CONSPECTUS:
Over the past decade, there has been growing interest in developing biosensors and devices with nanoscale and vertical topography. Vertical nanostructures induce spontaneous cell engulfment, which enhances the cell–probe coupling efficiency and the sensitivity of biosensors. Although local membranes in contact with the nanostructures are found to be fully fluidic for lipid and membrane protein diffusions, cells appear to actively sense and respond to the surface topography presented by vertical nanostructures. For future development of biodevices, it is important to understand how cells interact with these nanostructures and how their presence modulates cellular function and activities.
How cells recognize nanoscale surface topography has been an area of active research for two decades before the recent biosensor works. Extensive studies show that surface topographies in the range of tens to hundreds of nanometers can significantly affect cell functions, behaviors, and ultimately the cell fate. For example, titanium implants having rough surfaces are better for osteoblast attachment and host–implant integration than those with smooth surfaces. At the cellular level, nanoscale surface topography has been shown by a large number of studies to modulate cell attachment, activity, and differentiation. However, a mechanistic understanding of how cells interact and respond to nanoscale topographic features is still lacking.
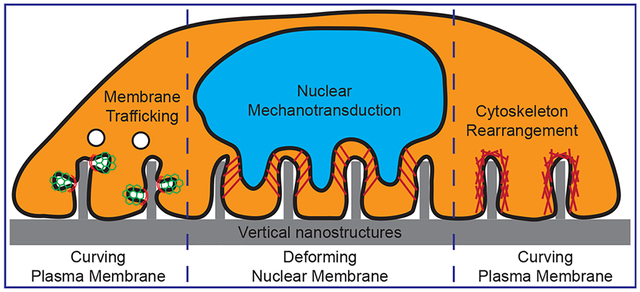
In this Account, we focus on some recent studies that support a new mechanism that local membrane curvature induced by nanoscale topography directly acts as a biochemical signal to induce intracellular signaling, which we refer to as the curvature hypothesis. The curvature hypothesis proposes that some intracellular proteins can recognize membrane curvatures of a certain range at the cell-to-material interface. These proteins then recruit and activate downstream components to modulate cell signaling and behavior. We discuss current technologies allowing the visualization of membrane deformation at the cell membrane-to-substrate interface with nanometer precision and demonstrate that vertical nanostructures induce local curvatures on the plasma membrane. These local curvatures enhance the process of clathrin-mediated endocytosis and affect actin dynamics. We also present evidence that vertical nanostructures can induce significant deformation of the nuclear membrane, which can affect chromatin distribution and gene expression. Finally, we provide a brief perspective on the curvature hypothesis and the challenges and opportunities for the design of nanotopography for manipulating cell behavior.
Graphical Abstract
1. INTRODUCTION
Nanostructures protruding from a flat surface have been utilized in the recent development of biosensors and biodevices. For example, vertical nanostructures have been used as electric probes for intracellular recording,1–3 mechanical probes for mechano-sensing4 and cell guidance,5 optical probes for fluorescence and Raman imaging,6,7 and tools for biomolecule delivery.8–12 These vertical nanostructures, including nanopillars, nanowires, nanoneedles, nanotubes, nanostraws, and nanocones, usually have a height of a few micrometers, a diameter of tens to hundreds of nanometers, and an aspect ratio of 1–200 (Figure 1). The use of vertical nanosensors for probing biological events have been summarized in a number of recent review articles.13–15 In addition to being used as biosensors, these vertical nanostructures were also found to affect cellular behaviors such as cell morphology, adhesion, migration, proliferation, and differentiation.16–18 Therefore, cells can actively respond to the presence of these vertical nanostructures. However, the molecular mechanism of how cells recognize these vertical nanostructures and induce intracellular processes remains poorly understood.18
Figure 1.
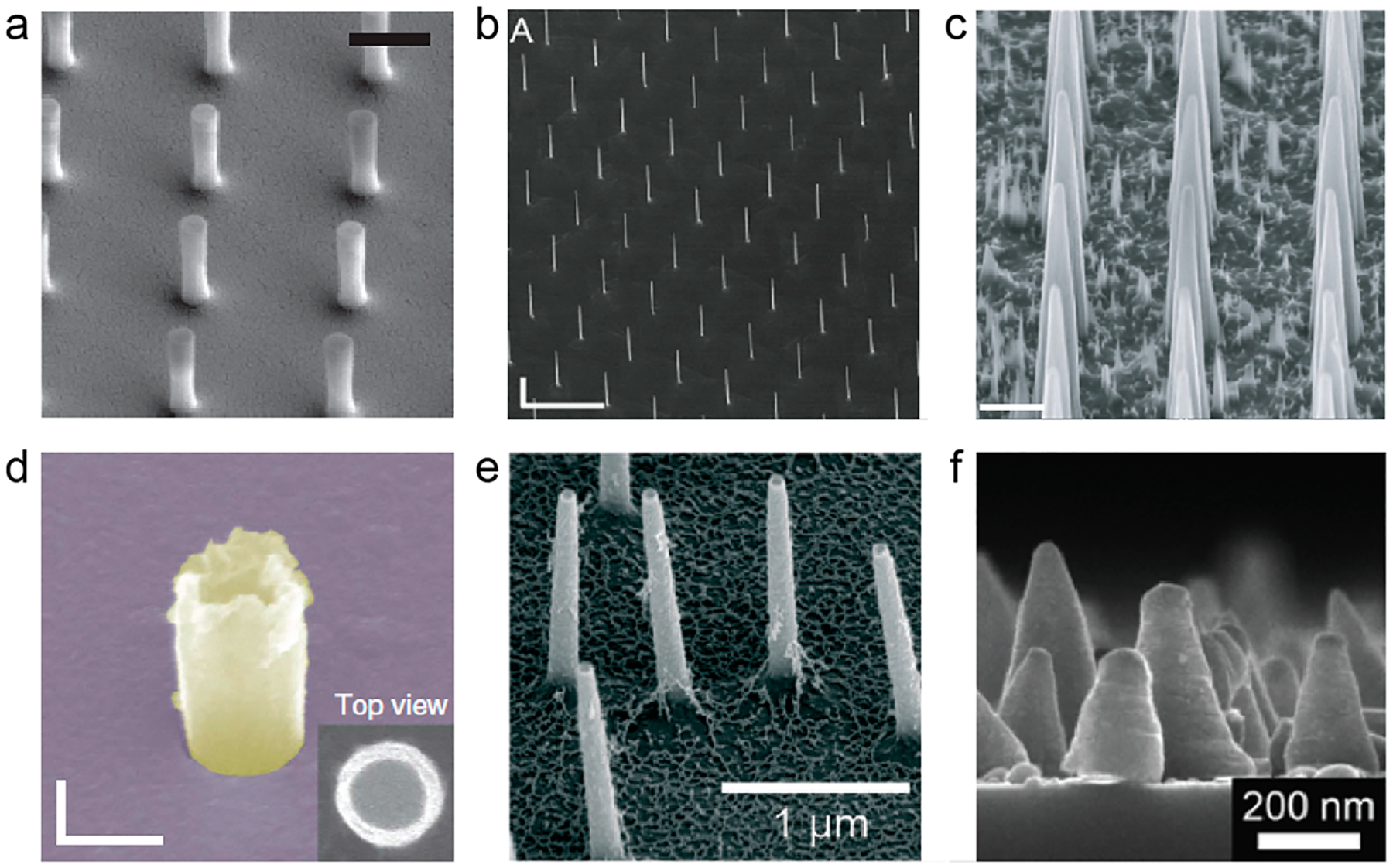
Overview of vertical nanostructures including (a) nanopillars,23 (b) nanowires,27 (c) nanoneedles,11 (d) nanotubes,55 (e) nanostraws,40 and (f) nanocones.57 The diameter, height, and aspect ratio of the vertical nanostructures are as follows: (a) 150 nm, 1.4 μm, 9; (b) 91 nm, 11 μm, 120; (c) 600 nm, 9 μm, 15; (d) 181 nm, 500 nm, 3; (e) 100 nm, 1.5 μm, 15; (f) 50 nm, 200 nm, 4. Scale bars are as follows: (a) 1 μm, (b) 500 nm, (c) 2 μm, (d) 200 nm. Reprinted with permission from the following: ref 23, Copyright 2015 Nature Publishing Group; ref 27, Copyright 2012 IOP Publishing Ltd.; ref 11, Copyright 2015 Nature Publishing Group; ref 55, Copyright 2014 Nature Publishing Group; ref 40, Copyright 2016 Royal Society of Chemistry; ref 57, Copyright 2014 Springer Nature.
Among all the cellular structures, the plasma membrane is in direct contact with vertical nanostructures. A number of recent studies show that the plasma membrane deforms and wraps around vertical nanostructures, which creates highly curved membranes at the nano–bio interface.19,20 In cells, curvature-sensitive proteins actively sense and modulate membrane curvatures for essential intracellular processes such as endocytosis and exocytosis.21,22 Therefore, vertical nanostructure induced membrane curvatures may hijack these curvature-sensitive proteins to modulate intracellular signaling. In addition to deforming the plasma membrane, some nanostructures also induce deformation of nuclear envelope membranes.23,24 Nuclear envelopes have double membranes with one side connecting to cytoskeletal networks for mechanotransduction and the other side linking with chromosomes for gene expression regulation.25 Thus, vertical nanostructure deformed nuclear envelope can activate nuclear mechanotransduction and alter gene expression and cell behavior. In this Account, we review recent studies on vertical nanostructure induced deformation of both the plasma membrane and the nuclear envelope, and their impacts on intracellular processes and cell behavior. We will focus on the topography of these nanostructures rather than their chemical compositions.
2. NANOSTRUCTURE-INDUCED PLASMA MEMBRANE DEFORMATIONS AND THEIR ROLES IN MODULATING INTRACELLULAR SIGNALING
The plasma membrane not only serves as a physical barrier that divides intracellular and extracellular spaces but also is the key location for highly regulated molecular trafficking through the membrane. Endocytosis is an essential membrane trafficking process that involves gradual bending of a flat plasma membrane to a highly curved membrane enclosing the budding vesicle. Recent studies suggest that the curved membrane itself can act as a biochemical signal to actively participate in intracellular signaling through a variety of curvature-sensing proteins.22,26
Vertical nanostructures induce local curvature on the plasma membrane right within the curvature range of the endocytosis process. Therefore, nanostructure-induced membrane curvatures may hijack curvature-sensing proteins and act as a biological signal to modulate intracellular signaling. Unlike membrane trafficking events such as endocytosis where the membrane curvature is transient and relaxes to flat after vesicle scission, nanostructure-induced membrane curvature is stable. Thus, curvature-induced signaling can be much stronger and longer lasting to affect cellular behavior and functions. In this section, we will review experimental evidence in support of the hypothesis that vertical nanostructures can modulate intracellular signaling by locally induced membrane curvatures.
2.1. Ultrastructural Imaging Reveals Membrane Curvatures around Vertical Nanostructures
Plasma membrane deformations on vertical nanostructures have been observed by many studies using both fluorescence and electron microscopic techniques. Confocal fluorescence imaging and the reconstruction of vertical cross sections shows the plasma membrane wrapping around nanopillars, some taller than the height of the cell (Figure 2a).27,28 Our fluorescence imaging of the plasma membrane, by either a membrane-staining dye or a fluorescence membrane protein, shows a brighter signal at vertical nanostructures, which indicates an increased membrane area when projected at the image plane.29 Scanning electron microscopy (SEM) imaging of cells on our nanopillars showed deformation of the apical plasma membrane at the cell edges (Figure 2b).24 Transmission electron microscopy (TEM) gives a much higher spatial resolution in visualizing the membrane deformation at the interface between the basal membrane and the vertical nanostructures (Figure 2c). We used TEM to show that the plasma membrane is pushed upward by vertical nanostructures.19,30 Instead of the tent-link elastic deformation observed on the apical membrane, the basal plasma membrane wraps tightly around the nanostructure with a gap (~18 nm) much smaller than that for a flat surface (~50 nm), which generates locally curved membranes with a curvature value determined by the size of the nanostructure.
Figure 2.
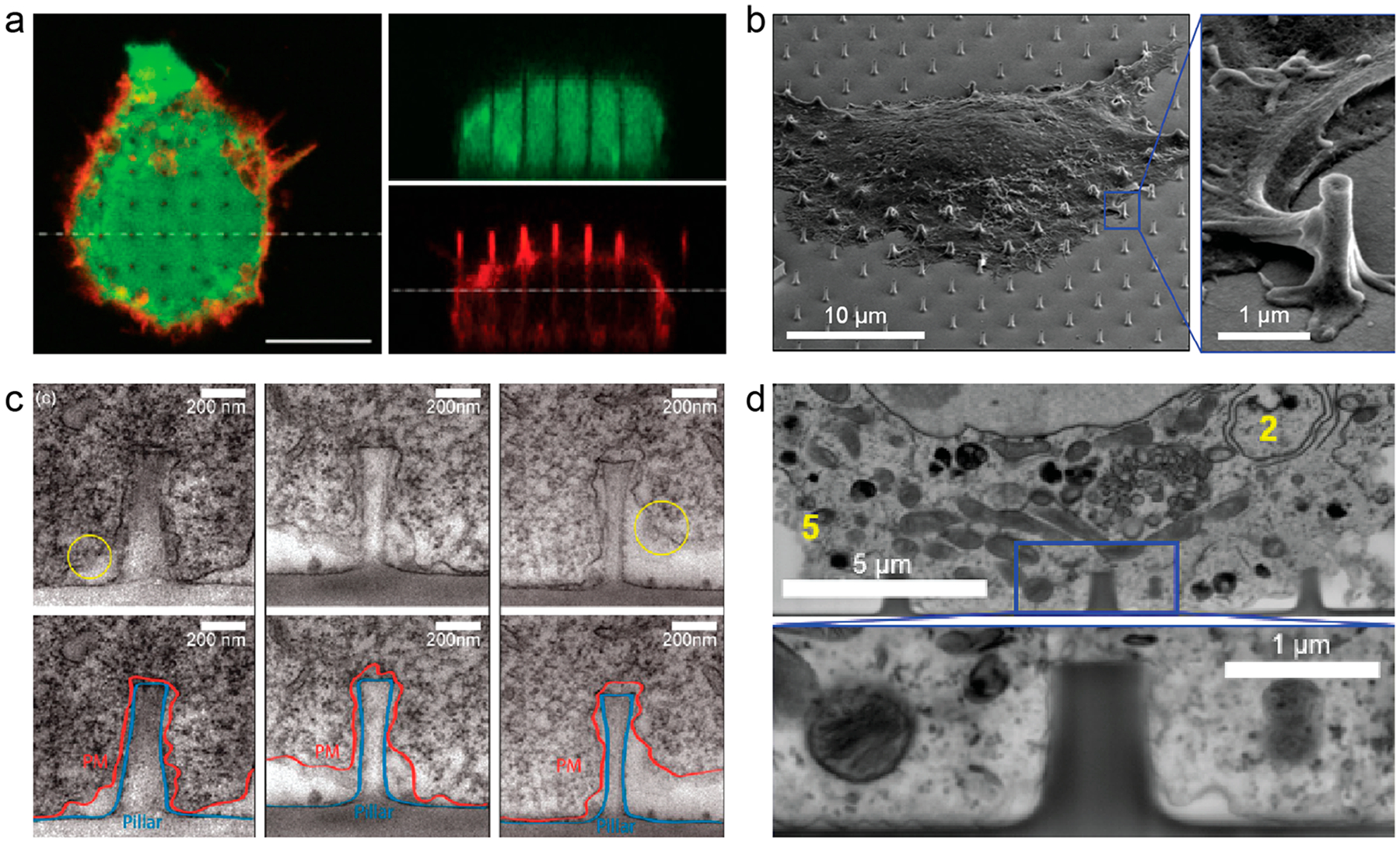
Vertical nanostructures induce plasma membrane wrapping. Plasma membrane deformation is observed by (a) confocal microscopy27 (scale bar, 10 μm), (b) SEM,24 (c) TEM,19 and (d) FIB/SEM.24 Reprinted with permission from the following: ref 27, Copyright 2012 IOP Publishing Ltd.; ref 24, Copyright 2017 American Chemical Society; ref 19, Copyright 2012 American Chemical Society.
The focus-ion-beam and scanning electron microscopy (FIB/SEM) method recently developed by us and others affords a new approach to examine the nano–bio interface with nanometer resolution without prior removal of the substrate with nanostructures, which is a necessary step for thin-slice preparation in TEM (Figure 2d).24,31 The FIB/SEM method preserves cells by ultrathin layer plastification with enhanced contrast by heavy metal staining. The FIB milling opens up the nano–bio interface at any desired location. Using this method, we showed that the distance between cell membrane and the nanopillar surfaces, including poly(L-lysine)-coated and fibronectin-coated, is usually within 10–30 nm on average, consistent with TEM studies.24 We will discuss in section 2.4 how nanostructure dimensions affect the membrane deformation.
2.2. Membrane Curvatures and Curvature-Sensitive Proteins Underlie the Enhanced Endocytosis by Nanostructures
As discussed in the last section, vertical nanostructures induce local membrane curvatures at the interface. Whether and how cells perceive these exogenous membrane curvatures with their intrinsic curvature-associated proteins and related signaling pathways was, however, not explored until recently. Dalby et al. first reported that nanocolumns (100 nm diameter) affected clathrin-mediated endocytosis.32 This study showed that the presence of nanocolumns caused global redistributions of dynamin and clathrin, two key regulatory proteins in clathrin-mediated endocytosis. Teo et al. reported that nanopillars, micro-pillars, and microgrooves increased the amount of endocytosed dextran in different cell types.33 Interestingly, they found that nanopillars of 200 nm diameter were more effective than micropillars of 2000 nm diameter in introducing GFP-encoding plasmid into cells. Galic et al. used substrates interlaced with strips of flat areas and strips of areas with cone-shaped nanostructures that deformed the plasma membrane (Figure 3a,b).34 The authors showed convincing evidence that, in the same cell, N-BAR protein nadrin-2 preferentially accumulates on the nanocone strips but not the flat strips (Figure 3c). However, the size and shape of nanocones vary greatly, and they were not individually discernible under the optical microscopy. Therefore, the curvature values induced by nanocones had a large uncertainty, making difficult to correlate membrane curvature values with protein responses.
Figure 3.
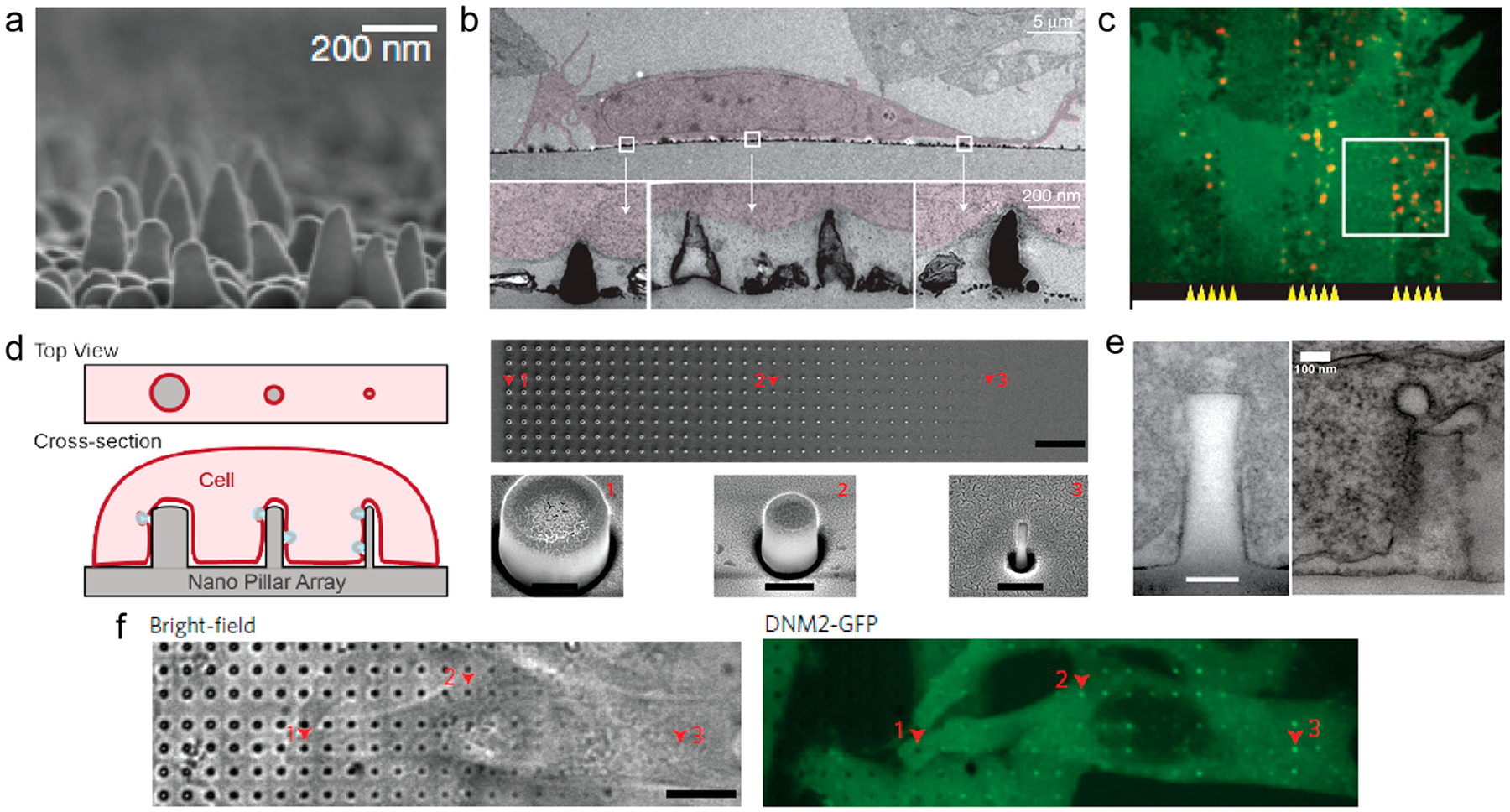
Vertical nanostructures induce local accumulation of proteins. (a) SEM image of the nanocone substrate.34 (b) TEM images of 3T3 cells on nanocones.34 (c) Fluorescent images show nadrin-2 (red) preferentially accumulated on nanocone strips compared with flat strips.34 (d) (left) Schematic illustration of gradient nanopillars deforming the plasma membrane. (right) SEM images of the gradient nanopillars.29 Scale bars, 10 μm (top), 400 nm (bottom). (e) TEM images of the membrane–nanopillar interface (left) and clathrin-coated pits (right) on the nanopillars.29 Scale bar, 100 nm. (f) A time-averaged image of dynamin2–GFP demonstrates that dynamin–GFP exhibits strong preference to sharp nanopillars.29 Scale bar, 10 μm. Reprinted with permission from the following: ref 34, Copyright 2012 Nature Publishing Group; ref 29, Copyright 2017 Nature Publishing Group.
We recently demonstrated that only a certain range of curvature values generated by nanostructures stimulate the recruitment of curvature-sensing proteins.29 This work used nanopillars of precisely controlled geometry and a gradient array with 32 different diameters ranging from 100 to 1000 nm (Figure 3d). The diameter of these nanopillars determines the value of the induced membrane curvature. These nanopillars were arranged in well-ordered arrays, and their sizes and locations were easily identified. A clathrin-coated pit budding off from the curved membrane around a nanopillar is visible in another TEM image (Figure 3e). Fluorescence microscopy studies showed that dynamin-2 only exhibits preferential accumulation when the diameter of the nanopillar is less than 500 nm (Figure 3f), indicating a curvature range for nanostructure-induced membrane deformation to modulate clathrin-mediated endocytosis.
We further confirmed the curvature effect by using nanobars that induce high curvature at two ends and are flat along the side walls (Figure 4a). Both clathrin and dynamin-2 show preferential accumulation at the two ends of nanobars with high curvatures (Figure 4b). In addition to clathrin and dynamin, we examined the curvature preference of ten different proteins (Figure 4c). Lipid dye or a membrane associated protein, mCherry-CAAX, is evenly distributed along the entire length of the nanobars. On the other hand, all proteins involved in clathrin-dependent endocytosis, including four curvature-sensing proteins, F-BAR protein FCHo1, N-BAR protein amphiphysin 1, and ENTH protein Epsin 1, show strong preference to the ends of nanobars. Interestingly, caveolin, a protein involved in caveolin-dependent endocytosis, does not show curvature-dependent accumulation, which indicates that cellular processes that are modulated by nanostructures are highly specific.29 This experimental evidence demonstrates that nanostructures enhance clathrin-mediated endocytosis by recruiting curvature-sensitive proteins.
Figure 4.
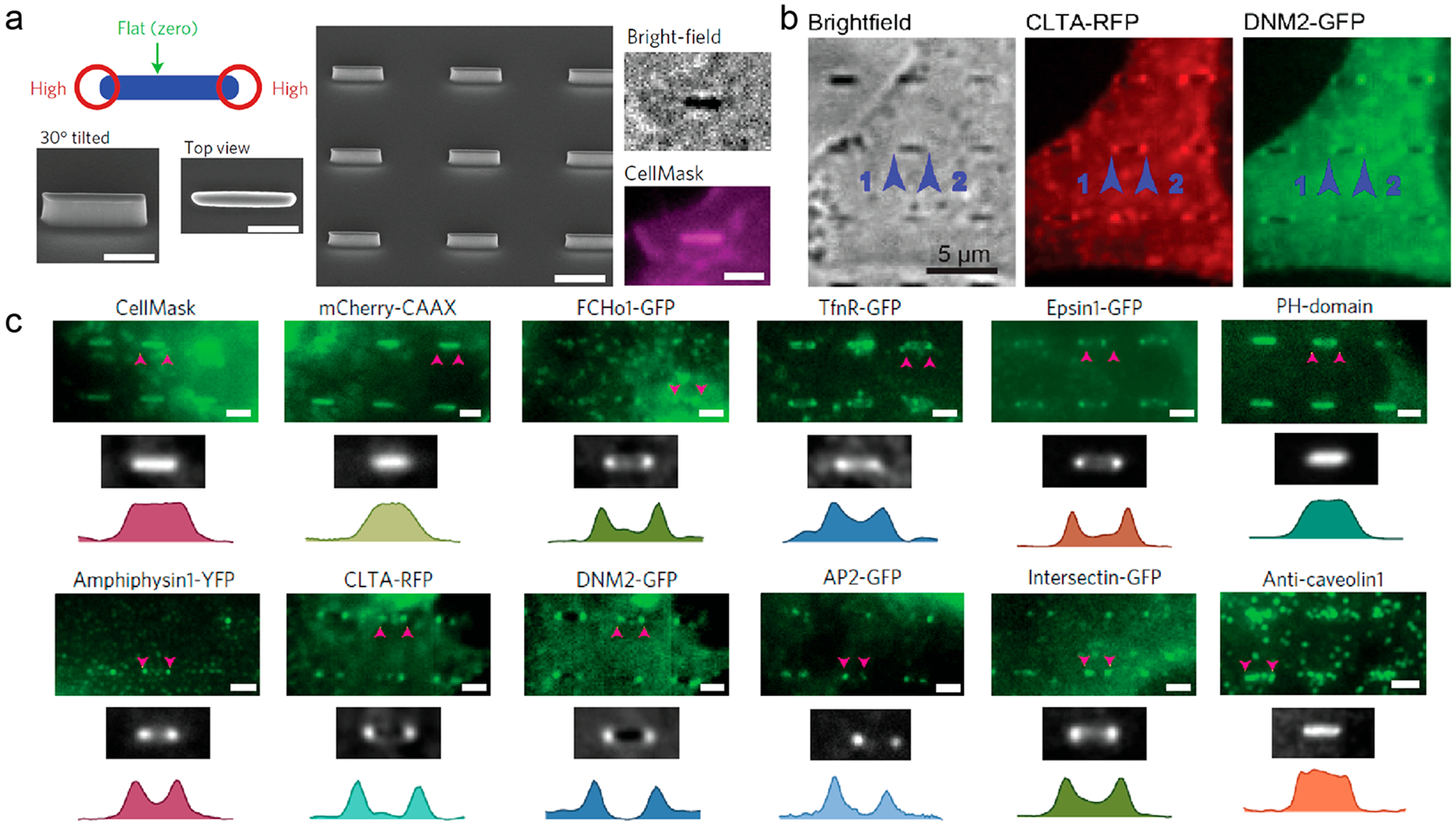
Clathrin-dependent endocytic proteins accumulate at nanobar ends in a curvature-dependent manner.29 (a) (left and middle) Schematic and SEM images of nanobar structure. Scale bars, 1 μm. (right) CellMask Deep Red staining of SK-MEL-2 cells on the nanobar arrays. Scale bar, 2 μm. (b) Averaged fluorescence images show that clathrin and dynamin-2 preferentially accumulate around the ends of nanobar structures. Scale bar, 5 μm. (c) The distribution of CellMask, mCherry-CAAX, and various endocytic proteins on the nanobar arrays. Scale bars, 2 μm. Reprinted with permission from ref 29. Copyright 2017 Nature Publishing Group.
We observed that nanopillars not only enhance the occurrence frequency of clathrin-mediated endocytosis but also accelerate the dynamics of the clathrin-mediated endocytosis process.29 The average lifetime of clathrin puncta shows a 40% reduction on nanopillars as compared with flat areas on the same substrate. As membrane bending is an energy consuming process, it is possible that nanopillar-induced membrane curvature reduces the membrane-bending energy and thus facilitates CME dynamics, but further evidence is needed to support the bending energy assumption.
2.3. Nanostructure-Induced Actin Polymerization and Its Potential Link to Membrane Curvature
Unlike the nanostructure-enhanced endocytosis that has only been investigated by a handful of studies, there are extensive studies on how nanostructures change the cell shape and modulate whole-cell actin cytoskeleton, which has been reviewed previously and is not a focus of this Account.14,35 Here we discuss studies that show local accumulation of actin filaments around nanostructures. By phalloidin staining, accumulation of fibrous actin (F-actin) was observed on various vertical nanostructures including IrOx nanotubes,36 InAs nanowires,28 CuO nanowires,30 SiO2 nanopillars,23 and SU-8 polymer nanopillars (Figure 5a).37 Using LifeAct-GFP, a protein marker for visualizing F-actin in live cells, we observed accumulation of actin fiber at SiO2 nanopillars in live cells.29 Therefore, local actin accumulation on nanostructures seems to be rather ubiquitous, observed by many research groups in different cell types and using nanostructures of different materials.
Figure 5.
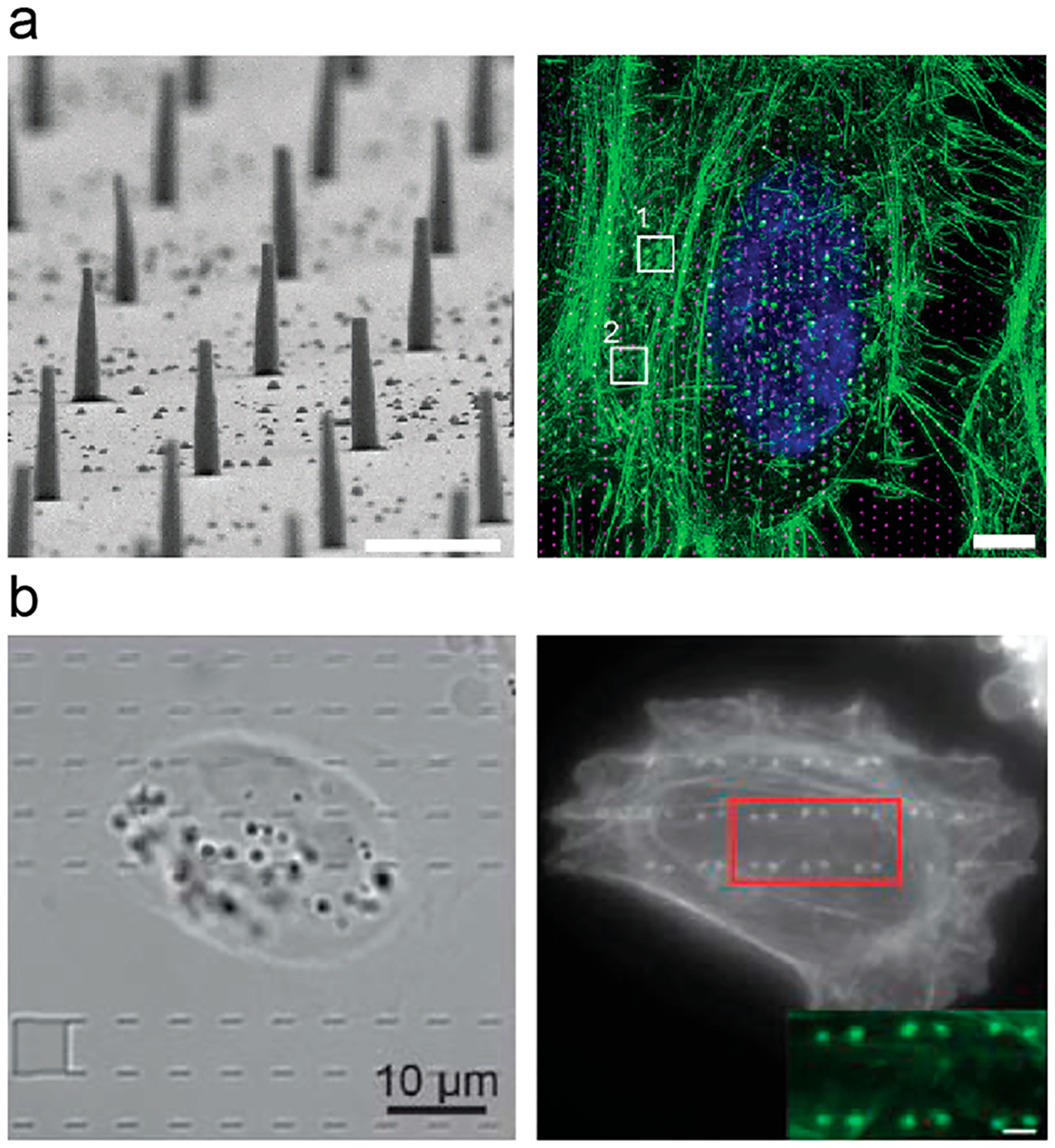
Vertical nanostructures induce local polymerization of F-actin. (a) (left) SEM image of SU-8 nanopillar arrays. Scale bar, 1 μm. (right) Maximum intensity projection of a 3D-SIM stack showing phalloidin–Alexa488-labeled F-actin (green), Hoechst 34580 labeled nucleus (blue), and 1 μm spaced nanopillars (magenta) in a hexagonal array.37 Scale bar, 5 μm. (b) Full frame images of F-actin shows its strong preference on two ends of nanobars, suggesting a curvature effect.29 Reprinted with permission from the following: ref 37, Copyright 2015 The Royal Society of Chemistry; ref 29, Copyright 2017 Nature Publishing Group.
Molecular mechanisms underlying local accumulation of F-actin on nanostructures remain poorly understood. The F-actin appears as distinct puncta or rings at the cell periphery, inside the cell, and underneath the nucleus. Most of the time, these puncta are not connected to linear stress fibers in the same cell and do not colocalize with focal adhesion kinase.28 Except at the cell edge, these actin puncta do not colocalize with focal adhesion probed by vinculin.28 Using nanobars that induce two different culture values, we showed that F-actin accumulates strongly at nanobar ends with high membrane curvature but very little along the flat sidewall (Figure 5b).29 This result suggests that F-actin accumulation at nanostructure locations is curvature dependent.29 However, how membrane curvature induces F-actin accumulation on nanostructures remains to be elucidated.
2.4. Nanostructure Geometry Effect on the Plasma Membrane Deformation and Membrane Integrity
Membrane curvature is affected by the diameter, density, and height of the nanostructures underneath. The diameter or shape of the nanostructures determines the curvature value of the membrane deformation for sparsely distributed or intermediate-density nanopillars.29 The density of nanostructures not only affects the total area of deformed membrane but also determines whether the plasma membrane can fully wrap around these structures. For example, NIH3T3 cells engulfed low density (3 NPs/100 μm2) nanopillars but grew on the top of high density (700 NPs/100 μm2) nanopillars.38 The height of nanostructures also plays a significant role in determining the area of the deformed membrane. When nanowires are much taller than the height of the cell, they may prevent cells from attaching to the bottom surface.39 Overall, nanostructures with small diameter (<500 nm), intermediate density, and intermediate height would induce the most significant effect in deforming the plasma membrane.
Many studies indicate an intact plasma membrane wrapping around vertical nanostructures, and therefore, electroporation,2 physical insertion,1,40 and other physical forces41 have been explored for these nanostructures to gain intracellular access. However, some studies suggest that sharp nanostructures may have spontaneous access to the intracellular domain. For example, vertical silicon nanowires and silicon nanoneedles were shown to deliver biological reagents such as peptides, siRNAs, and nucleic acids into the cells.8,11 In another example, nanostraws could deliver cobalt ions to quench green fluorescent proteins in the cytosol but only a small fraction of nanostraws were able to do so.42 The molecular mechanisms of how some vertical nanostructures spontaneously gain intracellular access remain unknown, but this topic has been explored by theoretical studies. Xie et al. suggested that nanowire penetration is unlikely to occur in the absence of external force unless the nanowire radius is as small as 10 nm.43 Their later study suggested that penetration is possible for 100 nm diameter nanopillars but only occurs in a limited time window such as shortly after cell adhesion.44
3. NANOSTRUCTURE-INDUCED NUCLEAR DEFORMATIONS AND THEIR POTENTIAL ROLES IN REGULATING GENE EXPRESSION
In addition to the plasma membrane deformation, some vertical nanostructures also induce nuclear deformation. The nucleus contains most of the cell’s genome and controls cell behaviors by regulating gene expression and protein synthesis.45 Furthermore, it is also the most rigid organelle and considered as the major contributor to the mechanical properties of the cell.46 It is shown that the nuclear shape, size, and mechanical rigidity respond to mechanical forces and pathological conditions.47 For example, a genetic mutation of lamin A, a nuclear lamina protein providing structural support for the nucleus, causes severe nuclear deformation and premature aging in Hutchinson–Gilford progeria syndrome.48 Therefore, it is important to understand how nanostructures deform the nuclear envelope and how these nuclear deformations contribute toward nanostructure-induced cell behaviors and gene expression.
3.1. Evidence of Nanostructure-Induced Nuclear Deformation
Several ultrastructural studies by TEM and FIB/SEM demonstrate that the cell nucleus can be deformed by nanostructures protruding from the surface. For example, high aspect ratio CuO nanowires caused bulging of the nuclear membrane,30 and GaP nanowires caused deep invagination of nuclear envelope (Figure 6a).49 We showed that vertical SiO2 nanopillars push nuclear envelope deep into the nucleus of 3T3 cells (Figure 6b).23 Two independent FIB/SEM studies both show that nanostructures not only deform the cell membrane but also cause significant upward bending of the nuclear envelope.24,50 Fluorescence studies also confirmed the TEM observation of nuclear deformation by nanostructures (Figure 6c).23 Large micropillars also induce dramatic nuclear deformation. However, unlike nanopillar-induced nuclear deformation, which is characterized by nucleoplasmic invaginations of the envelope membrane, micropost-induced nuclear deformation is often dramatic shape changes induced by confinement (Figure 6d).51,52
Figure 6.
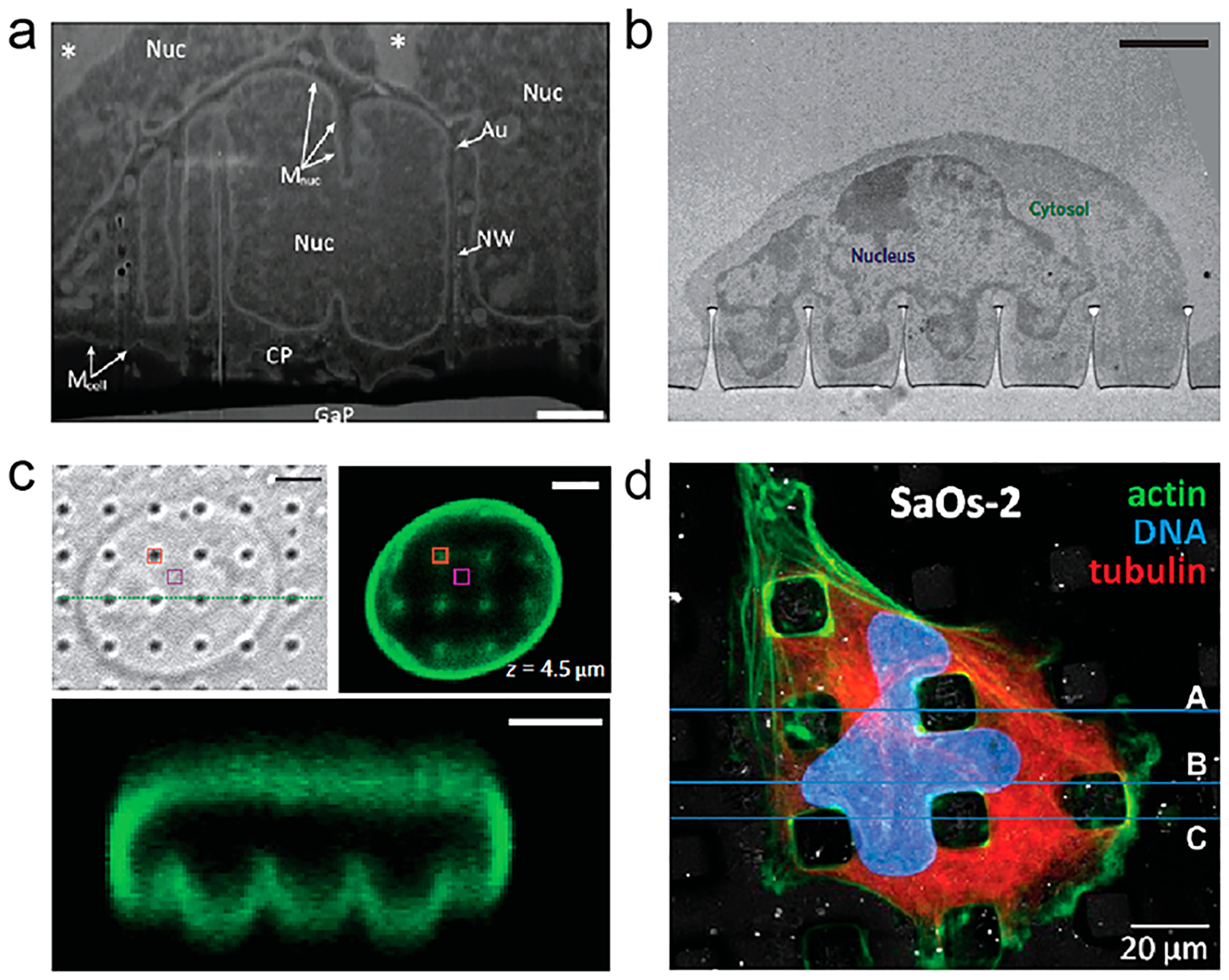
Vertical nanostructures induce nuclear membrane deformation. (a, b) TEM images of nuclear membrane deformation on vertical nanowires49 and nanopillars.23 Scale bars, (a) 1 μm and (b) 2 μm. (c) Confocal microscopy showed nuclear membrane deformation of 3T3 nucleus on nanopillars.23 Scale bars, 3 μm. (d) Nuclear deformation between PLLA micropillars showed by fluorescence microscopy.51 Reprinted with permission from the following: ref 49, Copyright 2013 Wiley; ref 23, Copyright 2015 Nature Publishing Group; ref 51, Copyright 2013 Elsevier.
Systematic studies have provided detailed investigation of how the depth of nuclear deformation depends on the spacing, the diameter, and the height of nanopillars. It was found that the spacing between nanopillars dramatically affects the extent of nuclear deformation. When the nanopillar spacing was increased from 2 to 6 μm, the depth of NIH3T3 nucleus deformation increased by 400% from 220 to 900 nm.23 The diameter of nanopillars is shown to mildly affect the nuclear deformation, with 300 nm diameter pillars inducing deeper nuclear deformation than 700 nm diameter pillars of the same height and spacing (260 and 160 nm) in 3T3 cells. The height of nanopillars also slightly affects nuclear deformation. It was found that 2 μm tall nanopillars induce slightly deeper deformation than 1.4 μm-tall nanopillars (290 vs 260 nm). Therefore, within the measurement range of the three geometric parameters, the spacing is the most effective in modulating nuclear deformation, while the diameter and height affect the nuclear deformation to a lesser extent.
3.2. Actin Cytoskeleton Is Critically Involved in Nanostructure-Induced Nuclear Deformation
Vertical nanostructures are not in direct contact with the nuclear membrane, so nanostructure-induced nuclear deformation must be mediated by intracellular forces. Cytoskeleton, including F-actin, intermediate filament, and microtubule, physically connects the nucleus to the plasma membrane and plays a critical role in mechanotransduction between the plasma membrane and the nucleus.53 To understand the mechanisms of nanostructure-induced nuclear deformation, it is important to investigate whether and which cytoskeletal filaments modulate nanostructure-induced nuclear deformation. We found that latrunculin B treatment, which disrupts actin filaments, significantly reduced the extent of nanopillar-induced nuclear deformation, which suggested that actin filaments are crucial for generating the contractile force necessary for the nanostructure-induced nuclear deformation in 3T3 cells.23 The intermediate filaments, however, seem to act against the contractile force from the F-actin. Meanwhile, the microtubule has no significant effect on pillar-induced nuclear deformation.23 These results indicate that the cytoskeleton, especially F-actin, regulates the force transmission between nano- or micropillars and nucleus.
3.3. Nanostructure-Induced Nuclear Deformation May Affect Gene Expression
Microposts often cause dramatic change of the nuclear shape. However, despite their severely misshapen nuclei, osteosarcoma cells on polymer microposts show the same level of viability as cells grown on flat surfaces of the same polymer.52 Furthermore, deformed nuclei enter into the proliferation cycle at a similar rate as non-deformed nuclei,52 which indicates that nuclear deformation did not impede DNA replication and cell proliferation. Similarly, nanopillars with low aspect ratios (height/diameter < 10) that induce mild nuclear deformation and do not affect cell viability or proliferation28,54 or their electrophysiological functions.2,55 Some studies even show that these nanopillars at low densities caused a slight increase in cell proliferation or the percentage of dividing cell.28 These results indicate that the nuclear shape change by microposts or low-aspect ratio nanopillars does not affect either viability, proliferation, or differentiation of cells.
However, sharp and tall nanopillars with high aspect ratios (height/diameter > 10) that induce deep nuclear invaginations are shown to decrease the cell proliferation rate and cause the occurrence of multinuclear cells.49 These high aspect ratio nanopillars also caused measurable generation of ROS and DNA damage. We note that nuclear deformations induced by microposts or short nanopillars usually have shallow curvatures, while nuclear deformations induced by sharp and tall nanopillars usually have high curvatures. These studies provide evidence that the curvature of local membrane deformations instead of the global nuclear shape may affect signaling and gene expression inside the nucleus. However, a direct link between nanostructure-induced nuclear membrane curvature and gene expression is yet to be established.
4. SUMMARY AND PERSPECTIVES
In a broader sense, this Account provides a new angle to understand the interactions between biological systems and nanotopography. Our curvature hypothesis proposes that nanotopography can directly affect intracellular signaling and functions by inducing local membrane curvatures. It includes but is not limited to nanostructure-generated membrane curvatures at the plasma membrane and the activation of curvature-related intracellular signaling pathways, for example, endocytosis and actin cytoskeleton rearrangement, nanostructure-induced nuclear envelope reshaping, and its possible impacts on both nuclear mechanotransduction and chromatin-organization-related epigenetic control.
A few pioneer studies have been discussed here, but the whole picture is far from clear. The role of membrane curvature at the nano–bio interface has been largely overlooked until we demonstrated its effect on endocytosis.29 Besides endocytosis, curvature sensitive proteins participate in many other cellular processes from exocytosis, filopodia generation, and adhesion to neuronal development and differentiation.56 Inside cells, there are still more curvature-sensitive proteins controlling the shaping of intracellular organelles and the membrane trafficking between different organelles. Whether and how nanostructures disrupt or reshape their behaviors are intriguing questions but yet to be investigated. Similarly, for the nanostructure induced nuclear deformation, whether there are curvature-related proteins involved in triggering signal transduction or chromatin repositioning is unknown but highly possible.
Membrane curvature is likely not the only mechanism underlying how cells recognize and sense nanotopography. Nevertheless, existing evidence suggests that membrane curvature is a major player participating in nanotopography-modulated intracellular signaling. We envision more research endeavors will be put into this curvature angle to understand how nanotopography impacts cellular behavior, such as cell migration54 and stem cell differentiation.18 The knowledge obtained can guide the design of biomaterials and biodevices that interface with cells.
ACKNOWLEDGMENTS
We thank NIH for supporting this Account (Grant no. 1R01GM125737).
Biographies
Hsin-Ya Lou received his Bachelor degree in chemistry from National Taiwan University and is currently a Ph.D. candidate at Stanford University in the United States.
Wenting Zhao is currently an assistant professor in the School of Chemical and Biomedical Engineering at Nanyang Technological University, Singapore. She obtained her Ph.D. degree from Hong Kong University of Science and Technology and then did her postdoctoral training at Stanford University. Her research interest is studying the interplay between biology and materials, with a specific focus on nanostructure design for the manipulation of nanoscopic cellular features.
Yongpeng Zeng obtained his Bachelor degree in materials science from Sichuan University and is currently a graduate student at Nanyang Technological University in Singapore.
Bianxiao Cui is an associate professor in the Department of Chemistry, Stanford University. She obtained a B.S. degree in Material Science and Engineering from University of Science and Technology of China, and a Ph.D. degree in Chemistry from the University of Chicago. Her research focuses on developing new tools, innovative methods, and emerging technologies for the study of intracellular processes.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Duan X; Gao R; Xie P; Cohen-Karni T; Qing Q; Choe HS; Tian B; Jiang X; Lieber CM Intracellular Recordings of Action Potentials by an Extracellular Nanoscale Field-Effect Transistor. Nat. Nanotechnol 2012, 7 (3), 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Xie C; Lin Z; Hanson L; Cui Y; Cui B Intracellular Recording of Action Potentials by Nanopillar Electroporation. Nat. Nanotechnol 2012, 7 (3), 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Robinson JT; Jorgolli M; Shalek AK; Yoon M-H; Gertner RS; Park H Vertical Nanowire Electrode Arrays as a Scalable Platform for Intracellular Interfacing to Neuronal Circuits. Nat. Nanotechnol 2012, 7 (3), 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Yang MT; Sniadecki NJ; Chen CS Geometric Considerations of Micro- to Nanoscale Elastomeric Post Arrays to Study Cellular Traction Forces. Adv. Mater 2007, 19 (20), 3119–3123. [Google Scholar]
- (5).Bucaro MA; Vasquez Y; Hatton BD; Aizenberg J Fine-Tuning the Degree of Stem Cell Polarization and Alignment on Ordered Arrays of High-Aspect-Ratio Nanopillars. ACS Nano 2012, 6 (7), 6222–6230. [DOI] [PubMed] [Google Scholar]
- (6).Xie C; Hanson L; Cui Y; Cui B Vertical Nanopillars for Highly Localized Fluorescence Imaging. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (10), 3894–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).De Angelis F; Das G; Candeloro P; Patrini M; Galli M; Bek A; Lazzarino M; Maksymov I; Liberale C; Andreani LC; Di Fabrizio E Nanoscale Chemical Mapping Using Three-Dimensional Adiabatic Compression of Surface Plasmon Polaritons. Nat. Nanotechnol 2010, 5 (1), 67–72. [DOI] [PubMed] [Google Scholar]
- (8).Shalek AK; Robinson JT; Karp ES; Lee JS; Ahn D-R; Yoon M-H; Sutton A; Jorgolli M; Gertner RS; Gujral TS; MacBeath G; Yang EG; Park H Vertical Silicon Nanowires as a Universal Platform for Delivering Biomolecules into Living Cells. Proc. Natl. Acad. Sci. U. S. A 2010, 107 (5), 1870–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Chen X; Kis A; Zettl A; Bertozzi CR A Cell Nanoinjector Based on Carbon Nanotubes. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (20), 8218–8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Kim W; Ng JK; Kunitake ME; Conklin BR; Yang P Interfacing Silicon Nanowires with Mammalian Cells. J. Am. Chem. Soc 2007, 129 (23), 7228–7229. [DOI] [PubMed] [Google Scholar]
- (11).Chiappini C; De Rosa E; Martinez JO; Liu X; Steele J; Stevens MM; Tasciotti E Biodegradable Silicon Nanoneedles Delivering Nucleic Acids Intracellularly Induce Localized in Vivo Neovascularization. Nat. Mater 2015, 14 (5), 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Shalek AK; Gaublomme JT; Wang L; Yosef N; Chevrier N; Andersen MS; Robinson JT; Pochet N; Neuberg D; Gertner RS; Amit I; Brown JR; Hacohen N; Regev A; Wu CJ; Park H Nanowire-Mediated Delivery Enables Functional Interrogation of Primary Immune Cells: Application to the Analysis of Chronic Lymphocytic Leukemia. Nano Lett. 2012, 12 (12), 6498–6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Angle MR; Cui B; Melosh NA Nanotechnology and Neurophysiology. Curr. Opin. Neurobiol 2015, 32, 132–140. [DOI] [PubMed] [Google Scholar]
- (14).Bonde S; Buch-Månson N; Rostgaard KR; Andersen TK; Berthing T; Martinez KL Exploring Arrays of Vertical One-Dimensional Nanostructures for Cellular Investigations. Nanotechnology 2014, 25 (36), 362001. [DOI] [PubMed] [Google Scholar]
- (15).Acaron Ledesma H; Tian B Nanoscale Silicon for Subcellular Biointerfaces. J. Mater. Chem. B 2017, 5 (23), 4276–4289. [DOI] [PubMed] [Google Scholar]
- (16).Qi S; Yi C; Ji S; Fong C-C; Yang M Cell Adhesion and Spreading Behavior on Vertically Aligned Silicon Nanowire Arrays. ACS Appl. Mater. Interfaces 2009, 1 (1), 30–34. [DOI] [PubMed] [Google Scholar]
- (17).Liu D; Yi C; Zhang D; Zhang J; Yang M Inhibition of Proliferation and Differentiation of Mesenchymal Stem Cells by Carboxylated Carbon Nanotubes. ACS Nano 2010, 4 (4), 2185–2195. [DOI] [PubMed] [Google Scholar]
- (18).Chen W; Shao Y; Li X; Zhao G; Fu J Nanotopographical Surfaces for Stem Cell Fate Control: Engineering Mechanobiology from the Bottom. Nano Today 2014, 9 (6), 759–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hanson L; Lin ZC; Xie C; Cui Y; Cui B Characterization of the Cell-Nanopillar Interface by Transmission Electron Microscopy. Nano Lett. 2012, 12 (11), 5815–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Santoro F; Dasgupta S; Schnitker J; Auth T; Neumann E; Panaitov G; Gompper G; Offenhausser A Interfacing Electrogenic Cells with 3D Nanoelectrodes: Position, Shape, and Size Matter. ACS Nano 2014, 8 (7), 6713–6723. [DOI] [PubMed] [Google Scholar]
- (21).McMahon HT; Gallop JL Membrane Curvature and Mechanisms of Dynamic Cell Membrane Remodelling. Nature 2005, 438 (7068), 590–596. [DOI] [PubMed] [Google Scholar]
- (22).Jarsch IK; Daste F; Gallop JL Membrane Curvature in Cell Biology: An Integration of Molecular Mechanisms. J. Cell Biol 2016, 214 (4), 375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hanson L; Zhao W; Lou H-Y; Lin ZC; Lee SW; Chowdary P; Cui Y; Cui B Vertical Nanopillars for in Situ Probing of Nuclear Mechanics in Adherent Cells. Nat. Nanotechnol 2015, 10 (6), 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Santoro F; Zhao W; Joubert L-M; Duan L; Schnitker J; van de Burgt Y; Lou H-Y; Liu B; Salleo A; Cui L; Cui Y; Cui B Revealing the Cell–Material Interface with Nanometer Resolution by Focused Ion Beam/Scanning Electron Microscopy. ACS Nano 2017, 11 (8), 8320–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Gupta S; Marcel N; Sarin A; Shivashankar GV Role of Actin Dependent Nuclear Deformation in Regulating Early Gene Expression. PLoS One 2012, 7 (12), e53031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Mim C; Unger VM Membrane Curvature and Its Generation by BAR Proteins. Trends Biochem. Sci 2012, 37 (12), 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Berthing T; Bonde S; Rostgaard KR; Madsen MH; Sørensen CB; Nygaård J; Martinez KL Cell Membrane Conformation at Vertical Nanowire Array Interface Revealed by Fluorescence Imaging. Nanotechnology 2012, 23 (41), 415102. [DOI] [PubMed] [Google Scholar]
- (28).Bonde S; Berthing T; Madsen MH; Andersen TK; Buch-Maånson N; Guo L; Li X; Badique F; Anselme K; Nygaård J; Martinez KL Tuning In As Nanowire Density for HEK293 Cell Viability, Adhesion, and Morphology: Perspectives for Nanowire-Based Biosensors. ACS Appl. Mater. Interfaces 2013, 5 (21), 10510–10519. [DOI] [PubMed] [Google Scholar]
- (29).Zhao W; Hanson L; Lou H-Y; Akamatsu M; Chowdary PD; Santoro F; Marks JR; Grassart A; Drubin DG; Cui Y; Cui B Nanoscale Manipulation of Membrane Curvature for Probing Endocytosis in Live Cells. Nat. Nanotechnol 2017, 12 (8), 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Mumm F; Beckwith KM; Bonde S; Martinez KL; Sikorski P A Transparent Nanowire-Based Cell Impalement Device Suitable for Detailed Cell-Nanowire Interaction Studies. Small 2013, 9 (2), 263–272. [DOI] [PubMed] [Google Scholar]
- (31).von Erlach TC; Bertazzo S; Wozniak MA; Horejs C-M; Maynard SA; Attwood S; Robinson BK; Autefage H; Kallepitis C; Del Río Hernández A; Chen CS; Goldoni S; Stevens MM Cell-Geometry-Dependent Changes in Plasma Membrane Order Direct Stem Cell Signalling and Fate. Nat. Mater 2018, 17 (3), 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Dalby MJ; Berry CC; Riehle MO; Sutherland DS; Agheli H; Curtis ASG Attempted Endocytosis of Nano-Environment Produced by Colloidal Lithography by Human Fibroblasts. Exp. Cell Res 2004, 295 (2), 387–394. [DOI] [PubMed] [Google Scholar]
- (33).Teo BKK; Goh S-H; Kustandi TS; Loh WW; Low HY; Yim EKF The Effect of Micro and Nanotopography on Endocytosis in Drug and Gene Delivery Systems. Biomaterials 2011, 32 (36), 9866–9875. [DOI] [PubMed] [Google Scholar]
- (34).Galic M; Jeong S; Tsai F-C; Joubert L-M; Wu YI; Hahn KM; Cui Y; Meyer T External Push and Internal Pull Forces Recruit Curvature-Sensing N-BAR Domain Proteins to the Plasma Membrane. Nat. Cell Biol 2012, 14 (8), 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Biggs MJP; Richards RG; Dalby MJ Nanotopographical Modification: A Regulator of Cellular Function through Focal Adhesions. Nanomedicine 2010, 6 (5), 619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Persson H; Beech JP; Samuelson L; Oredsson S; Prinz CN; Tegenfeldt JO Vertical Oxide Nanotubes Connected by Subsurface Microchannels. Nano Res. 2012, 5 (3), 190–198. [Google Scholar]
- (37).Beckwith KS; Cooil SP; Wells JW; Sikorski P Tunable High Aspect Ratio Polymer Nanostructures for Cell Interfaces. Nanoscale 2015, 7 (18), 8438–8450. [DOI] [PubMed] [Google Scholar]
- (38).Buch-Månson N; Kang D-H; Kim D; Lee KE; Yoon M-H; Martinez KL Mapping Cell Behavior across a Wide Range of Vertical Silicon Nanocolumn Densities. Nanoscale 2017, 9 (17), 5517–5527. [DOI] [PubMed] [Google Scholar]
- (39).Buch-Månson N; Bonde S; Bolinsson J; Berthing T; Nygård J; Martinez KL Towards a Better Prediction of Cell Settling on Nanostructure Arrays-Simple Means to Complicated Ends. Adv. Funct. Mater 2015, 25 (21), 3246–3255. [Google Scholar]
- (40).Xu AM; Kim SA; Wang DS; Aalipour A; Melosh NA Temporally Resolved Direct Delivery of Second Messengers into Cells Using Nanostraws. Lab Chip 2016, 16 (13), 2434–2439. [DOI] [PubMed] [Google Scholar]
- (41).McKnight TE; Melechko AV; Hensley DK; Mann DGJ; Griffin GD; Simpson ML Tracking Gene Expression after DNA Delivery Using Spatially Indexed Nanofiber Arrays. Nano Lett. 2004, 4 (7), 1213–1219. [Google Scholar]
- (42).Xu AM; Aalipour A; Leal-Ortiz S; Mekhdjian AH; Xie X; Dunn AR; Garner CC; Melosh NA Quantification of Nanowire Penetration into Living Cells. Nat. Commun 2014, 5, 3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Xie X; Xu AM; Angle MR; Tayebi N; Verma P; Melosh NA Mechanical Model of Vertical Nanowire Cell Penetration. Nano Lett. 2013, 13 (12), 6002–6008. [DOI] [PubMed] [Google Scholar]
- (44).Xie X; Aalipour A; Gupta SV; Melosh NA Determining the Time Window for Dynamic Nanowire Cell Penetration Processes. ACS Nano 2015, 9 (12), 11667–11677. [DOI] [PubMed] [Google Scholar]
- (45).Fedorchak GR; Kaminski A; Lammerding J Cellular Mechanosensing: Getting to the Nucleus of It All. Prog. Biophys. Mol. Biol 2014, 115 (2–3), 76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Caille N; Thoumine O; Tardy Y; Meister J-J Contribution of the Nucleus to the Mechanical Properties of Endothelial Cells. J. Biomech 2002, 35 (2), 177–187. [DOI] [PubMed] [Google Scholar]
- (47).Isermann P; Lammerding J Nuclear Mechanics and Mechanotransduction in Health and Disease. Curr. Biol 2013, 23 (24), R1113–R1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Taimen P; Pfleghaar K; Shimi T; Möller D; Ben-Harush K; Erdos MR; Adam SA; Herrmann H; Medalia O; Collins FS; Goldman AE; Goldman RD A Progeria Mutation Reveals Functions for Lamin A in Nuclear Assembly, Architecture, and Chromosome Organization. Proc. Natl. Acad. Sci. U. S. A 2009, 106 (49), 20788–20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Persson H; Købler C; Mølhave K; Samuelson L; Tegenfeldt JO; Oredsson S; Prinz CN Fibroblasts Cultured on Nanowires Exhibit Low Motility, Impaired Cell Division, and DNA Damage. Small 2013, 9 (23), 4006–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Wierzbicki R; Købler C; Jensen MRB; Lopacinśka J; Schmidt MS; Skolimowski M; Abeille F; Qvortrup K; Mølhave K Mapping the Complex Morphology of Cell Interactions with Nanowire Substrates Using FIB-SEM. PLoS One 2013, 8 (1), e53307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Badique F; Stamov DR; Davidson PM; Veuillet M; Reiter G; Freund J-N; Franz CM; Anselme K Directing Nuclear Deformation on Micropillared Surfaces by Substrate Geometry and Cytoskeleton Organization. Biomaterials 2013, 34 (12), 2991–3001. [DOI] [PubMed] [Google Scholar]
- (52).Davidson PM;Özçelik H; Hasirci V; Reiter G; Anselme K Microstructured Surfaces Cause Severe but Non-Detrimental Deformation of the Cell Nucleus. Adv. Mater 2009, 21 (35), 3586–3590. [Google Scholar]
- (53).Houben F; Ramaekers FCS; Snoeckx LHEH; Broers JLV Role of Nuclear Lamina-Cytoskeleton Interactions in the Maintenance of Cellular Strength. Biochim. Biophys. Acta, Mol. Cell Res 2007, 1773 (5), 675–686. [DOI] [PubMed] [Google Scholar]
- (54).Xie C; Hanson L; Xie W; Lin Z; Cui B; Cui Y Noninvasive Neuron Pinning with Nanopillar Arrays. Nano Lett. 2010, 10 (10), 4020–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Lin ZC; Xie C; Osakada Y; Cui Y; Cui B Iridium Oxide Nanotube Electrodes for Sensitive and Prolonged Intracellular Measurement of Action Potentials. Nat. Commun 2014, 5, 3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Coutinho-Budd J; Ghukasyan V; Zylka MJ; Polleux F The F-BAR Domains from srGAP1, srGAP2 and srGAP3 Regulate Membrane Deformation Differently. J. Cell Sci 2012, 125, 3390–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Jeong S; Galic M Nanocones to Study Initial Steps of Endocytosis. Methods Mol. Biol 2014, 1174, 275–284. [DOI] [PubMed] [Google Scholar]


