Abstract
Treatment duration for invasive mold disease (IMD) in patients with hematological malignancy is not standardized and is a challenging subject in antifungal stewardship. Concerns for IMD relapse during subsequent reinduction or consolidation chemotherapy or graft versus host disease treatment in hematopoietic stem cell transplant recipients often results in prolonged or indefinite antifungal treatment. There are no validated criteria that predict when it is safe to stop antifungals. Decisions are individualized and depend on the offending fungus, site and extent of IMD, comorbidities, hematologic disease prognosis, and future plans for chemotherapy or transplantation. Recent studies suggest that FDG-PET/CT could help discriminate between active and residual fungal lesions to support decisions for safely stopping antifungals. Validation of noninvasive biomarkers for monitoring treatment response, tests for quantifying the “net state of immunosuppression,” and genetic polymorphisms associated with poor fungal immunity could lead to a personalized assessment for the continued need for antifungal therapy.
Keywords: invasive mold infection, duration, treatment, leukemia, stem cell transplantation
The duration of antifungal therapy for invasive mold disease needs to be individualized according to improvement in or resolution of attributable signs and symptoms, follow-up radiology and fungal biomarkers, and future plans for treatment of underlying hematologic malignancy.
Invasive mold disease (IMD), once considered primarily an acute infection with high mortality rates in patients with hematological cancer, has increasingly evolved into a subacute or even chronic disease due to improvements in early diagnosis and the availability of more effective antifungal treatment options. As these infections often relapse in the setting of subsequent immunosuppression, an initial treatment phase followed by long-term secondary antifungal prophylaxis continued for months to even years has become an increasingly common clinical scenario. This approach has its pitfalls, as prolonged antifungal use increases the patient’s risk for cumulative toxicity, drug interactions, potential for selection of resistant fungi, compliance difficulties, and considerable cost. Importantly, the concern of potentially active IMD often delays consolidation chemotherapy or transplantation required to maintain complete remission and achieve cure of the underlying malignancy. The issue of the duration of antifungal therapy for IMD in high-risk patient populations has not been a focus of antifungal stewardship efforts to date, perhaps because of the complexity involved in treatment decisions on when to stop antifungal therapy.
The optimal duration of antifungal therapy for IMD depends on an individualized assessment of several complex, often interrelated factors (Table 1). Some, but not all, guidelines provide guidance about duration of antifungal therapy; however, the evidence supporting any particular recommendation is low [1]. In this review, we offer a conceptual framework on the key issues that shape decisions on when to stop all antifungals for IMD in patients who have undergone chemotherapy or transplantation for the treatment of a hematological malignancy and discuss the possible role of the novel imaging modalities and future questions in this increasingly common scenario.
Table 1.
Factors Influencing the Duration of Antifungals in Invasive Mold Disease in Patients With Hematologic Cancer
| Risk Factor | Type of Factor |
|---|---|
| Underlying disease and treatment-related factors | Cytopenia (granulocytopenia, lymphocytopenia, monocytopenia) |
| Relapsed or refractory leukemia | |
| HSCT and GvHD High-risk allogeneic HSCT Matched unrelated donor Cord stem cell T-cell depleted Haploidentical Mismatched CLL as the indication for HSCT Acute leukemia not in CR | |
| Small-molecule kinase inhibitors targeting immune-signaling pathways (eg, ibrutinib) | |
| Type of mold infection | Aspergillus |
| Mucorales | |
| Other molds | |
| Extent of lung infection | Bilateral versus unilateral disease |
| Number of lung lesions | |
| Type of lesion, especially cavitary masses | |
| Presence of residual sequestra of infected lung tissue | |
| Coinfections | CMV |
| Pseudomonas | |
| Comorbidities | Poorly controlled diabetes mellitus, malnutrition, iron overload |
Abbreviations: CLL, chronic lymphocytic leukemia; CMV, cytomegalovirus; CR, complete remission; GvHD, graft versus host disease; HSCT, hematopoietic stem cell transplant
The Pathophysiology of Persistent or Relapsed Invasive Mold Disease
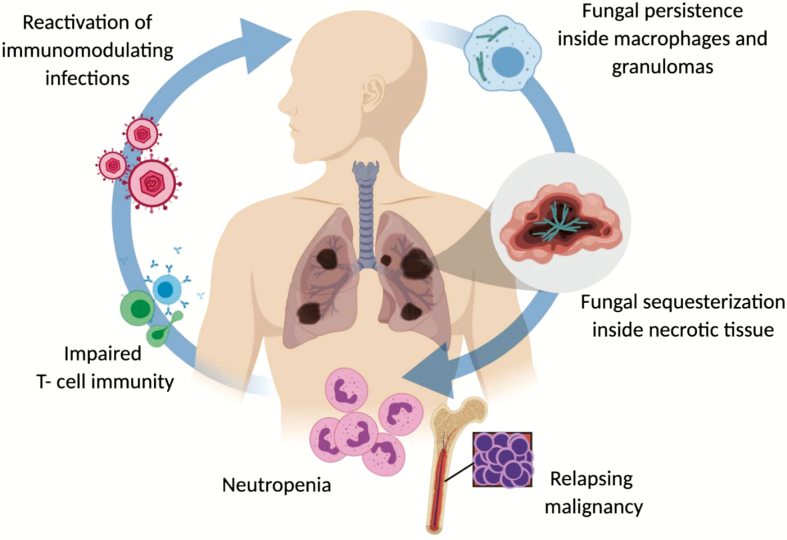
There is no validated definition of IMD “cure” in patients with hematological diseases. However, several aspects of the IMD pathobiology may contribute to the persistence as a latent, subclinical infection and relapses of the disease during subsequent periods of host immunosuppression [2] (Figure 1). Specifically, invasive molds invade blood vessels resulting in hemorrhage, occlusion and necrosis, and tissue sequestration [3]. Thus, there is limited access to both host immune cells and antifungals, which are often concentrated in the lipid-rich membranes of macrophages outside the necrotic centers of the lesions [4]. Moreover, fungi may remain dormant inside macrophages and subsequently reactivate during subsequent courses of immunosuppressive therapy [5]. Survival inside macrophages and granuloma formation may contribute to fungal latency, as the macrophages prevent spore germination but are unable to kill the spores, partially through iron restriction [6]. In addition, fungi have the ability to modulate their metabolism or to phenotypically change to survive inside the host [6], as a strategy to escape the immune system.
Figure 1.
Pathophysiology of relapsing or persistent invasive mold disease.
A compromised host immune status is widely recognized to be a central risk factor for relapse of previously controlled IMD. “Classical” host immunosuppression risk factors are typically linked to active hematologic malignancy and prolonged neutropenia associated with the underlying disease or salvage chemotherapy, high-risk allogeneic transplantation (Table 1), the use of high-dose corticosteroids for graft versus host disease (GvHD) treatment following allogeneic stem cell transplantation or the immunosuppressive effects of systemic reactivation of herpesviruses such as cytomegalovirus (CMV) [7]. Active leukemia has been reported to be more predictive of attributable mortality risk from IMD than neutrophil recovery [8]. While it is unclear how leukemia relapse affects antifungal immune responses, it is plausible that dysfunctional leukocytes and the cytokine milieu associated with relapsing hematologic malignancy affect immune cells’ antifungal capacity [9].
Although not directly studied, the type of mold might inform the propensity for tissue persistence and therefore the risk of relapse when discontinuing antifungals. For example, Mucorales are resistant, highly angioinvasive molds that cause extensive tissue necrosis and rapid dissemination to sanctuary sites [10, 11].
Systemic metabolic risk factors for relapse/reactivation (eg, iron overload, uncontrolled hyperglycemia) may also impair host immune mechanisms containing the fungus and directly facilitate fungal proliferation. Whether other local factors such as viral (eg, influenza or respiratory syncytial virus) or a bacterial (eg, Pseudomonas aeruginosa pneumonia) coinfection may exert local or systemic immunosuppressive effects that are permissive to IMD recurrence is unclear. Coinfection has been identified significantly more often in patients with probable pulmonary invasive aspergillosis (IA) than in those with proven pulmonary IA [12], increasing the diagnostic uncertainty and challenging the certainty of aspergillosis diagnosis. Evaluating the optimal duration of antifungal therapy in the context of coinfection is difficult, and the appropriateness of anti-infective therapy for coinfection should also be considered in assessing response and decisions on when to stop antifungals.
The extent of IMD also determines its “curability” and risk of relapse. Involvement of more than a single pulmonary lobe or dissemination outside the lung is associated with worse prognosis and higher risk of relapse [8]. Lung cavitation may foster the development of subacute expansion of existing fungal masses or nodules, where response to antifungal therapy may be difficult to ascertain [13]. In localized lung infections, surgical resection of residual fungal lesions could eradicate sequestered cavitary infection and reduce the incidence of relapse [14]. Moreover, increasing evidence suggests that, beyond the cystic fibrosis patient population, biofilms are present in chronic mold infections [15] and similar to bacterial biofilms can limit fungal clearance by immune cells as well as the penetration/activity of systemic antifungal therapy.
Fungal Biomarkers
Deciding when it is safe to stop antifungal therapy often depends on objective evidence of treatment response documented through serial computed tomography (CT) imaging, nonspecific markers of inflammation (C-reactive protein, fever), IMD-attributable symptoms, and a semiqualitative assessment of improved net state of immunosuppression of the patient. However, discriminating between an active infectious process and residual postinflammatory scarring with current CT imaging approaches is difficult in patients without clear IMD-attributable signs and symptoms [16].
Baseline galactomannan (GM) and GM kinetics seem to correlate with aspergillosis response and survival in patients with IA, regardless of neutropenia status [17], and clearly play a role in monitoring early response to therapy. ß-D-glucan seems to be less reliable than GM for predicting therapy response [18], and published experience with serial polymerase chain reaction is limited [19]. However, declines in serum GM decline and subsequent normalization occur early, usually within 7–14 days, in cases of IA that are responding. Although it is logical to recommend persistent negative serum GM levels as a prerequisite for stopping antifungals in patients with serum-GM positive IA, a negative GM does not indicate cure, and therefore cannot be used as a sole criterion to decide the timing of stopping antifungals.
Radiological Imaging to Guide Treatment Duration
Computed tomographic imaging is the most frequently used test to guide treatment decisions for pulmonary IMD [20]. Complete radiologic response may take weeks or months [21], and serial CT scans are recommended for follow-up, although the exact timing of subsequent CT images is not standardized and varies widely [22]. In neutropenic patients, the natural course of pulmonary IA includes a “paradoxical” increase in size and number of lesions on CT when imaging is performed shortly after the initiation of effective antifungal treatment that does not correlate with response to antifungals and/or surgery [23]. Changes in lesion volume between days 7 and 14 may correlate better with patient outcome of IA in these patients [21]. Notably, worsening of lesions can also occur simultaneously with neutrophil recovery due to an immune reconstitution inflammatory syndrome [24]. The natural course of other mold diseases such as mucormycosis is not as well described, but several small studies suggest similarities to IA [25].
Due to the scarcity of published serial CT studies, it is difficult to identify a prognostic “cutoff” for changes in lung lesion size during antifungal treatment that predict lack of IMD relapse following cessation of antifungal therapy. In a prospective study of patients with invasive pulmonary aspergillosis (IPA), the initial volume was reduced to 0.76 the baseline for a median follow-up of 17 days [23]. In a retrospective study of CT follow-up after IPA, in 62.5% of the patients the size of the lung lesions decreased to half from the maximum after a median of 31 days and 42.5% of patients showed a complete radiologic remission within a median of 80 days [26]. However, additional research is needed to better define thresholds of radiological response that support discontinuation of antifungal therapy. To add to the complexity, formation of cavitation prolongs time to resolution as much as 2.5 times; however, cavitation is associated with improved survival as it is a sign corresponding to neutrophil recovery [26]. In contrast to paradoxical increase in size of lung lesions following initiation of effective antifungal therapy, the number of lesions promptly decreases after the first week’s peak [26]. Lesion count was not associated with prognosis in a recent study examining prognostic significance of CT findings in IPA [21].
18Fluoro-2-deoxy-D-glucose positron emission tomography coupled with multidetector CT (FDG-PET/CT) can add important functional data compared with CT, even in cytopenic patients, as glucose uptake is probably carried out by other inflammatory cells (macrophages) or due to a stress response of the infected host cells [27]. FDG-PET/CT displays active metabolic changes resulting from inflammatory cell activity, allowing the distinction from postinflammatory scarring [5]. In addition, the ability of FDG-PET/CT for whole-body screening allows the detection of occult lesions that could influence both selection and duration of antifungal therapy. Several small studies suggest that, due to its functional imaging capacity, FDG-PET/CT has the potential to both “stage “ IMD and to differentiate active infection from residual scar tissue (as seen in chest CT), and therefore be used as a guide to the duration of antifungal therapy [28], although no comparative studies between FDG-PET/CT and conventional CT have been performed (Table 2).
Table 2.
FDG-PET/CT Studies for Monitoring Invasive Mold Disease in Patients With Hematologic Cancer
| Reference | Type of Study (N) | Fungus/Site | Aim | Target Population | Median Time to Follow-up PET | Results |
|---|---|---|---|---|---|---|
| Chamilos et al, 2008 [16] | Single-center retrospective (13) and literature review (n = 9) | Aspergillosis (73%), Mucorales (27%), mostly pneumonia (77%) | PET performed for restaging of malignancy. Evaluated for diagnosis and management of IMD. | Immunocompromised patients; underlying malignancy (73%), primarily hematological (55%) | N/A | Radiographic resolution of the mold infection in CT imaging was associated with scarring and lagged several weeks behind the FDG-PET imaging results. FDG-PET/CT was more useful than CT in 36% of patients in guiding the duration of treatment. In 19% of non-neutropenic patients FDG-PET/ CT revealed an occult IMD site. |
| Koh et al, 2012 [29] | Case-control study (37:76) | Not specified /mostly lungs (40.5%) and gastrointestinal (24%) | PET versus no PET in FN management. Evaluated the impact on diagnosis and antimicrobial utilization. | High-risk FN in patients with hematologic malignancy | N/A | An underlying cause for FN was determined in 94.6% of cases versus 69.7% of controls. FDG-PET was associated with shorter duration of liposomal amphotericin-B therapy for systemic fungal infection (median, 4.0 day cases vs 10.0 day controls; P = .001) |
| Leroy- Freschini et al, 2018 [30] | Single-center retrospective study (51) | Molds and yeasts (mainly Aspergillus [45%] and Candida [33%])/many different sites | Evaluate the impact of FDG-PET/CT in the management of IFI. | Immunocompromised (53% hematologic malignancy) | 4.4 ± 3.2 months | Results during treatment triggered antifungal drugs escalation in 15% and reduction of antifungal drugs dosage or treatment withdrawals in 31%. |
| Douglas et al, 2018 [28] | Retrospective single-center (48) | Molds and yeasts (mainly Aspergillus (76%) and Candida (7%))/lung (71%), lung + other (17%), other (12%) | Comparison to conventional CT imaging. Evaluate detecting and guiding management of IFIs. | Cancer patients (78% hematologic malignancy) | Follow-up FDG- PET/CT at a median of 2.5 months after initial FDG-PET/CT (0.5 to 13 months) | FDG-PET/CT located clinically occult infection or dissemination to another organ in 40% and 38% of IFI patients. Metabolically active changes allowed the distinction from postinflammatory scarring: discordant findings between the 2 imaging modalities were found in 61%. FDG-PET/CT performed well even in patients with severe neutropenia. |
| Schirmer et al, 2018 [31] | Single-center (10) | Fusariosis/blood or skin | Evaluate findings and clinical follow-up from a series of cases of Fusarium spp infections. | Hematopoietic stem cell transplant | N/A | Useful to discriminate uncomplicated cases of primary bloodstream infections, and to detect occult foci of metastatic infection in patients with positive cutaneous lesions. |
| Ankrah et al, 2019 [32] | Single-center retrospective (28) | Invasive aspergillosis (64%), invasive candidiasis (32%), Hormografiella aspergillata (4%)/ site not specified | Monitoring treatment response in patients with IFIs. All had PET at baseline and at 1 or more time points during treatment. | Immunocompromised (68% hematologic malignancy) | Median duration of therapy until the last FDG-PET/ CT scan was 33.5 (5–242) weeks | FDG-PET/CT altered management in 50% of the patients. The authors identified PET parameters that could discriminate between patients eventually having a CMR and patients who did not achieve CMR. Suboptimal performance in patients with predominantly cerebral and renal lesions (high FDG physiological uptake in those areas). |
Abbreviations: CMR, complete metabolic response; CT, computed tomography; IFI, invasive fungal infection; IMD, invasive mold infection; FDG-PET/CT, FDG-PET, PET, 18-fluoro-deoxy-glucose positron emission tomography; FN, febrile neutropenia; N/A, not available.
A drawback of FDG-PET/CT for IMD monitoring is its suboptimal performance in patients with predominantly cerebral and renal lesions, as the high FDG physiological uptake in areas such as the brain, urinary tract, and heart may impair visualization of IMD foci in these organs [33]. There are also differences in findings between children and adults; however, FDG uptake findings suggest it can be used as a tool in both populations [33]. Ideally, a baseline FDG-PET/CT would be performed to allow for comparison with subsequent scans, but at the expense of added cost and radiation. For clinical decision making, performing only a follow-up FDG-PET/CT might suffice. The optimal timing for FDG-PET/CT to evaluate IMD treatment remains unresolved. Different time frames for follow-up FDG-PET/CT have been used, from 2.5 months after initial FDG-PET/CT [28] to 4.4 ± 3.2 months [30]. Timing of a follow-up PET/CT should be dictated by the clinical context, specifically the type and extent of IMD and whether it is used either to assess treatment effectiveness or to confirm suspicion of a relapse. Large multicenter prospective studies are required to validate the prognostic value of FDG-PET/CT, as well as its cost-effectiveness for monitoring of prolonged antifungal treatment [16]. Of note, PET/CT has been shown to have value in evaluation and follow-up of other chronic lung diseases such as tuberculosis [34].
Adjunct Surgical Therapy
Setting aside surgical indications for emergency situations (eg, life-threatening pulmonary bleeding), surgical resection of residual pulmonary nodules or cavities, necrotic skin, and soft tissue lesions in primary cutaneous IMD or IMD of the sinuses has often been recommended to decrease risk of reactivation in patients who undergo subsequent chemotherapy or transplantation. Debulking of a dominant cavitating lung lesion that persists despite improvement in IMD elsewhere is more debatable.
However, the role of surgery for pulmonary IMD in the modern era of early diagnosis based on CT/biomarkers and potent antifungal drugs appears to be diminishing. Most of the studies evaluating risk–benefit of surgery in this setting are based on data from the pre–voriconazole era [35]. Nevertheless, surgery should be considered in selected patients (eg, patients with good performance status and absence of severe comorbidities who are to undergo curative chemotherapy and/or hematopoietic stem cell transplantation [HSCT] or reinduction or consolidation for hematologic cancer) and who have residual, ideally solitary, IMD lesions. The ideal surgical candidate for pulmonary IMD should be able to tolerate wedge resection or partial pneumonectomy, although the risk of hematologic relapse during the recovery period (typically 4–6 weeks) needs to be balanced against the potential benefits of surgical resection. Notwithstanding, it must be taken into account that postoperative recovery may delay chemotherapy or HSCT and thus the durability of hematological remission [4], which is the most important factor affecting long-term survival of the patient [14]. The most conservative surgical approach is preferred, making every effort to avoid pneumonectomy and to keep surgical impact as small as possible.
Conversely, surgery still plays an important role in rhino-orbito-cerebral IMD due to its different pathophysiology. Debulking surgery has shown to improve the outcome of central nervous system (CNS) and rhino-sinusal mold infection as it facilitates access of immune cells and better penetration of antifungals [36], thus allowing the cure of mold infections that would otherwise have a dismal outcome or require lifelong suppressive therapy. In particular, in IMD caused by resistant Aspergillus or difficult to treat non-Aspergillus molds, particularly Mucorales, repeated surgical debridements, when feasible, remain paramount to a favorable clinical outcome [37].
Risk of Relapse With Subsequent Chemotherapy/Hematopoietic Stem Cell Transplantation
Consideration should be given to stopping antifungal therapy in patients treated for IMD when all of the following conditions are met, including (1) negative follow-up cultures and/or biomarkers, (2) evidence of diminishing or resolved lesions by serial CT exam, and (3) patients in hematologic remission with adequate counts provided that no further chemotherapy or transplantation is planned and the patient can be closely followed. However, in the aforementioned patients whose antifungals were stopped, these agents should be resumed in the setting of subsequent relapse of hematologic disease requiring reinduction chemotherapy. This cautionary approach of restarting mold-active drugs as secondary prophylaxis should apply for patients without evidence of active IMD who are to undergo stem cell transplantation. The new mold-active triazoles have good success rates as secondary prophylaxis in patients with a history of IMD undergoing chemotherapy or transplantation.
Role of Long-term Antifungal Therapy
In selected cases, long-term (and possibly lifelong) antifungals should be considered. The benefits of antifungal suppressive therapy need be weighed against toxicities of chronic azole therapy, which include a variety of side effects from periostitis to skin cancer [38]. The presence of sequestrum (eg, lung cavity), difficult site involvement with poor source control (such as eye, CNS, or bone), or infection caused by a drug-resistant mold (especially Mucorales or mixed infections) would justify an extended course of antifungals, requiring highly motivated patients to ensure compliance and achieve prolonged survival [39].
Future Questions
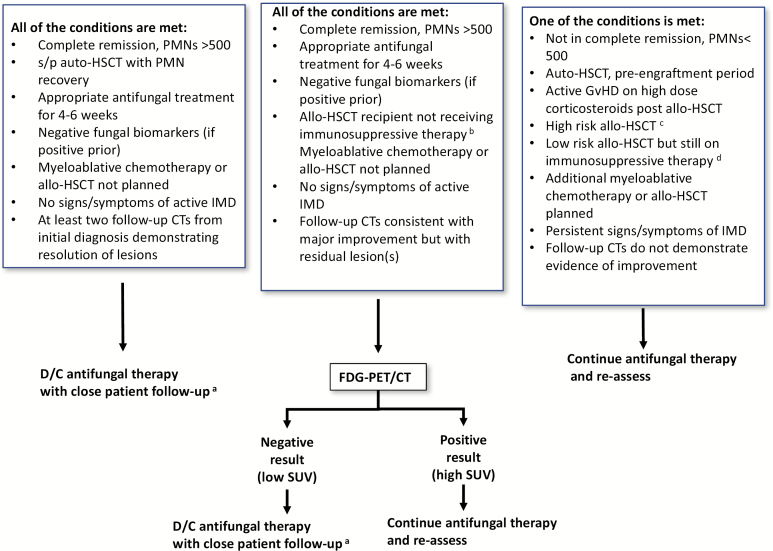
Our proposed algorithm to decide on when to stop antifungals in patients with the most common IMD, IA, is shown in Figure 2. However, several questions remain in this difficult-to-study clinical area (Table 3). Assessing the net state of immunosuppression of the patient and more specifically antimold immunity in patients on conventional chemotherapies is a key question. Even more difficult is assessment of risk for IMD relapse in patients with hematological cancers historically considered at low risk for IMD, such as those receiving the Bruton tyrosine kinase inhibitor ibrutinib and other small-molecule kinase inhibitors. A subset of these patients have a complex immunodeficiency, whose duration is hard to assess with conventional quantitative measures of immune recovery such as neutrophil or lymphocyte count [40]. Ideally, a validated test quantifying specific immunity against molds (equivalent to Quantiferon® CMV [Qiagen]) should be developed and inform decisions on when to stop antifungals. Aspergillus- and Mucorales-specific T cells in peripheral blood have been studied as a diagnostic marker [41, 42], but their study as a marker of specific immunity and their association with outcome deserve further research. In the absence of such a test, there are some promising data with the ImmuknowR CylexTM assay in solid organ transplantation patients, a technique that evaluates peripheral blood CD4(+) adenosine triphosphate activity as a surrogate marker of immunosuppression [43], allowing a more personalized adjustment of immunosuppressive agents. This assay could be adapted to identify patients at risk of developing IMD and provide a tool to optimize immunosuppressive therapy. Similar prognostic risk models could be developed and validated for hematology populations to more reliably estimate the cumulative risk of IMD relapse over time and support treatment assessments.
Figure 2.
Proposed algorithm to decide on when to stop antifungals in patients with invasive aspergillosis. aPatients with IMD who stop mold-active antifungal therapy need to resume mold-active antifungal if they undergo future chemotherapy or HSCT. bFor at least 2 months. cMatched unrelated donor allo-HSCT, cord stem cell HSCT, T-cell–depleted allo-HSCT, haplo-identical allo-HSCT, mismatched allo-HSCT, AML not in remission, CLL. dallo-HSCT, low-risk matched unrelated donor allo-HSCT, AML in first complete remission, CML, lymphoma, aplastic anemia. Abbreviations: allo-HSCT, allogenic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CT, computed tomography; D/C, discontinue; FDG-PET/CT, 18fluoro- 2-deoxy-D-glucose positron emission tomography coupled with multidetector computed tomography; GvHD, graft versus host disease; HSCT, hematopoietic stem cell transplantation; IMD, invasive mold disease; PMN, polymorphonuclear (cells/mm3); s/p, status post; SUV, standardized uptake value.
Table 3.
Future Questions Regarding Duration of Treatment for Invasive Mold Disease in Patients With Hematologic Cancer
| Risk Factor | Questions |
|---|---|
| Host | Should we routinely “stage” IMD by FDG-PET/CT to identify occult sites before stopping antifungal therapy? |
| Do all patients need to resume secondary antifungal prophylaxis after stopping antifungals if a patient is restarting chemotherapy? | |
| How do chronic comorbidities (eg, diabetes mellitus, iron overload) influence risk for IMD relapse? | |
| How do host immunogenetics impact risk of IMD relapse? | |
| Can we develop and validate generalizable prognostic models (risk scores) for predicting risk of IMD relapse? | |
| How are new targeted therapies and immunosuppressive but nonmyelotoxic therapies affecting the fungal immune responses? | |
| Laboratory/imaging | Does in vitro susceptibility of the offending mold correlate with IMD relapse risk? |
| In addition to neutrophils, how do lymphocyte, NK and monocyte counts impact decisions of when to stop antifungal therapy? | |
| Can the tempo or pattern for normalization of serum biomarkers (eg, Aspergillus GM) be used to determine duration of therapy? | |
| How frequently should FDG-PET/CT be optimally used to define treatment response in IMD? | |
| Can emerging point-of-care diagnostics or other noninvasive tests (eg, breath analysis of volatile organic compounds, qPCR) be used to detect early relapse? | |
| Treatment | Do chronic mold infections have a biofilm physiology that should be disrupted to reduce the risk of relapse? |
| What is the relationship of antifungal dosing for antifungal duration and risk of IMD relapse? | |
| Does combination therapy reduce the risk of relapse? | |
| How frequently does acquired antifungal resistance in vivo contribute to IMD relapse? | |
| Does the sequence of different antifungal classes impact the risk of IMD relapse? | |
| How does the timing and extent of surgery impact the risk for IMD relapse? | |
| Does surgical debulking of major lesions reduce the risk of relapse or shorten the duration of effective antifungal treatment needed? |
Abbreviations: IMD, invasive mold infection; FDG-PET/CT, 18-fluoro-deoxy-glucose positron emission tomography; GM, galactomannan; NK, natural killer; qPCR, quantitative polymerase chain reaction.
New experimental techniques based on specific molecular probes, such as ImmunoPET, an antibody-guided imaging that uses radiolabeled Aspergillus-specific monoclonal antibodies coupled with PET/magnetic resonance, or gallium-68–labeled siderophores microPET imaging [44], offer potential for a more precise differentiation of an expanding lesion of an increasing uptake to fungal proliferation versus malignancy relapse [45]. The correlation with activity of the disease of new point-of-care noninvasive tests that are being tested for aspergillosis diagnosis, such as the lateral flow dipstick assay which detects a urine Aspergillus fumigatus antigen [46] or breath detection of volatile organic compounds specific for Aspergillus [47], should be evaluated to determine their usefulness to assist in the assessment of antifungal therapy duration and early IMD relapse.
Antifungal therapy could potentially be shortened if, in addition to fighting the mold, the host immunity against it is enhanced. Among strategies to boost host immunity, adoptive mold-specific T-cell transfer and chimeric antigen receptor T cell or natural killer cell therapies have the potential to provide long-term control of nascent IMD. Adoptive T-cell therapy is already in use against viral infections, and is being developed for molds, with ongoing clinical trials [48].
Studies have shown that susceptibility to IA in allogenic-HSCT patients is influenced by polymorphisms in innate immunity genes (polymorphisms in cell-surface–expressed Toll-like receptors and dectin-1) [49]. The association between genetic polymorphisms such as these and the risk of IMD relapse after discontinuing antifungals needs to be studied to identify a subpopulation amenable to more prolonged antifungal therapy or secondary prophylaxis. Currently, genome-wide studies are in progress to uncover host susceptibility to mold invasive infections based on genetic variations.
In summary, duration of antifungal therapy for IMD needs to be individualized. In the future, prognostic risk models that take into account clinical, radiological, microbiological, genetic, and disease-related factors may allow for more precise identification of patients who can safely discontinue antifungal therapy, supporting antifungal stewardship goals in this high-risk population.
Note
Potential conflicts of interest. A. F.-C. reports honoraria for lectures from Astellas; Merck, Sharp and Dohme; and Gilead. R. E. L. received research support from Merck and has served on advisory boards for Gilead and Cidara and F2G. D. P. K. acknowledges the Texas 4000 Distinguished Professorship for Cancer Research and the National Institutes of Health–National Cancer Institute Cancer Center CORE Support grant number 16672. D. P. K. reports research support from Astellas Pharma and honoraria for lectures from Merck & Co, Gilead, and United Medical. He has served as a consultant for Astellas Pharma, Cidara, Amplyx, Astellas, Pulmocide, and Mayne and on the advisory board of Merck & Co. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Patterson TF, Thompson GR 3rd, Denning DW, et al. Executive summary: practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao Y, Prideaux B, Baistrocchi S, Sheppard DC, Perlin DS. Beyond tissue concentrations: antifungal penetration at the site of infection. Med Mycol 2019; 57:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ben-Ami R, Lewis RE, Kontoyiannis DP. Enemy of the (immunosuppressed) state: an update on the pathogenesis of Aspergillus fumigatus infection. Br J Haematol 2010; 150:406–17. [DOI] [PubMed] [Google Scholar]
- 4. Sipsas NV, Kontoyiannis DP. Clinical issues regarding relapsing aspergillosis and the efficacy of secondary antifungal prophylaxis in patients with hematological malignancies. Clin Infect Dis 2006; 42:1584–91. [DOI] [PubMed] [Google Scholar]
- 5. Brunet K, Alanio A, Lortholary O, Rammaert B. Reactivation of dormant/latent fungal infection. J Infect 2018; 77:463–8. [DOI] [PubMed] [Google Scholar]
- 6. Andrianaki AM, Kyrmizi I, Thanopoulou K, et al. Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species. Nat Commun 2018; 9:3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yong MK, Slavin MA, Kontoyiannis DP. Invasive fungal disease and cytomegalovirus infection: is there an association? Curr Opin Infect Dis 2018; 31:481–9. [DOI] [PubMed] [Google Scholar]
- 8. Nivoix Y, Velten M, Letscher-Bru V, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis 2008; 47:1176–84. [DOI] [PubMed] [Google Scholar]
- 9. Cunha C, Kurzai O, Löffler J, Aversa F, Romani L, Carvalho A. Neutrophil responses to aspergillosis: new roles for old players. Mycopathologia 2014; 178:387–93. [DOI] [PubMed] [Google Scholar]
- 10. Ben-Ami R, Luna M, Lewis RE, Walsh TJ, Kontoyiannis DP. A clinicopathological study of pulmonary mucormycosis in cancer patients: extensive angioinvasion but limited inflammatory response. J Infect 2009; 59:134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis RE, Lortholary O, Spellberg B, Roilides E, Kontoyiannis DP, Walsh TJ. How does antifungal pharmacology differ for mucormycosis versus aspergillosis? Clin Infect Dis 2012; 54(Suppl 1):S67–72. [DOI] [PubMed] [Google Scholar]
- 12. Georgiadou SP, Kontoyiannis DP. Concurrent lung infections in patients with hematological malignancies and invasive pulmonary aspergillosis: how firm is the Aspergillus diagnosis? J Infect 2012; 65:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peghin M, Ruiz-Camps I, Garcia-Vidal C, et al. Unusual forms of subacute invasive pulmonary aspergillosis in patients with solid tumors. J Infect 2014; 69:387–95. [DOI] [PubMed] [Google Scholar]
- 14. Reischies F, Hoenigl M. The role of surgical debridement in different clinical manifestations of invasive aspergillosis. Mycoses 2014; 57(Suppl 2):1–14. [DOI] [PubMed] [Google Scholar]
- 15. Kernien JF, Snarr BD, Sheppard DC, Nett JE. The interface between fungal biofilms and innate immunity. Front Immunol 2017; 8:1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chamilos G, Macapinlac HA, Kontoyiannis DP. The use of 18F-fluorodeoxyglucose positron emission tomography for the diagnosis and management of invasive mould infections. Med Mycol 2008; 46:23–9. [DOI] [PubMed] [Google Scholar]
- 17. Kontoyiannis DP, Selleslag D, Mullane K, et al. Impact of unresolved neutropenia in patients with neutropenia and invasive aspergillosis: a post hoc analysis of the SECURE trial. J Antimicrob Chemother 2018; 73:757–63. [DOI] [PubMed] [Google Scholar]
- 18. Neofytos D, Railkar R, Mullane KM, et al. Correlation between circulating fungal biomarkers and clinical outcome in invasive aspergillosis. PLoS One 2015; 10:e0129022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barnes RA, White PL, Morton CO, et al. Diagnosis of aspergillosis by PCR: clinical considerations and technical tips. Med Mycol 2018; 56:60–72. [DOI] [PubMed] [Google Scholar]
- 20. Georgiadou SP, Sipsas NV, Marom EM, Kontoyiannis DP. The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis 2011; 52:1144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vehreschild JJ, Heussel CP, Groll AH, et al. Serial assessment of pulmonary lesion volume by computed tomography allows survival prediction in invasive pulmonary aspergillosis. Eur Radiol 2017; 27:3275–82. [DOI] [PubMed] [Google Scholar]
- 22. Cochon LR, Kapoor N, Carrodeguas E, et al. Variation in follow-up imaging recommendations in radiology reports: patient, modality, and radiologist predictors. Radiology 2019; 291:700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caillot D, Latrabe V, Thiébaut A, et al. Computer tomography in pulmonary invasive aspergillosis in hematological patients with neutropenia: an useful tool for diagnosis and assessment of outcome in clinical trials. Eur J Radiol 2010; 74:e172–5. [DOI] [PubMed] [Google Scholar]
- 24. Miceli MH, Maertens J, Buvé K, et al. Immune reconstitution inflammatory syndrome in cancer patients with pulmonary aspergillosis recovering from neutropenia: proof of principle, description, and clinical and research implications. Cancer 2007; 110:112–20. [DOI] [PubMed] [Google Scholar]
- 25. Choo JY, Park CM, Lee HJ, Lee CH, Goo JM, Im JG. Sequential morphological changes in follow-up CT of pulmonary mucormycosis. Diagn Interv Radiol 2014; 20:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brodoefel H, Vogel M, Hebart H, et al. Long-term CT follow-up in 40 non-HIV immunocompromised patients with invasive pulmonary aspergillosis: kinetics of CT morphology and correlation with clinical findings and outcome. AJR Am J Roentgenol 2006; 187:404–13. [DOI] [PubMed] [Google Scholar]
- 27. Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992; 33:1972–80. [PubMed] [Google Scholar]
- 28. Douglas AP, Thursky KA, Worth LJ, et al. FDG PET/CT imaging in detecting and guiding management of invasive fungal infections: a retrospective comparison to conventional CT imaging. Eur J Nucl Med Mol Imaging 2019; 46:166–73. [DOI] [PubMed] [Google Scholar]
- 29. Koh KC, Slavin MA, Thursky KA, et al. Impact of fluorine-18 fluorodeoxyglucose positron emission tomography on diagnosis and antimicrobial utilization in patients with high-risk febrile neutropenia. Leuk Lymphoma 2012; 53:1889–95. [DOI] [PubMed] [Google Scholar]
- 30. Leroy-Freschini B, Treglia G, Argemi X, et al. 18F-FDG PET/CT for invasive fungal infection in immunocompromised patients. QJM 2018; 111:613–22. [DOI] [PubMed] [Google Scholar]
- 31. Schirmer MR, Carneiro MP, Machado LS, Chaves A, Lopes F. . Fluorine-18-fluorodeoxyglucose PET/CT in hematopoietic stem cell transplant patients with fusariosis: initial findings of a case series review. Nucl Med Commun 2018; 39:545–52. [DOI] [PubMed] [Google Scholar]
- 32. Ankrah AO, Span LFR, Klein HC, et al. Role of FDG PET/CT in monitoring treatment response in patients with invasive fungal infections. Eur J Nucl Med Mol Imaging 2019; 46:174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ankrah AO, Klein HC, Span LFR, et al. The role of PET in monitoring therapy in fungal infections. Curr Pharm Des 2018; 24:795–805. [DOI] [PubMed] [Google Scholar]
- 34. Sánchez-Montalvá A, Barios M, Salvador F, et al. Usefulness of FDG PET/CT in the management of tuberculosis. PLoS One 2019; 14:e0221516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matt P, Bernet F, Habicht J, et al. Predicting outcome after lung resection for invasive pulmonary aspergillosis in patients with neutropenia. Chest 2004; 126:1783–8. [DOI] [PubMed] [Google Scholar]
- 36. Walter RB, Kantarjian HM, Huang X, et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M.D. Anderson Cancer Center Study. J Clin Oncol 2010; 28:1766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chitasombat MN, Kontoyiannis DP. Treatment of mucormycosis in transplant patients: role of surgery and of old and new antifungal agents. Curr Opin Infect Dis 2016; 29:340–5. [DOI] [PubMed] [Google Scholar]
- 38. Benitez LL, Carver PL. Adverse effects associated with long-term administration of azole antifungal agents. Drugs 2019; 79:833–53. [DOI] [PubMed] [Google Scholar]
- 39. Davoudi S, Anderlini P, Fuller GN, Kontoyiannis DP. A long-term survivor of disseminated Aspergillus and mucorales infection: an instructive case. Mycopathologia 2014; 178:465–70. [DOI] [PubMed] [Google Scholar]
- 40. Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis 2018; 66:140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Potenza L, Vallerini D, Barozzi P, et al. Mucorales-specific T cells in patients with hematologic malignancies. PLoS One 2016; 11:e0149108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Potenza L, Vallerini D, Barozzi P, et al. Characterization of specific immune responses to different Aspergillus antigens during the course of invasive aspergillosis in hematologic patients. PLoS One 2013; 8:e74326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ravaioli M, Neri F, Lazzarotto T, et al. Immunosuppression modifications based on an immune response assay: results of a randomized, controlled trial. Transplantation 2015; 99:1625–32. [DOI] [PubMed] [Google Scholar]
- 44. Kaeopookum P, Summer D, Pfister J, et al. Modifying the siderophore triacetylfusarinine C for molecular imaging of fungal infection. Mol Imaging Biol 2019; 21:1097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thornton CR. Molecular imaging of invasive pulmonary aspergillosis using ImmunoPET/MRI: the future looks bright. Front Microbiol 2018; 9:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marr KA, Datta K, Mehta S, et al. Urine antigen detection as an aid to diagnose invasive aspergillosis. Clin Infect Dis 2018; 67:1705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koo S, Thomas HR, Daniels SD, et al. A breath fungal secondary metabolite signature to diagnose invasive aspergillosis. Clin Infect Dis 2014; 59:1733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sam QH, Yew WS, Seneviratne CJ, Chang MW, Chai LYA. Immunomodulation as therapy for fungal infection: are we closer? Front Microbiol 2018; 9:1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Al-Bader N, Sheppard DC. Aspergillosis and stem cell transplantation: an overview of experimental pathogenesis studies. Virulence 2016; 7:950–66. [DOI] [PMC free article] [PubMed] [Google Scholar]