Abstract
The fate of engineered zero-valent copper nanoparticles (Cu NPs) in soils collected from geographically-distinct regions of the continental United States and incubated under controlled conditions was investigated with respect to NP affinity for soil surfaces and changes in speciation, as well as their impact on bacterial communities. Soil geochemical properties had a great influence on Cu NP migration and transformation. Translocation of Cu NPs was low in soils enriched in organic matter and high in clay and sandy soils. X-ray absorption spectroscopic analysis showed that the highest rates for transformation to Cu ions and adsorption complexes was in acidic soils. Although there was some change in overall bacterial community richness at the level of order in experimental soil, the level of perturbation was evident in side-by-side comparisons of orders using a 50% microbial community change value (MCC50). This assessment revealed that generally, Sphingomonas, known for its importance for remediation, and Rhizobiales, symbiotic partners with certain plants appeared susceptible to Cu NPs and their transformation products. The ecological importance of organisms from these orders and its greater vulnerability to Cu NPs suggests need for future targeted studies.
Keywords: Copper nanoparticles, Environmental impact, Transport, Bacteria, Regression model
Graphical Abstract

Change in relative abundance of the most variable bacteria for layer-site from Control to NP-treated Soil.
1. Introduction
Over the past decade, numerous engineered nanomaterials with properties tailored for specific applications have been developed. One such nanomaterial, zero-valent copper nanoparticles (Cu NPs) have been widely used in electronics, ceramics, films, polymers, inks, metallic, lubricant oil, coatings, and health care products (Rispoli et al., 2010). In particular, due to their excellent electrical conductivity, it is forecasted that along with silver NPs, Cu NPs will capture a major share of the conductive ink market by 2018 (Ghaffarzadeh and Zervos, 2015), in addition to their utilization in environmental remediation and for drug-resistant pathogens (Li et al., 2015, Daniel et al., 2014, Huang et al., 2011, Wu et al., 2009, Pelgrift and Friedman, 2013). Similar to other industrial products, it is predicted that Cu NPs will enter the soil unintentionally through atmospheric emissions, domestic wastewater, agriculture, and accidental release during manufacture and transport, or directly during remediation projects (Stampoulis et al., 2009). Therefore, an understanding of the transport and impact of Cu NPs in soil is of considerable importance.
The transport of engineered NPs has primarily been assessed using well-defined, porous materials such as glass beads or homogenous quartz sand in column experiments (Mystrioti et al., 2015, Zhang et al., 2015, Li and Ghoshal, 2016, El Badawy et al., 2013, Flory et al., 2013, Seiyed Mossa and Tosco, 2013, Basnet et al., 2015). In contrast, there are only few reports on engineered NP transport in natural soils (Cornelis et al., 2013, Omer et al., 2012, Zhang et al., 2012, Cornelis et al., 2012, Fang et al., 2009). From this admittedly limited data set, there appears to be two primary mechanisms that constrain the movement of well-dispersed engineered NPs in soil: NP aggregation or agglomeration and interactions between the NPs and the soil matrix (Omer et al., 2012). As a consequence, soil pH, dissolved organic matter, ionic strength, clay content, and surface charge contribute to the movement of particles through soil (Fang et al., 2009). Previously, we have shown that Cu NPs travel through field soil as NPs, with that transport parallel to transformation and dissolution with ion leaching (Collins et al., 2012). It is not known, however, if this is a general phenomenon or if the fate of NPs is related to soil type. Different soils will have distinct microbiota, and thus the impact of Cu NPs, as well as movement, are likely to be influenced by soil type. Studying the fate and transport of Cu NPs in situ at different field sites is not practical since abiotic factors could confound such analyses, but microcosm experiments allow the assessment of particle transport and any Cu NP-mediated community ecotoxic effects under similar environmental conditions. Here we have sampled soils from 12 different locations across the continental United States and have used these in microcosms in order to obtain a broader appreciation of the range of mobility of Cu NPs in different soil matrices and their possible impact on soil health.
2. Materials and methods
2.1. Nanoparticles
Zero-valent copper NPs were purchased from Sun Innovations (Fremont, CA) and were extensively characterized for crystal structure using powder X-ray diffraction, oxidation state assessed using X-ray absorption fine structure (XAFS) spectroscopy, the K-edge spectral profile obtained using a synchrotron, and particle size and shape determined using transmission electron spectroscopy, all as reported earlier (Collins et al., 2012). Briefly, they were uncapped, spherical and with particle sizes of 10–200 nm. The particles consisted of a core of metallic copper with an outer layer of oxidized copper (Cu2O and CuO) (Collins et al., 2012). The point of zero net proton charge, measured by isoelectric point (iep) was pH 9.4 in a NaCl background electrolyte (ranging from pH 10–4.5) (Collins et al., 2012).
2.2. Soil characterization
Top soil samples used in the study were obtained from twelve property owners and public locations at various locations in continental USA and immediately sealed in plastic bags. No permits were required for collection. Upon receipt, visible debris was removed from the soil samples (plant matter, rocks, and wood chips). After mixing for 15 min, subsamples were commercially characterized (Long Island Analytical Laboratories, New York) using standard Environmental Protection Agency (EPA) methods for pH (EPA 9045C), ion concentration (sodium, calcium, copper, iron; EPA 3050B/6010C), nitrogen (nitrate, nitrite, ammonia; EPA 9056 A and SM 4500NH3 B-97–11/SM19–21 4500-NH3 C), total phosphate (EPA 9056 A), total organic carbon (TOC) (EPA 9060A), and the percentage of clay, sand, rock, vegetation and moisture (SM 18–20 2540B (97)).
2.3. Soil treatments
Screened soil was placed in 200 mL Styrofoam cups to a depth of 8 cm and was allowed to stabilize for one week prior to amendment. Cu NPs (50 mg) were dusted over a 2 cm diameter area located at the center of the soil surface. Control soil received no NPs. Soils were incubated inside at 22.2 °C, the mean summer temperature for the continental USA (Anon.). Distilled water (20 mL) was introduced every 48 h to the 2 cm diameter central surface area, dropwise from a 25 mL glass pipette, to a total of 520 mL. Both the dusting and addition of distilled water was done from a distance of 2 cm from the soil surface. After 55 days, the soils were cored from the center by pushing down an open-end PVC centrifuge tubes (50 mL) into the soil cups. The affinity of the Cu NPs for soil surfaces was determined by monitoring the quantity of NPs that migrated away from the central location where they were deposited. Significant migration of particles through the loosely packed soils would indicate that there was no high affinity of the Cu NPs for the soil surface or that the soil chemical conditions contributed to significant aggregation of the NPs. To evaluate migration, core samples were divided into two equal layers, the top 4 cm layer and the bottom 4 cm layer. Soil remaining in the cup was designated as the side layered soil. Fig. 1 illustrates an experimental microcosm, which shows how the experimental setup allowed us to follow the vertical and sideward movement of NPs. Each of the three layers from each cup were mixed for 5 min to decrease soil heterogeneity. Experiments were performed in duplicates and a total of 48 microcosms were set-up for the experiment (2 control and 2 nanoparticle amended microcosm × 12 soil samples).
Figure 1.
Illustration of experimental setup and soil fraction distribution used for sample collection and the location of the application site of the copper nanoparticles (NP).
2.4. DNA isolation and 454-pyrosequencing
Prior to DNA isolation, soil samples were first treated with ethidium monoazide (EMA) as described (Pisz et al., 2007). The treatment allows the preferential amplification of DNA from viable bacteria. DNA was extracted from the soil using PowerSoil™ DNA isolation kits (MO BIO Laboratories Inc., Carlsbad, CA). Bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) were performed as described previously using Gray28F 5′TTTGATCNTGGCTCAG and Gray519r 5′ GTNTTACNGCGGCKGCTG primers (Omer et al., 2012). Amplifications consisting of one-step polymerase chain reaction (PCR) of 30 cycles, were accomplished using a mixture of Hot Start and HotStar high fidelity Taq polymerases. Amplicons originating and extending from the primers were used for initial generation of the sequencing library. Pyrosequencing analyses utilized Roche 454 FLX instrument with Titanium reagents and Titanium procedures and were performed at the Research and Testing Laboratory (Lubbock, TX) based upon RTL protocols (www.researchandtesting.com). After sequencing, all failed sequence reads, low quality sequence ends, tags and primers as well as any non-bacterial ribosome sequences and chimeras were removed using the UCHIME chimera detection software in de novo mode (Edgar et al., 2011). To curate the short (< 150 bp) reads, sequences with ambiguous base calls, and sequences with relatively long homopolymers (> 6 bp) were also removed. To determine the identity of bacteria in the remaining sequences, sequences were denoised, assembled into OTU clusters (97% identity) using the UPARSE algorithm (Edgar, 2013), and then globally aligned using the USEARCH global algorithm (Edgar, 2010) against a database of high quality 16S rRNA bacterial gene sequences compiled by RTL to determine taxonomic classifications. After OTU selection was performed, a phylogenetic tree was constructed in Newick format from a multiple sequence alignment of the OTUs done in MUSCLE (Edgar, 2004a, Edgar, 2004b) and generated in FastTree (Price et al., 2009, Price et al., 2010). Based upon the generated OTU table and taxonomy file, the bacteria were classified at the appropriate taxonomic levels. The percentage of sequences assigned to each bacterial phylogenetic level were individually analyzed for each pooled sample providing relative abundance information within and among the individual samples.
2.5. Solid phase analysis
After sampling, samples were freeze dried (Millrock Technology, Bench Top Model, Kingston, NY) and stored in airtight containers prior to analysis. The concentration of Cu was determined by microwave-assisted acid digestion (USEPA, 1986). Briefly, a sample (0.2–0.5 g) of the homogenized, dried soil was extracted via microwave-assisted digestion and chemical extraction using boiling nitric and hydrochloric acid. The resulting extracts were analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES; Thermo Elemental IRIS Intrepid; Madison, WI). The accuracy and precision of the instrument were verified before and after analysis.
The copper K-edge spectra were collected at beam line 10-BM (Materials Research Collaborative Access Team, Advanced Photon Source, Argonne National Laboratory, Argonne, IL). In addition to the MCA, XAFS scans were collected for pure mineral forms of malachite (Cu2CO3(OH)2), copper hydroxide (Cu(OH)2), tentorite (CuO), cuprite (Cu2O), and pseudomalachite (Cu5(PO4)2(OH)2); aqueous copper solutions of copper chloride, copper acetate, copper oxalate, and copper EDTA; and Cu adsorbed to ferrihydrite, γAl2O3, smectite, and birnessite. Adsorption spectra were collected at the K-edge energies of 8979 eV and scans were collected from 8779 to 9979 eV. Data collection was done in fluorescence mode using a 4-element solid-state Si-detector. The synchrotron was operated at 7.0 GeV at a nominal 100 mA fill current. The energy of a Si (111) double crystal monochromator was calibrated using an elemental Cu foil. All spectra were collected under ambient conditions. A minimum of three scans (and up to 5) was collected for each sample. All spectra were processed using the Athena program in the IFEFFIT software package for analysis of X-ray absorption spectroscopy (Ravel and Newville, 2005). Speciation was determined through linear combination fitting of the first derivative of the X-ray absorption near edge structure (XANES) spectra. Prior to LCF analysis spectra were averaged and normalized. LCF analysis was conducted on both the normalized and first derivative of the normalized data.
2.6. Data analysis
DNA isolation was performed in duplicate for each sample and the two isolated DNA samples were pooled prior to pyrosequencing. To avoid including sequences that may have been generated by PCR errors, only those sequences present at ≥ 1% abundance were used for richness and MCC50 analysis. Thus microbial richness is defined in the study as the number of orders in soil occupying > 1% of the microbial population. A microbial richness ratio was calculated by dividing the richness in NP-treated soils by the richness in control soils for each corresponding layer. A Microbial Community Change (MCC50) value was calculated using Eq. (1).
| (1) |
The solid phase analysis of the soil was carried out in triplicate. It should be noted that the natural copper ions found in the control soils were accounted for prior to the calculation of the migration of the copper derived from the NP amendment. A Copper Retention Ratio (CRR) was calculated using the following Eq. (2).
| (2) |
A Nanoparticle Speciation Value (NSV) for each layer of soil analyzed was calculated using Eq. (3).
| (3) |
To further analyze pyrosequencing data, first a bar plot was generated using OTU relative abundances. UniFrac distances were calculated using the phyloseq package in R (McMurdie and Holmes, 2013) with multivariate differences among groups (location, treatment, and site) evaluated using methods described by Okansen et al. (Oksansen et al., 2011). For ADONIS, distances among samples were first calculated using unweighted (presence and absence of OTUs) or weighted (abundance of OTUs) UniFrac, and then an ANOVA-like simulation was conducted to test for group differences. Principal coordinates analyses (PCoA) were conducted and plotted from weighted and unweighted UniFrac distances. R statistical software was used for these analyses and in the creation of these figures (R Development Core Team, 2011).
To develop a linear regression model, 9 independent variables were selected from the chemical characteristics of each analyzed soil including pH, ion concentrations, TOC, and the proportion of clay and sand. Prior to developing the regression model, each of the independent variables was set with minimum and maximum values for each variable coding for 1.0 and 2.0, respectively (Table S1). Two separate regression models using Microsoft Excel® were developed with MCC50 for the top layer of soil and copper retention ratio (CRR) as dependent variables.
3. Results
3.1. Soil characteristics
Soil samples obtained from different locations across continental USA had chemical properties that varied widely (Table 1). For example, the pH ranged from 4.4–8.1, TOC ranged from 3970 to 37,500 mg/kg, and the major soil cations including calcium and iron, had a wide range distribution from almost 0–51,500 mg/kg and from 338 mg/kg–36,100 mg/kg, respectively. In contrast, nitrogen (ammonia, nitrate and nitrite) appeared to have a narrow range and phosphate did not vary widely (Table 1). Soils from two adjacent states (NY and PA) represented the extremes in the percentage of sand and clay, with soils from other states showing varying degrees of clay, sand and rock.
Table 1.
Physical and chemical properties of soil used in the study to understand the fate and impact of Cu NPs.
| State | pH | Ammonia | Na | Ca | Cu | Fe | Nitrate | Nitrite | Phosphate | TOC | Soil type | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg soil | Clay % | Rock % | Sand % | Others % | Vegetation % | Moisture % | ||||||||||
| AZ | 7.8 | < 1.00 | 285.0 | 51,500 | 29.4 | 18,400 | 16.0 | < 5.0 | 60.0 | 10,300 | 18 | 29 | 37 | 1 | 6 | 9 |
| CA | 7.9 | 11.2 | 464.0 | 4100 | 14.5 | 13,100 | 9.3 | < 5.0 | 99.9 | 12,300 | 16 | 18 | 45 | 10 | 1.2 | 9.8 |
| DE | 6.4 | 6.5 | 36.7 | 979 | 7.5 | 18,700 | 16.0 | < 4.6 | 38.4 | 7250 | 20 | 22 | 34 | 12 | < 1 | 11 |
| LA | 4.4 | 8.4 | 52.0 | 179 | 3.4 | 1020 | 12.0 | < 5.0 | 132.0 | 37,500 | 8.4 | 1.9 | 62 | 5 | 3.8 | 18.9 |
| MA | 5.1 | 7.5 | 80.0 | 8 | 1.7 | 383 | 13.0 | < 5.0 | 62.8 | 6510 | 12 | 34 | 35 | 2 | 6 | 11 |
| MD | 6.0 | 10.3 | 121.0 | 2550 | 47.0 | 36,100 | 16.0 | < 5.0 | 94.9 | 17,100 | 16.9 | 19 | 42 | 3 | 5 | 14.1 |
| MS | 6.7 | 5.6 | 13.2 | 1080 | 2.4 | 4400 | 10.0 | < 4.5 | 87.3 | 10,100 | 18 | 22 | 37 | 9 | 1.1 | 12.9 |
| ND | 8.1 | 8.4 | 123.0 | 34,300 | 33.8 | 11,000 | 23.0 | < 5.0 | 75.3 | 21,400 | 27 | 7.7 | 34 | 4 | 9.4 | 17.9 |
| NY | 4.9 | 6.5 | 8.1 | 57 | < 1.96 | 2980 | < 5.0 | < 5.0 | 78.7 | 3970 | 0 | 6.4 | 64 | 1 | 3.2 | 25.4 |
| NY (1) | 6.3 | 3.7 | 38.2 | 4810 | 17.4 | 29,100 | 18.0 | < 5.0 | 103.0 | 9980 | 16 | 27 | 39 | 3 | 7 | 8 |
| OH | 7.3 | 4.7 | 57.9 | 16,100 | 23.5 | 18,200 | 16.0 | < 4.9 | 120.0 | 15,300 | 21 | 25 | 27 | 15 | 1.5 | 10.5 |
| PA | 6.2 | 3.7 | 13.5 | 577 | 5.6 | 24,700 | 13.0 | < 5.0 | 144.0 | 11,700 | 51 | 9 | 11 | 3.8 | 9 | 16.2 |
(Abbreviations used are: AZ, Arizona; CA, California; DE, Delaware, LA, Louisiana; MA, Massachusetts; MD, Maryland; MS, Mississippi; NY, New York; OH, Ohio; PA, Pennsylvania for State and Na, Sodium; Ca, Calcium; Cu, Copper; Fe, Iron; TOC, Total Organic Carbon for soil properties).
3.2. NP affinity and speciation
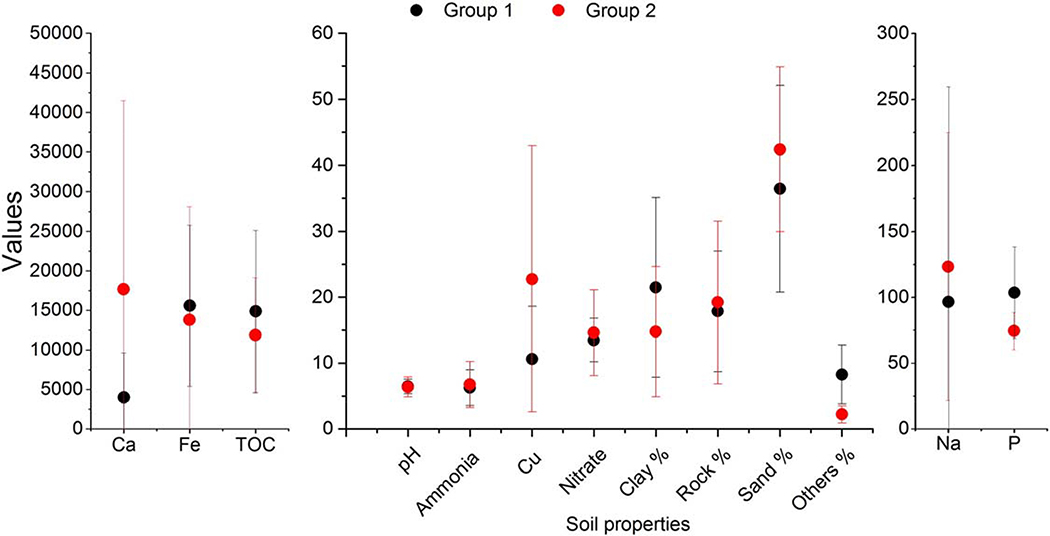
The transformation and affinity of Cu NPs for soil surfaces varied between soils. All soils showed evidence of Cu NP migration away from the initial depositional location, but this varied in magnitude (Fig. 2). Two distinct groups of soils were identified based on the migration of Cu NPs away from the depositional location. Group 1 (CA, DE, LA, MS, NY(1), PA and OH) showed little movement of the Cu NPs, indicating that the NPs had a high affinity for the soil surface. While for Group 2 (AZ, MA, MD, ND and NY), < 40% of the original NPs remained. T, the mean (and standard deviation) for each chemical and physical property was calculated to determine if there were specific differences between the two groups (Fig. 3). Overlapping error bars between the two indicated there was no statistical difference between any of the parameters measured. The biggest difference between means was associated with Ca, %Clay, and %Sand (3975 mg kg− 1, 22%, and 37% and 17,683 mg kg− 1, 14%, and 42% for Ca, Clay, and sand for Groups 1 and 2, respectively). It is formally possible that the increased concentration of Ca in Group 2 could result in some NP aggregation and thus a reduced interaction with the soil surface. However, closer inspection of the range of Ca concentrations in Group 2, along with the migration of Cu NPs does not support this hypothesis. Similarly, the increased clay content of the Group 1 may have resulted in reduced mobility of the Cu NPs through increased interaction/affinity with the clay fraction. As with the Ca data, closer inspection did not support the hypothesis. Although the differences in soil affinity could be associated with numerous parameters, no attempt was made to control physical factors that would govern solute movement through the soil (bulk density, porosity, particle size distribution, or minimization of macro pores). Therefore it is possible that migration or lack of migration was related to the physical condition of the soil along with the affinity of Cu NPs for the soil surface.
Figure 2.
Percentage of total copper after 55 days of incubation in different layers of NP treated soil (state abbreviations are as in Table 1).
Figure 3.
Average and standard deviation for soil chemical and physical properties for two soil groups based on the difference in the affinity of Cu NPs for the surface. Group 1 consists of CA, DE, OH, LA, MS, PA, and NY (1); Group 2 consists of AZ, MA, ND, NY, and MD. Units for Ca, Fe, TOC, Ammonia, Cu, Nitrate, Na, and P are presented as mg kg− 1; pH is present in pH units; and Clay, Rock, Sand, and Other are presented as percentages of the whole (abbreviations for state locations and soil properties are as in Table 1).
Calculations of Cu NP retention, CRR, allowed soil comparisons based solely on the ability of copper (in ion or NP form) to migrate through the soil. The highest retained copper ratios (values of 9.8 and 8.2) were in MS and DE soils, respectively (Table 2). In contrast, soils with high migration (MD and ND) had CRR values of 0.0. Again, for both extremes of the CRR ratio values there was no clear soil property or group of properties that were correlated with ion/particle mobility. It should be noted that CRR calculations treat each layer as individual blocks, and thus any migration within a particular layer would not be reflected in the ratio.
Table 2.
Microbial Community Change (MCC50), Nanoparticle Retention Ratio (NRR) and Nano Speciation Value (NSV) observed for various soil samples after 55 days of incubation.
| Soilb | MCC50 | Copper retention ratio (CRR) | Nano speciation value (NSV) | ||||
|---|---|---|---|---|---|---|---|
| Top | Bottom | Side | % MT/(%MS + %MB)a | Top | Bottom | Side | |
| AZ | 0.6 | 0.2 | 0.3 | 0.5 | 0.8 | 0.7 | 0.8 |
| CA | 0.7 | 0.4 | 0.3 | 6.0 | 0.3 | NA | 0.5 |
| DE | 0.6 | 0.3 | 0.4 | 8.2 | 0.6 | NA | 1.5 |
| LA | 0.1 | 0.3 | 0.8 | 6.2 | 1.1 | 6.1 | 15.0 |
| MA | 0.3 | 0.2 | 0.2 | 0.6 | 1.6 | 5.4 | 45.7 |
| MD | 0.5 | 0.4 | 0.6 | 0.0 | 1.1 | 7.0 | 1.8 |
| MS | 0.5 | 0.4 | 0.3 | 9.8 | 0.6 | NA | NA |
| ND | 0.1 | 0.2 | 0.3 | 0.0 | 0.4 | 1.3 | 0.6 |
| NY | 0.3 | 0.2 | 0.6 | 0.5 | 0.9 | 4.3 | NA |
| NY (1) | 0.1 | 0.2 | 0.2 | 1.9 | 0.7 | NA | 2.1 |
| OH | 0.3 | 0.1 | 0.1 | 4.8 | NA | NA | 1.4 |
| PA | 0.7 | 0.3 | 0.8 | 1.86 | 1.7 | NA | NA |
MT, Metal in the top layer; MS, Metal in the side layer; MB, metal in the bottom layer.
State abbreviations for the soil locations as in Table 1.
XAFS analysis provided insight into Cu NP degradation/dissolution in different soils. The linear combination fitting (LCF) results from the first derivative of the Cu Kα XANES data between − 20 to 30 eV over the edge energy are shown in Table 3. There was a difference in the relative abundance of Cu species present representing the added Cu NP (Cu0, Cu2O and CuO). The nano speciation value (NSV) denotes the distribution of copper in each layer of soil in both the nanoparticle and ionic form (Table 2). Since NSV represents the ratio of the percent copper in ionic form to percent copper in nanomaterial form, a higher NSV value indicates an increased transformation of NPs. The greatest transformations were seen in the side and bottom layers of LA and MA soils (Table 2). Both soils are acidic with a pH of 4.4 and 5.1 for LA and MA soil, respectively (Table 1), where an increased dissolution and transformation of the Cu NPs would be expected to occur (Adeleye et al., 2014, Conway et al., 2015). Although the pH of the NY soil was also acidic (4.9) and Cu would likely dissociate to cupric ions, the XANES data did not indicate that a significant portion of the Cu NPs had been ionized or transformed. However, the LA and NY soils were relatively deficient in Fe (Table 1) suggesting that Cu NPs could be used in chemical and biological reactions in the place of Fe and thus be more efficiently transformed (Conway et al., 2015, Kumar et al., 2011). In comparison, a low speciation value was seen for the AZ soils, where NPs migrated with minimum transformation. With a soil pH of 7.8, Cu ionization to cupric ions would not be expected. The relative abundance of the Cu NP species present in each soil as a function of pH underscores the association of low pH with dissolution of Cu NPs (Fig. 4). Similar analyses of other measured soil properties relative to the abundance of Cu NP species indicated that there was a significant relationship between the presence of Cu NP remaining after incubation and the Na and Ca ion concentration (mg·kg− 1). Elevated levels of soil Ca and Na in the soils may have resulted in the increased aggregation of the added Cu NPs thus reducing the available surface area for active dissolution and transformation of the NPs. However, the significantly lower concentrations of Na compared to Ca, would suggest another potential reason for the observation. Linear regression of Ca and Na as a function of other soil properties indicated that both NA and Ca were significantly correlated with pH (α = 0.05; p-value = 0.021 and 0.048 for Ca and Na, respectively). The history (fertilizer application, liming) of the soils used in the current study are unknown but the significant correlation between Ca and pH, and the weaker correlation between Na and pH, would suggest that some of the soils were limed in the past, resulting in an increase in pH. Calcium carbonate (calcite) in the form of crushed limestone is a common material used for raising soil pH in both agricultural and residential soil management. We suggest that the perceived relationship between Cu NP mobility between Na and Ca is potentially an artifact of soil management practices to alter soil pH.
Table 3.
Relative abundance of Cu species present in the different soils fractions after 55 days as determined from LCF analysis of the first derivative of the Cu kα XANES data (− 20 to 30 eV above the edge).
| Samplea | Cu2O | ± | CuO | ± | Cu | ± | Cu-Ferrb | ± | CuCl2aq | ± | Cu-Orgcd | ± | Red Chi2d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | ||||||||
| CA NT | 13 | 2 | 56 | 4 | 7 | 2 | 24 | 3 | 4.7 ∗ 10− 5 | ||||
| CA NS | 8 | 1 | 71 | 2 | 5 | 1 | 16 | 1 | 0 | 0 | 2.8 ∗ 10− 5 | ||
| DE NT | 8 | 2 | 51 | 5 | 4 | 2 | 11 | 3 | 26 | 3 | 6.6 ∗ 10− 5 | ||
| DE NS | 6 | 1 | 61 | 2 | 12 | 2 | 21 | 2 | 0 | 0 | 3.6 ∗ 10− 5 | ||
| OH NS | 4 | 1 | 62 | 3 | 9 | 3 | 25 | 3 | 0 | 0 | 6.9 ∗ 10− 5 | ||
| LA NT | 5 | 1 | 67 | 1 | 4 | 1 | 23 | 2 | 1.7 ∗ 10− 5 | ||||
| LA NS | 39 | 3 | 28 | 3 | 34 | 9 | 5.5 ∗ 10− 5 | ||||||
| LA NB | 49 | 2 | 25 | 2 | 26 | 2 | 3.7 ∗ 10− 5 | ||||||
| MS NT | 4 | 1 | 66 | 3 | 29 | 5 | 5.7 ∗ 10− 5 | ||||||
| PA NT | 48 | 4 | 16 | 2 | 35 | 5 | 2.6 ∗ 10− 5 | ||||||
| NY NT | 6 | 2 | 63 | 4 | 32 | 7 | 4.8 ∗ 10− 4 | ||||||
| NY NS | 48 | 2 | 20 | 1 | 31 | 4 | 2.6 ∗ 10− 5 | ||||||
| AZ NT | 5 | 1 | 65 | 3 | 30 | 6 | 2.8 ∗ 10− 5 | ||||||
| AZ NS | 5 | 1 | 61 | 3 | 4 | 1 | 30 | 5 | 1.8 ∗ 10− 5 | ||||
| AZ NB | 5 | 1 | 60 | 2 | 6 | 1 | 29 | 3 | 2.3 ∗ 10− 5 | ||||
| MA NT | 55 | 2 | 13 | 1 | 32 | 3 | 1.7 ∗ 10− 5 | ||||||
| MA NS | 34 | 2 | 28 | 1 | 38 | 5 | 1.9 ∗ 10− 5 | ||||||
| MA NB | 23 | 5 | 33 | 3 | 44 | 8 | 1.2 ∗ 10− 4 | ||||||
| ND NT | 9 | 1 | 58 | 3 | 5 | 2 | 28 | 2 | 4.7 ∗ 10− 5 | ||||
| ND NS | 1 | 1 | 71 | 3 | 28 | 5 | 3.3 ∗ 10− 5 | ||||||
| ND NB | 10 | 2 | 66 | 3 | 24 | 4 | 8.1 ∗ 10− 5 | ||||||
| NY(1) NT | 5 | 1 | 60 | 3 | 35 | 5 | 2.2 ∗ 10− 5 | ||||||
| NY(1) NB | 4 | 2 | 47 | 3 | 21 | 3 | 28 | 8 | 9.0 ∗ 10− 5 | ||||
| MD NT | 5 | 1 | 64 | 3 | 32 | 4 | 2.5 ∗ 10− 5 | ||||||
| MD NS | 46 | 2 | 22 | 1 | 32 | 3 | 1.3 ∗ 10− 5 | ||||||
| MD NB | 22 | 4 | 36 | 2 | 42 | 5 | 3.3 ∗ 10− 5 | ||||||
Results of the LCF data are presented only for samples where the concentration of Cu in the soil was great enough to obtain useable XANES data for LCF analysis of the first derivative of the normalized data; state locations are abbreviated as listed in Table 1 with Cu NP treated top (NT), side (NS) or bottom (NB) samples.
Cu-Ferr refers to Cu adsorbed onto ferrihydrite.
Cu-Org refers to the summation of the Cu organic complexes used in the LCF fit. Individual complexes included LCF fit included Cu-Acetate, Cu-Citrate, and Cu EDTA.
Red Chi2 refers to the reduced chi square value reported in the Iffefit software package as a goodness of fit parameter.
Figure 4.
Percentage of copper nanoparticles remaining after 55 days of incubation as a function of soil pH.
3.3. Microbial community changes after NP amendment
There appeared to be no decrease in community richness, at least as assessed to the level of order in all three layers of half of the NP-treated soils (ND and PA), compared to unamended controls (Fig. 5). Similarly, in another six soils (AZ, CA, DE, MA, MS, and NY(1)), there was no decrease in richness in two of the three layers. In seven NP-treated soils, at least one layer showed increases in microbial richness, compared to unamended controls. In contrast, three soil samples showed decreases in richness in two of the three layers (MD, NY, and OH). LA soil sample showed decreases in richness in all three layers of the NP-treated soil when compared to the corresponding control. There appeared to be no relationship between soil characteristics including texture, pH, iron or nitrogen concentration and TOC and the potential for reduction in the number of recovered communities after Cu NP amendment (Table 1 and Fig. 5).
Figure 5.
Change in richness ratio (richness in experimental soil/richness in control soil) in soils originating from different states (abbreviated as listed in Table 1) after incubation with copper nanoparticles for 55 days. State locations are abbreviated as listed in Table 1.
To facilitate a side-by-side comparison of the impact on communities in the 12 different soils, and examine more than simple community richness, a MCC50 value was calculated, which allows orders that differ by 50% or more between control and experimental microcosms to be identified (Table 2). MCC50 values are similar to MCT25 values proposed in our earlier study, except we are using data obtained from a PCR-pyrosequencing experimental protocol (USEPA, 1986). Also, by increasing the value from 25 to 50, only orders that differed by 50% in the two sets were calculated, making the values more stringent comparisons. Even by using this more stringent cutoff, the perturbation of the microbial community was still evident in majority of soil samples. The highest impact to community structure was observed in side layers of the treated LA and PA soil with an MCC50 value of 0.8. High values of 0.7 were seen for top layers in CA and PA treated soil (Table 2).
In our previous field study of Cu NPs (Collins et al., 2012), we had observed that bacterial orders associated with nitrogen cycling appeared to be particularly susceptible to these particles. In the absence of environmental-imposed abiotic stress and the range of soil types tested here, there was no marked reduction in the percentage of bacteria representing Nitrosomonadales or Nitrospirales (with members capable of nitrification and nitrate oxidation, respectively) as a proportion to the total recovered bacterial sequences (Table S2). Nevertheless, there was an apparent reduction in the recovery of Sphingomonadales (an order known for symbiotic relationships and remediation properties), relative to other bacteria in the Cu NP-treated microcosms containing DE soil (22% and 13% to 4% and 5% for the top and side layer, respectively), in the bottom layer of OH soil (from 7% to 3%), in the side layer of MS soil (from 10% to 5%), in the side layer of PA soil (from 9% to 3%), and in the side layer of the AZ soil (from 4% to 9%). An increase (7% to 13%) was only observed in the side layer of CA soil. Marked reductions were also detected in Rhizobiales, a known nitrogen fixing bacterial order, in the top layer of MD soil (20% to 16%), CA soil (20% and 22% to 15% and 17% for the bottom and side layer, respectively), in the top layer of OH soil (14% to 10%), in the side layer of LA soil (from 18% to 9%), in the bottom layer of MS soil (from 23% to 15%), in PA soil (16%, 23%, and 16% to 12%, 14%, and 11% for the bottom, side, and top layer, respectively), and the side layer of NY soil (18% to 8%). Again, this was not always the case and an increase in Rhizobiales was observed in a single sample, the top layer of CA soil (12% to 20%) (Fig. 6). Therefore under well-controlled conditions, the susceptibility of the communities (at least at the level of order) to Cu NPs appears to be soil-specific.
Figure 6.
Relative abundance of the top 30 bacterial orders in both control and treatment, faceted by location (see state abbreviations in Table 1) and soil layer.
The PCoAs, for both unweighted and weighted UniFrac distances also identify that the microbial community structure is more location dependent than anything, with treatments and soil sites within each location grouping well (Fig. 7). The PERMANOVA using these distance matrices (ADONIS) yielded significance (P = 0.001 for both unweighted and weighted UniFrac) among locations while also explaining a large proportion of the variation (R2 = 0.57 and 0.77 for unweighted and weighted UniFrac, respectively; Table 4).
Figure 7.
Principal Coordinates Analysis based on unweighted and weighted UniFrac distances.
Table 4.
Results of the ADONIS, based on unweighted and weighted UniFrac distances.
| Unweighted UniFrac | Weighted UniFrac | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Df | SumsOfSqs | MeanSqs | F·Model | R2 | Pr (> F) | Df | SumsOfSqs | MeanSqs | F·Model | R2 | Pr (> F) | ||
| Location | 11 | 9.424 | 0.857 | 7.227 | 0.568 | 0.001 | Location | 11 | 0.900 | 0.082 | 19.313 | 0.767 | 0.001 |
| Treatment | 1 | 0.129 | 0.129 | 1.088 | 0.008 | 0.315 | Treatment | 1 | 0.010 | 0.010 | 2.389 | 0.009 | 0.032 |
| Site | 2 | 0.264 | 0.132 | 1.114 | 0.016 | 0.218 | Site | 2 | 0.023 | 0.012 | 2.725 | 0.020 | 0.006 |
| Treatment:Site | 2 | 0.250 | 0.125 | 1.054 | 0.015 | 0.328 | Treatment:Site | 2 | 0.008 | 0.004 | 0.887 | 0.006 | 0.549 |
| Residuals | 55 | 6.520 | 0.119 | NA | 0.393 | NA | Residuals | 55 | 0.233 | 0.004 | NA | 0.199 | NA |
| Total | 71 | 16.59 | NA | NA | 1 | NA | Total | 71 | 1.173 | NA | NA | 1.000 | NA |
3.4. Linear regression model
Nine geochemical and other characteristics were selected for the development of a linear regression model in an effort to predict the impact of each variable on CRR and MCC50. Values obtained for the soil top layer (Table 2) were used to obtain a model for MCC50. The model shown in Eq. (4) has R2 value of 0.83 (standard error = 0. 21; F ratio = 1.08). The model shown in Eq. (5) has an R2 value of 0.78 (standard error = 3.78; F ratio = 0.81). We caution that each model has been based on a limited number of experiments, does not consider secondary or interactive parameters, and with independent variables close to number of experiments, it cannot be used as predictively. Despite these caveats, however, the models shown in Eq. (4), (5) can be used to screen for variables with positive, negative or a negligible effects on the dependent variables.
| (4) |
| (5) |
where x1 is soil pH, x2 is the Na ion concentration, x3 is Ca ion concentration, x4 is Cu species, x5 is Fe species, x6 is P concentration, x7 is TOC, x8 is % of clay, and x9 is % of sand in the soil.
Based on the positive coefficient values, an increased Na and copper ion concentration combined with TOC appears to result in increased impact on microbial communities when exposed to Cu NPs (Eq. (4)). However, microbial assemblages in soil with higher iron, sand or clay concentrations were less influenced by NP addition (Eq. (4)). Ranking the coefficients in Eq. (5), it further suggests that soils with increased pH or TOC are associated with decreased migration of copper in the soil. Further, when clay or sand concentration increases, Cu NPs can be predicted to migrate some distance from the application site.
4. Discussion
The soils used in this study were collected from geographically-distinct sites in order to include a range of geochemical properties and parent materials in order to better assess the fate and impact of Cu NPs. Our results indicate that NP transport and speciation is effected by multiple variables. As NPs migrate through the soil matrix, ion leaching occurs parallel to that of particle transport (Table 1, Fig. 5), consistent with a previous report (Collins et al., 2012). However, the NP migration rate and the particular transformation products vary significantly with soil type, as underscored by CRR values varying widely between 9.8 and 0.0 (Table 2). Indeed, certain soil conditions resulted in little NP transformation (low NSV values) even after migration to lateral and bottom layers (low CRR values, e.g. ND soil). In other soils where the top layer retained NPs, there was similarly insignificant transformation (NSV values < 1, e.g. in DE and MS soils).
The impact of NPs and/or their transformation products on the soil community was evident with high MCC50 values, reflecting changes in the assemblage of certain samples (e.g. 0.7 in CA and PA soils). An increase in richness (Fig. 5) likely reflects a change in conditions leading to the proliferation of species which were in low abundance in control pots as well as the inhibition of some formerly abundant species. The changes to the bacterial communities observed in this study (Table S2) and others reported in the literature, suggests that members of the orders Sphingomonas and Rhizobiales are among the more susceptible to Cu NPs. Bacteria from Sphingomonas are known to degrade and mineralize a wide range of soil organic pollutants (Böltner et al., 2008, Aylward et al., 2013). Should NPs inhibit these organisms, the ability of soil to remove organic pollutants from the soil could be compromised, resulting in secondary problems associated with the accumulation of organic pollutants. Plant symbiotic bacteria of the order Rhizobiales are capable of nitrogen fixation (Carvalho et al., 2010). Thus based on these observations we recommend that bacteria of these orders be used for further study to examine the potential for environmental toxicity mediated by Cu NPs.
We carefully chose to regulate the temperature and to ensure that the soils were not saturated with water to prevent any anaerobic conditions that certainly would have impacted the assemblage. The addition of only 20 mL water every 48 h resulted in cracks in soils with a high clay content after few days of incubation. These drier channels would have facilitated the migration of NPs laterally and vertically. Thus the use of Eq. (5) revealed clay as a significant factor in determining copper retention in soil. Porosity is higher in sandy soil and not surprisingly, Eq. (5) also showed that soils with a higher percentage of sand, there was increased particle migration. As a consequence, a decreased impact of NPs can be expected in soils with a high percentage of clay and sand. Concomitantly, the use of Eq. (4) showed that MCC50 was negatively proportional to clay and sand concentration, supporting our hypothesis.
A positive coefficient of pH in Eq. (5) further suggested that as soil pH increases, Cu was retained in increasing proportions in the top layer. The point of zero charge (PZC) for the Cu NPs used here was at pH 9.4, (IEP measured in 0.01 M NaCl) (Collins et al., 2012) and all the soil samples used the study had a lower pH. Thus the effect of pH on fate of NPs could be attributed to the increasing transformation of NPs under acidic conditions. The strength of the relationship between pH, and relative abundance of Cu NPs, highlights the importance of this variable in potentially predicting the persistence of Cu NPs in soil. Considering that pH is a relatively simple environmental property to measure that does not require extensive laboratory analysis, it could be used as an early screening tool to assess the impact of Cu NPs in soil.
TOC was also shown to increase Cu retention (Eq. (5)), and this phenomenon has been previously observed with Ag NPs, where particle migration was significantly lower in soil rich in organic matter (Coutris et al., 2012). Organic carbons including humic acids appear to coat metal NPs and increase their mobility through steric interactions and electrostatic stabilization, at least in saturated column studies. Under saturated conditions, NPs would move quickly with insufficient time to undergo transformation. In our drier mesocosms, there would be time for Cu ionization between wetting periods. Ionized Cu would then bind to soil organic carbon and be retained longer (Yin et al., 2002). Thus the prolonged presence of NPs and ions in soil in the presence of increased TOC should result in a greater impact on the microbial community. A positive coefficient for TOC in Eq. (4), supports this hypothesis.
Taken together this research shows that the fate and impact of Cu NPs are entwined. The bacterial community can be perturbed by varying degrees, depending on the geochemical properties of the soil, the parent material, and the layer analyzed. While we were heartened to see that there was not an obvious impact on several orders of bacteria associated with nitrogen cycling, the long term effects on soil fertility and productivity are currently unknown. Thus we advise that additional study is warranted to determine if the changes in bacterial community observed are temporary or permanent and if they can impact soil function or crop production.
Supplementary Material
Highlights.
Soil geochemical properties had a great influence on Cu NP migration and transformation.
Affinity of Cu NPs was greatest in organic soils with increased translocation seen in clay and sand-rich soils.
The highest rates for transformation to Cu ions and adsorption complexes was in acidic soils.
Cu NPs had little effect on overall bacterial community richness at the level of order.
Sphingomonas and Rhizobiales appeared susceptible to Cu NPs and their transformation products.
Acknowledgement
The work was funded by National Science Foundation grant # 966741 to VS and Natural Sciences and Engineering Research Council (Discovery; Canada) to VKW. TB and JY were sponsored by an NSF funded HBCU-UP E3MaS grant HRD - 0928797 of Southern University at New Orleans. This work has been subjected to EPA administrative review and approved for publication. Any opinions expressed in this paper are those of the author(s) and do not, necessarily, reflect the official positions and policies of the USEPA or the funding agencies. Any mention of the products or trade names does not constitute recommendations for use by the USEPA. The Advanced Photon Source (APS, Argonne, Illinois, USA) is acknowledged for the allocation of beam time. MRCAT operations are supported by the Department of Energy (DOE) and the MRCAT member institutions. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Conflict of interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
References
- Adeleye AS, Conway JR, Perez T, Rutten P, Keller AA, Influence of extracellular polymeric substances on the long-term fate, dissolution, and speciation of copper-based nanoparticles, Environ. Sci. Technol, 48 (2014), pp. 12561–12568 [DOI] [PubMed] [Google Scholar]
- Aylward FO, McDonald BR, Adams SM, et al. , Comparison of 26 Sphingomonad genomes reveals diverse environmental adaptations and Biodegradative capabilities, Appl. Environ. Microbiol, 79 (2013), pp. 3724–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basnet M, Tommaso CD, Ghoshal S, Tufenkji N, Reduced transport potential of a palladium-doped zero valent iron nanoparticle in a water saturated loamy sand, Water Res, 68 (2015), pp. 354–363 [DOI] [PubMed] [Google Scholar]
- Böltner D, Godoy P, Muñoz-Rojas J, et al. , Rhizoremediation of lindane by root-colonizing Sphingomonas, Microb. Biotechnol, 1 (2008), pp. 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho FM, Souza RC, Barcellos FG, Hungria M, Vasconcelos ATR, Genomic and evolutionary comparisons of diazotrophic and pathogenic bacteria of the order Rhizobiales, BMC Microbiol., 10 (2010), p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Luxton T, Kumar N, Shreya S, Walker VK, Shah V, Assessing the impact of copper and zinc oxide nanoparticles on soil: a field study, PLoS One, 7 (2012), p. e42663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JR, Adeleye AS, Gardea-Torresdey J, Keller AA, Aggregation, dissolution, and transformation of copper nanoparticles in natural waters, Environ. Sci. Technol, 49 (2015), pp. 2749–2756 [DOI] [PubMed] [Google Scholar]
- Cornelis G, Doolette C, Thomas M, McLaughlin MJ, Kirby JK, Beak DG, Chittleborough D, Retention and dissolution of engineered silver nanoparticles in natural soils, Soil Sci. Soc. Am. J, 76 (2012), pp. 891–902 [Google Scholar]
- Cornelis G, Pang L, Doolette C, Kirby JK, McLaughlin MJ, Transport of silver nanoparticles in saturated columns of natural soils, Sci. Total Environ, 463 (2013), pp. 120–130 [DOI] [PubMed] [Google Scholar]
- Coutris C, Joner EJ, Oughton DH, Aging and soil organic matter content affect the fate of silver nanoparticles in soil, Sci. Total Environ, 420 (2012), pp. 327–333 [DOI] [PubMed] [Google Scholar]
- Daniel SK, Malathi S, Balasubramanian S, Sivakumar M, Sironman TA, Multifunctional Silver, Copper and Zero Valent Iron Metallic Nanoparticles for Wastewater Treatment, Application of Nanotechnology in Water Research (2014), pp. 435–457 [Google Scholar]
- Edgar RC, MUSCLE: multiple sequence alignment with high accuracy and high throughput, Nucleic Acids Res, 32 (2004), pp. 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC, MUSCLE: a multiple sequence alignment method with reduced time and space complexity, BMC Bioinforma., 19 (2004), p. 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC, Search and clustering orders of magnitude faster than BLAST, Bioinformatics, 1–3 (2010) [DOI] [PubMed] [Google Scholar]
- Edgar RC, UPARSE: highly accurate OTU sequences from microbial amplicon reads, Nat. Methods, 10 (2013), pp. 996–998 [DOI] [PubMed] [Google Scholar]
- Edgar RC, Hass BJ, Clemente JC, Quince C, Knigh R, UCHIME improves sensitivity and speed of chimera detection, Oxf. J. Bioinforma, 16 (2011), pp. 2194–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Badawy AM, Aly Hassan A, Scheckel KG, Suidan MT, Tolaymat TM, Key factors controlling the transport of silver nanoparticles in porous media, Environ. Sci. Technol, 47 (2013), pp. 4039–4045 [DOI] [PubMed] [Google Scholar]
- Fang J, Shan X.-q., Wen B, Lin J-M, Owens G, Stability of titanium nanoparticles in soil suspensions and transport in saturated homogenous soil columns, Environ. Pollut, 157 (2009), pp. 1101–1109 [DOI] [PubMed] [Google Scholar]
- Flory J, Kanel SR, Racz L, Impellitteri CA, Silva RG, Goltz MN, Influence of pH on the transport of silver nanoparticles in saturated porous media: laboratory experiments and modeling, J. Nanopart. Res, 15 (2013), pp. 1–11 [Google Scholar]
- Ghaffarzadeh K, Zervos H, Conductive Ink Markets 2015–2025: Forecasts, Technologies, Players Silver Flake, Silver Nanoparticles, Copper, Graphene, PEDOT and beyond, IDTechEx (2015) [Google Scholar]
- Anon., Summer temperature averages for every state, Available online, http://www.currentresults.com/Weather/US/average-state-temperatures-in-summer.php, accessed on 17/2/2016, Huang et al., 2011
- Huang CC, Lo SL, Tsai SM, Lien HL, Catalytic hydrodechlorination of 1, 2-dichloroethane using copper nanoparticles under reduction conditions of sodium borohydride, J. Environ. Monit, 13 (2011), pp. 2406–2412 [DOI] [PubMed] [Google Scholar]
- Kumar N, Shah V, Walker VK, Perturbation of an arctic soil microbial community by metal nanoparticles, J. Hazard. Mater, 190 (2011), pp. 816–822 [DOI] [PubMed] [Google Scholar]
- Li J, Ghoshal S, Comparison of the transport of the aggregates of nanoscale zerovalent iron under vertical and horizontal flow, Chemosphere, 144 (2016), pp. 1398–1407 [DOI] [PubMed] [Google Scholar]
- Li P, Song Y, Wang S, Tao Z, Yu S, Liu Y, Enhanced decolorization of methyl orange using zero-valent copper nanoparticles under assistance of hydrodynamic cavitation, Ultrason. Sonochem, 22 (2015), pp. 132–138 [DOI] [PubMed] [Google Scholar]
- McMurdie PJ, Holmes S, Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data, PLoS One, 8 (2013), p. e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mystrioti C, Papassiopi N, Xenidis A, Dermatas D, Chrysochoou M, Column study for the evaluation of the transport properties of polyphenol-coated nanoiron, J. Hazard. Mater, 281 (2015), pp. 64–69 [DOI] [PubMed] [Google Scholar]
- Oksansen J, Blanchet FG, Kindt R, et al. , Permutational Multivariate Analysis of Variance Using Distance Matrices, Community Ecology Package (2011), R package version 1.17–8 [Google Scholar]
- Omer S, Dror I, Berkowitz B, Transport of silver nanoparticles (AgNPs) in soil, Chemosphere, 88 (2012), pp. 670–675 [DOI] [PubMed] [Google Scholar]
- Pelgrift RY, Friedman AJ, Nanotechnology as a therapeutic tool to combat microbial resistance, Adv. Drug Deliv. Rev, 65 (2013), pp. 1803–1815 [DOI] [PubMed] [Google Scholar]
- Pisz JM, Lawrence JR, Schafer AN, Siciliano SD, Differentiation of genes extracted from non-viable versus viable microorganisms in environmental samples using ethidium monoazide bromide, J. Microbiol. Methods, 71 (2007), pp. 312–318 [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP, FastTree: computing large minimum evolution trees with profiles instead of a distance matrix, Mol. Biol. Evol, 26 (2009), pp. 1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP, FastTree 2 – approximately maximum-likelihood trees for large alignments, PLoS One, 5 (2010), p. e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria: (2011), ISBN 3–900051-07–0 [Google Scholar]
- Ravel B, Newville M, ATHENA ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT, J. Synchrotron Radiat, 12 (2005), pp. 537–541 [DOI] [PubMed] [Google Scholar]
- Rispoli F, Angelov A, Badia D, Kumar A, Seal S, Shah V, Understanding the toxicity of zero valent copper nanoparticle against Escherichia coli, J. Hazard. Mater, 180 (2010), pp. 212–216 [DOI] [PubMed] [Google Scholar]
- Seiyed Mossa H, Tosco T, Transport and retention of high concentrated nano-Fe/Cu particles through highly flow-rated packed sand column, Water Res, 47 (2013), pp. 326–338 [DOI] [PubMed] [Google Scholar]
- Stampoulis D, Sinha KS, White JC, Assay-dependent phytotoxicity of nanoparticles to plant, Environ. Sci. Technol, 43 (2009), pp. 9473–9479 [DOI] [PubMed] [Google Scholar]
- USEPA, Metalitc Analytes. 3.2, Sample Preparation Methods. Method 3050/3051, Acid Digestion of Sediments, Sludges, and Soils, vol. 1, USEPA, Washington, DC: (1986), Section A. Part I. Chapter 3 [Google Scholar]
- Wu SJ, Liou TH, F.L. M, Synthesis of zero-valent copper-chitosan nanocomposites and their application for treatment of hexavalent chromium, Bioresour. Technol, 100 (2009), pp. 4348–4353 [DOI] [PubMed] [Google Scholar]
- Yin Y, Impellitteri CA, You SJ, Allen HE, The importance of organic matter distribution and extract soil: solution ratio on the desorption of heavy metals from soils, Sci. Total Environ, 287 (2002), pp. 107–119 [DOI] [PubMed] [Google Scholar]
- Zhang L, Hou L, Wang L, Kan AT, Chen W, Tomson MB, Transport of fullerene nanoparticles (n C60) in saturated sand and sandy soil: controlling factors and modeling, Environ. Sci. Technol, 46 (2012), pp. 7230–7238 [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang H, Tu C, Hu X, Li L, Luo Y, Christie P, Facilitated transport of titanium dioxide nanoparticles by humic substances in saturated porous media under acidic conditions, J. Nanopart. Res, 17 (2015), pp. 1–11 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.