | 2 | E2I1L2 | None |
| 10 | F | 6 | Genetic | ED | ILC + ED | TI, short, easily broken | Asthma, rhinitis, atop. dermatitis, food allergy | Not available | >50% | 3 | E2I3L3 | 1 y |
| 11 | F | 3 | Genetic | ED | ILC + ED | TI | Atop. dermatitis, food allergy | >5000 | <50% | 3 | E2I1L1 | 1 y |
| 12 | M | 39 | Genetic | ED | ILC + ED | TI, short easily broken | Asthma, rhinitis, atop. dermatitis, food allergy | 3522 | >50% | 3 | E1I2L3 | 12 y |
| 13 | M | 43 | Genetic | ED | ILC | TI | Asthma, rhinitis, atop. dermatitis, food allergy | 136 | <25% | Not available | Missing | 14 y |
| 14 | M | 36 | Genetic | ED | ILC | TI, easily broken | Asthma, rhinitis, atop. dermatitis, food allergy | >5000 | >50% | 2 | E2I2L0 | 25 y |
Abbreviations: BSA, body surface area; ED, erythroderma; IGA, Investigator's Global Assessment; ILC, ichthyotic linearis circumflexa; TI, trichorrhexis invaginata; TLSS, total lesional sign score (see also Table S1).
Ten patients were born with generalized erythroderma and two patients developed erythroderma within several hours after birth (Table 1). The skin severity varied between patients: Six patients had an affected body surface area (BSA) of 100% and 5 patients of >50% with a median Investigator Global Assessment (IGA)‐NS score of 3 (IQR 3‐4). All patients had trichorrhexis invaginata. Seven out of 14 patients noticed improvement of their skin over time with a median of 6.5 years. All patients reported daily invalidation on a scale of 1‐10 with a median of 5 (IQR 3‐6), a daily pain numerical rating scale (NRS) median of 7.0 (IQR 2.8‐8.0), and pruritus NRS median of 4.0 (IQR 3.0‐7.5; Table S2).
When compared to the normal growth chart for children and the average height for adults (data of the Netherlands Organization for Applied Scientific Research), patients seemed to be smaller than the average population (Table S3).
3.2. Atopic syndrome
Ten out of 14 patients had self‐reported hay fever, and 7 patients had self‐reported asthma. Thirteen out of 14 patients reported reactions to food (especially nuts, cow milk, eggs, and fish) with symptoms occurring within two hours after intake (nausea, stomach pain, or edema of the nasopharynx) (Table 1 and Table S4). Most patients had refused double‐blind food provocation tests due to severe acute reactions. Additional laboratory tests were performed to confirm IgE sensitization to inhalant and food allergens. All 10 patients who were tested had IgE sensitization to food allergens (Table S4). Eleven out of the 12 tested patients had elevated total immunoglobulin E (IgE) above 100 IE/mL, and one patient had a normal IgE level of 22 IE/mL (Table 1).
3.3. Treatment
All 14 patients used emollients for daily skin treatment, and 12 out of 14 patients needed topical corticosteroids, ranging from hydrocortisone acetate to clobetasol propionate. One patient intermittently used tacrolimus ointment based on the severity of complaints, and one patient used coal tar. Three patients received treatment with oral antihistamines, and all received treatment with inhalation medication. At the time of study inclusion, none of the patients received systemic immunosuppressive treatment.
3.4. Immunological evaluation
A significant number of infections were reported in 13 out of 14 patients, particularly recurrent skin infections being a common problem, with a median of 4.1 infections requiring treatment with antibiotics every year (IQR: 2.8‐5.0; Table 2). Seven patients reported recurrent ENT infections in early childhood for which repetitive antibiotic treatments were required. In one patient, recurrent ENT infections persisted throughout adulthood. Several patients reported the need of regular cleaning of the external auditory meatus every 4 weeks to prevent external ear infections. Before the age of 6 years, patients did not report frequent respiratory infections. Of the 12 patients above the age of 6 years, 7 patients suffered from recurrent skin infections in the previous 12 months. One patient reported recurrent gastrointestinal infections as a child, and none reported these during adulthood. Similarly, one patient reported severe systemic infections during childhood, but none were reported during adulthood. No patients described a period of persistent fever after vaccinations (Table 2).
Table 2.
Infectious manifestation and immunological characteristics (n = 2)
| Patient | Recurrent infections | Treatment with IVIG | Serum Ig levels (g/L) | Antibody titers against S pneumoniae | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Resp tract | ENT | Gastrointestinal | Severe invasive | IgM (0.45‐2.3)a | IgG (7.0‐16.0)a | IgA (0.76‐3.91)a | ||||||||
| ≤6 y | >6 y | ≤6 y | >6 y | ≤6 y | >6 y | ≤6 y | >6 y | ≤6 y | >6 y | ||||||
| 1 | Yes | Yes | — | — | — | — | — | — | — | — | — | 0.52 | 22.0 | 5.70 | Low |
| 2 | Yes | — | Yes | Yes | Yes | — | — | ‐ | — | — | 1.98 | 11.0 | 2.82 | Low | |
| 3 | Yes | Yes | — | — | Yes | ‐ | — | — | ‐ | — | — | 1.19 | 7.0 | 2.54 | n.d. |
| 4 | Yes | Yes | — | — | Yes | ‐ | Yes | — | ‐ | — | — | 0.97 | 12.2 | 1.80 | Normal |
| 5 | Yes | ‐ | Yes | — | Yes | Yes | — | — | Yes | — | — | 0.97 | 13.9 b | 1.84 | n.d. |
| 6 | Yes | Yes | — | — | — | — | — | — | ‐ | — | — | 0.92 | 9.6 | 1.24 | Low |
| 7 | Yes | Yes | — | — | Yes | — | — | — | ‐ | — | Yes | 1.36 | 16.8 | 2.11 | Low |
| 8 | — | — | — | — | ‐ | — | — | — | ‐ | — | — | 0.34 | 12.4 | 2.35 | Low |
| 9 | Yes | Yes | — | — | ‐ | — | — | — | ‐ | — | — | 0.47 | 13.7 | 3.87 | Low |
| 10 | Yes | Yes | — | — | ‐ | — | — | — | ‐ | — | Yes | 0.67 | 10.7 | 1.81 | Low |
| 11 | Yes | Yes | — | — | Yes | Yes | — | — | ‐ | — | — | 1.13 | 12.2 | 1.53 | Low |
| 12 | Yes | — | — | — | Yes | ‐ | — | — | ‐ | — | — | 0.26 | 14.2 | 1.71 | Low |
| 13 | Yes | — | — | — | — | — | — | — | ‐ | — | — | n.d. | n.d. | n.d. | n.d. |
| 14 | — | Yes | Yes | — | — | — | — | — | ‐ | — | — | 0.51 | 9.0 | 1.89 | Low |
Values below the normal range are depicted in bold font; above normal range in italics.
Abbreviation: n.d., not determined.
Normal values for adults.
Normal values children aged 5‐10 y are IgM 0.3‐1.8 g/L, IgG 5.0‐13.0 g/L, and IgA 0.5‐2.3 g/L.
None of the 13 tested patients showed an overt antibody deficiency. All patients had normal to high levels of IgG and IgA, and only 2 patients had reduced IgM serum levels (Table 2). In 10 out of 11 tested patients, levels of specific antibodies against S pneumoniae were low (Table 2).
3.5. Patients with NS have higher numbers of granulocytes
Blood leukocytes and their subsets were studied in 9 of the 10 adult patients (Figure 1). One patient had a leukocyte count above the normal range, which was caused by an elevated granulocyte count. Compared with the healthy individuals, patients with NS had a significantly higher granulocyte count (P = .04). Absolute numbers of total lymphocytes and monocytes were in the normal range. Within the granulocyte subset, mainly the numbers of neutrophils seemed higher, although this was not significantly different from controls. Two patients had an increased eosinophil count. All patients had a normal basophil count, yet the median count was significantly lower than that of the control group (P = .02). Numbers of plasmacytoid dendritic cells (pDCs) were dramatically decreased in the patient group (P < .001). Within the lymphocyte subset, one patient had an increased B‐cell count and one patient had an increased T‐cell count, yet as a group the median T‐cell count was significantly lower than that of healthy controls (P = .04). In our study cohort, NK‐cell numbers were quite diverse, but not statistically different from the control group. Still, one patient had increased NK‐cell numbers, whereas two patients had reduced NK‐cell numbers.
Figure 1.
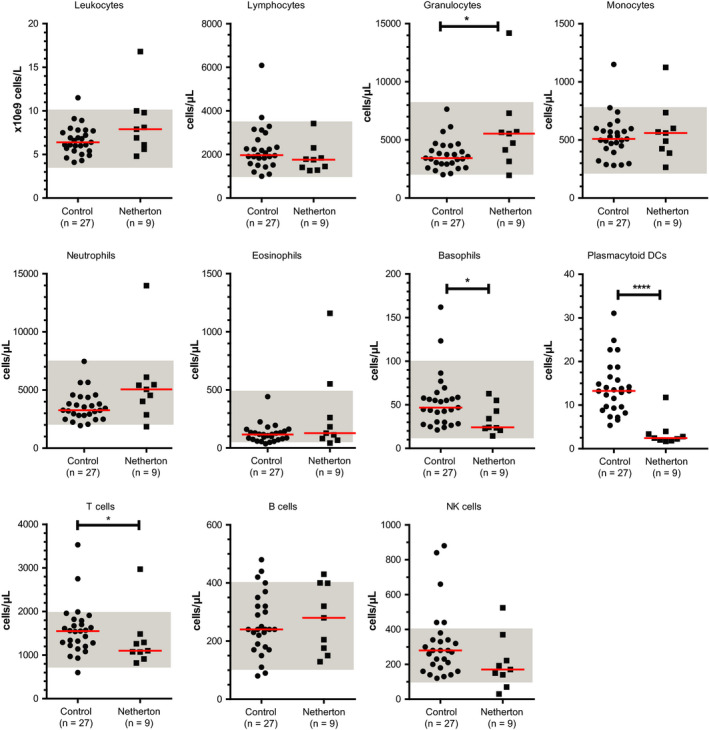
Absolute numbers of leukocytes and leukocyte subsets in healthy individuals and in adult patients with NS. Each symbol represents an individual with red lines indicating median values. Normal ranges are depicted in gray shades. Statistics, Mann‐Whitney U test; *P < .05; ****P < .0001 (n = 3)
3.6. Patients with NS have lower IgM‐only memory B‐cell numbers and increased IgE+ CD27− memory B‐cell numbers
Within the B‐cell compartment, we studied naive, memory, and effector B‐cell subsets (Figure 2). Absolute numbers of naive B‐cell subsets (transitional B cells and naive mature B cells) were comparable to that of healthy controls. In the memory compartment, median cell numbers of IgM‐only memory B cells (IgM+ IgD− CD27+) were significantly lower than that of healthy controls (P = .03), whereas cell numbers of other memory B‐cell subsets (IgM+ IgD+ CD27+ natural effector B cells, IgG+ and IgA+ memory B cells) were comparable to those of healthy controls (Figure 2A). In contrast, the median cell number of IgE + CD27− memory B cells was significantly higher in the patient group (P = .01; Figure 2B). Plasma blast numbers seemed much lower in patients with NS. One patient had a high plasma blasts count; therefore, as a group there was no significant difference between patients and controls.
Figure 2.
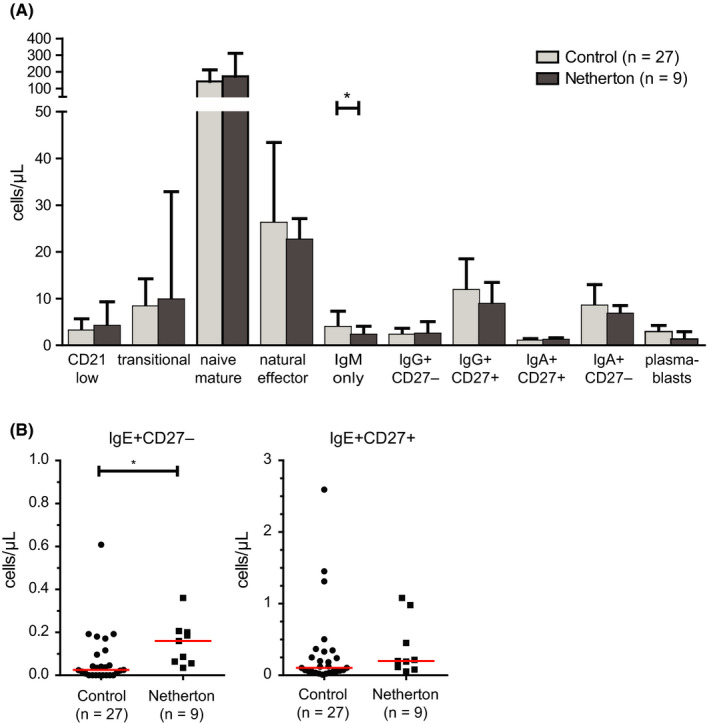
Absolute numbers of B‐cell subsets in healthy individuals and in adult patients with NS. A, Absolute numbers of naive and memory B‐cell subsets and plasma cells. Columns indicate median values with interquartile range. B, Absolute numbers of IgE + memory B cells. Each symbol represents an individual with red lines indicating median values. Statistics, Mann‐Whitney U test; *P < .05 (n = 3)
3.7. Patients with NS have lower Th1‐cell numbers
Since LEKTI is highly expressed in the thymus, the primary organ for T‐cell development, we performed detailed analysis of the T‐cell compartment (Figure 3). Although total T‐cell numbers were significantly lower in the total group of patients as compared to controls, all patients but one had T‐cell numbers still within the normal range. In line with this, total CD4+ and CD8+ T cells, as well as numbers of naive, central memory, and effector memory subsets within CD4+ and CD8+ T cells, were not different between patients and controls (Figure 3A and 3).
Figure 3.

Absolute numbers of T‐cell subsets in healthy individuals and in adult patients with NS. A, of total CD8 + T cells and CD8 + Tnaive, Tcm, TemRO, and TemRA. B, Total CD4+ T cells and CD4 + Tnaive, Tcm, TemRO, and TemRA. Columns represent median values with interquartile range. C, Counts of regulatory T cells, Th1, Th2, and Th17 T cells. Each symbol represents an individual with red lines indicating median values. Statistics, Mann‐Whitney U test; *P < .05 (n = 3)
Within the T‐helper cells, numbers of Th1 cells were significantly lower in patients with NS, whereas Th2 cell numbers were not affected (Figure 3C). Th17 nor regulatory T‐cell numbers were different between patients and controls (Figure 3C).
4. DISCUSSION
In this case series, 14 of the 16 known NS patients in the Netherlands were evaluated for their clinical phenotypes, atopic diathesis, and immunological characteristics. To our knowledge, this is the first described national NS patient cohort. Their history and clinical presentation are an illustration of the wide spectrum of disease in patients with NS, especially concerning clinical features, disease severity, and follow‐up.26
Immunological evaluation of our patients showed no evidence for a severe, clinically relevant systemic immunodeficiency, even in the more severely affected patients, which is in contrast to other studies.22, 26 Although low titers of specific antibodies against S pneumoniae were measured, the current evaluation could not confirm the presence of a clinically relevant humoral immunodeficiency, based on the fact that patients did not suffer from an increased number of severe infections that can be contributed to these findings.22 Functional assays evaluating potential NK‐cell disturbances were not performed, but NK‐cell numbers were not affected. This does not explain the difference between our observations with previous studies; however, an explanation might be that due to the national recruitment we experienced less selection bias resulting in more diversity in our cohort, hence giving a better reflection of the whole spectrum of NS, at least in the Netherlands. The dramatically decreased numbers of pDCs can be explained by the fact that these cells are highly sensitive to (cutaneous) corticosteroid therapy.35 One patient used tacrolimus ointment, which has been reported to result in increased systemic absorption in children with Netherton disease.36 The patient in our cohort was an adult with intermittent use, for whom no data on systemic side effects were acquired.
All patients in the Dutch cohort were below the age of 50 years. We hypothesized that NS patients may have an accelerated aging of the immune system due to the severe skin barrier defect and/or by the defective LEKTI expression in the thymus, which may result in a lower life expectancy. This was not confirmed by our data as the numbers of naive T cells as well as effector T‐cell subsets were within normal range. This study does not explain the young age of the adult patients.
The increased manifestation of skin and ENT infections, especially in childhood, observed in our cohort, may still imply an immunodeficiency according to the guidelines of European Society for Immunodeficiencies (ESID),37 although in our cohort it is not known by which pathogens the infections were caused. We did observe lower numbers of IgM‐only memory B cells (IgM+ IgD− CD27+); however, this has not been associated with immunodeficiency. Natural effector (IgM+ IgD+ CD27+) B cells are responsible for natural antibodies, whereas lower numbers of switched memory B cells (CD27+ IgM− IgD−) are found in patients with primary antibody deficiencies, including common variable immune deficiency (CVID).38 In our patients, switched memory B‐cell numbers were within the normal range. Moreover, no hypogammaglobulinemia was found in NS patients. The clinical relevance of the decreased IgM‐only memory B cells remains therefore undetermined.
The recurrent ear infections in children and adults with NS could well be caused by obstruction of the external auditory canal as a result of excessive skin scaling. As patients were born erythroderm, the observed change in skin phenotypes from erythroderma into ichthyosis linearis circumflexa (ILC) in some patients is in line with previously described NS patients. Our cohort included less severely affected NS patients and half of our patients reported improvement of their skin with age (median age: 6.5 years). This might explain why less skin infections were reported in adults. We also hypothesized an impact of natural maturation of the immune system. In general, an increased susceptibility to infections during childhood has previously been described in healthy children, especially for respiratory infections and nasal immune responses in the first 2 years of life.39
Evaluation of atopic manifestations showed a remarkable high percentage of sensitization to food allergens, which is in line with previous observations.40 The increased risk of an atopic constitution corresponded to an elevated total IgE in 11 of the 12 tested patients as well as increased IgE+ memory B‐cell numbers. Similar observations have been described in patients with atopic dermatitis.41 As described, the impaired epidermal skin barrier may open aberrant routes of entrance for allergens.42, 43 Combined with a different local immune response with enhanced risk of Th2 imprinting, this could lead to further sensitization to (food) allergens.20, 42, 44 In our cohort food allergies for nuts, cow milk, eggs, and fish were most common. The types of allergens do not differ from the general population in which 90% of the allergic responses to food are caused by cow's milk, soy, eggs, wheat, peanuts, tree nuts, fish, and shellfish.45, 46, 47, 48 This suggests that the role of the skin barrier and subepithelial environment with an increased Th2 profile might be greater than assumed for the atopic constitution in NS. Although Th2‐cell numbers were not affected and we did not measure specific cytokines such as TSLP and TARC (thymus and activation‐regulated chemokine), we speculate that Th2 cytokines are increased as well. In addition, possibly not only cell numbers but also balances between Th1/Th2 immunity are important which is in line with our observations which show significant lower Th1‐cell numbers implying a disturbed Th1/Th2 balance. However, since NS patients display ichthyosis also a Th17/IL23 pathway could be involved.49
To our knowledge, this national cohort of 14 NS patients is the largest described until now.22, 23, 26, 40, 50, 51, 52, 53, 54, 55 Because of active recruitment, we assume less selection bias and greater diversity in severity in our cohort. For example, two patients were not in care before active recruitment and received their topical treatment from their general practitioner. Another strength is the multidisciplinary approach of this study. Evaluation of anamnestic information was partially based on retrospective data and information on the type of microorganisms involved in previous infections was incomplete and can be considered as a limitation.
4.1. Clinical implications
Evaluation of patients with Netherton syndrome in the Dutch cohort showed no evidence for a severe, clinically relevant systemic immunodeficiency. The syndrome more likely seems a severe skin disease due to a local impaired skin barrier with an increased risk of infections and sensitization to food allergens, combined with reduced maturation of the immune system at a young age.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
AUTHOR CONTRIBUTIONS
KS, JJH, VASHD, MCvZ, and SGMAP designed research. KS, JJH, and RWJM performed research. KS, VASHD, EvH, SAMG, and SGMAP evaluated and included patients in the study. KS, JJH, VASHD, MCvZ, and SGMAP wrote the manuscript, and all authors commented on the manuscript and approved the final version.
Supporting information
ACKNOWLEDGMENTS
We would like to thank all patients and their family for their participation, their suggestions, and their enthusiasm. This work was financially supported through Grant S698 from the Sophia Children's Hospital Fund (SKF) and by NHMRC Fellowship GNT1117687 to MCvZ.
Stuvel K, Heeringa JJ, Dalm VASH, et al. Comel‐Netherton syndrome: A local skin barrier defect in the absence of an underlying systemic immunodeficiency. Allergy. 2020;75:1710–1720. 10.1111/all.14197
Stuvel and Heeringa contributed equally to this work.
REFERENCES
- 1. Comel M. Ichthyosis linearis circumflexa. Dermatologica 1949;98:133‐136. [PubMed] [Google Scholar]
- 2. Netherton EW. A unique case of trichorrhexis nodosa; bamboo hairs. AMA Arch Derm. 1958;78:483‐487. [DOI] [PubMed] [Google Scholar]
- 3. Bitoun E, Micheloni A, Lamant L, et al. LEKTI proteolytic processing in human primary keratinocytes, tissue distribution and defective expression in Netherton syndrome. Hum Mol Genet. 2003;12:2417‐2430. [DOI] [PubMed] [Google Scholar]
- 4. Chavanas S, Bodemer C, Rochat A, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141‐142. [DOI] [PubMed] [Google Scholar]
- 5. Magert HJ, Standker L, Kreutzmann P, et al. LEKTI, a novel 15‐domain type of human serine proteinase inhibitor. J Biol Chem. 1999;274:21499‐21502. [DOI] [PubMed] [Google Scholar]
- 6. Tartaglia‐Polcini A, Bonnart C, Micheloni A, et al. SPINK5, the defective gene in netherton syndrome, encodes multiple LEKTI isoforms derived from alternative pre‐mRNA processing. J Invest Dermatol. 2006;126:315‐324. [DOI] [PubMed] [Google Scholar]
- 7. Bonnart C, Deraison C, Lacroix M, et al. Elastase 2 is expressed in human and mouse epidermis and impairs skin barrier function in Netherton syndrome through filaggrin and lipid misprocessing. J Clin Invest. 2010;120:871‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitsudo K, Jayakumar A, Henderson Y, et al. Inhibition of serine proteinases plasmin, trypsin, subtilisin A, cathepsin G, and elastase by LEKTI: a kinetic analysis. Biochemistry 2003;42:3874‐3881. [DOI] [PubMed] [Google Scholar]
- 9. Fortugno P, Bresciani A, Paolini C, et al. Proteolytic activation cascade of the Netherton syndrome‐defective protein, LEKTI, in the epidermis: implications for skin homeostasis. J Invest Dermatol. 2011;131:2223‐2232. [DOI] [PubMed] [Google Scholar]
- 10. Hachem JP, Wagberg F, Schmuth M, et al. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Invest Dermatol. 2006;126:1609‐1621. [DOI] [PubMed] [Google Scholar]
- 11. Jayakumar A, Kang Y, Mitsudo K, et al. Expression of LEKTI domains 6–9' in the baculovirus expression system: recombinant LEKTI domains 6–9' inhibit trypsin and subtilisin A. Protein Expr Purif. 2004;35:93‐101. [DOI] [PubMed] [Google Scholar]
- 12. Deraison C, Bonnart C, Lopez F, et al. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH‐dependent interaction. Mol Biol Cell. 2007;18:3607‐3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egelrud T, Brattsand M, Kreutzmann P, et al. hK5 and hK7, two serine proteinases abundant in human skin, are inhibited by LEKTI domain 6. Br J Dermatol. 2005;153:1200‐1203. [DOI] [PubMed] [Google Scholar]
- 14. Schechter NM, Choi EJ, Wang ZM, et al. Inhibition of human kallikreins 5 and 7 by the serine protease inhibitor lympho‐epithelial Kazal‐type inhibitor (LEKTI). Biol Chem. 2005;386:1173‐1184. [DOI] [PubMed] [Google Scholar]
- 15. Descargues P, Deraison C, Bonnart C, et al. Spink5‐deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet. 2005;37:56‐65. [DOI] [PubMed] [Google Scholar]
- 16. Borgono CA, Michael IP, Komatsu N, et al. A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J Biol Chem. 2007;282:3640‐3652. [DOI] [PubMed] [Google Scholar]
- 17. Descargues P, Deraison C, Prost C, et al. Corneodesmosomal cadherins are preferential targets of stratum corneum trypsin‐ and chymotrypsin‐like hyperactivity in Netherton syndrome. J Invest Dermatol. 2006;126:1622‐1632. [DOI] [PubMed] [Google Scholar]
- 18. Meyer‐Hoffert U. Reddish, scaly, and itchy: how proteases and their inhibitors contribute to inflammatory skin diseases. Arch Immunol Ther Exp (Warsz). 2009;57:345‐354. [DOI] [PubMed] [Google Scholar]
- 19. Steinhoff M, Corvera CU, Thoma MS, et al. Proteinase‐activated receptor‐2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8:282‐294. [DOI] [PubMed] [Google Scholar]
- 20. Briot A, Deraison C, Lacroix M, et al. Kallikrein 5 induces atopic dermatitis‐like lesions through PAR2‐mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hosomi N, Fukai K, Nakanishi T, Funaki S, Ishii M. Caspase‐1 activity of stratum corneum and serum interleukin‐18 level are increased in patients with Netherton syndrome. Br J Dermatol. 2008;159:744‐746. [DOI] [PubMed] [Google Scholar]
- 22. Renner ED, Hartl D, Rylaarsdam S, et al. Comel‐Netherton syndrome defined as primary immunodeficiency. J Allergy Clin Immunol. 2009;124:536‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hannula‐Jouppi K, Laasanen SL, Ilander M, et al. Intrafamily and interfamilial phenotype variation and immature immunity in patients with Netherton syndrome and Finnish SPINK5 founder mutation. JAMA Dermatol. 2016;152:435‐442. [DOI] [PubMed] [Google Scholar]
- 24. Judge MR, Morgan G, Harper JI. A clinical and immunological study of Netherton's syndrome. Br J Dermatol. 1994;131:615‐621. [DOI] [PubMed] [Google Scholar]
- 25. Stryk S, Siegfried EC, Knutsen AP. Selective antibody deficiency to bacterial polysaccharide antigens in patients with Netherton syndrome. Pediatr Dermatol. 1999;16:19‐22. [DOI] [PubMed] [Google Scholar]
- 26. Van Gysel D, Koning H, Baert MR, Savelkoul HF, Neijens HJ, Oranje AP. Clinico‐immunological heterogeneity in Comel‐Netherton syndrome. Dermatology. 2001;202:99‐107. [DOI] [PubMed] [Google Scholar]
- 27. Lacroix M, Lacaze‐Buzy L, Furio L, et al. Clinical expression and new SPINK5 splicing defects in Netherton syndrome: unmasking a frequent founder synonymous mutation and unconventional intronic mutations. J Invest Dermatol. 2012;132:575‐582. [DOI] [PubMed] [Google Scholar]
- 28. Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997;195:10‐19. [DOI] [PubMed] [Google Scholar]
- 29. Yan AC, Honig PJ, Ming ME, Weber J, Shah KN. The safety and efficacy of pimecrolimus, 1%, cream for the treatment of Netherton syndrome: results from an exploratory study. Arch Dermatol. 2010;146:57‐62. [DOI] [PubMed] [Google Scholar]
- 30. A first‐in‐human study to evaluate safety and tolerability of repeated topical administrations of BPR277 ointment in healthy volunteers, and safety, tolerability and preliminary efficacy of multiple topical administrations of BPR277 in patients with atopic dermatitis and Netherton syndrome. BPR277, Protocol No. CBPR277X2101: Novartis Institutes for BioMedical Research, unpublished protocol.
- 31. Warning Signs of PID ‐ General. Available from https://esid.org/Working-Parties/Clinical-Working-Party/Resources/10-Warning-Signs-of-PID-General
- 32. Borgers H, Moens L, Picard C, et al. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol. 2010;134:198‐205. [DOI] [PubMed] [Google Scholar]
- 33. Heeringa JJ, Karim AF, van Laar JAM, et al. Expansion of blood IgG4(+) B, TH2, and regulatory T cells in patients with IgG4‐related disease. J Allergy Clin Immunol. 2018;141(5):1831‐1843. [DOI] [PubMed] [Google Scholar]
- 34. Kalina T, Flores‐Montero J, van der Velden VH, et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia 2012;26:1986‐2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shodell M, Siegal FP. Corticosteroids depress IFN‐alpha‐producing plasmacytoid dendritic cells in human blood. J Allergy Clin Immunol. 2001;108:446‐448. [DOI] [PubMed] [Google Scholar]
- 36. Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747‐750. [PubMed] [Google Scholar]
- 37. Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan‐American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93:190‐197. [DOI] [PubMed] [Google Scholar]
- 38. Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(‐)IgD(‐)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood 2002;99:1544‐1551. [DOI] [PubMed] [Google Scholar]
- 39. van Benten IJ, van Drunen CM, Koopman LP, et al. Age‐ and infection‐related maturation of the nasal immune response in 0‐2‐year‐old children. Allergy 2005;60:226‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hannula‐Jouppi K, Laasanen SL, Heikkila H, et al. IgE allergen component‐based profiling and atopic manifestations in patients with Netherton syndrome. J Allergy Clin Immunol. 2014;134:985‐988. [DOI] [PubMed] [Google Scholar]
- 41. Berkowska MA, Heeringa JJ, Hajdarbegovic E, et al. Human IgE(+) B cells are derived from T cell‐dependent and T cell‐independent pathways. J Allergy Clin Immunol. 2014;134:688‐697. [DOI] [PubMed] [Google Scholar]
- 42. Brough HA, Liu AH, Sicherer S, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2015;135:164‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nowak‐Wegrzyn A, Szajewska H, Lack G. Food allergy and the gut. Nat Rev Gastroenterol Hepatol. 2017;14:241‐257. [DOI] [PubMed] [Google Scholar]
- 44. Watanabe N, Wang YH, Lee HK, et al. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 2005;436:1181‐1185. [DOI] [PubMed] [Google Scholar]
- 45. Yu W, Freeland DM, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider‐diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berni Canani R, Di Costanzo M, Troncone R. The optimal diagnostic workup for children with suspected food allergy. Nutrition 2011;27:983‐987. [DOI] [PubMed] [Google Scholar]
- 48. Lehrer SB, Ayuso R, Reese G. Current understanding of food allergens. Ann N Y Acad Sci. 2002;964:69‐85. [DOI] [PubMed] [Google Scholar]
- 49. Paller AS, Renert‐Yuval Y, Suprun M, et al. An IL‐17‐dominant immune profile is shared across the major orphan forms of ichthyosis. J Allergy Clin Immunol. 2017;139:152‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yalcin AD. A case of netherton syndrome: successful treatment with omalizumab and pulse prednisolone and its effects on cytokines and immunoglobulin levels. Immunopharmacol Immunotoxicol. 2016;38:162‐166. [DOI] [PubMed] [Google Scholar]
- 51. Smith DL, Smith JG, Wong SW, deShazo RD. Netherton's syndrome: a syndrome of elevated IgE and characteristic skin and hair findings. J Allergy Clin Immunol. 1995;95:116‐123. [DOI] [PubMed] [Google Scholar]
- 52. Kogut M, Salz M, Hadaschik EN, Kohlhase J, Hartmann M. New mutation leading to the full variety of typical features of the Netherton syndrome. J Dtsch Dermatol Ges. 2015;13:691‐693. [DOI] [PubMed] [Google Scholar]
- 53. Sun JD, Linden KG. Netherton syndrome: a case report and review of the literature. Int J Dermatol. 2006;45:693‐697. [DOI] [PubMed] [Google Scholar]
- 54. Blaschke S, Moller R, Hausser I, Anton‐Lamprecht I, Paul E. [Comel‐Netherton syndrome]. Hautarzt 1998;49:499‐504. [DOI] [PubMed] [Google Scholar]
- 55. Singer R, Copur M, Yuksel EN, Kocatürk E, Erhan SŞ. Ichthyosis linearis circumflexa in a child. Response to narrowband UVB therapy. J Dermatol Case Rep. 2015;9:110‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


