Abstract
Proteolysis targeting chimeras (PROTACs) are heterobifunctional small molecules that utilize the ubiquitin proteasome system (UPS) to degrade proteins of interest (POI). PROTACs are potentially superior to conventional small molecule inhibitors (SMIs) because of their unique mechanism of action (MOA, i.e., degrading POI in a sub-stoichiometric manner), ability to target “undruggable” and mutant proteins, and improved target selectivity. Therefore, PROTACs have become an emerging technology for the development of novel targeted anticancer therapeutics. In fact, some of these reported PROTACs exhibit unprecedented efficacy and specificity in degrading various oncogenic proteins and have advanced to various stages of preclinical and clinical development for the treatment of cancer and hematologic malignancy. In this review, we systematically summarize the known PROTACs that have the potential to be used to treat various hematologic malignancies and discuss strategies to improve the safety of PROTACs for clinical application. Particularly, we propose to use the latest human pan-tissue single-cell RNA sequencing data to identify hematopoietic cell type-specific/selective E3 ligases to generate tumor-specific/selective PROTACs. These PROTACs have the potential to become safer therapeutics for hematologic malignancies because they can overcome some of the on-target toxicities of SMIs and PROTACs.
Keywords: PROTAC, Cell-specific E3 ligases, Small molecule inhibitor, Hematologic malignancy
Introduction
Proteolysis targeting chimeras (PROTACs) are bivalent small molecules consisting of a ligand that binds to a protein of interest (POI) connected via a linker to a recruitment moiety for an E3 ubiquitin ligase [1]. Such conjugates can recruit the POI to the E3 ligase, promote proximity-induced ubiquitination of the POI, and lead to its degradation through the ubiquitin proteasome system (UPS). Significant progress has been made in the development of antitumor PROTACs over the last 20 years, which demonstrates that PROTACs can degrade numerous oncogenic protein targets with unprecedented efficacy [1, 2].
PROTACs are potentially more advantageous to treat tumors compared to traditional small molecule inhibitors (SMIs) in several aspects (Table 1). First, PROTACs act catalytically to induce protein degradation in a sub-stoichiometric manner [1]. Because of this unique mechanism of action (MOA), PROTACs produce longer and stronger biological effects than SMIs on a target, which allow PROTACs to be used at a much less intensive dosing regimen to be therapeutically effective than SMIs and thus reduce the risk of undesirable side effects that usually result from off-target binding of SMIs when used at higher concentrations. Second, PROTACs can be developed to target “undruggable” or difficult-to-target proteins such as transcription factors (TFs) and scaffold proteins [3]. Third, PROTACs can be used to overcome drug resistance resulting from mutations of a POI or compensatory increase in POI induced by SMIs [3]. Fourth, it is possible to achieve tumor-specific/selective degradation of a POI with PROTACs by using ligands for cell type- and/or tumor-specific/selective E3 ligases [2, 4].
Table 1.
Comparison among PROTACs, small molecule inhibitors (SMIs), monoclonal antibodies, and therapeutic nucleic acids (TNAs)
| PROTACs | SMIs | Monoclonal antibodies | TNAs |
|---|---|---|---|
| Highly selective | Poor selectivity | Selective | Selective |
| Oral bioavailability can be achieved | Oral bioavailability is easy to achieve | Oral bioavailability is not achievable | Oral bioavailability is not achievable |
| Can target proteins on cell surface and inside a cell | Can target proteins on cell surface and inside a cell | Can only target proteins on cell surface, not inside a cell | Target DNA or RNA |
| Tissue penetration is good | Tissue penetration is good | Tissue penetration is poor | Tissue penetration is poor |
| Metabolic stability is good | Metabolic stability is good | Metabolic stability is poor | Metabolic stability is poor |
| Sub-stoichiometric concentrations are required | Stoichiometric concentrations are required | N/A | N/A |
| Can target proteins without an active binding site i.e. undruggable proteins | Difficult to target proteins without an active binding site | N/A | N/A |
| Can target mutated proteins | Cannot target mutated proteins | N/A | N/A |
| Degradation blocks both enzymatic and scaffolding functions | Inhibition blocks only enzymatic functions | N/A | N/A |
A number of PROTACs have been developed to target various POIs that are involved in the tumorigenesis and progression of hematologic malignancies, such as anaplastic lymphoma kinase (ALK) [5], Bcl-xL [4], BCR-ABL [6], Bruton’s tyrosine kinase (BTK) [7], BRD4 [8], CDK-6 [9], FMS-like tyrosine kinase-3 (FLT-3) [10], HDAC6 [11], and signal transducer and activator of transcription 3 (STAT3) [3]. Some of them have been shown to be extremely potent to kill leukemia and cancer cells in vitro, and can achieve complete tumor regression in vivo in animal models [3].
This review will discuss the advantages of PROTACs over other approaches for protein suppression, systematically summarize the POIs that have been targeted by PROTACs against various hematologic malignancies, and safety concerns of PROTACs and their potential of being translated into clinical applications. We further contemplate the concept of developing cell type- and tumor-specific/selective PROTACs to overcome on-target toxicities of PROTACs using the latest pan-tissue single-cell RNA sequencing (scRNA-seq) data of human adults to identify cell type-specific/selective E3 ligases [12].
A brief history of PROTACs
In 2001, Crews’ and Deshaies’ groups developed the first PROTAC [13], which recruits the SCFβ−TrCP E3 ligase to degrade methionine aminopeptidase-2 (MetAp-2) [14]. In 2003, Sakamoto et al. developed PROTACs that can degrade estrogen receptor-alpha (ERα) or androgen receptor (AR) in breast cancer and prostate cancer, respectively [15]. However, these PROTACs were peptide-based and had a very high molecular weight and poor cellular permeability. To overcome these shortcomings, they developed a cell-permeable PROTAC that was able to degrade selected proteins in cells via the Von Hippel-Lindau (VHL) E3 ligase [16]. In 2008, the first small molecule-based PROTAC was developed, which utilized nutlin-3a as the mouse double minute 2 (MDM2) E3 ligase ligand to recruit MDM2 to degrade AR [17], representing a significant advancement of the PROTAC technology. From then onwards, a series of new ligands of E3 ligases such as inhibitors of apoptosis proteins (IAPs), cereblon (CRBN), and VHL were discovered and used for PROTAC development [18–20]. This was followed by several innovative strategies in the PROTAC field, including the most recent development of homo-PROTACs [21, 22] and photo-PROTACs [23, 24]. In 2013, a PROTAC based on a peptide ligand for VHL was first demonstrated to inhibit tumor growth in murine models [25]. In 2015, small molecule-based PROTACs were first reported to have in vivo activity [26, 27]. In 2019, several PROTACs that have high in vivo antitumor potency were reported [3, 4]. Recently, PROTACs against AR and ER, named ARV-110 and ARV-471, respectively, were the first to enter phase-I clinical trials (Identifier: NCT03888612; NCT04072952).
Advantages of using PROTACs over other approaches to inhibit a POI
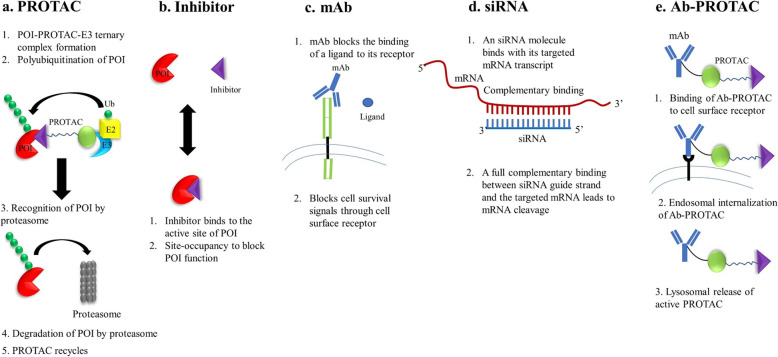
There are four main approaches to suppress a POI as shown in Fig. 1: (1) protein inhibition by conventional SMIs; (2) genetic manipulation to suppress a target protein, e.g., by RNAi and CRISPR-Cas9; (3) target neutralization using monoclonal antibodies; and (4) targeted protein degradation by PROTACs and other strategies. Each of these strategies has their own advantages and disadvantages. PROTACs have several potential advantages compared to the other three strategies (Table 1). The target inhibition by SMIs is the most commonly used therapeutic approach so far. The main advantage of PROTACs over SMIs is their catalytic mode of action, due to which PROTACs are required at significantly lower concentrations to exert a biological effect than SMIs. In addition, PROTACs have several other advantages including high tissue and target selectivity, and capacity to target undruggable and mutated proteins [28, 29]. Recently, homo-PROTACs were developed by tethering two E3 ligase ligands with a linker to induce self-degradation of E3 ligases [21, 22]. This approach can particularly be useful to target oncogenic E3 ligases such as MDM2. In addition, tissue/tumor-specific degradation of POIs can be achieved by using light-controllable PROTACs when combined with photodynamic therapy. In light-controllable PROTACs, a photo-removable group is attached to either a POI ligand or E3 ligand or linker. This photo-removable group is detached from the PROTAC upon light irradiation converting it to an active PROTAC [23, 30, 31]. The design and mechanisms of action of homo-PROTACs and light-controllable PROTACs are illustrated in Fig. 2. Recently, we and others have achieved tumor-selective degradation of a target protein by exploiting E3 ligases that are selectively expressed in tumors compared to normal tissues and cells such as platelets, an innovative strategy which is otherwise not achievable with conventional SMIs [2, 4, 32].
Fig. 1.
Schematic representation depicting different strategies of protein suppression. a PROTAC recruits an E3 ligase to a POI followed by polyubiquitination of the POI by an E2 conjugating enzyme. The polyubiquitinated POI is recognized and degraded by the proteasome. Once the POI is degraded, the PROTAC molecule can be recycled to induce the next round of POI degradation, thus working in a sub-stoichiometric manner. b A small molecule inhibitor (SMI) typically binds at the active site of a POI to inhibit the enzymatic functions of the POI. c A monoclonal antibody (mAb) binds to a cell surface receptor to block the signal transduction stimulated by a ligand, a growth factor or a cytokine. d siRNA binds to its targeted mRNA transcript in a complementary manner to induce mRNA cleavage and consequently translational suppression. e An antibody-PROTAC conjugate (Ab-PROTAC) is designed by linking a PROTAC to a mAb. Once an Ab-PROTAC binds to its cell surface receptor, it is internalized into the cytosol through endosomes. In the cytosol, an active PROTAC releases from Ab-PROTAC through lysosomal pathway which induces the degradation of POI
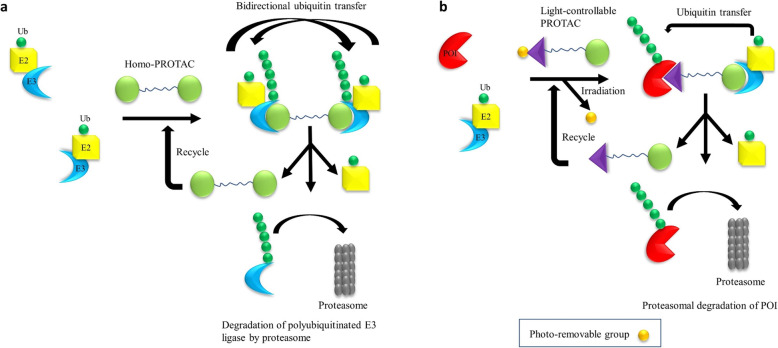
Fig. 2.
Design and mechanism of homo-PROTACs and light-controllable PROTACs. a Design and mechanism of homo-PROTACs. A homo-PROTAC is consisted of two E3 ligase ligand molecules connected via a linker. Homo-PROTAC recruits an E3 ligase molecule to another E3 ligase molecule followed by bidirectional polyubiquitination of E3 ligase molecules and subsequent degradation of the E3 ligase by the proteasome. b Design and mechanism of light-controllable PROTACs. In a light-controllable PROTAC, a photo-removable group is attached to the POI ligand or E3 ligand or linker. Upon light irradiation, the photo-removable group is detached from the light-controllable PROTAC converting it to an active PROTAC for the proteasomal degradation of POI
The other two strategies of target suppression, i.e., monoclonal antibodies and genetic manipulation, are limited in their scope. Thus far, monoclonal antibodies can only be used to effectively target cell surface proteins but not intracellular targets [14, 33]. On the other hand, genetic manipulation by therapeutic nucleic acids (TNAs) including antisense oligonucleotides (ASO), siRNA, miRNA, CRISPR-Cas9, etc. are still in their early developmental stage. TNAs also have several limitations including inefficient delivery to target cells, metabolic instability, and off-target effects that hinder their effective and safe use in vivo [1, 34, 35]. Some TNAs have been approved by the FDA for their use in different disease conditions, but none has been approved to treat cancers [36, 37]. Considering these limitations of using monoclonal antibodies and TNAs, PROTACs have a better chance for faster clinical development against a broader range of targets in multiple tumor types. Moreover, antibody-PROTAC conjugates (Ab-PROTACs) have been reported recently, in which a PROTAC is conjugated to a monoclonal antibody similar to antibody-drug conjugates. These Ab-PROTACs can selectively degrade POIs in targeted cells/tissues [38–40].
PROTACs against hematologic malignancies
In this section, we systematically summarize the reported oncogenic proteins that have been targeted by PROTACs for hematologic malignancies. The relevant information of representative PROTACs against these targets including the concentration required to achieve 50% degradation (DC50), and in vitro and in vivo activities is shown in Table 2.
Table 2.
Targets and efficacy of PROTACs in hematologic malignancies
| In vitro efficacy | In vivo efficacy | |||||||
|---|---|---|---|---|---|---|---|---|
| Target | E3 ligase | Compound | Disease | Efficacy (EC50 or other ) | Degradation (DC50/Dmax or other) | Model and efficacy | Degradation | Ref. |
| ALK | CRBN |
TL13-112 |
ALCL | Karpas299 (<50 nM); SU-DHL-1 (<50 nM) | Karpas299 (DC50: 40 nM) | NA | NA | [41] |
| Bcl-6 | CRBN |
PROTAC 15 |
DLBCL | PROTAC 15 showed weak antiproliferative activity in DLBCL cell lines (>5μM) | 1 μM of 15 caused 82% degradation of Bcl-6 in OCI-Ly1 | NA | NA | [42] |
| Bcl-xL | VHL |
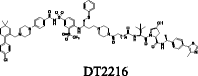
DT2216 |
T-ALL | MOLT-4 (52 nM) | MOLT-4 (DC50: 63 nM; Dmax: 90.8%) |
MOLT-4 xenograft: Once weekly dosing of DT2216 completely inhibited MOLT-4 xenograft CUL76 T-ALL PDX: Combination of DT2216 with chemotherapy drug substantially increased survival |
MOLT-4 xenograft: Single dose of DT2216 at 15 mg/kg caused sustained reduction in Bcl-xL in MOLT-4 xenograft | [4] |
| BCR-ABL | VHL |
SIAIS178 |
CML | K562 (24 nM) | K562 (DC50: 8.5 nM) | K562-Luc xenograft: SIAIS178 at 5 and 15 mg/kg/day, i.p. for 12 days dose-dependently inhibited tumor growth | K562 xenograft: SIAIS178 at 5 and 15 mg/kg/day, i.p. for 4 days dose-dependently depleted BCR-ABL levels in tumors 6 h after last dose | [6] |
| BRD4 | CRBN |
dBET6 |
T-ALL | In a set of 20 T-ALL lines, such as SUPT11(<10 nM) | MOLT4 (DC50< 10 nM) |
SUPT11 xenograft: dBET6 (7.5 mg/kg, twice daily) significantly reduced leukemic burden MOLT-4 xenograft: dBET6 (7.5 mg/kg BID) exhibited a significant survival benefit in mice |
SUPT11 xenograft: dBET6 led to degradation of BRD4 in leukemic bone marrow 3 h after treatment |
[43] |
| VHL |
ARV-771 |
MCL | Mino (<20 nM), Z238 (~200 nM) Primary MCL cells (NA) | Mino (NA); primary MCL cells (NA) | Z138 xenograft: ARV-771 (30 mg/kg) was significantly more effective in improving the median and overall survival |
Z138 xenograft: ARV-771 (30 mg/kg, daily for 5 days) resulted in the depletion of BRD4 from the spleen and bone marrow |
[8] | |
| BTK | CRBN |
DD-03-171 |
BCL | TMD8-BTK WT (29.2 nM); TMD8-BTK C481S (128 nM); Mino (12 nM) | Effectively degraded BTK at concentrations as low as 100 nM within 4 hours of treatment in Ramos B cells | DLBCL and MCL PDX models: DD-03-171 at 50 mg/kg/day, i.p. for 14 days significantly inhibited the tumor burden and enhanced the survival of DFBL-96069-engrafted mice | DLBCL and MCL PDX models: DD-03-171 at 50 mg/kg/day, i.p. for 3 days depleted BTK levels in the spleens of DFBL-96069-engrafted mice | [44] |
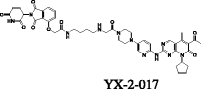
| CDK6 | CRBN |
YX-2-017 |
Ph+ ALL | BV173 (NA); SUP-B15 (NA) | BV173 (4 nM); MUTZ-5 (NA); MHH-CALL-4 (NA); SEM (NA); Jurkat (NA) |
Ph+ALL xenografts: YX-2-107 suppressed S-phase cell percentage and decreased phosphor-RB and FOXM1 expression in bone marrow cells Ph+ALL PDX: YX-2-107 suppressed peripheral blood leukemia burden |
Ph+ALL xenografts: NSG mice were given YX-2-107 at 150 mg/kg per day for 3 consecutive days. YX-2-107 reduced CDK6 level in bone marrow cells | [9] |
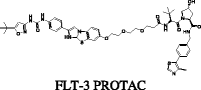
| FLT-3 | VHL |
FLT-3 PROTAC |
AML | MV4-11 (0.6 nM); MOLM-14 (NA); OCIAML-3 (>2.8 μM) | MV4-11 (NA) | NA | MV4-11 xenograft : FLT-3 PROTAC at 30 mg/kg daily for 3 d decreased FLT-3 by 60% in tumor | [10] |
| HDAC6 | CRBN |
12d |
MM | MM.1S (EC50=74.9 nM, Emax=63.1%) | MM.1S (DC50: 1.6 nM) | NA | NA | [11] |
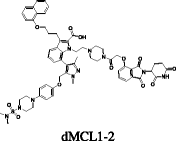
| Mcl-1 | CRBN |
dMCL1-2 |
MM | NA |
OPM2 (NA) MM.1S (NA) |
NA | NA | [45] |
| MDM2 | CRBN |
MD-224 |
ALL; AML |
RS4-11 (1.5 nM); MOLM-13 (7.3 nM); MOLM-14 (10.5 nM); SIG-M5 (19.8 nM); ML-2 (4.4 nM) OCL-AML-5 (33.1 nM) |
Effectively induced marked depletion of MDM2 at 1 nM in RS4-11 cells | RS4-11 xenograft: MD-224 at 25 mg/kg, daily, 5 days a week for 2 weeks, or at 50 mg/kg, every other day for 3 weeks achieved complete tumor regression | RS4-11 xenograft: Single intravenous dose of MD-224 effectively depleted MDM2 protein at 3 h, with the effect persisting for >24 h | [46] |
| PRC2 | VHL |
UNC6852 |
DLBCL |
DB (3.4 μM) Pfeiffer (0.41 μM) |
DB (DC50/Dmax): EED 0.61 μM/96%; EZH2 0.67 μM/94%; SUZ12 0.59 μM/82%) |
NA | NA | [47] |
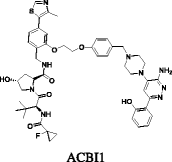
| SMARCA2/4, PBRM1PBRM1 | VHL |
ACBI1 |
AML | MV-4-11 (29 nM) | MV-4-11 cell (6 nM (SMARCA2), 11 nM (SMARCA4), 32 nM (PBRM1)), Dmax = 100% for SMARCA2/4 and PBRM1 | NA | NA | [48] |
| STAT3 | CRBN |
SD-36 |
AML; ALCL |
AML: MOLM-16 (0.035 μM) ALCL: SU-DHL-1 (0.25 μM); SUP-M2 (0.13 μM); KI-JK (0.18 μM); Karpas-299 (0.98 μM); DEL (1.48 μM); |
MOLM-16 (Dmax: 90%) | MOLM-16 xenograft: SD-36 at 100 mg/kg weekly or 50 mg/kg twice weekly for 4 weeks induced complete tumor regression; SUP-M2 xenograft: SD-36 at 50 mg/kg 3 times per week completely inhibited tumor growth. SD-36 at 100 mg/kg 3 times per week achieved complete tumor regression | MOLM-16 xenograft: Single dose of 25 mg/kg SD-36 depleted STAT3 protein by >80% in MOLM-16 engrafted tumors; SU-DHL-1 xenograft: Single dose of SD-36 near-completely depleted STAT3 protein in SU-DHL-1 engrafted tumor | [3] |
ALCL anaplastic large-cell lymphoma, ALL acute lymphoblastic leukemia, AML acute myeloid leukemia, BCL B cell lymphoma, CML chronic myelogenous leukemia, DLBCL diffused large B cell lymphoma, MCL mantle cell lymphoma, MM multiple myeloma, Ph+ ALL Philadelphia chromosome-positive acute lymphoblastic leukemia, T-ALL T cell acute lymphoblastic leukemia
ALK
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase which is activated in many cancers including several hematologic malignancies (e.g., anaplastic large-cell lymphoma (ALCL) and diffused large B cell lymphoma (DLBCL)) and solid tumors (e.g., non-small cell lung cancer (NSCLC)) due to chromosomal translocations, substitution mutations, and gene amplification [49]. Several ALK inhibitors (crizotinib, ceritinib, alectinib, and brigatinib) have been approved for the treatment of ALK-positive NSCLC [50], and some of them are undergoing clinical trials against ALCL and other lymphomas [Identifier: NCT02465060; NCT00939770; NCT03719898]. The efficacy of ALK inhibitors is hindered by the emergence of different resistance mechanisms [50]. Researchers have adopted PROTAC technology to overcome the resistance to ALK inhibitors. The first series of ALK PROTACs were reported by Gray’s group. These PROTACs were very efficient in degrading ALK (DC50 ~ 10 nM in H3122 NSCLC cells) and inhibiting the proliferation of ALK-dependent ALCL and NSCLC cells. However, these PROTACs were not specific to ALK and could not degrade a mutated ALK fusion protein EML4-ALK [51]. At the same time, another group reported two ALK PROTACs (MS4077 and MS4078) that efficiently degraded ALK fusion proteins NPM-ALK and EML4-ALK in SU-DHL-1 ALCL and NCI-H2228 NSCLC cells, respectively, and potently inhibited the proliferation of SU-DHL-1 cells [5]. Another VHL-based ALK PROTAC TD-004 efficiently induced ALK degradation and inhibited the proliferation of SU-DHL-1 and H3122 cells in vitro, and reduced H3122 xenografted tumor growth in vivo [41]. Recently, a VHL-recruiting ALK PROTAC based on brigatinib, named SIAIS117, was found to be more potent than brigatinib in inhibiting the growth of G1202R mutant ALK-expressing 293T cells by inducing G1202R mutant ALK degradation [52]. The PROTACs against ALK have also been briefly discussed in a review by Kong et al. [53].
Bcl-2 family proteins
Resistance to apoptosis plays a crucial role in tumorigenesis and is responsible for resistance to cancer therapies [54]. Therefore, targeting the apoptotic pathway becomes an attractive therapeutic strategy for cancer treatment. B cell lymphoma 2 (Bcl-2) proteins control the intrinsic mitochondria-mediated apoptotic pathway [55, 56]. SMIs targeting the anti-apoptotic Bcl-2 family proteins, including Bcl-2, Bcl-xL, and Mcl-1, have been developed for cancer treatment. Venetoclax (ABT-199), a highly selective inhibitor of Bcl-2, is the first FDA-approved Bcl-2 antagonist for the treatment of various hematologic malignancies including chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) as a single agent, and for acute myeloid leukemia (AML) in combination with chemotherapy [57]. Wang et al. reported a Bcl-2 PROTAC with a DC50 of 3.0 μM in NCI-H23 lung adenocarcinoma cell line [58]; however, neither degradation nor cellular cytotoxicity data was available in hematologic tumor cell lines. Navitoclax (ABT-263), a dual inhibitor of Bcl-2 and Bcl-xL, entered clinical trials in 2006. Unfortunately, the clinically effective dosage of ABT-263 is significantly limited by thrombocytopenia because platelets solely depend on Bcl-xL for survival [59]. Recently, we reported the first example of utilizing PROTAC technology to reduce on-target toxicity of SMIs [4]. DT2216, a representative PROTAC that hijacks the VHL E3 ligase to achieve tissue/cell-selective degradation of Bcl-xL, was found to be more potent against Bcl-xL-dependent tumor cells and less toxic against platelets in comparison to conventional Bcl-xL inhibitors. Moreover, DT2216 potently degraded Bcl-xL protein and inhibited MOLT-4 T-ALL in vitro and in vivo. The discovery of DT2216 provides a proof of principle to utilize PROTAC technology to rescue undruggable targets due to previously unmanageable on-target toxicities. Using a similar strategy, we reported several PROTACs that recruited CRBN or IAPs for Bcl-xL degradation [60–63]. These Bcl-xL degraders showed significantly reduced cytotoxicity against platelets but improved killing of various tumor cells and senescent cells due to differential expression of these E3 ligases in these cells, indicating that an improved therapeutic window can be achieved by converting a non-selective SMI to a tissue/cell-specific degrader.
Mcl-1 is upregulated in various hematologic malignancies (e.g., multiple myeloma (MM) and AML) and some solid tumors (e.g., hepatocellular carcinoma and NSCLC) [64]. Tremendous efforts in drug discovery programs have generated highly selective and potent Mcl-1 inhibitors with a binding affinity at picomolar levels. Some of them (AMG-176, AMG-397, AZD5991, S64315/MIK665, and ABBV-467) have entered phase I clinical trials [65]. Notably, in late 2019, the FDA and Amgen halted the clinical trials of AMG-176 and AMG-397 due to a “safety signal for cardiac toxicity,” which is likely to be an on-target toxicity of Mcl-1 inhibition. There are reported Mcl-1 PROTACs [45, 58], dMCL1-2 as a representative, which degrades Mcl-1 in OPM2 and MM.1S MM cell lines. It is worth looking into the possibility to design tissue-specific PROTACs to reduce the on-target toxicity of Mcl-1 inhibition in the heart.
Bcl-6
B cell lymphoma 6 (Bcl-6) is an emerging oncoprotein and therapeutic target for lymphoma. It is broadly expressed in many lymphomas and plays a significant role in lymphomagenesis by being involved in the formation of germinal centers (GCs) during the humoral immune response [66–69]. As a transcriptional repressor, Bcl-6 exerts its function by recruiting its corepressors to its N-terminal BTB domain to repress the expression of a broad set of genes involved in the proliferation and survival of GC B cells [70]. By targeting the interactions between the BTB domain of Bcl-6 and its corepressors, a variety of Bcl-6 inhibitors have been developed over the past decade, including peptide mimetics, small molecules, and natural compounds [71]. However, due to the challenge of disrupting PPI, these Bcl-6 inhibitors have suffered from low potency [72] and thus have limited clinical application. Chemically induced degradation of Bcl-6 by small molecules, such as BI-3802, have been found to have stronger effects than Bcl-6 inhibition and thus offer new routes to the development of lymphoma treatments [73]. By degrading Bcl-6 via the UPS, BI-3802 displayed stronger de-repression of target genes than Bcl-6 inhibitors and demonstrated antiproliferative effects in several DLBCL cell lines [73]. However, inducing Bcl-6 degradation through PROTAC strategy has met with limited success. McCoull et al. developed highly potent Bcl-6 inhibitors and converted them into PROTAC molecules by conjugating them with thalidomide [42]. Although the PROTAC molecules could degrade Bcl-6 in a number of cells, they did not improve antiproliferative activity over Bcl-6 inhibitors in DLBCL cells. Further investigation revealed that the weak antiproliferative response is due to the incomplete Bcl-6 degradation [42]. It remains unknown if complete degradation of Bcl-6 can be achieved through the exploration of the chemical space of their PROTAC molecules or through the recruitment of different E3 ligases.
BCR-ABL
BCR-ABL is an oncogenic fusion protein implicated in the development of chronic myelogenous leukemia (CML) [74]. Tyrosine kinase inhibitors (TKIs, e.g., imatinib, dasatinib, and bosutinib) that inhibit the kinase function of BCR-ABL by competitively binding at the ATP-binding site of ABL are the standard-of-care against BCR-ABL-driven CML [75]. However, TKIs need to be administered continuously for life to treat CML patients likely due to the scaffolding functions of BCR-ABL that activate compensatory signaling pathways to promote the survival of leukemic stem cells (LSCs) [76, 77]. Since PROTACs eliminate both enzymatic and scaffolding functions of targeted proteins by inducing its degradation, they can potentially overcome the abovementioned limitations of BCR-ABL inhibitors. This led to the development of the first BCR-ABL PROTAC by Crews’ group. In this study, a lead PROTAC based on dasatinib recruiting CRBN (DAS-6-2-2-6-CRBN) degraded both ABL and BCR-ABL, whereas one recruiting VHL (DAS-6-2-2-6-VHL) could only degrade ABL. DAS-6-2-2-6-CRBN effectively reduced the viability of BCR-ABL-dependent K562 CML cells with an EC50 of 4.4 nM [78]. In the same year, Naito’s and Kurihara’s groups developed the specific and non-genetic IAP-dependent protein erasers (SNIPERs) against BCR-ABL. The lead SNIPERs, SNIPER(ABL)-2 and SNIPER(ABL)-3, degraded both BCR-ABL and ABL leading to growth inhibition in K562 cells at concentrations of 30 μM or higher [79]. Subsequently, Naito’s group developed a more potent BCR-ABL SNIPER, SNIPER (ABL)-39 that degraded BCR-ABL and inhibited the proliferation of several BCR-ABL-dependent CML cells at lower nanomolar concentrations [80]. Recently, Crews’ group reported the development of BCR-ABL PROTACs based on an allosteric inhibitor (GNF-5) as the warhead linked to the VHL ligand. The lead PROTAC in this study (GMB-475) induced the degradation of both BCR-ABL1 and ABL1 in a concentration- and time-dependent manner and inhibited the proliferation of K562 cells with an EC50 of ~ 1 μM. Interestingly, GMB-475 inhibited the proliferation of imatinib-resistant murine Ba/F3 cells expressing mutant BCR-ABL1 (T315I) with similar potency as the cells expressing wild-type BCR-ABL1 [81]. Recently, another study reported the most potent BCR-ABL PROTAC to date, named SIAIS178. SIAIS178 potently degraded BCR-ABL protein (DC50 = 8.5 nM) and inhibited the proliferation of K562 cells (EC50 = 24 nM), and further efficiently inhibited tumor growth and induced BCR-ABL degradation in K562 xenografts. More importantly, SIAIS178 was able to degrade several mutant forms of BCR-ABL [6]. More recently, light-controllable Azo-PROTACs against BCR-ABL have been reported with high potency in K562 cells [82].
BRD4
The bromodomain and extra terminal domain (BET) family includes four bromodomain-containing proteins (BRDs): BRD2, BRD3, BRD4, and BRDT. Each BRD contains two bromodomains that serve as “epigenetic readers” to recognize acetylated lysine residues. In vitro and in vivo studies demonstrate that BET inhibitors (BETi), such as JQ1 and I-BET151, can suppress the proliferation of multiple types of tumors [83]. However, acquired resistance limits the efficacy of BETi treatment in clinical trials [84]. In addition, long-term dosing or high concentrations associated with BETi treatment also leads to obvious BRD4 protein accumulation and inefficient c-MYC suppression [85].
Recently, numerous BET PROTACs have been generated and tested in vitro and in vivo. Different E3 ligases including VHL [86–89], CRBN [43, 85, 90–93], IAP [94], MDM2 [95], AHR [96], DCAF16 [97], RNF114 [98], and RNF4 [99], were recruited to degrade BET proteins. Most of the reported PROTACs not only degrade BRD4, but also BRD2 and BRD3. However, simultaneous degradation of the three BRD proteins may increase drug toxicities [100–102]. To improve the specificity of the PROTACs, Ciulli’s group reported the first BRD4-specific PROTAC (ZXH-3-26). However, no cellular antiproliferative efficacy against cancer cells was reported for ZXH-3-26 [103].
In 2015, Bradner’s group reported the first small molecule-based BRD4 degrader, dBET1 [91]. Later, they optimized the linker and found a more potent PROTAC (dBET6) that showed better efficacy against T-ALL compared with JQ1 [43]. In the same year, Crews’ group reported a CRBN-based BET PROTAC, ARV-825, by utilizing a more potent BETi (OTX015) as the warhead for BRD4 [85]. In 2016, a VHL-based PROTAC (ARV-771) that also used OTX015 as the BRD4 warhead was generated [86]. Later, ARV-825 and ARV-771 were tested widely and displayed potent in vitro and in vivo efficacies against AML [104, 105], post-myeloproliferative neoplasm secondary AML [106, 107], MM [108], mantle cell lymphoma (MCL) [8], and DLBCL [109].
In 2018, Wang’s group reported two more potent CRBN-based BET PROTACs, BETd-260 [92] and QCA570 [93]. BETd-260 exerted high potency to rapidly degrade BRD2/3/4 and inhibit the growth of RS4-11 B cell acute lymphoblastic leukemia (B-ALL) cells in vitro and in a mouse xenograft model [92]. QCA570 is the most potent BET PROTAC that displays picomolar potency against human acute leukemia cell lines in vitro and in vivo [93]. In 2019, Crews’ group reported an MDM2-based BRD PROTAC (A1874), which not only degrades BRD4, but also stabilizes p53, and exhibited high anti-proliferative effect in several tumor cell lines including BL and myeloid leukemia [95]. Very recently, TD-428, a new BRD4 PROTAC comprised of a novel CRBN binding moiety (TD-106) and the BET binding moiety JQ1, was reported [110]. TD-428 possesses two unique properties: high specificity and lacking the Hook effect. Compared with ARV-825, TD-428 only induces moderate IKZF1/3 degradation at higher concentrations. Jiang et al. reported a new CRBN-based PROTAC (compound 15) which selectively degrades BRD2/4 over BRD3. Compound 15 suppresses the proliferation of different AML cells (MV4-11 and MOLM-13) as well as solid tumor cell lines in the NCI-60 cell line panel [111]. Interestingly, in a series of previously reported VHL-based PROTACs derived from a more potent BETi, tetrahydroquinoline (I-BET726), only selectively induce BRD3/4 degradation without any effect on BRD2, but still possess the anti-proliferation effect against AML cells [89].
BTK
Bruton’s tyrosine kinase (BTK) is involved in constitutively active B cell receptor (BCR) signaling in various B cell malignancies CLL, DLBCL, and MCL, and thus, is an important therapeutic target in these malignancies [112]. Ibrutinib, an irreversible covalent inhibitor of BTK, is an approved drug to treat CLL and other BTK-driven cancers. Although most of the CLL patients on ibrutinib therapy show durable responses, the resistance to ibrutinib emerges due to a cysteine to serine mutation (C418S) at the ibrutinib binding site in BTK [113, 114]. Therefore, researchers have aimed to employ PROTAC technology to overcome the resistance. Using a quantitative proteomic approach, Gray’s group reported the degradation of multiple kinases including BTK by a multi-kinase degrader, TL12-186. They also reported two selective BTK degraders, one based on a promiscuous inhibitor (bosutinib) and another based on a selective BTK inhibitor (RN486). The RN486-based degrader, named DD-04-015, efficiently and selectively degraded BTK and produced a similar antiproliferative activity as RN486 in BTK-dependent TMD8 DLBCL cells. However, the ability of these PROTACs to degrade mutant forms of BTK was not evaluated [115]. Later, MT-802, a lead BTK PROTAC developed by Crews’ group, was shown to be equally effective in degrading both wild-type and C418S mutated BTK. MT-802, but not ibrutinib, was found to reduce activated-BTK levels in patient-derived CLL cells harboring the C481S mutation [116]. Using a similar strategy, Rao’s group reported a BTK degrader, named P13I, which efficiently degraded both wild-type and C418S BTK with DC50 values of 10 nM and 28 nM, respectively. More importantly, P13I more effectively reduced the viability of BTK-dependent wild-type and C418S BTK-expressing HBL-1 DLBCL cells compared to ibrutinib [117]. Subsequently, several potent BTK PROTACs aimed at targeting mutant BTK in ibrutinib-resistant B cell malignancies with improved cellular activities and possessing in vivo potencies were reported by different groups [7, 44, 118–120]. Recently, a light-controllable PROTAC against BTK, named pc-PROTAC3, was reported to efficiently degrade BTK in Ramos Burkitt’s lymphoma (BL) cells upon light exposure. pc-PROTAC3 was designed by attaching a bulky photo-removable group, 4,5-dimethoxy-2-nitrobenzyl (DMNB), to the imide nitrogen of a previously described BTK PROTAC MT-802 [23].
CDK6
CDK6 is a member of cyclin-dependent kinases (CDKs) and regulates G1-S phase transition and drives cell proliferation by forming complexes with D-type cyclins to phosphorylate the tumor suppressor retinoblastoma (Rb) [121, 122]. Multiple types of leukemia cells are dependent on CDK6 for survival, including mixed-lineage leukemia (MLL) [123] and Philadelphia chromosome-positive ALL (Ph+ ALL) [9]. However, selectively targeting CDK6 with ATP-competitive SMIs has been challenging due to the presence of an identical ATP-binding domain in its close-related homology CDK4 [9]. Moreover, ATP-competitive inhibitors cannot disrupt the scaffolding functions of CDK6, which is important for the transcription function of CDK6 [124, 125]. Soon after the degradability of CDK4/6 and other CDK family kinase was demonstrated by Huang et al. with a multi-kinase PROTAC degrader TL12-186 [115], several groups have developed selective CDK6 PROTAC degraders by converting CDK4/6 dual inhibitor (palbociclib) to PROTAC molecules [9, 121, 126]. For example, BSJ-03-123, a CRBN-based CDK6 degrader, was reported to selectively degrade CDK6 but not CDK4 in AML cell lines because it can form a ternary complex with CDK6 and CRBN but not with CDK4 and CRBN [121]. Dominici et al. demonstrated that CDK6 degradation by PROTACs is more effective than treatment with the dual CDK4/6 inhibitor palbociclib in suppressing Ph+ ALL in mice [9], because PROTACs disrupted both the kinase-dependent and independent activities of CDK6. YX-2-107, a CRBN-recruiting PROTAC, degraded CDK6 selectively in Ph-like and MLL-rearranged cell lines, and inhibited their proliferation. Moreover, YX-2-107 is effective in a Ph+ ALL xenograft mouse model. However, the limited pharmacokinetic (PK) properties (e.g., short half-life) of YX-2-107 warrant its further optimization.
FLT-3
FMS-like tyrosine kinase-3 (FLT-3) belongs to the class III receptor tyrosine kinases, which is activated by a FLT-3 ligand and plays a major role in the regulation of hematopoiesis [127]. Mutations in the FLT-3 gene have been found in patients with ALL (1–3%), myelodysplasia (5–10%), and AML (15–35%), making it one of the most frequently mutated genes in hematologic malignancies [128]. Mutations in the FLT-3 gene, such as the most common internal tandem duplication (ITD), result in its constitutive activation in these malignancies. Thus, FLT-3 has been emerged as a therapeutic target for hematologic malignancies. Several FLT-3 inhibitors have been tested in clinical trials and two (midostaurin and gilteritinib) of them have been approved by FDA for the patients with FLT3-mutated AML [129]. However, clinical response duration to FLT3 inhibitor treatment remains short partially due to the development of secondary resistance [130]. Achieving a complete and sustained inhibition of FLT-3 ITD signaling to maintain effective clinical response requires a high dose of inhibitors, which leads to off-target toxicities [131].
In an early study, using a CRBN-recruiting PROTAC based on a promiscuous kinase inhibitor to target multiple kinases failed to significantly degrade FLT-3, likely due to its poor cell permeability [115]. In contrast, a VHL-recruiting FLT-3 PROTAC from Crews’ lab potently induced FLT-3 ITD degradation in MV4-11 and MOLM-14 AML cell lines in vitro [10]. They confirmed the ability of this FLT-3 PROTAC to degrade FLT-3 in MV4-11 mouse xenografts. This study demonstrated that conversion of an FLT-3 inhibitor into a PROTAC can result in the degradation of FLT-3 ITD. However, the in vitro anti-proliferative activity of the FLT-3 PROTAC varies among AML cells compared to the FLT-3 inhibitor that was used to develop the PROTAC. For example, the PROTAC exerted a significantly better activity against MV4-11 and wild-type MOLM-14 AML cells, but was less potent in OCI-AML3 cells, than the FLT-3 inhibitor, which is likely attributed to the extent of kinase engagement/selectivity of the PROTAC [10]. In addition, both the FLT-3 PROTAC and inhibitor were ineffective against other types of hematologic malignancies, such as K562 CML cells, suggesting that the antitumor effect of the FLT-3 PROTAC on different FLT-3 ITD mutated cells is cell-dependent.
HDAC family proteins
Histone deacetylases (HDACs) and histone acetyltransferase (HAT) are essential regulators of lysine acetylation which determines chromatin accessibility during transcription. Dysregulation of HDACs is commonly observed in both hematologic and solid malignancies, and inhibition of HDACs leads to apoptosis, differentiation, and growth arrest in tumor cells. There are 11 isoforms of zinc-dependent HDACs in humans, which can be divided into four classes: class I (HDACs 1, 2, 3, and 8); class IIa (HDACs 4, 5, 7, and 9); class IIb (HDACs 6 and 10); and class IV (HDAC 11) [132].
To date, four HDAC inhibitors (HDACi) have been approved by the FDA for the treatment of lymphomas, leukemias, and MM. Most of the approved drugs have limited isoform selectivity and none of the drug candidates have achieved clinical success for treating solid tumors as a single agent, which is probably due to their inability to achieve high concentrations in solid tumor tissues. Importantly, dose-limiting adverse effects such as cardiac toxicity associated with hERG K+ channel activation are hindering their progress in the clinic [133, 134].
By converting an HDACi into an isoform-selective HDAC PROTAC, it is possible to achieve improved potency and reduced off-target toxicity as reported recently [11, 135–138]. CRBN-based PROTACs recruiting a pan-HDAC inhibitor AB3 or a HDAC6 selective binder nexturastat A resulted in specific degradation of HDAC6. Wu et al. proposed that HDAC6-IKZFs dual degraders would achieve an enhanced anti-myeloma activity based on the synergistic effect of HDAC6 inhibitors and immunomodulatory imide drugs (IMiDs) [11]. Moreover, VHL-based HDAC6 degraders were capable of degrading HDAC6 without causing IKZFs degradation. HDAC6 seems to be very sensitive to PROTAC-mediated degradation, probably because of its dominant cytoplasmic localization, whereas most other isoforms are localized in the nucleus [139]. The degradation of class I HDACs was achieved by recruiting benzamide-based HDACi CI-994 as the warhead [140]. The degradation effect of this PROTAC was more significant on HDAC1-2 in comparison to HDAC3, which can probably be attributed to the HDAC binding preference of the warhead.
MDM2
MDM2 is a known E3 ubiquitin ligase that acts as a powerful inhibitor of the tumor suppressor P53 [141]. Overexpression of MDM2 has been often observed in various human cancers and can contribute to tumorigenesis [142, 143]. Therefore, activation of the p53 pathway by antagonizing MDM2 attracts great interest for developing targeted tumor therapeutics. Several MDM2 SMIs including nutlins (nutlin 1, nutlin 2, nutlin 3) and their derivatives have been developed and exhibit good activities against various solid and hematologic malignancies, but can also cause hematopoietic suppression and gastrointestinal toxicity [144]. In addition, MDM2 inhibitor can lead to a significant compensatory increase in MDM2 expression, which can lead rapid degradation of p53 upon clearance of the MDM2 [46]. In 2018, Li et al. employed the PROTAC strategy to design small-molecule degraders of MDM2, including MD-224 as a lead degrader that targets MDM2 to CRBN for degradation [46]. MD-224 potently induced rapid degradation of MDM2 in leukemia cells. Moreover, MD-224 is capable of achieving complete and durable tumor regression in a RS4-11 xenograft model by intravenous administration. As expected, MD-224 can only inhibit the growth of leukemia cells carrying the wild-type P53 but not those with P53 mutants [46].
PLK1
Polo-like kinase 1 (PLK1) is a Ser/Thr protein kinase which belongs to the CDC5/Polo subfamily. It is highly expressed in many different types of tumors, including hematologic malignancies such as AML and thus is an attractive tumor therapeutic targets. Mu et al. reported a CRBN-based BRD4/PLK1 dual degrader, HBL-4, which is more potent than its parental inhibitor BI2536 [145]. Interestingly, in an earlier report by the same group, a BI2536 derivative was used to build a PROTAC (compound 22f), which also displayed potent anti-proliferative activity in RS4-11 leukemia cells [146]. In that study, compound 22f only degraded BRD4. Therefore, it would be of great interest to test if compound 22f can also induce PLK1 degradation.
PRC2
Polycomb repressive complex 2 (PRC2) is one of the two major classes of polycomb proteins, which have been broadly linked to hematologic malignancies [147–149]. The PRC2 is composed of an enhancer of zeste homolog 2 (EZH2), EED, SUZ12, RBAP46/48, and AEBP2 [150]. EZH2 acts as the core catalytic subunit of PRC2, and EED and SUZ12 subunits are indispensable for EZH2 catalytic activity [151]. As PRC2 is highly associated with hematopoietic differentiation and proliferation [152], EZH2, EED, or SUZ12 loss-of-function mutations are related to the increased activity of hematologic stem cells (HSCs) and progenitor cells [153]. High frequency of mutations in EZH2, EED, and SUZ12 are observed in patients with early T cell precursor ALL (ETP-ALL) [154, 155]. Effective inhibition of PRC2 catalytic activity has been achieved by targeting both EED and EZH2. The discovery of deazaneplanocin A (DZNep) is the first attempt to inhibit EZH2 function, which could effectively deplete EZH2, SUZ12, and EED at cellular levels [156]. Later, a series of EZH2 inhibitors were developed for targeting wild-type and/or mutated EZH2 [157]. Due to the critical role of EED in EZH2 activity, the effect of EZH2 inhibitors could be phenocopied by SMIs targeting the EED [158, 159]. Although EZH2 inhibitors are promising antitumor agents, preclinical data suggest that drug resistance could be generated by secondary mutations in both wild-type and mutant EZH2 alleles [160].
In 2020, two separate research groups successfully converted the EED inhibitors into PROTACs [47, 161]. Interestingly, both groups used the VHL ligand to design the PROTACs, but used different EED inhibitors as the warhead, as well as different strategies for linker optimization. The most potent compound named PROTAC2 developed by Bloecher’s group selectively degraded EED, EZH2, and SUZ12, and reduced the proliferation of EZH-dependent tumor cells Karpas422 [161]. The most potent compounds named UNC6852 developed by James’ group also selectively degraded EED, EZH2, and SUZ12, and potently inhibited the proliferation of DLBCL cell lines with a wild-type and Y641N mutant EZH2 [47]. Although the EED-based PROTACs could degrade EZH2, so far no selective PROTACs based on EZH2 inhibitors were reported, until 2019 when Jian et al. developed MS1943, an EZH2 inhibitor-based degrader, via hydrophobic tagging strategy, which was designed by linking an EZH2 inhibitor to a bulky adamantyl group [162]. MS1943 could effectively degrade EZH2 and selectively kill EZH2-dependent TNBC cells, whereas EZH2 inhibitors are ineffective in blocking the proliferation of TNBC cells.
SMARCA2, SMARCA4, and PBRM1
ATP-dependent chromatin remodeling complexes (CRCs) or remodelers use the energy of ATP hydrolysis to change the packaging state of chromatin and thus regulate cellular processes related to chromatin structure such as transcription, DNA repair, DNA replication, and decatenation of chromosomes during mitosis [48, 163]. Among the four distinct families of CRCs (SWI/SNF, ISWI, CHD, INO80), SWI/SNF complexes are the most implicated in diseases with more than 20% of human cancers bearing mutations in the genes encoding mammalian SWI/SNF (mSWI/SNF) subunits [163]. mSWI/SNF complexes are separated into two forms: BRG-/BRM-associated factor (BAF) and polybromo-associated BAF (PBAF). BAF and PBAF complexes share numerous subunits, such as BRG1 (SMARCA4) and BRM (SMARCA2) ATPases, which differ by incorporating different peripheral subunits. Mutations in BAF and PBAF subunits appear to drive different malignancies [163, 164]. SMARCA4 and SMARCA2 are ATPases that are mutually exclusively present in BAF and PBAF complexes. A study found that leukemia cells rely on SMARCA4 to support their oncogenic transcriptional program [165]. Whereas SMARCA2 is essential for the growth of tumor cells that harbor loss of function mutations in SMARCA4 [166]. These findings have inspired great interest in suppressing the bromodomain of SMARCA2 and SMARCA4 through SMIs [166–168]. However, such inhibitors have failed to inhibit tumor cell growth [48] because the ATPase domain but not the bromodomain is essential for tumor cell growth [169]. By converting a non-functional bromodomain ligand to PROTAC molecules, Farnaby et al. successfully developed SMARCA2/4 degraders, including ACBI1. ACBI1 potently induced SMARCA2/4 and PBRM1 degradation in the AML cell line, and inhibited cell growth in multiple cancer cell lines [48]. Degradation of SMARCA2/4 and PBRM1 subunits may disrupt the integrity of the BAF and PBAF complexes, resulting in the dissociation of other subunits. Meanwhile, SMIs that target the allosteric binding site of the SMARCA2/4 ATPase domain and are orally bioavailable with in vivo antitumor activity have been reported [170]. However, due to the dual inhibition of SMARCA2 and SMARCA4, these allosteric inhibitors have a dose-limiting tolerability issue that hampered their further exploration. It would be interesting to see if converting these dual SMARCA2 and SMARCA4 inhibitors to PROTACs would result in the selective degradation of SMARCA2 or SMARCA4.
STAT3
Signal transducer and activator of transcription 3 (STAT3) belongs to the STAT family of proteins, which are both signal transducers and TFs [171]. Aberrant activation of STAT3 is associated with many human malignancies [172–174], because STAT3 regulates numerous genes involved in tumorigenesis, progression, and drug resistance, and thus it has become an attractive cancer target [172]. Several SMIs that block the dimerization of STAT3 have reached the clinical development stage, but only exhibited very limited clinical activity [173]. One major reason for their ineffectiveness is that monomeric STAT3 also has transcriptional activity [175]. To overcome the shortcoming, Wang’s group developed a series of STAT3 PROTACs, including SD-36 [3]. SD-36 efficiently degraded STAT3 protein in MOLM-16 AML cells and several anaplastic ALCL cell lines (SU-DHL-1, DEL, and KIJK). Interestingly, although SD-36 also binds to other STAT proteins, such as STAT1 and STAT4, it only degrades STAT3. In addition, SD-36 effectively degraded mutated STAT3 proteins in tumor cells. Further, SD-36 had desirable PK/pharmacodynamic (PD) properties, was well tolerated and achieved complete and long-lasting tumor regression in mice xenografted with AML and ALCL cell lines. More examples of STAT3 degraders were found in another study from Wang’s lab [176]. This study provides an excellent example that PROTAC technology can be used to target a previously undruggable target such as STAT3. However, STAT3 PROTACs may have some limitations in targeting leukemia than lymphoma, because SD-36 demonstrates potent activity in five of nine lymphoma cell lines, while showing activity in only one of nine AML cell lines [3]. Developing PROTACs that can target other STAT family proteins may add an additional layer of benefits in the treatment of hematologic malignancies.
Other targets
In addition to the above targets, there are also some less studied PROTAC targets (SIRT2, PCAF/GCN5, BRD7, BRD9, IRAK4, TRIM24, and RIPK2) that are implicated in various malignancies, including leukemia and lymphoma. Schiedel et al. reported a PROTAC against sirtuin 2 (SIRT2). This PROTAC selectively induced SIRT2 degradation in HeLa cells and led to more pronounced acetylation of the microtubule network than the corresponding inhibitor [177]. P300/CBP-associated factor (PCAF) and general control nonderepressible 5 (GCN5) are multidomain epigenetic proteins, containing an acetyltransferase and a bromodomain, which are involved in many cellular processes including proliferation, DNA damage repair, and inflammation [178]. A PCAF/GCN5 PROTAC, named GSK983, efficiently degraded PCAF and GCN5 in THP1 acute monocytic leukemia cells [179]. BRD7 and BRD9 are bromodomain-containing subunits of the BAF and PBAF complexes, respectively [164]. Brander’s group reported a selective degrader of BRD9, named dBRD9, which efficiently degraded BRD9 and exerted a 10–100-fold increase in antiproliferative activity than the corresponding inhibitor against MOLM-13 and other AML cell lines [180]. Subsequently, Ciulli’s group reported a VHL-based dual BRD7 and BRD9 degrader, VZ185, which was found to be more effective than the corresponding inhibitor in reducing the viability of EOL-1 acute myeloid eosinophilic leukemia cells and A-204 malignant rhabdoid tumor cells [181]. The hyperactivation of interleukin-1 receptor-associated kinase 4 (IRAK4), a key mediator of innate immunity, is linked with autoimmune diseases and cancer [182]. IRAK4 has both enzymatic (kinase-dependent) and scaffolding (kinase-independent) functions [183]. Nunes et al. reported a VHL-based PROTAC that was capable of degrading IRAK4 in peripheral blood mononuclear cells and dermal fibroblasts with DC50 values of 151 nM and 36 nM, respectively [184]. In addition, PROTACs targeting tripartite motif-containing protein 24 (TRIM24), receptor-interacting protein kinase 2 (RIPK2), and the Janus kinase family enzymes for degradation may also have the potential to be used to treat certain types of hematologic malignancies but require further studies [185–187].
Potential oncoproteins that can be targeted by PROTAC in hematologic malignancies
CALR
Calreticulin gene (CALR) mutations frequently occur in Philadelphia chromosome (Ph)-negative myeloproliferative neoplasms (MPNs), including essential thrombocythemia (ET), primary myelofibrosis (PMF), and polycythemia vera (PV) [188–190]. CALR mutations are mutually exclusive with JAK2 or MPL mutations in MPNs, suggesting common oncogenic pathways. CALR mutations are located in exon 9 and generate a + 1 base-pair translation frameshift. Although the extent of the C-terminal alterations vary among CALR mutations, all resulting variants share a loss of C-terminal 27 amino acids with a concomitant gain of a novel peptide consisting of 36 amino acids.
CALR mutations has been shown to be pathogenic and serve as a disease-initiating event to favor the expansion of the megakaryocytic lineage by specific activation of the thrombopoietin receptor and uncontrolled activation of the JAK2/STAT pathway [191, 192]. The oncogenic CALR mutant protein could be an excellent PROTAC target to treat MPN patients with CALR mutations. It would be ideal to design the PROTAC against all CALR mutant proteins using specific ligand for the unique 36 amino acids in the C-terminal of all CALR mutants and a megakaryocytic lineage specific E3 ligase.
NPM1
Nucleophosmin (NPM1) is one of the most commonly mutated genes in AML, occurring in approximately 50% of adult and 20% of childhood AML with normal karyotypes [193, 194]. Due to its clinical significance, NPM1-mutated AML was categorized as a distinct leukemic entity in the WHO-2016 classification [195]. NPM1 mutations are heterozygous and restricted to exon-12, causing a frameshift to eliminate the nucleolar localization sequence of NPM1 and to generate a new C-terminal nuclear export signal motif. Therefore, mutations in NPM1 result in mislocalization of NPM1 mutant proteins to the cytoplasm (NPM1c+). NPM1c+ acts as a dominant negative mutant for NPM1 function and has a gain-of-function role in pathogenesis of the AML phenotype [196, 197]. Although there are many different strategies in targeting NPM1 such as inhibition of translocation of NPM1c+ [198] and proteasomal degradation of NPM1c+ [199], the unmet needs to develop effective drugs targeting NPM1 is still growing. The development of a PROTAC specifically targeting NPM1c+ could be a superb strategy. Giving the distinct cellular localization of WT NPM1 and NPM1c+, a cytosolic E3 ligase would effectively eliminate the degradation of WT NPM1, if the ligand is not specific enough to NPM1c+. In addition, efforts could be directed to screen specific ligands for type A NPM1 mutation, which accounts for ~ 80% of the NPM1 mutations and has a unique 12 amino acid peptide in the C-terminal including a lysine.
Oncofusion proteins
Chromosomal translocations are very common in hematologic malignancies [200, 201]. It is well established that chromosomal translocations promote the development of hematologic malignancies either through the formation of oncogenic fusion protein or through oncogene activation by a stronger promoter or enhancer [200, 201]. Both the “alien” oncofusion proteins and the miss-activated oncoproteins are potential PROTAC targets. Indeed, several misactivated oncoproteins such as Bcl-2 and Bcl-6, and oncofusion proteins such as BCR-ABL have been targeted by PROTACs for specific subtypes of hematologic malignancies (see above sections in this review). Theoretically, these oncofusion proteins could be ideal PROTAC targets, since they are “alien” to human cells and the side effects would be easier to manage. The most challenging step for such PROTAC development will be the screening for relative specific ligands for such oncofusion proteins. The available crystal structures for some of these oncofusion proteins will aid this endeavor. The oncofusion proteins are often specific for subtype of hematologic malignancies, which also have potential value for PROTAC development in terms of E3 ligase selections, e.g., there are four most prevalent oncofusion proteins in AML: PML-RARα, AML1-ETO, CBFβ-MYH11, and MLL-fusions, while ETV6-RUNX1 and TCF3-PBX1 fusions are common in B-cell ALL [202].
Safety concerns of PROTACs
As discussed above, PROTACs have several advantages over SMIs [32]. It is encouraging that the PROTACs targeting AR and ER have advanced to the clinic and the preliminary results from these clinical studies reveal that the AR PROTAC ARV-110 appears clinically effective in a patient with metastatic castration-resistant prostate cancer (https://ir.arvinas.com/node/7666/pdf). However, there are still some safety concerns about PROTACs, which should be considered before advocating for their clinical translation. Although PROTACs can be more potent than SMIs against a POI, they might produce greater on-target toxicities than SMIs for several reasons. First, SMIs rarely inhibit the functions of their targets completely, while PROTACs can almost entirely deplete their targets. The incomplete inhibition of a POI with SMIs may be tolerable [203], while their complete depletion with a PROTAC might be detrimental if the POI is essential for normal cell function. Second, some POIs have both enzymatic and scaffold functions. The latter may be important for normal cell functions. In this case, in contrast to SMIs which only inhibit enzymatic function, PROTACs can completely deplete the protein, resulting in the loss of both enzymatic activity and scaffolding function, which can potentially cause some undesirable consequences. Third, SMIs may cause a transient inhibition of a protein function while PROTACs induce prolonged depletion of the target protein due to their unique MOA. The prolonged depletion of a target by PROTACs may cause on-target toxicities if a POI is indispensable for normal functions [4].
In addition, many PROTACs are not completely selective and can degrade proteins other than their desired targets [7, 90, 204, 205]. For example, proteins that are not directly bound to a PROTAC can be degraded if they are a part of the same complex or are in close proximity with the target protein [161]. An off-target effect can also arise from the neomorphic interactions with neosubstrates of an E3 ligase if the E3 ligase ligand used to generate the PROTAC or the PROTAC itself can bind the neosubstrates [206]. A well-characterized example of off-target activities of PROTACs are CRBN-based PROTACs because CRBN ligands, including thalidomide, pomalidomide, and lenalidomide, used for the CRBN-targeted PROTACs can recruit several neo-substrates such as SALL4, IKZF1, and IKZF3 to CRBN to induce their degradation [207, 208]. This can lead to some adverse effects including teratogenic effect and cardiovascular, hepatic, and neuronal toxicities. Similarly, the VHL E3 ligase is essential for the ubiquitination and degradation of hypoxia-inducible factor-1 alpha (HIF1a) [209], a TF that regulates many genes necessary for tumor growth. If VHL is overly engaged by a PROTAC, it can potentially inhibit the degradation of HIF1a to promote tumorigenesis. Therefore, profiling the changes in global protein expression by proteomics will be helpful to appreciate the potential off-target toxicities of PROTACs [3, 4, 43]. In addition, the formation of a stable ternary complex of the POI, PROTAC, and E3 ligase is essential for PROTACs to induce specific degradation of the POI. However, high concentrations of PROTACs produce the “Hook effect,” which may lead to the formation of binary complexes of PROTACs and E3 ligases [28]. These binary complexes can potentially recruit off-targets and lead to their degradation and off-target toxicities. Lastly, the UPS maintains the homeostasis of intracellular proteins [210]. If PROTACs cause disruption of the UPS homeostatic function, they can cause some safety concerns for clinical application. Therefore, strategies should be developed to reduce both the on-target and off-target toxicities of PROTACs in order to more rapidly translate PROTACs into the clinic. In the following sections, we will focus our discussion on the strategies that can potentially be used to reduce the on-target toxicities of PROTACs.
Cell type- and tumor-specific/selective PROTACs
One of the most important safety concerns about PROTACs discussed above are on-target toxicities, which may be avoided to a large extent by utilizing ligands against cell type- and tumor-specific/selective E3 ligases for designing PROTACs [2]. These PROTACs are expected to avoid the on-target degradation of POIs in cells or tissues where the specific/selective E3 ligases are not expressed. Although numerous PROTACs have been generated in the past few years, only a few of them are tumor-selective to avoid normal tissue toxicity, including platelet sparing Bcl-xL PROTACs reported by us recently by taking advantage of the E3 ligases that are barely expressed in platelets [4, 60–63]. These findings are in agreement with Crews’ recent suggestion that developing tumor-specific/selective PROTACs has the potential to reduce the on-target toxicities of PROTACs [2].
There are several strategies to achieve tumor-specific/selective degradation of tumor-associated POIs by PROTACs as discussed in a recent review [211]. First, if a POI is tumor-specific, we can generate a tumor-specific PROTAC by using any available E3 ligases in the tumor tissues to degrade this POI. One example is BCR-ABL1 PROTACs because BCR-ABL1 is specifically expressed in CML cells [81]. This strategy may be applicable to many of the oncofusion proteins specifically expressed in leukemia cells. Second, if a POI is not tumor-specific but is specific for a tumor-derived tissue or cell, we can still develop tumor-selective PROTACs by targeting the POI to any available E3 ligases in that tissue or cell, providing the POI is dispensable for the normal tissue function or the normal tissue function is nonessential. An example is BTK, that is important for B cell development and can promote the development of B cell lymphoma [118]. Third, if a POI is not tissue/tumor-specific, we can generate a tumor-selective PROTAC by targeting this POI to a tissue- and/or tumor-selective E3 ligase to limit its on-target toxicity. Bcl-xL PROTACs discussed above are an example of this approach [4, 61–63]. However, numerous POIs are ubiquitously expressed in both tumor and normal tissues and are indispensable for normal cell functions. To overcome the on-target toxicity of a PROTAC, targeting this kind of protein in tumor cells will require the identification of tumor-specific/selective E3 ligases [2, 211]. This can be accomplished by profiling E3 ligase expression at a cellular level as discussed below.
Identification of cell type-specific/selective E3 ligases
The recent advancement in the single-cell RNA-Seq (scRNA-Seq) technology has allowed us to profile gene expression at a single-cell level and deconvolute the cell-type heterogeneity in tissues. By leveraging a recent human pan-tissue scRNA-Seq data (a.k.a. Human Cell Landscape; HCL) [12], we can identify cell-type specific/selective E3 ligases to facilitate the development of tumor-specific/selective PROTACs. A detailed description of the HCL data, including sample preparation, data sequencing procedure, and batch removal were previously described [12]. We obtained the data of the batch-removed counts from https://figshare.com/articles/HCL_DGE_Data/7235471, including 37 tissues from human adults and a total of 315 different cell types, and employed R package Seurat for data processing and quality control [212]. To be more specific, the cells with low quality (i.e., unique transcript counts less than 300) or exhibiting an aberrantly high gene count (i.e., unique transcript counts over 2500), or those with mitochondrial contamination (i.e., > 20% mitochondrial counts) were filtered out. The transcript expression measurements of each cell were normalized against total expression using a global-scaling normalization method, followed by log-transformation. Since multiple cell samples existed in each cell type of a tissue, we calculated the mean expression value by taking their averages. Finally, transcripts for 574 from more than 600 putative E3 ligases were successfully identified in the HCL data [213]. The resulting transcript expression levels of the E3 ligases were visualized by heatmap [214], and presented in Additional file 1. Information including tissue name, cell types, and gene expression levels corresponding to Additional file 1 are provided in Additional file 2. There is an obvious cluster of cell-type non-specific E3 ligases consisting of ZFAND5, WSB1, and TNFAIP3, because they are expressed across different cell types in many tissues. Some E3 ligases are considered cell-type specific/selective because they are highly expressed in certain cell types. For example, PEX10, KBTBD4, PATZ1, RABGEF1, AREL1, LONRF2, FBXL16, ZBTB45, KCNB1, RNF2208, KCTD16, KCTD4, DPF1, MKRN3, and RHOBTB1 are highly expressed in neurons of the temporal lobe but lowly expressed in most cell types in other tissues; KRT8 is highly expressed in cells in gastrointestinal tract, prostate, and pancreas, but lowly expressed in cells of brain and hematopoietic system (Additional file 1).
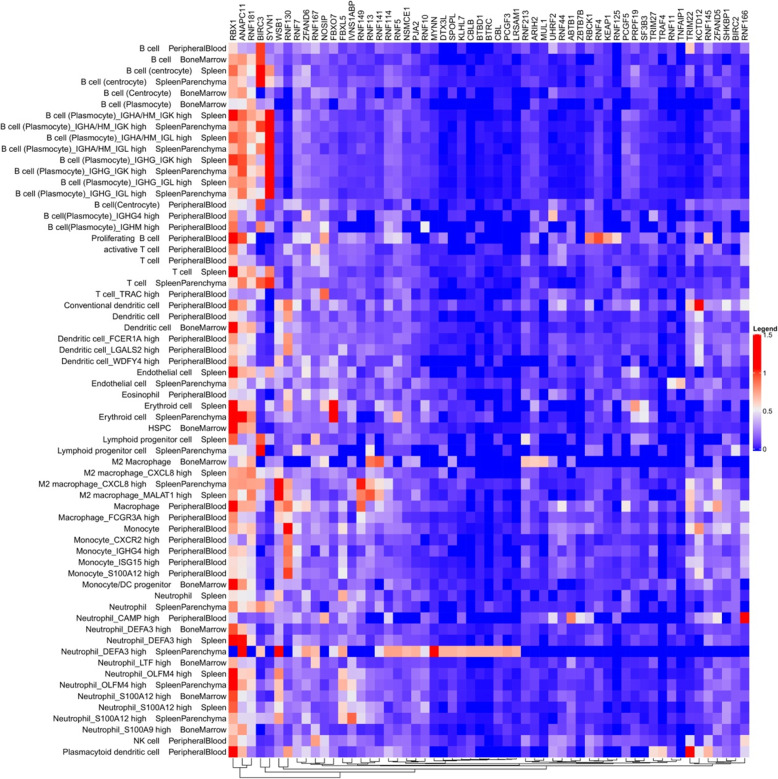
Furthermore, we analyzed the single-cell gene expression profile of E3 ligases in the hematopoietic system to identify E3 ligases differentially expressed in different types of hematopoietic cells (Additional file 3). The top 57 highly expressed E3 ligases in hematopoietic cells are presented in Fig. 3. Among them, RBX1, ZFAND5, WSB1, ANAPC11, SOCS3, KRT8, RNF181, LGALS3BP, BIRC3, and IVNS1ABP are the top 10 ubiquitously expressed E3 ligases in various hematopoietic cells, of which RBX1 [215], ZFAND5 [216], WSB1 [217], ANAPC11 [218], SOCS3 [219], RNF181 [220], and BIRC3 [221] have been reported to have E3 ligase activity.
Fig. 3.
Heatmap of single-cell expression of 57 E3 ligases in hematopoietic tissues. The levels of single-cell expression of 57 E3 ligases in different types of hematopoietic cells from the peripheral blood, bone marrow, and spleen in human adults were abstracted from the Human Cell Landscape (HCL) data. In the heatmap, each row represents a cell type, and each column represents an E3 ligase. The color legend shown on the right was determined by averaging the expression values of all cells within a specific cell type. These E3 ligases were clustered by the hierarchical clustering algorithm, and the resulting dendrogram was shown at the bottom. The 57 E3 ligases presented in the figure were chosen according to the criteria: E3 ligases with an average expression value > 0.5 in at least one cell type. Single cell RNA-sequencing (scRNA-seq) data were from the website: https://figshare.com/articles/HCL_DGE_Data/7235471.
Of these highly expressed E3 ligases in hematopoietic cells, BIRC3 (or cIAP2) is of particular interest for the design of hematopoietic cell-selective PROTACs. BIRC3 belongs to the IAP family proteins, of which cIAP1 and XIAP have been widely used to develop protein degraders named SNIPERs [18, 79, 80, 222–224]. Recently, we also developed IAP-recruiting PROTACs targeting Bcl-xL for degradation in lymphoma and a set of solid tumor cell lines [61]. BIRC3 has been reported to have E3 ligase activity [221], but has not been used to design PROTACs. This is likely attributable to the lack of specific ligands for BIRC3 [225, 226], as well as overlooked by its low expression in most tumor cells (detectable in only 8% of 60 tumor cell lines) compared to other IAP proteins, such as cIAP1 and XIAP [227]. Interestingly, although the levels of BIRC3 are low in the majority of cancers, it is highly expressed in many hematologic malignant cells, such as CLL, MCL, follicular lymphoma (FL), marginal zone lymphoma (MZL), splenic marginal zone lymphoma (SMZL), and DLBCL, compared to the other members of the IAP family proteins [228, 229]. We also found that BIRC3 is highly expressed in T cell lymphoma (TCL) cells compared to other IAP proteins [61]. Therefore, BIRC3 represents a promising E3 ligase that can be used to design cell-selective PROTACs targeting hematologic malignancies, especially TCL. Another advantage to using IAPs to design PROTACs is that their ligands are also able to kill hematologic malignant cells because some of them depend on IAPs for survival [230]. The shortcoming of IAP-recruiting PROTACAs is the induction of autoubiquitination and degradation of some IAP proteins such as cIAP1 [18]. This drawback can be avoided by modifying the linker attachment site of the E3 ligase ligand MeBS [231]. Compared to cIAP1, BIRC3 is relatively less sensitive to the ligand-induced autoubiquitination and degradation and thus may be more suitable for the design of PROTACs [225].
In addition to BIRC3, we found that SYVN1 (also known as HRD1) is a plasmocyte selective E3 ligase. SYVN1 is lowly expressed in proliferating B cells and moderately expressed in some differentiated B cells, i.e., centrocytes, but highly expressed in plasmocytes (Fig. 3), suggesting that its expression is upregulated during B cell differentiation. In addition, the expression of SYVN1 is low in other cell types in the hematopoietic system (Fig. 3). SYVN1 was originally identified as an E3 ligase within an endoplasmic reticulum (ER) membrane-anchored complex that targets luminal misfolded glycoproteins for degradation [232, 233]. It is a key regulator for B cell proliferation and development [234]. Loss of SYNV1 in B cell precursors leads to a severe developmental block at the transition from large to small pre-B cells [234]. SYVN1 can also regulate B cell immunity by protecting B cells from activation-induced cell death via degrading the death receptor Fas [235]. Moreover, SYVN1 can also mediate the degradation of P53 [236]. Therefore, SYVN1 has been implicated in the oncogenesis of activated B cell-like DLBCL (ABC-DLBCL) [237], and has been considered a potential therapeutic target for restoring the tumor suppressor activity of unstable lymphoma-associated Blimp-1 mutants [237]. In addition, MM cells express high levels of SYVN1 to cope with ER stress resulting from immunoglobulin hyperproduction [238]. Therefore, it would be of great interest to determine whether SYVN1 can be used to design cell type-specific PROTACs to target hematologic malignancies associated with B cells, particularly MM.
Besides the E3 ligases discussed above, there are many other cell type-specific E3 ligases identified at the single-cell level (Fig. 3), such as KCTD12 in blood dendritic cells, and TRIM22 in blood plasmacytoid dendritic cells. Gaining insight into the function and designing ligands for these cell type-specific E3 ligases would be greatly useful for the development of cell type- and/or tumor-specific/selective PROTACs in the future.
Clinical translation of PROTACs
To date, two PROTACs have entered into clinical trials for the treatment of prostate and breast cancers. As discussed above, although numerous PROTACs have been developed to target oncoproteins associated with hematologic malignancies, none has entered into the clinical trials for this indication thus far. In this section, we will briefly discuss the PROTACs currently undergoing in clinical trials, and those in preclinical development for the treatment of hematologic malignancies.
PROTACs in clinical trials
ARV-110 is an orally bioavailable AR PROTAC that entered into a clinical trial to treat patients with metastatic castrate-resistant prostate cancer (NCT03888612) in 2019. Recently, ARV-110 was reported to have an acceptable safety profile and appears clinically effective in a patient with metastatic castration-resistant prostate cancer (https://ir.arvinas.com/node/7296/pdf). ARV-471 is a PROTAC designed to specifically target ER. In preclinical studies, ARV-471 demonstrated near-complete ER degradation in tumor cells, and induced robust tumor shrinkage when dosed as a single agent in multiple ER-driven xenograft models. ARV-471 is currently in a phase I clinical trial (NCT04072952). Its safety, tolerability, pharmacokinetics, and anti-tumor activity in patients with ER+/HER2− locally advanced or metastatic breast cancer (https://ir.arvinas.com/node/7296/pdf) will likely be reported in the coming months. A positive result from these PROTAC clinical studies will provide proof-of-principle that PROTACs can be developed as effective antitumor therapeutics.
PROTACs in preclinical development for hematologic malignancies
Although PROTACs have been developed to successfully target a number of targets associated with hematologic malignancies, none has entered into the clinical trials. However, several PROTACs have shown promising preclinical results in the treatment of hematologic malignancies [3, 4, 44], and will likely be evaluated in the clinic in the near future. In addition, many pharmaceutical companies have invested heavily in the development of their antitumor PROTAC pipelines. For example, Nurix is going to file an IND application with the FDA at the end of this year to test its BTK PROTAC in patients with B cell malignancies (https://www.nurixtx.com/research-development/pipeline/), Kymera is advancing a STAT3 PROTAC as one of their drug candidates in oncology (https://www.kymeratx.com/pipeline/), and Dialectic Therapeutics (DT) is pursuing preclinical development of the Bcl-xL PROTAC DT2216 for the treatment of hematologic malignancies and solid cancer tumors in 2021 (https://www.dtsciences.com/#science). Therefore, we expect to witness more clinical studies on PROTACs for hematologic malignancies in the near future.
Conclusions and perspectives of PROTACs as therapeutics for hematologic malignancies
Targeted protein degradation using PROTACs has emerged as a novel therapeutic modality in drug discovery. Since the first PROTAC was reported, researchers have made remarkable progress in the field in the last 20 years. PROTACs have been shown to have unique advantages over conventional SMIs. However, the high molecular weight and complex structure of PROTACs makes some of the PROTACs have low in vivo efficacy. Cellular permeability, compound stability, tissue distribution, and other pharmaceutical properties need to be balanced during PROTAC optimization to generate a desirable therapeutic. In addition, PROTACs based on VHL and CRBN have some drawbacks associated with tumor-suppressor functions of these proteins. For example, VHL is frequently mutated in kidney cancer [239, 240]; therefore, VHL-based PROTACs cannot be used to treat kidney cancer patients with mutation or deletion in VHL gene. Moreover, resistance to both VHL- and CRBN-based PROTACs was reported in cancer cells following chronic treatment, which was primarily led by genomic alterations that compromise core components of the relevant E3 ligase complexes [241]. Also, careful optimization of dosing is needed to avoid tumor suppressor functions of VHL and CRBN.
To date, two PROTACs targeting AR and ER are already in phase I clinical trials to treat patients with prostate and breast cancers, respectively. Several PROTACs have shown promising preclinical results in the treatment of hematologic malignancies [3, 4, 46], which have a great potential of being evaluated in clinical trials in the near future. In the translational applications of PROTACs, safety concerns should be taken into consideration. Identifying more strategies to develop safer PROTACs will have greater potential for PROTACs to be successful in the clinic. The development of tumor-specific/selective PROTACs based on cell-type and tumor-specific/selective E3 ligases may revolutionize the field of PROTACs as antitumor therapeutics. Breakthroughs in scRNA-seq have greatly enhanced our ability to determine cell-type specific transcriptomes [12], and enable us to understand the profile of oncogenic targets and E3 ligases at the single-cell level. Based on this, we can choose more specific E3 ligases to target POIs to improve the efficiency of PROTACs and maximally reduce the toxicities to normal cells, which may be particularly applicable for hematologic malignancies.
Identifying appropriate ligands for E3 ligases and substrates is the most critical but challenging step for the design of tumor-specific/selective PROTACs. Among the hundreds of E3 ligases in the human genome, only a few have been used in PROTACs, Therefore, more candidate E3 ligases should be further explored to expand the choices for the rational design of PROTACs. In addition, recently researchers have developed similar strategies to induce targeted degradation of RNAs (e.g., oncogenic micro-RNAs) by recruiting nucleases using small-molecules. These RNA degraders are named as ribonuclease targeting chimeras (RIBOTACs), which also have the potential to be developed as novel anticancer therapeutics [242, 243].
Supplementary information
Additional file 1. Profiling E3 ligase expression at single cell level in different tissues. The heatmap shows the single-cell expression levels of 574 E3 ligases across all cell types and tissues in human adults from the Human Cell Landscape (HCL) data. In the heatmap, each row represents a cell type, and each column represents an E3 ligase. The color legend was shown on the right, which was determined by averaging the expression values of all cells within a tissue-specific cell type. These E3 ligases and the different cell types were clustered by the hierarchical clustering algorithm, and the resulting dendrograms were shown on bottom and right, respectively.
Additional file 2. List of 574 E3 ligases expressed in different cell types from all tissues.
Additional file 3. List of 574 E3 ligases expressed in different types of hematopoietic cells in the blood, spleen, and bone marrow.
Acknowledgements
The authors would like to thank the members of the Zheng and Zhou laboratories for their thoughtful discussion and assistance and Ms. Janet Wiegand for editorial assistance.
Abbreviations
- ABC-DLBCL
Activated B cell-like diffused large B cell lymphoma
- Ab-PROTACs
Antibody-PROTAC conjugates
- ADC
Antibody-drug conjugate
- ALK
Anaplastic lymphoma kinase
- AML
Acute myeloid leukemia
- AR
Androgen receptor
- BTK
Bruton’s tyrosine kinase
- BAF
BRG-/BRM-associated factor
- Bcl-2
B cell lymphoma 2
- Bcl-6
B cell lymphoma 6
- BET
Bromodomain and extra terminal domain
- BETi
Bromodomain and extra terminal domain inhibitor
- BL
Burkitt’s lymphoma
- BRDs
Bromodomain-containing proteins
- CALR
Calreticulin
- CDK
Cyclin-dependent kinase
- CLL
Chronic lymphocytic leukemia
- CML
Chronic myelogenous leukemia
- CRBN
Cereblon
- CRC
Chromatin remodeling complex
- DMNB
4,5-Dimethoxy-2-nitrobenzyl
- DLBCL
Diffused large B cell lymphoma
- DZNep
3-Deazaneplanocin A
- ERα
Estrogen receptor-alpha
- ET
Essential thrombocythemia
- ETP-ALL
Early T cell precursor ALL
- EZH2
Enhancer of zeste homolog 2
- FLT-3
FMS-like tyrosine kinase-3
- GC
Germinal center
- GCN5
General control nonderepressible 5
- HAT
Histone acetyltransferase
- HDACi
Histone deacetylase inhibitor
- HDAC
Histone deacetylase
- HIF1a
Hypoxia-inducible factor-1 alpha
- HSC
Hematologic stem cell
- IMiDs
Immunomodulatory imide drugs
- IRAK4
Interleukin-1 receptor-associated kinase 4
- LSC
Leukemic stem cell
- MDM2
Mouse double minute 2 homolog
- MCL
Mantle cell lymphoma
- MLL
Mixed-lineage leukemia
- MM
Multiple myeloma
- MPNs
Myeloproliferative neoplasms
- NPM1
Nucleophosmin
- NSCLC
Non-small cell lung cancer
- PCAF
P300/CBP-associated factor
- PD
Pharmacodynamic
- PDX
Patient-derived xenograft
- PK
Pharmacokinetic
- PLK1
Polo-like kinase 1
- PROTACs
Proteolysis targeting chimeras
- RIPK2
Receptor-interacting protein kinase 2
- PBAF
Polybromo-associated BAF
- Ph+ ALL
Philadelphia chromosome-positive acute lymphoblastic leukemia
- PMF
Primary myelofibrosis
- PV
Polycythemia vera
- SCLC
Small cell lung cancer
- scRNA-seq
Single-cell RNA sequencing
- SIRT2
Sirtuin 2
- SLL
Small lymphocytic lymphoma
- SMIs
Small molecule inhibitors
- SNIPER
Non-genetic IAP-dependent protein eraser
- STAT3
Signal transducer and activator of transcription 3
- T-ALL
T cell acute lymphoblastic leukemia
- TCL
T cell lymphoma
- TF
Transcription factor
- TNAs
Therapeutic nucleic acids
- TRIM24
Tripartite motif-containing protein 24
- UPS
Ubiquitin proteasome system
- VHL
Von Hippel-Lindau
Authors’ contributions
YH, SK, DL, XZ, XL, YY, and MX performed the literature search and wrote the manuscript. ZH and YH performed single-cell transcriptomic analyses. RH and GZ revised the manuscript. DZ conceived and supervised the review, and interpreted data, and wrote the manuscript. All authors have read and approved the final version of the manuscript.
Funding
This study was supported by US National Institutes of Health (NIH) grants R01CA211963 (D.Z.), R01CA219836 (D.Z.), R01CA242003 (D.Z. and G.Z.) and R21CA223371 (G.Z.).
Availability of data and materials
All data and materials supporting the conclusion of this study have been included within the article and the additional files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
This study does not include any individual person’s data in any form.
Competing interests
Y.H., S.K., X.Z, X.L., G.Z., and D.Z. are inventors of two pending patent applications for use of Bcl-xL PROTACs as senolytic and antitumor agents. G.Z., and D.Z. are co-founders of and have equity in Dialectic Therapeutics, which develops Bcl-xL PROTACs to treat cancer. The remaining authors declare no competing financial interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yonghan He and Sajid Khan contributed to this manuscript equally.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13045-020-00924-z.
References
- 1.Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16:101–114. doi: 10.1038/nrd.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schapira M, Calabrese MF, Bullock AN, Crews CM. Targeted protein degradation: expanding the toolbox. Nat Rev Drug Discov. 2019;18:949–963. doi: 10.1038/s41573-019-0047-y. [DOI] [PubMed] [Google Scholar]
- 3.Bai L, Zhou H, Xu R, Zhao Y, Chinnaswamy K, McEachern D, et al. A potent and selective small-molecule degrader of STAT3 achieves complete tumor regression in vivo. Cancer Cell. 2019;36:498–511.e17. doi: 10.1016/j.ccell.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan S, Zhang X, Lv D, Zhang Q, He Y, Zhang P, et al. A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat Med. 2019;25:1938–1947. doi: 10.1038/s41591-019-0668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Han X-R, Yang X, Jiang B, Liu J, Xiong Y, et al. Proteolysis targeting chimeras (PROTACs) of anaplastic lymphoma kinase (ALK) Eur J Med Chem. 2018;151:304–314. doi: 10.1016/j.ejmech.2018.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Q, Ren C, Liu L, Chen J, Shao Y, Sun N, et al. Discovery of SIAIS178 as an effective BCR-ABL degrader by recruiting Von Hippel-Lindau (VHL) E3 ubiquitin ligase. J Med Chem. 2019;62:9281–9298. doi: 10.1021/acs.jmedchem.9b01264. [DOI] [PubMed] [Google Scholar]
- 7.Zorba A, Nguyen C, Xu Y, Starr J, Borzilleri K, Smith J, et al. Delineating the role of cooperativity in the design of potent PROTACs for BTK. Proc Natl Acad Sci U S A. 2018;115:E7285–E7292. doi: 10.1073/pnas.1803662115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun B, Fiskus W, Qian Y, Rajapakshe K, Raina K, Coleman KG, et al. BET protein proteolysis targeting chimera (PROTAC) exerts potent lethal activity against mantle cell lymphoma cells. Leukemia. 2018;32:343–352. doi: 10.1038/leu.2017.207. [DOI] [PubMed] [Google Scholar]
- 9.De Dominici M, Porazzi P, Xiao Y, Chao A, Tang H-Y, Kumar G, et al. Selective inhibition of Ph-positive ALL cell growth through kinase-dependent and -independent effects by CDK6-specific PROTACs. Blood. 2020;135:1560–1573. doi: 10.1182/blood.2019003604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burslem GM, Song J, Chen X, Hines J, Crews CM. Enhancing antiproliferative activity and selectivity of a FLT-3 inhibitor by proteolysis targeting chimera conversion. J Am Chem Soc. 2018;140:16428–16432. doi: 10.1021/jacs.8b10320. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Yang K, Zhang Z, Leisten ED, Li Z, Xie H, et al. Development of multifunctional histone deacetylase 6 degraders with potent antimyeloma activity. J Med Chem. 2019;62:7042–7057. doi: 10.1021/acs.jmedchem.9b00516. [DOI] [PubMed] [Google Scholar]
- 12.Han X, Zhou Z, Fei L, Sun H, Wang R, Chen Y, et al. Construction of a human cell landscape at single-cell level. Nature. 2020;581:303–309. doi: 10.1038/s41586-020-2157-4. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci U S A. 2001;98:8554–8559. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mir O, Broutin S, Desnoyer A, Delahousse J, Chaput N, Paci A. Pharmacokinetics/pharmacodynamic (PK/PD) relationship of therapeutic monoclonal antibodies used in oncology: what’s new? Eur J Cancer. 2020;128:103–106. doi: 10.1016/j.ejca.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto KM, Kim KB, Verma R, Ransick A, Stein B, Crews CM, et al. Development of Protacs to target cancer-promoting proteins for ubiquitination and degradation. Mol Cell Proteomics. 2003;2:1350–1358. doi: 10.1074/mcp.T300009-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Schneekloth JS, Fonseca FN, Koldobskiy M, Mandal A, Deshaies R, Sakamoto K, et al. Chemical genetic control of protein levels: selective in vivo targeted degradation. J Am Chem Soc. 2004;126:3748–3754. doi: 10.1021/ja039025z. [DOI] [PubMed] [Google Scholar]
- 17.Schneekloth AR, Pucheault M, Tae HS, Crews CM. Targeted intracellular protein degradation induced by a small molecule: En route to chemical proteomics. Bioorg Med Chem Lett. 2008;18:5904–5908. doi: 10.1016/j.bmcl.2008.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh Y, Ishikawa M, Naito M, Hashimoto Y. Protein knockdown using methyl bestatin-ligand hybrid molecules: design and synthesis of inducers of ubiquitination-mediated degradation of cellular retinoic acid-binding proteins. J Am Chem Soc. 2010;132:5820–5826. doi: 10.1021/ja100691p. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 20.Buckley DL, Gustafson JL, Van Molle I, Roth AG, Tae HS, Gareiss PC, et al. Small-molecule inhibitors of the interaction between the E3 ligase VHL and HIF1α. Angew Chem Int Ed Engl. 2012;51:11463–11467. doi: 10.1002/anie.201206231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maniaci C, Hughes SJ, Testa A, Chen W, Lamont DJ, Rocha S, et al. Homo-PROTACs: bivalent small-molecule dimerizers of the VHL E3 ubiquitin ligase to induce self-degradation. Nat Commun. 2017;8:830. doi: 10.1038/s41467-017-00954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinebach C, Lindner S, Udeshi ND, Mani DC, Kehm H, Köpff S, et al. Homo-PROTACs for the chemical knockdown of Cereblon. ACS Chem Biol. 2018;13:2771–2782. doi: 10.1021/acschembio.8b00693. [DOI] [PubMed] [Google Scholar]
- 23.Xue G, Wang K, Zhou D, Zhong H, Pan Z. Light-induced protein degradation with photocaged PROTACs. J Am Chem Soc. 2019;141:18370–18374. doi: 10.1021/jacs.9b06422. [DOI] [PubMed] [Google Scholar]
- 24.Reynders M, Matsuura BS, Bérouti M, Simoneschi D, Marzio A, Pagano M, et al. PHOTACs enable optical control of protein degradation. Sci Adv. 2020;6:eaay5064. doi: 10.1126/sciadv.aay5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hines J, Gough JD, Corson TW, Crews CM. Posttranslational protein knockdown coupled to receptor tyrosine kinase activation with phosphoPROTACs. Proc Natl Acad Sci U S A. 2013;110:8942–8947. doi: 10.1073/pnas.1217206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bondeson DP, Mares A, Smith IED, Ko E, Campos S, Miah AH, et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol. 2015;11:611–617. doi: 10.1038/nchembio.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S, et al. DRUG DEVELOPMENT. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science. 2015;348:1376–1381. doi: 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettersson M, Crews CM. PROteolysis targeting chimeras (PROTACs)—past, present and future. Drug Discov Today Technol. 2019;31:15–27. doi: 10.1016/j.ddtec.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Churcher I. Protac-induced protein degradation in drug discovery: breaking the rules or just making new ones? J Med Chem. 2018;61:444–452. doi: 10.1021/acs.jmedchem.7b01272. [DOI] [PubMed] [Google Scholar]
- 30.Pfaff P, Samarasinghe KTG, Crews CM, Carreira EM. Reversible spatiotemporal control of induced protein degradation by bistable PhotoPROTACs. ACS Cent Sci. 2019;5:1682–1690. doi: 10.1021/acscentsci.9b00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Chen H, Ma L, He Z, Wang D, Liu Y, et al. Light-induced control of protein destruction by opto-PROTAC. Sci Adv. 2020;6:eaay5154. doi: 10.1126/sciadv.aay5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Ma J, Liu Y, Xia J, Li Y, Wang ZP, et al. PROTACs: a novel strategy for cancer therapy. Semin Cancer Biol. 2020. 10.1016/j.semcancer.2020.02.006. [DOI] [PubMed]
- 33.Yang X, Xie S, Yang X, Cueva JC, Hou X, Tang Z, et al. Opportunities and challenges for antibodies against intracellular antigens. Theranostics. 2019;9:7792–7806. doi: 10.7150/thno.35486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlgren M, Simoff I, Keiser M, Oswald S, Artursson P. CRISPR-Cas9: a new addition to the drug metabolism and disposition tool box. Drug Metab Dispos. 2018;46:1776–1786. doi: 10.1124/dmd.118.082842. [DOI] [PubMed] [Google Scholar]
- 35.Lee K, Jang B, Lee Y-R, Suh E-Y, Yoo J-S, Lee M-J, et al. The cutting-edge technologies of siRNA delivery and their application in clinical trials. Arch Pharm Res. 2018;41:867–874. doi: 10.1007/s12272-018-1069-4. [DOI] [PubMed] [Google Scholar]
- 36.Sinha G. Antisense battles small molecule for slice of rare lipid disorder market. Nat Biotechnol. 2013;31:179–180. doi: 10.1038/nbt0313-179. [DOI] [PubMed] [Google Scholar]
- 37.Yamakawa K, Nakano-Narusawa Y, Hashimoto N, Yokohira M, Matsuda Y. Development and clinical trials of nucleic acid medicines for pancreatic cancer treatment. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed]
- 38.Maneiro MA, Forte N, Shchepinova MM, Kounde CS, Chudasama V, Baker JR, et al. Antibody-PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem Biol. 2020;15:1306–12. [DOI] [PMC free article] [PubMed]
- 39.Pillow TH, Adhikari P, Blake RA, Chen J, Del Rosario G, Deshmukh G, et al. Antibody conjugation of a chimeric BET degrader enables in vivo activity. ChemMedChem. 2020;15:17–25. doi: 10.1002/cmdc.201900497. [DOI] [PubMed] [Google Scholar]
- 40.Dragovich PS, Adhikari P, Blake RA, Blaquiere N, Chen J, Cheng Y-X, et al. Antibody-mediated delivery of chimeric protein degraders which target estrogen receptor alpha (ERα) Bioorg Med Chem Lett. 2020;30:126907. doi: 10.1016/j.bmcl.2019.126907. [DOI] [PubMed] [Google Scholar]
- 41.Kang CH, Lee DH, Lee CO, Du Ha J, Park CH, Hwang JY. Induced protein degradation of anaplastic lymphoma kinase (ALK) by proteolysis targeting chimera (PROTAC) Biochem Biophys Res Commun. 2018;505:542–547. doi: 10.1016/j.bbrc.2018.09.169. [DOI] [PubMed] [Google Scholar]
- 42.McCoull W, Cheung T, Anderson E, Barton P, Burgess J, Byth K, et al. Development of a novel B-cell lymphoma 6 (BCL6) PROTAC to provide insight into small molecule targeting of BCL6. ACS Chem Biol. 2018;13:3131–3141. doi: 10.1021/acschembio.8b00698. [DOI] [PubMed] [Google Scholar]
- 43.Winter GE, Mayer A, Buckley DL, Erb MA, Roderick JE, Vittori S, et al. BET bromodomain proteins function as master transcription elongation factors independent of CDK9 recruitment. Mol Cell. 2017;67:5–18.e19. doi: 10.1016/j.molcel.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dobrovolsky D, Wang ES, Morrow S, Leahy C, Faust T, Nowak RP, et al. Bruton tyrosine kinase degradation as a therapeutic strategy for cancer. Blood. 2019;133:952–961. doi: 10.1182/blood-2018-07-862953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papatzimas JW, Gorobets E, Maity R, Muniyat MI, MacCallum JL, Neri P, et al. From inhibition to degradation: targeting the antiapoptotic protein myeloid cell leukemia 1 (MCL1) J Med Chem. 2019;62:5522–5540. doi: 10.1021/acs.jmedchem.9b00455. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Yang J, Aguilar A, McEachern D, Przybranowski S, Liu L, et al. Discovery of MD-224 as a first-in-class, highly potent, and efficacious proteolysis targeting chimera murine double minute 2 degrader capable of achieving complete and durable tumor regression. J Med Chem. 2019;62:448–466. doi: 10.1021/acs.jmedchem.8b00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potjewyd F, Turner A-MW, Beri J, Rectenwald JM, Norris-Drouin JL, Cholensky SH, et al. Degradation of polycomb repressive complex 2 with an EED-targeted bivalent chemical degrader. Cell Chem Biol. 2020;27:47–56.e15. doi: 10.1016/j.chembiol.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farnaby W, Koegl M, Roy MJ, Whitworth C, Diers E, Trainor N, et al. BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Nat Chem Biol. 2019;15:672–680. doi: 10.1038/s41589-019-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holla VR, Elamin YY, Bailey AM, Johnson AM, Litzenburger BC, Khotskaya YB, et al. ALK: a tyrosine kinase target for cancer therapy. Cold Spring Harb Mol Case Stud. 2017;3:a001115. doi: 10.1101/mcs.a001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin JJ, Riely GJ, Shaw AT. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017;7:137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powell CE, Gao Y, Tan L, Donovan KA, Nowak RP, Loehr A, et al. Chemically induced degradation of anaplastic lymphoma kinase (ALK) J Med Chem. 2018;61:4249–4255. doi: 10.1021/acs.jmedchem.7b01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun N, Ren C, Kong Y, Zhong H, Chen J, Li Y, et al. Development of a Brigatinib degrader (SIAIS117) as a potential treatment for ALK positive cancer resistance. Eur J Med Chem. 2020;193:112190. doi: 10.1016/j.ejmech.2020.112190. [DOI] [PubMed] [Google Scholar]
- 53.Kong X, Pan P, Sun H, Xia H, Wang X, Li Y, et al. Drug discovery targeting anaplastic lymphoma kinase (ALK) J Med Chem. 2019;62:10927–10954. doi: 10.1021/acs.jmedchem.9b00446. [DOI] [PubMed] [Google Scholar]
- 54.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 55.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, He N, Guo Z, Niu C, Song T, Guo Y, et al. Proteolysis targeting chimeras for the selective degradation of mcl-1/Bcl-2 derived from nonselective target binding ligands. J Med Chem. 2019;62:8152–8163. doi: 10.1021/acs.jmedchem.9b00919. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 60.He Y, Zhang X, Chang J, Kim H-N, Zhang P, Wang Y, et al. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat Commun. 2020;11:1996. doi: 10.1038/s41467-020-15838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, He Y, Zhang P, Budamagunta V, Lv D, Thummuri D, et al. Discovery of IAP-recruiting BCL-XL PROTACs as potent degraders across multiple cancer cell lines. Eur J Med Chem. 2020;199:112397. doi: 10.1016/j.ejmech.2020.112397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X, Thummuri D, Liu X, Hu W, Zhang P, Khan S, et al. Discovery of PROTAC BCL-XL degraders as potent anticancer agents with low on-target platelet toxicity. Eur J Med Chem. 2020;192:112186. doi: 10.1016/j.ejmech.2020.112186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Thummuri D, He Y, Liu X, Zhang P, Zhou D, et al. Utilizing PROTAC technology to address the on-target platelet toxicity associated with inhibition of BCL-XL, 55. Chem Commun (Camb). 2019:14765–8. [DOI] [PMC free article] [PubMed]
- 64.Fletcher S. MCL-1 inhibitors - where are we now (2019)? Expert Opin Ther Pat. 2019;29:909–919. doi: 10.1080/13543776.2019.1672661. [DOI] [PubMed] [Google Scholar]
- 65.Hird AW, Tron AE. Recent advances in the development of mcl-1 inhibitors for cancer therapy. Pharmacol Ther. 2019;198:59–67. doi: 10.1016/j.pharmthera.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 66.Cardenas MG, Oswald E, Yu W, Xue F, MacKerell AD, Melnick AM. The expanding role of the BCL6 oncoprotein as a cancer therapeutic target. Clin Cancer Res. 2017;23:885–893. doi: 10.1158/1078-0432.CCR-16-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 68.Fukuda T, Yoshida T, Okada S, Hatano M, Miki T, Ishibashi K, et al. Disruption of the Bcl6 gene results in an impaired germinal center formation. J Exp Med. 1997;186:439–448. doi: 10.1084/jem.186.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, et al. The BCL-6 proto-oncogene controls germinal-Centre formation and Th2-type inflammation. Nat Genet. 1997;16:161–170. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- 70.Cardenas MG, Yu W, Beguelin W, Teater MR, Geng H, Goldstein RL, et al. Rationally designed BCL6 inhibitors target activated B cell diffuse large B cell lymphoma. J Clin Invest. 2016;126:3351–3362. doi: 10.1172/JCI85795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leeman-Neill RJ, Bhagat G. BCL6 as a therapeutic target for lymphoma. Expert Opin Ther Targets. 2018;22:143–152. doi: 10.1080/14728222.2018.1420782. [DOI] [PubMed] [Google Scholar]
- 72.Sameshima T, Yamamoto T, Sano O, Sogabe S, Igaki S, Sakamoto K, et al. Discovery of an irreversible and cell-active BCL6 inhibitor selectively targeting Cys53 located at the protein-protein interaction interface. Biochemistry. 2018;57:1369–1379. doi: 10.1021/acs.biochem.7b00732. [DOI] [PubMed] [Google Scholar]
- 73.Kerres N, Steurer S, Schlager S, Bader G, Berger H, Caligiuri M, et al. Chemically induced degradation of the oncogenic transcription factor BCL6. Cell Rep. 2017;20:2860–2875. doi: 10.1016/j.celrep.2017.08.081. [DOI] [PubMed] [Google Scholar]
- 74.Hantschel O, Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5:33–44. doi: 10.1038/nrm1280. [DOI] [PubMed] [Google Scholar]
- 75.An X, Tiwari AK, Sun Y, Ding P-R, Ashby CR, Chen Z-S. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk Res. 2010;34:1255–1268. doi: 10.1016/j.leukres.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 76.Ichim CV. Kinase-independent mechanisms of resistance of leukemia stem cells to tyrosine kinase inhibitors. Stem Cells Transl Med. 2014;3:405–415. doi: 10.5966/sctm.2012-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2012;119:1501–1510. doi: 10.1182/blood-2010-12-326843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai AC, Toure M, Hellerschmied D, Salami J, Jaime-Figueroa S, Ko E, et al. Modular PROTAC design for the degradation of oncogenic BCR-ABL. Angew Chem Int Ed Engl. 2016;55:807–810. doi: 10.1002/anie.201507634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Demizu Y, Shibata N, Hattori T, Ohoka N, Motoi H, Misawa T, et al. Development of BCR-ABL degradation inducers via the conjugation of an imatinib derivative and a cIAP1 ligand. Bioorg Med Chem Lett. 2016;26:4865–4869. doi: 10.1016/j.bmcl.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 80.Shibata N, Miyamoto N, Nagai K, Shimokawa K, Sameshima T, Ohoka N, et al. Development of protein degradation inducers of oncogenic BCR-ABL protein by conjugation of ABL kinase inhibitors and IAP ligands. Cancer Sci. 2017;108:1657–1666. doi: 10.1111/cas.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burslem GM, Schultz AR, Bondeson DP, Eide CA, Savage Stevens SL, Druker BJ, et al. Targeting BCR-ABL1 in chronic myeloid leukemia by PROTAC-mediated targeted protein degradation. Cancer Res. 2019;79:4744–4753. doi: 10.1158/0008-5472.CAN-19-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin Y-H, Lu M-C, Wang Y, Shan W-X, Wang X-Y, You Q-D, et al. Azo-PROTAC: novel light-controlled small-molecule tool for protein knockdown. J Med Chem. 2020;63:4644–4654. doi: 10.1021/acs.jmedchem.9b02058. [DOI] [PubMed] [Google Scholar]
- 83.Andrieu G, Belkina AC, Denis GV. Clinical trials for BET inhibitors run ahead of the science. Drug Discov Today Technol. 2016;19:45–50. doi: 10.1016/j.ddtec.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu J, Qian Y, Altieri M, Dong H, Wang J, Raina K, et al. Hijacking the E3 ubiquitin ligase cereblon to efficiently target BRD4. Chem Biol. 2015;22:755–763. doi: 10.1016/j.chembiol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raina K, Lu J, Qian Y, Altieri M, Gordon D, Rossi AMK, et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2016;113:7124–7129. doi: 10.1073/pnas.1521738113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zengerle M, Chan K-H, Ciulli A. Selective small molecule induced degradation of the BET bromodomain protein BRD4. ACS Chem Biol. 2015;10:1770–1777. doi: 10.1021/acschembio.5b00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gadd MS, Testa A, Lucas X, Chan K-H, Chen W, Lamont DJ, et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol. 2017;13:514–521. doi: 10.1038/nchembio.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chan K-H, Zengerle M, Testa A, Ciulli A. Impact of target warhead and linkage vector on inducing protein degradation: comparison of bromodomain and extra-terminal (BET) degraders derived from triazolodiazepine (JQ1) and tetrahydroquinoline (I-BET726) BET inhibitor scaffolds. J Med Chem. 2018;61:504–513. doi: 10.1021/acs.jmedchem.6b01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bai L, Zhou B, Yang C-Y, Ji J, McEachern D, Przybranowski S, et al. Targeted degradation of BET proteins in triple-negative breast cancer. Cancer Res. 2017;77:2476–2487. doi: 10.1158/0008-5472.CAN-16-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S, et al. Drug development. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science. 2015;348:1376–1381. doi: 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou B, Hu J, Xu F, Chen Z, Bai L, Fernandez-Salas E, et al. Discovery of a small-molecule degrader of bromodomain and extra-terminal (BET) proteins with picomolar cellular potencies and capable of achieving tumor regression. J Med Chem. 2018;61:462–481. doi: 10.1021/acs.jmedchem.6b01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qin C, Hu Y, Zhou B, Fernandez-Salas E, Yang C-Y, Liu L, et al. Discovery of QCA570 as an exceptionally potent and efficacious proteolysis targeting chimera (PROTAC) degrader of the bromodomain and extra-terminal (BET) proteins capable of inducing complete and durable tumor regression. J Med Chem. 2018;61:6685–6704. doi: 10.1021/acs.jmedchem.8b00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ohoka N, Ujikawa O, Shimokawa K, Sameshima T, Shibata N, Hattori T, et al. Different degradation mechanisms of inhibitor of apoptosis proteins (IAPs) by the specific and nongenetic IAP-dependent protein eraser (SNIPER) Chem Pharm Bull. 2019;67:203–209. doi: 10.1248/cpb.c18-00567. [DOI] [PubMed] [Google Scholar]
- 95.Hines J, Lartigue S, Dong H, Qian Y, Crews CM. MDM2-recruiting PROTAC offers superior, synergistic antiproliferative activity via simultaneous degradation of BRD4 and stabilization of p53. Cancer Res. 2019;79:251–262. doi: 10.1158/0008-5472.CAN-18-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ohoka N, Tsuji G, Shoda T, Fujisato T, Kurihara M, Demizu Y, et al. Development of small molecule chimeras that recruit AhR E3 ligase to target proteins. ACS Chem Biol. 2019;14:2822–2832. doi: 10.1021/acschembio.9b00704. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X, Crowley VM, Wucherpfennig TG, Dix MM, Cravatt BF. Electrophilic PROTACs that degrade nuclear proteins by engaging DCAF16. Nat Chem Biol. 2019;15:737–746. doi: 10.1038/s41589-019-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spradlin JN, Hu X, Ward CC, Brittain SM, Jones MD, Ou L, et al. Harnessing the anti-cancer natural product nimbolide for targeted protein degradation. Nat Chem Biol. 2019;15:747–755. doi: 10.1038/s41589-019-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ward CC, Kleinman JI, Brittain SM, Lee PS, Chung CYS, Kim K, et al. Covalent ligand screening uncovers a RNF4 E3 ligase recruiter for targeted protein degradation applications. ACS Chem Biol. 2019;14:2430–2440. doi: 10.1021/acschembio.8b01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shang E, Wang X, Wen D, Greenberg DA, Wolgemuth DJ. Double bromodomain-containing gene Brd2 is essential for embryonic development in mouse. Dev Dyn. 2009;238:908–917. doi: 10.1002/dvdy.21911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Houzelstein D, Bullock SL, Lynch DE, Grigorieva EF, Wilson VA, Beddington RSP. Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol Cell Biol. 2002;22:3794–3802. doi: 10.1128/MCB.22.11.3794-3802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang F, Liu H, Blanton WP, Belkina A, Lebrasseur NK, Denis GV. Brd2 disruption in mice causes severe obesity without type 2 diabetes. Biochem J. 2009;425:71–83. doi: 10.1042/BJ20090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nowak RP, DeAngelo SL, Buckley D, He Z, Donovan KA, An J, et al. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nat Chem Biol. 2018;14:706–714. doi: 10.1038/s41589-018-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mill CP, Fiskus W, DiNardo CD, Qian Y, Raina K, Rajapakshe K, et al. RUNX1-targeted therapy for AML expressing somatic or germline mutation in RUNX1. Blood. 2019;134:59–73. doi: 10.1182/blood.2018893982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Piya S, Mu H, Bhattacharya S, Lorenzi PL, Davis RE, McQueen T, et al. BETP degradation simultaneously targets acute myelogenous leukemia stem cells and the microenvironment. J Clin Invest. 2019;129:1878–1894. doi: 10.1172/JCI120654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saenz DT, Fiskus W, Qian Y, Manshouri T, Rajapakshe K, Raina K, et al. Novel BET protein proteolysis-targeting chimera exerts superior lethal activity than bromodomain inhibitor (BETi) against post-myeloproliferative neoplasm secondary (s) AML cells. Leukemia. 2017;31:1951–1961. doi: 10.1038/leu.2016.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saenz DT, Fiskus W, Mill CP, Perera D, Manshouri T, Lara BH, et al. Mechanistic basis and efficacy of targeting the β-catenin-TCF7L2-JMJD6-c-Myc axis to overcome resistance to BET inhibitors. Blood. 2020;135:1255–1269. doi: 10.1182/blood.2019002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X, Lee HC, Shirazi F, Baladandayuthapani V, Lin H, Kuiatse I, et al. Protein targeting chimeric molecules specific for bromodomain and extra-terminal motif family proteins are active against pre-clinical models of multiple myeloma. Leukemia. 2018;32:2224–2239. doi: 10.1038/s41375-018-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jain N, Hartert K, Tadros S, Fiskus W, Havranek O, Ma MCJ, et al. Targetable genetic alterations of TCF4 (E2-2) drive immunoglobulin expression in diffuse large B cell lymphoma. Sci Transl Med. 2019;11. [DOI] [PMC free article] [PubMed]
- 110.Kim SA, Go A, Jo S-H, Park SJ, Jeon YU, Kim JE, et al. A novel cereblon modulator for targeted protein degradation. Eur J Med Chem. 2019;166:65–74. doi: 10.1016/j.ejmech.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 111.Jiang F, Wei Q, Li H, Li H, Cui Y, Ma Y, et al. Discovery of novel small molecule induced selective degradation of the bromodomain and extra-terminal (BET) bromodomain protein BRD4 and BRD2 with cellular potencies. Bioorg Med Chem. 2020;28:115181. doi: 10.1016/j.bmc.2019.115181. [DOI] [PubMed] [Google Scholar]
- 112.Woyach JA, Bojnik E, Ruppert AS, Stefanovski MR, Goettl VM, Smucker KA, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL) Blood. 2014;123:1207–1213. doi: 10.1182/blood-2013-07-515361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Woyach JA, Furman RR, Liu T-M, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang H-T, Dobrovolsky D, Paulk J, Yang G, Weisberg EL, Doctor ZM, et al. A chemoproteomic approach to query the degradable kinome using a multi-kinase degrader. Cell Chem Biol. 2018;25:88–99.e6. doi: 10.1016/j.chembiol.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buhimschi AD, Armstrong HA, Toure M, Jaime-Figueroa S, Chen TL, Lehman AM, et al. Targeting the C481S ibrutinib-resistance mutation in Bruton’s tyrosine kinase using PROTAC-mediated degradation. Biochemistry. 2018;57:3564–3575. doi: 10.1021/acs.biochem.8b00391. [DOI] [PubMed] [Google Scholar]
- 117.Sun Y, Zhao X, Ding N, Gao H, Wu Y, Yang Y, et al. PROTAC-induced BTK degradation as a novel therapy for mutated BTK C481S induced ibrutinib-resistant B-cell malignancies. Cell Res. 2018;28:779–781. doi: 10.1038/s41422-018-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun Y, Ding N, Song Y, Yang Z, Liu W, Zhu J, et al. Degradation of Bruton’s tyrosine kinase mutants by PROTACs for potential treatment of ibrutinib-resistant non-Hodgkin lymphomas. Leukemia. 2019;33:2105–2110. doi: 10.1038/s41375-019-0440-x. [DOI] [PubMed] [Google Scholar]
- 119.Jaime-Figueroa S, Buhimschi AD, Toure M, Hines J, Crews CM. Design, synthesis and biological evaluation of proteolysis targeting chimeras (PROTACs) as a BTK degraders with improved pharmacokinetic properties. Bioorg Med Chem Lett. 2020;30:126877. doi: 10.1016/j.bmcl.2019.126877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gabizon R, Shraga A, Gehrtz P, Livnah E, Shorer Y, Gurwicz N, et al. Efficient targeted degradation via reversible and irreversible covalent PROTACs. J Am Chem Soc. 2020. 10.1021/jacs.9b13907. [DOI] [PMC free article] [PubMed]
- 121.Brand M, Jiang B, Bauer S, Donovan KA, Liang Y, Wang ES, et al. Homolog-selective degradation as a strategy to probe the function of CDK6 in AML. Cell Chem Biol. 2019;26:300–306.e9. doi: 10.1016/j.chembiol.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Topacio BR, Zatulovskiy E, Cristea S, Xie S, Tambo CS, Rubin SM, et al. Cyclin D-Cdk4,6 drives cell-cycle progression via the retinoblastoma protein’s C-terminal helix. Mol Cell. 2019;74:758–770.e4. doi: 10.1016/j.molcel.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Placke T, Faber K, Nonami A, Putwain SL, Salih HR, Heidel FH, et al. Requirement for CDK6 in MLL-rearranged acute myeloid leukemia. Blood. 2014;124:13–23. doi: 10.1182/blood-2014-02-558114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kollmann K, Heller G, Schneckenleithner C, Warsch W, Scheicher R, Ott RG, et al. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell. 2013;24:167–181. doi: 10.1016/j.ccr.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tigan A-S, Bellutti F, Kollmann K, Tebb G, Sexl V. CDK6-a review of the past and a glimpse into the future: from cell-cycle control to transcriptional regulation. Oncogene. 2016;35:3083–3091. doi: 10.1038/onc.2015.407. [DOI] [PubMed] [Google Scholar]
- 126.Su S, Yang Z, Gao H, Yang H, Zhu S, An Z, et al. Potent and preferential degradation of CDK6 via proteolysis targeting chimera degraders. J Med Chem. 2019;62:7575–7582. doi: 10.1021/acs.jmedchem.9b00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosnet O, Schiff C, Pébusque MJ, Marchetto S, Tonnelle C, Toiron Y, et al. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood. 1993;82:1110–1119. [PubMed] [Google Scholar]
- 128.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 129.Antar AI, Otrock ZK, Jabbour E, Mohty M, Bazarbachi A. FLT3 inhibitors in acute myeloid leukemia: ten frequently asked questions. Leukemia. 2020;34:682–696. doi: 10.1038/s41375-019-0694-3. [DOI] [PubMed] [Google Scholar]
- 130.McMahon CM, Ferng T, Canaani J, Wang ES, Morrissette JJD, Eastburn DJ, et al. Clonal selection with RAS pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discov. 2019;9:1050–1063. doi: 10.1158/2159-8290.CD-18-1453. [DOI] [PubMed] [Google Scholar]
- 131.Grunwald MR, Levis MJ. FLT3 inhibitors for acute myeloid leukemia: a review of their efficacy and mechanisms of resistance. Int J Hematol. 2013;97:683–694. doi: 10.1007/s12185-013-1334-8. [DOI] [PubMed] [Google Scholar]
- 132.Yang X-J, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guha M. HDAC inhibitors still need a home run, despite recent approval. Nat Rev Drug Discov. 2015;14:225–226. doi: 10.1038/nrd4583. [DOI] [PubMed] [Google Scholar]
- 134.Gryder BE, Sodji QH, Oyelere AK. Targeted cancer therapy: giving histone deacetylase inhibitors all they need to succeed. Future Med Chem. 2012;4:505–524. doi: 10.4155/fmc.12.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang K, Wu H, Zhang Z, Leisten ED, Nie X, Liu B, et al. Development of selective histone deacetylase 6 (HDAC6) degraders recruiting Von Hippel-Lindau (VHL) E3 ubiquitin ligase. ACS Med Chem Lett. 2020;11:575–581. doi: 10.1021/acsmedchemlett.0c00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang K, Song Y, Xie H, Wu H, Wu Y-T, Leisten ED, et al. Development of the first small molecule histone deacetylase 6 (HDAC6) degraders. Bioorg Med Chem Lett. 2018;28:2493–2497. doi: 10.1016/j.bmcl.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 137.Yang H, Lv W, He M, Deng H, Li H, Wu W, et al. Plasticity in designing PROTACs for selective and potent degradation of HDAC6. Chem Commun (Camb) 2019;55:14848–14851. doi: 10.1039/c9cc08509b. [DOI] [PubMed] [Google Scholar]
- 138.An Z, Lv W, Su S, Wu W, Rao Y. Developing potent PROTACs tools for selective degradation of HDAC6 protein. Protein Cell. 2019;10:606–609. doi: 10.1007/s13238-018-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boyault C, Sadoul K, Pabion M, Khochbin S. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007;26:5468–5476. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- 140.Smalley JP, Adams GE, Millard CJ, Song Y, Norris JKS, Schwabe JWR, et al. PROTAC-mediated degradation of class I histone deacetylase enzymes in corepressor complexes, Chem Commun (Camb). 2020;56:4476–9. [DOI] [PMC free article] [PubMed]
- 141.Kruse J-P, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jones SN, Hancock AR, Vogel H, Donehower LA, Bradley A. Overexpression of Mdm2 in mice reveals a p53-independent role for Mdm2 in tumorigenesis. Proc Natl Acad Sci U S A. 1998;95:15608–15612. doi: 10.1073/pnas.95.26.15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Teoh G, Urashima M, Ogata A, Chauhan D, DeCaprio JA, Treon SP, et al. MDM2 protein overexpression promotes proliferation and survival of multiple myeloma cells. Blood. 1997;90:1982–1992. [PubMed] [Google Scholar]
- 144.Tisato V, Voltan R, Gonelli A, Secchiero P, Zauli G. MDM2/X inhibitors under clinical evaluation: perspectives for the management of hematological malignancies and pediatric cancer. J Hematol Oncol. 2017;10:133. doi: 10.1186/s13045-017-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mu X, Bai L, Xu Y, Wang J, Lu H. Protein targeting chimeric molecules specific for dual bromodomain 4 (BRD4) and polo-like kinase 1 (PLK1) proteins in acute myeloid leukemia cells. Biochem Biophys Res Commun. 2020;521:833–839. doi: 10.1016/j.bbrc.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 146.Wang S, Song Y, Wang Y, Gao Y, Yu S, Zhao Q, et al. Design and synthesis of novel bispecific molecules for inducing BRD4 protein degradation. Chem Res Chin Univ. 2018;34:67–74. [Google Scholar]
- 147.Martin-Perez D, Piris MA, Sanchez-Beato M. Polycomb proteins in hematologic malignancies. Blood. 2010;116:5465–5475. doi: 10.1182/blood-2010-05-267096. [DOI] [PubMed] [Google Scholar]
- 148.Takamatsu-Ichihara E, Kitabayashi I. The roles of Polycomb group proteins in hematopoietic stem cells and hematological malignancies. Int J Hematol. 2016;103:634–642. doi: 10.1007/s12185-016-2011-5. [DOI] [PubMed] [Google Scholar]
- 149.Iwama A. Polycomb repressive complexes in hematological malignancies. Blood. 2017;130:23–29. doi: 10.1182/blood-2017-02-739490. [DOI] [PubMed] [Google Scholar]
- 150.Herviou L, Cavalli G, Cartron G, Klein B, Moreaux J. EZH2 in normal hematopoiesis and hematological malignancies. Oncotarget. 2016;7:2284–2296. doi: 10.18632/oncotarget.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 152.Xie H, Xu J, Hsu JH, Nguyen M, Fujiwara Y, Peng C, et al. Polycomb repressive complex 2 regulates normal hematopoietic stem cell function in a developmental-stage-specific manner. Cell Stem Cell. 2014;14:68–80. doi: 10.1016/j.stem.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Majewski IJ, Ritchie ME, Phipson B, Corbin J, Pakusch M, Ebert A, et al. Opposing roles of polycomb repressive complexes in hematopoietic stem and progenitor cells. Blood. 2010;116:731–739. doi: 10.1182/blood-2009-12-260760. [DOI] [PubMed] [Google Scholar]
- 154.Simon C, Chagraoui J, Krosl J, Gendron P, Wilhelm B, Lemieux S, et al. A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia. Genes Dev. 2012;26:651–656. doi: 10.1101/gad.186411.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481:157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–1063. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lue JK, Amengual JE. Emerging EZH2 inhibitors and their application in lymphoma. Curr Hematol Malig Rep. 2018;13:369–382. doi: 10.1007/s11899-018-0466-6. [DOI] [PubMed] [Google Scholar]
- 158.Ueda T, Sanada M, Matsui H, Yamasaki N, Honda Z-I, Shih L-Y, et al. EED mutants impair polycomb repressive complex 2 in myelodysplastic syndrome and related neoplasms. Leukemia. 2012;26:2557–2560. doi: 10.1038/leu.2012.146. [DOI] [PubMed] [Google Scholar]
- 159.Qi W, Zhao K, Gu J, Huang Y, Wang Y, Zhang H, et al. An allosteric PRC2 inhibitor targeting the H3K27me3 binding pocket of EED. Nat Chem Biol. 2017;13:381–388. doi: 10.1038/nchembio.2304. [DOI] [PubMed] [Google Scholar]
- 160.Gibaja V, Shen F, Harari J, Korn J, Ruddy D, Saenz-Vash V, et al. Development of secondary mutations in wild-type and mutant EZH2 alleles cooperates to confer resistance to EZH2 inhibitors. Oncogene. 2016;35:558–566. doi: 10.1038/onc.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hsu JH-R, Rasmusson T, Robinson J, Pachl F, Read J, Kawatkar S, et al. EED-targeted PROTACs degrade EED, EZH2, and SUZ12 in the PRC2 complex. Cell Chem Biol. 2020;27:41–46.e17. doi: 10.1016/j.chembiol.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 162.Ma A, Stratikopoulos E, Park K-S, Wei J, Martin TC, Yang X, et al. Discovery of a first-in-class EZH2 selective degrader. Nat Chem Biol. 2020;16:214–222. doi: 10.1038/s41589-019-0421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.St Pierre R, Kadoch C. Mammalian SWI/SNF complexes in cancer: emerging therapeutic opportunities. Curr Opin Genet Dev. 2017;42:56–67. doi: 10.1016/j.gde.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kadoch C, Hargreaves DC, Hodges C, Elias L, Ho L, Ranish J, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet. 2013;45:592–601. doi: 10.1038/ng.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shi J, Whyte WA, Zepeda-Mendoza CJ, Milazzo JP, Shen C, Roe J-S, et al. Role of SWI/SNF in acute leukemia maintenance and enhancer-mediated Myc regulation. Genes Dev. 2013;27:2648–2662. doi: 10.1101/gad.232710.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hoffman GR, Rahal R, Buxton F, Xiang K, McAllister G, Frias E, et al. Functional epigenetics approach identifies BRM/SMARCA2 as a critical synthetic lethal target in BRG1-deficient cancers. Proc Natl Acad Sci U S A. 2014;111:3128–3133. doi: 10.1073/pnas.1316793111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gerstenberger BS, Trzupek JD, Tallant C, Fedorov O, Filippakopoulos P, Brennan PE, et al. Identification of a chemical probe for family VIII Bromodomains through optimization of a fragment hit. J Med Chem. 2016;59:4800–4811. doi: 10.1021/acs.jmedchem.6b00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sutherell CL, Tallant C, Monteiro OP, Yapp C, Fuchs JE, Fedorov O, et al. Identification and development of 2,3-dihydropyrrolo[1,2-a]quinazolin-5(1H)-one inhibitors targeting bromodomains within the switch/sucrose nonfermenting complex. J Med Chem. 2016;59:5095–5101. doi: 10.1021/acs.jmedchem.5b01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vangamudi B, Paul TA, Shah PK, Kost-Alimova M, Nottebaum L, Shi X, et al. The SMARCA2/4 ATPase domain surpasses the bromodomain as a drug target in SWI/SNF-mutant cancers: insights from cDNA rescue and PFI-3 inhibitor studies. Cancer Res. 2015;75:3865–3878. doi: 10.1158/0008-5472.CAN-14-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Papillon JPN, Nakajima K, Adair CD, Hempel J, Jouk AO, Karki RG, et al. Discovery of orally active inhibitors of brahma homolog (BRM)/SMARCA2 ATPase activity for the treatment of brahma related gene 1 (BRG1)/SMARCA4-mutant cancers. J Med Chem. 2018;61:10155–10172. doi: 10.1021/acs.jmedchem.8b01318. [DOI] [PubMed] [Google Scholar]
- 171.Darnell JE. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 172.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 173.Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 175.Yang J, Stark GR. Roles of unphosphorylated STATs in signaling. Cell Res. 2008;18:443–451. doi: 10.1038/cr.2008.41. [DOI] [PubMed] [Google Scholar]
- 176.Zhou H, Bai L, Xu R, Zhao Y, Chen J, McEachern D, et al. Structure-based discovery of SD-36 as a potent, selective, and efficacious PROTAC degrader of STAT3 protein. J Med Chem. 2019;62:11280–11300. doi: 10.1021/acs.jmedchem.9b01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schiedel M, Herp D, Hammelmann S, Swyter S, Lehotzky A, Robaa D, et al. Chemically induced degradation of sirtuin 2 (Sirt2) by a proteolysis targeting chimera (PROTAC) based on sirtuin rearranging ligands (SirReals) J Med Chem. 2018;61:482–491. doi: 10.1021/acs.jmedchem.6b01872. [DOI] [PubMed] [Google Scholar]
- 178.Nagy Z, Tora L. Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene. 2007;26:5341–5357. doi: 10.1038/sj.onc.1210604. [DOI] [PubMed] [Google Scholar]
- 179.Bassi ZI, Fillmore MC, Miah AH, Chapman TD, Maller C, Roberts EJ, et al. Modulating PCAF/GCN5 immune cell function through a PROTAC approach. ACS Chem Biol. 2018;13:2862–2867. doi: 10.1021/acschembio.8b00705. [DOI] [PubMed] [Google Scholar]
- 180.Remillard D, Buckley DL, Paulk J, Brien GL, Sonnett M, Seo H-S, et al. Degradation of the BAF complex factor BRD9 by heterobifunctional ligands. Angew Chem Int Ed Engl. 2017;56:5738–5743. doi: 10.1002/anie.201611281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zoppi V, Hughes SJ, Maniaci C, Testa A, Gmaschitz T, Wieshofer C, et al. Iterative design and optimization of initially inactive proteolysis targeting chimeras (PROTACs) identify VZ185 as a potent, fast, and selective von Hippel-Lindau (VHL) based dual degrader probe of BRD9 and BRD7. J Med Chem. 2019;62:699–726. doi: 10.1021/acs.jmedchem.8b01413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 183.Qin J, Jiang Z, Qian Y, Casanova J-L, Li X. IRAK4 kinase activity is redundant for interleukin-1 (IL-1) receptor-associated kinase phosphorylation and IL-1 responsiveness. J Biol Chem. 2004;279:26748–26753. doi: 10.1074/jbc.M400785200. [DOI] [PubMed] [Google Scholar]
- 184.Nunes J, McGonagle GA, Eden J, Kiritharan G, Touzet M, Lewell X, et al. Targeting IRAK4 for degradation with PROTACs. ACS Med Chem Lett. 2019;10:1081–1085. doi: 10.1021/acsmedchemlett.9b00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gechijian LN, Buckley DL, Lawlor MA, Reyes JM, Paulk J, Ott CJ, et al. Functional TRIM24 degrader via conjugation of ineffectual bromodomain and VHL ligands. Nat Chem Biol. 2018;14:405–412. doi: 10.1038/s41589-018-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mares A, Miah AH, Smith IED, Rackham M, Thawani AR, Cryan J, et al. Extended pharmacodynamic responses observed upon PROTAC-mediated degradation of RIPK2. Commun Biol. 2020;3:140. doi: 10.1038/s42003-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shah RR, Redmond JM, Mihut A, Menon M, Evans JP, Murphy JA, et al. Hi-JAK-ing the ubiquitin system: the design and physicochemical optimisation of JAK PROTACs. Bioorg Med Chem. 2020;28:115326. doi: 10.1016/j.bmc.2020.115326. [DOI] [PubMed] [Google Scholar]
- 188.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 190.Broséus J, Park J-H, Carillo S, Hermouet S, Girodon F. Presence of calreticulin mutations in JAK2-negative polycythemia vera. Blood. 2014;124:3964–3966. doi: 10.1182/blood-2014-06-583161. [DOI] [PubMed] [Google Scholar]
- 191.Marty C, Pecquet C, Nivarthi H, El-Khoury M, Chachoua I, Tulliez M, et al. Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood. 2016;127:1317–1324. doi: 10.1182/blood-2015-11-679571. [DOI] [PubMed] [Google Scholar]
- 192.Chachoua I, Pecquet C, El-Khoury M, Nivarthi H, Albu R-I, Marty C, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood. 2016;127:1325–1335. doi: 10.1182/blood-2015-11-681932. [DOI] [PubMed] [Google Scholar]
- 193.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 194.Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 195.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 196.Bolli N, Nicoletti I, De Marco MF, Bigerna B, Pucciarini A, Mannucci R, et al. Born to be exported: COOH-terminal nuclear export signals of different strength ensure cytoplasmic accumulation of nucleophosmin leukemic mutants. Cancer Res. 2007;67:6230–6237. doi: 10.1158/0008-5472.CAN-07-0273. [DOI] [PubMed] [Google Scholar]
- 197.Alcalay M, Tiacci E, Bergomas R, Bigerna B, Venturini E, Minardi SP, et al. Acute myeloid leukemia bearing cytoplasmic nucleophosmin (NPMc+ AML) shows a distinct gene expression profile characterized by up-regulation of genes involved in stem-cell maintenance. Blood. 2005;106:899–902. doi: 10.1182/blood-2005-02-0560. [DOI] [PubMed] [Google Scholar]
- 198.Etchin J, Montero J, Berezovskaya A, Le BT, Kentsis A, Christie AL, et al. Activity of a selective inhibitor of nuclear export, selinexor (KPT-330), against AML-initiating cells engrafted into immunosuppressed NSG mice. Leukemia. 2016;30:190–199. doi: 10.1038/leu.2015.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nabbouh AI, Hleihel RS, Saliba JL, Karam MM, Hamie MH, Wu H-CJM, et al. Imidazoquinoxaline derivative EAPB0503: a promising drug targeting mutant nucleophosmin 1 in acute myeloid leukemia. Cancer. 2017;123:1662–1673. doi: 10.1002/cncr.30515. [DOI] [PubMed] [Google Scholar]
- 200.Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer. 2007;7:233–245. doi: 10.1038/nrc2091. [DOI] [PubMed] [Google Scholar]
- 201.Fröhling S, Döhner H. Chromosomal abnormalities in cancer. N Engl J Med. 2008;359:722–734. doi: 10.1056/NEJMra0803109. [DOI] [PubMed] [Google Scholar]
- 202.Rowley JD. Chromosomal translocations: revisited yet again. Blood. 2008;112:2183–2189. doi: 10.1182/blood-2008-04-097931. [DOI] [PubMed] [Google Scholar]
- 203.Strelow JM. A perspective on the kinetics of covalent and irreversible inhibition. SLAS Discov. 2017;22:3–20. doi: 10.1177/1087057116671509. [DOI] [PubMed] [Google Scholar]
- 204.Bondeson DP, Smith BE, Burslem GM, Buhimschi AD, Hines J, Jaime-Figueroa S, et al. Lessons in PROTAC design from selective degradation with a promiscuous warhead. Cell Chem Biol. 2018;25:78–87.e5. doi: 10.1016/j.chembiol.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Matyskiela ME, Lu G, Ito T, Pagarigan B, Lu C-C, Miller K, et al. A novel cereblon modulator recruits GSPT1 to the CRL4(CRBN) ubiquitin ligase. Nature. 2016;535:252–257. doi: 10.1038/nature18611. [DOI] [PubMed] [Google Scholar]
- 206.Moreau K, Coen M, Zhang AX, Pachl F, Castaldi MP, Dahl G, et al. Proteolysis-targeting chimeras in drug development: a safety perspective. Br J Pharmacol. 2020;177:1709–1718. doi: 10.1111/bph.15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Donovan KA, An J, Nowak RP, Yuan JC, Fink EC, Berry BC, et al. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane radial ray syndrome. Elife. 2018;7. [DOI] [PMC free article] [PubMed]
- 208.Sievers QL, Petzold G, Bunker RD, Renneville A, Słabicki M, Liddicoat BJ, et al. Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science. 2018;362. [DOI] [PMC free article] [PubMed]
- 209.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 210.Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000;22:442–451. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 211.Khan S, He Y, Zhang X, Yuan Y, Pu S, Kong Q, et al. Proteolysis targeting chimeras (PROTACs) as emerging anticancer therapeutics. Oncogene. 2020;39:4909–24. [DOI] [PMC free article] [PubMed]
- 212.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, et al. Comprehensive integration of single-cell data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li W, Bengtson MH, Ulbrich A, Matsuda A, Reddy VA, Orth A, et al. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS One. 2008;3:e1487. doi: 10.1371/journal.pone.0001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847–2849. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 215.Xu J, Li L, Yu G, Ying W, Gao Q, Zhang W, et al. The neddylation-cullin 2-RBX1 E3 ligase axis targets tumor suppressor RhoB for degradation in liver cancer. Mol Cell Proteomics. 2015;14:499–509. doi: 10.1074/mcp.M114.045211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lee D, Takayama S, Goldberg AL. ZFAND5/ZNF216 is an activator of the 26S proteasome that stimulates overall protein degradation. Proc Natl Acad Sci U S A. 2018;115:E9550–E9559. doi: 10.1073/pnas.1809934115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim JJ, Lee SB, Jang J, Yi S-Y, Kim S-H, Han S-A, et al. WSB1 promotes tumor metastasis by inducing pVHL degradation. Genes Dev. 2015;29:2244–2257. doi: 10.1101/gad.268128.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shoji S, Muto Y, Ikeda M, He F, Tsuda K, Ohsawa N, et al. The zinc-binding region (ZBR) fragment of Emi2 can inhibit APC/C by targeting its association with the coactivator Cdc20 and UBE2C-mediated ubiquitylation. FEBS Open Bio. 2014;4:689–703. doi: 10.1016/j.fob.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Orr SJ, Morgan NM, Buick RJ, Boyd CR, Elliott J, Burrows JF, et al. SOCS3 targets Siglec 7 for proteasomal degradation and blocks Siglec 7-mediated responses. J Biol Chem. 2007;282:3418–3422. doi: 10.1074/jbc.C600216200. [DOI] [PubMed] [Google Scholar]
- 220.Pedersen SM. Chan W, Jattani RP, Mackie deMauri S, Pomerantz JL. Negative regulation of CARD11 signaling and lymphoma cell survival by the E3 ubiquitin ligase RNF181. Mol Cell Biol. 2015;36:794–808. doi: 10.1128/MCB.00876-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mica L, Härter L, Trentz O, Keel M. Endotoxin reduces CD95-induced neutrophil apoptosis by cIAP-2-mediated caspase-3 degradation. J Am Coll Surg. 2004;199:595–602. doi: 10.1016/j.jamcollsurg.2004.05.272. [DOI] [PubMed] [Google Scholar]
- 222.Okuhira K, Demizu Y, Hattori T, Ohoka N, Shibata N, Nishimaki-Mogami T, et al. Development of hybrid small molecules that induce degradation of estrogen receptor-alpha and necrotic cell death in breast cancer cells. Cancer Sci. 2013;104:1492–1498. doi: 10.1111/cas.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ohoka N, Morita Y, Nagai K, Shimokawa K, Ujikawa O, Fujimori I, et al. Derivatization of inhibitor of apoptosis protein (IAP) ligands yields improved inducers of estrogen receptor α degradation. J Biol Chem. 2018;293:6776–6790. doi: 10.1074/jbc.RA117.001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shibata N, Nagai K, Morita Y, Ujikawa O, Ohoka N, Hattori T, et al. Development of protein degradation inducers of androgen receptor by conjugation of androgen receptor ligands and inhibitor of apoptosis protein ligands. J Med Chem. 2018;61:543–575. doi: 10.1021/acs.jmedchem.7b00168. [DOI] [PubMed] [Google Scholar]
- 225.Vamos M, Welsh K, Finlay D, Lee PS, Mace PD, Snipas SJ, et al. Expedient synthesis of highly potent antagonists of inhibitor of apoptosis proteins (IAPs) with unique selectivity for ML-IAP. ACS Chem Biol. 2013;8:725–732. doi: 10.1021/cb3005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zobel K, Wang L, Varfolomeev E, Franklin MC, Elliott LO, Wallweber HJA, et al. Design, synthesis, and biological activity of a potent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPs. ACS Chem Biol. 2006;1:525–533. doi: 10.1021/cb600276q. [DOI] [PubMed] [Google Scholar]
- 227.Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6:1796–1803. [PubMed] [Google Scholar]
- 228.de Graaf AO, van Krieken JH, Tönnissen E, Wissink W, van de Locht L, Overes I, et al. Expression of C-IAP1, C-IAP2 and SURVIVIN discriminates different types of lymphoid malignancies. Br J Haematol. 2005;130:852–859. doi: 10.1111/j.1365-2141.2005.05690.x. [DOI] [PubMed] [Google Scholar]
- 229.Rosebeck S, Rehman AO, Apel IJ, Kohrt D, Appert A, O’Donnell MA, et al. The API2-MALT1 fusion exploits TNFR pathway-associated RIP1 ubiquitination to promote oncogenic NF-κB signaling. Oncogene. 2014;33:2520–2530. doi: 10.1038/onc.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fulda S. Inhibitor of apoptosis (IAP) proteins in hematological malignancies: molecular mechanisms and therapeutic opportunities. Leukemia. 2014;28:1414–1422. doi: 10.1038/leu.2014.56. [DOI] [PubMed] [Google Scholar]
- 231.Itoh Y, Ishikawa M, Kitaguchi R, Sato S, Naito M, Hashimoto Y. Development of target protein-selective degradation inducer for protein knockdown. Bioorg Med Chem. 2011;19:3229–3241. doi: 10.1016/j.bmc.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 232.Carvalho P, Goder V, Rapoport TA. Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell. 2006;126:361–373. doi: 10.1016/j.cell.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 233.Denic V, Quan EM, Weissman JS. A luminal surveillance complex that selects misfolded glycoproteins for ER-associated degradation. Cell. 2006;126:349–359. doi: 10.1016/j.cell.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 234.Ji Y, Kim H, Yang L, Sha H, Roman CA, Long Q, et al. The Sel1L-Hrd1 endoplasmic reticulum-associated degradation complex manages a key checkpoint in B cell development. Cell Rep. 2016;16:2630–2640. doi: 10.1016/j.celrep.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kong S, Yang Y, Xu Y, Wang Y, Zhang Y, Melo-Cardenas J, et al. Endoplasmic reticulum-resident E3 ubiquitin ligase Hrd1 controls B-cell immunity through degradation of the death receptor CD95/Fas. Proc Natl Acad Sci U S A. 2016;113:10394–10399. doi: 10.1073/pnas.1606742113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yamasaki S, Yagishita N, Sasaki T, Nakazawa M, Kato Y, Yamadera T, et al. Cytoplasmic destruction of p53 by the endoplasmic reticulum-resident ubiquitin ligase “Synoviolin.”. EMBO J. 2007;26:113–122. doi: 10.1038/sj.emboj.7601490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang W-F, Yan L, Liu Z, Liu L-X, Lin J, Liu Z-Y, et al. HSP70-Hrd1 axis precludes the oncorepressor potential of N-terminal misfolded blimp-1s in lymphoma cells. Nat Commun. 2017;8:363. doi: 10.1038/s41467-017-00476-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Harnoss JM, Le Thomas A, Shemorry A, Marsters SA, Lawrence DA, Lu M, et al. Disruption of IRE1α through its kinase domain attenuates multiple myeloma. Proc Natl Acad Sci U S A. 2019;116:16420–16429. doi: 10.1073/pnas.1906999116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Clifford SC, Prowse AH, Affara NA, Buys CH, Maher ER. Inactivation of the von Hippel-Lindau (VHL) tumour suppressor gene and allelic losses at chromosome arm 3p in primary renal cell carcinoma: evidence for a VHL-independent pathway in clear cell renal tumourigenesis. Genes Chromosomes Cancer. 1998;22:200–209. doi: 10.1002/(sici)1098-2264(199807)22:3<200::aid-gcc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 240.Clifford SC, Walsh S, Hewson K, Green EK, Brinke A, Green PM, et al. Genomic organization and chromosomal localization of the human CUL2 gene and the role of von Hippel-Lindau tumor suppressor-binding protein (CUL2 and VBP1) mutation and loss in renal-cell carcinoma development. Genes Chromosomes Cancer. 1999;26:20–28. [PubMed] [Google Scholar]
- 241.Zhang L, Riley-Gillis B, Vijay P, Shen Y. Acquired resistance to BET-PROTACs (proteolysis-targeting chimeras) caused by genomic alterations in core components of E3 ligase complexes. Mol Cancer Ther. 2019;18:1302–1311. doi: 10.1158/1535-7163.MCT-18-1129. [DOI] [PubMed] [Google Scholar]
- 242.Costales MG, Aikawa H, Li Y, Childs-Disney JL, Abegg D, Hoch DG, et al. Small-molecule targeted recruitment of a nuclease to cleave an oncogenic RNA in a mouse model of metastatic cancer. Proc Natl Acad Sci U S A. 2020;117:2406–2411. doi: 10.1073/pnas.1914286117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Costales MG, Childs-Disney JL, Haniff HS, Disney MD. How We Think about Targeting RNA with Small Molecules. J Med Chem. 2020. 10.1021/acs.jmedchem.9b01927. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Profiling E3 ligase expression at single cell level in different tissues. The heatmap shows the single-cell expression levels of 574 E3 ligases across all cell types and tissues in human adults from the Human Cell Landscape (HCL) data. In the heatmap, each row represents a cell type, and each column represents an E3 ligase. The color legend was shown on the right, which was determined by averaging the expression values of all cells within a tissue-specific cell type. These E3 ligases and the different cell types were clustered by the hierarchical clustering algorithm, and the resulting dendrograms were shown on bottom and right, respectively.
Additional file 2. List of 574 E3 ligases expressed in different cell types from all tissues.
Additional file 3. List of 574 E3 ligases expressed in different types of hematopoietic cells in the blood, spleen, and bone marrow.
Data Availability Statement
All data and materials supporting the conclusion of this study have been included within the article and the additional files.