Abstract
Across mammals, increased body size is positively associated with lifespan. However, within species, this relationship is inverted. This is well illustrated in dogs (Canis familiaris), where larger dogs exhibit accelerated life trajectories: growing faster and dying younger than smaller dogs. Similarly, some age-associated traits (e.g., growth rate and physiological pace of aging) exhibit accelerated trajectories in larger breeds. Yet, it is unknown whether cognitive performance also demonstrates an accelerated life course trajectory in larger dogs. Here, we measured cognitive development and aging in a cross-sectional study of over 4000 dogs from 66 breeds using nine memory and decision-making tasks performed by citizen scientists as part of the Dognition project. Specifically, we tested whether cognitive traits follow a compressed (accelerated) trajectory in larger dogs, or the same trajectory for all breeds, which would result in limited cognitive decline in larger breeds. We found that all breeds, regardless of size or lifespan, tended to follow the same quadratic trajectory of cognitive aging—with a period of cognitive development in early life and decline in later life. Taken together, our results suggest that cognitive performance follows similar age-related trajectories across dog breeds, despite remarkable variation in developmental rates and lifespan.
Keywords: Cognitive evolution, Cognitive aging, Breed differences, Citizen science, Executive function
Introduction
Across mammals, larger species tend to live longer than smaller species (Healy et al. 2014). Yet, within species, this pattern is reversed (Metcalfe and Monaghan 2003; Austad 2010; Bartke 2017). This pattern is well documented in domestic dogs (Galis et al. 2007; Kraus et al. 2013; Fan et al. 2016) where larger dog breeds (e.g., Bernese Mountain Dog, mean lifespan = 7 years) have an expected lifespan that is approximately half that of smaller breeds (e.g., Chihuahua, mean lifespan = 13 years; Jones et al. 2008). While large breeds take longer to mature than small breeds, they weigh disproportionately more, and, therefore, have faster growth rates and an accelerated pace of physiological aging (e.g., cellular damage; Fick et al. 2012; Kraus et al. 2013; Fan et al. 2016). Domestic dogs have been under strong artificial selection for at least 15,000 years (vonHoldt et al. 2010), which has driven extensive diversity in physical and life history traits (i.e., size, growth rate, and lifespan). The large variation in these life history traits, in particular, has made dogs an invaluable model species for studying the underpinnings of age-related changes in health (Hoffman et al. 2018).
Domestic dogs also provide a powerful model in which to explore intraspecific patterns of cognitive aging. In humans and other animals, including dogs, cognitive abilities, such as learning and memory, change throughout aging (Craik and Bialystok 2006; Bizon and Woods 2009; Harada et al. 2013; Chapagain et al. 2018). However, for some cognitive processes, dogs may even provide a better model for human cognition than rodents and nonhuman primates, potentially due to convergent evolution between humans and dogs (Miklósi et al. 2004; Hare and Tomasello 2005; Hare 2017; MacLean et al. 2017). For millennia, humans have selected dogs for both behavioral (i.e., herding and hunting) and physical traits (Ostrander et al. 2017; Parker et al. 2017), contributing to the extensive diversity seen across modern breeds. While the association between domestic dog cognition and other life history traits (e.g., age) remains largely unexplored, there is evidence that absolute brain size is associated with breed differences in executive function (Horschler et al. 2019)—a cognitive domain responsible for inhibitory control, mental flexibility, and decision-making (Alvarez and Emory 2006; Jurado and Rosselli 2007).
Executive functions typically include inhibition (i.e., self-control and selective memory), working memory, and cognitive flexibility (Diamond 2013). In humans, executive function, learning, and long-term memory have largely been found to increase in early life and decrease in late life (Craik and Bialystok 2006; Harada et al. 2013; but see Verhaeghen 2011). Other cognitive abilities (e.g., vocabulary and general knowledge) increase steadily throughout life (i.e., linearly) or increase rapidly and then plateau (i.e., resembling a positive logarithmic curve; Harada et al. 2013). Similar to humans, domestic dogs experience changes to critical cognitive functions across life (Chapagain et al. 2018). Recent studies from laboratory and pet dogs have demonstrated that learning, memory, and cognitive functions under executive control decrease in older dogs (Adams et al. 2000; Tapp et al. 2003; Szabo et al. 2016; Wallis et al. 2016). Older dogs also show greater variability in the extent of cognitive decline with age (Adams et al. 2000). One source of this variability may be lifelong behavioral training, which has been associated with greater sustained and selective attention in older dogs (Chapagain et al. 2017). Although domestic dogs exhibit age-related cognitive changes, we still know very little about how cognition changes with age, in large part due to sample size: collecting data from enough very young and very old dogs has been challenging (Szabó et al. 2016). To date, most studies of dog cognitive aging have focused on one breed or a small number of breeds, which, due to the limited variability in life history within individual breeds, has limited our ability to examine associations between cognitive aging and physiological pace of aging. Consequently, the extent to which cognitive changes throughout aging are associated with life history traits that covary with physiological pace of aging (as measured by average breed lifespan) remains unknown.
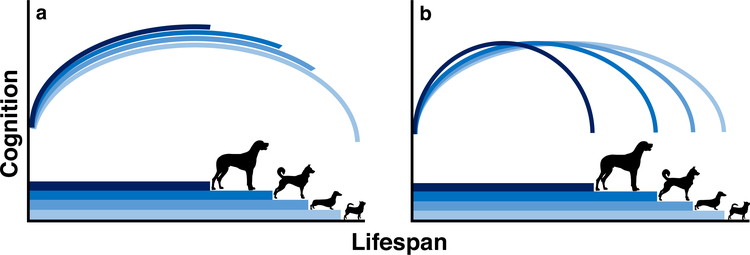
Here, we investigated the associations between lifespan and cognitive traits in dogs, to test the hypothesis that animals with faster life histories also exhibit earlier onset of and/or more rapid cognitive decline. We addressed two questions using a cross-sectional dataset of more than 4000 dogs from 66 breeds collected from participants of the Dognition project, a citizen science initiative in which owners perform simple cognitive tests with their dogs at home (Stewart et al. 2015). First, we tested how cognition changes across the lifespan of domestic dogs. To date, many studies of dog cognition have modeled cognition as a linear process throughout the lifespan, yet data from humans, apes, and several within-breed studies of domestic dogs suggest the likelihood of non-linear changes across development and senescence (Craik and Bialystok 2006; Harada et al. 2013; Wallis et al. 2014; Manrique and Call 2015). Second, we tested if and how a key life history trait, expected breed lifespan, affected the trajectory of cognition across the lifespan. We explicitly tested two alternative hypotheses: (1) truncation: that all breeds have similar cognitive trajectories throughout aging with larger breeds having a limited period of cognitive decline (Fig. 1a), and (2) compression: that changes in cognitive abilities scale with lifespan such that larger dogs have a compressed (i.e., accelerated) cognitive trajectory (Fig. 1b).
Fig. 1.
Alternative models of cognitive aging in dogs. a Schematic of the truncation hypothesis in which larger and smaller dog breeds have similar cognitive trajectories throughout aging. Under this hypothesis, large dog breeds experience limited cognitive decline because they typically die before the more precipitous cognitive decline experienced by longer-lived, smaller breeds. b The compression hypothesis in which cognitive performance scales with lifespan, such that larger breeds will have an accelerated cognitive trajectory
Methods
Data sources
Cognitive performance data were collected from Dognition. com, a citizen science website which provides users with video instructions for completing simple cognitive experiments at home with their dogs. Owners entered data into the website by answering simple questions about their dog’s behavior during the cognitive tests (e.g., which location did your dog approach?). Importantly, results from citizen scientists using Dognition recapitulate results from professional scientists working in controlled laboratory settings (Stewart et al. 2015). Here, we restricted our analysis to data collected prior to April 2018 from purebred dogs with known sex, age, reproductive alteration status (i.e., spayed/neutered vs. intact), and breed (n=4419). Dogs in the study represented 66 breeds and ranged in age from < 1 to 14.2 years with a mean age of 4.78 years (standard deviation ± 3.13; Supplemental Fig. 1). To ensure representative sampling, only breeds with ten or more individuals were retained for analysis (Supplemental Table 1). We used data from purebred dogs to ensure that we could estimate mean breed lifespan and to control for relatedness among breeds based on breed-averaged genotypic data (Parker et al. 2017; Supplemental Table 2). We used estimates of mean breed lifespan from Jones et al. (2008; Supplemental Table 3). Because recent studies have found behavioral modifications correlated with reproductive alteration (Hart 2001; Mongillo et al. 2017; Scandurra et al. 2019), we also included reproductive alteration status as a covariate in all models.
We included data from nine cognitive tasks measuring diverse processes involving aspects of executive function, such as memory, reasoning, decision-making, self-control, as well as measures of social cognition (Stewart et al. 2015; Horschler et al. 2019; Table 1). Sample size varied across tasks due to participant attrition across the series of experiments. We focused our analyses on tasks involving executive function, as it is one of the cognitive domains most susceptible to effects of aging (Jurado and Rosselli 2007). Seven of the Dognition tasks involved an object-choice paradigm in which the dog had to choose one of two possible options, across a series of trials (range = 1–6, mode=4; Table 1). One of the other two tasks was the eye contact task, in which the owner held a piece of food up to their face and recorded the time until the dog broke eye contact (up to 90 s). This task was designed as a measure of dog’s social engagement. In the remaining task, the owner set a treat before the dog, instructed the dog not to take a treat, and recorded the time until the dog took the treat (up to 90 s) while the owner (1) was visibly watching the dog, (2) had their back turned to the dog, or (3) faced the dog with their eyes closed (detailed in Stewart et al. 2015). Although originally developed as a measure of social cognition (sensitivity to cues about the human’s visual perspective), recent analyses have focused on the executive function component of this task, which requires dogs to delay gratification (Horschler et al. 2019). Following this approach, we considered the latency to take the forbidden treat as a measure of executive function in our analyses. To summarize performance in this task, we performed a principal component analysis on the latencies to take the treat across conditions. This analysis yielded one principal component which explained 88% of the variance and is subsequently referred to as ‘delay of gratification’. The Dognition battery also includes a contagious yawning task which we did not include because preliminary analyses showed minimal evidence for contagious yawning in this sample.
Table 1.
Description of cognitive tasks, cognitive processes the task was designed to test, number of trials conducted per task, number of individuals included in analysis, and number of breeds included in analysis
| Task | Description | Cognitive processes | Trials | Total dogs | Total breeds |
|---|---|---|---|---|---|
| Eye contact | The owner holds a treat to their face and records if and when the dog breaks eye contact within 90 s | Social engagement | 3 | 4359 | 65 |
| Arm pointing | The owner places one treat to their right and left, points to one treat location, and records the location the dog first approaches | Social cognition/communication | 6 | 4367 | 65 |
| Foot pointing | The owner places one treat to their right and left, extends their foot toward one treat location, and records the location the dog first approaches | Social cognition/communication | 6 | 4071 | 63 |
| Delay of gratification | Inhibition/self-control | 6 | 2826 | 51 | |
| Watching condition | The owner places the treat in front of the dog and verbally commands the dog not to take the treat. The owner records the duration of time until the dog takes the treat, up to 90 s | 2 | 2826 | 51 | |
| Closed eyes condition | Same as above, with the owner closing their eyes | 2 | 2826 | 51 | |
| Turned back condition | Same as above, with the owner turning their back | 2 | 2826 | 51 | |
| Memory vs. pointing | In view of the dog, the owner places a treat under one of two cups, then proceeds to point to the other cup. The owner records which location the dog first approaches | Bias for information from memory vs. communication | 6 | 2346 | 48 |
| Memory vs. smell | Allowing the dog to see, the owner places a treat under one of two cups, then blocks the dog's view while switching the position of the treat. The owner records which location the dog first approaches | Bias for information from memory vs. olfaction | 4 | 2187 | 47 |
| Delayed memory | In full view of the dog, the owner places a treat under one of two cups and then waits 60, 90, 120, and 150 s (across four trials) before releasing the dog. Then the owner records which location the dog first approaches | Short-term memory/sustained attention | 4 | 2124 | 47 |
| Inferential reasoning | The owner appears to place treats under two cups, while only baiting one. The owner raises the empty cup to show it is empty and records which location the dog first approaches | Inferential reasoning/reasoning by exclusion | 4 | 1737 | 42 |
| Physical reasoning | Blocking the dog's view, the owner places two pieces of folded paper on the floor. The owner places a treat under one paper so that the paper is elevated by the treat while the other paper is flat and records which location the dog first approaches | Physical causality/inferential reasoning | 4 | 1654 | 40 |
Statistical analysis
To address the questions of (1) how cognitive performance changes across domestic dog lifespan (Eqs. 1, 2, and 3) and (2) whether cognition scales with mean breed lifespan (Eqs. 3 and 4), we compared the fit of all four mixed effects models for each of the seven tasks with a binomial response and the two linear response variables (eye contact and the principal component scores reflecting delay of gratification; Supplemental Tables 4, 5). Because many cognitive abilities in humans exhibit a negative quadratic relationship (an inverted U-shape) with age (in particular those associated with executive processes), while others tend to increase throughout life or increase quickly during development and then plateau (Craik and Bialystok 2006; Harada et al. 2013; Wallis et al. 2014), we tested if cognitive performance followed these trajectories by modeling age as a quadratic, linear, and logarithmic predictor (Eqs. 1, 2, 3; Supplemental Table 4).
| (1) |
| (2) |
| (3) |
| (4) |
Equations 1, 2, 3, and 4 Eqs. 1, 2, and 3 represent hypothesized trajectories of cognitive aging, based on previously described patterns of cognitive aging. Equations 3 and 4 represent the truncation and compression hypotheses.
In all models, the age and mean breed lifespan predictors were mean centered and scaled to a standard deviation of one. Age and mean breed lifespan were in units of years and, therefore, one order of magnitude larger than the predictors of sex and reproductive alteration. Scaling the predictors of age and mean breed lifespan made them similar in magnitude to the predictors of sex and reproductive alteration, facilitating interpretation of the model results (Harrison et al. 2018). All models of the quadratic trajectory included orthogonal linear and quadratic predictors of age. Mixed-effects models of the seven binomial measures were carried out using the ‘PQLseq’ package, which implements the mixed modeling framework MACAU in the R environment (Lea et al. 2015; Sun et al. 2019). This allowed us to model a binomial outcome variable (i.e., number of times dog chose the left cup out of six) while controlling for background genetic similarity among the breeds, which was calculated from a recent genomic analysis of 150,067 SNPs in 1346 dogs representing 161 breeds (Parker et al. 2017; Supplemental Table 2). The eye contact and delay of gratification measures were modeled controlling for breed relatedness using the ‘EMMREML’ package (Akdemir and Okeke 2015) in the R environment. Only breeds for which we had both cognitive and genetic data were included in our analyses (Supplemental Table 1). Model fits were compared using Akaike information criterion (AIC; Akaike 1974). To test the sensitivity of our models, we also repeated these analyses excluding overrepresented breeds, which were breeds which each constituted over 5% of the dataset (Supplemental Table 6). Five breeds fit this criterion (Australian Shepherds, Border Collies, German Shepherd Dogs, Golden Retrievers, and Labrador Retrievers) and together comprised over 45% of the dataset. For models of the three tasks involving gesture following (arm pointing, foot pointing, and memory vs. pointing), we included behavioral training history as a predictor in the truncation and compression models to ensure that our results were not confounded by the correlation between dog size and training history (training history was rated on a scale of 1 [none] - 4 [substantial]; rs = 0.12, p < 0.001; Supplemental Table 7; Horschler et al. 2019).
Delayed memory task
We considered the delayed memory task to be the clearest test of basic memory because other tests involving memory measured preferences and biases, where memory was pitted against other information sources (e.g., memory of treat location vs. owner’s pointing, memory of treat location vs. scent of the treat). Although designed and interpreted as a measure of memory, it is possible that the delayed memory task also reflects variation in sustained attention, since the hiding locations were not out of the dog’s view during the delay. However, unlike traditional sustained attention tasks, there was no cue provided at the end of the delay, and thus subjects would most likely have been reliant on memory to motivate their search for food at the baited location. We modeled all trials from this task to test the truncation and compression hypotheses; however, we focused our analyses on the 150 s trial, as this trial was the longest time delay and likely the most cognitively challenging. Importantly, the results of the 60, 90, and 120 s delayed memory trials were very similar to the 150 s trial (Supplemental Table 8).
Results
Cognition across the lifespan
For all tasks, the best models (ΔAIC > 2) included the quadratic predictor of age, compared to linear or logarithmic, meaning that cognitive performance followed the expected inverted U-shaped trajectory—increasing in early life and declining in late life (Figs. 2, 3; Supplemental Fig. 2; Supplemental Table 4). Although the best models included quadratic functions of age, the coefficient for the quadratic term was significant in only six tasks (eye contact, delayed memory, memory vs. pointing, memory vs. smell, and inferential reasoning tasks, as well as the delay of gratification score; Figs. 2, 3; Supplemental Tables 4, 5, 8). Models without a significant age2 term (arm pointing, foot pointing, and physical reasoning tasks) showed an increase in cognitive performance throughout aging (Supplemental Table 5).
Fig. 2.
Age has both linear and quadratic associations with cognitive function. Effect sizes and estimated confidence intervals (beta ± 1.96 X SE) of age and age2 predictor variables for the additive (truncation) models for each cognitive task. Estimated confidence intervals that do not cross the line of null effect (x = 0) are statistically significant. For ease of interpretation and visualization, and to keep all variables on a similar scale, we converted the beta for the eye contact task to minutes
Fig. 3.
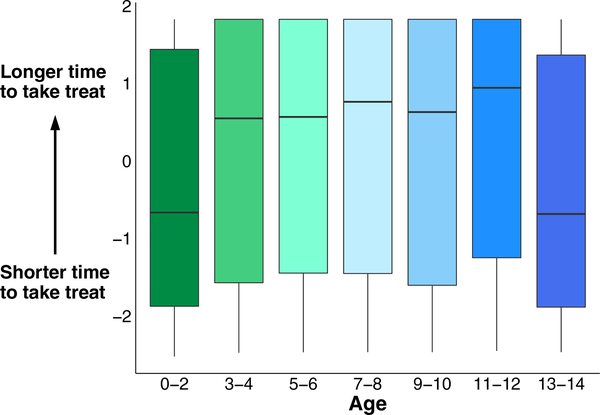
Self-control changes with age. Delay of gratification principal component 1 values for 2-year age groups. The delay of gratification task measures the time until the dog takes a treat (latency) under conditions of the owner watching, closing their eyes, and turning their back. Increasing y-axis values indicate greater performance in prolonging gratification (i.e., increased latency to take the treat)
Truncation vs. compression models
Models testing the truncation hypothesis fit the data better than or as well as models of the compression hypothesis for seven of the nine tasks (Table 2; Supplemental Table 5). The two tasks for which the model of the compression hypothesis had a better model fit were the eye contact task and delay of gratification score. However, neither of these models had significant interactions with mean breed lifespan, between age and mean breed lifespan, nor between age2 and mean breed lifespan. For models of the compression hypothesis, only the arm pointing (β = 2.196, SE = 0.996, p = 0.028, n=4367) and foot pointing tasks (β = 2.018, SE = 1.028, p = 0.049, n=4071) showed significant interaction effects between age2 and mean breed lifespan, while the memory vs. pointing task had a significant effect of mean breed lifespan in models of the truncation and compression hypotheses (β = 0.087, SE = 0.042, p = 0.037, n = 2346; Supplemental Table 5). These associations would support the compression hypothesis; however, interactive models of these tasks did not fit the data better than additive models representing the truncation hypothesis (ΔAIC < 2; Table 2; Supplemental Table 5). Our sensitivity analyses that excluded overrepresented breeds generally recapitulated results of the comparison between truncation and compression hypothesis models (Supplemental Table 6). In these analyses, fewer tasks had significant effects of age and age2, likely due to reduced power; however, the results were very similar to analyses with the entire dataset and did not suggest that our results were being driven by breeds that were overrepresented in the data. While we focused our analyses on the 150 s delayed memory trial, we evaluated how age affected cognitive performance across all delayed memory trials by modeling delay-specific accuracies in young (0–5 years), middle-aged (6–10 years), and old dogs (11 + years). Comparing the slope of the delay function, we found that dogs 11 years and older performed lower across all delayed memory trials (Supplemental Fig. 3).
Table 2.
Comparing the fit of models of the truncation hypothesis and the compression hypothesis for each cognitive task
| Task | ΔAIC of truncation hypothesis models and compression hypothesis models (compression—truncation) | Model with better fit |
|---|---|---|
| Eye contact | − 13.0 | Compression hypothesis |
| Arm pointing | 1.2 | No difference |
| Foot pointing | − 0.3 | No difference |
| Delay of gratification | − 7.5 | Compression hypothesis |
| Memory vs. pointing | 0.1 | No difference |
| Memory vs. smell | 0.8 | No difference |
| Delayed memory 150 s | − 0.2 | No difference |
| Inferential reasoning | 2.4 | Truncation hypothesis |
| Physical reasoning | 1.7 | No difference |
Lower AIC value indicates a better model fit. ∆AIC > 2 is considered a difference in model fit. The numbers in bold denote a difference in model fit between truncation and compression models
Gesture following
We observed associations between predictors involving breed mean lifespan for the three tasks involving gesture following (arm pointing, foot pointing, and memory vs. pointing), supporting the compression hypothesis, although model fit did not differ substantially between models of the truncation and compression hypotheses (Table 2; Supplemental Table 5). Additionally, in all models of gesture following, the age coefficient indicated increases in performance with aging, rather than late-life deterioration of cognitive performance, suggesting that these associations are not likely to support accelerated cognitive deterioration in faster aging breeds. To ensure that our results were not confounded by the correlation between dog size and training history, we included training as a predictor in the truncation and compression models for all gesture following models. The proportion of dogs in each category of training history was similar between intact and spayed and neutered individuals, males and females, and across dogs of all ages (in 1-year age groups). Effects of breed lifespan were reduced to statistically indistinguishable from zero when we included training history in the models of gesture following tasks (arm pointing [mean breed lifespan x age2]: β = - 0.64, SE = 0.915, p = 0.485, n = 1105; foot pointing [mean breed lifespan x age2 ]: β = 0.275, SE = 0.936, p = 0.769, n = 1057; memory vs. pointing [mean breed lifespan]: β= 0.071, SE = 0.083, p = 0.394 n = 720; Supplemental Table 7). However, because not all owners reported their dog’s training history—resulting in reduced power for this analysis—we could not rule out the possibility that this reduction in significance was due to a smaller sample size. We, therefore, compared this analysis to an analysis of the same subsample excluding training history as a predictor (Supplemental Table 7) and found that the effects of mean breed lifespan were not significant.
Associations of reproductive alteration vary across gesture following tasks
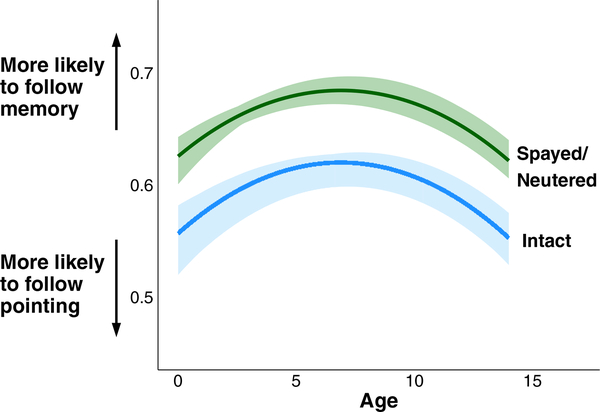
Reproductive alteration was associated with decreased gesture following throughout aging (Fig. 4; arm pointing: β = - 0.117, SE = 0.039, p < 0.01, n = 4367; memory vs. pointing: β = 0.379, SE = 0.097, p < 0.001, n = 2346; Supplemental Table 5). After controlling for training history in analyses of the arm pointing and memory vs. pointing tasks, reproductive alteration was still significantly associated with decreased gesture following (arm pointing: β = - 0.272, SE = 0.073, p < 0.001, n = 1105; memory vs. pointing: β = 0.527, SE = 0.164, p < 0.01, n = 720), indicating that spayed or neutered animals were less likely to follow social cues from their owner, even after controlling for training history (Supplemental Table 7).
Fig. 4.
Gesture following differs between intact and spayed and neutered dogs. Predicted cognitive trajectory for the memory vs. pointing task throughout aging (years) of an average dog in the dataset whether spayed or neutered (green) or intact (blue), with bootstrapped 95% confidence intervals
Sex differences
In the eye contact task, males maintained eye contact with the experimenter longer than females (β = 2.26, SE = 0.738, p = 0.002, n=4359), but this effect size was small (males held eye contact for an average of 2.26 s longer than females; Supplemental Table 5). In the delayed memory task, males were more likely than females to locate a treat after the 150 s delayed memory trial (β = 0.204, SE = 0.101, p = 0.043, n = 2124), but not any other delayed memory trial; again this effect was small, but statistically significant (males had 22% higher odds of choosing the cup with the treat; Supplemental Table 8). After performing our sensitivity analyses by excluding overrepresented breeds, males still showed longer social engagement in the eye contact task (β = 2.323, SE = 1.008, p = 0.021, n = 2318) but sex differences for the 150 s delayed memory trial were no longer significant (Supplemental Table 6).
Discussion
We investigated age-related changes in dog cognition and found that all cognitive measures changed across the lifespan, with most measures following a clear negative quadratic trajectory across the lifespan. For each of the nine cognitive tasks we evaluated, models with a quadratic term for age better fit the data than linear and logarithmic terms and six of these cognitive tasks showed a distinct inverted U-shape across aging (Fig. 2; Supplemental Fig. 2; Supplemental Tables 4, 5), indicating that a broad suite of cognitive processes in domestic dogs increase in early life, peak in midlife, and decrease in late life. Additionally, we found that cognitive performance in tasks testing physical reasoning and the propensity to follow owners’ pointing gestures (without competing sources of information) increase throughout aging.
Tasks directly testing memory and self-control were the clearest tests of executive function and had particularly robust quadratic curves throughout aging (Figs. 2, 3; Supplemental Table 5). In humans, executive function follows a similar negative quadratic trajectory throughout the lifespan and is one of the cognitive domains most susceptible to aging. Declines in executive function greatly impact daily life by reducing cognitive performance in domains such as decision-making, memory, and self-control (Jurado and Rosselli 2007; Alvarez and Emory 2006; Bizon and Woods 2009; Harada et al. 2013). Similar declines have been reported in nonhuman primate and rodent models (Moore et al. 2006; Rodefer and Nguyen 2008; Beas et al. 2013), and recently described for domestic dog attention (Wallis et al. 2014). Our findings extend these similarities to dogs across a range of cognitive processes, thus building on previous laboratory work with dogs (Milgram et al. 1994; Head et al. 2001; Tapp et al. 2003) and advancing companion dogs as a useful model for human cognitive aging.
To assess associations between cognition and aging, we tested models representing what we have termed the ‘truncation hypothesis’, in which all dogs have similar cognitive trajectories, and the ‘compression hypothesis’, in which the timing of decline is accelerated in shorter lived breeds. Models representing the truncation hypothesis fit the data better than or as well as models representing the compression hypothesis across seven of the nine cognitive tasks (Table 2; Supplemental Table 5). The two tasks for which models representing the compression hypothesis fit the data better than models representing the truncation hypothesis did not have significant associations of mean breed lifespan or between mean breed lifespan and linear or quadratic terms of age. Additionally, all models representing the compression hypothesis lacked significant interactions between age and mean breed lifespan after controlling for training history. Thus, we conclude that there is not sufficient evidence for the compression hypothesis, and that these results most strongly support the truncation hypothesis. Together, our results suggest that all dog breeds, regardless of average breed lifespan or rate of physiological aging, exhibit similar cognitive aging trajectories such that larger dogs may experience a limited cognitive decline at the end of their shorter lives.
Although the pace of physiological aging varies with lifespan, there is evidence that the age of onset of senescence does not differ among breeds, except, perhaps, in very large breeds (Kraus et al. 2013). If general onset of senescence is similar among breeds, larger breeds will tend to have an abnormally shortened senior period, while smaller breeds will likely undergo a protracted decline. Our findings indicate a similar pattern in cognitive performance and are concordant with recent work finding similar prevalence of canine dementia across breeds of varying size (Salvin et al. 2010, 2012). However, Kraus et al. (2013) suggest that large breeds physiologically deteriorate rapidly, which we did not detect for cognitive aging. Together, these results may suggest that the pathways which influence cognitive aging may be partially decoupled from those which affect the pace of physiological aging. While further investigation is clearly warranted, these findings have important implications for dog owners considering the quality of life during the senior period in terms of both cognitive and physiological health.
We also observed effects of reproductive alteration on tasks involving responses to pointing gestures. Intact dogs were more likely than spayed and neutered dogs to follow owners’ cues across two tasks involving arm pointing even after controlling for training history (Supplemental Table 7). These findings are consistent with results from a recent study that demonstrated reduced tendency of gonadectomized female dogs to follow human pointing gestures compared to intact females (Scandurra et al. 2019). Neutering increases food motivation and decreases metabolic rate, which can lead to lower energy levels and increased risk of obesity (Duffy and Serpell 2006; German 2006). It is, therefore, possible that neutered dogs had a greater motivation to obtain the food than intact dogs and were less attentive to the cue given by the owner. It is unclear how sex hormones alter cognition and behavior in dogs; however, there is some evidence that intact male dogs may exhibit slower cognitive decline than neutered males (Hart 2001) which may be due to neuroprotective activity of sex hormones (Zárate et al. 2017). Additionally, our findings demonstrated few and inconsistent sex-related differences in cognitive performance. Sex was significantly associated with performance on two measures, duration of eye contact, in which male dogs held slightly longer eye contact than females, and the 150 s delayed memory trial, in which males had a greater propensity than females to remember treat location after this time delay (although this effect was not detected for the sensitivity analyses with reduced sample size; Supplemental Tables 5, 6). Sex differences in dog cognition have been reported across a variety of measures including looking times in violation of expectation tasks, gaze at human faces following oxytocin administration, and speed and accuracy in spatial memory tasks (Müller et al. 2011; Nagasawa et al. 2015; Mongillo et al. 2017). However, these studies found that female dogs tended to show longer looking times compared to males, and better performance on spatial memory, effects in the opposite direction to those we observed. Given the inconsistency across studies, it will be important for future research to assess the robustness of sex differences on these measures, as well as specific factors that may determine the nature of these effects (Miller and Halpern 2014).
One limitation of this study stems from a lack of very old dogs in our sample. We had relatively few dogs of very old age: dogs aged 11 and older comprised 5% of our dataset. We observed greater variation in cognitive performance in older dogs, particularly among the oldest dogs in the study. For many tasks, the performance of the oldest aged dogs in our study ranged greatly, often spanning the full spectrum of the dependent variable’s range. The relatively small number of very old dogs means that assessing cognitive performance in this group is more susceptible to the influence of a small number of individuals. Future studies further establishing how cognition changes and varies among this demographic would be valuable. We suspect that the limited number of very old dogs may result from a selection bias in which owners of older, potentially highly impaired dogs, may have been less likely to pursue participation in these activities. Thus, active recruitment of the oldest dogs will be an important priority for future research. While collecting self-reported data from dog owners enabled the relatively large sample size of this study, we had variation in the reporting of information such as training history, which decreased power to detect effects of aging and possible associations with mean breed lifespan within this group. It will be important to generate larger datasets in the future that can include other potentially relevant covariates. We also collected data from dog owners at a single time point which limited the degree to which we could evaluate individual variation in cognitive performance throughout aging. Lastly, the cognitive assessment we used included diverse tasks, but the particular cognitive processes measured by each specific task were not unambiguous.
Due to extraordinary intraspecific phenotypic diversity, dogs present a unique model for investigating how age-related traits vary with cognition across the lifespan. Our findings suggest that age-related changes in executive function in domestic dogs follow patterns similar to those in humans and provide insight regarding the relationships (or lack thereof) between life history and cognitive trajectories in a species characterized by extensive intraspecific diversity. An important priority for future work will be to determine whether dogs and humans share similar aging trajectories in other cognitive domains such as long-term memory, cognitive processing speed, and episodic-like memory. Using consistent and readily deployable cognitive assessments, such as the ones presented here, future studies could evaluate longitudinal changes in cognitive performance of the same cohort of dogs across various timepoints to gain a finer grained understanding of dog cognitive aging.
Supplementary Material
Acknowledgements
We thank Laurie Santos, Richard Wrangham, and all members of the Dognition team for helping create Dognition.com; the citizen scientists who contributed in collecting these data; Sarah Converse for helpful insight with statistical questions; Daniel Promislow for valuable feedback and sharing data vital to this project; Kenny Chiou, India Schneider-Crease, Corbin Johnson, Ian Dowsett, Sierra Sams, Lia Koklic, Grace DeCastro, and Matthew Harrington for their feedback on previous drafts.
Funding This work was supported by the National Institute of Health Grants R00AG051764, U19AG057377, R01AG060931, R01HD097732. AM was supported by the National Brain Research Program (2017-1.2.1-NKP-2017-00002) and from the ELTE Institutional Excellence Program supported by the National Research, Development and Innovation Office (NKFIH-1157-8/2019-DT).
Footnotes
Compliance with ethical standards
Conflict of interest BH is the founder of Dognition.com and is a member of the Dognition.com Scientific Advisory Board along with JC, JK, and ÁM.
Data accessibility The dataset analyzed during the current study is not publicly available due to third party restrictions, but is available from the corresponding author on reasonable request and permission of Canines Inc. Supplementary Materials associated with this article are available online. Code used to complete the described analyses is available at https://github.com/mwatowich/Dog-cognition-across-aging.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10071-020-01385-0) contains supplementary material, which is available to authorized users.
References
- Adams B, Chan A, Callahan H, Milgram NW (2000) The canine as a model of human cognitive aging. Prog Neuro Psychopharmacol Biol Psychiat 24:675–692 [DOI] [PubMed] [Google Scholar]
- Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Control 19:716–723. 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- Akdemir D, Okeke UG (2015) EMMREML: fitting mixed models with known covariance structures. R package version 31 https://cran.r-project.org/package=EMMREML Accessed 12 Jun 2019 [Google Scholar]
- Alvarez JA, Emory E (2006) Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev 16:17–42. 10.1007/s11065-006-9002-x [DOI] [PubMed] [Google Scholar]
- Austad S (2010) Animal size, metabolic rate, and survival, among and within species In: Wolf NS (ed) The comparative biology of aging. Springer, Dordrecht, pp 27–41 [Google Scholar]
- Bartke A (2017) Somatic growth, aging, and longevity. NPJ Aging Mech Dis 3:1–6. 10.1038/s41514-017-0014-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Bizon JL (2013) Distinct manifestations of executive dysfunction in aged rats. Neurobiol Aging 34:2164–2174. 10.1016/j.neurobiolaging.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Woods AG (2009) Animal models of human cognitive aging. Humana Press, Totowa [Google Scholar]
- Chapagain D, Virányi Z, Wallis LJ et al. (2017) Aging of attentiveness in border collies and other pet dog breeds: the protective benefits of lifelong training. Front Aging Neurosci 9:1–14. 10.3389/fnagi.2017.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapagain D, Range F, Huber L, Virányi Z (2018) Cognitive aging in dogs. Gerontology 64:165–171. 10.1159/000481621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Bialystok E (2006) Cognition through the lifespan: mechanisms of change. Trends Cogn Sci 10:131–138. 10.1016/j.tics.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Diamond A (2013) Executive functions. Annu Rev Psychol 64:135168 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DL, Serpell JA (2006) Center for the Interaction of Animals and Society, School of Veterinary Medicine, University of Pennsylvania Non-reproductive Effects of Spaying and Neutering on Behavior in Dogs Proceedings of the Third International Symposium on Non-Surgical Contracepti. In: Proceedings of the Third International Symposium on Non-Surgical Contraceptive Methods for Pet Population Control [Google Scholar]
- Fan R, Olbricht G, Baker X, Hou C (2016) Birth mass is the key to understanding the negative correlation between lifespan and body size in dogs. Aging (Albany NY) 8:3209–3222. 10.18632/aging.101081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick LJ, Fick GH, Li Z et al. (2012) Telomere length correlates with life span of dog breeds. Cell Rep 2:1530–1536. 10.1016/j.celrep.2012.11.021 [DOI] [PubMed] [Google Scholar]
- Galis F, Van Der Sluij I, Van Dooren TJM et al. (2007) Do large dogs die young? J Exp Zool Mol Dev Evol 308:119–126. 10.1002/jez.b [DOI] [PubMed] [Google Scholar]
- German AJ (2006) The growing problem of obesity in dogs and cats. Am Soc Nutr 136:1940–1946. 10.1093/jn/136.7.1940S [DOI] [PubMed] [Google Scholar]
- Harada CN, Love MCN, Triebel K (2013) Normal cognitive aging. Clin Geriatr Med 29:737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B (2017) Survival of the friendliest: Homo Sapiens evolved via selection for prosociality. Annu Rev Psychol 68:155–185. 10.1146/annurev-psych-010416-044201 [DOI] [PubMed] [Google Scholar]
- Hare B, Tomasello M (2005) Human-like social skills in dogs? Trends Cogn Sci 9:439–444. 10.1016/j.tics.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Harrison XA, Donaldson L, Correa-Cano ME et al. (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 10.7717/peerj.4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL (2001) Effect of gonadectomy on subsequent development of age-related cognitive impairment in dogs. J Am Vet Med Assoc 219:51–56 [DOI] [PubMed] [Google Scholar]
- Head E, Milgram NW, Cotman C (2001) Neurobiological models of aging in the dog and other vertebrate species Functional neurobiology of aging. Elsevier, Amsterdam, pp 457–468 [Google Scholar]
- Healy K, Guillerme T, Cooper N et al. (2014) Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc R Soc B Biol Sci 281:20140298–20140298. 10.1098/rspb.2014.0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, Creevy KE, Franks A et al. (2018) The companion dog as a model for human aging and mortality. Aging Cell 10.1111/acel.12737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horschler DJ, Hare B, Call J et al. (2019) Absolute brain size predicts dog breed differences in executive function. Anim Cogn 10.1007/s10071-018-01234-1 [DOI] [PubMed] [Google Scholar]
- Jones P, Chase K, Martin A et al. (2008) Single-nucleotide-polymorphism-based association mapping of dog stereotypes. Genetics 179:1033–1044. 10.1534/genetics.108.087866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M (2007) The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev 17:213–233. 10.1007/s11065-007-9040-z [DOI] [PubMed] [Google Scholar]
- Kraus C, Pavard S, Promislow DEL (2013) The size-life span trade-off decomposed: why large dogs die young. Am Nat 181:492–505. 10.1086/669665 [DOI] [PubMed] [Google Scholar]
- Lea AJ, Tung J, Zhou X (2015) A flexible, efficient binomial mixed model for identifying differential DNA methylation in bisulfite sequencing data. PLoS Genet 11:1–31. 10.1371/journal.pgen.1005650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Herrmann E, Suchindran S, Hare B (2017) Individual differences in cooperative communicative skills are more similar between dogs and humans than chimpanzees. Anim Behav 126:41–51. 10.1016/j.anbehav.2017.01.005 [DOI] [Google Scholar]
- Manrique HM, Call J (2015) Age-dependent cognitive in flexibility in great apes. Anim Behav 102:1–6. 10.1016/j.anbehav.2015.01.002 [DOI] [Google Scholar]
- Metcalfe NB, Monaghan P (2003) Growth versus lifespan: perspectives from evolutionary ecology. Exp Gerontol 38:935–940. 10.1016/S0531-5565(03)00159-1 [DOI] [PubMed] [Google Scholar]
- Miklósi Á, Topál J, Csányi V (2004) Comparative social cognition: what can dogs teach us? Anim Behav 67:995–1004. 10.1016/j.anbehav.2003.10.008 [DOI] [Google Scholar]
- Milgram NW, Head E, Weiner E, Thomas E (1994) Cognitive functions and aging in the dog: acquisition of nonspatial visual tasks. Behav Neurosci 108:57–68. 10.1037/0735-7044.108.1.57 [DOI] [PubMed] [Google Scholar]
- Miller DI, Halpern DF (2014) The new science of cognitive sex differences. Trends Cogn Sci 18:37–45. https://doi.Org/10.1016/j.tics.2013.10.011 [DOI] [PubMed] [Google Scholar]
- Mongillo P, Scandurra A, Aniello BD, Marinelli L (2017) Effect of sex and gonadectomy on dogs’ spatial performance. Appl Anim Behav Sci 191:84–89. 10.1016/j.applanim.2017.01.017 [DOI] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG et al. (2006) Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging 27:1484–1493. 10.1016/j.neurobiolaging.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Müller CA, Mayer C, Dorrenberg S et al. (2011) Female but not male dogs respond to a size constancy violation. Biol Lett 10.1098/rsbl.2011.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa M, Mitsui S, En S et al. (2015) Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Sci Rep 348:333–336 [DOI] [PubMed] [Google Scholar]
- Ostrander EA, Wayne RK, Freedman AH, Davis BW (2017) Demographic history, selection and functional diversity of the canine genome. Nat Rev Genet 18:705–720. 10.1038/nrg.2017.67 [DOI] [PubMed] [Google Scholar]
- Parker HG, Dreger DL, Rimbault M et al. (2017) Genomic analyses reveal the influence of geographic origin, migration, and hybridization on modern dog breed development. Cell Rep 19:697–708. 10.1016/j.celrep.2017.03.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodefer JS, Nguyen TN (2008) Naltrexone reverses age-induced cognitive deficits in rats. Neurobiol Aging 29:309–313. 10.1016/j.neurobiolaging.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Salvin HE, Mcgreevy PD, Sachdev PS, Valenzuela MJ (2010) Under diagnosis of canine cognitive dysfunction: a cross-sectional survey of older companion dogs. Vet J 184:277–281. 10.1016/j.tvjl.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Salvin HE, McGreevy PD, Sachdev PS, Valenzuela MJ (2012) The effect of breed on age-related changes in behavior and disease prevalence in cognitively normal older community dogs, Canis lupus familiaris. J Vet Behav 7:61–69 [Google Scholar]
- Scandurra A, Alterisio A, Di Cosmo A et al. (2019) Ovariectomy impairs socio-cognitive functions in dogs. Animals 10.3390/ani9020058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L, Rodriguez K, Call J et al. (2015) Citizen science as a new tool in dog cognition research. PLoS ONE 10:e0135176 10.1371/journal.pone.0135176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Zhu J, Mozaffari S et al. (2019) Heritability estimation and differential analysis of count data with generalized linear mixed models in genomic sequencing studies. Bioinformatics 35:487496 10.1093/bioinformatics/bty644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó D, Gee NR, Miklósi Á (2016) Natural or pathologic? Discrepancies in the study of behavioral and cognitive signs in aging family dogs. J Vet Behav Clin Appl Res 11:86–98. 10.1016/j.jveb.2015.08.003 [DOI] [Google Scholar]
- Tapp PD, Siwak CT, Estrada J et al. (2003) Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learn Mem 10:64–73. 10.1101/lm.54403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P (2011) Aging and executive control: reports of a demise greatly exaggerated. Curr Dir Psychol Sci 20:174–180. 10.1177/0963721411408772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VonHoldt BM, Pollinger JP, Lohmueller KE et al. (2010) Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 464:898–902. 10.1038/nature08837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis LJ, Range F, Müller CA et al. (2014) Lifespan development of attentiveness in domestic dogs: drawing parallels with humans. Front Psychol. 10.3389/fpsyg.2014.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis LJ, Virányi Z, Müller CA et al. (2016) Aging effects on discrimination learning, logical reasoning and memory in pet dogs. Age (Omaha) 38:1–18. 10.1007/s11357-015-9866-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárate S, Stevnsner T, Gredilla R (2017) Role of estrogen and other sex hormones in brain aging. Neuroprotection and DNA repair. Front Aging Neurosci 9:1–22. 10.3389/fnagi.2017.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.