Abstract
Background
Patients living with human immunodeficiency virus (PLWH) with low CD4 counts are at high risk for immune reconstitution inflammatory syndrome (IRIS) and death at antiretroviral therapy (ART) initiation.
Methods
We investigated the clinical impact of IRIS in PLWH and CD4 counts <100 cells/μL starting ART in an international, prospective study in the United States, Thailand, and Kenya. An independent review committee adjudicated IRIS events. We assessed associations between baseline biomarkers, IRIS, immune recovery at week 48, and death by week 48 with Cox models.
Results
We enrolled 506 participants (39.3% were women). Median age was 37 years, and CD4 count was 29 cells/μL. Within 6 months of ART, 97 (19.2%) participants developed IRIS and 31 (6.5%) died. Participants with lower hemoglobin at baseline were at higher IRIS risk (hazard ratio [HR], 1.2; P = .004). IRIS was independently associated with increased risk of death after adjustment for known risk factors (HR, 3.2; P = .031). Being female (P = .004) and having a lower body mass index (BMI; P = .003), higher white blood cell count (P = .005), and higher D-dimer levels (P = .044) were also significantly associated with increased risk of death. Decision-tree analysis identified hemoglobin <8.5 g/dL as predictive of IRIS and C-reactive protein (CRP) >106 μg/mL and BMI <15.6 kg/m2 as predictive of death.
Conclusions
For PLWH with severe immunosuppression initiating ART, baseline low BMI and hemoglobin and high CRP and D-dimer levels may be clinically useful predictors of IRIS and death risk.
Keywords: HIV, AIDS, immune reconstitution inflammatory syndrome (IRIS), death, biomarkers
In this prospective, international study, we found that immune reconstitution and female sex were independently associated with death after antiretroviral therapy initiation in people living with human immunodeficiency virus and CD4 counts <100 cells/µL. Hemoglobin, C-reactive protein, body mass index, and D-dimer levels could be used for risk stratification.
Randomized, controlled clinical trials have demonstrated that initiation of combination antiretroviral therapy (ART) upon diagnosis of human immunodeficiency virus (HIV) regardless of CD4 T-cell count results in improved morbidity and mortality [1, 2]. The World Health Organization and others moved quickly to support “treatment for all” [3–5]; however, CD4 counts remain low at the time of diagnosis worldwide, not infrequently at immunologic AIDS (CD4 <200 cells/µL) or with an AIDS-defining disease [6–9]. People living with HIV who start ART with severe immunosuppression are at higher risk of death and morbidity compared to those who start at earlier stages [10–12].
The immune recovery associated with ART is crucial for the survival of severely immunocompromised patients but can be complicated by immune reconstitution inflammatory syndrome (IRIS). IRIS is an often inflammatory deterioration of clinical manifestations of an infection or tumor that follows successful suppression of HIV viremia with ART in 5%–50% of patients who have developed AIDS [13, 14]. IRIS is particularly common in patients who have underlying opportunistic infections, especially tuberculosis (TB), Mycobacterium avium complex (MAC), cryptococcosis, and herpesviruses including varicella zoster virus (VZV), Kaposi sarcoma herpes virus (KSHV), and cytomegalovirus (CMV) [15, 16]. IRIS in the setting of a recognized and appropriately treated infection is called paradoxical. Early initiation of ART is now recommended in severely immunosuppressed patients (CD4 <50 cells/µL) before opportunistic infections are adequately treated, except in patients with central nervous system TB and cryptococcal meningitis [17, 18, 19], to improve mortality despite higher incidence of paradoxical IRIS. Unmasking IRIS can occur in patients with subclinical infections that only become clinically apparent after ART is initiated.
Despite the high frequency of IRIS among severely immunosuppressed patients, the clinical predictors of IRIS and its impact on immune recovery and mortality remain incompletely understood [18, 19, 21–23]. In addition, because most prospective studies of IRIS have focused on a particular pathogen in a specific country, it is unclear to what degree these relationships apply in different geographical settings due to differences in opportunistic infection prevalence and healthcare systems. A better understanding of IRIS and its potential relationship to mortality is essential to helping clinicians predict, mitigate, and manage IRIS without negatively impacting immune recovery or mortality.
We conducted a large international study in the United States, Kenya, and Thailand to study IRIS incidence and predictors and the associations between IRIS, immune recovery, and death in persons living with HIV with CD4 counts <100 cells/µL who were treatment naive and initiated ART at study enrollment.
METHODS
Study Design
A prospective, observational study (NCT00286767) of adults living with HIV who were naive to ART and had CD4 counts <100 cells/µL was conducted at 3 clinical research sites in the United States, Kenya, and Thailand (Supplementary Methods). Inclusion criteria were age ≥18 years, documentation of HIV, no previous ART (or restricted use for ≤12 weeks more than 24 weeks prior to enrollment), CD4 count ≤100 cells/µL, and residence within a 120-mile radius of the clinical sites. Exclusion criteria were pregnancy and active substance abuse. Consecutive eligible inpatients and outpatients were enrolled. The protocol was approved by the ethics committees of the participating sites, and all study participants signed informed consent.
Participants were followed prospectively from ART initiation (week 0) and at weeks 2, 4, 8, 12, 24, 36, and 48. ART regimens and timing of initiation were chosen according to local treatment guidelines and clinicians’ preferences. The primary objective was to estimate incidence and investigate predictors of IRIS events by week 24 and to observe clinical outcomes including death following ART initiation.
The clinical teams at study sites prospectively identified IRIS events and collected relevant clinical information that was presented to an endpoint review committee (Supplementary Table 1) via conference call. The committee, chaired by M. F., determined whether the events were consistent with IRIS using the following AIDS Clinical Trials Group IRIS definition criteria [24] (Supplementary Table 2): evidence of ART initiation with resultant increase in CD4 count (≥50 cells/µL or a ≥2-fold rise) and/or virologic suppression (>0.5 log10 decrease in plasma HIV viremia), clinical presentation consistent with an infectious or inflammatory condition, and the absence of an alternative etiology such as the expected course of a previously recognized infection or side effects of medications.
Laboratory Evaluations
Plasma HIV viral load, CD4 counts, and routine safety laboratory evaluations (hemoglobin, white blood cell count [WBC], platelets, glucose) were performed in real time by each site’s clinical laboratory using US Food and Drug Administration–approved assays. Batched cryopreserved plasma samples from all participants at the time of ART initiation were tested in the same laboratory using electrochemiluminescence for C-reactive protein (CRP; MesoScale Discovery, Rockville, MD) and using enzyme linked fluorescent assay on a VIDAS instrument (bioMerieux, Marcy l’Etoile, France) for D-dimer levels, according to the manufacturer’s instructions.
Statistical Methods
Wilcoxon rank-sum tests were used for the preliminary univariate analysis considering each of the biomarkers grouped by IRIS or death, ignoring censoring.
To investigate the relationship between IRIS and CD4 T-cell recovery among participants who survived for 12 months after starting ART, we ran 2 separate analyses. First, we ran 2 Cox models for time to first IRIS event. In these models, we included time-varying covariates of change in CD4 from last measure, change in CD4 from baseline, and current CD4 measurement again adjusted for baseline CD4, up to the time of IRIS or censoring by drop-out, death, or month 6. Although death is a competing risk, IRIS-specific hazard ratios (HRs) are a valid form of inference [25, 26]. Second, we ran a linear generalized estimating equation (GEE) model for the outcome of log CD4, which included all available data for the first 6 months of ART for each patient for the outcome of log CD4. The model included an indicator for IRIS after it occurred, time in days, and their interaction, using R package gee.
To investigate baseline biomarkers as predictors of IRIS risk, we fit multivariate Cox regression with IRIS as the outcome, censoring those patients who died or dropped out prior to 6 months, using the Survival package in R [27, 28]. We investigated the association of IRIS with death using a Cox model stratified by site for time to death adjusted for demographic characteristics (age and sex) and baseline biomarkers (CD4 and CD8 T-cell counts, HIV RNA, WBC, glucose, platelets, D-dimer, CRP). In the model, we accounted for IRIS as a time-varying, binary covariate indicating the occurrence of an IRIS-defining event or history of such an event. We used a similar Cox model to investigate the association of baseline biomarkers (WBC, glucose, platelets, D-dimer, CRP) and risk of death within 6 months after ΑRT initiation. All Cox models were stratified by site. Finally, we investigated the potential of baseline biomarkers to predict IRIS or death by performing conditional inference tree analysis using the R party package (version 3.5) [29].
RESULTS
We enrolled 506 participants at the 3 sites (206 in the United States, 200 in Kenya, and 100 in Thailand) out of 540 screened between December 2006 and March 2013 (Supplementary Results). The median age was 37 years (interquartile range [IQR], 31–45), the median body mass index (BMI) was 20.24 kg/m2 (IQR, 17.90–23.48), and 39.3% of study participants were women (Table 1). The median CD4 count was 29 cells/µL (IQR, 11–56), and the median CD8 count was 458 cells/µL (IQR, 273–740). The median plasma HIV viral load was 5.30 log10 copies/mL (IQR, 4.89–5.70), and median hemoglobin was 10.90 mg/dL (IQR, 9.60–12.40; Table 1). The majority of participants (98.5% in Kenya, 89% in Thailand, and 64.1% in the United States) started a nonnucleoside reverse transcriptase inhibitor–based regimen (Supplementary Figure 1). An integrase inhibitor regimen was started in 14.6% of US participants only. Sixteen dropped out of the study prior to week 24 and 28 dropped out prior to week 48.
Table 1.
Demographics and Baseline Biomarkers Overall and by Immune Reconstitution Inflammatory Syndrome Event
| Demographic | All Participants | IRIS | Non-IRIS | P Value | |
|---|---|---|---|---|---|
| Number of participants (%) | 506 (100) | 97 (19.2) | 409 (80.8) | ||
| Site | Kenya | 200 (39.5) | 33 (34.0) | 167 (40.8) | .1621 |
| Thailand | 100 (19.8) | 16 (16.5) | 84 (20.5) | ||
| United States | 206 (40.7) | 48 (49.5) | 157 (38.6) | ||
| Sex | Female | 199 (39.3) | 37 (38.1) | 162 39.6) | .8181 |
| Male | 307 (60.7) | 60 (61.9) | 247 (60.4) | ||
| Age, y | 37 (31–45) | 37 (30–44) | 37 (32–46) | .3662 | |
| Body mass index (kg/m2) | 20.24 (17.90–23.48) | 19.70 (17.80, 23.09) | 20.49 (17.90, 23.49) | .4171 | |
| White blood cell count (106/µL) | 3.25 (2.47–4.30) | 3.21 (2.37–4.98) | 3.25 (2.5–4.3) | .4535 | |
| Hemoglobin (g/dL) | 10.9 (9.6–12.4) | 10.0 (8.80–11.2) | 11.20 (9.8–12.7) | <.0001 | |
| Platelets (106/µL) | 221 (162, 289) | 219 (172.0, 303.5) | 221 (161.0, 285.0) | .7233 | |
| Glucose (mg/dL) | 85 (76.86, 95.00) | 85 (76.32, 96.15) | 85 (77.00, 94.68) | .7534 | |
| CD4 T cells/µL | 29 (11–56) | 22 (7–49) | 30 (12–58) | .0358 | |
| CD8 T cells/µL | 458 (273–740) | 384 (237, 656) | 476 (279, 747) | .0456 | |
| Human immunodeficiency virus RNA (copies/mL) | 5.30 (4.89–5.70) | 5.37 (5.06–5.70) | 5.28 (4.87–5.70) | .0491 | |
| D-dimer (µg/mL) | 1.04 (0.62, 2.12) | 1.56 (0.89, 3.32) | 0.99 (0.58, 1.84) | <.0001 | |
| C-reactive protein (µg/mL) | 5.01 (1.26, 16.69) | 9.14 (4.19, 30.06) | 4.07 (1.17, 14.57) | .0003 |
Median values are included with interquartile range or percentage. For continuous variables, we used the Wilcoxon rank sum test. For discrete variables, we used the Fisher exact test.
Abbreviation: IRIS, immune reconstitution inflammatory syndrome.
IRIS Events
Ninety-seven (19.2%) participants experienced 110 IRIS events by month 6. For participants who experienced 2 distinct IRIS events, only the first was included in the analyses. Similar to previous studies, the majority of IRIS events were related to TB (n = 32, 32.7% of participants with IRIS) and MAC infection (n = 17, 17.3% of participants with IRIS; Table 2). The incidence of IRIS did not differ significantly between any of the participating sites. Twenty-nine participants (29.6%) experienced IRIS related to viruses (eg, VZV and CMV) and 16 (16.5%) experienced IRIS related to fungi (eg, Cryptococcus neoformans and Pneumocystis jiroveci; Table 2). IRIS events related to Toxoplasmosis gondii were uncommon (n = 3 patients, 3.1% of participants with IRIS; Table 2).
Table 2.
IRIS Events by Associated Pathogen and by Site
| Type of IRIS | IRIS-related Organism | Number of Initial IRIS Events* (Percent of Events) | |||
|---|---|---|---|---|---|
| All sites (506 participants) | Kenya (200 participants) | Thailand (100 participants) | US (206 participants) | ||
| Any IRIS Type | 97 events | 33 events | 16 events | 48 events | |
| Mycobacterial IRIS | All mycobacterial IRIS events | 48 (49.5%)* | 17 (51.5%) | 8 (50.0%) | 23 (47.9%)* |
| Mycobacterium avium | 17 (17.5%)* | 0 | 2 (12.5%) | 15 (31.3%)* | |
| Mycobacterium tuberculosis | 32 (33.0%)* | 17 (51.5%) | 6 (37.5%) | 9 (18.8%)* | |
| Viral IRIS | All viral IRIS events | 29 (29.6%) | 9 (27.3%) | 2 (12.5%) | 18 (37.5%) |
| Cytomegalovirus | 7 (7.2%) | 0 | 2 (12.5%) | 5 (10.4%) | |
| Hepatitis B Virus | 2 (2.1%) | 1(3.0%) | 0 | 1 (2.1%) | |
| Hepatitis C Virus | 1 (1.0%) | 0 | 0 | 1 (2.1%) | |
| Kaposi Sarcoma Herpes Virus | 4 (4.1%) | 2 (6.1%) | 0 | 2 (4.2%) | |
| Varicella Zoster Virus | 15 (15.5%) | 6 (18.2%) | 0 | 9 (18.8%) | |
| Fungal IRIS | All fungal IRIS Events | 16 (16.5%)* | 4 (12.1%) | 6 (37.5%) | 6 (12.5%)* |
| Cryptoccocus neoformans | 8 (8.2%) | 1 (3.0%) | 5 (31.2%) | 2 (4.2%) | |
| Histoplasma capsulatum | 3 (3.1%) | 0 | 0 | 3 (6.3%) | |
| Pneumocystis jirovecii | 5 (5.2%) | 3 (9.1%) | 1 (6.2%) | 1 (2.1%) | |
| Other | All other IRIS events | 5 (5.2%) | 3 (9.1%) | 0 | 2 (4.2%) |
| Toxoplasma gondii | 3 (3.1%) | 3 (9.1%) | 0 | 0 | |
| Strongyloides | 1 (1.0%) | 0 | 0 | 1 (2.1%) | |
| Hodgkin lymphoma (flare) | 1 (1.0%) | 0 | 0 | 1 (2.1%) | |
*For patients who experienced two or more distinct immune reconstitution inflammatory syndrome (IRIS) events, only the first event is included in the analyses and in this table. The additional events were related to the following organisms: varicella zoster virus (n = 2), Candida (n = 1), Mycobacterium avium complex (n = 1), Mycobacterium tuberculosis (n = 2), Pneumocystis jirovecii (n = 1), toxoplasma gondii (n = 1), and Kaposi sarcoma herpes virus (n = 1), Two patients at the US site experienced single IRIS events potentially related to two different pathogens at the same time (M. tuberculosis and M. avium complex in one case, M. avium complex and Histoplasmosis in another). We have included both pathogens for both events in this table. For this reason, the numbers of pathogen-specific events add up to more than numbers of total, bacterial, and fungal events where indicated.
IRIS and Immune Recovery
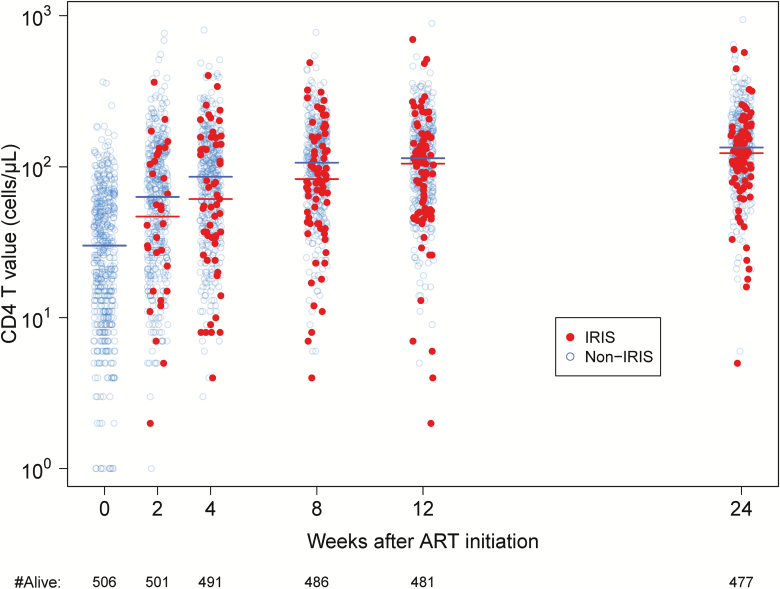
Overall, robust CD4 recovery was observed after ART initiation, regardless of whether or not participants experienced IRIS (Figure 1). We sought to determine if there was a relationship between CD4 restoration and IRIS events. First, we investigated whether participants who experienced better CD4 recovery were at higher risk of IRIS using 2 separate Cox models, as described in the Methods section. We found no association between IRIS and CD4 change or CD4 value at any time point, including baseline, demonstrating that participants with better CD4 recovery were not at a higher risk of IRIS. Second, we sought to determine if IRIS events had an impact on subsequent CD4 recovery over the first 6 months of ART using a GEE model. We found that CD4 increased with time in all participants; however, those who experienced IRIS had overall greater CD4 increases over time (1.45-fold change in the geometric mean of CD4 for a 30-day change in time in the presence of IRIS vs without; P < .001).
Figure 1.
Immune recovery in participants who experienced IRIS compared with participants who did not experience IRIS. CD4 T-cell counts of the surviving participants are displayed for each study time point, with color differentiating those who are still at risk of IRIS (blue) from those who were experiencing or had experienced IRIS (red) at that time point. Lines indicate median values of the 2 groups. Abbreviations: ART, antiretroviral therapy; IRIS, immune reconstitution inflammatory syndrome.
Risk Factors for IRIS
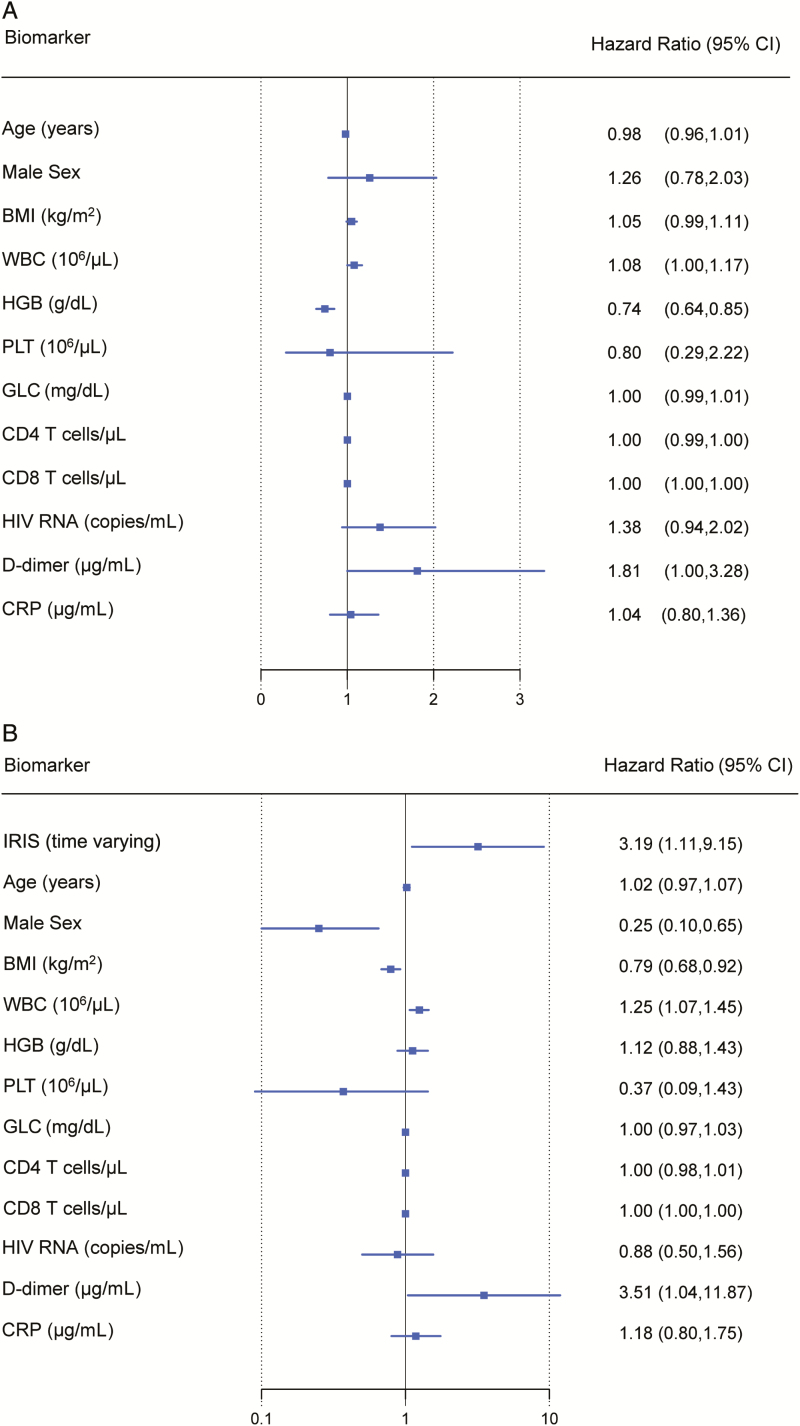
As shown in Table 1, participants who later developed IRIS had lower hemoglobin prior to ART initiation compared to those who did not develop IRIS (10.00 vs 11.20 mg/dL) and were slightly more lymphopenic (CD4 count 22 vs 30 cells/µL and CD8 count 384 vs 476 cells/µL). The baseline clinical characteristics of participants by IRIS outcome and site are shown in Supplementary Table 3. In our multivariate Cox regression model with IRIS as the outcome, only levels of hemoglobin were significantly associated with risk of an IRIS event (HR, 0.74; P < .001), with lower hemoglobin levels associated with increased risk of IRIS (Figure 2A).
Figure 2.
Results of multivariate Cox regression models with IRIS and death as outcomes. A, HRs with 95% CIs for IRIS before 6 months of antiretroviral therapy (ART). Participants who died or dropped out prior to 6 months were censored at time of death or dropout. B, HRs with 95% CIs for death before 6 months of ART. Participants who dropped out prior to 6 months were excluded from this model. Abbreviations: BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; GLC, glucose; HGB, hemoglobin; HIV, human immunodeficiency virus; IRIS, immune reconstitution inflammatory syndrome; PLT, platelets; WBC, white blood cell count.
Risk Factors for Death
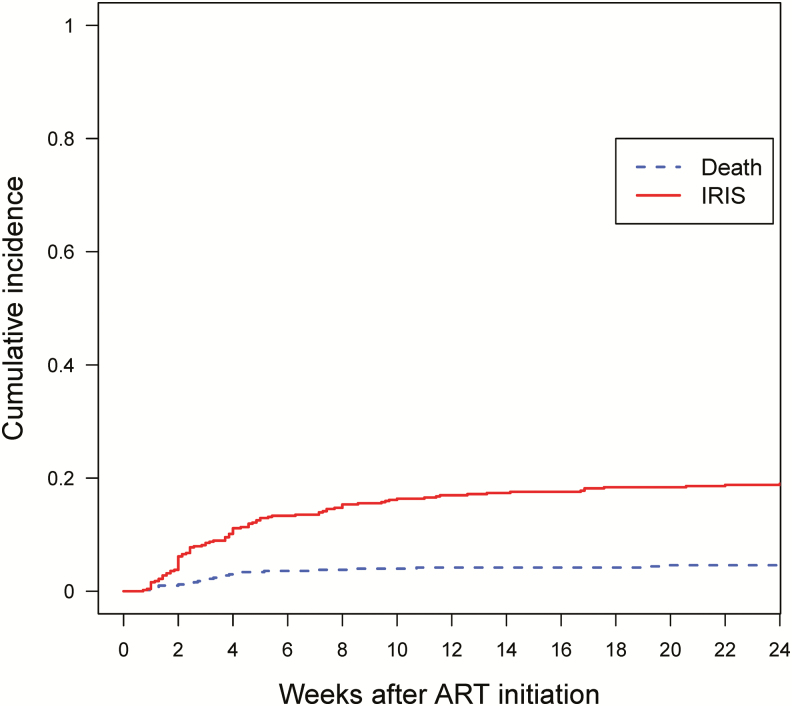
Thirty-one (6.1%) participants died within the first 6 months after ART initiation. The cumulative incidence of IRIS and death is shown in Figure 3. The incidence of death by 6 months was higher in Kenya (n = 21 [10%], P < .001) and Thailand (n = 7 [7%], P = .015) compared to the United States (n = 3 [1.5%]). Seven (22.6%) of the 31 participants who died had a diagnosis of IRIS (3 with TB IRIS, 1 with CMV hepatitis IRIS, 1 with VZV IRIS, 1 with Pneumocystis jirovecii pneumonia (PCP) IRIS, and 1 with PCP IRIS, KS IRIS, and a flare of hepatitis B). In nearly all instances, the participants who died had multiple HIV/AIDS-related comorbidities and the direct causes of death were not determined.
Figure 3.
Cumulative incidence of IRIS or death by weeks since ART initiation through week 24. Abbreviations: ART, antiretroviral therapy; IRIS, immune reconstitution inflammatory syndrome.
In the multivariate Cox model for time to death stratified by site, we found that IRIS was associated with an increased risk of death (HR, 3.2; P = .011). When we adjusted this same model for sex and baseline biomarkers, IRIS was still highly associated with increased risk of death (HR, 3.2; P = .031). In addition, higher WBC, higher D-dimer, lower BMI, and female sex were also significantly associated with an increased risk of death (Figure 2B).
Prediction of IRIS and Death Events by Baseline Biomarkers
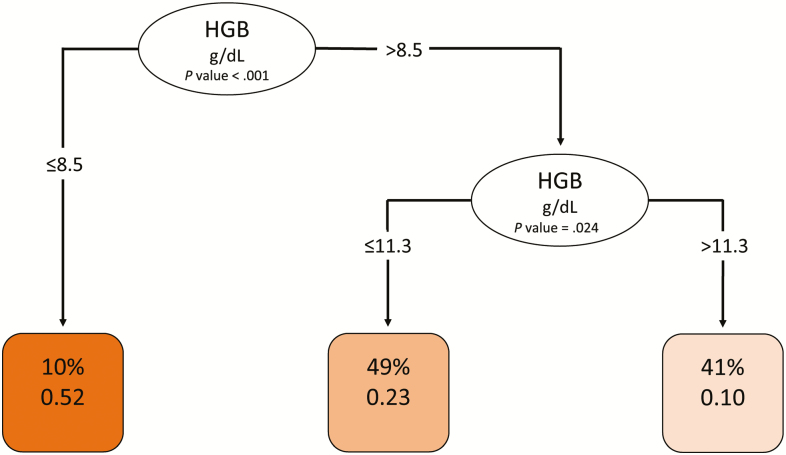
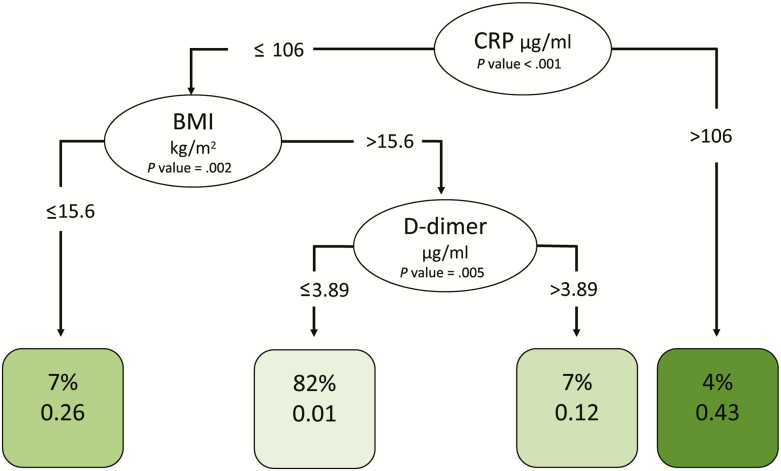
Having determined that low hemoglobin was a risk factor for IRIS and that higher WBC, higher D-dimer levels, lower BMI, female sex, and IRIS events were risk factors for death, we next investigated whether any baseline risk factors might have clinical utility for predicting IRIS or death. We investigated age, sex, BMI, CD4 count, viral load, baseline hemoglobin, WBC, platelets, CRP, and D-dimer levels using a tree-based analysis. We also assessed the baseline biomarker levels for their predictions of death by week 24 and a composite outcome of death and IRIS. Figures 4 and 5 display the trees for IRIS prediction and death, respectively. As shown in Figure 4, hemoglobin was the only notable predictor of IRIS, with cutoffs at 8.5 g/dL and then again at 11.3 g/dL being highly predictive; this was consistent with our Cox model. As shown in Figure 5, very high CRP levels (>106 µg/mL) and very low BMI (<15.6 kg/m2) were both highly predictive of death, while among those with higher BMI and lower CRP, D-dimer levels >3.89 µg/mL were also highly predictive of death.
Figure 4.
Decision tree analysis for predicting immune reconstitution inflammatory syndrome (IRIS). To create the conditional inference decision tree for predicting IRIS, we used 10 baseline biomarkers (body mass index, CD4 and CD8 T-cell counts, human immunodeficiency virus RNA, HGB, white blood cell count, platelets, glucose, D-dimer, and C-reactive protein) and site excluding patients who died without experiencing IRIS, using R the party package (version 3.5). Potential splits are included in the tree model if they met a Bonferroni-adjusted threshold for statistical significance. Ovals indicate a split in the prediction rule on a specific variable, along with the corresponding P value. Each square shows the percentage of observations within that branch that met the decisional criteria (in this case HGB value) followed by the proportion of those patients who experienced IRIS. The darkest shaded square indicates a higher proportion of patients with IRIS. The lightest shade shaded square indicates the lowest proportion of patients with IRIS. Abbreviation: HGB, hemoglobin.
Figure 5.
Decision tree analysis for predicting death. To create the conditional inference tree for predicting death, we used 10 baseline biomarkers (BMI, CD4 and CD8 T-cell counts, human immunodeficiency virus RNA, hemoglobin, white blood cell count, platelets, glucose, D-dimer, and CRP) and site, using the R party package (version 3.5). Potential splits are included in the tree model if they met a Bonferroni-adjusted threshold for statistical significance. Ovals indicate a split in the prediction rule on a specific variable, along with the corresponding P value. Each square shows the percentage of observations within that branch that met the decisional criteria followed by the proportion of those patients who subsequently died. The shading of the squares indicates the proportion of patients who died, with darker shading indicating the highest proportion. Abbreviations: BMI, body mass index; CRP, C-reactive protein.
DISCUSSION
Despite tremendous advancements in HIV care over the past 30 years, many new HIV diagnoses continue to occur late in people who have already progressed to immunologic AIDS and opportunistic infections [6, 30]. Although restoration of immunity with ART is essential to improved survival in these patients, it can also be associated with IRIS and death [31]. In this large, prospective, observational study conducted in 3 countries, representing diverse geographic and clinical environments in which IRIS may occur, we evaluated IRIS and death incidence, IRIS predictors, and the associations between IRIS, immune recovery, and death in participants living with HIV who started ART with CD4 count <100 cells/µL.
The incidence of IRIS was similar in the United States, Kenya, and Thailand despite differences in the prevalence of opportunistic diseases, healthcare systems, and participant socioeconomic factors. TB and MAC were the main drivers of IRIS events, with TB-related events occurring primarily in Thailand and Kenya and MAC-related events occurring in the United States. Viral IRIS, associated most frequently with VZV and CMV, was also frequent at all 3 sites. Cryptococcus neoformans was the most important fungal pathogen associated with fungal IRIS events across sites.
Consistent with the results of other trials that enrolled patients with severe immunosuppression [20, 34], mortality was high in this study, with 6% of participants dying within the first 6 months of starting ART. Nearly one-quarter of the participants who died had experienced IRIS prior to death. Because causes of death were often unknown and IRIS is a clinical diagnosis, this figure could be a conservative estimate of IRIS-associated mortality. In addition, mortality was higher in Kenya and Thailand than in the United States. This suggests that although IRIS occurs at a similar frequency in diverse settings, outcomes are affected by the clinical environment and available diagnostic and treatment resources in which the IRIS diagnosis and management are undertaken. A recent trial of severely immunosuppressed patients who initiated ART in 4 African countries showed that enhanced prophylaxis led to a substantial reduction in mortality compared to standard trimethoprim-sulfamethoxazole prophylaxis alone, demonstrating that management of subclinical infections may have a significant impact on clinical outcomes [19, 20]. Future studies should investigate how to enhance diagnostic monitoring and clinical care of immunosuppressed people who are starting ART in resource-constrained settings.
In contrast to other studies that showed higher mortality in men [35, 36], we found that female sex was independently associated with mortality irrespective of IRIS; this was mostly driven by the Kenyan site. It is unclear what the underlying mechanism may be, but recent studies have suggested a higher level of inflammation in women living with HIV that may increase mortality risk in specific geographic settings [37]. Finally, low BMI was associated with death, and this could possibly underscore more severe underlying opportunistic disease or immune suppression.
Anemia was a clear risk factor for IRIS in our study. Low hemoglobin, which has been associated with incident TB and TB-IRIS in previous studies [38, 39], may be an important surrogate marker, reflecting more advanced HIV or the presence of disseminated opportunistic infection, such as MAC or TB, or more severe inflammation that can be associated with low hemoglobin production [38, 40, 41]. Because hemoglobin is widely assessed using a laboratory test, the clinical utility of assaying hemoglobin levels prior to commencing ART should be further studied as a potential clinical predictor for IRIS. D-dimer levels were also independently associated with death, even after adjustment for other baseline factors. These associations are consistent with those found in several other studies that have concluded that D-dimer level is an important independent predictor of mortality in people living with HIV [42, 43].
A tool that could help predict and distinguish a patient’s risk of IRIS vs death using relatively readily available baseline biomarkers could be valuable to clinicians who initiate ART in severely immunosuppressed patients, as interventions may have different benefit-to-risk profiles for these pathologically distinct outcomes. Our decision tree models suggest that hemoglobin for IRIS and BMI, CRP, and D-dimer for death may be the most useful biomarkers. We note that a recent study conducted in Uganda successfully implemented point-of-care CRP testing; consistent with our findings, elevated baseline CRP was strongly associated with mortality [44]. Future research should confirm the predictive value of these biomarkers and assess how predictive biomarkers may be used to optimize interventions, including corticosteroids or antibiotics, to prevent IRIS and death in immunocompromised patients initiating ART. Risk stratification may be particularly important clinically when immunosuppressive treatments such as prednisone are used to prevent IRIS [45]. Predictors of risk may also improve the cost-effectiveness of efforts to reduce mortality with enhanced antimicrobial prophylaxis [19, 20].
Our study had several limitations. Some IRIS diagnoses may have been missed, especially among patients who died before a proper workup could be performed. IRIS events, regardless of associated pathogen, were combined in our analysis and some predictors could differ significantly by pathogen. In addition, by selecting severely lymphopenic patients, we may have underestimated the impact of baseline CD4 counts on the incidence of IRIS. Last, although diversity of clinical sites was also a strength, we may have underestimated site-specific factors or impact of specific antiretrovirals. Despite these limitations, we believe our study results provide valuable information about the risks and consequences of IRIS among patients living with HIV and severe immunosuppression.
In conclusion, in our international study, IRIS was observed at similar rates in all 3 countries upon ART initiation in patients living with HIV and CD4 counts <100 cells/µL and was strongly associated with anemia. Importantly, IRIS was an independent risk factor for death, along with female sex. Future prospective studies should further validate the potential predictive value, feasibility, and cost-effectiveness of hemoglobin, BMI, CRP, and D-dimer levels in estimating IRIS and death risk in severely immunosuppressed people initiating ART.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. I. S., F. S., D. S., and J. A. were the principal investigators. I. S. designed the trial with contributions from D. S., A. P., M. F., J. A., and F. S. G. R., N. C., B. A., and H. N. provided regulatory and project support during the course of the study. I. S., V. S., D. S., N. P., G. R., H. N., F. K., A. P., J. M., R. B., N. C., N. T., S. T., D. L., J. K., and F. S. provided clinical care and collected the data. M. F. chaired the endpoint review committee. A. R. performed laboratory assays. E. G. and J. W. performed the statistical analysis. M. N. assisted with the decision tree analysis. I. S., V. S., E. G., and J. Y. interpreted results and prepared the manuscript with contributions from all the authors.
Acknowledgments. First and foremost, the authors thank our study participants. The authors also thank our clinical and support staff at the National Institutes of Health (NIH) outpatient clinic 8 (Bethesda, Maryland, USA), the NIH Clinical Center inpatient ward (Bethesda), the Unilever and Kericho District Hospitals (Kenya), the Bamrasnaradura Infectious Diseases Institute (Nonthaburi, Thailand), and the Thai Red Cross AIDS Research Centre (Bangkok). The authors are grateful for their Endpoint Review Committee members for their expert opinion. The authors thank their colleagues Dean Follmann, Rebecca DerSimonian, H. Clifford Lane, and Anthony Fauci for their advice and consultation. The authors also thank the members of the institutional review boards at the National Institute of Allergy and Infectious Diseases (NIAID), Uniformed Services University of the Health Sciences (USUHS), Thailand, and Kenya.
Disclaimer. The views expressed are those of the authors and do not necessarily reflect the official views or policies of the USUHS; the NIH; the Department of Health and Human Services; the Department of Defense; the Departments of the Army, Navy, and Air Force; or the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government. The investigators have adhered to the policies for protection of human subjects as prescribed in AR-7.
Financial support. The trial was supported in part by federal funds from the Intramural Research Program of the NIAID/NIH and from the Office of AIDS Research at the NIH. The project was funded in whole or in part with federal funds from the National Cancer Institute, NIH (under contract HHSN261200800001E) and the NIAID, NIH (under interagency agreement Y1-AI-5072). This project was also partially funded by the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences through a cooperative agreement with the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.
Potential conflicts of interest. J. A. has received honoraria from ViiV Healthcare, Gilead, Merck, Roche, and AbbVie. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lundgren JD, Babiker AG, Gordin F, et al. ; INSIGHT START Study Group Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danel C, Moh R, Gabillard D, et al. ; TEMPRANO ANRS 12136 Study Group A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
- 3. Recommendations regarding provision of ART for all persons living with HIV (Test and START): PEPFAR Scientific Advisory Board. 2015. Available at: https://www.pepfar.gov/documents/organization/250048.pdf. [Google Scholar]
- 4. Prevent HIV, Test and Treat All. Progress Report 2016. WHO Support for Country Impact. Geneva, Switzerland: World Health Organization, 2016. [Google Scholar]
- 5. PoAGfAaA GftUoAAiA, Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescentarv/10/initiation-of-antiretroviral-therapy. Accessed 13 September 2019.
- 6. Mocroft A, Lundgren JD, Sabin ML, et al. ; Collaboration of Observational HIV Epidemiological Research Europe Study in EuroCoord Risk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE). PLoS Med 2013; 10:e1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis 2015; 60:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. IeDea, Collaborations CC. Global trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis 2018; 66:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data— United States and 6 dependent areas— 2013. Suppl Rep 2015; 20 Available at: www.cdc.gov/hiv/library/reports/surveillance. Accessed 7 March 2017. [Google Scholar]
- 10. Boulle A, Schomaker M, May MT, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med 2014; 11:e1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. May MT, Vehreschild JJ, Trickey A, et al. ; Antiretroviral Therapy Cohort Collaboration Mortality according to CD4 count at start of combination antiretroviral therapy among HIV-infected patients followed for up to 15 years after start of treatment: collaborative cohort study. Clin Infect Dis 2016; 62:1571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braitstein P, Brinkhof MW, Dabis F, et al. ; Antiretroviral Therapy in Lower Income Countries Collaboration; ART Cohort Collaboration Groups Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet 2006; 367:817–24. [DOI] [PubMed] [Google Scholar]
- 13. Boulougoura A, Sereti I. HIV infection and immune activation: the role of coinfections. Curr Opin HIV AIDS 2016; 11:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M; IeDEA Southern and Central Africa Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang CC, Sheikh V, Sereti I, French MA. Immune reconstitution disorders in patients with HIV infection: from pathogenesis to prevention and treatment. Curr HIV/AIDS Rep 2014; 11:223–32. [DOI] [PubMed] [Google Scholar]
- 16. Achenbach CJ, Harrington RD, Dhanireddy S, Crane HM, Casper C, Kitahata MM. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clin Infect Dis 2012; 54:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boulware DR, Meya DB, Muzoora C, et al. ; COAT Trial Team Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Török ME, Yen NT, Chau TT, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)–associated tuberculous meningitis. Clin Infect Dis 2011; 52:1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hakim J, Musiime V, Szubert AJ, et al. ; REALITY Trial Team Enhanced prophylaxis plus antiretroviral therapy for advanced HIV infection in Africa. N Engl J Med 2017; 377:233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Post FA, Szubert AJ, Prendergast AJ, et al. ; Reduction of EArly mortaLITY in HIV-infected adults and children starting antiretroviral therapy (REALITY) Trial Team Causes and timing of mortality and morbidity among late presenters starting antiretroviral therapy in the REALITY trial. Clin Infect Dis 2018; 66:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eshun-Wilson I, Okwen MP, Richardson M, Bicanic T. Early versus delayed antiretroviral treatment in HIV-positive people with cryptococcal meningitis. Cochrane Database Syst Rev 2018; 7:CD009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Havlir DV, Kendall MA, Ive P, et al. ; AIDS Clinical Trials Group Study A5221 Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365:1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Volkow P, Cesarman-Maus G, Garciadiego-Fossas P, Rojas-Marin E, Cornejo-Juárez P. Clinical characteristics, predictors of immune reconstitution inflammatory syndrome and long-term prognosis in patients with Kaposi sarcoma. AIDS Res Ther 2017; 14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Immune reconstitution inflammatory syndrome (IRIS) case definitions. Revised 2009. AIDS Clinical Trials Group, 2009. Available at: https://actgnetwork.org/IRIS_Case_Definitions. [Google Scholar]
- 25. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017; 36:4391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geskus RB. Cause-specific cumulative incidence estimation and the Fine and Gray model under both left truncation and right censoring. Biometrics 2011; 67:39–49. [DOI] [PubMed] [Google Scholar]
- 27.Grambsch PM, Therneau TM. Modeling survival data: extending the Cox model. New York: Springer, 2000. [Google Scholar]
- 28. Therneau T. A Package for Survival Analysis in S. Version 2.38, 2015. Available at: https://CRAN.R-project.org/package=survival. [Google Scholar]
- 29. Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat 2006; 15:651–74. [Google Scholar]
- 30. Ravimohan S, Tamuhla N, Steenhoff AP, et al. Early immunologic failure is associated with early mortality among advanced HIV-infected adults initiating antiretroviral therapy with active tuberculosis. J Infect Dis 2013; 208:1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barber DL, Andrade BB, Sereti I, Sher A. Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nat Rev Microbiol 2012; 10:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ford ES, Magaret AS, Spak CW, et al. Increase in HSV shedding at initiation of antiretroviral therapy and decrease in shedding over time on antiretroviral therapy in HIV and HSV-2 infected persons. AIDS 2018; 32:2525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nason MC, Patel EU, Kirkpatrick AR, et al. Immunological signaling during herpes simplex virus-2 and cytomegalovirus vaginal shedding after initiation of antiretroviral treatment. Open Forum Infect Dis 2016; 3:ofw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bisson GP, Ramchandani R, Miyahara S, et al. ; Adult AIDS Clinical Trials Group A5274 (REMEMBER) Study Team Risk factors for early mortality on antiretroviral therapy in advanced HIV-infected adults. AIDS 2017; 31:2217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coelho L, Grinsztejn B, Castilho JL, et al. Mortality in HIV-infected women, heterosexual men, and men who have sex with men in Rio de Janeiro, Brazil: an observational cohort study. Lancet HIV 2016; 3:e490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Croxford S, Kitching A, Desai S, et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health 2017; 2:e35–46. [DOI] [PubMed] [Google Scholar]
- 37. Siedner MJ, Zanni M, Tracy RP, et al. Increased systemic inflammation and gut permeability among women with treated HIV infection in rural Uganda. J Infect Dis 2018; 218:922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Narendran G, Andrade BB, Porter BO, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One 2013; 8:e63541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mupfumi L, Moyo S, Molebatsi K, et al. Immunological non-response and low hemoglobin levels are predictors of incident tuberculosis among HIV-infected individuals on Truvada-based therapy in Botswana. PLoS One 2018; 13:e0192030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Musselwhite LW, Andrade BB, Ellenberg SS, et al. Vitamin D, D-dimer, interferon γ, and sCD14 levels are independently associated with immune reconstitution inflammatory syndrome: a prospective, international study. EBioMedicine 2016; 4:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hella J, Cercamondi CI, Mhimbira F, et al. Anemia in tuberculosis cases and household controls from Tanzania: contribution of disease, coinfections, and the role of hepcidin. PLoS One 2018; 13:e0195985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuller LH, Tracy R, Belloso W, et al. ; INSIGHT SMART Study Group Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boulware DR, Hullsiek KH, Puronen CE, et al. ; INSIGHT Study Group Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chaisson LH, Semitala FC, Asege L, et al. Point-of-care C-reactive protein and risk of early mortality among adults initiating antiretroviral therapy. AIDS 2019; 33:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meintjes G, Stek C, Blumenthal L, et al. ; PredART Trial Team Prednisone for the prevention of paradoxical tuberculosis-associated IRIS. N Engl J Med 2018; 379:1915–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.