Abstract
Genetic defects in telomere maintenance result in stem cell exhaustion and a spectrum of telomere biology diseases. Systemic treatments beyond organ transplantation are lacking for these diseases. Nagpal and colleagues identified small molecules that restore telomere maintenance in patient-derived stem cells, offering a promising therapy for telomere biology diseases.
Keywords: dyskeratosis congenita (DC), telomerase, TERC, PAPD5, PARN, bone marrow failure (BMF)
Telomeres shorten as a result of incomplete replication by DNA polymerases, limiting the number of times a cell can divide. However, stem cells require extensive proliferation to drive tissue development and maintenance. To circumvent this, stem cells activate telomerase, an enzyme that elongates telomeres to extend replicative lifespan. Telomerase activity is determined by the telomerase reverse transcriptase (TERT) protein, which utilizes a template on the telomerase RNA component (TERC) to synthesize telomeric DNA at chromosome ends. Mutations in genes that affect telomerase function cause a spectrum of diseases that vary in severity and affected organs but share the common diagnostic trait of extremely short telomeres. One prominently severe telomere biology disease is dyskeratosis congenita (DC), a stem cell disorder that displays symptoms in multiple organs requiring tissue regeneration. Mortalities in DC are often due to bone marrow failure (BMF) caused by hematopoietic stem cell depletion resulting from defective telomere maintenance. Currently, the only curative treatment option for BMF in DC is bone marrow transplantation, which fails to treat DC symptoms that manifest in organs outside of the hematopoietic system, resulting in poor long-term outcomes [1]. In a recent break-through study, Nagpal and colleagues identified small molecules with the potential to restore telomerase in stem cell compartments throughout the body, offering a much-needed systemic therapy for treatment of DC [2].
Therapies that restore telomerase at the molecular level have been sought-after because they have the potential to cure symptoms across multiple organ systems. However, telomerase activation confers immortality to somatic cells, making them highly permissive to tumorigenesis [3]. While TERT is normally silenced upon differentiation, it is inappropriately reactivated in cancer cells. In contrast, TERC is expressed ubiquitously across cell types [4]. As such, the authors reasoned that targeting TERC rather than TERT would allow them to specifically restore telomerase in DC-relevant stem cells without risking tumorigenic activation. Recent discovery of telomere disease mutations in the poly(A)-specific ribonuclease (PARN) has revealed an RNA processing pathway that could be pharmacologically targeted to increase TERC [5–7]. Immediately following transcription, TERC is adenylated at its 3’ end by the noncanonical polymerase PAPD5, which marks the RNA for degradation (Figure 1) [8]. Formation of mature TERC requires removal of this tail by PARN, as loss of PARN function causes accumulation of unstable, oligo-adenylated TERC transcripts targeted for degradation by the RNA exosome, culminating in reduced steady-state levels [9]. PARN and PAPD5 compete for RNA processing to control the availability of mature TERC and, by extension, regulate telomerase activity (Figure 1) [8, 9]. Previous reports have demonstrated that genetic reduction of PAPD5 can compensate for loss of PARN and restore telomere maintenance in vitro, making PAPD5 a promising target for therapeutic intervention of TERC degradation [10–12]. In the highlighted report, Nagpal and colleagues validate this approach by identifying small molecule PAPD5 inhibitors that increase TERC accumulation and telomere length in DC patient stem cells in vivo. This study is the first to describe easily administered, bioavailable compounds that specifically restore telomere maintenance in human stem cells. This work represents a major advancement towards developing systemic treatments for DC and other genetic telomere diseases.
Figure 1. TERC Processing and degradation determine telomerase activity.
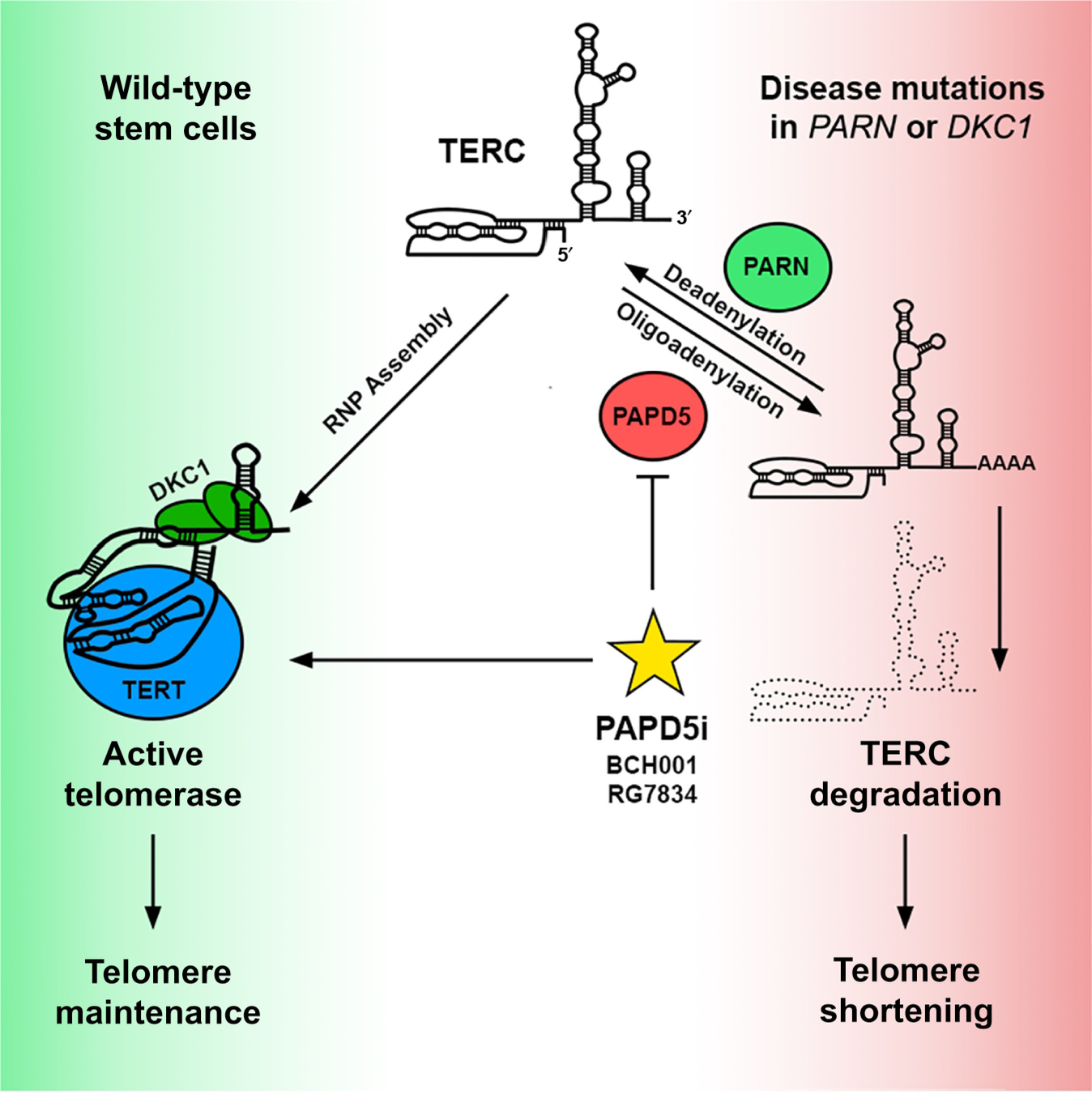
Telomerase assembly and activation require stabilization of TERC by dyskerin (DKC1) and stem cell-specific expression of TERT. Non-templated adenosine addition and removal by PAPD5 and PARN, respectively, establish a steady-state TERC level to maintain telomere lengths in wild type stem cells (left panel, green). Patients harboring mutations in PARN or DKC1 alter the balance of TERC processing, resulting in excessive TERC degradation, drastic telomere shortening, and stem cell exhaustion (right panel, red). Nagpal and colleagues identify small molecule PAPD5 inhibitors (PAPD5i, yellow star) that inhibit TERC degradation and restore telomere maintenance in patient stem cells, offering a systemic approach for treating telomere biology diseases.
Nagpal and colleagues highlight two small molecule PAPD5 inhibitors: BCH001, which the authors identified in a high-throughput screen, and RG7834, a hepatitis B surface antigen suppressor previously reported to inhibit PAPD5 in the context of viral infection [13]. The authors found that BCH001 and RG7834 specifically inhibited PAPD5 activity at low micromolar and nanomolar concentrations, respectively. Both molecules prevented oligoadenylation of TERC in induced pluripotent stem cells (iPSCs) derived from DC patients harboring PARN mutations, culminating in increased TERC accumulation and telomerase activity. Remarkably, they found that continuous treatment with these compounds was sufficient to elongate and eventually stabilize telomeres in patient iPSCs at lengths comparable to wild-type cells, indicating that PAPD5 inhibitors can reverse the molecular cause of DC. Despite an increase in TERC accumulation in TERT-negative patient fibroblasts treated with these inhibitors, telomerase was not activated in these cells, verifying that targeting of TERC biogenesis does not immortalize somatic cells and can be used to specifically target disease-relevant adult stem cells.
While PARN mutations associated with DC have only been identified within the last few years, a majority of DC mutations arise in the gene coding for the protein dyskerin (DKC1) [14,15]. Dyskerin, like PARN and PAPD5, is an integral component of TERC biogenesis and stability (Figure 1). Previous studies demonstrated that genetic PAPD5 depletion partially restores TERC accumulation in cells deficient for dyskerin, indicating that these two proteins function in establishing steady-state levels of TERC [11, 12]. Nagpal and colleagues show that pharmacological inhibition of PAPD5 increases TERC levels and lengthens telomeres in iPSCs harboring DKC1 mutations similarly to the effects observed in PARN mutant cells. This indicates that drugs that inhibit PADP5 could be effective in DC patients across multiple genetic backgrounds, which greatly expands the applicability of this therapeutic approach.
As DC is characterized as a BMF disease, the authors next examined the efficacy of BCH001 and RG7834 in hematopoietic stem and progenitor cells (HSPCs). They found that both molecules restored TERC processing and accumulation in PARN-deficient human HSPCs, demonstrating that PAPD5 inhibitors can restore telomerase in DC-relevant human adult stem cells in vitro.
Investigating telomere biology diseases in whole organisms is complicated by the fact that mice maintain much longer telomeres than humans [2]. To circumvent this, the authors transplanted PARN-deficient human HSPCs into immunodeficient mice and treated them orally with RG7834, as this was the more potent and bioavailable compound. Mice did not exhibit any indication of drug toxicity. After six weeks of treatment, engrafted human cells in the mouse bone marrow displayed a detectable increase in bulk telomere length, demonstrating that RG7834 can rescue telomere maintenance within the hematopoietic compartment, in vivo, thereby reversing the molecular cause of DC. These results validate the use of PAPD5 inhibitors as a promising and now clinically achievable approach for systemic treatment of DC and other telomere biology diseases. As it is uncertain whether TERC is the sole RNA that relies on PARN/PAPD5 processing, it will be critical to assess potential side effects of PAPD5 inhibitors as these promising molecules move forward into clinical trials.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants R01GM120094 and R01AG050509, and the American Cancer Society Research Scholar grant RSG-17-037-01-DMC (to J.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLAIMER STATEMENT
The authors have no conflicts of interest to declare.
REFERENCES:
- [1].Niewisch MR and Savage SA, “An update on the biology and management of dyskeratosis congenita and related telomere biology disorders.,” Expert Rev. Hematol, vol. 12, no. 12, pp. 1037–1052, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nagpal N et al. , “Small-Molecule PAPD5 Inhibitors Restore Telomerase Activity in Patient Stem Cells,” Cell Stem Cell, April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim NW et al. , “Specific association of human telomerase activity with immortal cells and cancer.,” Science, vol. 266, no. 5193, pp. 2011–5, December 1994. [DOI] [PubMed] [Google Scholar]
- [4].Feng J et al. , “The RNA component of human telomerase.,” Science, vol. 269, no. 5228, pp. 1236–41, September 1995. [DOI] [PubMed] [Google Scholar]
- [5].Stuart BD et al. , “Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening.,” Nat. Genet, vol. 47, no. 5, pp. 512–7, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moon DH et al. , “Poly(A)-specific ribonuclease (PARN) mediates 3’-end maturation of the telomerase RNA component.,” Nat. Genet, vol. 47, no. 12, pp. 1482–8, December 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tummala H et al. , “Poly(A)-specific ribonuclease deficiency impacts telomere biology and causes dyskeratosis congenita,” J. Clin. Invest, vol. 125, no. 5, pp. 2151–2160, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Roake CM, Chen L, Chakravarthy AL, Ferrell JE, Raffa GD, and Artandi SE, “Disruption of Telomerase RNA Maturation Kinetics Precipitates Disease,” Mol. Cell, vol. 74, no. 4, pp. 688–700.e3, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tseng C-K, Wang H-F, Burns AM, Schroeder MR, Gaspari M, and Baumann P, “Human Telomerase RNA Processing and Quality Control,” Cell Rep, vol. 13, no. 10, pp. 2232–2243, December 2015. [DOI] [PubMed] [Google Scholar]
- [10].Boyraz B et al. , “Posttranscriptional manipulation of TERC reverses molecular hallmarks of telomere disease.,” J. Clin. Invest, vol. 126, no. 9, pp. 3377–82, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shukla S, Schmidt JC, Goldfarb KC, Cech TR, and Parker R, “Inhibition of telomerase RNA decay rescues telomerase deficiency caused by dyskerin or PARN defects,” Nat. Struct. Mol. Biol, vol. 23, no. 4, pp. 286–292, April 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fok WC et al. , “Posttranscriptional modulation of TERC by PAPD5 inhibition rescues hematopoietic development in dyskeratosis congenita,” Blood, vol. 133, no. 12, pp. 1308–1312, March 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mueller H et al. , “PAPD5/7 Are Host Factors That Are Required for Hepatitis B Virus RNA Stabilization,” Hepatology, vol. 69, no. 4, pp. 1398–1411, April 2019. [DOI] [PubMed] [Google Scholar]
- [14].Heiss NS et al. , “X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions,” Nat. Genet, vol. 19, no. 1, pp. 32–38, May 1998. [DOI] [PubMed] [Google Scholar]
- [15].Mitchell JR, Wood E, and Collins K, “A telomerase component is defective in the human disease dyskeratosis congenita.,” Nature, vol. 402, no. 6761, pp. 551–5, December 1999. [DOI] [PubMed] [Google Scholar]


