Abstract
After allogeneic hematopoietic cell transplantation (HCT), the minimal myeloid chimerism required for full T and B cell reconstitution in patients with severe combined immunodeficiency (SCID) is unknown. We retrospectively reviewed our experience with low-exposure busulfan (cumulative area under the curve, 30 mg.hr/L) in 10 SCID patients undergoing either first or repeat HCT from unrelated or haploidentical donors. The median busulfan dose required to achieve this exposure was 5.9 mg/kg (range, 4.8 to 9.1). With a median follow-up of 4.5 years all patients survived, with 1 requiring an additional HCT. Donor myeloid chimerism was generally >90% at 1 month post-HCT, but in most patients it fell during the next 3 months, such that 1-year median myeloid chimerism was 14% (range, 2% to 100%). Six of 10 patients had full T and B cell reconstitution, despite myeloid chimerism as low as 3%. Three patients have not recovered B cell function at over 2 years post-HCT, 2 of them in the setting of treatment with rituximab for post-HCT autoimmunity. Low-exposure busulfan was well tolerated and achieved sufficient myeloid chimerism for full immune reconstitution in over 50% of patients. However, other factors beyond busulfan exposure may also play critical roles in determining long-term myeloid chimerism and full T and B cell reconstitution.
Keywords: Severe combined, immunodeficiency, Busulfan, Chimerism
INTRODUCTION
Severe combined immunodeficiency (SCID) is a heterogeneous group of genetic disorders with the shared phenotype of profoundly deficient T cells and absent B lymphocyte function. For long-term survival, patients with SCID require restoration of T cell immunity via allogeneic hematopoietic cell transplantation (HCT), gene therapy, or enzyme replacement therapy in adenosine deaminase deficiency. There is considerable debate regarding the role and intensity of conditioning before allogeneic HCT. Depending on genotype and donor, many patients with SCID will engraft donor T cells, and occasionally B cells, without any cytotoxic conditioning [1–3]. Data suggest that in genotypes with no B cells or intrinsically defective B cells, the use of conditioning intended to open hematopoietic stem cell niches in the marrow improves the likelihood of B cell engraftment and reconstitution [4,5] and may improve some elements of T cell reconstitution [4–6]. However, the use of conditioning does not improve overall survival in SCID patients [4,5] and may increase the risk of graft-versus-host disease (GVHD) [5]. Furthermore, the long-term effects of conditioning in infants are largely unknown [7], except in patients with DNA-repair defects where alkylator exposure is associated with the development of significant late effects [8].
Although unconditioned HCT allows donor T cell engraftment for certain SCID genotypes, patients with natural killer (NK) cell-positive forms of SCID have high rates of graft rejection with this approach, in particular, when nonmatched sibling donors are used [5,9]. Therefore, beginning in 2011, for patients with these “graft-resistant” forms of SCID, the standard procedure at our center has been to administer busulfan, pharmacokinetically targeted to achieve a low cumulative exposure, plus agents designed to provide additional immunoablation when needed.
METHODS
Study Population
All patients who received low-exposure targeted busulfan from 2011 to 2017 at the University of California San Francisco (UCSF) were included in this retrospective report. Patients included those with NK cell-positive SCID or leaky SCID, as defined by Primary Immunodeficiency Treatment Consortium criteria [10], who either required a first HCT or a repeat HCT due to rejection or poor immunity after the first HCT. The patient with a mutation in BCL11B causing leaky SCID has been previously reported [11]. This retrospective evaluation was approved by the UCSF Institutional Review Board in accordance with the Declaration of Helsinki.
Donors and Graft Manipulations
No patient in this report had an HLA-matched sibling donor. When an unrelated donor (URD) was identified, bone marrow was the preferred stem cell source and was infused without manipulation, other than RBC or plasma depletion as necessary. If a URD was not identified, a haploidentical parent was used to donate granulocyte colony-stimulating factor-mobilized peripheral blood stem cells that were CD34-selected using the CliniMACs Plus machine (IND14442; Bergisch, Gladbach, Germany), as previously described [12,13].
Transplant Regimens
Initially, the standard procedure at UCSF was to administer busulfan every 6 hours for 16 doses. Given the very low absolute doses and volumes of busulfan administered to infants, the dosing was changed to every 12 hours for 8 doses and subsequently to every 24 hours for 4 doses. The target busulfan exposure was a cumulative area under the curve of 30 mg.hr/L, chosen to be 50% of the UCSF standard myeloablative target [14–16]. Two patients received plerixafor (240 μg/kg s.c.) 9 hours before each dose of busulfan in an attempt to enhance clearance of stem cells.
If undergoing first HCT or repeat HCT from a new donor, patients with any T cells were also administered fludarabine (160 mg/m2 total dose for URDs, 200 mg/m2 total dose for haploidentical donors). Patients with NK cells expected to mediate rejection (ie, in the setting of HLA-mismatched donors) were also administered thiotepa (10 mg/kg total dose), given that fludarabine has no reported efficacy against NK cells [17]. Serotherapy was also typically administered, either thymoglobulin at 3.5 (T cell depleted) or 8 mg/kg (T cell replete) total dose on days −4 to −1 or alemtuzumab at 1.5 mg/kg total dose on days −12 to −10.
Analysis of Engraftment and Immunologic Parameters
Donor chimerism was determined on bead-selected CD3+, CD19+, and CD14/15+ cells using short tandem repeats markers, as previously described [18]. The purity of cell subpopulations was determined by flow cytometry, and the interassay variation was ±1%. Lymphocyte subsets, including naïve and memory markers CD45RA+ and CD45RO+,wereassessedbyflow cytometry. T cell function was assessed by response to phytohemagglutinin and reported as a percentage of stimulated immunologically competent control CD45+ lymphocytes tested simultaneously (Mayo Medical Laboratories, Rochester, MN). T cell receptor excision circles were measured in a research laboratory, as previously described [19]. Normal T cell reconstitution was defined as a CD4 count >500× 109/L and CD4/CD45RA counts >200× 109/L [5]. Ig infusions were discontinued when IgM and IgA concentrations were within the normal range for age and IgM isohemagglutinin titer(s) were at ≥1:8 dilution or normal class-switched memory B cells (CD17+IgD−IgM−) were observed. Ig infusions were restarted if specific antibodies did not rise by 3-fold after vaccination or if the patient experienced recurrent infections.
Definitions and Statistics
Events were repeat conditioned HCT or death. Sinusoidal obstruction syndrome was diagnosed according to European Society for Blood and Marrow Transplantation criteria [20]. Mucositis was graded according to Common Terminology Criteria for Adverse Events v.5.0. Acute and chronic GVHD were graded on standard Mount-Sinai Acute GVHD International Consortium and National Institutes of Health criteria [21]. The medians and ranges of each continuous measurement were described and compared between groups in a univariate analysis with the Kruskal-Wallis test. Data were analyzed as of February 1, 2019.
RESULTS
Patient Characteristics
Ten patients (4 with typical and 6 with leaky SCID) received low-exposure busulfan, 5 for first HCT and 5 for repeat HCT (Table 1). The median age at time of HCT was 5 months (range, 2 to 108). Two of the repeat HCT patients were born before the implementation of T cell receptor excision circles for newborn screening. Four patients experienced severe viral or fungal infections before HCT, and 3 were still actively infected at the time of HCT. Four patients had idiopathic neutropenia (absolute neutrophil count < .5 × 109/L) before administration of busulfan. Of the 5 patients receiving second or greater transplant, 3 patients had transplacental maternally engrafted T cells at initial diagnosis with maternal donors for first HCT, 1 patient (UPN 2046) had previously rejected 2 maternal haploidentical grafts with serotherapy-only conditioning, and 1 patient (UPN 1799) had previously rejected a URD graft with serotherapy-only conditioning.
Table 1.
Baseline Patient Characteristics
| UPN | Transplant # (prior conditioning) | Age at HCT | Sex | Race / Ethnicity | Mutation | CD3 (x10^6/L) | CD4 (x10^6/L) | CD4 / CD45RA (%) | CD8 (x10^6/L) | CD19 (x10^6/L) | CD16/56 (x10^6.L) | PHA | ANC (x10^6/L) | Pre-HCT Infection |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1533 | 2 (Flu) | 10 mo | M | Caucasian | IL2RG (c.868G>A)1 | 869* | 404 | 2% | 273 | 51 | 81 | ND | 440 | PCP (pre-NBS) |
| 1713 | 2 (None) | 9 yo | M | Native American | DCLRE1C (c.597C>A, homozygous)1 | 324* | 195 | 0% | 140 | 0 | 66 | 85% | 750 | Noro (NBS) |
| 1740 | 1 | 3 mo | F | Hispanic | RAG1 (c,1420C>T; c.1267T>C) | 166 | 116 | 3% | 33 | 183 | 1262 | 47% | 470 | None (NBS) |
| 1799 | 2 (aCD52) | 6 mo | M | Hispanic | BCL11B (c.l323T>G) | 27 | 13 | 17% | 0 | 958 | 306 | 37% | 3830 | None (NBS) |
| 1835 | 1 | 2.5 mo | F | Caucasian | RAG1 (c.2258A>T; c2522G>A)) | 110 | 92 | 0% | 18 | 156 | 635 | 19% | 3210 | CMV (NBS) |
| 1837 | 1 | 4 mo | F | Caucasian | RAG2 (c,1583A>G; c,104G>C) | 46 | 32 | 33% | 13 | 0 | 135 | 100% | 1550 | None (NBS) |
| 1875 | 2 (None) | 13 mo | F | Native American | DCLRE1C (c.597C>A, homozygous)1 | 44* | 36 | 3% | 2 | 0 | 130 | 1% | 1610 | Noro (pre-NBS) |
| 1947 | 1 | 3 mo | F | Hispanic | RAG1 (c,1210C>T; c.2867T>C) | 67 | 50 | 16% | 17 | 0 | 409 | 12% | 150 | None (NBS) |
| 2046 | 3 (None; rATG) | 6 mo | F | Native American | DCLRE1C (c.597C>A, homozygous)1 | 0 | 0 | 0% | 0 | 0 | 202 | 0% | 970 | None (NBS) |
| 2078 | 1 | 4 mo | M | Hispanic | Unknown2 | 128 | 115 | 17% | 0 | 819 | 205 | 70% | 280^ | None (NBS) |
UPN, Unique Patient Number; PHA, phytohemagglutinin; ANC, absolute neutrophil count; Flu, Fludarabine; aCD52, Alemtuzumab; rATG, Rabbit Anti-Thymocyte Globulin; CMV, cytomegalovirus; NBS, newborn screening; PCP, Pneumocystis carinii pneumonia; Noro, Norovirus.
Transplacental Maternal Engraftment noted.
On granulocyte colony-stimulating factor pre-HCT.
Typical SCID (per Primary Immunodeficiency Treatment Consortium definitions).
Whole Genome Sequencing pending.
Busulfan Dosing and Exposure
As seen in Table 2, the targeted busulfan exposure achieved was a median of 29 mg.hr/L (range, 27 to 35). The median cumulative dose required to achieve this narrow exposure range was 5.9 mg/kg, with a wide dose range from 4.8 to 9.1 mg/kg, attributed to significant interpatient differences in pharmacokinetics. Significant toxicities included 2 patients with grade 3 oral mucositis and 3 patients with bacteremia before day 100. There was 1 case of severe sinusoidal obstruction syndrome requiring defibrotide in a patient with prior liver injury from maternal GVHD. There were no apparent differences in short-term toxicities between patients who did or did not receive thiotepa, although numbers are small.
Table 2.
Transplant Characteristics and Clinical Outcomes
| UPN | Busulfan cAUC (mg*hr/L) | Cumulative Busulfan Dose (mg/kg) | Other Agents | Donor & Stem Cell Source | GVHD Prophylaxis | Significant Toxicities | ANC Recovery (Day post BMT) | Myeloid Chimerism at Day 30 | Acute GVHD Grade | Autoimmunity (time to event) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1533 | 31 | 9.1 (8 doses) | Flu(160) + aCD52 (1.5) | 9/10 URD BM1 | CSA/MTX | SOS | 16 | 100% | IV (Skin, Gut, Liver) | None |
| 1713 | 29 | 4.8 (8 doses) | Plerixafor | 7/10 Mother PBSC2 | CD34-Selection | None | 23 | 70% | None | None |
| 1740 | 29 | 8.1 (8 doses) | Flu(160) + aCD52 (1.5) | 9/10 URD BM | CSA/MTX | Mucositis | 16 | 91% | None | None |
| 1799 | 35 | 5.8 (16 doses) | Flu(160) + rATG(8) | 10/10 URD BM2 | CSA/MTX | Mucositis | 16 | 80% | None | None |
| 1835 | 27 | 7.2 (16 doses) | Flu(200) + TT (10) + rATG(3.5) | 7/12 Father PBSC | CD34-Selection | None | 15 | 98% | None | Myositis (3.2 yrs) |
| 1837 | 30 | 7.0 (8 doses) | Flu(160) + aCD52 (1.5) | 10/10 URD BM | CSA/MTX | None | 19 | 85% | None | None |
| 1875 | 29 | 5.6 (8 doses) | Plerixafor | 5/10 Mother PBSC2 | CD34-Selection | Bacteremia | 14 | 59% | II (Skin) | None |
| 1947 | 35 | 5.1 (8 doses) | Flu(200) + TT (10) + rATG(3.5) | 6/12 Mother PBSC | CD34-Selection | None | 17 | 99% | None | Thyroiditis (3.1 yrs) |
| 2046 | 29 | 4.9 (4 doses) | TT(10) + rATG(3.5) | 6/10 Father PBSC1 | CD34-Selection | Bacteremia | 15 | 98% | None | None |
| 2078 | 29 | 5.9 (4 doses) | Flu(200) + TT (10) + rATG(3.5) | 6/12 Father PBSC | CD34-Selection | Bacteremia | 15 | 100% | None | AIHA (0.8 yrs) |
cAUC, Cumulative Area Under the Curve; Flu, Fludarabine; TT, Thiotepa; rATG, Rabbit Anti-Thymocyte Globulin; aCD52, Alemtuzumab’ PBSC, Peripheral Blood Stem Cells; BM, Bone Marrow; URD, Unrelated Donor; GVHD, Graft-versus-Host Disease; CSA, Cyclosporine; MTX, Methotrexate; SOS, Sinusoidal Obstruction Syndrome; ANC, Absolute Neutrophil Count; AIHA, Autoimmune Hemolytic Anemia.
New Donor.
Original Donor.
Engraftment and T Cell Chimerism
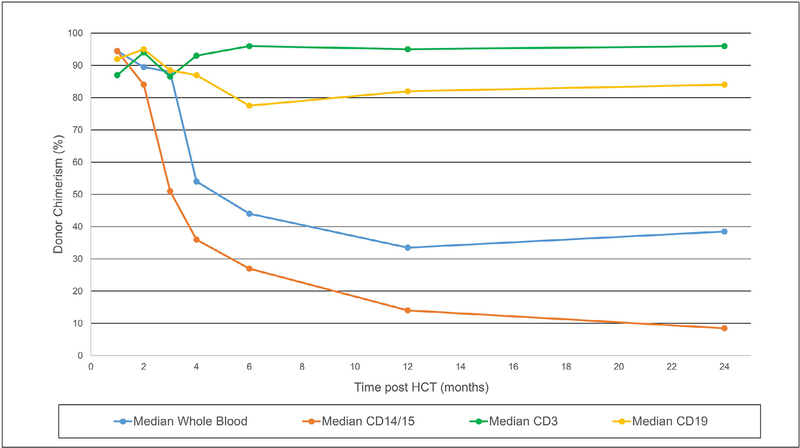
Low-exposure busulfan led to an expected fall in the absolute neutrophil count and subsequent recovery at a median of 16 days post-HCT (range, 14 to 23). In the first 100 days post-HCT, patients required a median of 4 PRBC (range, 1 to 6) and 4 platelet (range, 0 to 10) transfusions. All patients had evidence of donor engraftment post-HCT on whole blood chimerism. As expected, in patients with minimal to no baseline production of T cells, median donor T cell chimerism post-HCT started high and continued throughout follow-up (Figure 1).
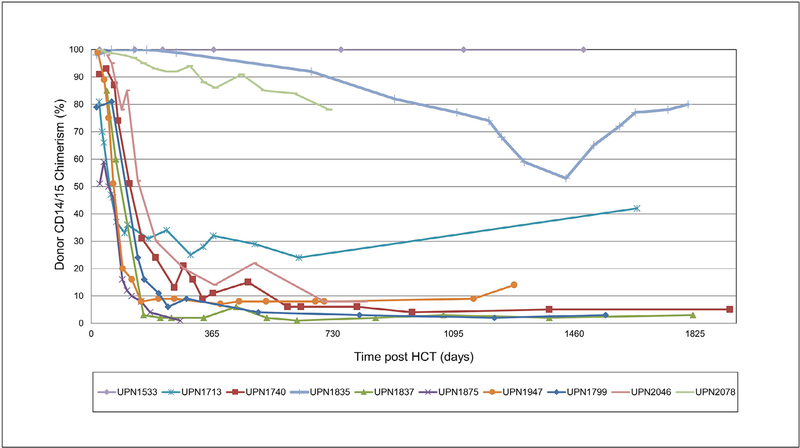
Figure 1.
Median whole blood and lineage-specific donor chimerism after low-exposure busulfan.
Myeloid and B Cell Chimerism
The median myeloid chimerism at day 30 (±7 days) was 96% (range, 59% to 100%) (Table 2, Figure 2). Three patients had ongoing persistence of predominantly donor myeloid chimerism. One patient (UPN 1533), with significant bone marrow dysfunction pre-HCT attributed to a graft-versus-marrow reaction from dysregulated transplacental maternally engrafted T cells, achieved 100% donor engraftment, which has persisted until the most recent follow-up at 7.5 years post-HCT. Two other patients had >80% myeloid chimerism at 1 year post-HCT. One patient (UPN 1835) had received 3 weeks of ganciclovir for treatment of CMV viremia and the other (UPN 2078, with unknown genotype) who was profoundly neutropenic pre-HCT due to unknown causes required intermittent granulocyte colony-stimulating factor for 7 weeks before HCT.
Figure 2.
Individual patient-level long-term myeloid chimerism after low-exposure busulfan.
In the remaining 7 patients, there was a significant fall in myeloid chimerism between 2 and 4 months post-HCT, such that by 1 year post-HCT the median myeloid chimerism of the entire cohort was 14% (range, 2% to 100%) (Figure 1). As shown in Figure 2, after 2 years post-HCT myeloid chimerism generally remained stable, except in 1 patient (UPN 1835), suggesting that long-term monitoring of lineage-specific chimerism may be necessary. At the last follow-up, 6 patients had <10% (range, 3% to 8%) donor myeloid chimerism. Four patients who underwent haploidentical HCT (including thiotepa as part of conditioning) had myeloid chimerism at 1 year post-HCT of 7%, 14%, 86%, and 99%, respectively, whereas the 6 patients who did not receive thiotepa had myeloid chimerism at 1 year post-HCT of 1%, 2%, 9%, 11%, 32%, and 100%, respectively.
Two patients had marrow obtained for clinical reasons, allowing assessment of bone marrow chimerism. At 3 months post-HCT, 1 patient (UPN 1713) had 52% donor chimerism in the CD34+ cell fraction, higher than the 33% donor CD14/15 chimerism noted in simultaneous peripheral blood. At 10 months post-HCT, 1 patient (UPN 1875) had only 2% donor chimerism in the CD34+ bone marrow cell fraction, corresponding closely to the 1% donor CD14/15 chimerism noted in peripheral blood.
As shown in Figure 1, median B cell chimerism started high and generally remained so. However, in 2 patients with baseline autologous B cells (UPN 1740 and UPN 1799) and low post-HCT myeloid chimerism, there was evidence of significant host B cells (only 17% and 3% donor, respectively) by 2 years post-HCT. Complete details on chimerism up to 2 years post-HCT can be found in Supplementary Table 1.
GVHD and Autoimmunity
Grades II to IV acute GVHD was seen in 2 patients. There were no cases of chronic GVHD. Three patients developed B cell-mediated autoimmunity at 9, 37, and 38 months post-HCT. Therefore, 50% of patients required immunosuppressive therapy at some point post-HCT (Table 2).
Immune Reconstitution and Survival
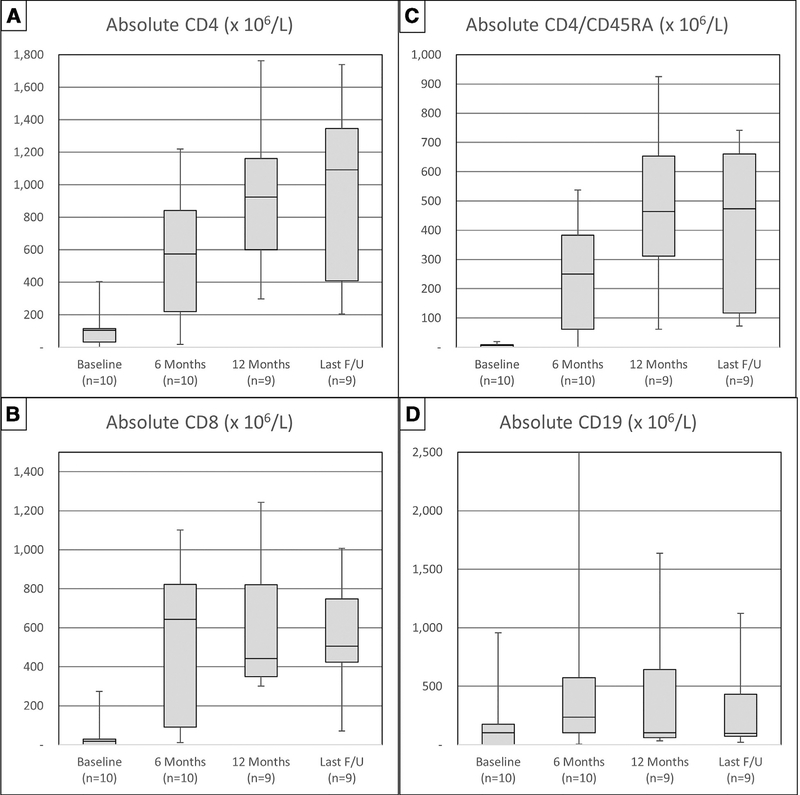
As shown in Figure 3A–C, the median CD4, CD4/CD45RA, and CD8 counts at 6 months post-HCT were 573 × 106/L (range, 17 to 1220), 250 × 106/L (range, 0 to 537), and 643 × 106/L (range, 11 to 1102), respectively. CD4 and CD4/ CD45RA counts continued to rise at 12 months and time of last follow-up, whereas CD8 numbers tended to stabilize after 6 months (Table 3). There was no apparent difference in median CD4 counts between those who did or did not receive thiotepa (6-month median, 799 versus 437 × 106/L [P = .4]; 12 months median, 910 versus 1104 × 106/L [P = .82]; last follow-up, 818 versus 1092 × 106/L [P = .68]), although numbers are small.
Figure 3.
Immune reconstitution over time in CD4+ T cells (A), CD8+ T cells (B), CD4/CD45RA+ naïve T cells (C), and CD19+ B cells (D).
Table 3.
Immune Reconstitution at 6 months, 12 months, and Last Follow Up
| 6 month Evaluation | 12 month Evaluation | Time to off IVIG(years) | At Last F/U Evaluation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN | CD4 (x10^6/L) | CD4/CD45RA (x10^6/L) | CD4 (x10^6/L) | CD4/CD45RA (x10^6/L) | TRECs | Last F/U (years) | T cellChimerism | B cell Chimerism | Myeloid Chimerism | CD4 (x10^6/L) | CD4/CD45RA (x10^6/L) | |
| 33 | 570 | 125 | 1567 | 925 | 99 | 1.0 | 7.5 | 100% | 100% | 100% | 1092 | 699 |
| 1713 | 191 | 42 | 385 | 128 | ND (14 @ 20 mo) | NR | 4.2 | 99% | 94% | 42% | 324 | 117 |
| 1740 | 576 | 374 | 1161 | 824 | 64 | 0.8 | 5.9 | 95% | 8% | 5% | 1378 | 661 |
| 1799 | 1220 | 537 | 1104 | 464 | ND | 0.6 | 5.0 | 98% | 2% | 5% | 1122 | 292 |
| 1835 | 1307 | 384 | 1438 | 820 | ND (476 @ 24 mo) | 0.8 | 5.0 | 93% | 99% | 83% | 1739 | 742 |
| 1837 | 304 | 119 | 600 | 312 | 17 | 1.4 | 4.9 | 95% | 76% | 3% | 408 | 73 |
| 1875 | 17 | 0 | NR* | NR* | NR* | NR* | 0.9* | 80% | 50% | 1%* | 18* | 0* |
| 1947 | 894 | 378 | 923 | 443 | ND | NR^ | 3.5 | 98% | 82% | 14% | 818 | 622 |
| 2046 | 683 | 423 | 897 | 601 | 117 | 1.7 | 2.0 | 97% | 92% | 8% | 1345 | 474 |
| 2078 | 87 | 27 | 297 | 95 | 5 | NR^ | 2.0 | 92% | 33% | 78% | 204 | 82 |
Bolded indicates a value considered to be ‘normal’ per institutional definition (see text).
TRECs, T cell receptor excision circles; IVIG, Intravenous Immunoglobulin; NR, Not Reached; ND, Not Done.
Received rituximab for treatment of autoimmunity.
Underwent repeat conditioned HCT from a new donor and is now 100% donor in all cell lines with normal T and B cell numbers & function at 2.9 years from most recent HCT.
At 6 and 12 months post-HCT 60% and 70% of patients, respectively, had full T cell reconstitution. Of the 3 patients without T cell reconstitution, 1 patient (UPN 1875) underwent a third HCT from a new URD at 10.5 months post-HCT due to continued poor immunity. Another subject (UPN 1713) experienced late improvement in T cell reconstitution and significant improvement in his severe norovirus-related diarrhea. Both patients were >12 months old at the time of HCT. One patient with an unknown genotype (UPN 2078) did not recover T cells at 2 years post-HCT, albeit in the setting of ongoing immunosuppressive treatment for autoimmune hemolytic anemia.
As shown in Figure 3D, the median CD19 count at 6 months was 235 × 106/L (range, 6 to 2644), with falling median counts at 12 months (103 × 106/L; range, 34 to 1636) and at time of last follow-up (98 × 106/L; range, 21 to 1122). Furthermore, in patients with baseline pre-HCT presence of B cells, the median final B cell numbers were 431 × 106/L (range, 33 to 1122) compared with 86 × 106/L (range, 21 to 112) in those with baseline absence of B cells (P = .16), suggesting that in patients with mixed myeloid and B cell chimerism, final B cell count may partly depend on residual endogenous B cell production.
Six patients recovered B cell immunity at a median of .9 years post-HCT (range, .6 to 1.7), and 1 additional patient eventually recovered B cells after repeat HCT. The median myeloid chimerism for the 6 patients with B cell reconstitution was 7% (range, 3% to 100%), 3 of whom have RAG or DCLRE1C mutations. One patient (UPN 1799) had a mutation in BCL11B, in which host B cells may not be defective [11]. Three patients remain on Ig at last follow-up, 2 of whom received rituximab for treatment of B cell mediated autoimmunity. With a median of 4.8 years of follow-up (range, 2.0 to 7.8), all patients are alive for an estimated 2-year event-free survival of 90% (95% confidence interval, 57% to 99%) and overall survival of 100% (95% confidence interval, 68% to 100%).
DISCUSSION
This retrospective report of low-exposure busulfan (cumulative area under the curve, 30 mg.hr/L) demonstrates that most patients experience significant autologous reconstitution of their marrow and only a small fraction of donor stem cells (as measured by blood myeloid chimerism) are required to restore functional long-term donor B cells in most patients. These data underscore the importance of careful monitoring of busulfan exposure in very young children, because drastically different doses (approximately 2-fold) were required to achieve comparable exposure, likely due to rapid changes in physiologic processes and maturation of liver metabolism in the first year of life as well as genetic variations in metabolic enzymes [15]. These data also demonstrate that busulfan dose does not adequately describe conditioning intensity and identifies the limitations of the current Center for International Blood and Marrow Transplant Research criteria for defining the intensity of a conditioning regimen [22]. Despite equivalent exposure throughout the cohort, 2 of 10 patients had total busulfan dosing > 8.0 mg/kg, classifying them per Center for International Blood and Marrow Transplant Research criteria as receiving “myeloablative conditioning.” This supports the decision of the Primary Immunodeficiency Treatment Consortium to apply an alternate definition of dose intensity for myeloablation (± 12 mg/kg total dose) [4,5]. This variability in exposure with a given dose also has implications beyond allogeneic HCT, becasue many gene therapy trials also use low-dose busulfan to open bone marrow niches for engraftment of gene-corrected cells [23].
As demonstrated by high levels of donor myeloid chimerism early post-HCT, low-exposure busulfan appears to effectively facilitate engraftment of committed myeloid progenitors. However, in most patients, between 2 and 4 months post-HCT there is a significant fall in donor myeloid chimerism. This suggests that low-exposure busulfan opens up a small number of marrow stem cell niches. One patient with significant pre-HCT marrow dysfunction achieved sustained 100% donor engraftment in the setting of severe acute GVHD, whereas 2 additional patients have had relatively stable myeloid chimerism of >80% at 2 and 5 years of follow-up. Most patients treated with this regimen stabilize with 3% to 10% long-term myeloid chimerism, which generally proved sufficient to restore B cell immunity and thereby prevent the long-term burden and costs of Ig replacement.
These data also suggest a potentially minimal contribution of thiotepa to the degree of myeloablation. This is supported by mouse models which demonstrate that although thiotepa improves allogeneic stem cell engraftment due to its immunoablative properties against T cells and NK cells, it has relatively limited effect against host primitive stem cells [24]. The ongoing C-SIDE trial (NCT03619551), which is comparing 2 different dose exposures of busulfan, may help determine the contribution of thiotepa to myeloid engraftment and toxicities, as patients with IL2RG or JAK3 mutations will only get busulfan, whereas those with RAG1/2 mutations will also receive thiotepa. The use of filgrastim and plerixafor before conventional-dosed conditioning has been reported to improve myeloid chimerism [25,26]; however, it is difficult to determine from these limited data whether plerixafor alone played any role in augmenting the effect of low-exposure busulfan, especially as 1 of 2 recipients of plerixafor was the patient ultimately with the poorest level of chimerism.
As long as they are not rejected, donor T cells will develop in a SCID patient even in the absence of any bone marrow engraftment, possibly from engraftment of long-term progenitors in the thymus [27]. However, in the absence of a graft-versus-marrow effect, space-making conditioning is required to achieve a bone marrow graft capable of producing functional donor B cells. Perhaps due to a selective advantage for the lymphocytes derived from a normal stem cell, it appears that only a small fraction of donor stem cells, in the range of 3% to 5%, may be sufficient to restore functional donor B cells in most patients, although additional studies are required to definitively determine the threshold amount. In contrast, other nonmalignant diseases typically require much higher levels of myeloid chimerism for clinical resolution of disease. For example, whole blood chimerism of >20% to 30% is protective against reactivation in patients with hemophagocytic lymphohistiocytosis [28], 20% donor myeloid chimerism is necessary to reverse the symptoms of sickle cell anemia [29], and as low as 10% may be sufficient for correcting thalassemia [30]. Therefore, patients with SCID represent optimal candidates for testing of novel approaches to facilitating engraftment of low amounts of stable donor myeloid engraftment, such as antibodies targeting CD45 or CD117 [23]. However, we also note that not all patients with levels of donor myeloid chimerism expected to be sufficient are guaranteed to have full T and B cell reconstitution. Possible hypotheses for this finding include alloreactive bone marrow dysfunction or thymic defects.
Although it appears that SCID infants can be effectively reconstituted using significantly reduced alkylator exposure, a potential downside is that mixed myeloid chimerism may predispose to the development of autoimmunity, especially in recipients of mismatched donors. This has previously been reported in post-HCT patients with Wiskott-Aldrich syndrome [31]. Wiskott-Aldrich syndrome carries a baseline risk of autoimmunity, and post-HCT autoimmunity in SCID patients may potentially be genotype dependent, as 2 of our 3 cases were in patients with mutations in RAG, which also carries a baseline risk of autoimmunity [32], and the third was in a patient of unknown genotype.
This report is limited by small numbers of patients, mixed molecular subtypes of SCID, and differing stem cell sources. However, we demonstrate that with precise targeting of busulfan, even low cumulative exposure accomplished multilineage engraftment and full immune reconstitution in patients with SCID. Remarkably, the long-term myeloid chimerism with this approach was extremely variable (ranging from 1% to 100%). This suggests that other factors, including the baseline health of the marrow, administration of secondary agents (eg, thiotepa or plerixafor), degree of donor matching, and post-HCT alloreactivity, all may play important roles in determining the degree of long-term bone marrow hematopoietic stem cell engraftment. The ongoing C-SIDE trial will help further clarify the role of low-exposure busulfan in establishing the minimal threshold of myeloid chimerism required to guarantee complete T and B cell reconstitution for patients with SCID.
Supplementary Material
ACKNOWLEDGMENTS
Conflict of interest statement: C.C.D., J.L.-B., and M.J.C. have received support from the California Institute of Medicine (DR2A-05365; PI: J. Shizuru). M.J.C., J.L.-B., and J.M.P. have received support from the California Institute of Medicine (CLIN2-10830 [PI: M.J. Cowan] and CLIN2-09504 [PI: M.J. Cowan]). C.C.D., J.M.P., and M.J.C. are supported by the Division of Allergy, Immunology and Transplantation, National Institute of Allergy and Infectious Diseases and the Office of Rare Diseases Research, National Center for Advancing Translational Sciences, National Institutes of Health, Public Health Service grant/cooperative agreements U54-AI082973 (PI: J.M. Puck), and U54-NS064808 and U01-TR001263 (PI: J.P. Krischer) and R13-AI094943 (PI: J.M. Puck).
Footnotes
Financial disclosure: The authors have nothing to disclose.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.bbmt.2019.03.008.
REFERENCES
- 1.Haddad E, Leroy S, Buckley RH. B-cell reconstitution for SCID: Should a conditioning regimen be used in SCID treatment? J Allergy Clin Immunol. 2013;131:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dvorak CC, Hassan A, Slatter MA, et al. Comparison of outcomes of hematopoietic stem cell transplantation without chemotherapy conditioning by using matched sibling and unrelated donors for treatment of severe combined immunodeficiency. J Allergy Clin Immunol. 2014;134:935–943. e915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dvorak CC, Patel K, Puck JM, et al. Unconditioned unrelated donor bone marrow transplantation for IL7Rα- and Artemis-deficient SCID. Bone Marrow Transplant 2017;52:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heimall J, Logan BR, Cowan MJ, et al. Immune reconstitution and survival of 100 SCID patients post-hematopoietic cell transplant: a PIDTC natural history study. Blood. 2017;130:2718–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haddad E, Logan B, Griffith L, et al. SCID genotype and 6-month posttransplant CD4 count predict survival and immune recovery: a PIDTC retrospective study. Blood. 2018;132:1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai S-Y, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371:434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heimall J, Puck J, Buckley R, et al. Current knowledge and priorities for future research in late effects after hematopoietic stem cell transplantation (HCT) for severe combined immunodeficiency patients: a consensus statement from the Second Pediatric Blood and Marrow Transplant Consortium international conference on late effects after pediatric HCT. Biol Blood Marrow Transplant 2017;23:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuetz C, Neven B, Dvorak CC, et al. SCID patients with ARTEMIS vs RAG deficiencies following HCT: increased risk of late toxicity in ARTEMIS-deficient SCID. Blood. 2014;123:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvorak CC, Hung G-Y, Horn B, Dunn E, Oon C-Y, Cowan MJ. Megadose CD34+ cell grafts improve recovery of T cell engraftment but not B cell immunity in patients with severe combined immunodeficiency disease undergoing haplocompatible nonmyeloablative transplantation. Biol Blood Marrow Transplant. 2008;14:1125–1133. [DOI] [PubMed] [Google Scholar]
- 10.Shearer WT, Dunn E, Notarangelo LD, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133:1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Punwani D, Zhang Y, Yu J, et al. Multisystem anomalies in severe combined immunodeficiency with mutant BCL11B. N Engl J Med. 2016;375: 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dvorak CC, Gilman AL, Horn B, et al. Haploidentical related-donor hematopoietic cell transplantation in children using megadoses of CliniMACs-selected CD34+ cells and a fixed CD3+ dose. Bone Marrow Transplant 2012;48:508. [DOI] [PubMed] [Google Scholar]
- 13.Wahlstrom J, Patel K, Eckhert E, et al. Transplacental maternal engraftment and posttransplantation graft-versus-host disease in children with severe combined immunodeficiency. J Allergy Clin Immunol. 2017;139: 628–633. e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartelink IH, Lalmohamed A, van Reij EML, et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. Lancet Haematol 2016;3:e526–e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savic RM, Cowan MJ, Dvorak CC, et al. Effect of weight and maturation on busulfan clearance in infants and small children undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:1608–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long-Boyle JR, Savic R, Yan S, et al. Population pharmacokinetics of busulfan in pediatric and young adult patients undergoing hematopoietic cell transplant: a model-based dosing algorithm for personalized therapy and implementation into routine clinical use. Ther Drug Monitor. 2015;37:236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson LE, Denny AW, Huh YO, Plunkett W, Keating MJ, Nelson JA. Natural killer cell activity in chronic lymphocytic leukemia patients treated with fludarabine. Cancer Chemother Pharmacol. 1996;37: 445–450. [DOI] [PubMed] [Google Scholar]
- 18.Ozyurek E, Cowan MJ, Koerper MA, Baxter-Lowe LA, Dvorak CC, Horn BN. Increasing mixed chimerism and the risk of graft loss in children undergoing allogeneic hematopoietic stem cell transplantation for non-malignant disorders. Bone Marrow Transplant. 2008;42:83. [DOI] [PubMed] [Google Scholar]
- 19.Puck JM. Laboratory technology for population-based screening for severe combined immunodeficiency in neonates: the winner is T-cell receptor excision circles. J Allergy Clin Immunol. 2012;129:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbacioglu S, Carreras E, Ansari M, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2017;53:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018;53:1401–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowan M, Dvorak C, Long-Boyle J. Opening marrow niches in patients undergoing autologous hematopoietic stem cell gene therapy. Hematol Oncol Clin North Am. 2017;31:809–822. [DOI] [PubMed] [Google Scholar]
- 24.Down JD, Westerhof GR, Boudewijn A, Setroikromo R, Ploemacher RE. Thiotepa improves allogeneic bone marrow engraftment without enhancing stem cell depletion in irradiated mice. Bone Marrow Transplant. 1998;21:327. [DOI] [PubMed] [Google Scholar]
- 25.Konopleva M, Benton CB, Thall PF, et al. Leukemia cell mobilization with G-CSF plus plerixafor during busulfan-fludarabine conditioning for allogeneic stem cell transplantation. Bone Marrow Transplant. 2015;50:939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balashov D, Laberko A, Shcherbina A, et al. A conditioning regimen with plerixafor is safe and improves the outcome of TCR alpha beta+ and CD19 + cell-depleted stem cell transplantation in patients with Wiskott-Aldrich syndrome. Biol Blood Marrow Transplant. 2018;24:1432–1440. [DOI] [PubMed] [Google Scholar]
- 27.Cavazzana-Calvo M, Carlier F, Le Deist F, et al. Long-term T-cell reconstitution after hematopoietic stem-cell transplantation in primary T-cell immunodeficient patients is associated with myeloid chimerism and possibly the primary disease phenotype. Blood. 2007;109:4575–4581. [DOI] [PubMed] [Google Scholar]
- 28.Hartz B, Marsh R, Rao K, et al. The minimum required level of donor chimerism in hereditary hemophagocytic lymphohistiocytosis. Blood. 2016;127:3281–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzhugh CD, Cordes S, Taylor T, et al. At least 20% donor myeloid chimerism is necessary to reverse the sickle phenotype after allogeneic HSCT. Blood. 2017;130:1946–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh MM, Wu CJ, Tisdale JF. In mixed hematopoietic chimerism, the donor red cells win. Haematologica. 2011;96:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moratto D, Giliani S, Bonfim C, et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980–2009: an international collaborative study. Blood. 2011;118:1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YN, Frugoni F, Dobbs K, et al. A systematic analysis of recombination activity and genotype-phenotype correlation in human recombination-activating gene 1 deficiency. J Allergy Clin Immunol. 2014;133:1099–1108. e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.