Abstract
Since William Coley utilized bacterial immunotherapy to treat sarcomas in the late nineteenth century, an association between infection and improved survival has been reported for human and canine osteosarcoma patients. One of the reasons for this improved survival is likely a reactivation of the host immune system towards an inflammatory anti-tumour response, and one of the key players is the macrophage. Yet, despite their importance, the response of macrophages to infectious agents in the context of osteosarcoma has not been thoroughly evaluated. The aim of this study was to evaluate how in vitro exposure to a bacterial agent (Staphylococcus aureus) influenced canine and human macrophage differentiation in the presence of osteosarcoma. Our hypothesis was that S. aureus would, in the presence of osteosarcoma, induce a macrophage phenotype with significantly increased inflammatory signatures. Consistent with our hypothesis, human macrophages co-cultured with osteosarcoma and S. aureus exhibited increased IFN-γ, TNF-α and IL-12p70 cytokine secretion, decreased TGF-β cytokine secretion, and increased mRNA expression of TNF-α when compared to macrophages co-cultured with osteosarcoma and to macrophages cultured alone. Canine macrophages similarly exhibited increased IFN-γ and TNF-α cytokine secretion, decreased TGF-β cytokine secretion, increased mRNA expression of TNF-α, and increased surface receptor expression of CD80 when co-cultured with osteosarcoma and S. aureus. Collectively, the findings of this study suggest that infection upregulates the inflammatory immune response to counteract osteosarcoma-induced immune suppression. This work informs a potential therapeutic strategy to optimize inflammatory stimuli for triggering an anti-osteosarcoma macrophage response.
Introduction:
Osteosarcoma (OS), a malignant primary tumour of bone, is a devastating disease for both human and canine patients. It is the most common primary bone tumour in children and adolescents, and in the dog, and arises from mesenchymal cells that produce osteoid.1,2 Treatment of OS involves removal of the primary tumour, usually with limb amputation or limb-salvage surgery, and chemotherapy. Adjuvant and neoadjuvant chemotherapeutic regimens are used in OS to target metastatic disease; however, metastases remain the main cause of death in human and canine OS.2,3
Of the many similarities between human and canine OS such as biological behavior, histological characteristics, and gene expression signatures, perhaps the most dismal similarity is the lack of significant improvement in survival over the past three decades. Despite attempts with various permutations of chemotherapeutics, the median survival in dogs with OS remains between 10–12 months, and the 5-year disease-free interval for human patients with non-metastatic OS remains around 70%, with only 20–30% long-term survival expected in patients that present with metastases.2,4–6 To improve the clinical outcomes for this disease, investigations into novel avenues of treating OS are sorely needed.
One promising new therapeutic strategy to treat osteosarcoma is immunotherapy. Immunotherapy aims to restore and / or upregulate the anti-tumour functions of the innate and adaptive immune systems. Macrophages are innate immune cells that play an important role in anti-tumour immunity. Macrophages respond to different environments by readily polarizing to functionally appropriate phenotypes, with M1 (pro-inflammatory) and M2 (anti-inflammatory) macrophages being the most commonly recognized phenotypes. Macrophages within a tumour microenvironment are known as tumour-associated macrophages (TAMs), and they can be subsumed by a tumour, which initiates an M2-like phenotype exhibiting pro-tumorigenic properties.7 Reversing the tumour-induced immunopathology in TAMs and restoring anti-tumour macrophage function is a critical component of immunotherapeutic strategies against OS.
One mechanism that can restore macrophage anti-tumour immunity is infection.8 In the late nineteenth century William Coley utilized bacterial immunotherapy to treat sarcomas.9 Surgical site infections after limb-salvage surgery are associated with improved survival in both canine and human OS patients.10–14 Additionally, a murine study demonstrated induction of anti-OS activity in tumour-bearing mice with concurrent bacterial osteomyelitis, and demonstrated that one of the key players in the anti-tumour response is the macrophage.8 Similarly, liposomal encapsulated muramyl tripeptide phosphatidyl ethanolamine (L-MTP-PE) has been shown to promote anti-OS activity.15–18 L-MTP-PE is a synthetic derivative of muramyl dipeptide (MDP), which is a cell wall component in both gram-positive and gram-negative bacteria. One mechanism of the anti-OS effect of L-MTP-PE is the upregulation of the cytotoxic properties of alveolar macrophages.16
The clinical observations of improved survival in OS in patients with a deep surgical site infection, together with the potential therapeutic efficacy of L-MTP-PE, provide strong support to the general hypotheses that OS: 1) can be targeted by an immune response upregulated via a bacterial stimulus and 2) that macrophages play a critical role in the anti-tumour response. The aim of this study was to evaluate how in vitro exposure to a bacterial agent (Staphylococcus aureus) influenced canine and human macrophage differentiation in the presence of OS. Our study hypothesis was that S. aureus induces a significantly heightened inflammatory macrophage phenotype (M1-like) compared to macrophages cultured alone or with OS.
Materials and Methods:
Blood collection – canine and human
A healthy population of owned pet large and giant breed dogs (n=36) over one year of age was recruited. Written owner consent was obtained for all dogs, and peripheral blood samples were collected under an approved IACUC protocol (NCSU #13–122-O). A healthy population of humans between ages 18 and 25 years (n=17) was recruited. Written consent was obtained for all humans, HIPAA regulations were strictly enforced, and peripheral blood samples were collected under an approved IRB protocol (NCSU #9124; Duke University #PRO00050745). Both populations of dogs and humans were reported to be free from disease and were not taking any prescription or over-the-counter medications (reported by owners for dog; self-reported by humans).
Cell cultures
The Abrams canine OS cell line was kindly provided by Dr. Nicole Ehrhart (Fort Collins, CO), and grown in media containing Dulbecco’s Modification of Eagle’s Medium with 4.5 g/L glucose and sodium pyruvate (DMEM, Corning Life Sciences, Tewksbury, MA) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Gibco®, Langley, OK), 1% antibiotic (penicillin/streptomycin) (Corning Life Sciences), 4mM L-glutamine (Corning Life Sciences). The 143B human OS cell line was grown in media containing DMEM supplemented with 10% heat inactivated FBS, 1% antibiotic (penicillin/streptomycin). Both cell lines were incubated at 37°C and 5% CO2 in a humidified incubator.
Primary macrophage cultures – canine and human
A minimum of 50 mls of peripheral blood were collected into EDTA blood collection tubes via venipuncture from normal canines and humans. Canine and human PBMCs were isolated by density gradient centrifugation using Histopaque®−1077 (Millipore Sigma, St. Louis, MO). The PBMCs were resuspended in macrophage culture media containing DMEM supplemented with 10% heat inactivated FBS, 1% antibiotic (penicillin/streptomycin), 1% L-glutamine, 1% essential amino acids (Corning Life Sciences), 1% non-essential amino acids (Corning Life Sciences), 7.5% sodium bicarbonate solution (Gibco®), divided evenly in 12-well cell culture plates, and incubated at 37°C and 5% CO2. After overnight incubation, the culture media was changed to remove non-adherent cells. The adherent cells were then incubated for another 96 hours to allow differentiation into macrophages, with an average confluency of 33% per well of a 12-well cell culture plate. For co-culture with OS cells, Abrams canine OS cells and 143B human OS cells were added at 36 hours to the canine and human macrophage cultures, respectively. A total of 200,000 OS cells suspended in 500 μl culture media were added to each 0.4 μm transwell insert and placed within each 12-well culture well for co-culture of OS cells with macrophages.
For addition of live S. aureus to the macrophage-OS co-culture, antibiotic-free culture media was used to establish primary macrophage cultures, and at 48 hours, S. aureus was added as follows. A frozen aliquot of XEN36 S. aureus (PerkinElmer, Waltham, MA) was thawed, and 100 μl was added to 25 mls LB broth in an Erlenmeyer flask. The flask was placed in a 37°C shaker at 200 rpm until an O.D. of 0.8 was reached. The bacteria were then washed twice with 25 mls PBS and pelleted by centrifugation at 8,000 rpm for 10 minutes. The pelleted bacteria were resuspended in antibiotic-free media and diluted 1:10 for addition of 1 ml (resulting in approximately 2 × 107 cfu/ml S. aureus to achieve a MOI of 1:50) to each well of a 12-well culture plate containing primary macrophages. The plates were incubated for 30 minutes for macrophage phagocytosis of bacteria at 37°C, 5% CO2, after which the media containing XEN36 S. aureus was removed from the wells. The wells were washed twice with HBSS with calcium and magnesium, followed by the addition of media containing 50 μg/ml concentration of Gentamicin (Thermo Fisher Scientific, Waltham MA) to kill the extracellular bacteria. The plates were incubated for 60 minutes at 37°C, 5% CO2, after which the media was removed and plated onto blood agar plates to confirm the absence of extracellular bacteria. The cells were washed twice with HBSS with calcium and magnesium. Media containing 10 μg/ml concentration of Gentamicin was added to the wells, and the plates cultured for an additional 48 hours at 37°C, 5% CO2. Two wells were used for each plate to measure the number of phagocytosed bacteria – 1 ml 0.1% Triton™ X (Millipore Sigma) was added to each well to lyse the macrophages, and the Triton™ X solution plated on blood agar plates for counting number of bacteria phagocytosed. Intracellular bacterial counts were determined to be within a logarithmic difference between samples.
Total RNA extraction, reverse transcription, and quantitative RT-PCR
Macrophages exclusively were collected from culture wells by addition of Tri-Reagent (Zymo Research, Irvine, CA) directly into the wells to lyse the cells, and stored at −80°C for subsequent analysis. The Direct Zol RNA Microprep™ kit (Zymo Research) was used to extract total RNA from macrophages according to the manufacturer’s protocol. The concentration and quality of the extracted RNA was determined using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific) as well as the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA), and RNA samples were stored at −80°C until processing. Reverse transcription was performed using 100 ng RNA per sample with the qScript™ cDNA synthesis kit (QuantaBio, Beverly, MA) according to the manufacturer’s directions, and the RT reactions were carried out in a GeneAmp®PCR System 9700 thermal cycler (Applied Biosystems, Thermo Fisher Scientific). Gene expression was determined with SYBR Green qPCR using the QuantaBio PerfeCTa® SYBR Green FastMix® per the manufacturer’s directions, and reactions were cycled in a Roche Lightcycler 480 (Roche Diagnostics, Basel, Switzerland). All samples were run in triplicate. PrimePCR™ (Bio-Rad, Hercules, CA) PCR primers were used for detection of mRNA expression levels of Arginase, IL-10, TNF-α, β-actin, and GAPDH (Table 1). The qPCR cycling conditions were as follows: activation at 95°C for 2 minutes, followed by 45 cycles of denaturation at 95°C for 5 seconds and annealing at 60°C for 30 seconds. Expression of the target genes was normalized to expression of the housekeeping genes GAPDH (human) and β-actin (canine). The 2−ΔΔCT method was used to calculate the normalized relative mRNA expression of the target genes.
Table 1:
PCR primers for human and canine macrophage qPCR
| Primers (human) | Unique ID |
| Arginase | qHsaCED0043785 |
| IL-10 | qHsaCED0003369 |
| TNF-α | qHsaCED003746 |
| GAPDH | qHsaCED0038674 |
| Primers (canine) | Unique ID |
| Arginase | qCfaCED0035802 |
| IL-10 | qCfaCED0031300 |
| TNF-α | qCfaCED0030703 |
| β-actin | qCfaCED0037901 |
Magnetic bead panel assay
Supernatants from primary macrophage cultures were collected when the macrophages were harvested for assays and stored at −20°C for batched cytokine analysis. Supernatants were also collected from OS cells cultured alone to serve as a control. Canine and human macrophage supernatants were assessed for IL-10, IFN-γ, MCP-1, and TNF-α levels, and additionally, IL-12p40, and IL-12p70 levels from human macrophages using commercially available canine and human cytokine magnetic bead panel kits, MILLIPLEX® MAP, (Millipore Sigma) according to the manufacturer’s directions.
TGF-β ELISA
Supernatants from primary macrophage cultures were collected when the macrophages were harvested for assays, stored at −20°C for batched cytokine analysis. Supernatants were also collected from OS cells cultured alone to serve as a control. Supernatants were assessed for TGF-β levels using a commercially available TGF-β ELISA kit (R&D, Minneapolis, MN) according to manufacturer’s directions.
Flow cytometry (FACS)
Cultured primary macrophages were lifted from cell culture wells using Accutase® (Millipore Sigma) and resuspended in 100 μl FACS buffer (1X HBSS with 2% FBS) per tube for staining with the following antibodies against cell surface receptors listed in Table 2. Since OS cells were contained in transwell inserts, they were readily excluded from the cell population used for flow cytometric analysis. DAPI viability stain was used for canine macrophages. A fixable viability stain (eFluor 660, eBioscience) was used for human macrophages. Normal donkey serum (Jackson ImmunoResearch, West Grove, PA) was added to the FACS buffer for blocking against non-specific staining. The cells were stained with antibodies at 2–8°C for 30 minutes, washed with FACS buffer, and the human macrophages were fixed with fixation buffer (eBioscience) prior to analysis. Samples were analyzed immediately after cell staining using a LSRII (BD Biosciences, Franklin Lakes, NJ) flow cytometer, and data analysis was performed using the Kaluza® (Beckman Coulter, Brea, CA) software. Single color controls and compensation were performed using compensation beads, and positively staining cells were gated based on fluorescence-minus-one cellular controls.
Table 2:
List of antibodies used for human and canine macrophage flow cytometry
| Antibodies (anti-human) | Clone | Fluorescence Labeling | Manufacturer |
| CD11b | M1/70 | Pacific Blue | BioLegend, San Diego, CA |
| CD14 | TÜK4 | FITC | AbD Serotec, Raleigh, NC |
| CD5 | L17F12 | APC | BioLegend |
| CD11c | Bu15 | APC-Cy7 | BioLegend |
| CCR2 | K036C2 | BV510 | BioLegend |
| Antibodies (anti-canine) | Clone | Fluorescence Labeling | Manufacturer |
| CD11b | M1/70 | PE-Cy5 | AbCam, Cambridge, UK |
| CD80 | 16–10A1 | PE | eBioscience |
| CD11c | BU15 | APC-eFluor 780 | eBioscience |
Statistical analyses
All statistical analyses were performed using Prism version seven (GraphPad, San Diego, CA). Kruskal-Wallis and Dunn’s multiple comparisons test were used to compare canine and human macrophage cell surface receptor expression, cytokine levels, and relative mRNA expression of each gene between groups. All statistical tests were carried out as two-sided tests, and a p-value of <0.05 was considered statistically significant. All statistical analyses were performed in consultation with the NCSU Biostatistics consulting group.
Results:
Macrophages co-cultured with OS and S. aureus demonstrate heightened inflammatory mRNA signatures.
TNF-α and arginase are expressed differentially depending on the inflammatory activation status of macrophages.19 We compared relative macrophage mRNA expression of IL-10, TNF-α and arginase between the three culture conditions for canine and human macrophages. While the levels of arginase mRNA remained unchanged among the groups, the mRNA expression of TNF-α was significantly increased in both human (Figure 1A) and canine (Figure 1B) macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone. TNF-α mRNA was also significantly increased in human macrophages co-cultured with OS + S. aureus compared to macrophages co-cultured with OS (Figure 1A), whereas in canine macrophages, TNF-α was significantly increased when macrophages were co-cultured with OS compared to macrophages cultured alone (Figure 1B). The mRNA expression of IL-10 was significantly increased in human macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone (Figure 1C), whereas in canine macrophages, the mRNA expression of IL-10 was significantly increased in macrophages co-cultured with OS compared to macrophages cultured alone (Figure 1D).
Figure 1: Human and canine macrophage increase inflammatory mRNA profile in the presence of OS and S. aureus.
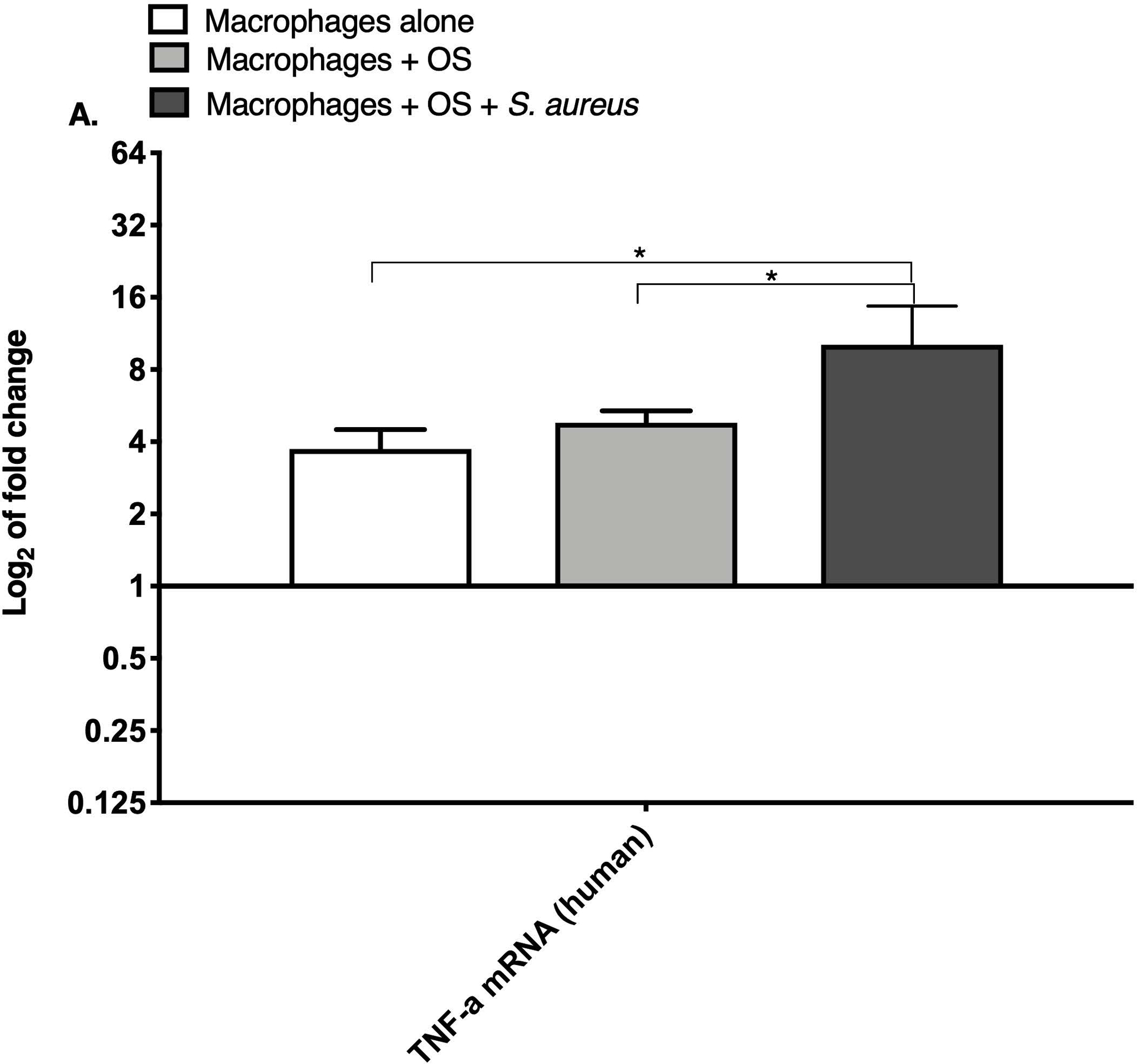
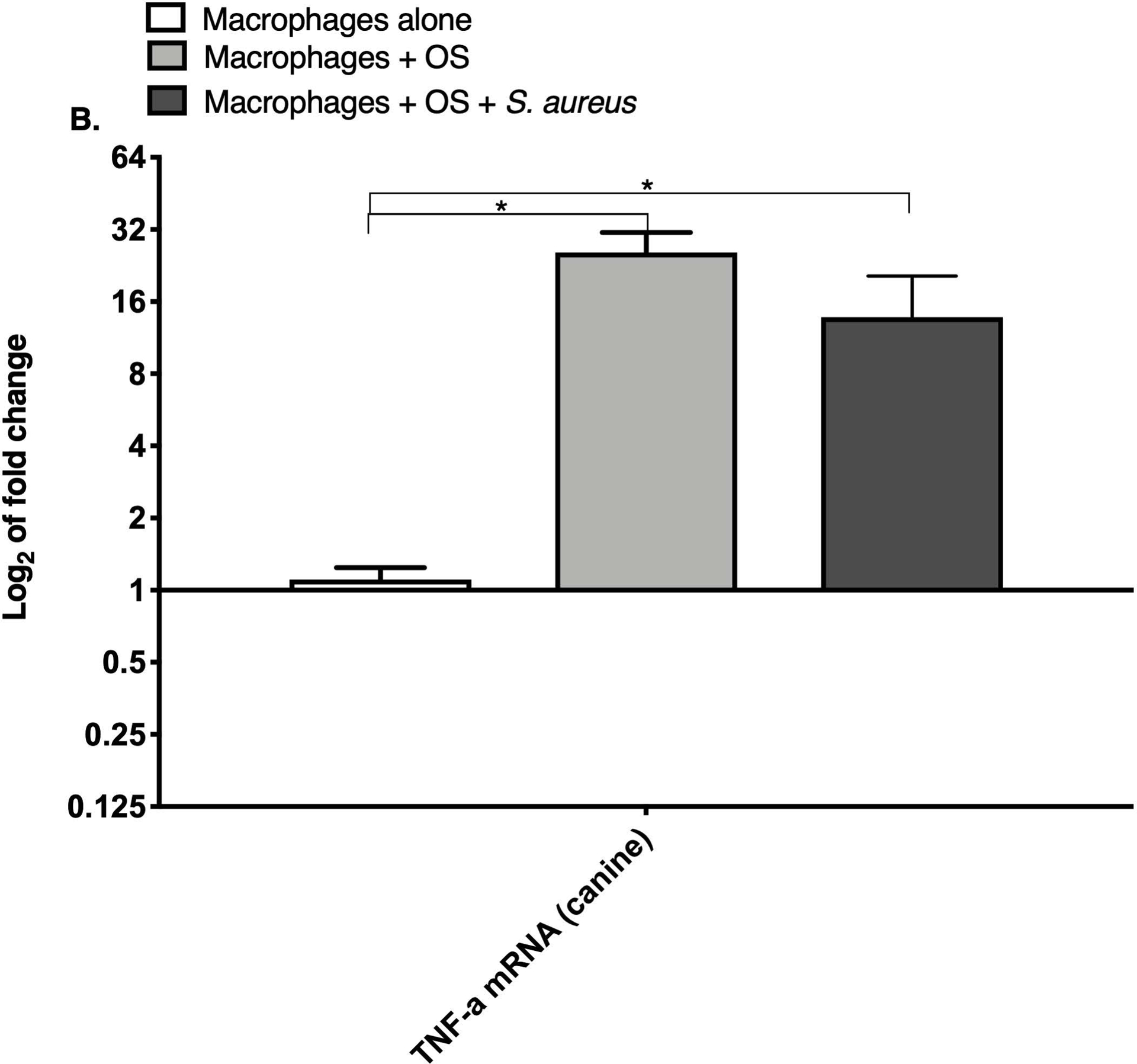
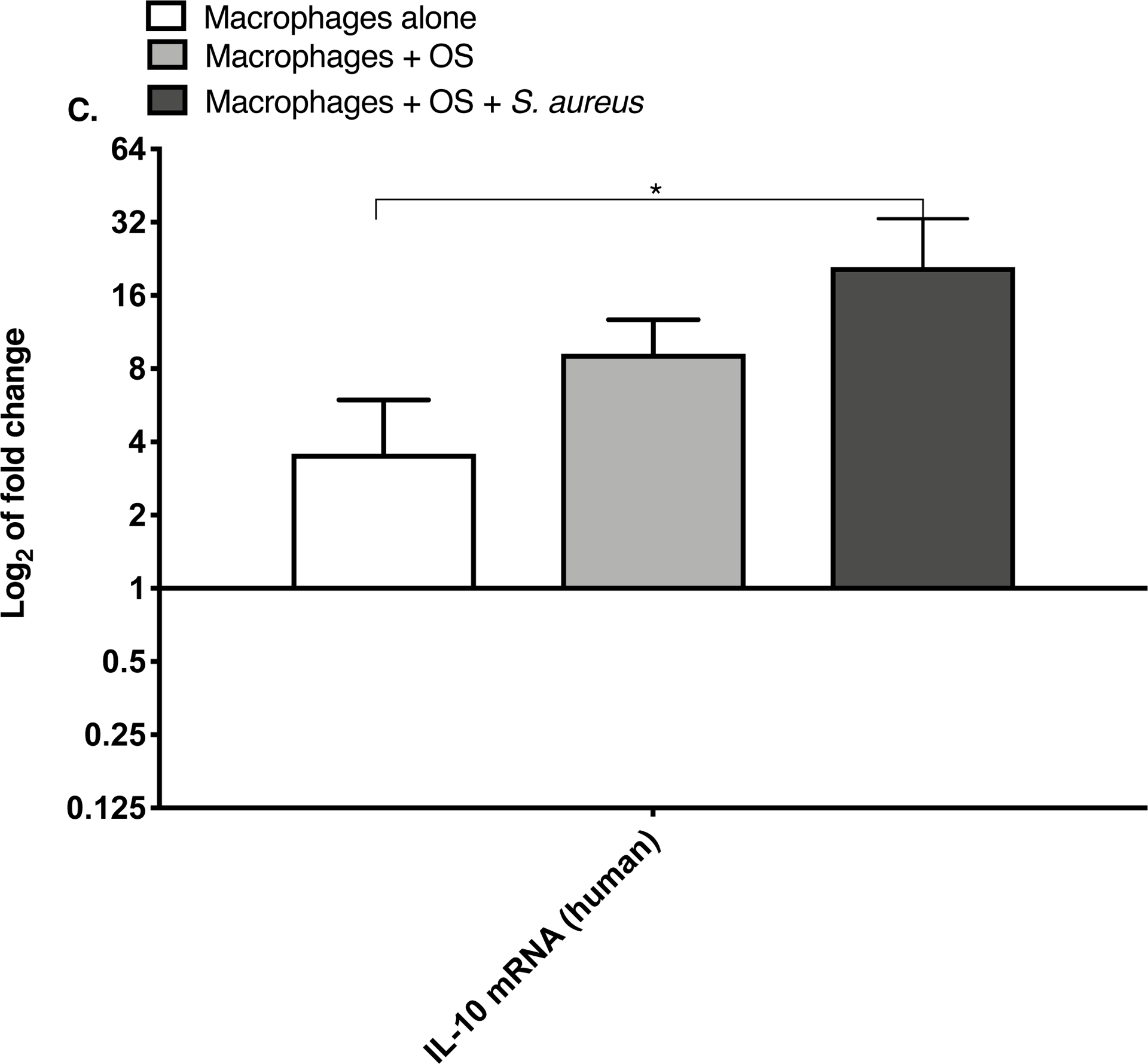
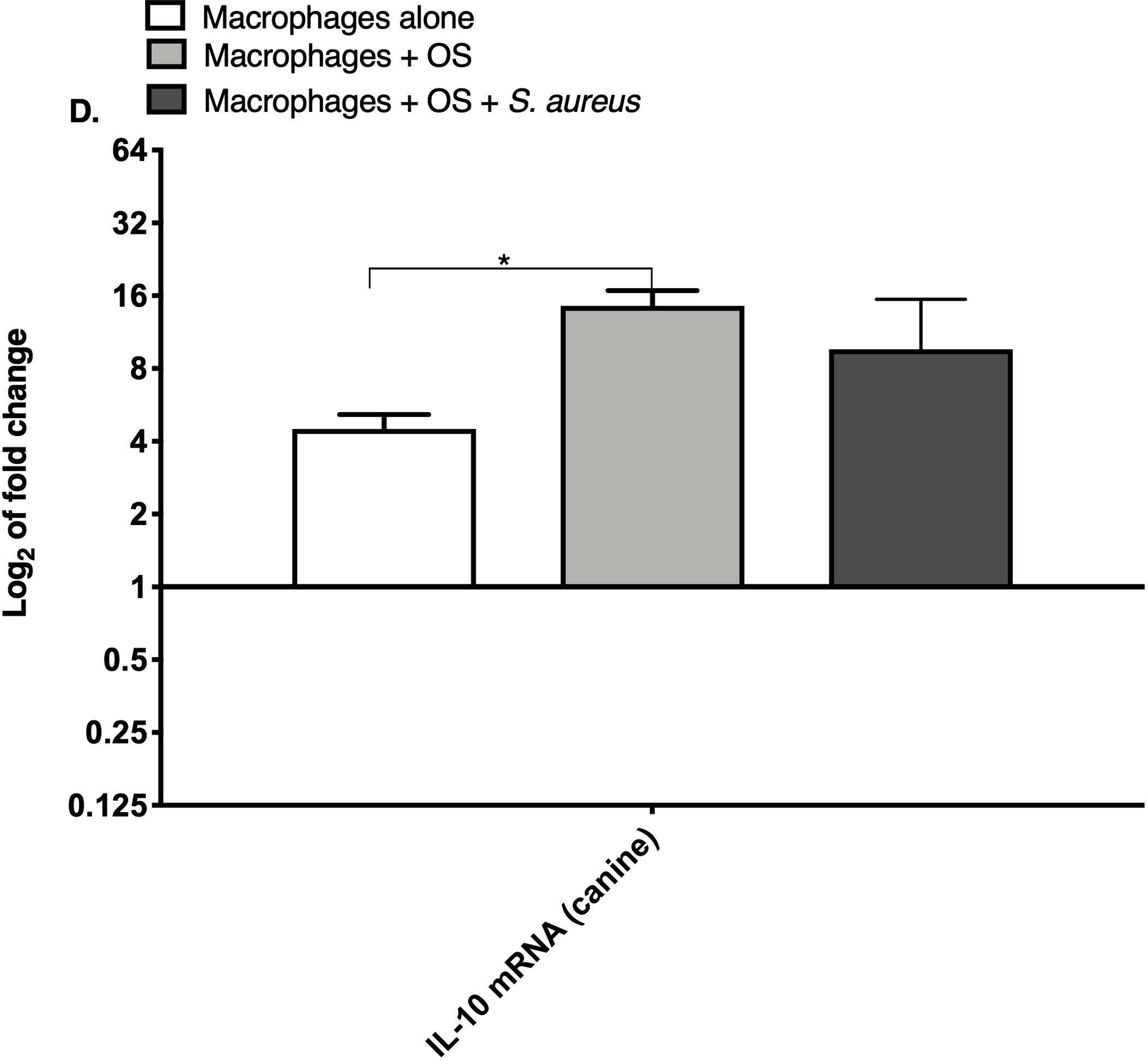
Bar graphs displaying the level of mRNA expression of (A) TNF-α in human macrophages cultured alone (n = 5), macrophages co-cultured with OS (n = 8), and macrophages co-cultured with OS + S. aureus (n = 9); (B) TNF-α in canine macrophages cultured alone (n = 12), macrophages co-cultured with OS (n = 14), and macrophages co-cultured with OS + S. aureus (n = 10); (C) IL-10 in human macrophages under the same culture conditions as (A); and (D) IL-10 in canine macrophages under the same culture conditions as (B). TNF-α mRNA expression was increased in macrophages co-cultured with OS and S. aureus compared to macrophages cultured alone (p = 0.0105) and to macrophages co-cultured with OS (p = 0.0368). TNF-α mRNA expression was significantly increased in canine macrophages co-cultured with OS (p<0.0001), and in macrophages co-cultured with OS and S. aureus (p = 0.0057) compared to macrophages cultured alone. IL-10 expression was significantly increased in human macrophages co-cultured with OS and S. aureus compared to macrophages cultured alone (p=0.0025). IL-10 expression was significantly increased in canine macrophages co-cultured with OS compared to macrophages cultured alone (p = 0.0023). Data shown as mean ± SEM of triplicate wells, asterisks indicate statistical significance (p<0.05).
Macrophages co-cultured with OS and S. aureus exhibit enhanced inflammatory cytokine secretion along with decreased TGF-β secretion.
We next asked if exposure to S. aureus led to an upregulation of the inflammatory cytokine signature in macrophages under the stated culture conditions. The MILLIPLEX® assay revealed significantly higher IFN-γ levels in culture supernatants of human (Figure 2A) and canine (Figure 2B) primary macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone. Human macrophages also secreted significantly higher IFN-γ levels when co-cultured with OS + S. aureus compared to macrophages co-cultured with OS (Figure 2A). TNF-α levels were significantly increased in culture supernatants of both human (Figure 2C) and canine (Figure 2D) primary macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone, and to macrophages co-cultured with OS. Additionally, canine macrophages co-cultured with OS secreted significantly higher TNF-α levels compared to macrophages cultured alone (Figure 2D). IL-12p70 levels were significantly increased in culture supernatants of human primary macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone and to macrophages co-cultured with OS (Figure 2E). IL-12p40 levels were significantly higher in culture supernatants of human macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone (Figure 2F). IL-12p40 levels were higher in macrophages co-cultured with OS + S. aureus compared to macrophages co-cultured with OS, but the difference was not significant (p=0.059). MCP-1 levels were significantly lower in supernatants of both human (Figure 2G) and canine (Figure 2H) macrophages cultured alone compared to macrophages co-cultured with OS and to macrophages co-cultured with OS + S. aureus. IL-10 levels were significantly lower in culture supernatants of human macrophages cultured alone compared with macrophages co-cultured with OS, and macrophages co-cultured with OS + S. aureus (Figure 2I). IL-10 levels from canine macrophages co-cultured with OS + S. aureus were significantly higher compared to levels from macrophages cultured alone and from macrophages co-cultured with OS (Figure 2J). IL-10 levels from canine macrophages co-cultured with OS were also significantly higher than levels from macrophages cultured alone (Figure 2J). For the controls, IFN-γ and IL-10 levels from canine and human OS cells cultured alone, TNF-α, IL-12p40 and IL-12p70 levels from human OS cells cultured alone were below detection limits. TNF-α levels from canine OS cells cultured alone were similar to macrophages cultured alone (OS cells: median 7.2 pg/mL, macrophages: median 6.33 pg/mL). MCP-1 levels from human OS cells cultured alone were higher than levels secreted by macrophages cultured alone but lower than levels secreted when macrophages were cultured with OS (OS cells: median 4475 pg/mL, macrophages: median 1323.9 pg/mL, macrophages + OS: median 85,250 pg/mL). MCP-1 levels from canine OS cells cultured alone were not recorded due to experimental error.
Figure 2: Human and canine macrophage inflammatory cytokine secretion is increased while TGF-β levels are decreased with exposure to S. aureus.
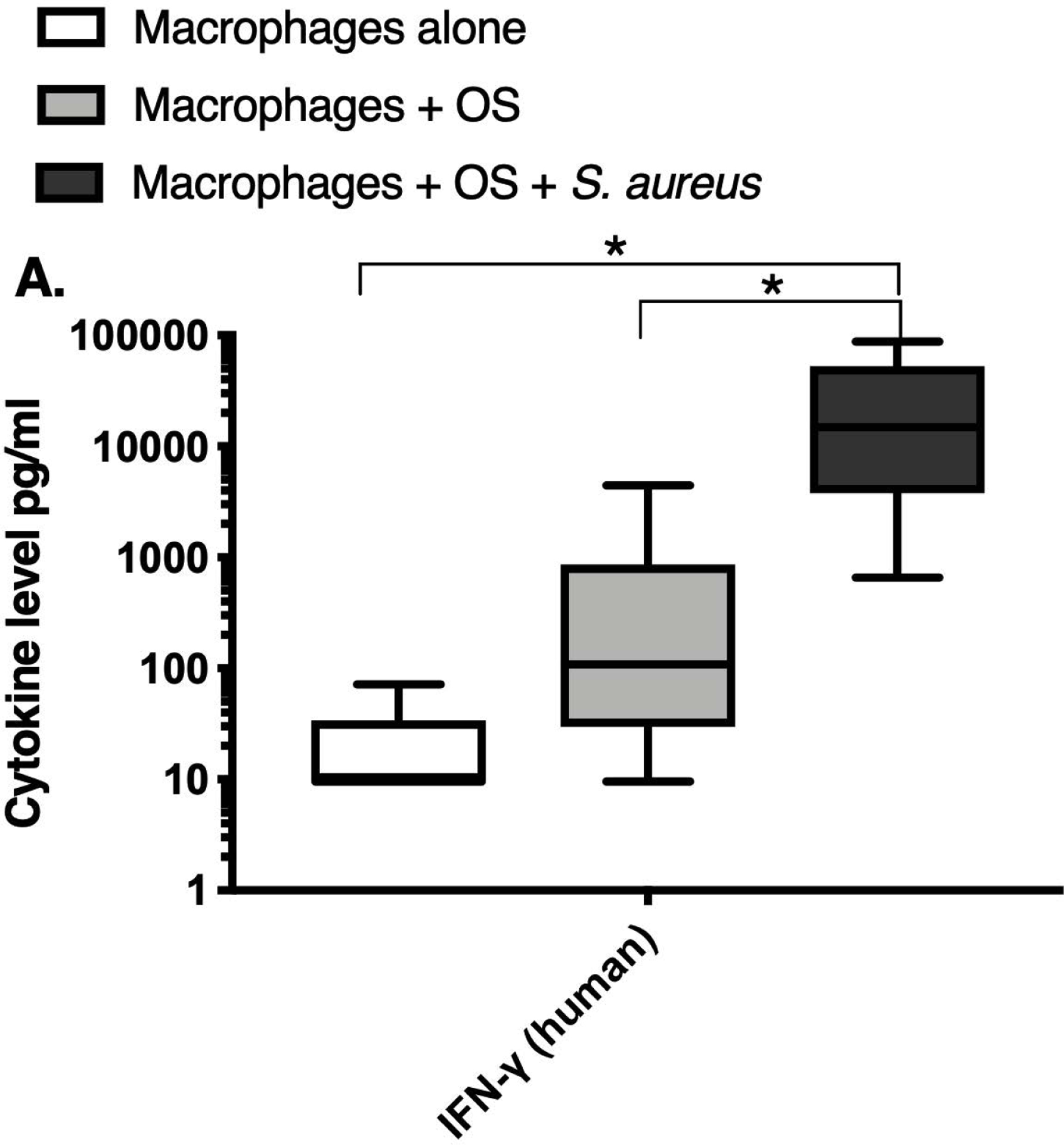
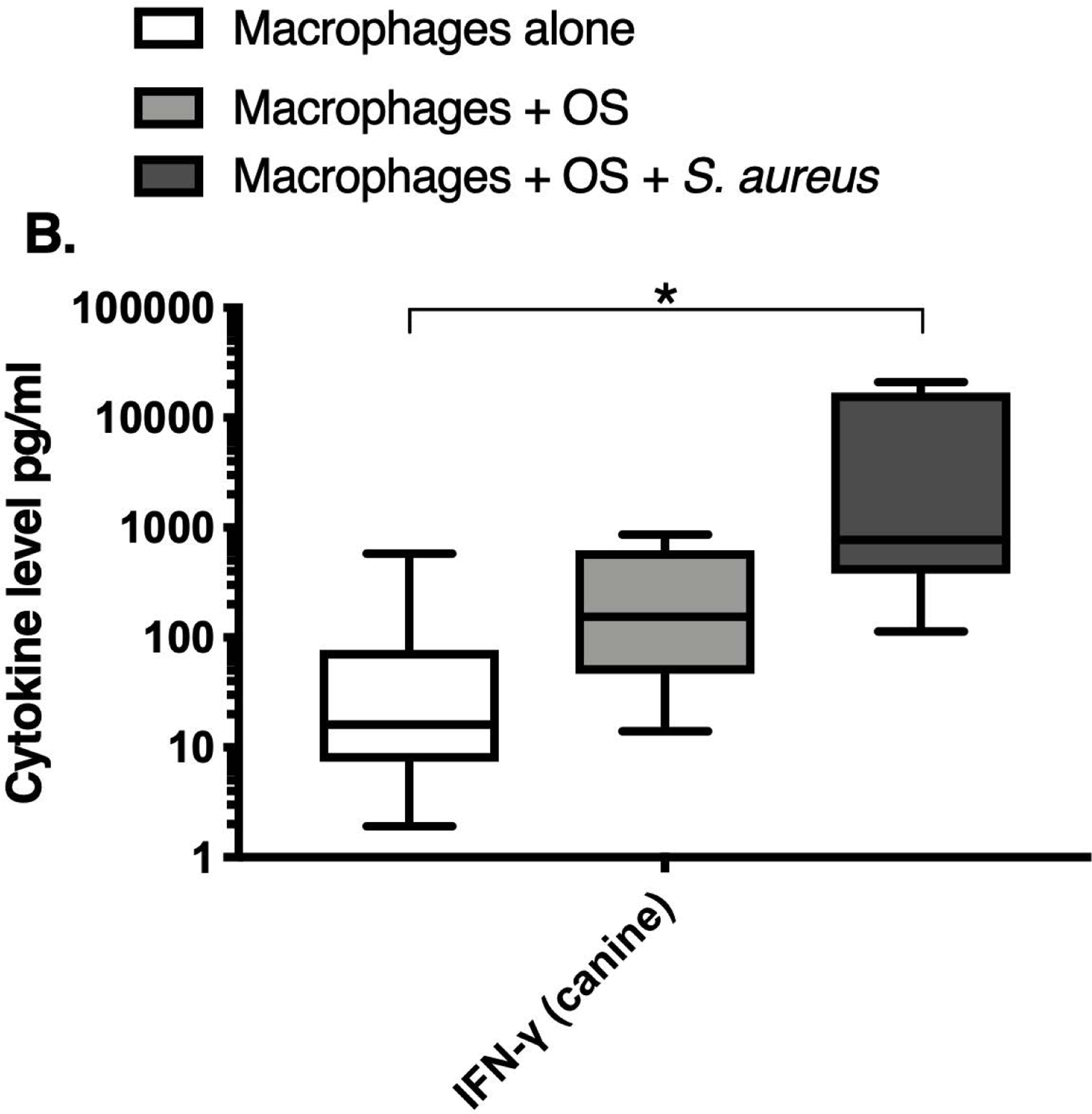
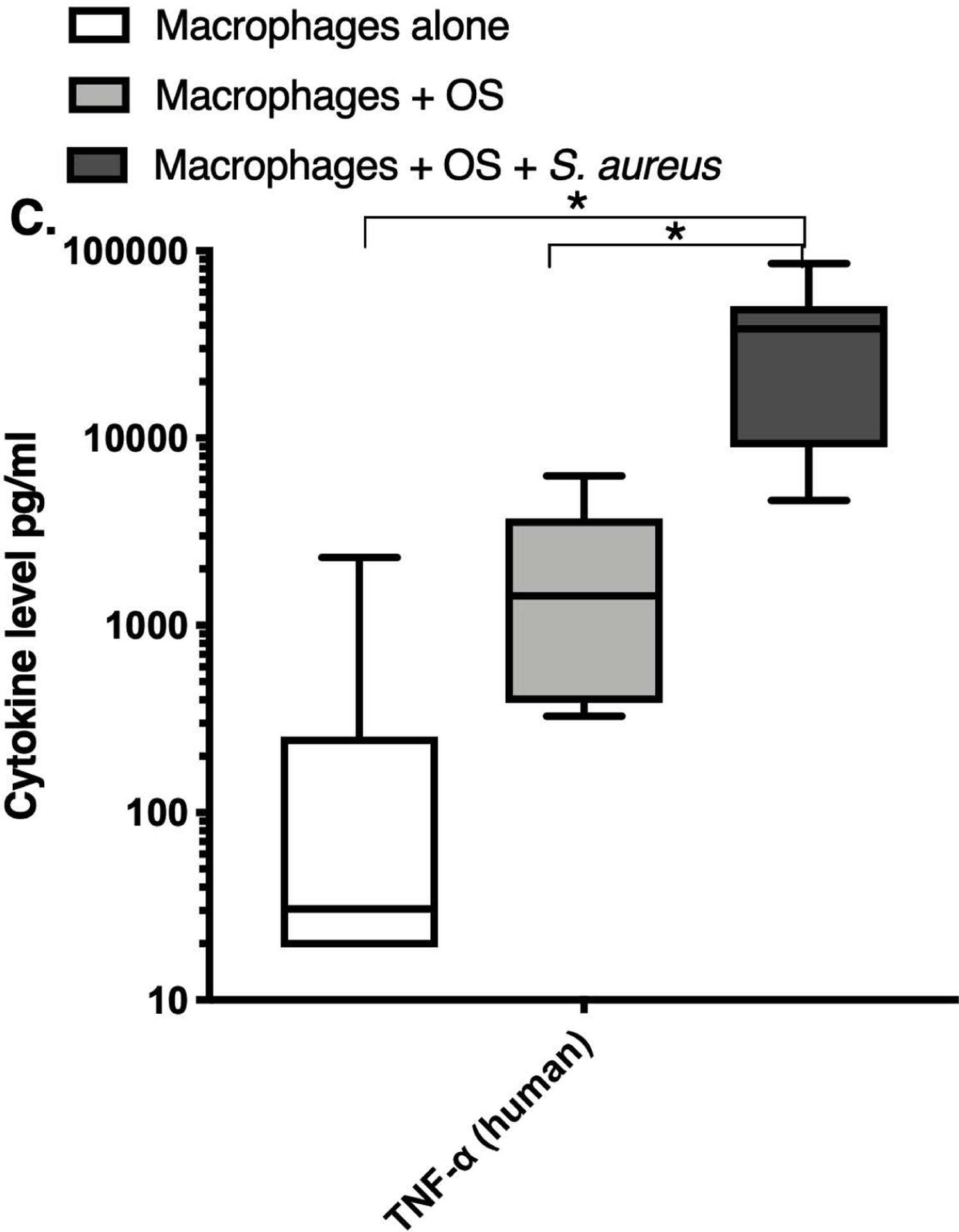
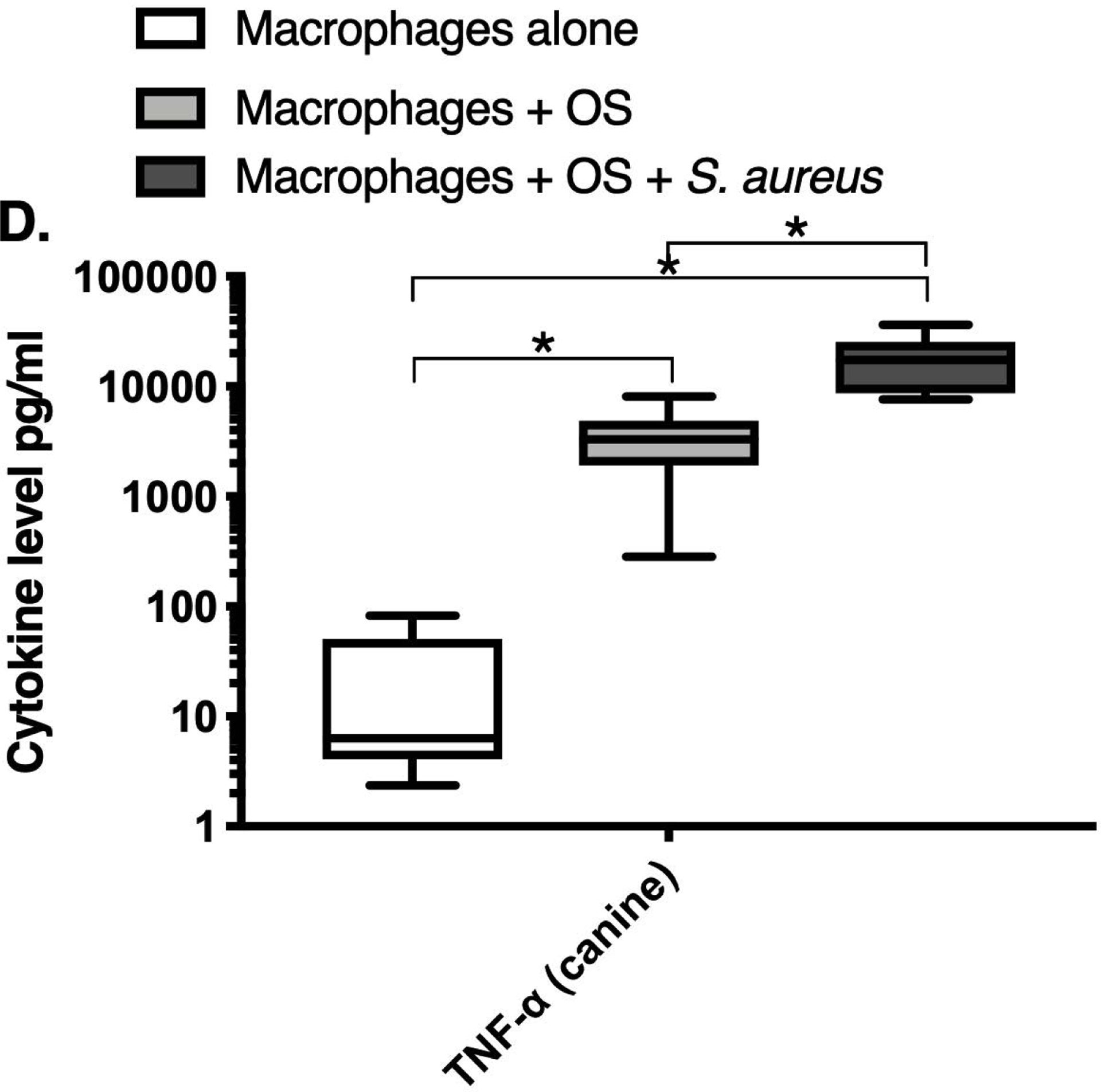
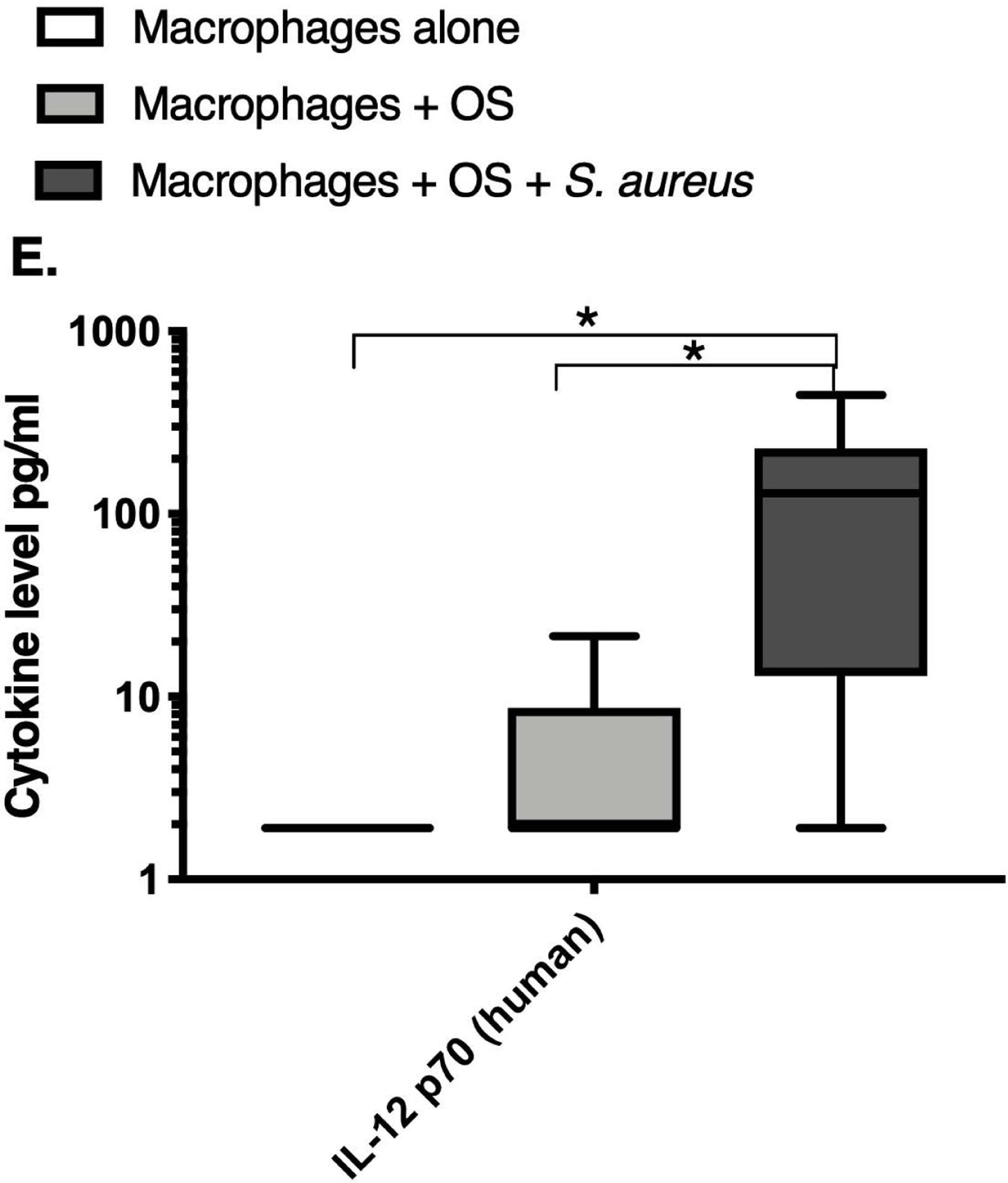
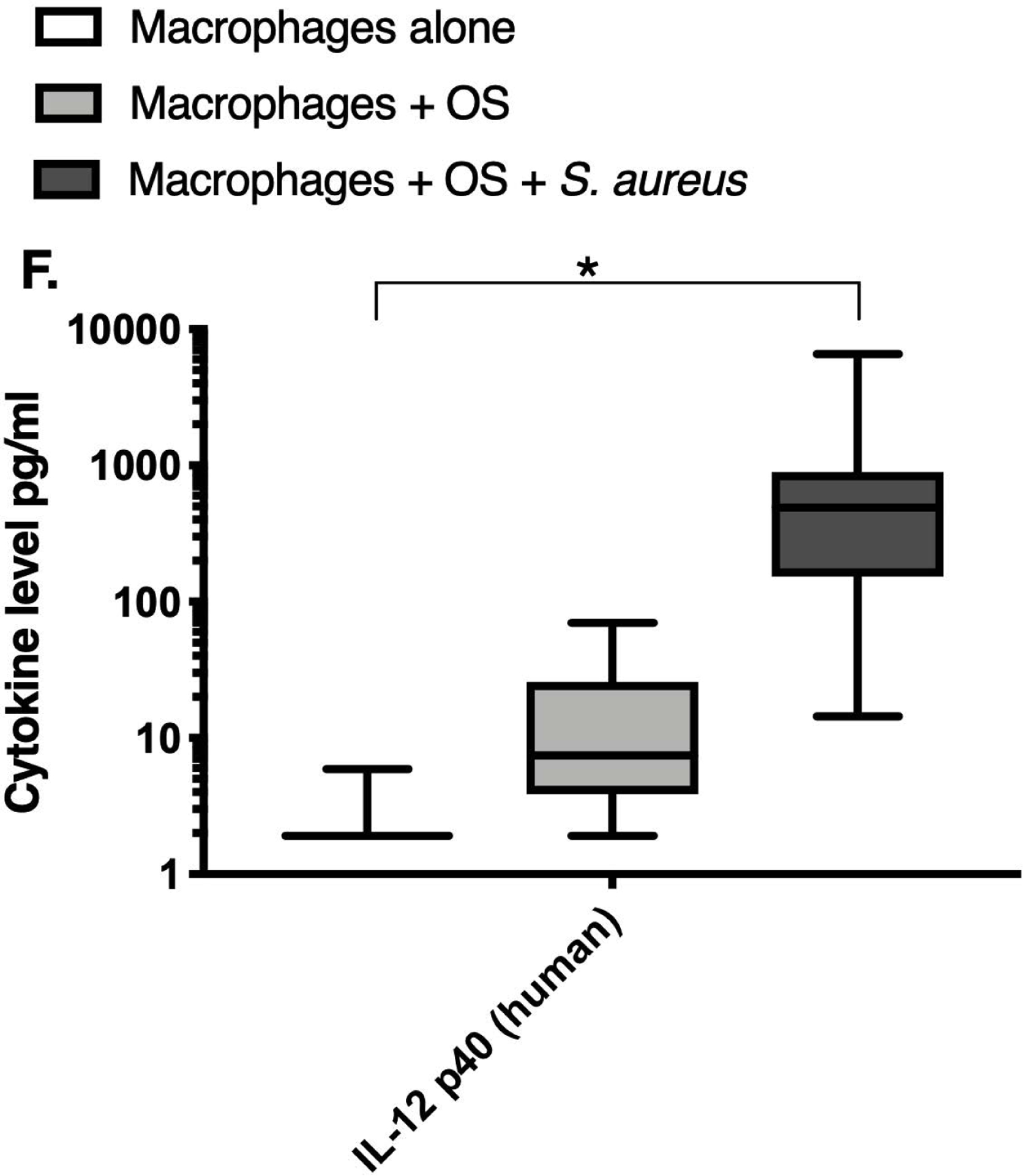
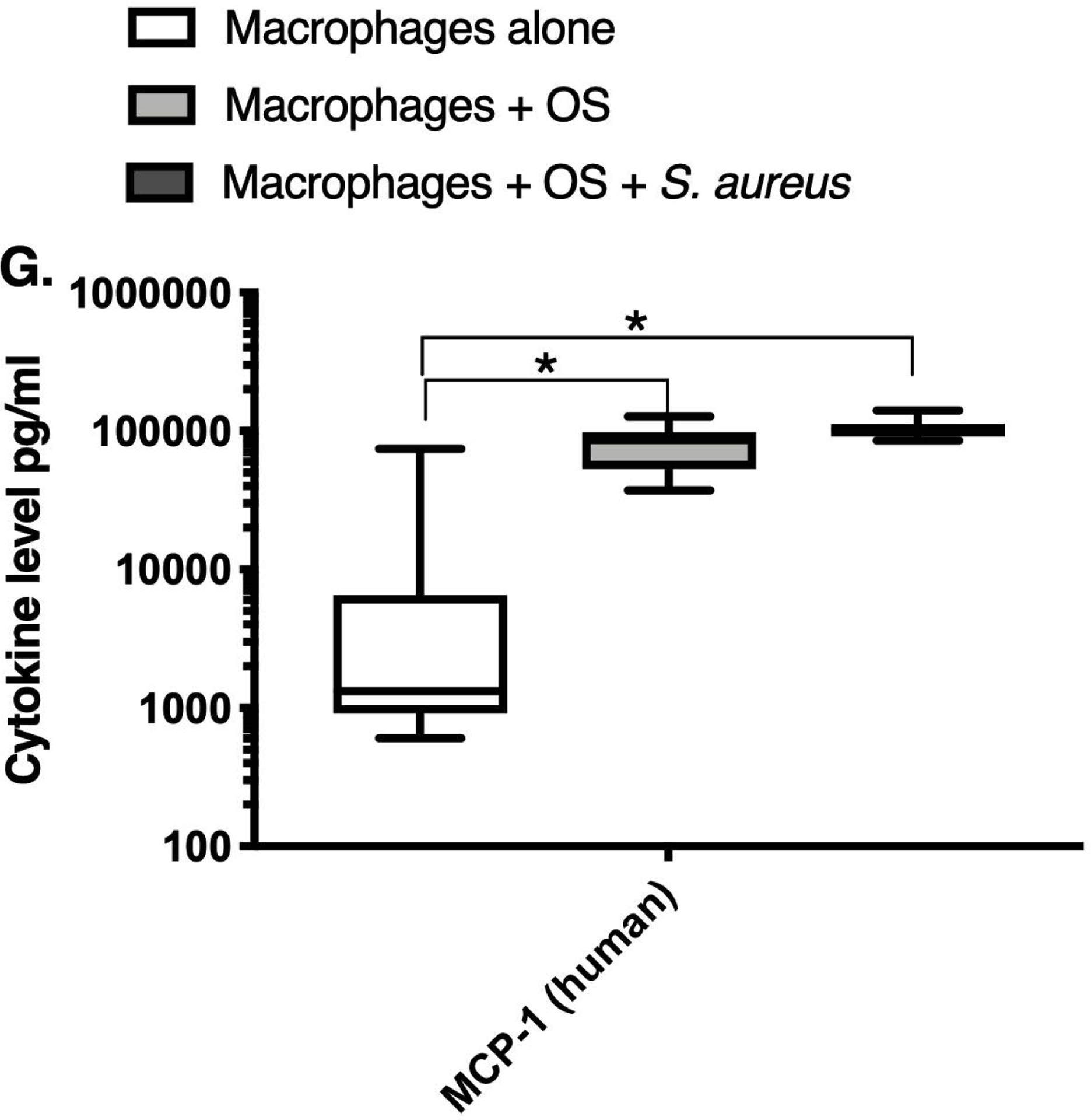
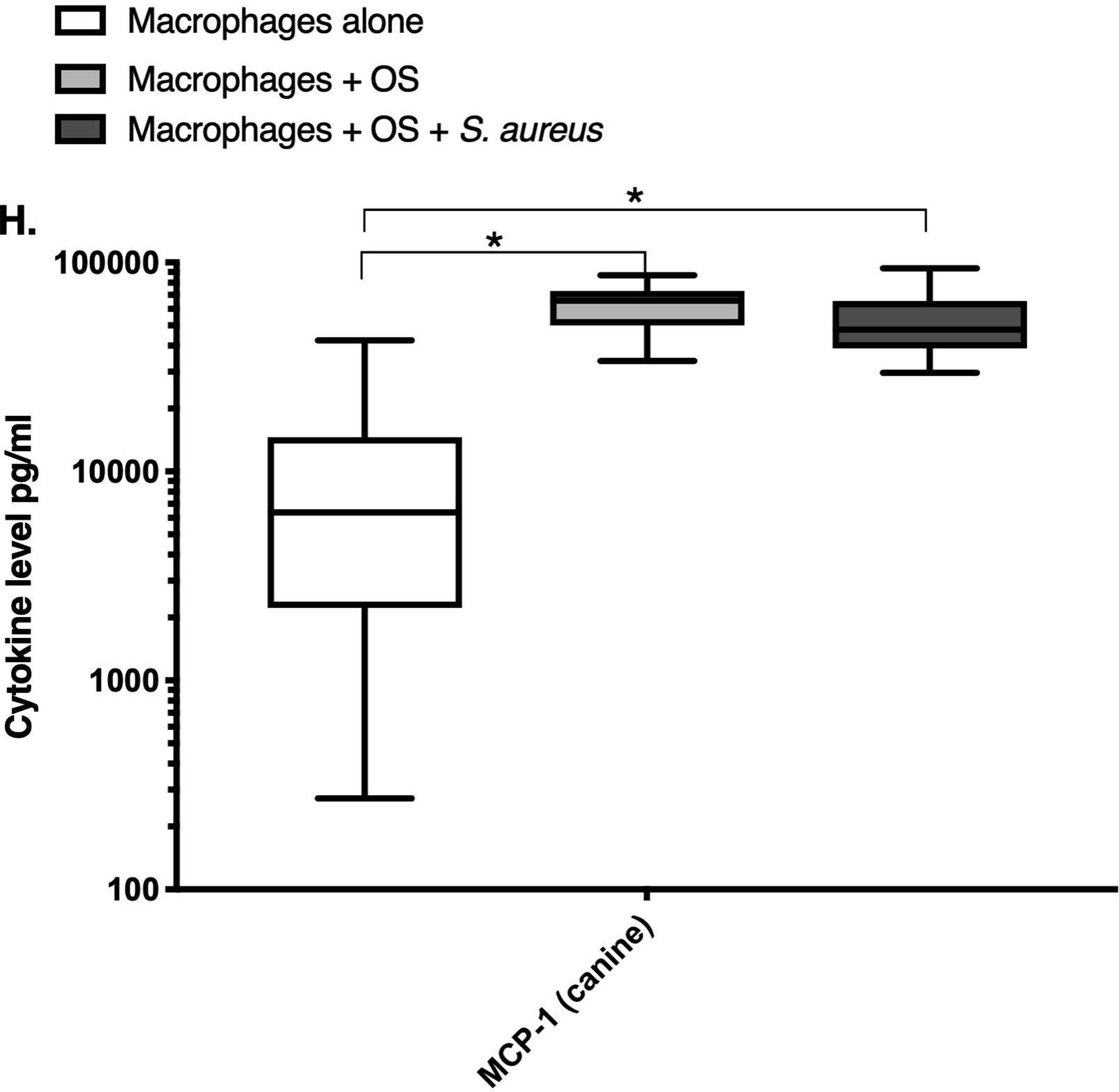
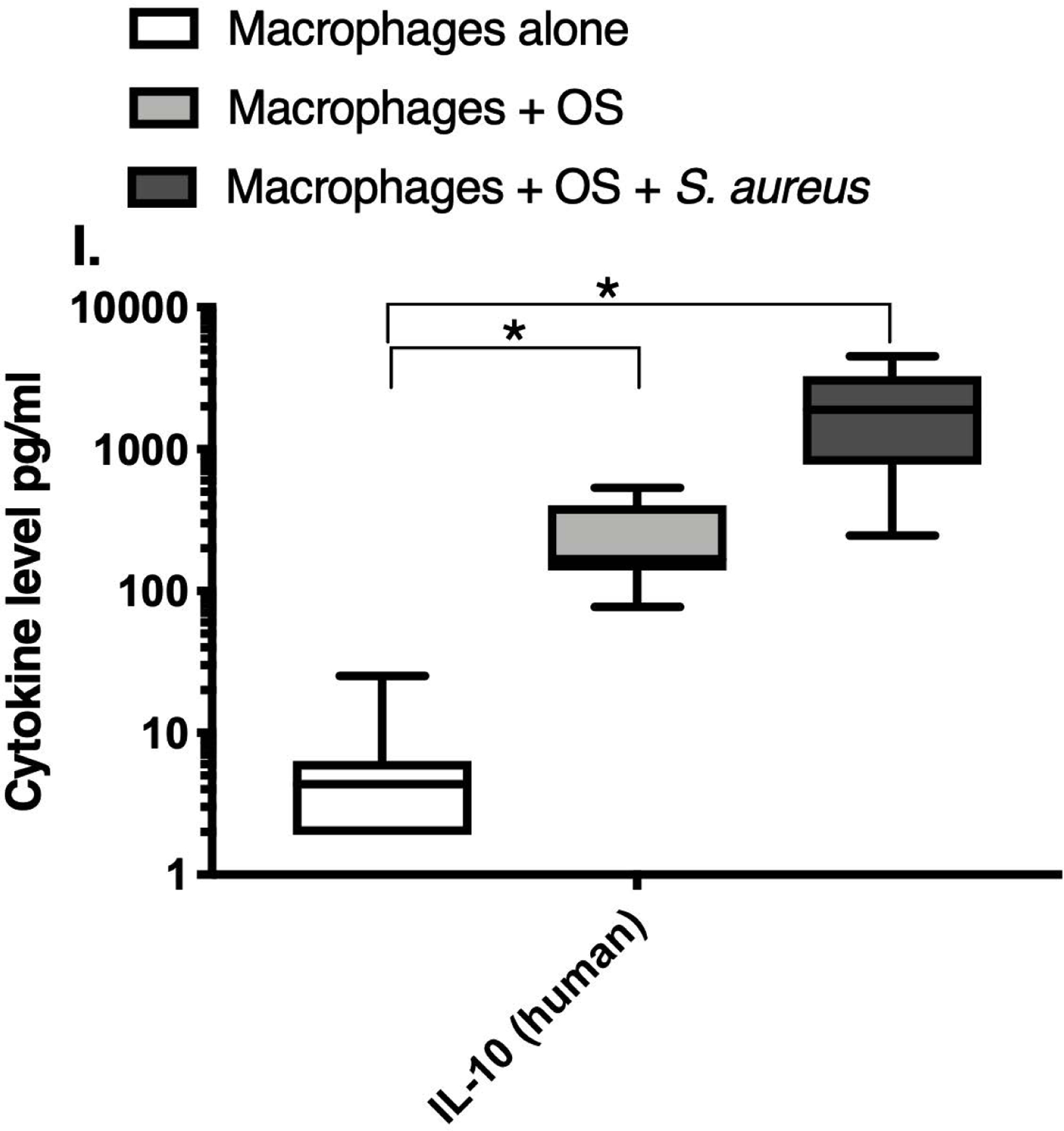
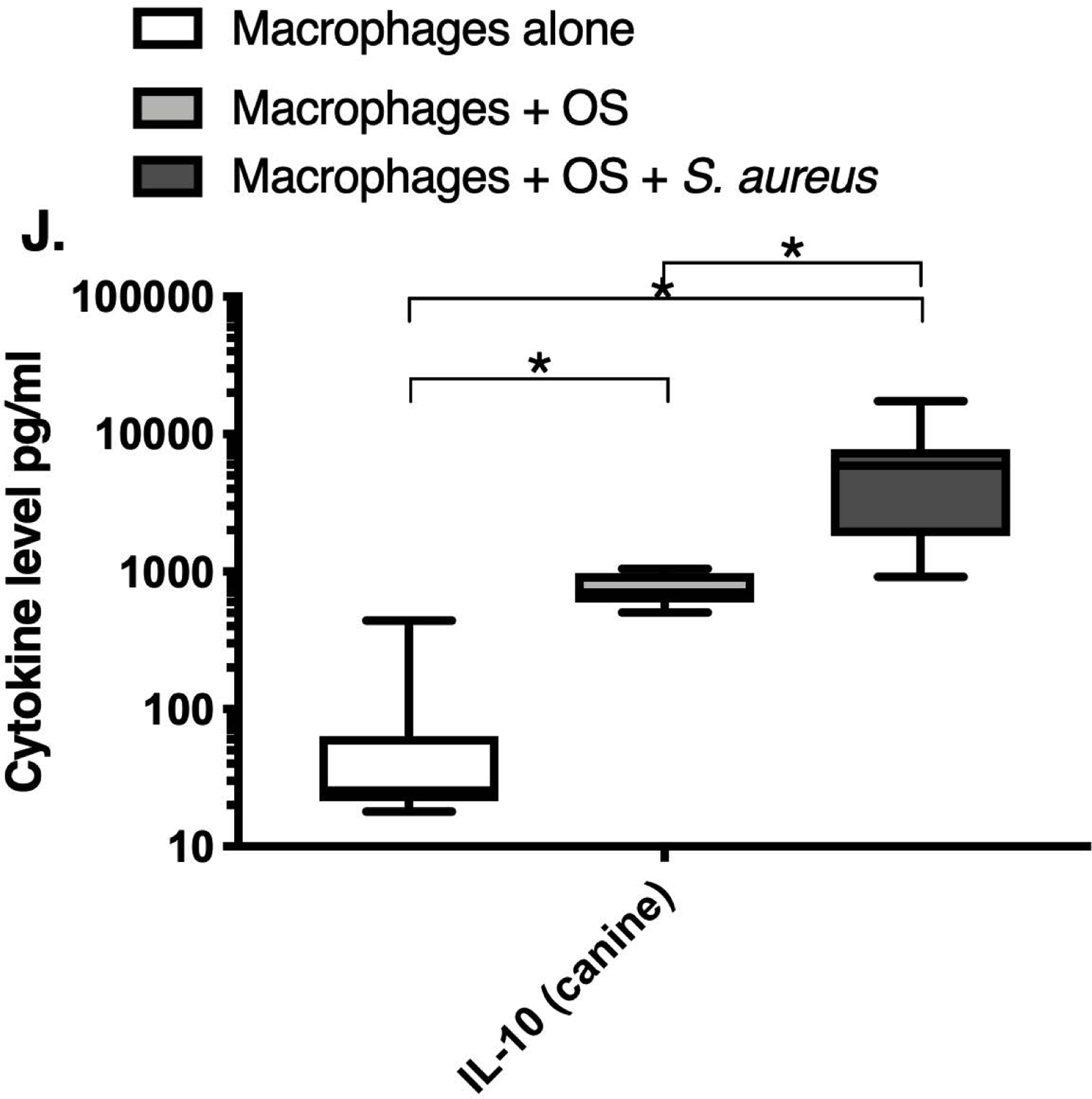
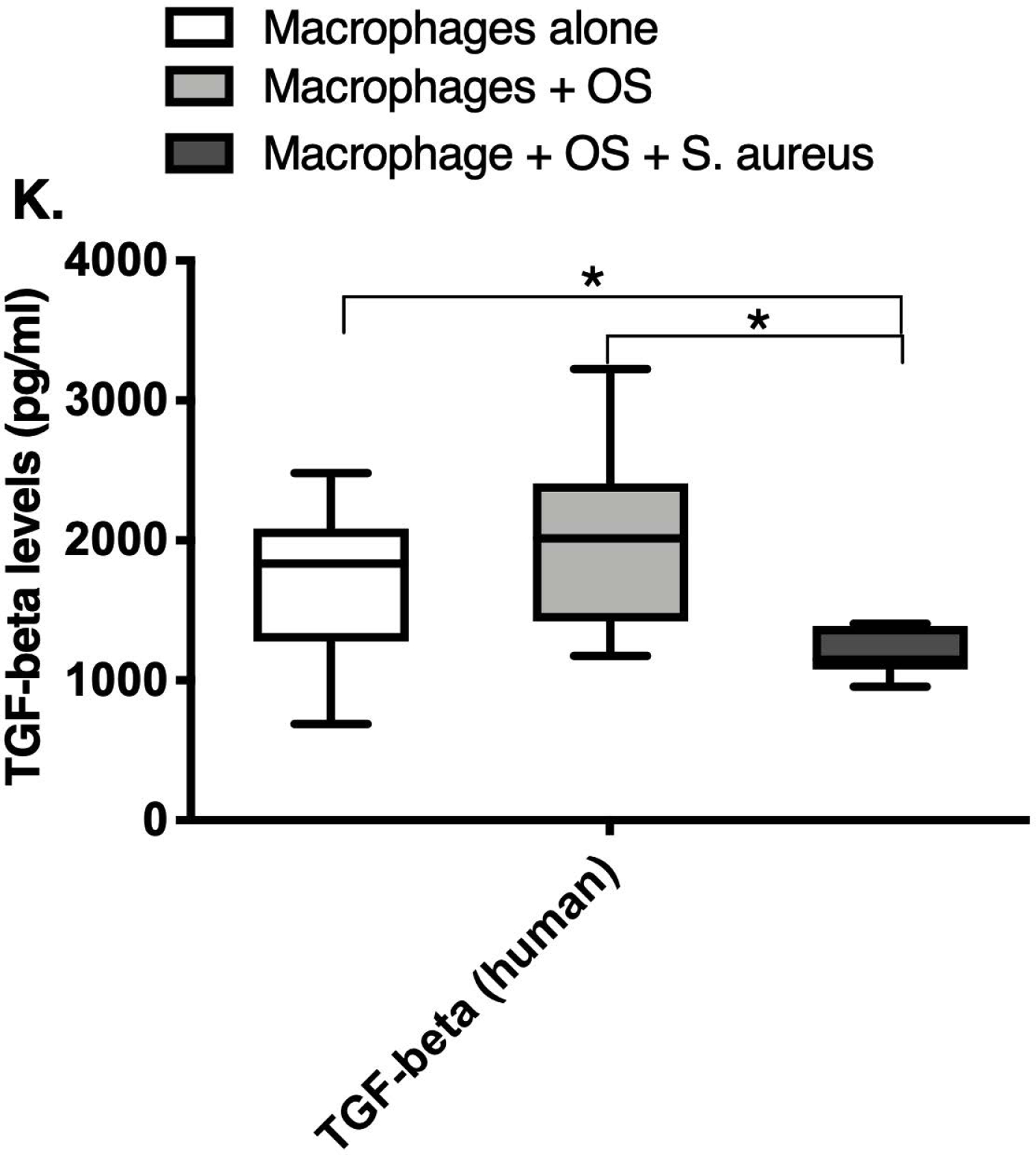
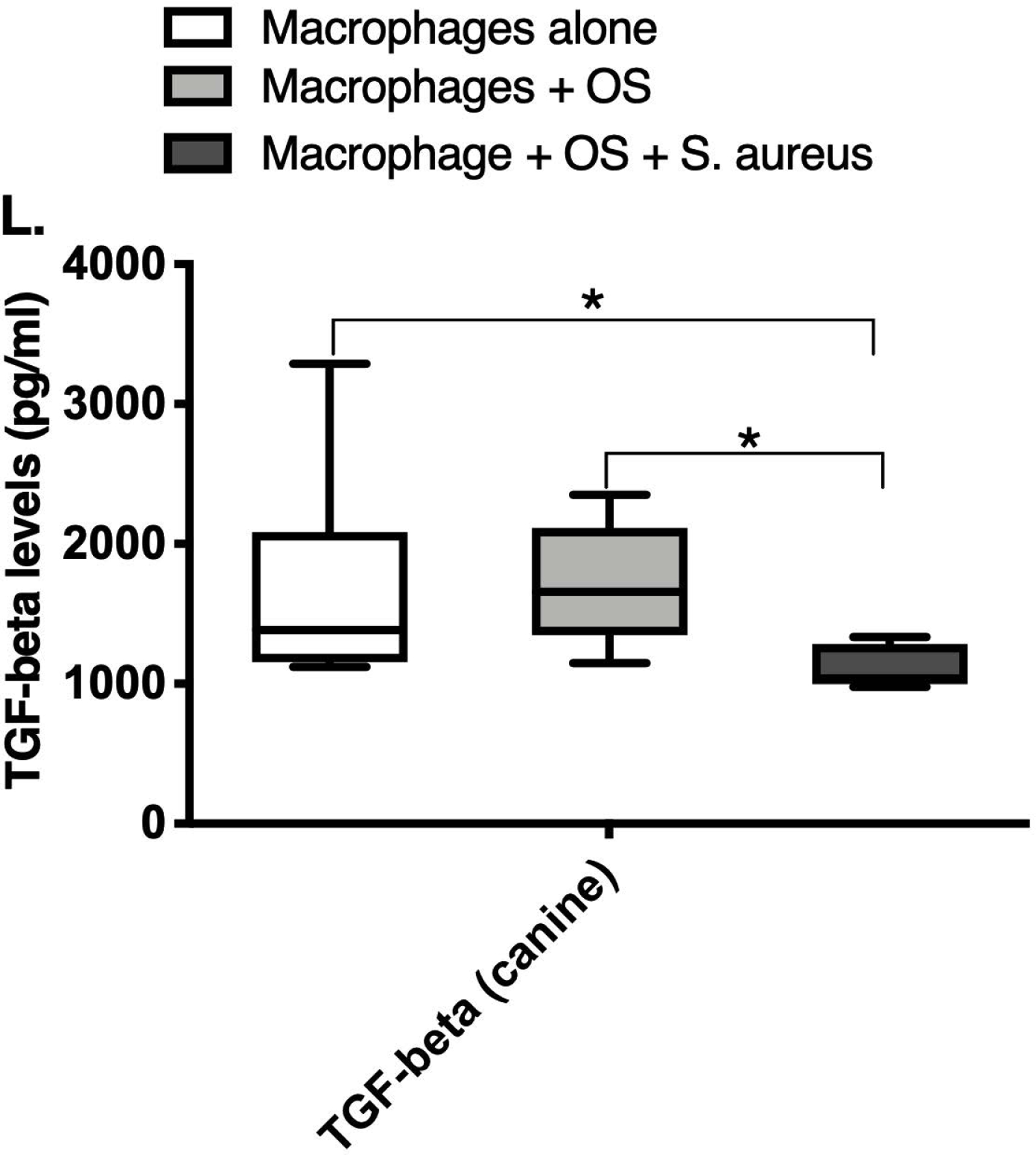
Box-and-whisker plots displaying the levels of (A) IFN-γ (human), (B) IFN-γ (canine), (C) TNF-α (human), (D) TNF-α (canine), (E) IL-12p70 (human), (F) IL-12p40 (human), (G) MCP-1 (human), (H) MCP-1 (canine), (I) IL-10 (human), (J) IL-10 (canine), (K) TGF-β (human), and (L) TGF-β (canine) in culture supernatants of macrophages cultured alone, macrophages co-cultured with OS, and macrophages co-cultured with OS + S. aureus. The sample numbers for human macrophage cultures are as follows: macrophages cultured alone (n = 10), macrophages co-cultured with OS (n = 9), and macrophages co-cultured with OS + S. aureus (n = 9) for all cytokines except for TGF-β, where macrophages cultured alone (n = 10), macrophages co-cultured with OS (n = 10), and macrophages co-cultured with OS + S. aureus (n = 9). The sample numbers for canine macrophage cultures are as follows: macrophages cultured alone (n = 11), macrophages co-cultured with OS (n = 13), and macrophages co-cultured with OS + S. aureus (n = 12) for all cytokines except for TGF-β, where macrophages cultured alone (n = 11), macrophages co-cultured with OS (n = 11), and macrophages co-cultured with OS + S. aureus (n = 8).
(A) IFN-γ levels were significantly higher in culture supernatants of human macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone (p<0.0001) and macrophages co-cultured with OS (p=0.0465). (B) IFN-γ levels were significantly higher in culture supernatants of canine macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone (p<0.0001). (C) TNF-α levels were significantly higher in culture supernatants of human primary macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone (p<0.0001) and macrophages co-cultured with OS (p=0.0404). (D) TNF-α levels were significantly higher in culture supernatants of canine macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone (p<0.0001) and macrophages co-cultured with OS (p=0.0131). TNF-α levels from macrophages co-cultured with OS were significantly higher than those from macrophages cultured alone (p=0.0138). (E) IL-12p70 levels were significantly higher in culture supernatants of human macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone (p=0.0002) and macrophages co-cultured with OS (p=0.0127). (F) IL-12p40 levels were significantly higher in culture supernatants of human macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone (p<0.0001). IL-12p40 levels were higher in macrophages co-cultured with OS + S. aureus compared to macrophages co-cultured with OS, but the difference was not significant (p=0.059 (G) MCP-1 levels were significantly lower in culture supernatants of human macrophages cultured alone compared to macrophages co-cultured with OS (p=0.0100) and macrophages co-cultured with OS + S. aureus (p<0.0001). (H) MCP-1 levels from culture supernatants of canine macrophages cultured alone were significantly lower compared to macrophages co-cultured with OS (p<0.0001), and macrophages co-cultured with OS + S. aureus (p=0.0015). (I) IL-10 levels were significantly lower in culture supernatants of human macrophages cultured alone compared with human macrophages co-cultured with OS (p=0.0232), and macrophages co-cultured with OS + S. aureus (p<0.0001). (J) IL-10 levels were significantly higher in culture supernatants of canine macrophages co-cultured with OS + S. aureus compared to levels from macrophages cultured alone (p<0.0001) and from macrophages co-cultured with OS (p=0.0281). IL-10 levels from macrophages co-cultured with OS were significantly higher than those from macrophages cultured alone (p=0.0223). (K) TGF-β levels were significantly decreased in culture supernatants of human macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone (p = 0.0466), and to macrophages co-cultured with OS (p = 0.0033). (L) TGF-β levels were significantly decreased in culture supernatants of canine macrophages co-cultured with OS + S. aureus compared to macrophages cultured alone (p = 0.0375), and to macrophages co-cultured with OS (p = 0.0034). Whiskers for box plot indicate minimum and maximum values. Asterisks indicate statistical significance (p<0.05).
Medians and ranges of cytokine concentrations can be found in Supplemental Table 1.
Most importantly, TGF-β levels in culture supernatants of both human (Figure 2K) and canine (Figure 2L) macrophages co-cultured with OS + S. aureus were significantly decreased compared to macrophages cultured alone, and to macrophages co-cultured with OS. For the controls, TGF-β levels from canine OS cells cultured alone were similar to levels secreted by macrophages cultured alone (OS cells: median 1388.3 pg/mL; macrophages: median 1381.13 pg/mL). TGF-β levels from human OS cells cultured alone were higher than levels secreted by macrophages cultured alone (OS cells: >detection limit; macrophages: median 1836.08 pg/mL). Table 3 provides a summary of the cytokine profiles between human and canine macrophages when cultured alone, or with OS +/− S. aureus.
Table 3:
Comparison of cytokine profiles from human and canine macrophages under different culture conditions.
| Macrophages alone (M) | Macrophages + OS (M+O) | Macrophages + OS + S. aureus (M+O+SA) | Macrophage subset predominantly secreting cytokine listed | |
|---|---|---|---|---|
| IFN-γ (human) | ↑ vs. M |
*↑ vs. M *↑ vs. M+O |
M1 | |
| IFN-γ (canine) | ↑ vs. M |
*↑ vs. M ↑ vs. M+O |
M1 | |
| TNF-α (human) | ↑ vs. M |
*↑ vs. M *↑ vs. M+O |
M1 | |
| TNF-α (canine) | *↑ vs. M |
*↑ vs. M *↑ vs. M+O |
M1 | |
| MCP-1 (human) |
*↓ vs. M+O *↓ vs. M+O+SA |
↓ vs. M+O+SA |
M1 | |
| MCP-1 (canine) |
*↓ vs. M+O *↓ vs. M+O+SA |
↑ vs. M+O+SA | M1 | |
| Il-12p70 (human) | No difference from M |
*↑ vs. M *↑ vs. M+O |
M1 | |
| IL-12p40 (human) | ↑ vs. M |
*↑ vs. M ↑ vs. M+O |
M1 | |
| IL-10 (human) |
*↓ vs. M+O *↓ vs. M+O+SA |
↓ vs. M+O+SA |
M2 | |
| IL-10 (canine) | *↑ vs. M |
*↑ vs. M *↑ vs. M+O |
M2 | |
| TGF-β (human) | ↑ vs. M |
*↓ vs. M *↓ vs. M+O |
M2 | |
| TGF-β (canine) | ↑ vs. M |
*↓ vs. M *↓ vs. M+O |
M2 |
Arrows indicate an increase or decrease when comparing macrophages from the indicated culture group to another culture group (significant differences marked with an asterisk). M = macrophages cultured alone, M+O = macrophages cultured with OS, M+O+SA – macrophages cultured with OS and S. aureus.
Co-Culture with S. aureus promotes an inflammatory macrophage surface receptor phenotype.
Given the variations in macrophage mRNA and cytokine profiles between the 3 culture conditions, we wished to test if differences existed in cell surface receptor expression of human macrophages using flow cytometry. We focused on receptors with known activity in sensing of bacterial infections and inflammation (CD80, CD11c), and macrophage migration (CCR2). Macrophages were gated based on forward and side scatter characteristics, and on positive expression of CD11b and CD14 after gating in the singlet cells (Figure 3). Any remaining lymphocytes were gated out using positive CD5 expression. The percentage of CCR2 expression was significantly lower in human macrophages co-cultured with OS and S. aureus compared to macrophages cultured alone (Figure 4A). The percentage of CD11c expression was significantly lower in human macrophages co-cultured with OS and S. aureus compared to macrophages cultured alone, and macrophages co-cultured with OS (Figure 4B). The percentage of CD80 expression was significantly higher in canine macrophages co-cultured with OS and in macrophages co-cultured with OS and S. aureus compared to macrophages cultured alone. (Figure 4C). These results suggest that exposure to S. aureus alters macrophage surface receptor expression towards an inflammatory phenotype.
Figure 3: Gating strategies for macrophage flow cytometry.
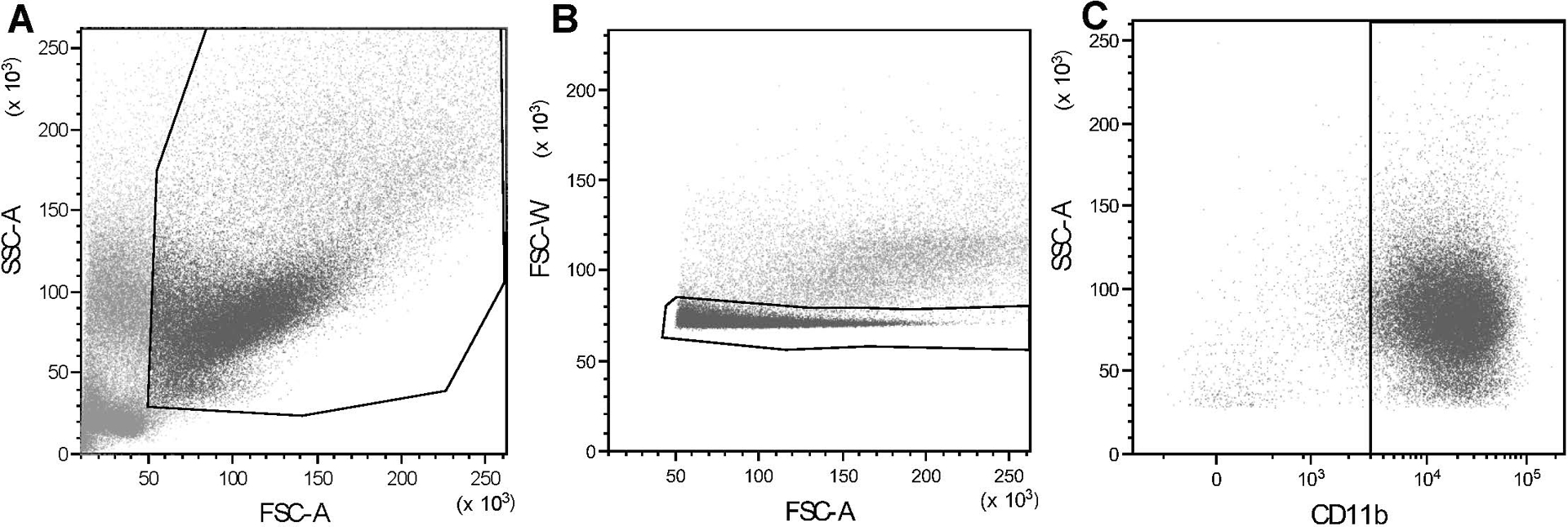
Representative dot plot showing canine macrophage gating using (A) typical forward and side scatter characteristics; (B) gating in of singlet cells (noted within the outlined area) to exclude doublets; (C) gating of CD11b+ macrophages (noted in the gate on the right side of the plot). Human macrophage gating (not shown here) was performed in similar fashion. The average percentages of macrophages of all the cells (including viable and non-viable) present prior to the application of gating strategies were as follows: canine macrophages 20.35%, human macrophages 20.82%. The average percentages of macrophages of the viable cells present after application of gating strategies were as follows: canine macrophages 93.24%, human macrophages 84.96%.
Figure 4: Exposure to S. aureus induces an inflammatory surface receptor expression profile in human and canine macrophages.
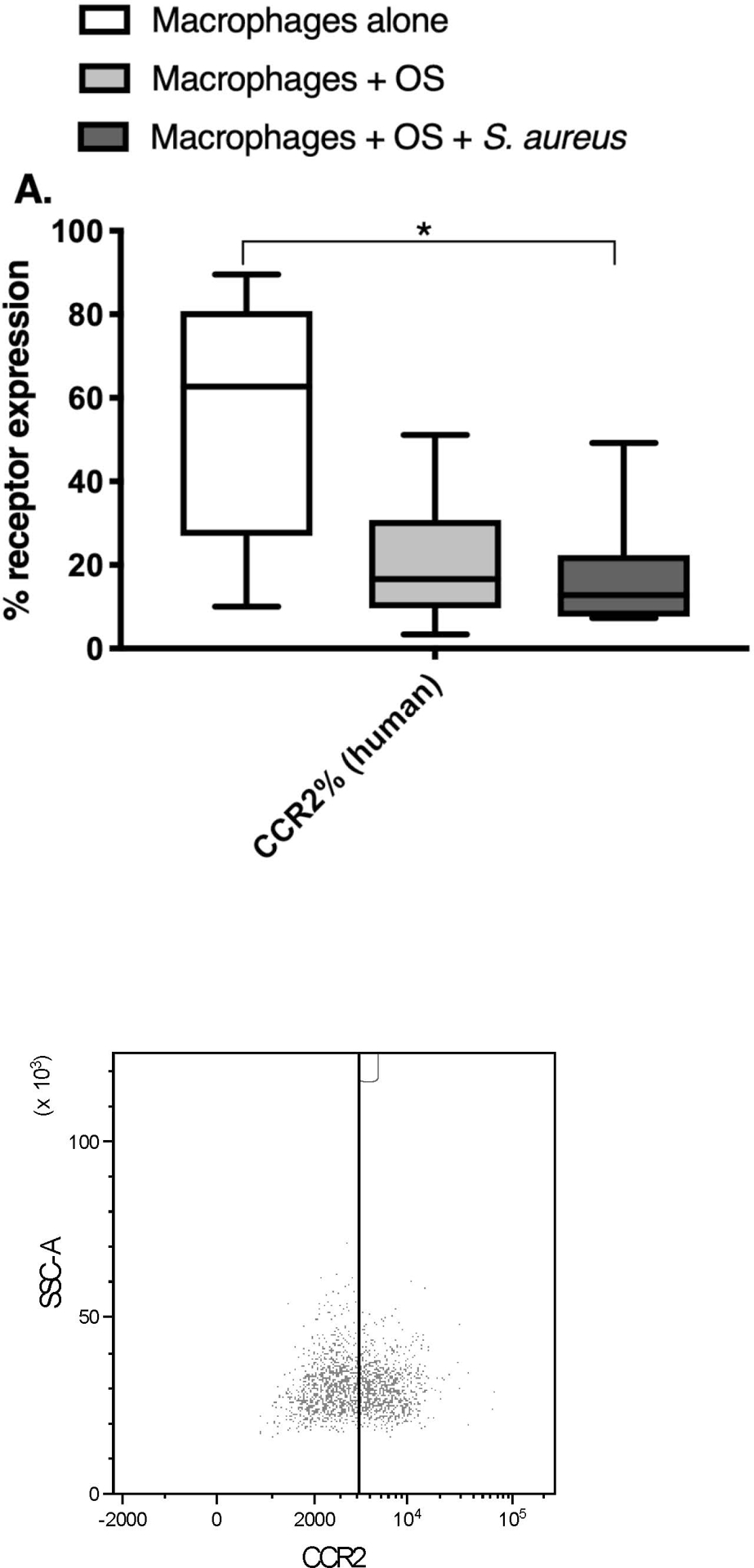
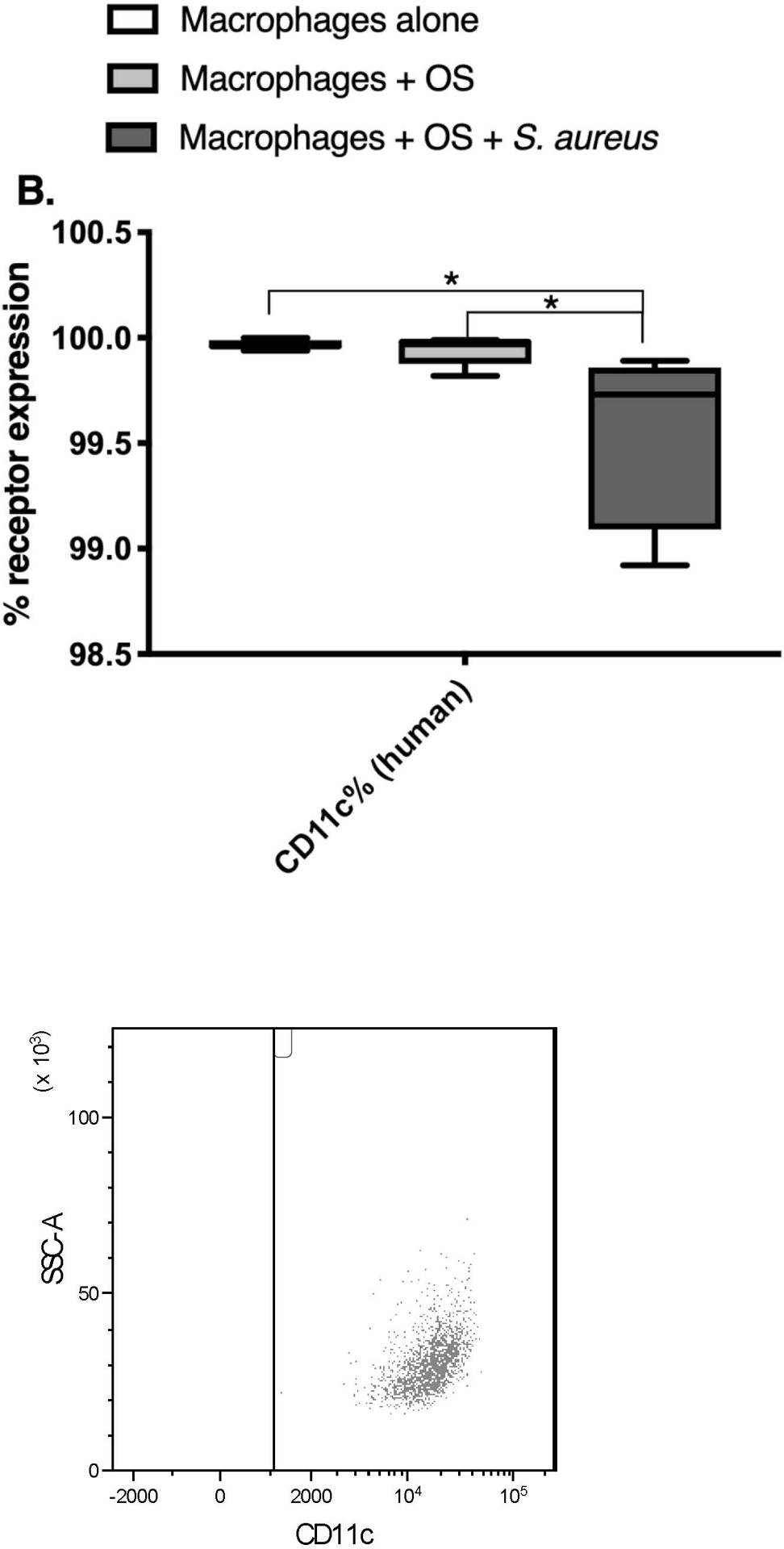
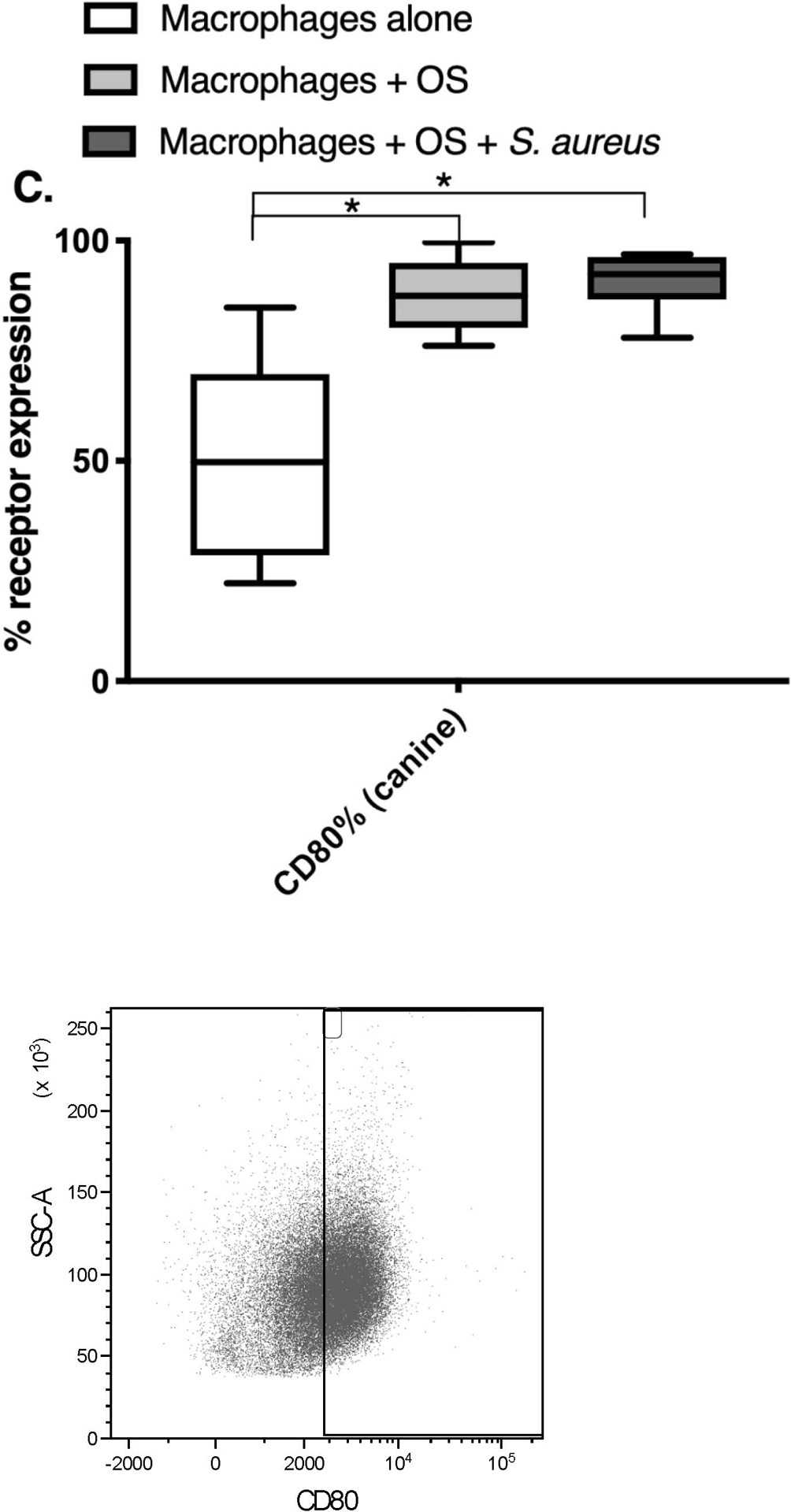
Box-and-whisker plots and accompanying FACS plots illustrating cell surface receptor expression in primary human macrophages from macrophages cultured alone (n = 7), macrophages co-cultured with OS (n = 9), and macrophages co-cultured with OS and S. aureus (n = 7); and in primary canine macrophages from macrophages cultured alone (n = 9), macrophages co-cultured with OS (n = 12), and macrophages co-cultured with OS and S. aureus (n = 6). (A) The percentage of CCR2 expression was significantly lower in human macrophages co-cultured with OS and S. aureus compared to macrophages cultured alone (p = 0.0391). (B) The percentage of CD11c expression was significantly lower in human macrophages co-cultured with OS and S. aureus compared to macrophages cultured alone (p = 0.0063), and macrophages co-cultured with OS (p = 0.0114). Note that the Y-axis value range had to be adjusted to reflect the small range of values for CD11c% expression. (C) The percentage of CD80 expression was significantly higher in canine macrophages co-cultured with OS (p = 0.0026) and in macrophages co-cultured with OS and S. aureus (p = 0.0021) compared to macrophages cultured alone. Asterisks indicate statistical significance (p<0.05). Whiskers for box plot indicate minimum and maximum values. Asterisks indicate statistical significance (p<0.05).
Medians and ranges of percent expression of the receptors can be found in Supplemental Table 2.
Discussion:
Suppression of an anti-tumour inflammatory macrophage phenotype is one way in which tumours such as OS evade the host immune response. Infection has the potential to restore anti-OS immunity. Understanding the macrophage response to OS and a bacterial agent such as S. aureus establishes the foundation upon which new immunostimulatory strategies can be explored. The aim of this study was to evaluate the influence of in vitro exposure to S. aureus on macrophage differentiation in the presence of OS across two species. Our hypothesis was that in dogs and humans, S. aureus induces an intensified inflammatory macrophage phenotype (M1-like) compared to macrophages cultured alone or with OS. Consistent with this hypothesis, we demonstrated that the addition of S. aureus to human and canine macrophages co-cultured with OS induced an inflammatory macrophage phenotype, with upregulation of inflammatory gene expression and cytokine signatures, and inflammatory cell surface receptor expression.
Macrophage polarization occurs along a spectrum, with M1 and M2 macrophages representing both ends of the spectrum. However, M1 and M2 macrophages are not discrete subsets; instead, they represent macrophages over the spectrum of continuum of activation and polarization. Canonical M1 macrophages are induced by bacterial products, such as lipopolysaccharide (LPS), cytokines such as IFN-γ, and they metabolize arginine to microbicidal nitric oxide (NO) and citrulline, enabling their killing function.20 M1 macrophages display increased expression of markers such as MHCII, CD80/86, and efficiently produce inflammatory cytokines (e.g. TNF-α, IFN-γ, IL-12) and cytotoxic mediators (e.g. reactive oxygen species, NO).20 M2 macrophages are induced by cytokines like IL-10, IL-4, and TGF-β, and they metabolize arginine to ornithine and polyamines, molecules that assist in tissue healing and remodeling.20 M2 macrophages display increased expression of mannose and scavenger receptors, secrete TGF-β and other anti-inflammatory molecules.20 The tremendous plasticity of macrophages affords them a broad functional range. However, this plasticity can also be subverted by neoplastic cells and contribute to disease progression.21,22 Tumour microenvironments can signal macrophages to induce polarization towards an M2-like phenotype.21,22 Additionally, tumours can maintain cancer-related inflammation, which is a chronic low-grade level of inflammation that is immunosuppressive and fosters a tolerogenic tumour microenvironment.23 TAMs have been associated with the promotion of cancer-related inflammation and the suppression of a robust anti-tumour inflammatory response.7,21–23 The key is to identify a consistent stimulus to reverse the TAM phenotype and induce a tumour-destructive inflammatory response. As a controlled exploration of macrophage polarization, the present work demonstrates specific changes in macrophage phenotypes when macrophages are co-cultured either with OS alone or with OS and S. aureus. These changes include 1) an upregulation of the inflammatory phenotype, such as an increase in pro-inflammatory cytokine secretion, in the presence of S. aureus, and 2) a decrease in immunosuppressive TGF-β in the presence of S. aureus. These changes suggest the restoration of the macrophage inflammatory response to an anti-neoplastic level.
The changes in macrophage phenotypes in this study share areas of similarity across species. Specifically, the present work demonstrated that human macrophages co-cultured with OS and S. aureus exhibit an inflammatory phenotype with increased IFN-γ, TNF-α and IL-12p70 cytokine secretion, and increased mRNA expression of TNF-α when compared to macrophages cultured alone or with OS. When exposed to S. aureus, human macrophages also exhibited decreased TGF-β cytokine secretion. Canine macrophages exhibited a similar trend when co-cultured with OS and S. aureus: increased IFN-γ and TNF-α cytokine secretion, increased mRNA expression of TNF-α, decreased TGF-β cytokine secretion, and increased receptor expression of CD80.
The macrophage inflammatory cytokine profile was intensified with exposure to S. aureus. IL-12p70 is the bioactive portion of the IL-12 cytokine, made up of p35 and p40 subunits, and it upregulates an inflammatory macrophage response largely through inducing IFN-γ secretion and subsequent T-helper 1 cell signaling.24 IFN-γ activates macrophages and is secreted in response to stimuli such as viral, bacterial pathogens. Binding of IFN-γ induces heightened release of macrophage inflammatory cytokines such as TNF-α primarily through STAT1 activation.25 Consistent with an increase in IFN-γ secretion, TNF-α levels were also increased when macrophages were exposed to S. aureus. TNF-α is one of the dominant cytokines in an inflammatory response to lipopolysaccharide and other bacterial products. A murine study reported that Salmonella enterica colonization of colon carcinoma lesions leading to tumour necrosis and growth inhibition was mediated, in large part, by TNF-α.26 Notably, with exposure to S. aureus, TNF-α, IFN-γ, and IL-12p70 levels from human macrophages and TNF-α levels from canine macrophages were not only significantly upregulated from macrophages cultured alone, they were also significantly increased compared to macrophages co-cultured with OS. These inflammatory cytokine levels were increased when macrophages were co-cultured with OS, most likely reflecting the induction of cancer-related inflammation. Even though cancer-related inflammation and a protective anti-tumour response are apparently discordant effects, both have been demonstrated to occur within a single murine tumour model.27 The significant upregulation of TNF-α, IFN-γ, and IL-12p70 secretion after exposure to S. aureus in this study indicates an intensified inflammatory response, and this heightened response potentially is now effective against the tumour.
Importantly, both canine and human macrophages co-cultured with OS also exhibited decreased secretion of the immunosuppressive cytokine, TGF-β, when exposed to S. aureus. TGF-β is an important regulator of inflammation, and physiologically inhibits macrophage activation to achieve a balanced inflammatory response.28 Decreased TGF-β secretion supports the inflammatory profile noted in macrophages exposed to S. aureus. Since TGF-β promotes OS metastasis, these results support our hypothesis that exposure to S. aureus induces a heightened and potentially anti-tumour inflammatory macrophage response.29 Interestingly, canine and human macrophages co-cultured with OS + S. aureus had significantly higher IL-10 secretion compared to macrophages cultured alone and macrophages co-cultured with OS. IL-10, like TGF-β, is an immunosuppressive cytokine. This increased secretion of IL-10 was paralleled by increased IL-10 mRNA expression from macrophages co-cultured with OS and S. aureus. Macrophages can secrete inflammatory cytokines to promote an inflammatory immune response as well as anti-inflammatory cytokines to prevent or minimize collateral damage.30,31 It is possible that the increased IL-10 level from macrophages were co-cultured with OS and S. aureus reflects a physiologic negative feedback, in which IL-10 is secreted to prevent the inflammatory response from becoming overly exuberant.31 Such a response may account for the differential levels of IL-10 and TGF-β when macrophages were co-cultured with S. aureus and OS, even though they both have suppressive effects.31,32
Flow cytometric analyses indicated that canine macrophages upregulated CD80 expression when co-cultured with S. aureus in the presence of OS. CD80 is a costimulatory molecule induced on macrophages in response to pathogen recognition, and functions to activate T cells in an inflammatory response.33 Upregulation of CD80 in macrophages co-cultured with OS and S. aureus indicates the ability of a bacterial agent to induce and intensify a macrophage inflammatory response that can have anti-tumour efficacy. In the presence of OS and S. aureus, CCR2 expression was significantly lower in human macrophages compared to macrophages cultured alone. CCR2 is the receptor for the chemokine CCL2 or MCP-1, and MCP-1 secretion was increased when macrophages were co-cultured with OS and S. aureus. It is possible the observed decrease in CCR2 expression was a negative feedback response to the increase in MCP-1 induced upon the inflammatory reaction to bacterial agents. The high level of macrophage CD11c expression observed in all experimental groups may be indicative of high baseline expression of CD11c that has been previously reported, and could potentially indicate development towards a dendritic morphology along the spectrum of mononuclear phagocyte differentiation.34
One potential limitation of the experiments outlined in this manuscript is the exclusion of other cells that reside within a tumor microenvironment such as fibroblasts. The tumor microenvironment consists of various non-malignant cell types apart from the tumor cells, and these cell types include immune cells, stromal cells, among others. Interactions between cells of the tumor microenvironment create a dynamic environment, and this manuscript focused on understanding the effects of OS cells on macrophages. Such an understanding will inform future studies that incorporate the evaluation of other cell types within the tumor microenvironment such as fibroblasts. Another potential limitation is the evaluation of mRNA expression in macrophages exclusively without evaluation of mRNA expression in OS cells in our qPCR studies. However, as only the mRNA expression in macrophages exclusively was compared between experimental groups, mRNA expression in OS cells was not evaluated. Another potential limitation is the possible influence of OS cells on the level of cytokine secretion within the co-culture experiments. However, most cytokine levels were below detection limits from canine and human OS cells with the exception of TNF-α levels from canine OS cells, MCP-1 levels from human OS cells, and TGF-β levels from human and canine OS cells. However, the levels of these cytokines were either similar to baseline macrophage levels or different enough from co-culture groups that the secretion from OS cells alone of these cytokines would not likely affect the overall results. A potential limitation in our flow cytometric analyses was the use of CD11b as a macrophage marker. CD11b can serve as a pan-macrophage marker, but is also expressed on other myeloid lineage cells. CD68, another macrophage marker, was not utilized in this study due to the lack of availability of a canine-specific anti-CD68 antibody, and CD11b was utilized to maintain consistency between canine and human studies.
Bacteria-based anti-tumour therapies have been investigated over time, most notably by William Coley, thus understanding of the mechanistic interplay between the tumour and the immune system, specifically macrophages, in response to bacteria will help advance our progression towards positively manipulating the anti-tumour immune response.9 Collectively, the findings in this study demonstrated intensification of an inflammatory macrophage signature when canine and human macrophages were exposed to S. aureus in the presence of OS, with strong similarities between the two species. The clear and specific alterations in macrophage inflammatory profiles presented in this work likely promote the infection-associated anti-tumour immune responses that have been previously observed in patients.9 With a goal of developing new treatment paradigms for OS, the results of this study are crucial to informing subsequent work that will identify the optimal stimuli necessary to trigger a robust and consistent inflammatory anti-tumour macrophage response.
Supplementary Material
Footnotes
Conflicts of Interest: The authors do not declare any conflicts of interest.
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Contributor Information
Joanne L. Tuohy, Departments of Population Health and Pathobiology, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607.
Jason A. Somarelli, Department of Medicine, Duke Medical Center and Duke Cancer Institute, Duke University, Durham, NC 27708.
Luke B. Borst, Departments of Population Health and Pathobiology, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607
William C. Eward, Department of Orthopaedic Surgery, Duke Cancer Institute, Duke University, Durham, NC 27708.
B. Duncan X. Lascelles, Departments of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607
Jonathan E. Fogle, Departments of Population Health and Pathobiology, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607
References:
- 1.Fenger JM, London CA, Kisseberth WC. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J 2014;55:69–85. [DOI] [PubMed] [Google Scholar]
- 2.Friebele JC, Peck J, Pan X, et al. Osteosarcoma: A meta-analysis and review of the literature. Am J Orthop (Belle Mead NJ) 2015;44:547–553. [PubMed] [Google Scholar]
- 3.Ehrhart NR S; Fan T Tumors of the Skeletal System In: Vail DM, ed. Withrow and MacEwen’s Small Animal Clinical Oncology. 5th ed: Saunders Elsevier, 2012;463–503. [Google Scholar]
- 4.Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther 2016;16(5):543–556. [DOI] [PubMed] [Google Scholar]
- 5.Selmic LE, Burton JH, Thamm DH, et al. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med 2014;28:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frimberger AE, Chan CM, Moore AS. Canine osteosarcoma treated by post-amputation sequential accelerated doxorubicin and carboplatin chemotherapy: 38 Cases. J Am Anim Hosp Assoc 2016;52:149–156. [DOI] [PubMed] [Google Scholar]
- 7.Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235–271. [DOI] [PubMed] [Google Scholar]
- 8.Sottnik JL, U’Ren LW, Thamm DH, et al. Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol Immunother 2010;59:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 2006;26:154–158. [PMC free article] [PubMed] [Google Scholar]
- 10.Thrall DE, Withrow SJ, Powers BE, et al. Radiotherapy prior to cortical allograft limb sparing in dogs with osteosarcoma: a dose response assay. Int J Radiat Oncol Biol Phys 1990;18:1351–1357. [DOI] [PubMed] [Google Scholar]
- 11.Lascelles BD, Dernell WS, Correa MT, et al. Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol 2005;12:1073–1083. [DOI] [PubMed] [Google Scholar]
- 12.Culp WT, Olea-Popelka F, Sefton J, et al. Evaluation of outcome and prognostic factors for dogs living greater than one year after diagnosis of osteosarcoma: 90 cases (1997–2008). J Am Vet Med Assoc 2014;245:1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YU, Xu SF, Xu M, et al. Postoperative infection and survival in osteosarcoma patients: Reconsideration of immunotherapy for osteosarcoma. Mol Clin Oncol 2015;3:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeys LM, Grimer RJ, Carter SR, et al. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol 2007;14:2887–2895. [DOI] [PubMed] [Google Scholar]
- 15.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J Clin Oncol 2008;26:633–638. [DOI] [PubMed] [Google Scholar]
- 16.Kurzman ID, Shi F, Vail DM, et al. In vitro and in vivo enhancement of canine pulmonary alveolar macrophage cytotoxic activity against canine osteosarcoma cells. Cancer Biother Radiopharm 1999;14:121–128. [DOI] [PubMed] [Google Scholar]
- 17.Kurzman ID, MacEwen EG, Rosenthal RC, et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res 1995;1:1595–1601. [PubMed] [Google Scholar]
- 18.MacEwen EG, Kurzman ID, Rosenthal RC, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst 1989;81:935–938. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A, Biswas SK, Galdiero MR, et al. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013;229:176–185. [DOI] [PubMed] [Google Scholar]
- 20.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 2014;5:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol 2019;40:310–327. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol 2013;33:1478–1483. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–444. [DOI] [PubMed] [Google Scholar]
- 24.Gee K, Guzzo C, Che Mat NF, et al. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets 2009;8:40–52. [DOI] [PubMed] [Google Scholar]
- 25.Hamidzadeh K, Christensen SM, Dalby E, et al. Macrophages and the recovery from acute and chronic inflammation. Annu Rev Physiol 2017;79:567–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leschner S, Westphal K, Dietrich N, et al. Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha. PLoS One 2009;4:e6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swann JB, Vesely MD, Silva A, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A 2008;105:652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li MO, Wan YY, Sanjabi S, et al. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006;24:99–146. [DOI] [PubMed] [Google Scholar]
- 29.Lamora A, Talbot J, Mullard M, et al. TGF-beta signaling in bone remodeling and osteosarcoma progression. J Clin Med 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siewe L, Bollati-Fogolin M, Wickenhauser C, et al. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur J Immunol 2006;36:3248–3255. [DOI] [PubMed] [Google Scholar]
- 31.Bogdan C, Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann N Y Acad Sci 1993;685:713–739. [DOI] [PubMed] [Google Scholar]
- 32.Schroder M, Meisel C, Buhl K, et al. Different modes of IL-10 and TGF-beta to inhibit cytokine-dependent IFN-gamma production: consequences for reversal of lipopolysaccharide desensitization. J Immunol 2003;170:5260–5267. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013;13:227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol 2008;181:5829–5835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


