Abstract
Diabetic foot ulcers (DFUs) have significant clinical impact and carry a substantial economic burden. Patients with DFUs that are refractory to standard wound care are at risk for major complications, including infection and amputation and have an increased risk of mortality. This study evaluated the safety and preliminary efficacy of a novel decellularised purified reconstituted bilayer matrix (PRBM) in treating DFUs. Ten diabetic patients with refractory wounds that failed to heal after at least 4 weeks of standard wound care were studied in this Institutional Review Board approved trial. Ten consecutive wounds were treated weekly with the PRBM for up to 12 weeks. At each weekly visit, the wound was evaluated, photographed, and cleaned, followed by application of new graft if not completely epithelialised. Assessment included measurement of the wound area and inspection of the wound site for signs of complications. The primary outcome measure was wound closure, as adjudicated by independent reviewers. Secondary outcomes included assessment of overall adverse events, time to closure, percent area reduction, and the cost of product(s) used. Nine of 10 patients achieved complete wound closure within 4 weeks, and 1 did not heal completely within 12 weeks. The mean time to heal was 2.7 weeks. The mean wound area reduction at 12 weeks was 99%. No adverse events nor wound complications were observed. These early clinical findings suggest that the PRBM may be an effective tool in the treatment of diabetic foot ulcers.
1. INTRODUCTION
Patients with diabetic foot ulcers (DFUs) face serious risks of additional severe complications. Rates of emergency department visits and hospitalisations related to DFUs exceed those associated with other common critical conditions, such as congestive heart failure, renal disease, and most cancers. 1 The annual incidence of an active DFU is approximately 6% among persons with diabetes globally, and the lifetime risk of a DFU is in the range of 19% to 34% in those patients. 1 In the United States, DFU treatment is responsible for $9 to $13 billion in annual direct health care costs. 2
Wound treatment should aim to maintain a moist healing environment, manage infection and wound exudate, protect the surrounding skin, and leverage advanced therapies when necessary, such as topical agents or devices. 3 Currently accepted first‐line treatment consists of wound debridement and cleansing, application of traditional dressings, and offloading.3, 4 When a reduction in ulcer size of at least 50% cannot be achieved within 4 weeks, advanced treatment methods should be considered. 3 Compromised healing is influenced by a complex interplay of physiologic and biochemical factors, including comorbidities that are commonly associated with diabetes.5, 6, 7 Wounds in diabetic patients present an altered milieu of matrix metalloproteinases, cytokines, and reactive oxygen species that can impede angiogenesis, degrade the native extracellular matrix (ECM), and lead to failed healing.8, 9, 10 Evidence indicates that the ECM in non‐healing DFUs significantly differs from that of normal wounds.11, 12 Thus, a number of treatment approaches have emerged that utilise ECM‐based materials to modulate the local wound environment, notably decellularised xenografts 13 and allografts.14, 15 These advanced wound care matrices presumably contribute an exogenous matrix, locally attenuate inflammatory cytokines and proteinases, support cell ingrowth and revascularisation, and eventually establishing a supportive bed for reepithelialisation.8, 16 In practice, certain advanced ECM‐based matrices have indeed shown clinical promise in successful healing of DFUs and other challenging chronic wounds.17, 18, 19, 20
In this study, a novel ECM‐based grafting material, PRBM (purified reconstituted bilayer matrix; Geistlich Derma‐Gide, Geistlich Pharma AG), was hypothesised to be an effective graft for the treatment of non‐healing DFUs. This PRBM is derived from two separate porcine connective tissues, which are thoroughly extracted and purified in a proprietary process that removes cells, lipids, and potential antigens to provide a non‐immunogenic and non‐inflammatory scaffold. The PRBM graft is formed by combining the two purified ECM constituents into a 3D construct. The result is an organotypic bilayer dermis‐like graft comprising an upper compact layer and a lower porous layer (Figure 1). The matrix architecture consists of interconnected pores approximately 10 to 200 μm in diameter with a porosity of approximately 90% void volume (Figure 1). The PRBM is designed to be flexible and conformable with hydrophilic properties that facilitate adaptation to the wound surface and immediate fluid uptake. Each bilayer matrix is capable of absorbing 9 times its own mass in fluid (Figure 1). The upper layer is compact, providing support and coverage of the wound site and is designed to guide reepithelialisation by mimicking the basement membrane. The porous lower layer allows for absorption of blood and wound exudate and is intended to serve as a scaffold for cellular ingrowth and eventual revascularisation.
FIGURE 1.
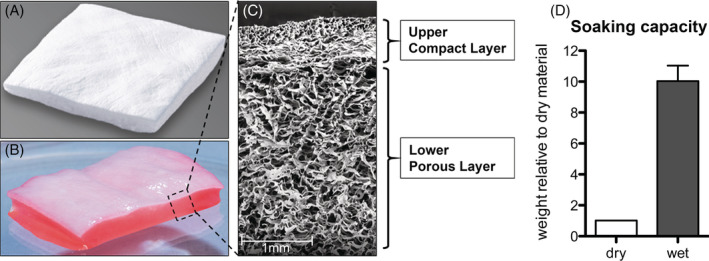
Purified reconstituted bilayer matrix (PRBM) in its dry state, A and hydrated state, B. Electron microscopy shows the porous bilayer architecture of the PRBM, C. Soaking capacity of the matrix, D
The current study aimed to collect data describing the initial clinical experience with PRBM in the treatment of non‐healing DFUs and to evaluate potential for a larger randomised controlled trial. The primary outcome measure of this study was wound closure at 12 weeks.
2. METHODS
Ten consecutive Wagner grades 1 and 2 21 pedal wounds from DFU patients were enrolled and treated with the PRBM (Table 1). The protocol for this study was approved by the Western Institutional Review Board (20182784), and activities were conducted in conformance with the ethical guidelines of the Declaration of Helsinki. All 10 patients were treated at a single site between March 2018 and August 2018. Study eligibility required that DFUs had failed to heal after a minimum of 4 weeks of standard wound care regimens, such as collagen alginate dressings, negative pressure therapy, and off‐loading. During the course of treatment, one patient did not return for the 8‐, 10‐, and 11‐week scheduled follow up visit(s) and did not comply with the treatment plan. Data from this patient met inclusion criteria and are included in this study.
TABLE 1.
Patient and wound characteristics
| Patient | Sex | Age (y) | Weight (lb) | Height (ft/in) | BMI | Duration preexisting DFU (w) | Wagner grade | DFU location |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 64 | 223 | 6′0″ | 30.2 | 10 | 1 | Plantar distal central metarsal |
| 2 | M | 81 | 273 | 6′4″ | 33.2 | 4 | 1 | Plantar distal lateral metarsal |
| 3 | M | 64 | 250 | 5′11″ | 47.2 | 6 | 1 | Plantar 2nd toe |
| 4 | F | 57 | 200 | 5′7″ | 30.4 | 40 | 1 | Dorsal hallux toe |
| 5 | M | 43 | 280 | 6′4″ | 34.1 | 8 | 2 | Plantar medial Metatarsal |
| 6 | M | 73 | 200 | 6′0″ | 27.1 | 14 | 1 | Plantar medial metatarsal |
| 7 | F | 63 | 190 | 5′4″ | 32.6 | 24 | 2 | Plantar distal central metatarsal |
| 8 | M | 68 | 210 | 6′0″ | 28.5 | 4 | 1 | Medial metatarsal |
| 9 | F | 75 | 200 | 5′3″ | 35.4 | 8 | 1 | Plantar midfoot |
| 10 | M | 59 | 290 | 5′8″ | 42.8 | 5 | 1 | Plantar midfoot |
| Mean | ‐ | 64.7 | 231.6 | 5′8″ | 34.2 | 12.3 | n.a. | ‐ |
| SD | ‐ | 10.6 | 38.1 | 0.4 | 6.3 | 11.5 | n.a. | ‐ |
Abbreviations: BMI, body mass index; DFU, diabetic foot ulcer; n.a., not applicable.
2.1. Treatment and evaluation
After failing at least 4 weeks of standard wound care, patients were treated with placement of the PRBM on a weekly basis until complete wound healing was observed for a maximum of 12 weeks. The PRBM was stored at ambient room temperature and applied to patients' wounds according to the instructions for use (IFU). The first application of each PRBM included cleaning of the wound site, sharp debridement, and graft placement. Subsequent reapplication of the PRBM included cleaning, light debridement, and application of a new graft. The PRBM was trimmed to match the respective wound size using scissors and applied dry with its compact side up. In situations when the PRBM was not fully hydrated by wound fluids in situ, saline was added to achieve complete hydration. The entire site was then covered with a non‐adherent dressing (Adaptic Touch, 3M Corporation) followed by a standard three‐layer wound dressing, including soft roll and two‐layer self‐adherent wraps (Dynaflex, 3M Corporation). Patients were instructed to off‐load the limb, given a diabetic offloading boot and returned weekly for wound evaluation and dressing change. At each weekly visit, the wound area was examined to identify indicators of complications such as infection or necrosis. The wounds were photographed and measured for area using acetate tracing and 2D analysis. A new PRBM was applied to non‐healed wounds at each visit. Complete healing was defined as 100% reepithelialisation without drainage or need for dressing. The primary outcome measure was the proportion of patients healed at or before 12 weeks. The treating clinician evaluated each DFU for closure each week, and wound healing was further adjudicated by three plastic surgeons based on the acquired photographic images. The time to heal and percent wound area reduction were recorded for each patient. Time to heal was evaluated through Kaplan‐Meier analysis using the Prism software (Prism 8, GraphPad; San Diego, California). The product cost for the PRBM was recorded using the manufacturer's list prices and the total of number and size of grafts used during treatment.
3. RESULTS
The average patient age was 65 ± 11 years (range: 43‐81 years). Seven patients were male and three were female. Average body mass index (BMI) was 34 ± 6 (range: 27‐47) with 80% of patients characterised as obese or morbidly obese (BMI > 30). Concomitant to diabetes, each patient had diverse comorbidities, such as hypertension, coronary artery disease, and smoking‐related pathologies. DFU anatomical locations included the plantar surface (7/10), medial/lateral surface (1/10), plantar digit (1/10), and dorsal digit (1/10). Prior to treatment with PRBM, the mean wound size was 3.3 cm2, and the mean initial wound depth was 0.3 cm. Eight patients had a DFU with Wagner grade 1 and two patients with Wagner grade 2. Patients had been previously treated with other wound care regimens for an average of 12.3 weeks prior to treatment with the PRBM. Study patient and wound characteristics are summarised in Table 1.
Upon application, it was observed in all instances that the PRBM immediately conformed to the wound surface and absorbed wound fluid, blood, and any added saline. A direct apposition between the PRBM and the wound bed was achieved, maintaining the position of the graft in the bed. During follow‐up visits, the superficial dressings were easily removed without adherence to the underlying newly formed tissue. Inspection of the wounds revealed no signs of infection or necrosis in any patient at any time point. In plantar DFUs, the graft was consistently observed to be completely integrated, replaced by tissue, or resorbed at 1 week after each application, while some residual wound matrix was consistently detectable in DFU sites in the lateral, posterior, and toe locations.
Wound closure was observed in 9 of 10 patients (90%) by the conclusion of the 12‐week period with a mean time to closure of 2.7 weeks (Figure 2). Complete closure was achieved at 1 week in 3 patients, at 3 weeks in 3 patients, and at 4 weeks in 3 patients. In summary, nine of 10 DFUs healed within 4 weeks after beginning PRBM treatment. Notably, after 1 week, the mean wound area reduction was 71 ± 26%. Further incremental wound area reduction was observed at 4 weeks (98 ± 6%) and 6 weeks (99 ± 2%). At the 12‐week study endpoint, the mean wound area reduction for all 10 patients was 99 ± 2% (Figure 3). Both Wagner 2 wounds healed over the study period (Figure 4). Seven out of eight of the Wagner 1 wounds healed (Figure 5). The wound of one patient did not heal after 12 weeks of treatment with PRBM. The initial wound area of 6 cm2 was reduced by 90% over the first 6 weeks. The patient did not participate in the scheduled 8‐, 10‐ and 11‐week visit but did return at 12 weeks for continued observation and treatment. The patient was also observed to be non‐compliant with instructions for home care and off‐loading. Eventually, this patient did achieve healing but required operating room treatment with a split thickness skin graft.
FIGURE 2.
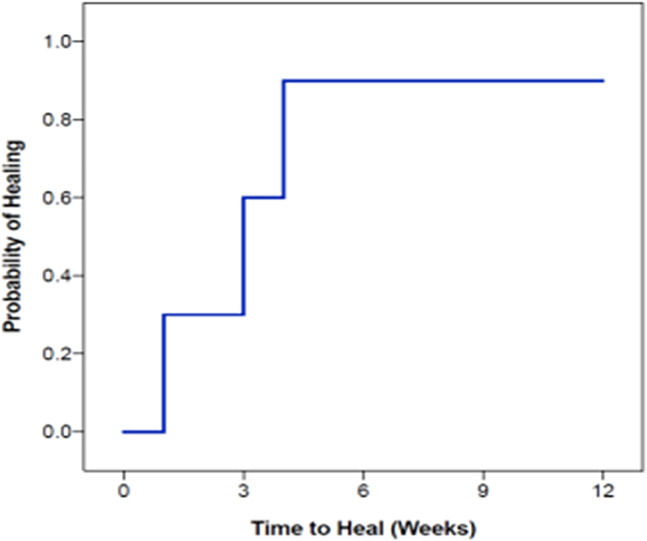
Kaplan‐Meier plot of time to heal; 90% of patients healed within 4 weeks of initiating purified reconstituted bilayer matrix treatment
FIGURE 3.
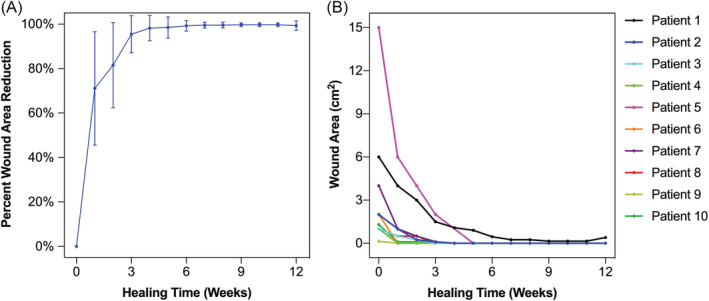
The mean percent wound area reduction, A and measured wound area by patient, B, plotted over the 12‐week treatment course. In A, error bars represent SD
FIGURE 4.
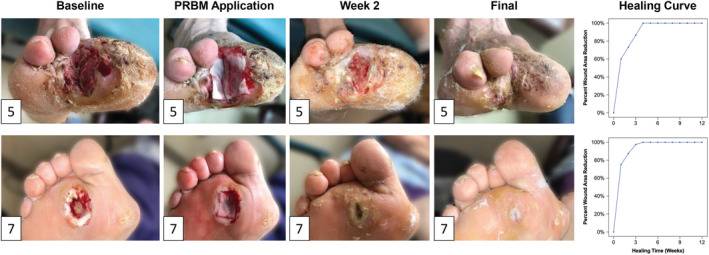
Representative images of the healing time course for the two study patients with Wagner 2 wounds and their respective progression to closure (patients 5 and 7; Table 1). The patient in the top row had the largest wound in the pilot study and healed in 4 weeks. The patient in the lower row is representative of the median, also with complete healing in 4 weeks
FIGURE 5.
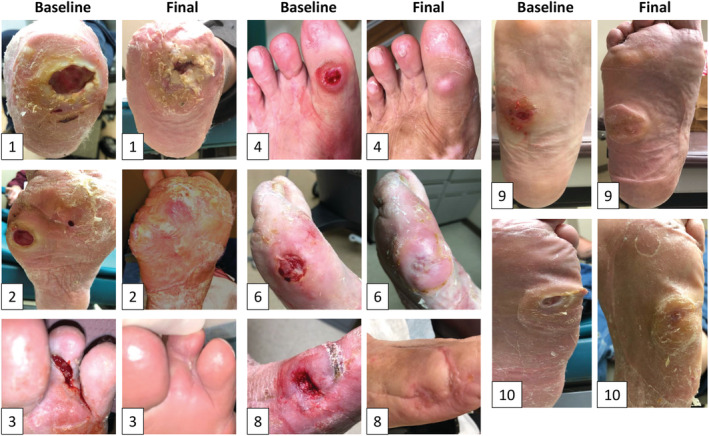
Images of all Wagner 1 wounds at baseline and at closure or at 12‐week study conclusion (subjects 1‐4, 6, 8‐10; demographic detailed in Table 1). Note that patient 1 failed to heal over 12 weeks but substantially reduced in wound size during the treatment period
Between 1 and 4 PRBM grafts were applied to the 9 patients who achieved complete wound closure, with a mean application of 2.7 grafts per patient. The mean per‐patient cost for PRBM grafts in the 9 healed patients was $1203.
No serious adverse events or complications were reported during the study, neither systemically nor specifically related to the PRBM grafts under evaluation.
4. DISCUSSION
Because of the serious medical risks and socioeconomic burden of DFUs, there is a critical unmet need for effective DFU treatment. Today, this need is not adequately addressed by standard‐of‐care (SOC) wound therapies, which have been demonstrated to yield 12‐week DFU closure in less than 50% of patients. 22
The 90% healing rate and 2.7‐week average time to closure of DFUs treated with PRBM in the current study falls within the published range of outcomes for other advanced wound care treatments.14, 15, 18, 19, 20, 23, 24, 25, 26 In a 40‐patient study investigating dehydrated human amnion and chorion allograft (dHACA), 85% of wounds were closed at 12 weeks with a mean time to heal of 5.3 weeks. 14 Similarly, a study of weekly application of human reticular acellular dermal matrix (HR‐ADM) resulted in an 80% wound closure rate with a mean time to heal of 5.4 weeks, 15 and an evaluation of human acellular dermal matrix (HADM) resulted in a 70% closure rate at 12 weeks. 17 Additionally, a randomised, controlled study of bioengineered skin substitute (BSS) and dehydrated human amnion/chorion membrane (dHACM) observed 73% and 97% wound closure rates, respectively. 23 Reported times to heal ranged from 1.9 to 3.4 weeks for dHACM to 6.8 weeks for BSS.23, 26
The PRBM used in this series is porcine derived. Previous randomised, controlled studies have evaluated other porcine‐derived materials for DFU treatment, namely urinary bladder matrix (UBM) 27 and small intestine submucosa (SIS). 13 The published time to closure of DFUs was 8.9 weeks for UBM 27 and 9 weeks for SIS. 13 In comparison, the current study demonstrated that treatment with PRBM resulted in an average of 2.7‐week time to closure. We hypothesise that these observed differences in time to closure may be related to differences in anatomical tissue source or differences in the processing methods for decellularisation and purification. SIS, in particular, has been described as a minimally processed porcine tissue, which in addition to inherent ECM components contains other cell and tissue remnants, including measurable levels of DNA and cellular debris, which have been associated with adverse clinical sequalae.28, 29 The PRBM manufacturing process utilises proprietary methods, which remove cellular material and potential antigens, resulting in a highly purified non‐immunogenic and non‐inflammatory matrix.
In this study, one treated patient did not achieve complete wound closure within 12 weeks. This patient did not attend all weekly visits and did not comply with recommended home care nor with the follow‐up plan, which may have contributed to the treatment failure. Numerous studies and clinical practice guidelines have stressed the importance of consistent and timely patient compliance with treatment schedules and appropriate self‐care techniques to support healing.1, 3, 30 Interventional studies consistently report that compliant patients who follow recommendations have better outcomes than noncompliant patients. 1
Rising medical treatment costs for high‐risk populations such as diabetics are an increasing societal and governmental concern. 31 Treatment‐related financial considerations, such as private insurance coverage, medicare/medicaid relevance, and socioeconomic status of the patient, must often be weighed by the clinician during the planning of an appropriate therapeutic approach.31, 32 Because the cost of treatment is directly affected by overall duration of patient care and the number of treatment visits needed, the time to closure is a key metric for evaluating the economic impact of a given treatment modality. The cumulative cost of the product used during treatment is also relevant. In this series, the direct mean cost of the PRBM grafts applied was $1203 in patients that achieved closure. In larger studies evaluating advanced wound care products, the mean total graft cost has been reported at nearly $9000 for product containing allogeneic viable cells 23 and has been reported between $1200 and $1800 for non‐viable allogeneic tissue.14, 15 Although the population of patients in this observational study is not sufficient to draw conclusions, the product cost trend falls at the low end of published literature and warrants further investigation.
5. CONCLUSIONS
These are the first published data describing the effective treatment of non‐healing DFUs using a PRBM. Despite the relatively small number of patients, the current study's 90% healing rate, relatively early wound reepithelialisation, freedom from adverse events, ease of use, and cost per patient suggest that this PRBM is a suitable option for DFU treatment. This study suggests that a larger, randomised study would be valuable in investigating whether the observed trends would be validated.
CONFLICT OF INTEREST
There are no other conflict of interests with any of the authors in relationship to this study, or with regard to Geistlich Pharma. IRB conflict of interest statements are on file with PERI.
DISCLOSURES OF INTEREST
This study was funded through a research grant from Geistlich Pharma AG, provided to the Professional Education and Research Institute (PERI), which Charles M Zelen, DPM, is medical director. David Armstrong, DPM, MD, PhD, received research funds from PERI to design and administrate the trial and also assist with the writing and review of the manuscript. Dennis P. Orgill, MD, PhD, received research funds to serve as a validating/adjudicating plastic surgeon to review study photos and assist with the writing and review of the manuscript. Robert D. Galiano, MD, received research funds to serve as a validating/adjudicating plastic surgeon to review study photos and assist with the writing and review of the manuscript. Paul M. Glat, MD, received research funds to serve as a validating/adjudicating plastic surgeon to review study photos and assist with the writing and review of the manuscript. Jarrod P. Kauffman, MD, received research funds to assist in study design and in manuscript preparation. Marissa Carter, PhD, received research funds to provide the statistical analysis plan, and provide the statistical analysis for this trial and assist with writing of the result section of the manuscript. Charles M Zelen, DPM, is the medical director of the PERI and his company received research funds to administrate the clinical trial and write the paper for publication.
Armstrong DG, Orgill DP, Galiano RD, et al. An observational pilot study using a purified reconstituted bilayer matrix to treat non‐healing diabetic foot ulcers. Int Wound J. 2020;17:966–973. 10.1111/iwj.13353
Funding information Geistlich Pharma AG, Grant/Award Number: 0001
REFERENCES
- 1. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367‐2375. [DOI] [PubMed] [Google Scholar]
- 2. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37(3):651‐658. [DOI] [PubMed] [Google Scholar]
- 3. Lavery LA, Davis KE, Berriman SJ, et al. WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen. 2016;24(1):112‐126. [DOI] [PubMed] [Google Scholar]
- 4. Blume P, Wu S. Updating the diabetic foot treatment algorithm: recommendations on treatment using advanced medicine and therapies. Wounds. 2018;30(2):29‐35. [PubMed] [Google Scholar]
- 5. Andrews KL, Houdek MT, Kiemele LJ. Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prosthet Orthot Int. 2015;39(1):29‐39. [DOI] [PubMed] [Google Scholar]
- 6. Uccioli L, Izzo V, Meloni M, Vainieri E, Ruotolo V, Giurato L. Non‐healing foot ulcers in diabetic patients: general and local interfering conditions and management options with advanced wound dressings. J Wound Care. 2015;24(4 Suppl):35‐42. [DOI] [PubMed] [Google Scholar]
- 7. Musa HG, Ahmed ME. Associated risk factors and management of chronic diabetic foot ulcers exceeding 6 months' duration. Diabetes Foot Ankle. 2012;3(10):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kasiewicz LN, Whitehead KA. Recent advances in biomaterials for the treatment of diabetic foot ulcers. Biomater Sci. 2017;5(10):1962‐1975. [DOI] [PubMed] [Google Scholar]
- 9. Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix‐metalloproteinases and their inhibitors in the wounds of diabetic and non‐diabetic patients. Diabetologia. 2002;45(7):1011‐1016. [DOI] [PubMed] [Google Scholar]
- 10. Xu F, Zhang C, Graves DT. Abnormal cell responses and role of TNF‐alpha in impaired diabetic wound healing. Biomed Res Int. 2013;2013:754802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol. 1998;111(5):850‐857. [DOI] [PubMed] [Google Scholar]
- 12. Sutcliffe JES, Thrasivoulou C, Serena TE, et al. Changes in the extracellular matrix surrounding human chronic wounds revealed by 2‐photon imaging. Int Wound J. 2017;14(6):1225‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cazzell SM, Lange DL, Dickerson JE Jr, Slade HB. The management of diabetic foot ulcers with porcine small intestine submucosa tri‐layer matrix: a randomized controlled trial. Adv Wound Care. 2015;4(12):711‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DiDomenico LA, Orgill DP, Galiano RD, et al. Use of an aseptically processed, dehydrated human amnion and chorion membrane improves likelihood and rate of healing in chronic diabetic foot ulcers: a prospective, randomised, multi‐centre clinical trial in 80 patients. Int Wound J. 2018;15(6):950‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zelen CM, Orgill DP, Serena TE, et al. An aseptically processed, acellular, reticular, allogenic human dermis improves healing in diabetic foot ulcers: a prospective, randomised, controlled, multicentre follow‐up trial. Int Wound J. 2018;15(5):731‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kunkemoeller B, Kyriakides TR. Redox signaling in diabetic wound healing regulates extracellular matrix deposition. Antioxid Redox Signal. 2017;27(12):823‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reyzelman A, Crews RT, Moore JC, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J. 2009;6(3):196‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao M, Attalla K, Ren Y, French MA, Driver VR. Ease of use, safety, and efficacy of integra bilayer wound matrix in the treatment of diabetic foot ulcers in an outpatient clinical setting: a prospective pilot study. J Am Podiatr Med Assoc. 2013;103(4):274‐280. [DOI] [PubMed] [Google Scholar]
- 19. Lullove EJ. Use of ovine‐based collagen extracellular matrix and gentian violet/methylene blue antibacterial foam dressings to help improve clinical outcomes in lower extremity wounds: a retrospective cohort study. Wounds. 2017;29(4):107‐114. [PubMed] [Google Scholar]
- 20. Guo X, Mu D, Gao F. Efficacy and safety of acellular dermal matrix in diabetic foot ulcer treatment: a systematic review and meta‐analysis. Int J Surg. 2017;40:1‐7. [DOI] [PubMed] [Google Scholar]
- 21. Wagner FW Jr. The diabetic foot. Orthopedics. 1987;10(1):163‐172. [DOI] [PubMed] [Google Scholar]
- 22. Warriner RA, Snyder RJ, Cardinal MH. Differentiating diabetic foot ulcers that are unlikely to heal by 12 weeks following achieving 50% percent area reduction at 4 weeks. Int Wound J. 2011;8(6):632‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zelen CM, Serena TE, Gould L, et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi‐centre comparative study examining clinical efficacy and cost. Int Wound J. 2016;13(2):272‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J. 2014;11(2):122‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zelen CM, Gould L, Serena TE, Carter MJ, Keller J, Li WW. A prospective, randomised, controlled, multi‐centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2015;12(6):724‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alvarez OM, Smith T, Gilbert TW, et al. Diabetic foot ulcers treated with porcine urinary bladder extracellular matrix and total contact cast: interim analysis of a randomized, controlled trial. Wounds. 2017;29(5):140‐146. [PubMed] [Google Scholar]
- 28. Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73(1):61‐67. [DOI] [PubMed] [Google Scholar]
- 29. Petter‐Puchner AH, Fortelny RH, Mittermayr R, Walder N, Ohlinger W, Redl H. Adverse effects of porcine small intestine submucosa implants in experimental ventral hernia repair. Surg Endosc. 2006;20(6):942‐946. [DOI] [PubMed] [Google Scholar]
- 30. Bus SA, van Deursen RW, Armstrong DG, Lewis JE, Caravaggi CF, Cavanagh PR. International working group on the diabetic F. footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(1):99‐118. [DOI] [PubMed] [Google Scholar]
- 31. Robbins JM, Dillon J. Evidence‐based approach to advanced wound care products. J Am Podiatr Med Assoc. 2015;105(5):456‐467. [DOI] [PubMed] [Google Scholar]
- 32. de Leon J, Bohn GA, DiDomenico L, et al. Wound care centers: critical thinking and treatment strategies for wounds. Wounds. 2016;28(10):S1‐S23. [PubMed] [Google Scholar]


