Abstract
Treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute leukemia (Ph+ ALL) has been revolutionized with the advent of tyrosine kinase inhibitors (TKIs). Most patients with CML achieve long-term survival similar to individuals without CML due to treatment with TKIs not only in frontline but also in further lines of therapy. The third-generation TKI ponatinib has demonstrated efficacy in patients with refractory CML and Ph+ ALL. Ponatinib is currently the most potent TKI in this setting demonstrating activity against T315I mutant clones. However, ponatinib's safety data revealed a dose-dependent, increased risk of serious cardiovascular (CV) events. Guidance is needed to evaluate the benefit–risk profile of TKIs, such as ponatinib, and safety measures to prevent treatment-associated CV events. An expert panel of German hematologists and cardiologists summarize current evidence regarding ponatinib's efficacy and CV safety profile. We propose CV management strategies for patients who are candidates for ponatinib.
Keywords: Chronic myeloid leukemia, Philadelphia chromosome-positive acute leukemia, Tyrosine kinase inhibitor, Ponatinib, Cardiovascular management, Consensus paper
Introduction
The pan-BCR-ABL1 kinase inhibitor ponatinib exhibits potent activity against both unmutated and mutated BCR-ABL1, including the prognostically unfavorable T315I mutation [1]. Ponatinib is the only approved tyrosine kinase inhibitor (TKI) with clinically relevant activity against the T315I mutation to date. According to the label ponatinib can be used for the second- or later-line treatment of patients with chronic myeloid leukemia (CML) in any phase of the disease who are resistant or intolerant (R/I) to dasatinib and/or nilotinib (second-generation/2G TKIs) and for whom imatinib (first-generation/TKI) is not clinically appropriate [1]. In addition, ponatinib is approved for the treatment of CML patients with the T315I mutation in all lines of therapy [1]. Ponatinib is also approved for the treatment of Philadelphia chromosome-positive acute leukemia (Ph+ ALL) patients who are R/I to dasatinib and for whom imatinib is not appropriate (i.e., for third-line treatment, as dasatinib is only approved for therapy after imatinib or another first-line treatment), as well as for Ph+ ALL patients harboring the T315I mutation, regardless of treatment line [1].
Ponatinib is highly effective in patients with resistance to a 2G TKI, often caused by BCR-ABL1 point mutations. To date, only the single ATP-binding site mutation T315M has been identified to confer resistance to ponatinib [2]. In vitro data suggest that some compound mutations (≥2 mutations in the same BCR-ABL1 molecule, thus in the same clone) involving T315I potentially impact ponatinib sensitivity and clinical response to ponatinib [3]. However, a reanalysis of heavily pretreated CML patients in chronic phase-CML (CP-CML) with next-generation sequencing did not identify any single or compound mutation consistently conferring resistance to ponatinib [2]. More recently, a prospective evaluation of routine next-generation sequencing-based BCR-ABL1 mutation screening in 751 TKI-resistant CML or Ph+ ALL patients showed that compound mutations are relatively infrequent in CP-CML, but may be relevant in accelerated or blast phase CML (AP/BP-CML) and Ph+ ALL [4]. T315I+E255V was the only compound mutant constantly associated with ponatinib resistance in this study population [4]. However, there are also BCR-ABL1-independent mechanisms of TKI resistance, such as additional chromosomal abnormalities, which may play an important role. There is evidence from a comparative analysis suggesting that in CP-CML patients failing a 2G TKI, treatment responses with ponatinib are likely 2-fold higher compared to sequential 2G TKI treatment (complete cytogenetic response, CCyR: 60 vs. 22–26%) [5].
The T315I-directed activity of ponatinib is especially important for non-transplantable patients at risk, patients with relapse after transplantation, and patients in advanced CML as a bridge to allogeneic stem cell transplantation (alloSCT). Ponatinib is active in inducing a second CP [6]. This significantly improves the prospects for long-term survival in CML even after SCT [7]. In a retrospective comparison between the European Bone Marrow Transplant registry and the ponatinib Ph+ ALL and CML evaluation (PACE) clinical trial, ponatinib in T315I-mutant CP-CML was associated with significantly longer overall survival (OS) than alloSCT [8]. Therefore, ponatinib may be an alternative in this setting, especially in older patients unsuitable for SCT or patients without a matched donor. However, interpretations must be made cautiously because of generally small sample sizes. Lastly, the recently approved dose reduction of ponatinib from 45 to 15 mg in CP-CML patients once major cytogenetic response (MCyR) has been achieved (see Section 4, “Recommendations for treatment with Ponatinib”) may improve tolerability and toxicity while also maintaining efficacy and could therefore represent an effective alternative for patients intolerant to other TKIs [9].
Efficacy of Ponatinib
Efficacy of Ponatinib in CML
Ponatinib demonstrated its potent antileukemic efficacy in heavily pretreated patients with CML or Ph+ ALL in the pivotal phase 2 PACE trial, which was conducted in 449 subjects who were either R/I to dasatinib or nilotinib (R/I cohort) or had developed a T315I mutation [10]. The CML cohort was composed of 270 patients in CP-CML, 85 in accelerated phase (AP-CML) and 62 in BP-CML. Totally, 32 patients were in the Ph+ ALL cohort. In total, 29% of all patients were T315I-positive at study entry [6].
Efficacy in CP-CML [6, 10]
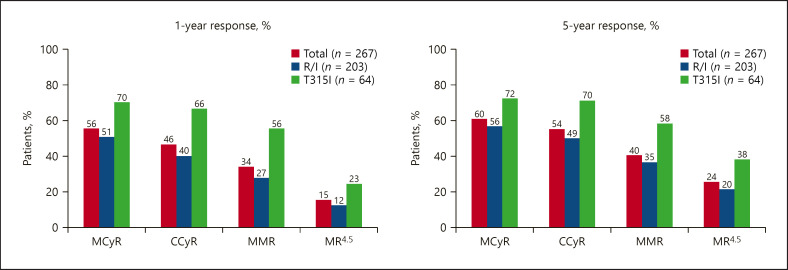
The median age in the CP-CML cohort was 60 years, about 40% were aged 65 or over. 93% had received ≥2 prior TKIs, and 60% ≥3 TKIs. Half of the patients had BCR-ABL1 mutations and 24% were T315I-positive. Median follow-up for CP-CML patients was 56.8 months, and median duration of ponatinib treatment was 32.1 months. Figure 1 shows response rates at 1 year and at the final 5-year update in the CP-CML cohort. The 12-month progression-free survival (PFS) and OS were 80 and 94%, respectively. The 5-year PFS was 53%, and OS was 73%. Median time to CCyR was 2.9 months, and median time to major molecular response (MMR) was 5.5 months. Median duration of MCyR and MMR was not reached by the time of publication. Eighty-three percent of patients were still in MCyR after 5 years. Response rates in the T315I cohort were higher than in the R/I cohort across all levels (Fig. 1). In a post hoc landmark analysis, cytogenetic responses (MCyR, CCyR) and molecular responses (BCR-ABL1 ≤0.1% = MMR, >0.1–1%, >1–10% and >10% = lack of molecular response) at 3, 6, and 12 months were significantly positively correlated with PFS and OS: Achievement of cytogenetic response and deep, early reductions in BCR-ABL1 levels at most landmark time points were associated with improved PFS and OS 4 years post-landmark [11]. Deeper responses at all landmarks correlated with achievement of MR4.5 over time.
Fig. 1.
PACE: 1- and 5-year response at any time in patients with CP-CML, overall, and in patients resistant/intolerant to previous treatment with dasatinib or nilotinib or with T315I mutation (adapted from [6, 10]). R/I resistant or intolerant; MCyR, major cytogenetic response; CCyR, complete cytogenetic response; MMR, major molecular response; MR4.5, 4.5-log reduction or ≤0.0032% BCR-ABL.
Efficacy in AP-/BP-CML [6, 10]
The median age for AP-CML and BP-CML was 60 and 53 years, respectively. Twenty-one percent of AP-CML patients and 39% of BP-CML patients were tested positive for T315I mutation at baseline. For patients with advanced CML, initial responses with ponatinib were rapid and the durability of response was similar to that observed with 2G TKIs for advanced disease in second-line after imatinib [12, 13, 14]. Median follow-up for AP-CML patients was 32.3 months and for BP-CML patients 6.2 months. Median treatment duration was 19.4 and 2.9 months, respectively. In the AP-CML cohort, 57% achieved major hematologic response (MHR) within the first 6 months, the median time to MHR being 3 weeks, and the median duration of response being 12.9 months. In the BP-CML cohort, 31% achieved MHR within the first 6 months with a median time to MHR of 1 month and a median duration of response of 6 months. Response rates, PFS, and OS are shown in Table 1
Table 1.
| AP-CML (n = 83) | BP-CML (n = 62) | |
|---|---|---|
| 6-month response; BP-CML 1-year response; AP-CML 5-year response; AP/BP-CML PFS OS |
MHR: 55%, MCyR: 39%, CCyR: 24%, MMR: 16% MHR: 61%, MCyR: 49%, CCyR: 31%, MMR: 22% 55% (1-year), 22% (5-year) 84% (1-year), 49% (5-year) |
MHR: 31%, MCyR: 23%, CCyR: 18% MHR: 31%, MCyR: 23%, CCyR: 18%, MMR: 13% 19% (1-year), 3.7 mo (5-year) 29% (1-year), 9% (3-year) |
CCyR, complete cytogenetic response; MCyR, major cytogenetic response; MHR, major hematologic response; MMR major molecular response; OS, overall survival; PFS, progression-free survival; BP-CML, blast phase chronic myeloid leukemia; AP-CML, accelerated phase; PACE, Ph+ ALL and CML Evaluation.
Real-world data confirm the high efficacy of ponatinib in patients with CP-CML. In an Italian multicenter observational study ponatinib showed deep and durable responses in heavily pretreated CML patients. Sixteen out of 49 patients (32.6%) obtained molecular response, almost all maintained it while on treatment despite dose reductions [15]. The French observational ponatinib evaluation and safety in real-life chronic myelogenous leukemia patients failing >2 TKIs PEARL study with 48 CP-CML patients similar to the PACE trial showed an OS rate of 80.5% at 3 years and a cumulative MMR of 81.8% at 18 months [16]. Real-world ponatinib prescribing data from the US showed that ponatinib is prescribed across all disease phases, therapy lines, and any mutational status. While a majority of patients (66–79%) were in their third-line of therapy or later, a substantial proportion of patients, especially in AP-CML (34%) and Ph+ ALL (31%), received ponatinib in second-line. Physicians appear to be selecting patients who are younger than those enrolled in registrational trials for ponatinib (median 55 vs. 64 years in PACE) and mitigating against potential risks using lower starting doses and proactive dose reduction [17]. Efficacy and safety data of ponatinib in 34 patients (21 CP-CML, 13 Ph+ ALL) R/I to 2G TKI or with the T315I mutation in routine clinical practice in Belgium showed that the majority of patients obtained deep molecular responses [17]. Median ponatinib treatment duration was 17.5 months for CP-CML patients and 4 months for Ph+ ALL patients. Using lower starting doses than the currently authorized (45 mg once daily) and dose modification allowed effective treatment without exposing patients to a high risk of adverse events, such as CV complications [18].
Ponatinib as Second-Line Treatment in CP-CML
Currently, about 50% of CP-CML patients do not achieve CCyR when receiving a 2G TKI as second-line treatment [19, 20, 21]. Moreover, patients with primary cytogenetic resistance to first-line (or second-line) therapy seem not to benefit as much from sequential therapy with 2G TKIs as from ponatinib [22]. Retrospective real-world studies showed favorable outcome with second-line ponatinib in CP-CML after previously failing one 2G TKI: an Italian case series reported outcomes in 29 patients with R/I to first-line TKI treatment who switched to ponatinib 45 mg, or to 30 mg or 15 mg in the presence of CV risk factors [23]. After a median of 12 months, response versus baseline was improved in 85.7% of patients. Eleven patients achieved MMR, 10 patients achieved deep molecular response of whom 78% achieved early molecular response (BCR-ABL1 <10% within 3 months), 78.5% achieved MMR within 6 months, and 32% MR4.5 within 12 months, suggesting ponatinib could be of benefit in this setting. In another small series with 7 CP-CML patients who switched to ponatinib 15 mg because of intolerance to first- or second-line dasatinib, all patients maintained previously achieved response and 2 patients increased the depth of molecular response, reaching MR4.5 [24].
The efficacy of ponatinib as first-line therapy in CP-CML was evaluated in the phase 3 EPIC trial, which compared ponatinib 45 mg daily versus imatinib 400 mg daily with MMR at 12 months as the primary endpoint [25]. Three hundred and 7 patients were enrolled, 155 received ponatinib and 152 Imatinib. In October 2013, the trial was terminated early at a median follow-up of 5.1 months due to the emerging safety concerns around the time the study was initiated. Thus, the efficacy of frontline ponatinib in CP-CML remains to be established. However, among patients who had evaluable molecular response assessments before study termination, ponatinib appeared to offer improved efficacy over imatinib in patients with newly diagnosed CP-CML. Eight of 10 patients (80%) treated with ponatinib and 5 of 13 patients (38%) treated with imatinib achieved MMR at 12 months (p = 0.074).
Efficacy of Ponatinib in Ph+ ALL
In Ph+ ALL, the incorporation of TKIs in conventional chemotherapeutic approaches has significantly improved depth and duration of responses and is now considered standard of care. With improved remission rates, a higher proportion of patients can be referred to alloSCT in first remission, and the 5-year OS in Ph+ ALL has increased with rates ranging from 38 to 71% depending on age group [26]. Relapses are often attributed to TKI resistance mutations, which are found in approximately 70–80% of imatinib-resistant patients [27, 28, 29]. Mutation T315I is the most frequently detected locus, accounting for about 37% [27]. In patients rescued with a 2G TKI after progression on imatinib, T315I prevalence increased to 65% [27]. Additionally, compound mutations occurred at higher rate. As these mutations confer a high degree of resistance, it is crucial to treat patients with an effective TKI early on to avoid expansion of resistant clones harboring highly resistant clones.
In the PACE trial, ponatinib demonstrated a substantial efficacy in the treatment of relapsed or refractory Ph+ ALL [6, 10]. Forty-one percent of the 32 heavily pretreated Ph+ ALL patients achieved MHR, on average after 0.7 months. Forty-one percent achieved MCyR and 38% CCyR, respectively. However, the duration of response was limited in later lines of treatment due to resistance mentioned above [3, 4]. Median duration of MHR was 3.2 months. Median PFS was 3 months, and the 3-year OS rate was 12% [10]. Ponatinib demonstrated off-label in the frontline setting in combination with hyper-CVAD chemotherapy (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and high efficacy in achieving long-term remission in newly diagnosed Ph+ ALL patients [30]. Seventy-six patients were enrolled and received 4 cycles of hyper-CVAD alternating with 4 cycles of high-dose methotrexate and cytarabine every 21 days. Ponatinib was given at 45 mg daily for the first 14 days of cycle 1 then continuously for the subsequent cycles. After 37 patients were treated, the protocol was amended due to the emergence of CV toxicity to reduce ponatinib to 30 mg daily starting at cycle 2, then 15 mg daily in patients with complete molecular response (CMR). Patients in complete response received ponatinib maintenance daily (with vincristine/prednisone monthly) for 2 years, followed by ponatinib indefinitely. After dose optimization, no further serious vascular events were reported. Ninety-seven percentage of patients achieved MMR and 83% CMR. With a median follow-up of 36 months, the 3-year event-free survival (EFS) and OS rates were 70 and 76%, respectively. Only 15 patients underwent alloSCT during the observation phase, with no difference in OS whether or not patients were transplanted in first remission. Eleven patients switched to alternative TKIs due to safety concerns at the time of the amendment. Four of these patients relapsed after a median of 19 months, in 2 the T315I mutation was found.
In an indirect comparison with dasatinib plus hyper-CVAD in first-line Ph+ ALL, ponatinib plus hyper-CVAD was associated with significantly higher 3-year EFS and OS rates, based on propensity-score matched analysis of 41 patients from each cohort (EFS: 69 vs. 46%; p = 0.04; OS: 83 vs. 56%; p = 0.03), probably resulting mainly from faster achievement of deep molecular response [31]. In a recent meta-analysis of studies in newly diagnosed Ph+ ALL patients, ponatinib in combination with chemotherapy was significantly more likely to obtain CMR than first-generation/2G TKIs in combination with chemotherapy (79 vs. 34%; OR 6.09; p = 0.034) [32]. Achieving CMR was coupled with a significantly higher 3-year OS (79 vs. 50%; OR 4.49; p = 0.050).
However, many groups still consider imatinib as the standard of care for Ph+ ALL and recommend change of TKI based on tolerability, minimal residual disease response, and detection of resistance mutations. Furthermore, alloSCT is considered to offer the best chance of long-term survival in younger and fit Ph+ ALL patients [27]. Patients without a donor are not eligible for an alloSCT, such as older patients have a large unmet need for new treatment concepts with reduced toxicity although long-term survival has been reported with combinations of chemotherapy with 2G TKIs.
The European Working Group on Adult ALL (EWALL) conducted 2 phase 2 trials with low-intensity induction (vincristine and steroids) and consolidation combined with dasatinib (EWALL-PH-01 study; n = 71) or nilotinib (EWALL-PH-02 study; n = 79) in elderly patients with newly diagnosed Ph+ ALL [29, 33]. Note that nilotinib is not approved in Ph+ ALL. Both studies reported high CR rates after induction of 96–97%. With dasatinib, the 1-year relapse-free survival was 58% and 5-year OS 36% [29]. With nilotinib, the 1-year EFS (resistant disease, relapse, or death) was 74%, and OS at 4 years was 47% [33]. Of note, the T315I mutation was a frequent cause of relapse in dasatinib-treated Ph+ ALL patients [29]. Despite the very high CR rates and low or no induction mortality, the majority of patients will not achieve long-term remission with low-intensity induction combined with 2G TKIs. Whether the introduction of third-generation TKIs such as ponatinib enables higher and more durable responses with comparable tolerability remains to be established. The EWALL group is initiating a 3-arm phase 3 trial comparing low-intensity chemotherapy plus ponatinib or imatinib versus ponatinib plus immunotherapy with blinatumomab, a bispecific antibody, in elderly Ph+ ALL patients (EWALL-Ph-03 study).
A number of trials are currently investigating whether a combination of TKI plus steroids or TKI plus immunotherapy might even render chemotherapy and SCT superfluous in some cases in the future, with the aim to reduce overall toxicity and to further improve prognosis. Initial results of a phase 2 trial by the Italian GIMEMA group with ponatinib in combination with steroids (plus intrathecal prophylaxis) as frontline therapy in 42 elderly or unfit Ph+ ALL patients (median age 68 years) showed a CR rate of 95% with 45.8% of patients achieving CMR and a 1-year OS of 87.5% [34]. Ponatinib effectively prevented the onset of BCR-ABL1 mutations in this setting. Full publication of this trial is awaited. The same group had evaluated dasatinib with steroids as induction in 60 Ph+ ALL patients (median age 42 years), but added chemotherapy and/or alloSCT if no sustained CMR was achieved [35]. They reported a CHR of 97% and CMR of 18.6%. DFS was 48.9% at 30 months and OS 58.3% at 36 months.
In relapsed/refractory ALL blinatumomab and inotuzumab showed promising response rates [36, 37]. Furthermore, first trials with the combination of TKIs (ponatinib, dasatinib, or bosutinib) plus blinatumomab have been started [38].
CV Safety Profile
CV Risks of TKIs
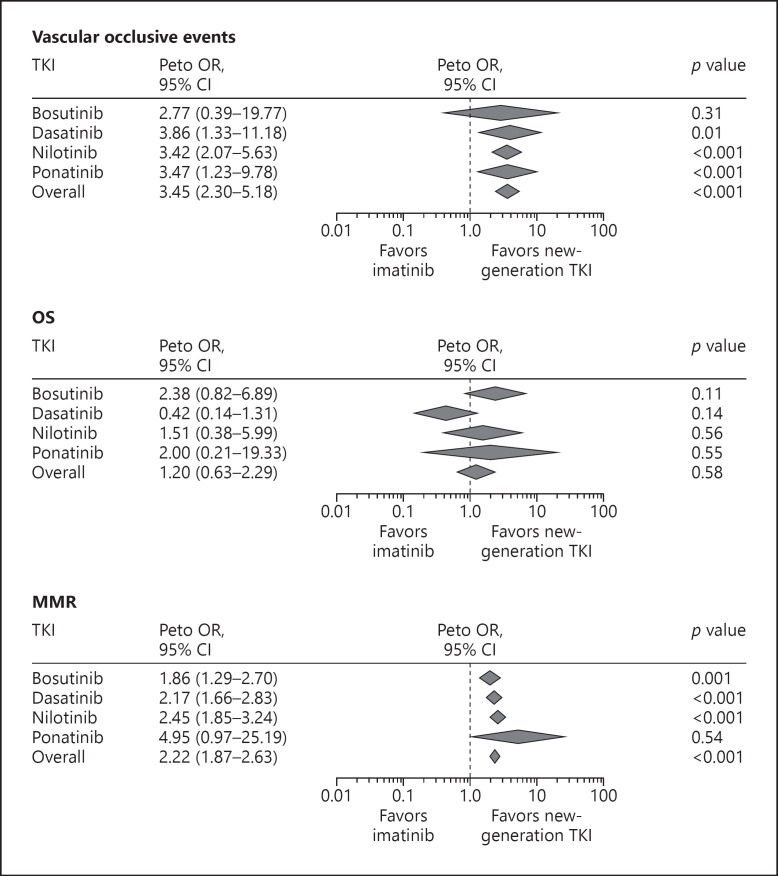
All new-generation TKIs may potentially increase the risk for CV disease (CVD) to different extents [22, 39]. A meta-analysis of all randomized clinical trials comparing imatinib with new-generation TKIs indicated significantly increased risk for vascular occlusive events with dasatinib, nilotinib, and ponatinib and a trend but nonsignificant risk for bosutinib (Fig. 2) [40]. Vascular occlusive events comprised a list of 49 terms specifically related to arterial or venous ischemia or thrombosis [40, see www.karger.com/doi/10.1159/000501927 for online suppl. Methods 2]. The meta-analyses also focused on efficacy (MMR) and OS. The MMR rate at 1 year was significantly higher with new-generation TKIs compared with imatinib; however, there was no difference in 1-year OS (Fig. 2). The potential CV risk needs to be balanced against efficacy considerations regarding 2G/third-generation TKIs versus imatinib.
Fig. 2.
Forest plots of vascular occlusive events, OS, and MMR in patients with Ph+ ALL treated with new-generation TKIs vs. imatinib (adapted from [40]). MMR, major molecular response; TKI, tyrosine kinase inhibitor; OS, overall survival. Diamonds reflect the summary effect for each TKI. The summary effect size was derived from the following trials: Bosutinib: BELA (NCT00574873); Dasatinib: SWOG-S0325 (NCT00070499), START-R (NCT00103844), DASISION (NCT00481247), NordCML006 (NCT00852566), NCT00320190; Nilotinib: RE-NICE (NCT01400074), ENESTnd (NCT00471497), ENESTcmr (NCT00760877); Ponatinib: EPIC (NCT01650805). No forest plot contains all available 10 trials because for each analysis (overall survival, major molecular response, and vascular occlusive events), at least one study did not report the event of interest. 95% CI, 95% confidence interval.
The precise extent of the CV risk during treatment with ponatinib or other TKIs is difficult to assess as vascular events were not systematically defined or collected prospectively in the registration trials. The available data are based on post hoc analysis. Moreover, adverse events in oncology trials are reported using the Common Terminology Criteria for Adverse Events, which differs from the way outcomes are measured in cardiology trials. CV events are often inconsistently defined, and a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, and CV death are often used. Therefore, a comparison between different oncology trials or with the general population may be difficult if not impossible [40, 41]. From the cardiologist's point of view, it is important to distinguish between nonfatal CV events (CV morbidity) and CV mortality. To facilitate evaluation and comparison of the CV risk during TKI treatment, future studies should apply standardized endpoints and definitions [41].
Possible Pathophysiological Mechanisms of Vascular Complications with TKI
The pathophysiological mechanisms underlying the vascular toxicity of TKIs have not been clearly established but are believed to overlap to some extent. In a mouse model of atherosclerosis, nilotinib was shown to have directly proatherogenic effects on endothelial cells [42]. Nilotinib also caused metabolic changes including elevation of cholesterol and fasting glucose levels [42]. Studies using aortic preparations indicate that ponatinib-related vascular side effects are mainly caused by vaso-spasms following endothelial damage [43]. The calcium channel blocker (CCB) nifedipine effectively inhibited these effects and might help to protect patients from vascular damage [41]. In a mouse model of ischemia, both ponatinib [43] and nilotinib [42] reduced proliferation and survival of endothelial cells after endothelial damage and hence reduced vascular regeneration and the repair processes that restore blood flow. These effects were found to be dose related for ponatinib (50–100 nM) [43] at concentrations reached in patients, which is also supported by the clinical observation that reducing ponatinib dose is an effective strategy to lower the risk of vascular disorders [44]. One recent study evaluating the cardiotoxicity associated with ponatinib and potential rescue methods in zebrafish and isolated neonatal rat cardiomyocytes showed that ponatinib inhibits the essential cardiomyocyte prosurvival AKT/ERK signaling pathway, resulting in cardiomyocyte apoptosis [43]. Neuregulin-1β treatment prevented ponatinib-induced cardiotoxicity by supplementing this signaling pathway [45].
Cardiovascular Toxicity of Ponatinib
Due to the occurrence of CV, cerebrovascular and peripheral arterial occlusive events (artery occlusive events, AOEs), and some venous thromboembolisms in the PACE-trial, therapeutic management strategies including ponatinib dose reductions (30 mg or 15 mg instead of 45 mg) were implemented in October 2013. Most patients treated with ponatinib who developed vascular disorders tended to have additional CV risk factors. Overall, the cumulative incidence of AOEs increased over time, while the exposure-adjusted incidence of newly occurring AOEs did not increase with longer duration of ponatinib treatment [6]. This can be explained in part by the reduction of the mean ponatinib dose administered. In contrast to AOEs, venous thromboembolic events (VTE) do not appear to be dose related. The cumulative and exposure-adjusted incidence rates of AOEs and VTE are summarized in Table 2 [6].
Table 2.
Cumulative and exposure-adjusted incidences of treatment-emergent AOEs and VTEs (adapted from [6])
| CP-CML (n = 270) |
Total (n = 449) |
|||
|---|---|---|---|---|
| AE | SAE | AE | SAE | |
| AOEs, n (%) | 84 (31)a | 69 (26)b | 111 (25)c | 90 (20)d |
| CV, n (%) | 42 (16) | 33 (12) | 59 (13) | 44 (10) |
| Cerebrovascular, n (%) | 35 (13) | 28 (10) | 41 (9) | 33 (7) |
| Peripheral vascular, n (%) | 38 (14) | 31 (11) | 48 (11) | 38 (8) |
| Exposure-adjusted AOEs, number of patients with events per 100 patient-years | 14.1 | 10.9 | 13.8 | 19.6 |
| VTEs, n (%) | 15 (6) | 13 (5) | 27 (6) | 23 (5) |
| Exposure-adjusted VTEs, number of patients with events per 100 patient-years | 2.1 | 1.8 | 2.8 | 2.4 |
46 patients had >1 AOE
31 patients had >1 serious AOE
57 patients had >1 AOE
38 patients had >1 serious AOE. Categorization of AOEs and VTEs is based on a broad collection of >400 MedDRA preferred terms related to vascular ischemia or thrombosis; AE, adverse event; AOE, arterial occlusive event; MedDRA, medical dictionary for regulatory activities; SAE, serious adverse event only; VTE, venous thromboembolic event; CV, cardiovascular; CP-CML, chronic phase-chronic myeloid leukemia.
Benefit–Risk Assessment of Ponatinib in CML
Successful treatment with ponatinib requires careful balancing of efficacy versus risk of transformation to BP-CML and potential CV risk. Factors such as age, disease phase, treatment line, mutation status, reason for switching TKI therapy, and comorbidities should be considered as well as strategies to minimize the CV risk by dose modification and CV management including close monitoring. In CP-CML, the effectiveness of preemptive ponatinib dose modification is well supported by clinical data [6, 15, 46, 47] and is recommended in the summary of product characteristics [1] (see Section 4, “Recommendations for treatment with Ponatinib”).
Clinical data support ponatinib as a highly effective treatment option for patients with CML (Fig. 2). The drug's importance increases with the resistance of the BCR-ABL1-positive clone and the transformation state of CML. The more advanced the CML, the more important efficacy becomes: in AP/BP-CML, mortality risk outweighs the CV risk. However, CP-CML patients with first-line failure on imatinib or a 2G TKI are also at higher risk of disease progression to AP/BP-CML. The latest revision to the WHO classification of CML 2016 reflects this by adding provisional “response to TKI therapy” criteria of AP-CML, rating resistance to 2 sequentially administered TKIs or occurrence of 2 or more BCR-ABL1 mutations during TKI therapy of CP-CML patients as constituting progression to AP [48]. Acquisition of additional chromosomal abnormalities (ACAs) as evidence of clonal evolution and atypical BCR-ABL1 transcripts have also been variably associated with an unfavorable course of disease [49]. Another aspect to be considered in the benefit–risk evaluation is the desired goal of treatment (e.g., short-term bridge to SCT or long-term treatment or treatment-free remission [TFR]). The CV risk probably carries less weight in short-term use of ponatinib than in long-term treatment as the median time to onset of AOEs was about 1 year [6]. Regarding TFR, the upcoming PONTrack (With ponatinib on track for TFR in CML) study of the German CML-study group (EudraCT number: 2018-004564-59) is investigating whether the earlier use of ponatinib may enable more patients to reach eligibility for attempting and achieving TFR.
Benefit–Risk Assessment of Ponatinib in Ph+ ALL
Treatment urgency is much higher in Ph+ ALL than in CML because of the significantly worse long-term prognosis. TKI resistance mutations are common, occur at a very early stage, and are a hallmark of this biologic instable disease. The incidence of T315I is high, as is the incidence of compound mutations [27]. Benefit–risk assessment is different in patients with an option for SCT and those without this option. It remains open whether and how different TKIs should be used in combination with chemotherapy and in which sequence. Several trials are ongoing to answer this question. Although different phase-2 studies have demonstrated preliminary efficacy of ponatinib in frontline therapy of ALL (extensively reviewed in Section 2.2, “Efficacy of Ponatinib in Ph+ ALL”), many groups still consider imatinib as an effective first-line strategy and consider changing the TKI in defined situations, for example, persistent MRD. More studies are needed also investigating potential dose reduction in good responders and the optimal use of ponatinib in Ph+ ALL.
Recommendations for Treatment with Ponatinib
Preemptive Dose Modification
The currently recommended ponatinib starting dose is 45 mg once daily [1]. Based on positive PACE long-term data, the summary of product characteristics reads: “…reducing the dose of ponatinib to 15 mg should be considered in CP-CML patients who have achieved MCyR in order to optimize CV safety” [1]. The median time to CCyR and MMR in PACE was 2.9 and 5.5 months, respectively, and the median time to onset of an AOE was about 1 year [6]. Hence, preemptive dose reductions within the first 6 months after optimal response (CCyR or MMR) may improve CV safety while also maintaining long-term response [6]. In addition to response status and the patient's CV risk profile, the assessment should also consider other factors including time to cytogenetic response and possible side effects of ponatinib treatment [1]. Close monitoring of response is recommended after a dose reduction [1]. Regarding the situation in Ph+ ALL, the little evidence available from the first-line study (off-label) in combination with hyper-CVAD likewise indicates that reducing the dose from 45 to 30 mg starting at cycle 2 with further reduction to 15 mg after achievement of CMR is an effective strategy for preventing AOEs [30].
Considerations of Lower Starting Doses of Ponatinib
The OPTIC trial comparing 15, 30, and 45 mg in patients with R/I CP-CML is currently evaluating whether a lower ponatinib starting dose would be noninferior and safer (ClinicalTrials.gov Identifier: NCT02467270). To date, there are only retrospective data available from an Italian case series which evaluated ponatinib in second-line [23]. Eleven of 29 patients were started on lower-dose ponatinib because of older age or other risk factors such as hypertension, diabetes, or hypercholesterolemia. However, this was a highly selected patient population with an overall relatively low CV risk profile. A lower starting dose is off-label, the opinions and recommendations of the expert panel in this regard are based entirely on clinical experience and the current body of evidence. Additional prospective data from ongoing or planned trials, such as PONS (ClinicalTrials.gov Identifier: NCT03807479; ponatinib 30 mg starting dose in second-line after nilotinib or dasatinib) will add further evidence.
In the panel's opinion, the possible criteria to support a ponatinib starting dose of 30 mg in CML might include chronic phase (as opposed to AP/BP), good response status (at least in MCyR), absence of mutations (as opposed to detected mutations), resistance to only 1 TKI (as opposed to >1 TKI), intolerance despite good response, and increased CV risk (in particular, diabetes and ischemia [50, 51]) and in Ph+ ALL poor general health and or significant CV risk such as coronary artery disease. In CV-risk patients, urgent need for ponatinib treatment due to disease risk is a clear indication to prescribe ponatinib (see risk categories, Table 3 below). A starting dose of 15 mg may be considered for instance in patients with very good response but severe intolerance to other TKIs switching to ponatinib or in patients with high CV risk. In Ph+ ALL, a 15 mg starting dose may well be associated with reduced efficacy, for example, in the presence of highly expressed compound mutations [27].
Table 3.
| Low to moderate risk | |
|---|---|
| − ESC-SCORE <5% | |
| − Many middle-aged subjects belong in this category; strongly influenced by obesity, total cholesterol and triglycerides, familiar history of coronary disease. |
Use of ponatinib possible Risk factor modification and lifestyle changes based on guidelines; consider statin therapy (e.g., atorvastatin) to keep (fasting) LDL-C <115 mg/dL (<3 mmol/L)a |
|
Monitoring: − History-taking and clinical examination, lab tests including diabetes and serum lipid profile (every 3 months in year 1, then every 6–12 months) −BP monitoring (every 3 months in year 1, then semiannually); self-monitoring with documentation as appropriate (20–30% incidence of hypertension during ponatinib treatment) − ECG (semiannually); ABI, stress ECG/alternative stress test (annually) | |
| High-risk | |
|---|---|
| Subjects with any of the following: − ESC-SCORE between 5% and <10% − Markedly elevated single risk factors, such as cholesterol >310 mg/dL (8 mmol/L) or severe hypertension (≥180/110 mm Hg) − Moderate CKD (GFR 30–59 mL/min) |
Use of ponatinib possible; alternative TKIs should be considered (benefit-risk assessment) Prospective dose reduction; Risk factor modification and lifestyle changes based on guidelines; consider statin therapy (e.g., atorvastatin) to keep (fasting) LDL-C <100 mg/dL (<2.6 mmol/L) |
|
Intensified monitoring: As indicated for low-/moderate-risk group, plus: − Regular blood pressure self-monitoring (documentation), − History-taking and clinical examination, lab tests including diabetes and lipid profile (quarterly) − ABI (semiannually) | |
| Very high-risk | |
|---|---|
| Subjects with any of the following: − ESC-SCORE ≥10% − Documented CVD (previous AMI, ACS, coronary revascularization, stroke, TIA or PAD) − DM with target organ damage, for example, proteinuria or with a major risk factor (smoking, hypertension, high cholesterol) − Severe CKD (GFR <30 mL/min) |
Ponatinib should be used only if strictly indicated; use of alternative TKI when possible Prospective dose reduction; risk factor modification and lifestyle changes; more aggressive treatment of dyslipidemia with target (fasting) LDL <70 mg/dL (<1.8 mmol/L) |
| Intensified monitoring: as indicated for high-risk group | |
Having considered the very low risk introduced by statin therapy, the panel felt that low- to medium-risk patients treated with ponatinib should probably merit earlier or more vigorous statin control of LDL-cholesterol. ABI, ankle-brachial index; ACS, acute coronary syndrome; AMI, acute myocardial infarction; BP, blood pressure; CKD, chronic kidney disease; DM, diabetes mellitus; ECG, electrocardiogram; ESC, European Society of Cardiology; GFR, glomerular filtration rate; PAD, peripheral artery disease; TIA, transient ischemic attack; SCORE, systematic coronary risk evaluation.
Prevention and Management of CV Risk
Hematologists should devote careful attention to the potential risk of CV events and to the monitoring of patients treated with ponatinib in order to get the full benefit of ponatinib's potency and minimize the risk to the fullest possible extent. The following aspects of CV management apply in principle to all TKIs. In at-risk individuals in particular, CV management should always be premised on close collaboration between hematologists and other specialists (angiologists, cardiologists, etc.), with additional involvement of the primary care physician. Patients should be made aware of potential CV symptoms before starting treatment and told to see a doctor immediately (ideally an angiologist or cardiologist at a specialist facility) if acute signs and symptoms occur. Most of those potential complications can be treated effectively and safely. As far as the diagnosis and management of CV risk factors are concerned, the same guidelines and recommendations may apply to CML and Ph+ALL patients as to the general population since no large prospective trials on CV event prevention in CML or Ph+ ALL patients have been conducted to date.
Risk Classification and Monitoring during Ponatinib Treatment
Each patient's individual CV risk should be determined before starting treatment with ponatinib. A number of broadly accepted risk scores are available for that purpose [52], which however were developed for the general population and have not yet been validated prospectively for CML or Ph+ ALL patients. Hence, individual patient's risk assessment is ultimately based on the physician's clinical judgment. In Europe, CV risk assessment is commonly done using the systematic coronary risk evaluation (SCORE) system developed by the European Society of Cardiology (ESC) based on the 5 classical risk factors age, sex, smoking, systolic blood pressure (BP), and serum cholesterol to estimate the 10-year risk of a fatal CV or cerebrovascular event [52]. The online risk calculator tool can be found at www.HeartScore.org [52]. It is important to note that CV risk factors are dynamic by nature and may change during the course of treatment. As such, the ESC-SCORE should be regularly recalculated over time with intervals depending on the individual patient's risk (e.g., more frequently in patients with moderate or greater risk or when risk factors change).
Regarding procedures in patients undergoing ponatinib treatment, the expert panel decided to simplify the SCORE system by using a 3-group classification – low to moderate risk, high risk, and very high risk – with consequences for the use of ponatinib and for risk-adjusted monitoring (Tables 3, 4). This is intended to ensure that blood glucose disorders, dyslipidemia, and the potential occurrence of peripheral artery disease or other CVD can be detected in a timely manner [53]. Patients who have documented CVD, diabetes, moderate or severe chronic kidney disease, or markedly raised single risk factors are already at very high or high risk and automatically qualify for intensive risk factor evaluation and management [52]. The assessments and procedures recommended for each risk group are given in Table 4
Table 4.
Recommended screening and assessment before starting treatment with ponatinib (adapted from [53])
| Assessments | Comment | |
|---|---|---|
| History-taking |
CV risk factors: Age, gender, BMI, smoking, hypertension, diabetes, hyperlipidemia, family history CV comorbidities/interventions: PAD, CAD, TIA, stroke, atrial fibrillation, and so on CV symptoms (past/current): CAD (angina pectoris, dyspnea, palpitations, syncope), CAS (visual symptoms, TIA, PRIND), PAD (intermittent claudication) |
|
| Clinical examination |
Vascular assessment: pulse status, edema, carotid bruit Heart/lungs BP (both arms), heart rate, arrhythmia, heart murmurs |
|
| Diagnostic imaging | ECG Echo ABI Carotid ultrasound |
The diagnostic imaging required depends on the clinical findings/risk |
| Laboratory tests | Serum: fasting blood glucose, HbA1C, LDL-C, HDL-C, total cholesterol, (fasting) triglycerides, potassium, creatinine, GFR, aminotransferases Urine: microalbumin, protein (quantitative) | |
ABI, ankle-brachial index; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CAS, cerebral artery stenosis; ECG, electrocardiogram; Echo, echocardiogram; GFR, glomerular filtration rate; HBAlc, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PAD, peripheral artery disease; PRIND, prolonged reversible ischemic neurological deficit; TIA, transient ischemic attack.
Management of CV Risk Factors
Alongside preemptive ponatinib dose reduction, additional CV risk reduction may be possible by systematic management of risk factors, especially in high-risk and very high-risk patients. Lifestyle changes (dietary modification, weight reduction, physical activity, abstaining from smoking) are crucial, but not usually sufficient on their own.
Hypertension
Hypertension is a common ponatinib side effect and known to increase the risk of AOEs [54]. Therefore, blood pressure (BP) needs to be checked in all patients before, during, and after discontinuation of ponatinib use, in particular in patients with known hypertension. Before starting ponatinib, BP should be normalized. BP goals are shown in Table 5. The recently updated ESC Guidelines for the management of arterial hypertension recommend starting the majority of patients on 2 antihypertensive drugs rather than one [53]. Exceptions are frail older patients and those at low risk and with grade 1 hypertension. For most patients, this will be a combination of an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker with a CCB or a thiazide/thiazide-like diuretic. For those requiring 3 drugs, a combination of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker with a CCB and a thiazide/thiazide-like diuretic should be used [55].
Table 5.
Therapeutic target levels for blood pressure, serum lipids, and parameters of glucose metabolism, depending on ESC-SCORE risk profile (adapted from [52, 53])
| Parameter | Low to moderate risk | High risk | Very high risk |
|---|---|---|---|
| SBP, mm Hg | <140 (150–140 in patients >80 years) | <140 | <140 |
| DBP, mm Hg | <90 | <90 (< 85 mm Hg in patients with DM) | <90 (< 85 mm Hg in patients with DM) |
| LDL-Ca, mg/dL | <115 (<3.0 mmol/L) | <100 (<2.6 mmol/L) | <70 (<1.8 mmol/L) |
| Triglyceridesa, b, mg/dL | − | <500 (<5.65 mmol/L) | <500 (<5.65 mmol/L) |
| HbA1c | <7% (<8% in older or unfit patients) | <7% (<8% in older or unfit patients) | <7% (<8% in older or unfit patients) |
Serum lipids should always be tested in the fasted patient
LDL-C and blood glucose should be well controlled beforehand in order to identify treatment-relevant hypertriglyceridemia. DBP, diastolic blood pressure; DM, diabetes mellitus; ESC, European Society of Cardiology; HBA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; SCORE, systematic coronary risk evaluation.
Hyperlipidemia
Low-density lipoprotein cholesterol should be tested in all patients, even if ponatinib in itself does not raise cholesterol [54]. In addition to lifestyle changes, the guidelines recommend ESC-SCORE-based statin treatment [52]. The relevant low-density lipoprotein target levels that also indicate limit levels for starting lipid-lowering therapy are given in Table 5. Drug interactions should be considered as ponatinib inhibits the activity of the transporter proteins P-glycoprotein and breast cancer resistance protein. Therefore, ponatinib has the potential to increase the plasma concentrations of coadministered drugs that are substrates of these transporter proteins such as pravastatin and rosuvastatin [1]. Atorvastatin can be used relatively safely with ponatinib (both CYP3A4 substrates, possible interaction through competitive metabolism). In patients with high or very high CV risk and significantly elevated triglyceride levels, drug therapy should be considered. Preference should be given to omega-3 fatty acids (fish oil) as TKIs may interact with fibrates. In addition to regular exercise and a low-calorie diet, reduced alcohol intake is advisable. Referral to a metabolic disease specialist should be considered if necessary.
Prediabetes/Diabetes
Unlike nilotinib, ponatinib has not been directly linked with hyperglycemia. However, diabetes and glucose intolerance are believed to increase the risk of CV events in individuals treated with ponatinib [54]. The clinical cutoffs for prediabetes are a fasting blood glucose level of 100–126 mg/dL (5.6–7 mmol/L), HbA1c of 5.7–6.5%, and 2-h oral glucose tolerance test levels of 140–199 mg/dL (7.8–11 mmol/L). Cutoff for fasting blood glucose level defining diabetes is >126 mg/dL (>7 mmol/L) and for HbA1c >6.5% (Table 5). In patients with type 1 or 2 diabetes, an HbA1c <7% is a generally accepted target level, however [52]. In some cases (older or unfit patients), a less stringent target HbA1c of up to 7.5–8% may be acceptable [52]. For more differentiated diabetes management, a diabetes specialist should be consulted. Blood glucose control can generally be achieved with a balanced diet, moderate exercise, and antidiabetic medication (metformin ± other oral antidiabetic drugs ± insulin) [52].
Primary and Secondary Prevention of Vascular Damage
Given that ponatinib increases the risk for vascular damage, primary prophylaxis should be considered. Statins are effective in the management of arteriosclerotic changes, generally well tolerated and have pleiotropic effects on the vascular system (e.g., anti-inflammatory activity), in addition to lowering cholesterol levels. In secondary prevention, consideration should be given to the possibility of replacing ponatinib with another TKI, especially in the presence of severe vascular side effects such as myocardial infarction or stroke. Otherwise, at least a highly potent statin (atorvastatin 40 mg) and a platelet aggregation inhibitor such as aspirin 100 mg or clopidogrel 75 mg should be prescribed. In certain situations, such as acute coronary syndrome, dual platelet inhibition for a certain period (usually 1 year) is also indicated. If vasoconstriction is suspected, a CCB such as nifedipine should be used, at least in individuals with concomitant hypertension (as is common with ponatinib).
Conclusions
Ponatinib is a potent therapeutic option in patients with CML or Ph+ ALL intolerant or resistant to other TKIs, and the benefit–risk balance should always be evaluated for each individual patient. Treatment with ponatinib (and other TKIs) poses an increased CV risk, which appears to be dose-dependent and can therefore be mitigated by dose modification and adequate CV management. This report provides recommendations on monitoring and comedication with ponatinib therapy. European guidelines on CVD prevention in clinical practice can be applied for CV risk assessment and management in CML and Ph+ ALL patients. For high- and very-high-risk patients, other therapeutic options should be considered before starting treatment with ponatinib. When alternative options are not available, treatment with ponatinib is indicated but requires a close collaboration between hematologists and cardiologists in the best interest of the patient.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
S.S.: advisory role for Novartis, Bristol-Myers Squibb, Pfizer, and Incyte; received research funding from Novartis, Bristol-Myers Squibb, and Incyte. F.L.: advisory role for Novartis, Ariad, Incyte, Celgene, Sanofi Aventis, and Bristol-Myers Squibb; receives support from the Frankfurter Förderung “Nachwuchswissenschaftler” and the EUTOS funding program; received funding from Novartis. S.K.: advisory board: Pfizer, Incyte, Ariad, Novartis, and Bristol-Myers Squibb; received honoraria from Pfizer, Incyte, Ariad, and Novartis; received research funding from Novartis, Pfizer, and Bristol-Myers Squibb and travel support from Pfizer, Incyte, Ariad, Novartis, and Bristol-Myers Squibb. W.H.: received honoraria for lectures from Ariad. A.K.: received honoraria for lectures and consulting services for Novartis, Bristol-Myers Squibb, and Pfizer. K.J.-U. and C.F.W.: no conflicts of interest to declare. F.S.: advisory board: Incyte, Novartis, and Pfizer; received speaker honoraria from Bristol-Myers Squibb, Novartis, and Pfizer and travel support from Bristol-Myers Squibb and Novartis. H.P.: advisory board: Incyte; received travel support and honoraria from Incyte, Amgen, Novartis, and Jazz Pharmaceuticals. P.R.: advisory board: Novartis, Bristol-Myers Squibb, Incyte, and Pfizer; received research funding and congress/travel support from Novartis and Bristol-Myers Squibb. N.G.: advisory board: Incyte and Novartis; received research support from Incyte and Novartis. C.R.: received honoraria for consulting services from AbbVie, Astellas, Basilea, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline Germany, Incyte, Janssen-Cilag, Pfizer, Merck, Novartis, Roche, Takeda, and Shire. G.-N.F.: received honoraria and research funding from Novartis and honoraria from Incyte and Pfizer. P.C.: received speaker honoraria from Incyte, Novartis, Bristol-Myers Squibb, and Pfizer. R.K.: received research grant from Ariad. C.J.: received financial support for research, honoraria for scientific advisory activity, and speaker honoraria from Incyte.
Funding Sources
Medical writing assistance was provided by Gerhard Emrich and funded by Incyte Corporation.
Author Contributions
All authors provided suggestions concerning the content and concept of the article and contributed to the writing and revision of all drafts, read and approved the final manuscript for submission. S.S., P.C., and S.K.: were primarily responsible for the CML part. F.L. and C.J.: for the Ph+ ALL part. S.K. and R.K. for the section on prevention and management of CV risk.
References
- 1.Iclusig® Summary of Product Characteristics dated September. 2018.
- 2.Deininger MW, Hodgson JG, Shah NP, Cortes JE, Kim DW, Nicolini FE, et al. Compound mutations in BCR-ABL1 are not major drivers of primary or secondary resistance to ponatinib in CP-CML patients. Blood. 2016 Feb;127((6)):703–12. doi: 10.1182/blood-2015-08-660977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA, Estrada J, Nicolini FE, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. 2014 Sep;26((3)):428–42. doi: 10.1016/j.ccr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soverini S, Bavaro L, Martelli M, De Benedittis C, Iurlo A, Orofino N, et al. Compound BCR-ABL1 Kinase Domain Mutants: Prevalence, Spectrum and Correlation with Tyrosine Kinase Inhibitor Resistance in a Prospective Series of Philadelphia Chromosome-Positive Leukemia Patients Analyzed By Next Generation Sequencing [ASH 2018, abstract 789] Blood. 2018;132(suppl 1):789. [Google Scholar]
- 5.Lipton JH, Bryden P, Sidhu MK, Huang H, McGarry LJ, Lustgarten S, et al. Comparative efficacy of tyrosine kinase inhibitor treatments in the third-line setting, for chronic-phase chronic myelogenous leukemia after failure of second-generation tyrosine kinase inhibitors. Leuk Res. 2015 Jan;39((1)):58–64. doi: 10.1016/j.leukres.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018 Jul;132((4)):393–404. doi: 10.1182/blood-2016-09-739086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury HJ, Kukreja M, Goldman JM, Wang T, Halter J, Arora M, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012 Jun;47((6)):810–6. doi: 10.1038/bmt.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolini FE, Basak GW, Kim DW, Olavarria E, Pinilla-Ibarz J, Apperley JF, et al. Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome-positive leukemias with the T315I mutation. Cancer. 2017 Aug;123((15)):2875–80. doi: 10.1002/cncr.30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breccia M, Efficace F, Iurlo A, Luciano L, Abruzzese E, Gozzini A, et al. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: the possible role of ponatinib. Expert Opin Drug Saf. 2018 Jun;17((6)):623–8. doi: 10.1080/14740338.2018.1480719. [DOI] [PubMed] [Google Scholar]
- 10.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. PACE Investigators A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013 Nov;369((19)):1783–96. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller M, Baccarani M, Deiniger MW, Guilhot F, Hochhaus A, Hughes TP, et al. Impact of early landmark responses with ponatinib on 4-yr outcomes in CP-CML patients (pts) in PACE, a pivotal phase II trial [ASCO 2017, abstract 7050] J Clin Oncol. 2017;35((15suppl)):7050. [Google Scholar]
- 12.le Coutre PD, Giles FJ, Hochhaus A, Apperley JF, Ossenkoppele GJ, Blakesley R, et al. Nilotinib in patients with Ph+ chronic myeloid leukemia in accelerated phase following imatinib resistance or intolerance: 24-month follow-up results. Leukemia. 2012 Jun;26((6)):1189–94. doi: 10.1038/leu.2011.323. [DOI] [PubMed] [Google Scholar]
- 13.Saglio G, Hochhaus A, Goh YT, Masszi T, Pasquini R, Maloisel F, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010 Aug;116((16)):3852–61. doi: 10.1002/cncr.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambacorti-Passerini C, Kantarjian HM, Kim DW, Khoury HJ, Turkina AG, Brümmendorf TH, et al. Long-term efficacy and safety of bosutinib in patients with advanced leukemia following resistance/intolerance to imatinib and other tyrosine kinase inhibitors. Am J Hematol. 2015 Sep;90((9)):755–68. doi: 10.1002/ajh.24034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luciano L, Specchia G, Martino B, Accurso V, Santoro M, Malato A, et al. A Real Life Evaluation of Efficacy and Safety of Ponatinib Therapy in CML Patients. (ASH 2017, abstract 2905] Blood. 2017;130(suppl 1):2905. [Google Scholar]
- 16.Heiblig M, Rea D, Chrétien ML, Charbonnier A, Rousselot P, Coiteux V, et al. Ponatinib evaluation and safety in real-life chronic myelogenous leukemia patients failing more than two tyrosine kinase inhibitors: the PEARL observational study. Exp Hematol. 2018 Nov;67:41–8. doi: 10.1016/j.exphem.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Mauro MJ, McGarry LJ, Yang M, Lustgarten S, Huang H. Real-World Ponatinib Prescribing Patterns and Outcomes in US Patients [ASH 2015, abstract 1591] Blood. 2017;126((23)):1591. [Google Scholar]
- 18.Devos T, Theunissen K, Benghiat FS, Gadisseur A, Meers S, Selleslag D, et al. Efficacy and Safety of Ponatinib in CML and Ph+ ALL Patients in Real-World Clinical Practice: data from a Belgian Registry [ASH 2018, abstract 1744] Blood. 2018;132(suppl 1):1744. [Google Scholar]
- 19.Shah NP, Kim DW, Kantarjian H, Rousselot P, Llacer PE, Enrico A, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010 Feb;95((2)):232–40. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz J, Larson RA, Gattermann N, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011 Jan;117((4)):1141–5. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortes JE, Kantarjian HM, Brümmendorf TH, Kim DW, Turkina AG, Shen ZX, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011 Oct;118((17)):4567–76. doi: 10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabbour E, Kantarjian H, Cortes J. Use of second- and third-generation tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia: an evolving treatment paradigm. Clin Lymphoma Myeloma Leuk. 2015 Jun;15((6)):323–34. doi: 10.1016/j.clml.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breccia M, Abruzzese E, Castagnetti F, Bonifacio M, Gangemi D, Sorà F, et al. Ponatinib as second-line treatment in chronic phase chronic myeloid leukemia patients in real-life practice. Ann Hematol. 2018 Sep;97((9)):1577–80. doi: 10.1007/s00277-018-3337-2. [DOI] [PubMed] [Google Scholar]
- 24.Iurlo A, Cattaneo D, Orofino N, Bucelli C, Molica M, Breccia M. Low-Dose Ponatinib in Intolerant Chronic Myeloid Leukemia Patients: A Safe and Effective Option. Clin Drug Investig. 2018 May;38((5)):475–6. doi: 10.1007/s40261-018-0623-7. [DOI] [PubMed] [Google Scholar]
- 25.Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Assouline S, et al. EPIC investigators Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2016 May;17((5)):612–21. doi: 10.1016/S1470-2045(16)00080-2. [DOI] [PubMed] [Google Scholar]
- 26.Soverini S, Bassan R, Lion T. Treatment and monitoring of Philadelphia chromosome-positive leukemia patients: recent advances and remaining challenges. J Hematol Oncol. 2019 Apr;12((1)):39–52. doi: 10.1186/s13045-019-0729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soverini S, De Benedittis C, Papayannidis C, Paolini S, Venturi C, Iacobucci I, et al. Drug resistance and BCR-ABL kinase domain mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia from the imatinib to the second-generation tyrosine kinase inhibitor era: the main changes are in the type of mutations, but not in the frequency of mutation involvement. Cancer. 2014 Apr;120((7)):1002–9. doi: 10.1002/cncr.28522. [DOI] [PubMed] [Google Scholar]
- 28.Soverini S, Colarossi S, Gnani A, Rosti G, Castagnetti F, Poerio A, et al. GIMEMA Working Party on Chronic Myeloid Leukemia Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006 Dec;12((24)):7374–9. doi: 10.1158/1078-0432.CCR-06-1516. [DOI] [PubMed] [Google Scholar]
- 29.Rousselot P, Coudé MM, Gokbuget N, Gambacorti Passerini C, Hayette S, Cayuela JM, et al. European Working Group on Adult ALL (EWALL) group Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016 Aug;128((6)):774–82. doi: 10.1182/blood-2016-02-700153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jabbour E, Short NJ, Ravandi F, Huang X, Daver N, DiNardo CD, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol. 2018 Dec;5((12)):e618–27. doi: 10.1016/S2352-3026(18)30176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki K, Jabbour EJ, Ravandi F, Short NJ, Thomas DA, Garcia-Manero G, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A propensity score analysis. Cancer. 2016 Dec;122((23)):3650–6. doi: 10.1002/cncr.30231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jabbour E, DerSarkissian M, Duh MS, McCormick N, Cheng WY, McGarry LJ, et al. Efficacy of Ponatinib Versus Earlier Generation Tyrosine Kinase Inhibitors for Front-line Treatment of Newly Diagnosed Philadelphia-positive Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2018 Apr;18((4)):257–65. doi: 10.1016/j.clml.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Ottmann OG, Pfeifer H, Cayuela JM, Spiekermann K, Jung W, Becket J, et al. Nilotinib and Low Intensity Chemotherapy for First-Line Treatment of Elderly Patients with BCRABL1-Positive Acute Lymphoblastic Leukemia: Final Results of a Prospective Multicenter Trial (EWALL-PH02) [ASH 2018, abstract 31] Blood. 2018;132(suppl 1):31. [Google Scholar]
- 34.Martinelli G, Piciocchi A, Papayannidis C, Paolini S, Robustelli V, Soverini S, et al. First report of the Gimema LAL1811 phase II prospective study of the combination of steroids with ponatinib as frontline therapy of elderly or unfit patients with Philadelphia chromosome-positive acute lymphoblastic leukemia [ASH 2017, abstract 99] Blood. 2017;130(suppl 1):99. [Google Scholar]
- 35.Chiaretti S, Vitale A, Elia L, Fedullo AL, Albino S, Piciocchi A, et al. Multicenter Total Therapy Gimema LAL 1509 Protocol for De Novo Adult Ph+ Acute Lymphoblastic Leukemia (ALL) Patients. Updated Results and Refined Genetic-Based Prognostic Stratification [ASH 2015, abstract 81] Blood. 2015;126((23)):81. [Google Scholar]
- 36.Martinelli G, Boissel N, Chevallier P, Ottmann O, Gökbuget N, Topp MS, et al. Complete Hematologic and Molecular Response in Adult Patients With Relapsed/Refractory Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia Following Treatment With Blinatumomab: Results From a Phase II, Single-Arm, Multicenter Study. J Clin Oncol. 2017 Jun;35((16)):1795–802. doi: 10.1200/JCO.2016.69.3531. [DOI] [PubMed] [Google Scholar]
- 37.Kantarjian HM, DeAngelo DJ, Stelljes M, Liedtke M, Stock W, Gökbuget N, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019 Jul;125((14)):2474–87. doi: 10.1002/cncr.32116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Assi R, Kantarjian HM, Short NJ, Daver N, Takahashi K, Garcia-Manero G, et al. Safety and Efficacy of Blinatumomab in Combination with a Tyrosine Kinase Inhibitor for the Treatment of Relapsed Philadelphia Chromosome-Positive Leukemia [ASH 2017 abstract 2598] Blood. 2017;130(suppl 1):2598. doi: 10.1016/j.clml.2017.08.101. [DOI] [PubMed] [Google Scholar]
- 39.Orphanos GS, Ioannidis GN, Ardavanis AG. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol. 2009;48((7)):964–70. doi: 10.1080/02841860903229124. [DOI] [PubMed] [Google Scholar]
- 40.Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogné JM. Association Between BCR-ABL Tyrosine Kinase Inhibitors for Chronic Myeloid Leukemia and Cardiovascular Events, Major Molecular Response, and Overall Survival: A Systematic Review and Meta-analysis. JAMA Oncol. 2016 May;2((5)):625–32. doi: 10.1001/jamaoncol.2015.5932. [DOI] [PubMed] [Google Scholar]
- 41.Aghel N, Delgado DH, Lipton JH. Cardiovascular events in chronic myeloid leukemia clinical trials. Is it time to reassess and report the events according to cardiology guidelines? Leukemia. 2018 Oct;32((10)):2095–104. doi: 10.1038/s41375-018-0247-1. [DOI] [PubMed] [Google Scholar]
- 42.Hadzijusufovic E, Albrecht-Schgoer K, Huber K, Hoermann G, Grebien F, Eisenwort G, et al. Nilotinib-induced vasculopathy: identification of vascular endothelial cells as a primary target site. Leukemia. 2017 Nov;31((11)):2388–97. doi: 10.1038/leu.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirchmair R, Theurl M, Lener D, Stanzl U, Gutmann C, Hadzijusufovic E, et al. Ponatinib Inhibits Endothelial Function, Induces Vasoconstriction and Effects Blood Flow Recovery in the Hindlimb Ischemia Model. AHA. 2016 poster presentation P1093. [Google Scholar]
- 44.Knickerbocker R, Dorer DJ, Haluska FG, Baccarani M, Cortes JE, Hochhaus A, et al. Impact of dose intensity of ponatinib on selected adverse events: multivariate analysis from a pooled population of clinical trial patients [ASH 2014, poster presentation P4546] Blood. 2014;124((21)):4546. doi: 10.1016/j.leukres.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Singh AP, Glennon MS, Umbarkar P, Gupte M, Galindo CL, Zhang Q, et al. Ponatinib-induced cardiotoxicity: delineating the signalling mechanisms and potential rescue strategies. Cardiovasc Res. 2019 Apr;115((5)):966–77. doi: 10.1093/cvr/cvz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valentin GG, Hernandez-Boluda JC, Osorio S, Correa JG, Sanchez-Guijo F, Gómez-Casares MT, et al. Safety and efficacy of ponatinib in real world clinical practice. Results from the Spanish Compassionate Use Program. A GELMC study. EHA 2016, abstract 2407. [Google Scholar]
- 47.Knoops L, Verhoef G, Berneman Z, Selleslag D, Straetmans N, Noens L, et al. Real-world data from 54 Belgian patients from the ponatinib named patient programme (NPP) EHA. 2016 abstract PB1822. [Google Scholar]
- 48.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May;127((20)):2391–405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 49.Lang F, Wunderle L, Pfeifer H, Schnittger S, Bug G, Ottmann OG. Dasatinib and Azacitidine Followed by Haploidentical Stem Cell Transplant for Chronic Myeloid Leukemia with Evolving Myelodysplasia: A Case Report and Review of Treatment Options. Am J Case Rep. 2017 Oct;18:1099–109. doi: 10.12659/AJCR.904956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dorer DJ, Knickerbocker RK, Baccarani M, Cortes JE, Hochhaus A, Talpaz M, et al. Impact of dose intensity of ponatinib on selected adverse events: multivariate analyses from a pooled population of clinical trial patients. Leuk Res. 2016 Sep;48:84–91. doi: 10.1016/j.leukres.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Khoury HJ, Cortes JE, Kim DW, Pinilla-Ibarz J, Le Coutre PD, Paquette R, et al. Analysis of the Cardiovascular Risk Profile of Ph+ Leukemia Patients Treated with Ponatinib [ASCO 2013, poster presentation & abstract 7048] J Clin Oncol. 2013;31((15suppl)):7048. [Google Scholar]
- 52.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. ESC Scientific Document Group 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2016 Aug;37((29)):2315–81. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiani A, Kuhlencordt P, Hochhaus A, Tesch H, Saussele S, Le Coutre P. Prävention und Management kardiovaskulärer Erkrankungen mit Nilotinib: empfehlungen einer interdisziplinären Expertengruppe. Onkologe. 2015;21((8)):724–31. [Google Scholar]
- 54.Breccia M, Pregno P, Spallarossa P, Arboscello E, Ciceri F, Giorgi M, et al. Identification, prevention and management of cardiovascular risk in chronic myeloid leukaemia patients candidate to ponatinib: an expert opinion. Ann Hematol. 2017 Apr;96((4)):549–58. doi: 10.1007/s00277-016-2820-x. [DOI] [PubMed] [Google Scholar]
- 55.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. ESC Scientific Document Group 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018 Sep;39((33)):3021–104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]