Abstract
Background
Human immunodeficiency virus (HIV) may affect the risk of death due to cardiovascular disease (CVD) differently in men versus women.
Methods
We examined CVD mortality rates between 2007 and 2017 among all New York City residents living with HIV and aged 13+ by sex, using data from city HIV surveillance and vital statistics and the National Death Index. Residents without HIV were enumerated using modified US intercensal estimates. We determined associations of HIV status with CVD mortality by sex and neighborhood poverty, defined as the percent of residents living below the federal poverty level, after accounting for age, race/ethnicity, and year.
Results
There were 3234 CVD deaths reported among 147 915 New Yorkers living with HIV, with the proportion of deaths due to CVD increasing from 11% in 2007 to 22% in 2017. The age-standardized CVD mortality rate was 2.7/1000 person-years among both men and women with HIV. The relative rate of CVD mortality associated with HIV status was significantly higher among women (adjusted rate ratio [aRR] 1.7, 95% confidence interval [CI] 1.6–1.8) than men (aRR 1.2, 95% CI 1.1–1.3) overall, and within strata defined by neighborhood poverty. Sex differences in CVD mortality rates were the greatest when comparing individuals living with HIV and having detectable HIV RNA and CD4+ T-cell counts <500 cells/uL with individuals living without HIV.
Conclusions
Among people with HIV, 1 in 5 deaths is now associated with CVD. HIV providers should recognize the CVD risk among women with HIV, and reinforce preventive measures (eg, smoking cessation, blood pressure control, lipid management) and viremic control among people living with HIV regardless of neighborhood poverty to reduce CVD mortality.
Human immunodeficiency virus (HIV) increases cardiovascular disease mortality risks to a greater degree among women than men, even after accounting for neighborhood poverty. HIV providers should emphasize cardiovascular disease prevention (eg, smoking cessation, hypertension control, lipid management) and viremic control.
Keywords: HIV infection, cardiovascular disease, mortality, women, poverty areas
Cardiovascular disease (CVD) is now well established as a major contributor to morbidity and mortality among people living with human immunodeficiency virus (HIV). Many people with HIV have multiple risk factors for CVD, including both traditional factors (eg, smoking, hypercholesterolemia) [1] and nontraditional factors (eg, hepatitis C coinfection, immune activation, and inflammation) [2–5]. While early studies in the antiretroviral therapy (ART) era focused on atherosclerotic vascular disease processes [6, 7], other manifestations of CVD, including myocardial abnormalities, have emerged as increasingly common in people living with treated HIV [8], with heart failure and stroke affecting people living with HIV at rates higher than in the general population [9, 10]. Furthermore, there is increasing evidence of sex differences in CVD risks in people with HIV that go beyond those in the general population, potentially owing to differences in immunologic profiles, the effects of sex hormones, and underlying comorbidities, such as obesity and depression [11, 12]. It remains unclear how much these differences result from disparities in socioeconomic profiles of men and women with HIV, versus underlying biological mechanisms.
We previously examined CVD mortality rates among people living with HIV, using HIV surveillance data in New York City though 2012 [13]. In that study, we documented consistent increases in the proportion of deaths due to CVD, and found that the association between HIV status and CVD mortality was much more pronounced among women. Our current analysis extends follow-up through 2017 to update trends in CVD mortality among people with HIV, as more events have accumulated. Because socioeconomic status may confound the association between sex and CVD mortality among people with HIV, we also reassessed the association of HIV status with CVD mortality after accounting for neighborhood-level poverty.
METHODS
Data Sources and Study Population
Our source for mortality rates among individuals diagnosed with HIV was the New York City HIV Surveillance Registry [14]. Briefly, name-based reporting of cases of HIV infection was implemented in New York in 2000, and all HIV-related laboratory tests have been reportable since 2005. Deaths among reported HIV cases in New York City are ascertained by quarterly linkages with the New York City Vital Statistics Registry, supplemented by record linkages with the National Death Index [15]. We determined denominators for CVD mortality rates among New Yorkers with HIV by assessing the person-years spent living with a diagnosis of HIV for anyone in the Surveillance Registry between 2007 and 2017, including both individuals with prevalent infection and those newly diagnosed. To focus on adults and adolescents, we excluded person-years for children <13 years old.
Our sources for mortality rates in those without HIV were the New York City Vital Statistics Registry and US Census Bureau interpolated intercensal population estimates modified by the New York City Department of Health and Mental Hygiene. For comparisons between people living with and without HIV, population denominator person-years were obtained by creating bins (strata) of city residents based on sex, age group, race/ethnicity, and neighborhood poverty. Bins were created because census data are not publicly available at the individual level. To limit bin denominators to individuals living without HIV, we subtracted the number of known person-years in people living with HIV from each bin, as determined from the HIV Surveillance Registry.
Outcomes
We defined deaths due to CVD based on underlying cause-of-death International Classification of Diseases, 10th Revision, codes representing “major cardiovascular diseases” (I00-I78) [16], derived from causes listed on the death certificate [13]. We also examined more specific underlying cardiovascular causes of death, including chronic ischemic heart disease, acute myocardial infarction, hypertensive diseases, cerebrovascular diseases, cardiomyopathy, arrhythmias, valvular heart disease, and pulmonary heart disease [17]. To capture conditions identified as contributing to the sequence of events leading to death, rather than classified as the underlying cause, we examined up to 20 contributing causes listed on the death certificate, focusing on the same categories, as well as heart failure. Heart failure was examined as a contributing but not underlying cause, because it is usually categorized as the consequence of an earlier health condition (eg, hypertension) [18].
Variables of Interest
The primary variables were HIV status and sex assigned at birth, as reported in the Registry. Neighborhood poverty was defined based on the percentage of the population living below the federal poverty level (FPL) within an individual’s residential ZIP code in a given year. In 2017, the US FPL was $12 488 for a single adult and $25 094 for a family of 4 [19]. The most recently available residential information for each individual was used, and we categorized neighborhood poverty as low (<10% below FPL), medium (10 to <20%), high (20 to <30%), and very high (≥30%). Other variables included current age; race/ethnicity; year; and HIV RNA level and CD4+ count, using the most recent value reported in each year. HIV RNA level was categorized as suppressed or unsuppressed, and we used <400 copies/mL to define suppression, to account for the lowest detection limit of multiple assays used during the period.
Statistical Methods
We calculated mortality rates per 1000 person-years using direct age standardization to the 2000 US standard population. Negative binomial regression tested for associations of HIV status with CVD mortality in models stratified by sex. We used 2 approaches to account for neighborhood poverty: regression adjustment and stratification. Regression models also controlled for confounding by age, race/ethnicity, and calendar year. We tested 2-way interactions between HIV status and sex. We performed secondary analyses that further stratified HIV status into groups defined by HIV RNA level and CD4+ count. These analyses were limited to person-years with at least 1 RNA measurement or CD4+ count, to focus on persons in care living with HIV. Analyses were conducted in SAS 9.4 and used an α of 0.05 to determine statistical significance for both main effects and interactions.
RESULTS
Study Population
The number of people reported to be living with HIV in New York City increased from 110 970 in 2007 to 127 713 in 2017. This corresponded to 108 083 men (937 818 person-years) and 39 832 women (359 013 person-years) over 11 years, taking into account both new diagnoses of HIV and deaths (Table 1). The median age in both men and women living with HIV increased, from 45 to 52. By race/ethnicity, 39% of men and 58% of women with HIV were Black (non-Hispanic), with Hispanic ethnicity comprising an additional one-third of both men and women. Among men, the most frequently reported HIV transmission risk was sex with men (53%); among women, 78% reported heterosexual transmission or an unknown risk. A history of injection drug use was reported among almost 20% of men and women. At the most recent measurement, the median CD4+ count was >500 cells/uL in both men and women, and about 80% had suppressed HIV RNA.
Table 1.
Characteristics of Persons Living with Human Immunodeficiency Virus in New York City, 2007–2017
| Males, n (%) | Females, n (%) | |
|---|---|---|
| Most recent age, years, median (IQR) | 52 (41–59) | 52 (44–59) |
| 13–24 | 2226 (2) | 909 (2) |
| 25–34 | 14 174 (13) | 3058 (8) |
| 35–44 | 17 327 (16) | 6437 (16) |
| 45–54 | 31 107 (29) | 12 795 (32) |
| 55–64 | 29.171 (27) | 11 642 (29) |
| 65–74 | 11 310 (10) | 3995 (10) |
| 75–84 | 2402 (2) | 862 (2) |
| 85+ | 366 (<1) | 134 (<1) |
| Race/ethnicity | ||
| Black | 42 571 (39) | 23 247 (58) |
| Hispanic | 35 724 (33) | 12 904 (32) |
| White | 26 341 (24) | 2950 (7) |
| Asian/Pacific Islander | 2627 (2.4) | 501 (1.3) |
| Native American | 267 (<1) | 91 (<1) |
| Other/Unknown | 553 (<1) | 139 (<1) |
| HIV transmission risk factor | ||
| Men who have sex with men | 57 607 (53) | … |
| Injection drug use history | 18 658 (17) | 7265 (18) |
| Perinatal | 1279 (1.2) | 1348 (3) |
| Heterosexual, other, and unknown | 30 539 (28) | 31 219 (78) |
| Neighborhood poverty level | ||
| Low poverty: <10% below FPL | 12 235 (12) | 2235 (6) |
| Medium poverty: 10-<20% below FPL | 32 973 (33) | 10 795 (29) |
| High poverty: 20 to <30% below FPL | 25 765 (26) | 10 472 (28) |
| Very high poverty: ≥30% below FPL | 27 675 (28) | 14 170 (38) |
| Most recent CD4+ T-cell count, median (IQR) | 536 (320–761) | 559 (298–830) |
| <200 cells/uL | 12 663 (15) | 5554 (17) |
| 200–349 cells/uL | 11 538 (13) | 4108 (13) |
| 350–499 cells/uL | 15 102 (17) | 4632 (14) |
| 500+ cells/uL | 47 241 (55) | 18 191 (56) |
| Most recent HIV-1 RNA level | ||
| Unsuppressed: 400+ copies/mL | 16 530 (19) | 7225 (22) |
| Suppressed: <400 copies/mL | 70 023 (81) | 25 163 (78) |
Data are from 108 083 males and 39 832 females. Data were reported to the New York City Department of Health and Mental Hygiene by 31 December 2018. There were 11 595 participants (8%) with unknown neighborhood poverty level, 28 886 (20%) with unknown CD4+ counts, and 28 974 (20%) with unknown HIV-1 RNA levels.
Abbreviations: FPL, federal poverty level; HIV, human immunodeficiency virus; IQR, interquartile range.
More than half of New Yorkers with HIV lived in very high– or high-poverty neighborhoods, which had higher proportions of women living with HIV (34% and 29%, respectively), compared with medium or low poverty neighborhoods (25% and 15%, respectively). Those living in higher-poverty neighborhoods were also younger and were more likely to be Black or Hispanic, have a history of injection drug use, and have unsuppressed HIV RNA (data not shown).
Trends in Cardiovascular Disease as Underlying Cause of Death
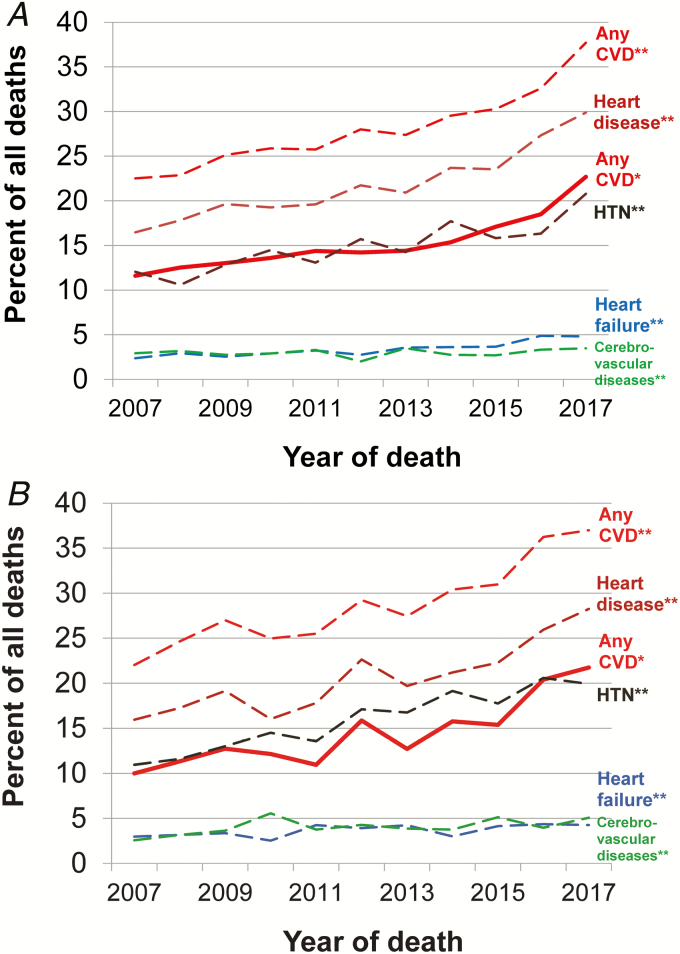
Between 2007 and 2017, 21 980 deaths due to any cause occurred among persons with HIV (Supplemental Table 1). The number of deaths declined over time, from 2343 in 2007 to 1757 in 2017, driven by a decrease in HIV-related deaths. During the study period, 15% of deaths were attributed to major cardiovascular diseases as the underlying cause of death, and this percentage was similar by sex (15% in men and 14% in women; Table 2; Supplemental Table 2). The numbers and percentages of deaths due to CVD among individuals with HIV increased steadily during the period, both overall and by sex (Figure 1). For example, among men and women combined, the percentage increased from 11% (n = 260) in 2007 to 22% (n = 394) in 2017 (P < .001). The median age of death due to CVD among adults living with HIV was 57 years, compared with 82 years among adults living without HIV.
Table 2.
Cardiovascular Disease Deaths Among Persons Living With Human Immunodeficiency Virus, New York City, 2007–2017
| Males | Females | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n, CVD Deaths | % of All Deaths | % of CVD Deaths | P for Trend | n, CVD Deaths | % of All Deaths | % of CVD Deaths | P for Trend | P for Difference by Sex | P for Trend | |
| Total deaths | 15 420 | 100.0 | … | … | 6560 | 100.0 | … | … | … | … |
| Underlying causes of death | ||||||||||
| Total CVD deaths | 2314 | 15 | 100 | <.001 | 920 | 14 | 100 | <.001 | .06 | <.001 |
| Diseases of heart | 1939 | 13 | 84 | <.001 | 697 | 11 | 76 | <.001 | <.001 | <.001 |
| Chronic ischemic heart disease | 1069 | 7 | 46 | <.001 | 354 | 5 | 38 | <.001 | <.001 | <.001 |
| Acute myocardial infarction | 196 | 1.3 | 8 | .002 | 68 | 1.0 | 7 | .24 | .14 | <.001 |
| Cardiomyopathy | 42 | 0.3 | 2 | .54 | 8 | 0.1 | 0.9 | .23 | .03 | .27 |
| Valvular heart disease | 24 | 0.2 | 1 | .31 | 11 | 0.2 | 1.2 | .63 | .84 | .27 |
| Pulmonary heart disease | 17 | 0.1 | 1 | .89 | 21 | 0.3 | 2.3 | .02 | <.001 | .08 |
| Arrhythmia | 17 | 0.1 | 1 | .46 | 5 | 0.1 | 0.5 | .02 | .47 | .08 |
| Hypertensive diseases | 625 | 4 | 27 | <.001 | 286 | 4 | 31 | <.001 | .27 | <.001 |
| Cerebrovascular diseases | 170 | 1.1 | 7 | .49 | 117 | 1.8 | 13 | .45 | <.001 | .37 |
| Contributing causes of death | ||||||||||
| Total mentions of CVD | 4261 | 28 | 100 | <.001 | 1844 | 28 | 100 | <.001 | .47 | <.001 |
| Diseases of heart | 3317 | 22 | 78 | <.001 | 1320 | 20 | 72 | <.001 | .02 | <.001 |
| Chronic ischemic heart disease | 1648 | 11 | 39 | <.001 | 549 | 8 | 30 | <.001 | <.001 | <.001 |
| Heart failure | 514 | 3 | 12 | <.001 | 236 | 4 | 13 | .07 | .32 | <.001 |
| Acute myocardial infarction | 325 | 2.1 | 8 | .07 | 120 | 1.8 | 7 | .16 | .18 | .002 |
| Arrhythmia | 211 | 1.4 | 5 | .002 | 72 | 1.1 | 4 | <.001 | .10 | <.001 |
| Pulmonary heart disease | 146 | 0.9 | 3 | .11 | 128 | 2.0 | 7 | .31 | <.001 | .09 |
| Cardiomyopathy | 140 | 0.9 | 3 | .12 | 38 | 0.6 | 2.1 | .50 | .01 | .26 |
| Valvular heart disease | 103 | 0.7 | 2 | .23 | 41 | 0.6 | 2.2 | .27 | .72 | .11 |
| Hypertensive diseases | 2261 | 15 | 53 | <.001 | 1012 | 15 | 55 | <.001 | .15 | <.001 |
| Cerebrovascular diseases | 460 | 3 | 11 | .55 | 261 | 4 | 14 | .03 | <.001 | .10 |
Data were reported to the New York City Department of Health and Mental Hygiene by 31 December 2018. Each reported death had a single underlying cause assigned but may have had >1 contributing cause. All trends were increasing over time. The ICD-10 codes used were I00–I78 for cardiovascular diseases (I46 was excluded for contributing causes); I00–I09, I11, I13, and I20–I51 for diseases of the heart (I46 was excluded for contributing causes); I20 and I25 for chronic ischemic heart disease; I21–I22 for acute myocardial infarction; I26–I28 for pulmonary heart disease; I47–I49 for arrhythmia; I42 for cardiomyopathy; I00–I09 and I34–I38 for valvular heart disease; I10–I13 and I15 for hypertensive diseases; I60–I69 for cerebrovascular diseases; and I50.0, I50.1, I50.9, I11.0, I13.0, I13.2, and I13.9 for heart failure.
Abbreviations: CVD, cardiovascular disease; ICD-10, International Classification of Diseases, 10th Revision.
Figure 1.
Deaths due to cardiovascular disease for (A) men and (B) women living with HIV, by percentage, New York City, 2007–2017. Based on *underlying or **contributing cause of death. Abbreviations: CVD, cardiovascular disease; HIV, human immunodeficiency virus; HTN, hypertension.
Among more specific CVD-related causes of death were chronic ischemic heart disease (44% of CVD deaths), hypertensive diseases (28%), and cerebrovascular diseases (9%). The percentage of deaths due to chronic ischemic heart disease was higher among men than women (46% vs 38%, respectively; P < .001), whereas the percentages due to either cerebrovascular diseases or pulmonary heart disease were higher among women than men (13% vs 7% and 2.3% vs 1%, respectively; both P values < .001).
Supplemental Figures 1–2 show that increases in CVD deaths that we observed overall in the city were generally maintained when stratifying by neighborhood poverty.
Trends in Cardiovascular Disease as Contributing Cause of Death
Among persons with HIV, a CVD condition was listed as a contributing cause on 28% of all death certificates, regardless of the underlying cause. The percentages were similar among both men and women (Table 2) and increased over time (Figure 1). The most common cardiovascular conditions were hypertensive diseases (54% of those with CVD listed as a contributing cause), chronic ischemic heart disease (36%), heart failure (12%), and cerebrovascular diseases (12%). Total mentions of CVD increased from 22% in 2007 to 38% in 2017, with hypertensive diseases, chronic ischemic heart disease, heart failure, and arrhythmias increasing over time (all P values < .001). Women had more mentions of cerebrovascular diseases and pulmonary heart disease than men (4.0% vs 3.0% and 2.0% vs 0.9%, respectively; both P values < .001).
Cardiovascular Mortality Rates By Sex and Human Immunodeficiency Virus Status
Overall, we found HIV status to be associated with a 32% increased rate of CVD death (rate ratio [RR] 1.32, 95% confidence interval [CI] 1.27–1.37), after adjustments for age, year, sex, race/ethnicity, and neighborhood poverty. In stratified analyses by sex, we found that the relative rate of CVD mortality associated with HIV was more pronounced in women (RR 1.73, 95% CI 1.62–1.85) than in men (RR 1.20, 95% CI 1.15–1.26; P for interaction <.001; Table 3).
Table 3.
Absolute and Relative Cardiovascular Disease Mortality Rates, New York City, 2007–2017
| Males | Females | |||||
|---|---|---|---|---|---|---|
| CVD Deaths, n | Age-standardized CVD Mortality Rate, Per 1000 Person-years (95% CI) | Adjusted Rate Ratio for CVD Death vs Persons Without HIV (95% CI) | CVD Deaths, n | Age-standardized CVD Mortality Rate, Per 1000 Person-years (95% CI) | Adjusted Rate Ratio for CVD Death vs Persons Without HIV (95% CI) | |
| Reference group: persons without HIV | 106 261 | 3.55 (3.53–3.57) | 1.00 (Ref.) | 123 469 | 2.70 (2.68–2.71) | 1.00 (Ref.) |
| Comparison 1: persons with HIV | 2314 | 2.71 (2.53–2.88) | 1.20 (1.15–1.26) | 920 | 2.70 (2.41–2.98) | 1.73 (1.62–1.85) |
| Comparison 2: persons with HIV, by HIV RNA level and CD4+ T-cell count | ||||||
| Suppressed HIV RNA and 500+ cells/uL | 482 | 3.17 (2.67–3.67) | 1.01 (.92–1.11) | 196 | 2.01 (1.52–2.49) | 1.25 (1.08–1.44) |
| Unsuppressed and 500+ cells/uL | 86 | 7.45 (4.29–10.60) | 1.55 (1.25–1.93) | 32 | 2.73 (.74–4.72) | 1.31 (.92–1.87) |
| Suppressed HIV RNA and <500 cells/uL | 860 | 4.84 (4.35–5.33) | 1.99 (1.85–2.14) | 285 | 3.91 (3.21–4.60) | 3.04 (2.69–3.44) |
| Unsuppressed and <500 cells/uL | 469 | 9.05 (7.32–10.77) | 3.41 (3.09–3.75) | 250 | 8.46 (6.05–10.88) | 5.64 (4.96–6.41) |
Data were reported to the New York City Department of Health and Mental Hygiene by 31 December 2018. Rate ratios were adjusted for age, race/ethnicity, neighborhood poverty level, and calendar time.
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HIV, human immunodeficiency virus; Ref., reference group.
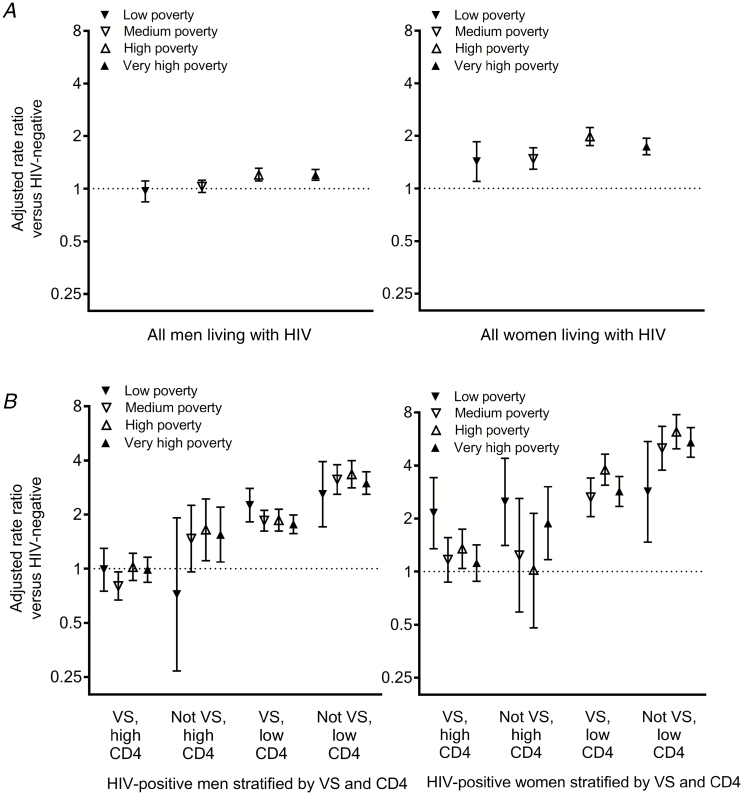
We also stratified analyses by neighborhood poverty. Within each level (low, medium, high, and very high poverty), there were differences by sex in the association between HIV and CVD mortality (P values for interaction by sex <.01 within all levels; Figure 2A). For example, in very high–poverty neighborhoods, the adjusted CVD mortality rate ratio for HIV status was 1.74 (95% CI 1.56–1.94) in women and 1.20 (95% CI 1.12–1.29) in men. Yet even among those living in low- and medium-poverty neighborhoods, the differences in relative rates for HIV status by sex were statistically significant. In contrast to findings among women, there was no increased CVD mortality risk among men with HIV who were living in low- or medium-poverty neighborhoods (RR 0.97 [95% CI 0.84–1.11] and RR 1.03 [95% CI 0.95–1.12] respectively).
Figure 2.
Adjusted association of (A) HIV status and (B) HIV RNA and CD4+ count with cardiovascular disease mortality rate, stratified by sex and neighborhood poverty level, New York City, 2007–2017. Low poverty, <10% below FPL; medium poverty, 10 to <20% below FPL; high poverty, 20 to <30% below FPL; very high poverty, ≥30% below FPL. Rate ratios were adjusted for calendar year, age, and race/ethnicity. Models excluded persons living outside New York City at diagnosis, persons whose residence at diagnosis was unknown, and (B) persons with missing HIV RNA or CD4+ counts. The rate ratio was not shown for low-poverty women with VS and low CD4, due to small counts. Abbreviations: FPL, federal poverty level; HIV, human immunodeficiency virus; VS, virologically suppressed.
Cardiovascular Disease Mortality Rates by Viral Suppression and CD4+ T-Cell Count
We further stratified individuals living with HIV into the following groups by HIV RNA suppression and CD4+ T-cell counts at the last available test: (1) suppressed and ≥500+ cells/uL; (2) unsuppressed and ≥500 cells/uL; (3) suppressed and <500 cells/uL; and (4) unsuppressed and <500 cells/uL. Men and women who were both unsuppressed and had CD4+ counts <500 cells/uL had the highest CVD mortality rates, relative to uninfected individuals; the magnitude was greater in women (RR 5.64, 95% CI 2.96–6.41), compared with men (RR 3.41, 95% CI 3.09–3.75). In contrast, men with the best-controlled HIV disease (ie, suppressed and ≥500 cells/uL) had CVD mortality rates that were similar to those of uninfected men (RR 1.01, 95% CI 0.92–1.11). Among women with similarly controlled HIV disease, CVD mortality rates were slightly elevated, compared with uninfected women (RR 1.25, 95% CI 1.08–1.44).
We found relatively little variation in RRs by neighborhood poverty after including viral suppression and CD4+ counts in statistical models (Figure 2B). For example, among men, the relative rate of CVD mortality in those who were both unsuppressed and had CD4+ counts <500 cells/uL, compared with men living without HIV, ranged from 2.59 (95% CI 1.71–3.94) to 3.35 (95% CI 2.81–3.99) by neighborhood poverty level. Among women, the same relative rate ranged from 2.84 (95% CI 1.47–5.47) to 5.41 (95% CI 4.46–6.56).
DISCUSSION
Cardiovascular disease continues to increase as a major cause of death among New Yorkers with HIV, and at younger ages. During the study period, the proportion of deaths attributed to CVD in people with HIV doubled from 11% in 2007 to 22% in 2017. While ischemic disease appeared to be the underlying cause for most cardiovascular deaths among men and women alike, cerebrovascular diseases were more common among women, consistent with recent studies of HIV-associated stroke incidence [10, 20]. As the average age of people with HIV increases beyond 50, it is expected that these trends will continue, although the increases could be mitigated by a more complete implementation of prevention and treatment of CVD as part of HIV clinical care.
We previously found that the relative rates of CVD deaths associated with HIV were 1.23 in men and 2.24 in women, suggesting that HIV increases the CVD mortality risk to a greater degree among women than men. A similarly greater association in women versus men has been observed in cohort studies comparing myocardial infarction risks between people living with and without HIV [11]. In addition, women living with HIV in New York City are more likely than men to live in higher-poverty neighborhoods, and it is possible that behavioral risk factors that often cluster in the setting of social and economic disparities [21], including illicit drug use, smoking, hypertension, and obesity from a poor diet and low physical activity, may amplify the CVD risk to a greater extent in women than in men. For example, the prevalences of many traditional CVD risk factors are higher in higher-poverty neighborhoods in New York City (Supplemental Figure 3). Therefore, we accounted for neighborhood-level poverty using both regression adjustment and stratification. We found that disparities by sex were reduced but still evident; the relative rate of CVD mortality remained similar in men (from 1.23 to 1.20), but was attenuated in women (from 2.24 to 1.73). Sex differences were observed across all poverty levels, but were more pronounced in higher-poverty neighborhoods. CVD mortality appears to disproportionately affect women living with HIV, especially those with uncontrolled viremia and lower CD4+ counts.
Why is the association between HIV status and CVD mortality greater in women than in men? Factors related to socioeconomic status have already been mentioned, and other structural-level factors, such as racism and sexism, may have also contributed to our findings, but were unmeasured [22]. Beyond these potential determinants, among people with HIV, obesity is more prevalent among women than men [23] and, among women, weight gain after starting ART may intensify existing levels of immune activation and inflammation, increasing the likelihood of conditions like atherosclerosis and stroke [24]. Furthermore, it is well known that estrogen plays a protective role in CVD in younger women, but that the risk of CVD increases after menopause [25]. HIV itself, as well as greater immunosuppression and higher HIV RNA levels, have been associated with earlier-onset menopause [26, 27], which may hasten the postmenopausal CVD risk. Reproductive aging in HIV is understudied [11, 28], despite the fact that the median age of women living with HIV now pushes beyond the menopausal transition. Understanding the excess CVD risk associated with weight gain, adiposity, sex hormones, inflammation, and other biological changes related to HIV in women will be increasingly important.
In lower-poverty neighborhoods, men living with HIV did not appear to have an elevated CVD mortality risk. This may be indicative of access to health care among these men, leading to better uptake of ART, as well as primary and secondary prevention of CVD. Indeed, a study based in Kaiser Permanente found that the risk of myocardial infarction among people with HIV (91% of whom were men) has decreased over time to levels similar to those in people without HIV, and this was attributed to their system’s integrated care setting [29]. When we examined New York City men with HIV living in low-poverty areas, only those with suppressed viremia and high CD4 counts maintained CVD mortality rates that were similar to those in individuals living without HIV. This suggests that the prevention of CVD mortality requires resources that can facilitate not only access to care, but also durable virologic suppression to sustain long-term health.
Our study has some limitations. First, there was likely residual confounding and misclassification due to the limited information available from our data sources. For example, HIV surveillance does not typically collect information on smoking, blood pressure, cholesterol levels, diabetes, and pregnancy-related stroke [30], which would be useful to better characterize CVD risks. Census data contain even less health-related information. Nonetheless, both sources are population-based and, therefore, may provide more generalizable information than health-care record studies or traditional cohort studies. Second, information on ART is not systematically collected by HIV surveillance, but this information would have been useful to better understand previously reported ART drug class associations with CVD [31, 32]. However, we did have information on HIV RNA and CD4+ counts, which provide a good proxy for ART use, and we found a generally well-treated HIV population, based on viral suppression rates. Third, there are well-known limitations to the accuracy of causes of death listed on death certificates [33] and, therefore, causes may have been misclassified, particularly for some categories, like sudden cardiac death [34]. Finally, while we derived neighborhood poverty levels based on residential ZIP codes, we did not have individual-level data on socioeconomic status.
CVD continues to be a major complication in the lives of contemporary women and men living with HIV, with 1 in 5 deaths among New Yorkers with HIV now associated with CVD. Even after accounting for socioeconomic status, the association of HIV with CVD mortality is higher in women than men, which may stem from a combination of differences in risk behaviors, lifestyle factors, and biology. Given that ischemic heart disease is already under-recognized in middle-aged women in the general population, and especially in minority women [35], CVD risks should be better evaluated in women with HIV. In addition to traditional CVD prevention measures, like smoking cessation, blood pressure control, and lipid management, HIV providers should emphasize durable virologic suppression and maintenance of CD4+ counts to reduce the risk or progression of CVD, especially among those living in higher-poverty areas. As HIV increasingly becomes a disease of aging, future work should better characterize how these factors interact in order to reduce CVD mortality in those living with HIV.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work draws on data from human immunodeficiency virus surveillance, which is supported by a cooperative agreement with the Centers for Disease Control and Prevention (grant number PS18-1802/#NU62PS924575), and the National Institutes of Health (grant numbers K01-HL-137557 to D. B. H. and P30-AI-124414 to the Einstein-Rockefeller-CUNY Center for AIDS Research).
Potential conflicts of interest. J. R. K. reports stock ownership in Bristol-Myers Squibb, Gilead, Amgen, Merck, Medtronic, Johnson & Johnson, and Pfizer. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kaplan RC, Kingsley LA, Sharrett AR, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis 2007; 45:1074–81. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS 2008; 22:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tien PC, Schneider MF, Cole SR, et al. Association of hepatitis C virus and HIV infection with subclinical atherosclerosis in the Women’s Interagency HIV Study. AIDS 2009; 23:1781–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis 2015; 61:640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stein JH, Currier JS, Hsue PY. Arterial disease in patients with human immunodeficiency virus infection: what has imaging taught us? JACC Cardiovasc Imaging 2014; 7:515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaplan RC, Hanna DB, Kizer JR. Recent insights into cardiovascular disease (CVD) risk among HIV-infected adults. Curr HIV/AIDS Rep 2016; 13:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnes RP, Lacson JC, Bahrami H. HIV infection and risk of cardiovascular diseases beyond coronary artery disease. Curr Atheroscler Rep 2017; 19:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med 2011; 171:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012; 60:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raghavan A, Rimmelin DE, Fitch KV, Zanni MV. Sex differences in select non-communicable HIV-associated comorbidities: exploring the role of systemic immune activation/inflammation. Curr HIV/AIDS Rep 2017; 14:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scully EP. Sex differences in HIV infection. Curr HIV/AIDS Rep 2018; 15:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanna DB, Ramaswamy C, Kaplan RC, et al. Trends in cardiovascular disease mortality among persons with HIV in New York City, 2001–2012. Clin Infect Dis 2016; 63:1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Implementation of named HIV reporting--New York City, 2001. MMWR Morb Mortal Wkly Rep 2004; 52:1248–52. [PubMed] [Google Scholar]
- 15. Hanna DB, Pfeiffer MR, Sackoff JE, Selik RM, Begier EM, Torian LV. Comparing the National Death Index and the Social Security Administration’s Death Master File to ascertain death in HIV surveillance. Public Health Rep 2009; 124:850–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. International statistical classification of diseases and related health problems 10th revision. Available at: http://apps.who.int/classifications/icd10/browse/2010/en. Accessed 17 July 2013.
- 17. Zimmerman R, Li W, Begier E, et al. Summary of vital statistics, 2011: appendix A: supplemental population, mortality and pregnancy outcome data tables. Available at: http://www.nyc.gov/html/doh/downloads/pdf/vs/vs-appendix-a-2011.pdf. Accessed 17 July 2013.
- 18. Stevens GA, King G, Shibuya K. Deaths from heart failure: using coarsened exact matching to correct cause-of-death statistics. Popul Health Metr 2010; 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fontenot K, Semega J, Kollar M. Income and poverty in the United States: 2017. Available at: https://www.census.gov/content/dam/Census/library/publications/2018/demo/p60-263.pdf. Accessed 19 March 2019.
- 20. Chow FC, Wilson MR, Wu K, Ellis RJ, Bosch RJ, Linas BP. Stroke incidence is highest in women and non-Hispanic Blacks living with HIV in the AIDS Clinical Trials Group Longitudinal Linked Randomized Trials Cohort. AIDS 2018; 32:1125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mendenhall E, Kohrt BA, Norris SA, Ndetei D, Prabhakaran D. Non-communicable disease syndemics: poverty, depression, and diabetes among low-income populations. Lancet 2017; 389:951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krieger N. Discrimination and health inequities. Int J Health Serv 2014; 44:643–710. [DOI] [PubMed] [Google Scholar]
- 23. Thompson-Paul AM, Wei SC, Mattson CL, et al. Obesity among HIV-infected adults receiving medical care in the United States: data from the cross-sectional Medical Monitoring Project and National Health and Nutrition Examination Survey. Medicine 2015; 94:e1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bares SH, Smeaton LM, Vu V, et al. The impact of weight gain and sex on immune activation following initiation of ART [abstract 673]. Conference on Retroviruses and Opportunistic Infections (Seattle, WA). 2019. Available at: https://www.croiconference.org/sessions/impact-weight-gain-and-sex-immune-activation-following-initiation-art. Accessed 10 September 2019.
- 25. Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol 2009; 6:532–42. [DOI] [PubMed] [Google Scholar]
- 26. Schoenbaum EE, Hartel D, Lo Y, et al. HIV infection, drug use, and onset of natural menopause. Clin Infect Dis 2005; 41:1517–24. [DOI] [PubMed] [Google Scholar]
- 27. Scherzer R, Greenblatt RM, Merhi ZO, et al. Use of antimullerian hormone to predict the menopausal transition in HIV-infected women. Am J Obstet Gynecol 2017; 216:46.e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Imai K, Sutton MY, Mdodo R, Del Rio C. HIV and menopause: a systematic review of the effects of HIV infection on age at menopause and the effects of menopause on response to antiretroviral therapy. Obstet Gynecol Int 2013; 2013:340309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klein DB, Leyden WA, Xu L, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis 2015; 60:1278–80. [DOI] [PubMed] [Google Scholar]
- 30. van Alebeek ME, de Heus R, Tuladhar AM, de Leeuw FE. Pregnancy and ischemic stroke: a practical guide to management. Curr Opin Neurol 2018; 31:44–51. [DOI] [PubMed] [Google Scholar]
- 31. Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–35. [DOI] [PubMed] [Google Scholar]
- 32. Elion RA, Althoff KN, Zhang J, et al. ; North American AIDS Cohort Collaboration on Research and Design of International Epidemiology Databases to Evaluate AIDS Recent abacavir use increases risk of Type 1 and Type 2 myocardial infarctions among adults with HIV. J Acquir Immune Defic Syndr 2018; 78:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Naghavi M, Makela S, Foreman K, O’Brien J, Pourmalek F, Lozano R. Algorithms for enhancing public health utility of national causes-of-death data. Popul Health Metr 2010; 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol 2012; 59:1891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mehta PK, Wei J, Wenger NK. Ischemic heart disease in women: a focus on risk factors. Trends Cardiovasc Med 2015; 25:140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.