Abstract
BACKGROUND
Reluctance to use intravenous (IV) iron for the treatment of iron deficiency continues due to a perceived high risk of severe hypersensitivity reactions (HSRs). Additionally, it has been hypothesized that ‘dextran‐derived’ IV iron products (e.g., ferumoxytol [FER] and ferric derisomaltose/iron isomaltoside 1000 [FDI]) have a higher risk of severe HSRs than ‘non‐dextran‐derived’ products (e.g., ferric carboxymaltose [FCM] and iron sucrose [IS]). In the present analysis, HSR data from head‐to‐head randomized controlled trials (RCTs) with IV iron products were evaluated to determine if differences in safety signals are present among these IV iron formulations.
STUDY DESIGN AND METHODS
Reported serious or moderate‐to‐severe HSR incidence data from five RCTs (FIRM; FERWON‐NEPHRO/‐IDA; PHOSPHARE‐IDA04/‐IDA05) were used to calculate risk differences with 95% confidence intervals (CIs) for FER, FCM, FDI, and IS. The rates and risk differences for these HSRs were compared.
RESULTS
The analysis included data for 5247 patients: FER (n = 997), FCM (n = 1117), FDI (n = 2133) and IS (n = 1000). Overall rates of serious or moderate to severe HSRs were low (0.2%‐1.7%). The risk differences (95% CIs) showed small differences between the IV iron formulations: FER versus FCM, −0.1 (−0.8 to 0.6); FDI versus IS, 0.1 (−0.3 to 0.5); FDI versus FCM, −0.9 (−3.7 to 1.9).
CONCLUSION
RCT evidence confirms a low risk of serious or moderate to severe HSRs with newer IV iron formulations and no significant differences among existing commercially available products. Thus, RCT data show that the supposed classification of dextran‐derived versus non‐dextran‐derived IV iron products has no clinical relevance.
ABBREVIATIONS
- AESIs
adverse events of special interest
- CARPA
complement activation–related pseudo‐allergy
- FCM
ferric carboxymaltose
- FDA
US Food and Drug Administration
- FDI
ferric derisomaltose/iron isomaltoside 1000
- FER
ferumoxytol
- HSR
hypersensitivity reaction
- IS
iron sucrose
- MedDRA
Medical Dictionary for Regulatory Activities
- RCT
randomized controlled trial
For decades, intravenous (IV) iron has been successfully used for the treatment of iron deficiency, in diverse patient populations and across a wide range of diagnoses associated with iron deficiency. Increasingly, parenteral iron is also playing a role in patient blood management. 1 Early parenteral IV iron products, most notably high‐molecular‐weight iron dextran, were associated with higher rates of severe hypersensitivity reactions (HSRs) and restricted to use with caution or considered unsuitable for use. 2 The improved tolerability of newer formulations of IV iron 2 has been attributed to the structure of the molecule and, more specifically, to two key features: tighter binding of elemental iron in the iron–carbohydrate complex, 3 and low immunogenic potential of the carbohydrate moiety. 3
However, despite the widespread use of IV iron and the improved safety profile of newer products, there remains a degree of reluctance among the medical community to use IV iron due to a perceived high risk of severe HSRs. 2 All IV iron medications have the potential to cause minor infusion reactions, but severe HSRs with IV iron are extremely rare. 4 This perpetual reluctance may be due to misperceptions surrounding the management of minor acute infusion reactions to IV iron, such as Fishbane reactions (arthralgias, myalgias, and flushing), 4 and mild manifestations of nonallergic complement activation–related pseudo‐allergy reactions, 5 which can mimic the early symptoms of a more severe reaction.
Recently, there has been an insidious drive to categorize IV iron products as either ‘dextran‐based/derived’ or ‘non‐dextran‐based/derived’ which, given the historical evidence for high‐molecular‐weight iron dextran, has led to the misbelief that all products with dextran‐derived carbohydrate components are associated with a higher risk of severe HSRs. Based on in vitro tests of possible antigens with monoclonal antibodies, Neiser and colleagues 6 hypothesized that IV iron products with dextran‐derived carbohydrate moieties (e.g., ferumoxytol [FER] and ferric derisomaltose/iron isomaltoside 1000 [FDI]) have a higher risk of severe HSRs than non‐dextran‐derived products (e.g., ferric carboxymaltose [FCM] and iron sucrose [IS]). However, “…in vitro tests of possible antigens with monoclonal antibodies can generally neither assess the risk of anaphylaxis in an individual patient, nor can they predict the numerical risk of such anaphylaxis in the clinical setting.” 7 Recently conducted, ‘gold‐standard’ randomized controlled trials (RCTs) now provide the high‐quality evidence for clinical outcome that is needed to reveal any differences between IV iron drugs with respect to risk of HSRs.8, 9, 10, 11, 12
The objective of the analysis presented here is to use reported data for serious or moderate to severe HSRs from head‐to‐head RCTs to determine if differences in safety signals are present among the four most widely used IV iron products currently available on the market in Europe and the United States: FDI, FCM, IS, and FER. In particular, the aim of the analysis is to determine if differences exist between the nominal classifications of dextran‐derived and non‐dextran‐derived IV iron products.
MATERIALS AND METHODS
Relevant studies were identified through a search of the US National Library of Medicine clinical trials database, ClinicalTrials.gov. 13 The advanced search function was used (on September 4, 2019) with the following terms: ‘condition or disease’: anemia, iron deficiency; ‘study type’: interventional studies (clinical trials); ‘status, recruitment’: completed; ‘intervention/treatment’: (ferric derisomaltose) OR (iron isomaltoside) OR (ferumoxytol) OR (ferric carboxymaltose) OR (iron sucrose) OR (iron polymaltose) OR (sodium ferric gluconate) OR (saturated iron oxide). Defaults were used for all other fields. The search returned 86 records, which were screened for inclusion. The ClinicalTrials.gov records or, if more information was required, publications were reviewed. Trials were selected on the basis that they were RCTs:
Comparing dextran‐derived IV iron drugs (FER or FDI) to non‐dextran‐derived IV iron drugs (FCM or IS).
Reporting on serious and/or moderate to severe HSRs, based on a Medical Dictionary for Regulatory Activities (MedDRA) definition, as prespecified primary or secondary endpoints.
Four trials (NCT01114204; NCT01052779; NCT01227616; NCT02130063)14, 15, 16, 17 were excluded due to event relatedness not being judged by an investigator or an adjudicated blinded committee and/or the HSRs not being specified. Of these, three trials were in favor of the dextran‐derived comparator (FER 2.7% vs. IS 5.0% [adverse events of special interest, AESIs: moderate to severe hypersensitivity, and moderate to severe hypotension]; FER 1.3% vs. IS 6.1% [AESIs: including anaphylaxis/anaphylactoid reactions, and milder symptoms of hypersensitivity, moderate or severe hypotension, acute decreases in systolic blood pressure, and hypotension associated with symptoms]; FER 12.8% vs. IS 26.8% [AESIs: including hypersensitivity reactions and moderate to severe hypotension)14, 15, 16 and in one trial there was no difference (FDI 0.6% [severe dyspnea and severe pruritic rash, and moderate syncope] vs. IS 0.6% [severe anaphylactic reaction]). 17
Five RCTs were identified and included:
FIRM: a Phase III, randomized, double‐blind trial comparing FCM with FER in iron deficiency anemia of any etiology (NCT02694978) 9
FERWON‐NEPHRO and FERWON‐IDA: two similarly designed Phase III, randomized, open‐label trials comparing FDI with IS in non‐dialysis‐dependent chronic kidney disease, and iron deficiency anemia of mixed etiologies, respectively (NCT02940860, NCT02940886)10, 11, 13
PHOSPHARE‐IDA04 and PHOSPHARE‐IDA05: two identically designed Phase III, randomized, open‐label clinical trials comparing FDI with FCM in iron deficiency anemia of mixed etiologies (NCT03238911, NCT03237065).12, 13
The included studies are summarized in Appendix S1, available as supporting information in the online version of this paper. The FIRM study was designed in concert with the US Food and Drug Administration (FDA) to evaluate the incidence of moderate to severe HSRs, including anaphylaxis (or moderate to severe hypotension), as the primary endpoint. 9 A prespecified HSR questionnaire was used to capture information on HSRs (for criteria, see Appendix S2, available as supporting information in the online version of this paper).
The FERWON studies (also designed in concert with the FDA) evaluated the incidence of serious or severe HSRs as a coprimary endpoint, alongside the change in hemoglobin.10, 11 In the PHOSPHARE studies, the incidence of serious or severe HSRs was evaluated as a secondary endpoint. 12 The hypersensitivity terms in the FERWON and PHOSPHARE studies were defined by a standardized set of MedDRA terms (see Appendix S3, available as supporting information in the online version of this paper) based on the method used by the FDA during the new drug approval process for FCM. 18
In the FIRM and FERWON studies, the HSRs reported were confirmed and adjudicated by blinded independent committees.9, 10, 11
Reported serious or moderate to severe HSR incidence data from these five RCTs were used to calculate risk differences and 95% confidence intervals (CIs) for FER and FCM (FIRM study), for FDI and IS (FERWON studies, pooled), and for FDI and FCM (PHOSPHARE studies, pooled). For the individual analyses, the unadjusted risk difference and Wald‐based 95% CI is presented. For the pooled analysis, the risk difference and 95% CI was estimated by the weighted inverse variance method.
RESULTS
The analysis included data for 5247 patients: FER (n = 997), FCM (n = 1117), FDI (n = 2133), and IS (n = 1000). Rates of serious or moderate to severe HSRs were low (0.2%‐1.7%) for all IV iron products evaluated, with no significant risk differences among the four formulations (Fig. 1).
Fig. 1.
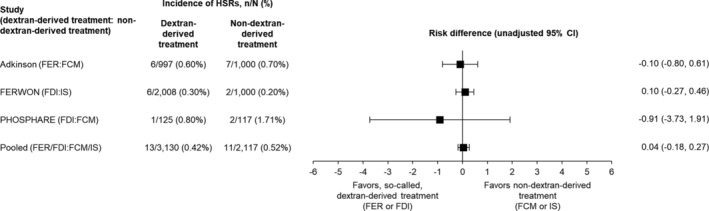
The risk of serious or moderate to severe HSRs associated with the use of IV iron products – data from the FIRM, FERWON, and PHOSPHARE studies. CI = confidence interval; FCM = ferric carboxymaltose; FDI = ferric derisomaltose/iron isomaltoside 1000; FER = ferumoxytol; HSR = hypersensitivity reaction; IS = iron sucrose.
The nature of HSRs was reported differently between studies. In FIRM, for FCM and FER, respectively, ‘moderate HSRs’ were reported by 0.6% and 0.3%, ‘severe HSRs’ by 0.0% and 0.1%, and ‘moderate hypotension’ by 0.1% and 0.2%. 9 In FERWON, HSRs were experienced by six patients in the FDI group: nausea and abdominal pain; shortness of breath and tightness in the chest; acute asthma exacerbation; complement activation–related pseudo‐allergy; flushing, bronchospasm, angina pectoris, hypotension, dyspnea, and leukocytosis; and infusion reaction (each 0.05%), and two patients in the IS group: anaphylactic reaction (0.2%).10, 11 In PHOSPHARE, HSRs in the FCM group were dyspnea and swelling (each 0.85%), and the HSR in the FDI group was swollen eyelid unilaterally (0.8%). 12
DISCUSSION
Our analysis identified five head‐to‐head RCTs that included combinations of FER, FDI, FCM, and IS, which were designed and powered to evaluate serious or moderate to severe HSRs as prespecified primary or secondary endpoints. This highest‐level RCT data enabled comparison of HSR incidence and risk differences for the newer IV iron drugs. The findings of low rates of serious or moderate to severe HSRs for IV iron and small differences among the four IV iron formulations in a large number of patients align with a previous meta‐analysis of 103 RCTs in which more than 10,000 patients were treated with IV iron. 19 Aside from ferric gluconate, none of the IV iron products included in this previous analysis had a statistically significant increase in severe infusion reactions compared to any comparator. 19 Additionally, a recent comprehensive meta‐analysis of 21 prospective, regulatory‐standard clinical trials in 8599 patients with iron deficiency anemia receiving FDI, FCM, or IS found rates of serious or severe HSRs (defined using standardized MedDRA terms) to be low and similar to those in our analysis. 20 A lack of understanding of the true nature of minor acute infusion reactions and their management has resulted in the misperception that IV iron products are associated with a high risk of severe HSRs. If minor acute reactions occur during an infusion of IV iron, they are usually self‐limiting, with symptoms abating within minutes of the infusion being interrupted, and patients can often be successfully rechallenged, going on to receive the remaining infusion of iron. 4 In clinical practice, however, the situation can be markedly different. Antihistamines are frequently administered as prophylaxis against HSRs. Antihistamines can cause vasoactive reactions, prompting inappropriate intervention with vasopressors, and consequently more antihistamines. 21 Such inappropriate intervention may escalate minor self‐limiting infusion reactions into hemodynamically significant serious adverse events ostensibly due to the iron. 2
A proposed algorithm is provided (Fig. 2) to assist health care personnel in the management of acute infusion reactions. In the management of HSRs experienced with IV iron, key aspects include alleviating patient and health care provider anxiety, using a slow infusion rate, recognizing anaphylaxis and treating it appropriately, and recognizing Fishbane reactions and isolated symptoms. 22 There is no role for premedication with diphenhydramine or steroids; antihistamines should be used only for symptomatic urticaria. 22 IV iron should be administered only when staff trained to evaluate and manage anaphylactic reactions are immediately available (as well as resuscitation facilities), 25 and it is recommended that administration teams should receive regular training in the management of IV infusions and associated adverse reactions. 4 Patients should be informed that delayed reactions, for example, flulike symptoms, characterized by arthralgia, myalgia, and sometimes fever, can occur from several hours up to a day after administration.22, 26 Delayed reactions are usually mild and self‐limited, and the symptoms can be relieved with simple analgesics. 26 In the case of ongoing fatigue, muscle weakness, and bone pain (symptoms possibly related to hypophosphatemia), it is recommended to measure phosphate, since hypophosphatemia is a known risk with some IV iron drugs.12, 27, 28 For example, in the PHOSPHARE studies presented here, hypophosphatemia (serum phosphate <2.0 mg/dL) occurred in 74.4% of patients receiving FCM and in 8.0% of those receiving FDI. 12
Fig. 2.
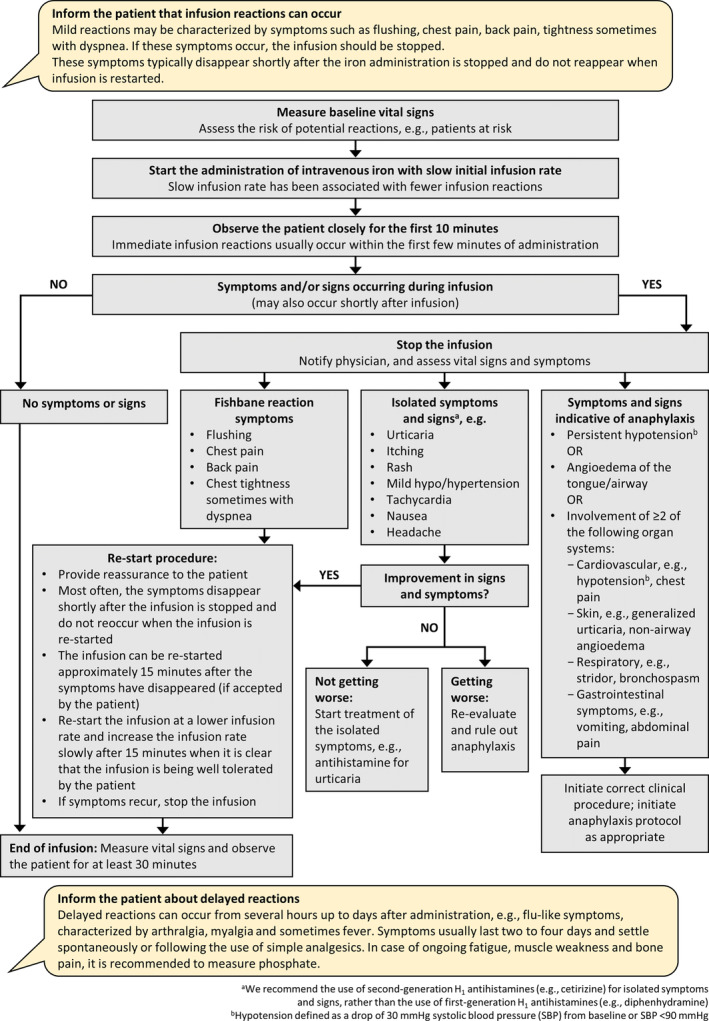
Algorithm for the management of immediate infusion reactions. Adapted from: Rampton et al.,4 Lim et al.,22 Macdougall et al.,23 and Simons et al. 24 [Color figure can be viewed at wileyonlinelibrary.com]
The present analysis of high‐quality data from head‐to‐head RCTs confirmed that there is a low risk of serious or moderate to severe HSRs with newer IV iron products and no statistically significant difference in HSR rates between products. Thus, RCT data show that the supposed classification of so‐called dextran‐derived versus non‐dextran‐derived IV iron products in relation to rates of HSRs has no clinical relevance.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
Writing support was provided by Cambridge Medical Communication Ltd (Cambridge, UK), and funded by Pharmacosmos A/S, Holbæk, Denmark.
REFERENCES
- 1. Mueller MM, Van Remoortel H, Meybohm P, et al. ICC PBM Frankfurt 2018 Group. Patient blood management: recommendations from the 2018 Frankfurt Consensus Conference. JAMA 2019;321:983‐97. [DOI] [PubMed] [Google Scholar]
- 2. Auerbach M, DeLoughery T. Single‐dose intravenous iron for iron deficiency: a new paradigm. Hematology Am Soc Hematol Educ Program 2016;2016:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jahn MR, Andreasen HB, Fütterer S, et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer), a new intravenous iron preparation and its clinical implications. Eur J Pharm Biopharm 2011;78:480‐91. [DOI] [PubMed] [Google Scholar]
- 4. Rampton D, Folkersen J, Fishbane S, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica 2014;99:1671‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macdougall IC, Vernon K. Complement activation‐related pseudo‐allergy: a fresh look at hypersensitivity reactions to intravenous iron. Am J Nephrol 2017;45:60‐2. [DOI] [PubMed] [Google Scholar]
- 6. Neiser S, Wilhelm M, Schwarz K, et al. Assessment of dextran antigenicity of intravenous iron products by an immunodiffusion assay. Port J Nephrol Hypert 2011;25:219‐24. [Google Scholar]
- 7. Neiser S, Schwarz K, Wilhelm M, et al. Reply to the letter to the editor by Johannes Ring and Rudi Valenta on the article “Assessment of dextran antigenicity of intravenous iron products by an immunodiffusion assay”. Port J Nephrol Hypert 2012;26:311‐2. [Google Scholar]
- 8. OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Available at: https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf.
- 9. Adkinson NF, Strauss WE, Macdougall IC, et al. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose in iron deficiency anemia: a randomized trial. Am J Hematol 2018;93:683‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Auerbach M, Henry D, Derman RJ, et al. A prospective, multi‐center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON‐IDA trial. Am J Hematol 2019;94:1007‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhandari S, Kalra PA, Berkowitz M, et al. Safety and efficacy of iron isomaltoside 1000/ferric derisomaltose versus iron sucrose in patients with chronic kidney disease: the FERWON‐NEPHRO randomized, open‐label, comparative trial. Nephrol Dial Transplant 2020. pii: gfaa011. 10.1093/ndt/gfaa011 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolf M, Rubin J, Achebe M, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron deficiency anemia: two randomized clinical trials. JAMA 2020;323:432‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Available from: www.clinicaltrials.gov.
- 14. Hetzel D, Strauss W, Bernard K, et al. A phase III, randomized, open‐label trial of ferumoxytol compared with iron sucrose for the treatment of iron deficiency anemia in patients with a history of unsatisfactory oral iron therapy. Am J Hematol 2014;89:646‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macdougall IC, Strauss WE, McLaughlin J, et al. A randomized comparison of ferumoxytol and iron sucrose for treating iron deficiency anemia in patients with CKD. Clin J Am Soc Nephrol 2014;9:705‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macdougall IC, Strauss WE, Dahl NV, et al. Ferumoxytol for iron deficiency anemia in patients undergoing hemodialysis. The FACT randomized controlled trial. Clin Nephrol 2019;91:237‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Derman R, Roman E, Modiano MR, et al. A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia. Am J Hematol 2017;92:286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Center for Drug Evaluation and Research . Medical review of Injectafer (VIT‐45, ferric carboxymaltose injection; FCM) for the treatment of iron deficiency anemia (203565Orig1s000). 2013. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203565Orig1s000MedR.pdf.
- 19. Avni T, Bieber A, Grossman A, et al. The safety of intravenous iron preparations: systematic review and meta‐analysis. Mayo Clin Proc 2015;90:12‐23. [DOI] [PubMed] [Google Scholar]
- 20. Pollock RF, Biggar P. Indirect methods of comparison of the safety of ferric derisomaltose, iron sucrose and ferric carboxymaltose in the treatment of iron deficiency anemia. Expert Rev Hematol 2020;13:187‐95. [DOI] [PubMed] [Google Scholar]
- 21. Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol 2016;91:31‐8. [DOI] [PubMed] [Google Scholar]
- 22. Lim W, Afif W, Knowles S, et al. Canadian expert consensus: management of hypersensitivity reactions to intravenous iron in adults. Vox Sang 2019;114:363‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macdougall IC, Bircher AJ, Eckardt KU, et al. Conference Participants. Iron management in chronic kidney disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2016;89:28‐39. [DOI] [PubMed] [Google Scholar]
- 24. Simons FE, Ardusso LR, Bilò MB, et al. World Allergy Organization. World Allergy Organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J 2011;4:13‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. European Medicines Agency . New recommendations to manage risk of allergic reactions with intravenous iron‐containing medicines. 2013. [cited 2020 Jan 21]. Available from: https://www.ema.europa.eu/en/medicines/human/referrals/intravenous-iron-containing-medicinal-products.
- 26. Bircher AJ, Auerbach M. Hypersensitivity from intravenous iron products. Immunol Allergy Clin North Am 2014;34:707‐23 x‐xi. [DOI] [PubMed] [Google Scholar]
- 27. Zoller H, Schaefer B, Glodny B. Iron‐induced hypophosphatemia: an emerging complication. Curr Opin Nephrol Hypertens 2017;26:266‐75. [DOI] [PubMed] [Google Scholar]
- 28. Detlie TE, Lindstrøm JC, Jahnsen ME, et al. Incidence of hypophosphatemia in patients with inflammatory bowel disease treated with ferric carboxymaltose or iron isomaltoside. Aliment Pharmacol Ther 2019;50:397‐406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


