Abstract
Background
Cryptococcal meningitis and tuberculosis are both important causes of death in persons with advanced human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS). Cytomegalovirus (CMV) viremia may be associated with increased mortality in persons living with HIV who have tuberculosis. It is unknown whether concurrent CMV viremia is associated with mortality in other AIDS-related opportunistic infections.
Methods
We prospectively enrolled Ugandans living with HIV who had cryptococcal meningitis from 2010–2012. Subsequently, we analyzed stored baseline plasma samples from 111 subjects for CMV DNA. We compared 10-week survival rates among those with and without CMV viremia.
Results
Of 111 participants, 52% (58/111) had detectable CMV DNA (median plasma viral load 498 IU/mL, interquartile range [IQR] 259–2390). All samples tested were positive on immunoglobin G serology. The median CD4+ T cell count was 19 cells/µL (IQR 9–70) and did not differ by the presence of CMV viremia (P = .47). The 10-week mortality rates were 40% (23/58) in those with CMV viremia and 21% (11/53) in those without CMV viremia (hazard ratio 2.19, 95% confidence interval [CI] 1.07–4.49; P = .03), which remained significant after a multivariate adjustment for known risk factors of mortality (adjusted hazard ratio 3.25, 95% CI 1.49–7.10; P = .003). Serum and cerebrospinal fluid cytokine levels were generally similar and cryptococcal antigen-specific immune stimulation responses did not differ between groups.
Conclusions
Half of persons with advanced AIDS and cryptococcal meningitis had detectable CMV viremia. CMV viremia was associated with an over 2-fold higher mortality rate. It remains unclear whether CMV viremia in severely immunocompromised persons with cryptococcal meningitis contributes directly to this mortality or may reflect an underlying immune dysfunction (ie, cause vs effect).
Clinical Trials Registration
Keywords: cryptococcus, cytomegalovirus, HIV, CMV, cryptococcal meningitis
Cytomegalovirus viremia was associated with increased mortality in persons with acquired immunodeficiency syndrome and cryptococcal meningitis. It is unclear whether cytomegalovirus reactivation is driving the hazard or is a sensitive marker of declining immune function.
Cryptococcal meningitis is the most common form of adult meningitis in people with human immunodeficiency virus (HIV), with a large burden in sub-Saharan Africa [1]. In Uganda, cryptococcal meningitis is more common than all other causes of meningitis combined [2]. Despite increased access to antiretroviral therapy (ART) and an increased emphasis on early ART initiation, the World Health Organization acknowledges that up to half of people presenting to care with HIV have advanced disease [3]. Cryptococcal meningitis most often occurs in the setting of acquired immunodeficiency syndrome (AIDS), and such persons are at risk for having multiple, concurrent opportunistic infections, including cytomegalovirus (CMV) [4].
CMV is a human herpesvirus that typically causes asymptomatic infections in immunocompetent hosts but may cause end-organ disease among immunocompromised persons, in sites such as the gastrointestinal tract, retina, or central nervous system [4, 5]. CMV seroprevalence, as defined by immunoglobin G (IgG) antibody positivity, is typically >90% in African populations living with HIV [6–8]. CMV viremia, likely a better indicator of active disease, has a prevalence of 1.8% to 22.6% in African cohorts living with HIV [6, 9, 10]. The CMV viremia prevalence may be higher in those with advanced disease; for example, Micol et al [11] reported that 55% of Cambodians with CD4 counts <100 cells/µL had detectable CMV viremia. Active CMV replication is associated with increased levels of plasma HIV DNA [12]. Moreover, those with detectable plasma CMV DNA have a 2.2-fold increased hazard of developing an AIDS-defining diagnosis, even when controlling for CD4 count and plasma HIV RNA concentration [13]. Fielding et al [10] found that subclinical CMV viremia was an independent risk factor for mortality among adult males living with HIV in South Africa, while other studies have noted that higher CMV viral loads were associated with greater hazards toward mortality in the pre-ART and early-ART eras [13–15].
Little is known regarding whether active CMV replication contributes to an increased risk of death in persons living with HIV who have other high-mortality opportunistic infections, such as cryptococcal meningitis. Interestingly, Ward et al [16] found that CMV viremia was associated with a trend toward increased mortality in persons living with HIV who had tuberculosis, and particularly in older patients. Both tuberculosis and cryptococcosis are caused by intra-cellular pathogens requiring Type 1 T helper (Th1) cell immunity for protection. Viral infections can increase the production of Type 1 interferons (eg, interferon-alpha), which may subsequently impair Th1 cytokine (eg, interferon-gamma) responses [17]. The stimulation of peripheral blood mononuclear cells with CMV antigens results in lower interferon-gamma and tumor necrosis factor alpha levels in CMV-seropositive versus CMV-seronegative kidney transplant patients [18]. Thus, a concomitant infection with CMV could potentially counteract protective Th1 immune responses against Cryptococcus. Given that CMV viremia has been associated with mortality in the aforementioned populations, we conducted this retrospective analysis to determine whether CMV viremia was associated with increased mortality in cryptococcal meningitis. We additionally explored the cytokine profile of our participants to assess whether CMV viremia was associated with impaired Th1 immune responses.
METHODS
Study Design
We analyzed samples that had been prospectively collected from participants enrolled in the Cryptococcal Optimal ART Timing (COAT) trial (ClinicalTrials.gov: NCT01075152), a randomized, clinical trial testing early ART initiation at 1–2 weeks versus deferred ART initiation at 4–6 weeks after cryptococcal meningitis [19]. Briefly, ART-naive adults living with HIV who had first-episode cryptococcal meningitis were enrolled at either Mulago Hospital or Mbarara Hospital in Uganda or at GF Jooste Hospital in Cape Town, South Africa, from November 2010 to April 2012. Induction therapy consisted of amphotericin B deoxycholate (0.7–1.0 mg/kg/day) plus fluconazole (800 mg/day) for 2 weeks, followed by “enhanced consolidation,” consisting of fluconazole at 800 mg/day therapy for ~3 weeks, until cerebrospinal fluid (CSF) cultures were sterile [20], and then at 400 mg/day through 12 weeks. All participants also received supportive care with therapeutic lumbar punctures, intravenous fluids, electrolyte management, and trimethoprim-sulfamethoxazole prophylaxis. Survival at 26 weeks was the primary endpoint of the parent trial.
All participants enrolled into the COAT trial who had stored plasma available were potentially eligible for this study. We excluded persons screened for the trial who were ineligible (eg, receiving ART) or who died <7 days from diagnosis and prior to trial enrollment. The parent trial received Institutional Review Board approval from United States, Ugandan, and South African institutions. All participants provided written informed consent for the storage and future testing of samples for research purposes.
Laboratory Methods
DNA Extraction and Polymerase Chain Reaction
DNA was extracted from 200 µL of plasma using the QIAcube HT (QIAGEN, Hilden, Germany) and QIAamp 96 Virus DNA QIAcube kit, according to the manufacturer’s instructions. All samples were eluted in 100 μL of polymerase chain reaction (PCR)-grade water and were stored at −20°C. Multiplex quantitative PCR was performed using the LightCycler 480 System (Roche, Basel, Switzerland). Detailed PCR methods can be found in the Supplementary Methods. Laboratory staff processed coded samples and were blinded to participant data and outcomes. CMV quantification of copies/mL values were converted to international units (IU) using the World Health Organization International Standard for Human Cytomegalovirus for Nucleic Acid Amplification Techniques (National Institute for Biological Standards and Control code 09/162) [21].
Serological Assessment
CMV IgG serostatuses were assessed using CMV IgG enzyme-linked immunosorbent assay (Diamedix, Miami Lakes, FL), following the manufacturer’s instructions. Specimens were run in triplicate at 1:100 dilution, with absorbance measured at 450 nm. Specimens with a measured enzyme-linked immunosorbent assay unit level of ≥10 (IgG index ≥1.0) were considered positive.
Immune Profiling
To assess whether active CMV was associated with differential immune responses, we performed a secondary analysis of previously measured serum and CSF cytokines [22]. First, we analyzed 19 measured cytokines and chemokines (see Supplementary Table 2) using Luminex magnetic bead technology (Bio-Rad Laboratories, Hercules, CA), according to the manufacturers’ protocols.
Second, we assessed whether cryptococcal antigen–specific immune responses differed among participants by their CMV status. We created a novel, whole-blood interferon-gamma release assay stimulating against: (1) cryptococcal glucuronoxylomannan polysaccharide (ie, “cryptococcal antigen”); (2) cryptococcal mannoprotein; and (3) phosphate-buffered saline negative control. For antigen-stimulation assays, we calculated the net cytokine production (antigen to phosphate-buffered saline negative control stimulation). Refer to the Supplementary Methods for assay details. Assessing the immune response was an exploratory, post hoc analysis to determine whether CMV was potentially affecting cryptococcal-specific immune responses.
Statistical Analysis
For the purposes of this study, someone with CMV viremia was defined as anyone with detectable CMV DNA in plasma. A subset of participants had paired samples from different time points tested for the longitudinal concordance of CMV DNA in plasma. Persons with multiple samples were counted as a single negative result if both samples were negative for CMV DNA. Conversely, if both were positive, the CMV viral load was averaged between the 2 samples and counted as a single positive result. There were 4 subjects missing baseline CSF white cell count values and 1 missing a baseline hemoglobin value, so the next available value was used (within ~3 days of baseline).
Baseline characteristics were compared by CMV viremia using the Chi-square test for proportions, the t-test for normally distributed data, or the Mann-Whitney U test for non-normally distributed data. The primary outcome in our analysis was 10-week survival. Survival was calculated in days from the date of diagnosis, until either death or 10 weeks. No participants were right-hand censored. A Kaplan-Meier plot with a log rank test compared the 10-week mortality rates by CMV status. Subjects were additionally stratified by CMV viral load as either low (<1000 IU/mL), high (≥1000 IU/mL), or no CMV, to assess whether the magnitude of viremia impacted mortality. Univariate Cox proportional hazards models evaluated the risk factors for 10-week mortality. A fully adjusted Cox model was used to control for confounders of age, sex, CD4 count, Glasgow coma scale score, hemoglobin, CSF white cells, and trial randomization arm. Hazard ratios (HRs), 95% confidence intervals (CIs), and P values were reported for each model. Serum, CSF, and antigen-stimulation cytokines were compared by CMV viremia using Wilcoxon rank sum tests. The analysis was performed in SPSS version 23 (IBM, Inc., Armonk, NY) and SAS version 9.4 (SAS Institute, Cary, NC). A P value of ≤.05 was considered statistically significant. The cytokines were studied as post hoc analyses that were exploratory for an immune signature as an explanation for differential clinical outcomes in participants with CMV viremia. No formal adjustments were made for multiple testing in the analyses of serum or CSF biomarkers or of antigen-stimulation assays; thus, readers may consider a more extreme P value of <.01 to be statistically significant.
RESULTS
We enrolled 177 participants with cryptococcal meningitis in the COAT trial, and we performed CMV DNA quantification on available stored plasma in 111 participants. This study population was comprised of 54% men and 46% women. The mean age was 35 years (standard deviation ±7.8). The median CD4+ T cell count was 19 cells/µL (interquartile range [IQR] 9–70). The mean HIV RNA plasma level was 5.38 log10 copies/mL (standard deviation ±0.55). The plasma samples tested for CMV DNA were taken a median of 6 days (IQR 0–11) from cryptococcal meningitis diagnosis. Baseline characteristics stratified by CMV status are shown in Table 1. CMV viremia was present in 52% (58/111) of subjects. CMV IgG serology was positive on all 109 participants who had sufficient plasma for testing. The median plasma CMV viral load was 498 IU/mL (IQR 259–2390) in those with viremia.
Table 1.
Baseline Characteristics by Cytomegalovirus Viremia Status
| Variable | No CMV Viremia | CMV Viremia | P Value |
|---|---|---|---|
| Number per group | 53 (48%) | 58 (52%) | … |
| Deferred HIV therapy | 22 (42%) | 31 (53%) | .21 |
| Women | 28 (53%) | 23 (40%) | .16 |
| Age, years | 37 [±8] | 34 [±7] | .06 |
| CD4 count, cells/µL | 18 (10–75) | 20 (9–52) | .53 |
| HIV plasma RNA, log10 copies/mL | 5.40 (5.13–5.74) | 5.45 (5.19–5.78) | .59 |
| CMV plasma DNA, IU/mL | … | 498 (259–2390) | … |
| Glasgow coma scale score <15 | 14 (26%) | 16 (28%) | .89 |
| Hemoglobin, g/dL | 11.4 [±2.8] | 10.6 [±2.5] | .09 |
| CSF white cells <5 cells/µL | 13 (25%) | 23 (40%) | .09 |
| C-reactive protein, mg/L | 47.0 (8.7–80.2) | 35.3 (18.5–76.0) | .79 |
Data are shown as n (%), mean [±SD], and median (IQR), with P values derived from the Chi-square test, t-test, or Mann-Whitney U test, respectively. Abbreviations: CMV, cytomegalovirus; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation.
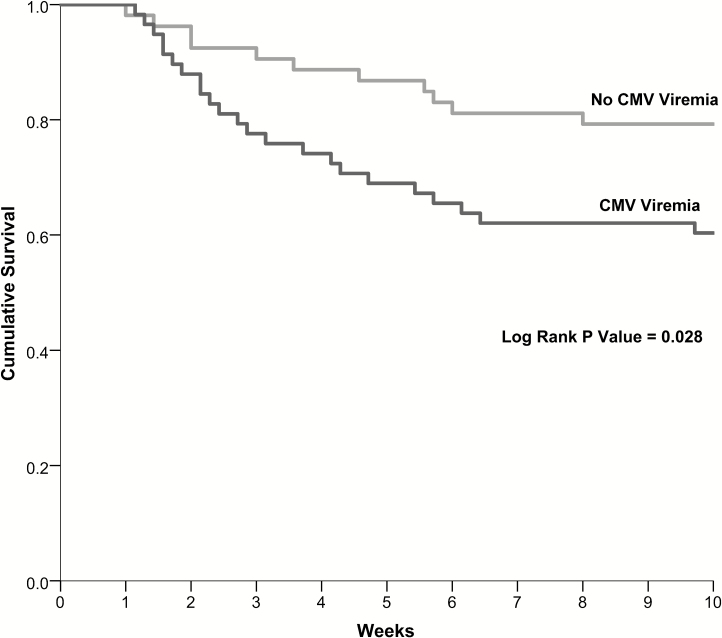
The mortality rates at 10 weeks were 40% (23/58) in those with CMV viremia and 21% (11/53) in those without CMV viremia (P = .03). Persons with CMV viremia had an approximately 2-fold higher risk of death (HR 2.19, 95% CI 1.07–4.49; P = .033; Figure 1). The hazard increased to over 3-fold and remained significant after adjusting for age, sex, CD4 count, Glasgow coma scale score, hemoglobin, CSF white cells, and trial randomization arm (adjusted HR 3.25, 95% CI 1.49–7.10; P = .003; Table 2). This mortality difference continued through 26 weeks, with mortality rates of 41% (24/58) in those with CMV viremia versus 30% (16/53) in those without CMV viremia (adjusted HR 2.18, 95% CI 1.09–4.37; P = .003). Thus, persons with CMV viremia had a 2-fold higher overall mortality rate and, when adjusted for potential confounders of cryptococcal or HIV severity, those with CMV viremia had a 3.3-fold higher risk of mortality at 10 weeks.
Figure 1.
Survival at 10 weeks by cytomegalovirus (CMV) viremia status after human immunodeficiency virus-associated cryptococcal meningitis in Kaplan-Meier model. Mortality by subgroup was as follows: no CMV viremia and early antiretroviral therapy (ART) (23%, 7/31); no CMV viremia and deferred ART (19%, 4/22); CMV viremia and early ART (56%, 15/27); and CMV viremia and deferred ART (26%, 8/31). Abbreviation: CMV, cytomegalovirus.
Table 2.
Hazard Ratios for 10-Week Mortality From Cox Regression Model
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value |
| CMV viremia | 2.19 | 1.07–4.49 | .033 | 3.25 | 1.49–7.10 | .003 |
| Women | 2.15 | 1.08–4.29 | .03 | 2.66 | 1.25–5.62 | .01 |
| Age, per year | 1.01 | .97–1.05 | .71 | 1.04 | .99–1.09 | .12 |
| CD4 count, per 10 cells/µL | 1.00 | .97–1.05 | .70 | 1.00 | .99–1.01 | .97 |
| Glasgow coma scale <15 | 1.71 | .84–3.45 | .14 | 2.07 | .97–4.41 | .06 |
| Hemoglobin, per g/dL | 0.90 | .79–1.02 | .10 | 0.93 | .80–1.09 | .38 |
| CSF white cells <5 cells/µL | 0.75 | .35–1.62 | .47 | 0.76 | .33–1.73 | .51 |
| Early HIV therapy trial arm | 1.90 | .94–3.83 | .07 | 2.50 | 1.21–5.81 | .01 |
Bold values indicate to highlight the primary variable of interest in context of outcome. Abbreviations: CI, confidence interval; CMV, cytomegalovirus; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus.
To test whether mortality was associated with the magnitude of CMV viremia, we stratified CMV viral loads into low (<1000 IU/mL) and high (≥1000 IU/mL) groups. Of the 58 participants with CMV viremia, 37 had low CMV viral loads (median 295 IU/mL, IQR 198–427) and 21 had high CMV viral loads (median 3520 IU/mL, IQR 2210–5240). Baseline characteristics were similar between the groups with low and high viral loads (Supplementary Table 1). While both groups with CMV viremia had lower survival rates than the CMV-negative group, the mortality rates did not significantly differ between those with low CMV viral loads (43%, 16/37) and those with high CMV viral loads (33%, 7/21; P = .46). CMV-viremic participants developed paradoxical immune reconstitution inflammatory syndrome at similar rates (21%, 11/53) as non-viremic participants (17%, 10/58; P = .64). The 10-week mortality rate was higher in the CMV-viremic participants receiving early ART (56%, 15/27), compared to in those who deferred ART (26%, 8/31; P = .02), consistent with the overall COAT trial results. Therefore, the randomization arm was included our multivariate analysis.
We assessed for risk factors associated with CMV viremia. The median CD4+ T cell count did not differ between 20 cells/µL (IQR 9–52) in those with CMV viremia versus 18 cells/µL (IQR 10–75) in those without CMV viremia (P = .53). Age, sex, HIV RNA plasma level, and trial arm randomization data did not differ between groups with and without CMV viremia, nor did baseline Glasgow Coma Scale scores, hemoglobin levels, or CSF white cell counts (Table 1).
In assessing the dynamics of detectable CMV DNA in plasma, 9 participants had two different, blinded samples tested for longitudinal concordance (n = 18 samples). Baseline samples and the subsequent samples were taken a median of 7 days (IQR 5–17) apart. There were 7 subjects who had qualitative concordance of detectable CMV DNA. There were 2 subjects who had no CMV detected in their first sample, but had measurable CMV DNA in their second sample (7 and 8 days later); both subjects were considered CMV positive for our study.
To determine whether CMV viremia was associated with an impaired Th1 response or was a surrogate marker of more severe global immune dysfunction, we assessed the cytokines present in the serum and CSF of 104 participants. We found that 2-fold higher median serum interleukin (IL)-12 (a Th1-related cytokine, protective in cryptococcosis; P = .035) and 35% higher median IL-13 (a Th2-related cytokine, nonprotective in cryptococcosis; P = .028) measurements were both positively associated with CMV viremia. In the CSF, CMV viremia was marginally associated with a 25% higher median granulocyte macrophage colony stimulating factor (GM-CSF) level, a 35% increase in macrophage inflammatory protein-1 beta (MIP-1β) (CCL4), and a 2-fold increase in monocyte chemoattractant protein-1 (MCP-1) (CCL2; all P values < .05) (Supplementary Table 2). Antigen stimulation responses to cryptococcal glucuronoxylomannan or mannoprotein did not differ by CMV status (Supplementary Table 3). These post hoc exploratory analyses revealed some marginal statistical associations with components of the host immune response, but did not reveal markedly impaired Th1 responses or significant immune signature differences among participants with CMV viremia.
DISCUSSION
Our results demonstrate that active CMV replication with viremia is associated with an over 2-fold mortality increase by 10 weeks in severely immunocompromised persons living with HIV who have cryptococcal meningitis. This is a novel finding that supports CMV viremia as a detrimental biomarker in persons with cryptococcal meningitis, irrespective of the presence or absence of end-organ CMV disease. and when combined with similarly observed trends by Ward et al in persons with HIV-associated TB [16], our results posit that CMV may be a risk factor for death in persons with advanced HIV disease. The increased risk for mortality remained significant when adjusting for variables previously shown to be associated with death in cryptococcal meningitis, such as baseline altered mental status and anemia [23–25]. Compared to the cohort living with HIV and with tuberculosis in Ward et al [16], our cryptococcal meningitis cohort had a lower median CD4+ T cell count (64 vs 19, respectively cells/µL) and higher prevalence of CMV viremia (31% vs 52%, respectively). We hypothesize that CMV replication could influence AIDS-related mortality among the severely immunocompromised, and that CD4+ T cell counts >100 cells/µL likely attenuate CMV effects. Interestingly, mortality rates did not correlate with the magnitude of CMV viremia, suggesting the absence of a dose-response relationship. This raises the fundamental question of whether CMV is solely a marker of immune dysfunction or whether CMV contributes (directly or indirectly) to mortality in persons with advanced HIV and concomitant opportunistic infections.
It has been hypothesized that CMV exerts detrimental effects without causing end-organ disease by modulating the host immune system. For example, an increased risk of kidney graft rejection in the setting of active CMV replication is thought to be instigated by immune dysregulation [26]. Interestingly, CMV has been linked to the development of invasive fungal infections in solid organ transplant recipients [27–29]. Invasive fungal infections, including Cryptococcus, often occur in the setting of T-cell depletion or dysfunction. CD4+ helper T cells play a critical role in mediating the adaptive immune response to Cryptococcus, which is highly predicated on helper T cell polarization to either a Th1 or Th2 phenotype. Studies in mice examining Cryptococcus-specific CD4+ T cell polarization demonstrated that Th1-mediated responses are protective and are associated with improved Cryptococcus clearance from the lung, whereas Th2-mediated responses are associated with decreased yeast clearance from the CSF and with poorer overall outcomes [30–33].
We assessed cytokine profiles in blood and CSF to explore whether CMV exerted a notable pattern of altered immune response. We did not find any marked differences in baseline immune responses, as assessed by measurements of soluble cytokine or antigen-specific responses. Our results identified some marginal statistical associations with higher levels of both Th1 (IL-12) and Th2 (IL-13) cytokines in the serum of participants with CMV viremia. Measuring the cytokine milieu in persons with AIDS can be problematic, in that multiple infections or immune processes could be contributing to dysregulation. In particular, tuberculosis coinfection is present in about 15% of first-episode cryptococcal meningitis cases in Uganda [19] and likely influences the cytokine profile. We also assessed the immune responses in the CSF, where CMV viremia was only marginally associated with increases in the chemokines MCP-1 (CCL2) and MIP-1β (CCL4) and the glycoprotein GM-CSF. To better assess Cryptococcus-specific immune responses, we developed a whole-blood antigen-stimulation assay. When we stimulated with Cryptococcus-specific antigens, we noted no differences in antigen-specific cytokine responses in CMV viremic versus nonviremic participants. Based on our study results, it is unlikely that the increased mortality conferred by active CMV replication is mediated by any attenuating effect of CMV on protective anti-cryptococcal Th1 responses.
CMV has been associated with increased mortality rates in numerous other clinical settings, including systemic lupus erythematosis [34], head and neck cancers [35], and solid organ transplant recipients [36, 37]. Treating symptomatic CMV disease decreases the mortality rate in solid organ transplants patients [38, 39]. CMV prevention in transplant patients demonstrates a survival benefit—using either prophylaxis or preemptive therapy—where treatment is initiated based on the blood CMV DNA concentration [40]. These observations raise the question of whether CMV treatment or prophylaxis has a role in advanced AIDS. We found that half of our severely immunosuppressed participants had active CMV replication and viremia. The high prevalence of CMV viremia, combined with the observed large effect size in mortality, provides a rationale supporting the consideration of a randomized, clinical trial testing CMV antivirals in persons with advanced HIV disease and opportunistic coinfections. The availability of new drugs, such as letermovir, with oral, less toxic formulations further bolsters the feasibility of a clinical trial [41].
Our study had several limitations. First, this study was a retrospective analysis of a randomized, clinical trial in which mortality rates differed by trial arm; however, the mortality association with CMV was present with and without an adjustment for trial arm. Second, we only determined CMV viremia at one given point in time (baseline sample). Future studies should measure CMV plasma DNA across multiple longitudinal time points to better evaluate the kinetics, persistence, and magnitude of viremia over time. Third, our study was not designed to identify and characterize CMV end-organ disease, so we were unable to stratify the differential risks between CMV viremia and confirmed CMV end-organ disease. Lastly, the generalizability of our findings to all persons with AIDS remains unclear, though persons with cryptococcosis and tuberculosis are important subsets in themselves.
In summary, our study demonstrated that the presence of CMV viremia is associated with an over 2-fold greater risk of death in persons with cryptococcal meningitis and, when these data were adjusted for known risk factors, the mortality hazard increased 3.3-fold. The etiology behind the increased risk of death is unclear, but these effects are unlikely to be mediated by attenuation of a beneficial helper T cell response. The lack of a dose-response relationship between the magnitude of CMV viremia and mortality is notable, and perhaps argues for CMV replication as a sensitive marker of severe immune dysfunction, rather than playing a causative role in the increased rate of death. Ultimately, a randomized, clinical trial is required to determine whether treatment of CMV is a modifiable risk factor contributing to mortality in persons with advanced AIDS.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study team and hospital staff working continuously to provide compassionate care to the participants and patients at Mulago National Referral Hospital.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant numbers T32AI055433, U01AI089244, and K23AI138851); the Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant number R01HD079918]); the National Institute of Neurologic Disorders and Stroke (grant number R01NS086312); the Fogarty International Center (grant numbers K01TW010268 and K43TW010718); and a combined National Institute of Neurologic Disorders and Stroke and Fogarty International Center award (grant number D43TW009345) via the Northern Pacific Global Health Fellows Program.
Potential conflicts of interest. G. M. has received grants from the Wellcome Trust and South African Government. M. R. S. has received consulting fees from Sanofi Vaccines and GSK Vaccines, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Durski KN, Kuntz KM, Yasukawa K, Virnig BA, Meya DB, Boulware DR. Cost-effective diagnostic checklists for meningitis in resource-limited settings. J Acquir Immune Defic Syndr 2013; 63:e101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Guideline for managing advanced HIV disease and the timing for initiating antiretroviral therapy. Available at: http://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/. Accessed 31 July 2017 [PubMed]
- 4. Springer KL, Weinberg A. Cytomegalovirus infection in the era of HAART: fewer reactivations and more immunity. J Antimicrob Chemother 2004; 54:582–6. [DOI] [PubMed] [Google Scholar]
- 5. Gianella S, Letendre S. Cytomegalovirus and HIV: a dangerous pas de deux. J Infect Dis 2016; 214(Suppl 2): S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gronborg HL, Jespersen S, Honge BL, Jensen-Fangel S, Wejse C. Review of cytomegalovirus coinfection in HIV-infected individuals in Africa. Rev Med Virol 2017; 27:e1907. [DOI] [PubMed] [Google Scholar]
- 7. Compston LI, Li C, Sarkodie F, Owusu-Ofori S, Opare-Sem O, Allain JP. Prevalence of persistent and latent viruses in untreated patients infected with HIV-1 from Ghana, West Africa. J Med Virol 2009; 81:1860–8. [DOI] [PubMed] [Google Scholar]
- 8. Chakraborty R, Rees G, Bourboulia D, et al. Viral coinfections among African children infected with human immunodeficiency virus type 1. Clin Infect Dis 2003; 36:922–4. [DOI] [PubMed] [Google Scholar]
- 9. Brantsaeter AB, Johannessen A, Holberg-Petersen M, et al. Cytomegalovirus viremia in dried blood spots is associated with an increased risk of death in HIV-infected patients: a cohort study from rural Tanzania. Int J Infect Dis 2012; 16:e879–85. [DOI] [PubMed] [Google Scholar]
- 10. Fielding K, Koba A, Grant AD, et al. Cytomegalovirus viremia as a risk factor for mortality prior to antiretroviral therapy among HIV-infected gold miners in South Africa. PLOS One 2011; 6:e25571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Micol R, Buchy P, Guerrier G, et al. Prevalence, risk factors, and impact on outcome of cytomegalovirus replication in serum of Cambodian HIV-infected patients (2004–2007). J Acquir Immune Defic Syndr 2009; 51:486–91. [DOI] [PubMed] [Google Scholar]
- 12. Gianella S, Massanella M, Richman DD, et al. ; California Collaborative Treatment Group 592 Team Cytomegalovirus replication in semen is associated with higher levels of proviral HIV DNA and CD4+ T cell activation during antiretroviral treatment. J Virol 2014; 88:7818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deayton JR, Prof Sabin CA, Johnson MA, Emery VC, Wilson P, Griffiths PD. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet 2004; 363:2116–21. [DOI] [PubMed] [Google Scholar]
- 14. Spector SA, Wong R, Hsia K, Pilcher M, Stempien MJ. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J Clin Invest 1998; 101:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Durier N, Ananworanich J, Apornpong T, et al. Cytomegalovirus viremia in Thai HIV-infected patients on antiretroviral therapy: prevalence and associated mortality. Clin Infect Dis 2013; 57:147–55. [DOI] [PubMed] [Google Scholar]
- 16. Ward A, Barr B, Schutz C, et al. Oral abstracts of the 21st International AIDS Conference 18–22 July 2016, Durban, South Africa. J Intern AIDS Soc 2016; 19:21264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moreira-Teixeira L, Mayer-Barber K, Sher A, O’Garra A. Type I interferons in tuberculosis: foe and occasionally friend. J Exp Med 2018; 215:1273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Essa S, Pacsa A, Raghupathy R, et al. Low levels of Th1-type cytokines and increased levels of Th2-type cytokines in kidney transplant recipients with active cytomegalovirus infection. Transplant Proc 2009; 41:1643–7. [DOI] [PubMed] [Google Scholar]
- 19. Boulware DR, Meya DB, Muzoora C, et al. ; Cryptococcal Optimal Antiretroviral Therapy Timing (COAT) Trial Team Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rolfes MA, Rhein J, Schutz C, et al. Cerebrospinal fluid culture positivity and clinical outcomes after amphotericin-based induction therapy for cryptococcal meningitis. Open Forum Infect Dis 2015; 2:ofv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayden RT, Sun Y, Tang L, et al. Progress in quantitative viral load testing: variability and impact of the WHO quantitative international standards. J Clin Microbiol 2017; 55:423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scriven JE, Rhein J, Hullsiek KH, et al. ; Cryptococcal Optimal Antiretroviral Therapy Timing (COAT) Team Early ART after cryptococcal meningitis is associated with cerebrospinal fluid pleocytosis and macrophage activation in a multisite randomized trial. J Infect Dis 2015; 212:769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hakyemez IN, Erdem H, Beraud G, et al. Prediction of unfavorable outcomes in cryptococcal meningitis: results of the multicenter infectious diseases international research initiative (ID-IRI) cryptococcal meningitis study. Eur J Clin Microbiol Infect Dis 2018; 37:1231–40. [DOI] [PubMed] [Google Scholar]
- 25. Tugume L, Morawski BM, Abassi M, et al. Prognostic implications of baseline anaemia and changes in haemoglobin concentrations with amphotericin B therapy for cryptococcal meningitis. HIV Med 2017; 18:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leeaphorn N, Garg N, Thamcharoen N, Khankin EV, Cardarelli F, Pavlakis M. Cytomegalovirus mismatch still negatively affects patient and graft survival in the era of routine prophylactic and preemptive therapy: a paired kidney analysis. Am J Transplant 2019; 19:573–84. [DOI] [PubMed] [Google Scholar]
- 27. George MJ, Snydman DR, Werner BG, et al. The independent role of cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG-Study Group. Cytogam, MedImmune, Inc. Gaithersburg, Maryland. Am J Med 1997; 103:106–13. [DOI] [PubMed] [Google Scholar]
- 28. Yong MK, Ananda-Rajah M, Cameron PU, et al. Cytomegalovirus reactivation is associated with increased risk of late-onset invasive fungal disease after allogeneic hematopoietic stem cell transplantation: a multicenter study in the current era of viral load monitoring. Biol Blood Marrow Transplant 2017; 23:1961–7. [DOI] [PubMed] [Google Scholar]
- 29. Yong MK, Slavin MA, Kontoyiannis DP. Invasive fungal disease and cytomegalovirus infection: is there an association? Curr Opin Infect Dis 2018; 31:481–9. [DOI] [PubMed] [Google Scholar]
- 30. Decken K, Köhler G, Palmer-Lehmann K, et al. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun 1998; 66:4994–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uicker WC, McCracken JP, Buchanan KL. Role of CD4+ T cells in a protective immune response against Cryptococcus neoformans in the central nervous system. Med Mycol 2006; 44:1–11. [DOI] [PubMed] [Google Scholar]
- 32. Wiesner DL, Specht CA, Lee CK, et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLOS Pathog 2015; 11:e1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wormley FL Jr, Perfect JR, Steele C, Cox GM. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect Immun 2007; 75:1453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsai WP, Chen MH, Lee MH, Yu KH, Wu MW, Liou LB. Cytomegalovirus infection causes morbidity and mortality in patients with autoimmune diseases, particularly systemic lupus: in a Chinese population in Taiwan. Rheumatol Int 2012; 32:2901–8. [DOI] [PubMed] [Google Scholar]
- 35. Kiprian D, Czarkowska-Paczek B, Wyczalkowska-Tomasik A, Paczek L. Human cytomegalovirus and Epstein-Barr virus infections increase the risk of death in patients with head and neck cancers receiving radiotherapy or radiochemotherapy. Medicine 2018; 97:e13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schnitzler MA, Lowell JA, Hardinger KL, Boxerman SB, Bailey TC, Brennan DC. The association of cytomegalovirus sero-pairing with outcomes and costs following cadaveric renal transplantation prior to the introduction of oral ganciclovir CMV prophylaxis. Am J Transplant 2003; 3:445–51. [DOI] [PubMed] [Google Scholar]
- 37. Hakimi Z, Aballea S, Ferchichi S, et al. Burden of cytomegalovirus disease in solid organ transplant recipients: a national matched cohort study in an inpatient setting. Transpl Infect Dis 2017; 19:e12732. [DOI] [PubMed] [Google Scholar]
- 38. Dunn DL, Mayoral JL, Gillingham KJ, et al. Treatment of invasive cytomegalovirus disease in solid organ transplant patients with ganciclovir. Transplantation 1991; 51:98–106. [DOI] [PubMed] [Google Scholar]
- 39. Asberg A, Humar A, Rollag H, et al. ; VICTOR Study Group. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 2007; 7:2106–13. [DOI] [PubMed] [Google Scholar]
- 40. Witzke O, Nitschke M, Bartels M, et al. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: long-term results after 7 years of a randomized clinical trial. Transplantation 2018; 102:876–82. [DOI] [PubMed] [Google Scholar]
- 41. Meesing A, Razonable RR. New developments in the management of cytomegalovirus infection after transplantation. Drugs 2018; 78:1085–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.