Abstract
Local glucocorticosteroid (“steroid”) therapy is widely used to treat the inner ears of patients with Meniere’s disease, idiopathic sudden sensorineural hearing loss and in combination with cochlear implants. Applied steroids have included dexamethasone, methylprednisolone and triamcinolone. In reality, however, this is often not true and the steroid forms commonly applied are dexamethasone-phosphate, methylprednisolone-hemisuccinate or triamcinolone-acetonide. In each case, the additional component is not a counter-ion but is covalently bound to the molecule to increase aqueous solubility or potency. These drug forms are approved for intravenous or intramuscular delivery and are used “off-label” in the ear. When given systemically, the molecular form of the drug is of minor importance as the drugs are rapidly metabolized. In contrast, when administered intratympanically, the exact form of the drug has a major influence on entry into perilymph and elimination from perilymph, which in turn influences distribution along the cochlear scalae. Dexamethasone-phosphate has completely different molecular properties to dexamethasone and has different pharmacokinetic properties entering and leaving perilymph. Molecular properties and perilymph pharmacokinetics also differ markedly for triamcinolone and triamcinolone-acetonide. Methylprednisolone-hemisuccinate has completely different molecular properties to methylprednisolone. In the ear, different steroid forms cannot therefore be regarded as equivalent in terms of pharmacokinetics or efficacy. This presents a terminology problem, where in many cases the drug stated in publications may not be the form actually administered. The lack of precision in nomenclature is a serious problem for the inner ear drug delivery field and needs to be recognized.
Keywords: intratympanic therapy, steroid, round window membrane, inner ear drug delivery
1. Applied Steroids
This analysis focuses on glucocorticosteroids (“steroids”) that have been used to treat disorders of the inner ear, primarily “dexamethasone”, “methylprednisolone” and “triamcinolone”. Each of these names refers to a specific compound, the free alcohol form of the drug, as indicated by the rows shaded gray in Table 1. Each has a unique “International Chemical Identifier” (InChI) key. Modified forms of the drugs (unshaded rows in Table 1) have higher formula weights and different InChI keys. Dexamethasone has been used intratympanically as it is a constituent of Otonomy’s OTO 104 and Otividex formulations which are both suspensions of powdered (micronized) drug having low aqueous solubility (~ 90 μg/mL (1). In more widespread clinical use is the more soluble form of the drug, dexamethasone-phosphate. Dexamethasone-phosphate is a different molecule with considerably higher formula weight. Dexamethasone-phosphate is a pro-drug and is inactive until metabolized to its active moiety, dexamethasone, by phosphatases which are ubiquitous in the body. While many physicians believe, or report in publications, they are administering dexamethasone intratympanically, they typically are not. In the vast majority of cases they are giving dexamethasone-phosphate. If the drug is given as a clear solution with a concentration above 90 μg/mL (commonly 4 to 24 mg/mL) the drug must be dexamethasone-phosphate as dexamethasone is not sufficiently soluble to reach such concentrations. The situation is exacerbated by misleading package labeling by some manufacturers, in which “dexamethasone” may be printed in larger or brightly-colored font while “sodium phosphate” is printed in a smaller, less-noticeable font. Some drug database websites have a similar problem where the title page may use the base form of the steroid and the actual form is stated elsewhere in small print.
Table 1:
Glucocorticoids applied intratympanically
| Drug | Example Brand Names | Formula Weight (g/mol) | InChI Key |
|---|---|---|---|
| Dexamethasone | Decadron (oral), Otividex (IT), OTO 104 (IT), OZURDEX (intravitreal) | 392.467 | UREBDLICKHMUKA-CXSFZGCWSA-N |
| Dexamethasone-phosphate | Decadron (IV), Fortecortin (IV) | 472.446 | VQODGRNSFPNSQE-CXSFZGCWSA-N |
| Methylprednisolone | Medrol, Urbason | 374.477 | VHRSUDSXCMQTMA-PJHHCJLFSA-N |
| Methylprednisolone-succinate | Solu-Medrol, Urbason-Solubile | 496.532 | FQISKWAFAHGMGT-SGJOWKDISA-M |
| Methylprednisolone-hemisuccinate | Solu-Medrol, Urbason-Solubile | 474.55 | IMBXEJJVJRTNOW-XYMSELFBSA-N |
| Triamcinolone | (No IV formulation available) | 394.439 | GFNANZIMVAIWHM-OBYCQNJPSA-N |
| Triamcinolone-acetonide | Kenalog, Volon A crystal suspension, Triamgalen (lotion/creme/ointment) | 434.504 | YNDXUCZADRHECN-JNQJZLCISA-N |
| Triamcinolone acetonide 21-dihydrogen phosphate | Volon A Solubile | 514.5 | NXLTXRVPGUCAHF-JNQJZLCISA-N |
| Prednisolone | Orapred, Decortin H | 360.45 | OIGNJSKKLXVSLS-VWUMJDOOSA-N |
| Prednisolone 21-hemisuccinate | Solu-Decortin H, | 482.5 | FKKAEMQFOIDZNY-CODXZCKSSA-M |
| Prednisolone-succinate | Prednisolut (IV) | 460.5 | APGDTXUMTIZLCJ-UHFFFAOYSA-N |
Different forms of steroids for four base forms (shaded gray). Modified forms of the drugs (unshaded) typically have higher formula weights than the base form. All have a unique InChI (International Chemical identifier). Data were obtained from the PubChem website (https://pubchem.ncbi.nlm.nih.gov).
The situation is similar, but more complex, for methylprednisolone, which is available in two forms that have higher solubility, methylprednisolone succinate and methylprednisolone hemisuccinate. Both forms are pro-drugs and inactive until metabolized to methylprednisolone. Metabolism is thought to occur predominantly by carboxylesterases (2). However, the base form of methylprednisolone is relatively soluble (~120 mg/mL) so it is not possible to distinguish the form applied based on the stated concentration.
The use of triamcinolone for systemic delivery or in creams and ointments has been largely replaced by a less-soluble form, triamcinolone-acetonide, which is preferred as it has higher potency. The original base form of triamcinolone is no longer available as a commercial product for intravenous or intramuscular injection in patients. The solubility of both compounds is low and both are delivered as suspensions.
2. Pharmacokinetics
Extensive work on drug passage through the gut and the blood-brain barrier brain has shown that the ability of drugs to pass through boundaries depends on specific molecular properties. Small, lipophilic molecules with few polar groups pass through membranous barriers more readily than larger, polar, hydrophilic molecules (3). The polar properties of any molecule can now be calculated using standardized algorithms on such websites as SwissADME (4). Figure 1 shows a “boiled egg plot” formed by plotting the calculated lipophilicity (WLOGP) against the topological polar surface area (TPSA) of the molecule. WLOGP is a calculated form of the logarithm of the partition coefficient of the molecule between octanol and water, LOG(P), while TPSA is the calculated area of the molecular surface occupied by polar groups (5). The ellipses on the plot show the statistical boundaries of molecular properties that allow passage through the blood-brain barrier (yellow ellipse) and passage through the gut (gray ellipse) (5). The blood-brain barrier is “tighter” than the gut, allowing entry of molecules with a narrower range of properties. The plot shows, for instance, that the calculated physical properties (lipophilicity and polarity) of dexamethasone and dexamethasone-phosphate are completely different, influencing their ability to pass through membranous boundaries. The polar phosphate group adds to the calculated polarity (TPSA) of the molecule, changing properties to a degree where dexamethasone-phosphate will pass less-readily though membranous boundaries. Pharmacokinetic studies in guinea pigs have shown that dexamethasone-phosphate is lost more slowly from perilymph than dexamethasone (6) and that dexamethasone-phosphate enters perilymph considerably more slowly from the middle ear than dexamethasone (7). The pharmacokinetic differences are consistent with the calculated properties of the two molecules. A number of studies have measured both forms of the drug in perilymph and have shown that dexamethasone-phosphate is metabolized to the active form, dexamethasone, within the ear (7, 8, 9).
Figure 1:
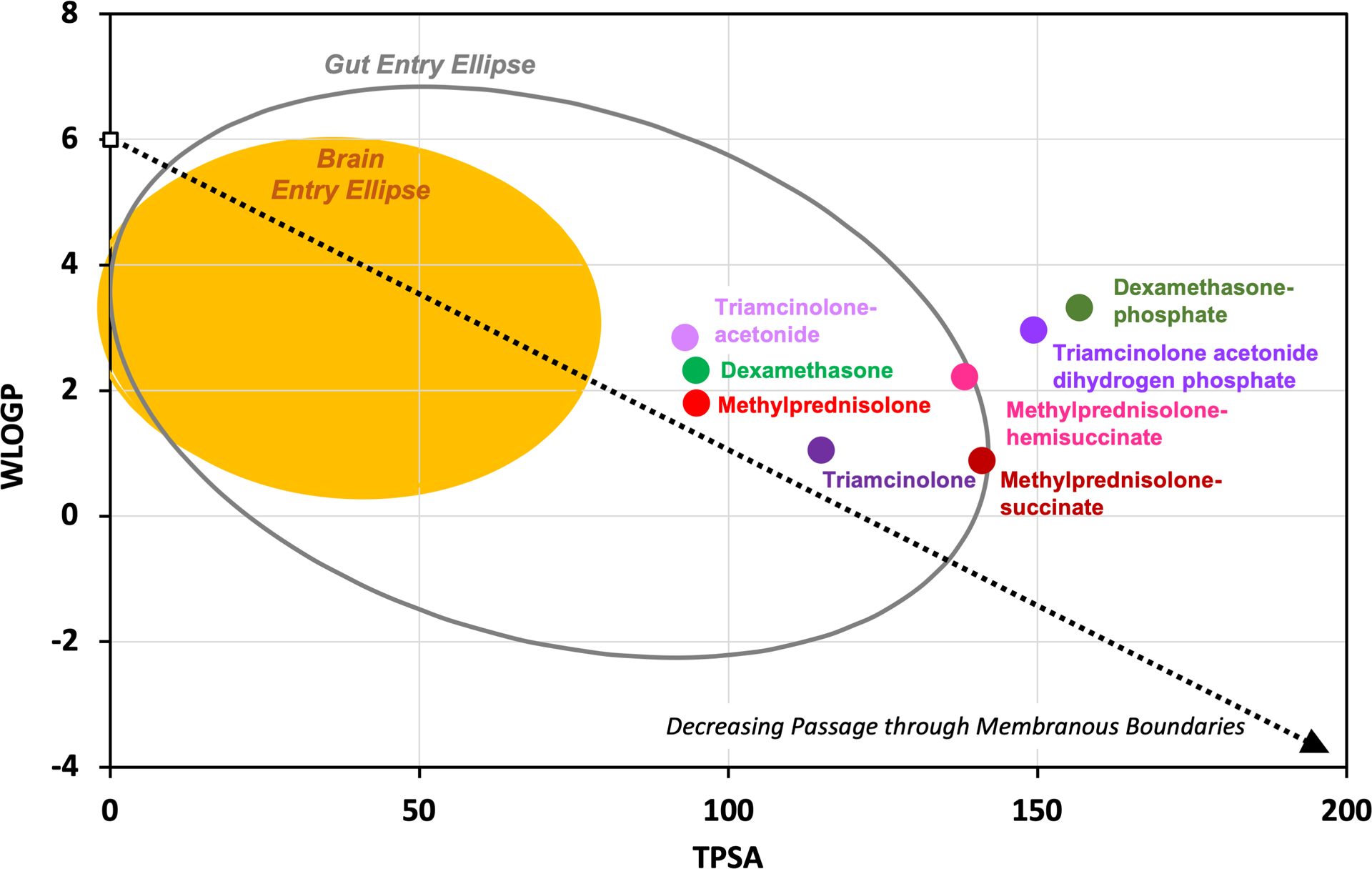
A “boiled egg plot”, adapted from Daina & Zoete (5), relating lipophilicity (WLOGP) to polar properties (TPSA) of molecules as calculated by the SwissADME website (4). Different forms of steroids have markedly different physical properties which influence their passage through membranous boundaries of the body. For intratympanic applications, these properties affect both entry into the ear and elimination from the ear to the vasculature, both of which involve passage through membranous cellular boundaries.
Triamcinolone-acetonide is less polar and more lipophilic molecule than triamcinolone, as shown in Figure 1. Pharmacokinetic studies in guinea pigs have found triamcinolone-acetonide to be rapidly eliminated from perilymph while triamcinolone had completely different characteristics and was retained well (10). The difference in pharmacokinetic properties is again consistent with the calculated molecular properties shown in Figure 1.
The pharmacokinetics of different forms of methylprednisolone in perilymph have not yet been compared experimentally. Further, it remains to be demonstrated whether the succinate esters of methylprednisolone are metabolized to the active form in the ear.
3. Published Studies with Steroids in the Ear
The examples cited here are not exhaustive but are presented as the “tip-of-the-iceberg” to illustrate the recently-identified nomenclature problem. The problem was unrecognized at the time of publication of the studies.
A recent survey of physician practices included a question concerning which steroid was preferred for intratympanic administration. Responses were presented as only the base forms of the steroids (dexamethasone, methylprednisolone, triamcinolone) but not the actual molecular form clinically applied (11, 12). A review of steroid use in Meniere’s disease also did not distinguish which forms of each steroid were used (13). Similarly, the steroid forms were not distinguished in meta-analyses of steroid use for ISSNHL (14, 15) and in a review of steroid use in combination with cochlear implants (16).
3.1. Dexamethasone
The problem in the literature is that some studies correctly report they have used dexamethasone while others report they have used dexamethasone when they actually used dexamethasone-phosphate. The two compounds are different substances, with different pharmacokinetic properties in the ear, and are not equivalent. This presents a major problem as the two substances are also unlikely to be equivalent in terms of patient outcome.
The documentation of which drug was used in publications comes in many forms.
Many studies report that dexamethasone was used when it is clearly incorrect. If the stated concentration exceeds the solubility limit for dexamethasone of about 90 μg/mL then dexamethasone cannot have been used. Instead, the study must have used dexamethasone-phosphate (17, 18, 19, 20).
Some studies correctly report the use of dexamethasone when they indeed used dexamethasone (21, 22, 23). Some state in the title that dexamethasone was used but in the abstract and the body of the paper confirm that dexamethasone-phosphate was used (8, 24). Some state in the title and/or body of the paper that dexamethasone was used but detail in the methods that dexamethasone-phosphate was used (25, 26).
Some appropriately state throughout the title and the body of the paper that dexamethasone-phosphate was used (27, 28).
3.2. Methylprednisolone
The high solubility of all three forms, methylprednisolone, methylprednisolone-succinate and methylprednisolone-hemisuccinate, makes it more difficult to ascertain which form of the drug was actually applied. If given systemically this would be relatively unimportant as methylprednisolone-succinate is rapidly metabolized (in rabbits) to methylprednisolone with a half time of about 10 min (29).
A number of studies report the use of intratympanic methylprednisolone (30, 31). The actual form applied in these studies carries a degree of uncertainty. Some reported the use of methylprednisolone when the drug brand used (SoluMedrol) indicated the applied drug was methylprednisolone-succinate (32). Others reported the use of methylprednisolone but detailed in the methods that methylprednisolone-succinate was applied (25, 33).
Of further concern is that the carboxylesterases responsible for metabolizing the inactive pro-drug methylprednisolone-succinate to the active form, methylprednisolone, are active in the liver and possibly in plasma (2). To date there are no studies showing carboxylesterases are active in the cochlea. The time course of metabolism of the succinate esters to active methylprednisolone in the ear remains unknown.
3.3. Triamcinolone
There are a number of reports of the use of local triamcinolone-acetonide in the ear (34, 35, 36). Some reported the use of triamcinolone where the actual use of triamcinolone-acetonide was detailed in the methods (26, 37). Others report the use of triamcinolone when the recency of the publication make it more likely triamcinolone-acetonide was applied (20, 38). This was confirmed by the authors for one of the studies (20).
4. Discussion
More than forty companies and numerous academic research groups are currently working on the development on targeted inner ear therapeutics. Many substances will soon be entering the clinic in the form of phase I or II trials. We now appreciate that the exact chemical composition of a substance used for local drug delivery to the inner ear is of enormous importance with respect to its pharmacokinetics, its pharmacodynamics and, thus, its efficacy in treating inner ear disorders. Minor changes in the drug molecule can make huge differences in the physical properties which influence pharmacokinetics, undoubtedly affecting the efficacy of the substance when applied to the ear. Published reports of steroid use in the ear have become almost “chaotic” as a result of widespread imprecise nomenclature. It is unacceptable for readers to be put in the position of guessing which drug was actually applied. Such determinations will be error-prone.
This situation has arisen because different steroid forms are of lesser importance when administered systemically, Nevertheless, with intratympanic and intracochlear applications the use of different forms of the same steroid critically influences pharmacokinetics and therefore will potentially affect the outcome for the patient. In none of the studies published to date has the possibility of different outcomes with different forms of the same steroid been considered or discussed. Adding further to the confusion, manufacturers have used the same brand names to represent multiple forms of a given steroid (Decadron, Urbason, Medrol, Volon, etc.). The naming of steroids reflects marketing decisions, where dexamethasone and triamcinolone have closely-related structures yet different names are used, while dexamethasone and dexamethasone-phosphate differ markedly in structure, yet both are described as dexamethasone. Nevertheless, it is becoming increasingly important to the intratympanic drug delivery field to use precise drug nomenclature for all substances applied. A similar situation has been observed for controlled release drug delivery systems for inner ear therapy. Mäder et al. (39) raised the issue that the reporting quality is low and it is often difficult to judge the results of published studies with drug delivery systems because important information related to the delivery systems is missing.
5. Recommendations
Publications should make it clear exactly which form of drug (the exact chemical composition) was used in the study. This should apply to any drug applied, not just steroids. There needs to be consensus on exactly how drugs should be specified in publications. Is giving details only in the methods section acceptable? Alternatively, should the full, complete drug name be used throughout the publication, including the title? Are abbreviations and acronyms acceptable?
Manufacturers need to label their product clearly with the specific chemical substance name, using uniform text size and color for the entire label.
Acknowledgements
This analysis was supported by the National Institutes on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health (NIH) under award number R01 DC001368 (A.N.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the German Ministry of Education and Research (BMBF) grant number 01KG1427 (S.K.P.).
Sources of Support: Supported by the National Institutes on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health (NIH) under award number R01 DC001368 (A.N.S.) and by Federal Ministry of Science and Research Germany (BMBF) grant number 01KG1427 (S.K.P.).
Disclosure of Funding NIH
Financial Disclosure: ANS is a paid consultant to Cochlear Corp., Sydney, Australia, Decibel Therapeutics Inc. Boston, USA and Otonomy, Inc., San Diego, USA. Stefan K. Plontke is a paid consultant to AudioCure Pharma GmbH, Berlin, Germany and Boehringer Ingelheim, Ingelheim, Germany.
References
- 1).PubChem. Available at https://pubchem.ncbi.nlm.nih.gov. Accessed August 7, 2019.
- 2).Furihata T, Hosokawa M, Fujii A, Derbel M, Satoh T, Chiba K. Dexamethasone-induced methylprednisolone hemisuccinate hydrolase: its identification as a member of the rat carboxylesterase 2 family and its unique existence in plasma. Biochem Pharmacol. 2005; 69:1287–1297. [DOI] [PubMed] [Google Scholar]
- 3).Egan WJ, Merz KM Jr, Baldwin JJ. Prediction of drug absorption using multivariate statistics. J Med Chem. 2000; 43:3867–7387. [DOI] [PubMed] [Google Scholar]
- 4).Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017; 7:42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Daina A, Zoete V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem. 2016; 11:1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Salt AN, Hartsock JJ, Gill RM, Piu F, Plontke SK. Perilymph pharmacokinetics of markers and dexamethasone applied and sampled at the lateral semi-circular canal. J Assoc Res Otolaryngol. 2012; 13: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Salt AN, Hartsock JJ, Piu F, Hou J. Dexamethasone and Dexamethasone Phosphate Entry into Perilymph Compared for Middle Ear Applications in Guinea Pigs. Audiol Neurootol. 2018; 23:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Hargunani CA, Kempton JB, DeGagne JM, Trune DR. Intratympanic injection of dexamethasone: time course of inner ear distribution and conversion to its active form. Otol Neurotol. 2006; 27:564–569. [DOI] [PubMed] [Google Scholar]
- 9).Plontke SK, Biegner T, Kammerer B, Delabar U, Salt AN. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol Neurotol. 2008; 29:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Salt AN, Hartsock JJ, Piu F, Hou J. Comparison of the pharmacokinetic properties of triamcinolone and dexamethasone for local therapy of the inner ear. Frontiers in Cellular Neuroscience 2019; 13, 347 https://www.frontiersin.org/article/10.3389/fncel.2019.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Sutton L, Schartinger V, Url C, Schmutzhard J, Lechner D, Kavasogullari C, Sandhu JS, Shaida A, Laszig R, Loehler J, Plontke S, Riechelmann H, Lechner M. Intratympanic steroid use for idiopathic sudden sensorineural hearing loss: current otolaryngology practice in Germany and Austria. Eur Arch Otorhinolaryngol. 2018; 275:1103–1110. [DOI] [PubMed] [Google Scholar]
- 12).Lechner M, Sutton L, Ferguson M, Abbas Y, Sandhu J, Shaida A. Intratympanic Steroid Use for Sudden Sensorineural Hearing Loss: Current Otolaryngology Practice. Ann Otol Rhinol Laryngol. 2019; 128:490–502. [DOI] [PubMed] [Google Scholar]
- 13).Patel M Intratympanic corticosteroids in Ménière’s disease: A mini-review. J Otol. 2017; 12:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Liebau A, Pogorzelski O, Salt AN Plontke SK Hearing changes after intratympanically applied steroids for primary therapy of sudden hearing loss: a meta-analysis using mathematical simulations of drug delivery protocols Otol Neurotol. 2017; 38:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Liebau A, Pogorzelski O, Salt AN, Plontke SK. Hearing Changes After Intratympanic Steroids for Secondary (Salvage) Therapy of Sudden Hearing Loss: A Meta-Analysis Using Mathematical Simulations of Drug Delivery Protocols. Otol Neurotol. 2018; 39:803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Plontke SK, Götze G, Rahne T, Liebau A Intracochlear drug delivery in combination with cochlear implants: Current aspects. HNO 2017; 65, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).McRackan TR, Best J, Pearce EC, Bennett ML, Dietrich M, Wanna GB, Haynes DS, Labadie RF. Intratympanic dexamethasone as a symptomatic treatment for Ménière’s disease. Otol Neurotol. 2014; 35:1638–40. [DOI] [PubMed] [Google Scholar]
- 18).Chu CH, Chiou SR, Wang MC, Shiao AS, Tu TY, Lin LY, Huang CY, Liao WH. The Efficacy of Concurrent or Sequential Intravenous and Intratympanic Steroid for Idiopathic Sudden Sensorineural Hearing Loss. Audiol Neurootol. 2018; 23:277–284. [DOI] [PubMed] [Google Scholar]
- 19).Wang Y, Han L, Diao T, Jing Y, Wang L, Zheng H, Ma X, Qi J, Yu L. A comparison of systemic and local dexamethasone administration: From perilymph/cochlea concentration to cochlear distribution. Hear Res. 2018; 370:1–10. [DOI] [PubMed] [Google Scholar]
- 20).Jumaily M, Faraji F, Mikulec AA. Intratympanic Triamcinolone and Dexamethasone in the Treatment of Ménière’s Syndrome. Otol Neurotol. 2017. March;38(3):386–391. [DOI] [PubMed] [Google Scholar]
- 21).Wang X, Dellamary L, Fernandez R, Harrop A, Keithley EM, Harris JP, Ye Q, Lichter J, LeBel C, Piu F. Dose-dependent sustained release of dexamethasone in inner ear cochlear fluids using a novel local delivery approach. Audiol Neurootol. 2009;14:393–401. [DOI] [PubMed] [Google Scholar]
- 22).Lambert PR, Nguyen S, Maxwell KS, Tucci DL, Lustig LR, Fletcher M, Bear M, Lebel C. A randomized, double-blind, placebo-controlled clinical study to assess safety and clinical activity of OTO-104 given as a single intratympanic injection in patients with unilateral Ménière’s disease. Otol Neurotol. 2012;33: 1257–1265. [DOI] [PubMed] [Google Scholar]
- 23).Fernandez R, Harrop-Jones A, Wang X, Dellamary L, LeBel C, Piu F. The Sustained-Exposure Dexamethasone Formulation OTO-104 Offers Effective Protection against Cisplatin-Induced Hearing Loss. Audiol Neurootol. 2016; 21:22–29. [DOI] [PubMed] [Google Scholar]
- 24).Plontke SK, Löwenheim H, Mertens J, Engel C, Meisner C, Weidner A, Zimmermann R, Preyer S, Koitschev A, Zenner HP. Randomized, double blind, placebo controlled trial on the safety and efficacy of continuous intratympanic dexamethasone delivered via a round window catheter for severe to profound sudden idiopathic sensorineural hearing loss after failure of systemic therapy. Laryngoscope. 2009; 119:359–369. [DOI] [PubMed] [Google Scholar]
- 25).Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope. 1999; 109:1–17. [DOI] [PubMed] [Google Scholar]
- 26).Braun S, Ye Q, Radeloff A, Kiefer J, Gstoettner W, Tillein J. Protection of inner ear function after cochlear implantation: compound action potential measurements after local application of glucocorticoids in the guinea pig cochlea. ORL J Otorhinolaryngol Relat Spec. 2011; 73:219–228. [DOI] [PubMed] [Google Scholar]
- 27).Bird PA, Murray DP, Zhang M, Begg EJ. Intratympanic versus intravenous delivery of dexamethasone and dexamethasone sodium phosphate to cochlear perilymph. Otol Neurotol. 2011; 32:933–936. [DOI] [PubMed] [Google Scholar]
- 28).Mamelle E, El Kechai N, Adenis V, Nguyen Y, Sterkers O, Agnely F, Bochot A, Edeline JM, Ferrary E. Assessment of the efficacy of a local steroid rescue treatment administered 2 days after a moderate noise-induced trauma in guinea pig. Acta Otolaryngol. 2018; 138:610–616. [DOI] [PubMed] [Google Scholar]
- 29).Ebling WF, Szefler SJ, Jusko WJ. Methylprednisolone disposition in rabbits. Analysis, prodrug conversion, reversible metabolism, and comparison with man. Drug Metab Dispos. 1985; 13:296–304. [PubMed] [Google Scholar]
- 30).Arslan N, Oguz H, Demirci M, et al. Combined intratympanic and systemic use of steroids for idiopathic sudden sensorineural hearing loss. Otol Neurotol 2011; 32:393–397. [DOI] [PubMed] [Google Scholar]
- 31).Dallan I, De Vito A, Fattori B, Casani AP, Panicucci E, Berrettini S et al. Intratympanic methylprednisolone in refractory sudden hearing loss: a 27 patient case-series with univariate and multivariate analysis. Otol Neurotol 2010;31:25–30. [DOI] [PubMed] [Google Scholar]
- 32).Herr BD, Marzo SJ. Intratympanic steroid perfusion for refractory sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2005. April;132(4):527–531. [DOI] [PubMed] [Google Scholar]
- 33).Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: A randomized trial. JAMA 2011; 305:2071–2079. [DOI] [PubMed] [Google Scholar]
- 34).Honeder C, Engleder E, Schöpper H, Gabor F, Reznicek G, Wagenblast J, Gstoettner W, Arnoldner C. Sustained release of triamcinolone acetonide from an intratympanically applied hydrogel designed for the delivery of high glucocorticoid doses. Audiol Neurootol. 2014; 19:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Engleder E, Honeder C, Klobasa J, Wirth M, Arnoldner C, Gabor F. Preclinical evaluation of thermoreversible triamcinolone acetonide hydrogels for drug delivery to the inner ear. Int J Pharm. 2014; 471:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Dahm V, Nieratschker M, Riss D, Kaider A, Auinger A, Honeder C, Arnoldner C. Intratympanic Triamcinolone Acetonide as Treatment Option for Idiopathic Sudden Sensorineural Hearing Loss. Otol Neurotol. 2019; 40:720–727. [DOI] [PubMed] [Google Scholar]
- 37).Ye Q, Tillein J, Hartmann R, Gstoettner W, Kiefer J. Application of a corticosteroid (Triamcinolon) protects inner ear function after surgical intervention. Ear Hear. 2007; 28:361–369. [DOI] [PubMed] [Google Scholar]
- 38).Loader B, Seemann R, Atteneder C, Sterrer E, Franz P, Lill C. Sealing of the round and oval window niches with triamcinolone-soaked fascia as salvage surgical therapy in sudden sensorineural hearing loss. Acta Otolaryngol. 2017; 137:923–927. [DOI] [PubMed] [Google Scholar]
- 39).Mäder K, Lehner E, Liebau A, Plontke SK. Controlled drug release to the inner ear: Concepts, materials, mechanisms, and performance. Hear Res. 2018; 368:49–66. [DOI] [PubMed] [Google Scholar]


