Abstract
Background:
The mechanisms responsible for the variable manifestations of chronic cutaneous graft-vs-host disease (cGVHD) are poorly understood. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury (isomorphic and isotopic responses), a recognized phenomenon in morphea, suggests a potential common pathway between cGVHD and other sclerotic skin conditions.
Observations:
Four cases of sclerotic-type cGVHD developed at the site of disparate skin injuries (ionizing radiotherapy, repeated needle sticks, central catheter site, and varicella-zoster virus infection). We review the spectrum of previously reported cases of sclerotic and nonsclerotic cGVHD relating to external forces on the skin.
Conclusions:
Localization of sclerotic-type cGVHD may occur after many types of skin injury, including UV and ionizing radiotherapy, needle sticks, viral infection, and pressure or friction. Recognition of this phenomenon may be helpful for the early diagnosis of sclerotic disease. Recent insights into the immunological consequences of minor skin injury may provide important clues to the underlying pathogenesis of cGVHD-mediated skin disease.
THE CUTANEOUS MANIFESTA-tions of chronic graft-vs-host disease (cGVHD) are polymorphic, ranging from epidermal disease mimicking lichen planus to sclerotic involvement of the dermis and deep connective tissue resembling morphea, systemic sclerosis, and eosinophilic fasciitis. Despite recent efforts to establish a single nomenclature of the diverse cutaneous manifestations of cGVHD,1 however, the mechanisms responsible for differing cutaneous presentations remain unknown. Nonetheless, similarities between cGVHD and well-described dermatologic diseases associated with dermal and subcutaneous fibrosis suggest that a common pathogenesis may be shared by these disorders.
Previously, we described the occurrence of sclerotic-type cGVHD at sites of skin friction (eg, waistband, brassiere line) in a large series of patients who underwent allogeneic hematopoietic cell transplantation (HCT),2 consistent with the isomorphic response of Koebner, a phenomenon that has been described in morphea3 and a number of other dermatologic conditions.4 Lichenoid-type cGVHD has also been reported at the site of previous varicella-zoster virus (VZV) infection,5–8 consistent with the isotopic phenomenon, a process first proposed by Wolf et al9 in 1995. However, evidence of the isotopic phenomenon associated with sclerotic-type cGVHD is not well described. Herein we report 4 additional cases that expand the spectrum of external factors associated with sclerotic-type cGVHD and suggest that the phenomenon of cGVHD at the site of skin injury may provide an important clue to the pathogenesis of cutaneous cGVHD.
REPORT OF CASES
CASE 1
A 34-year-old man with a history of acute myelogenous leukemia, treated with a matched allogeneic peripheral blood HCT, presented for evaluation of skin sclerosis approximately 3 years after HCT. Before HCT, chemotherapy was infused through a right subclavian venous catheter. This was complicated by cellulitis at the site of the catheter, and the line was removed. After HCT, he developed acute cutaneous GVHD that resolved with oral prednisone therapy.
On examination, a well-circumscribed 2×3-cm hypopigmented sclerotic plaque with a peripheral rim of pigmentation surrounded a small scar at the insertion site of the catheter on the lateral right side of the neck (Figure 1). Scattered indurated plaques with overlying hyperpigmentation were also present on the chest, arms, abdomen, flanks, and right ankle. A groove between fascial bundles was detected on the right forearm, as was a small sclerotic plaque above the right ankle. Biopsy findings of a plaque from the left abdomen demonstrated thickened collagen in the deep dermis and superficial subcutaneous tissue, consistent with sclerotic-type cGVHD.
Figure 1.
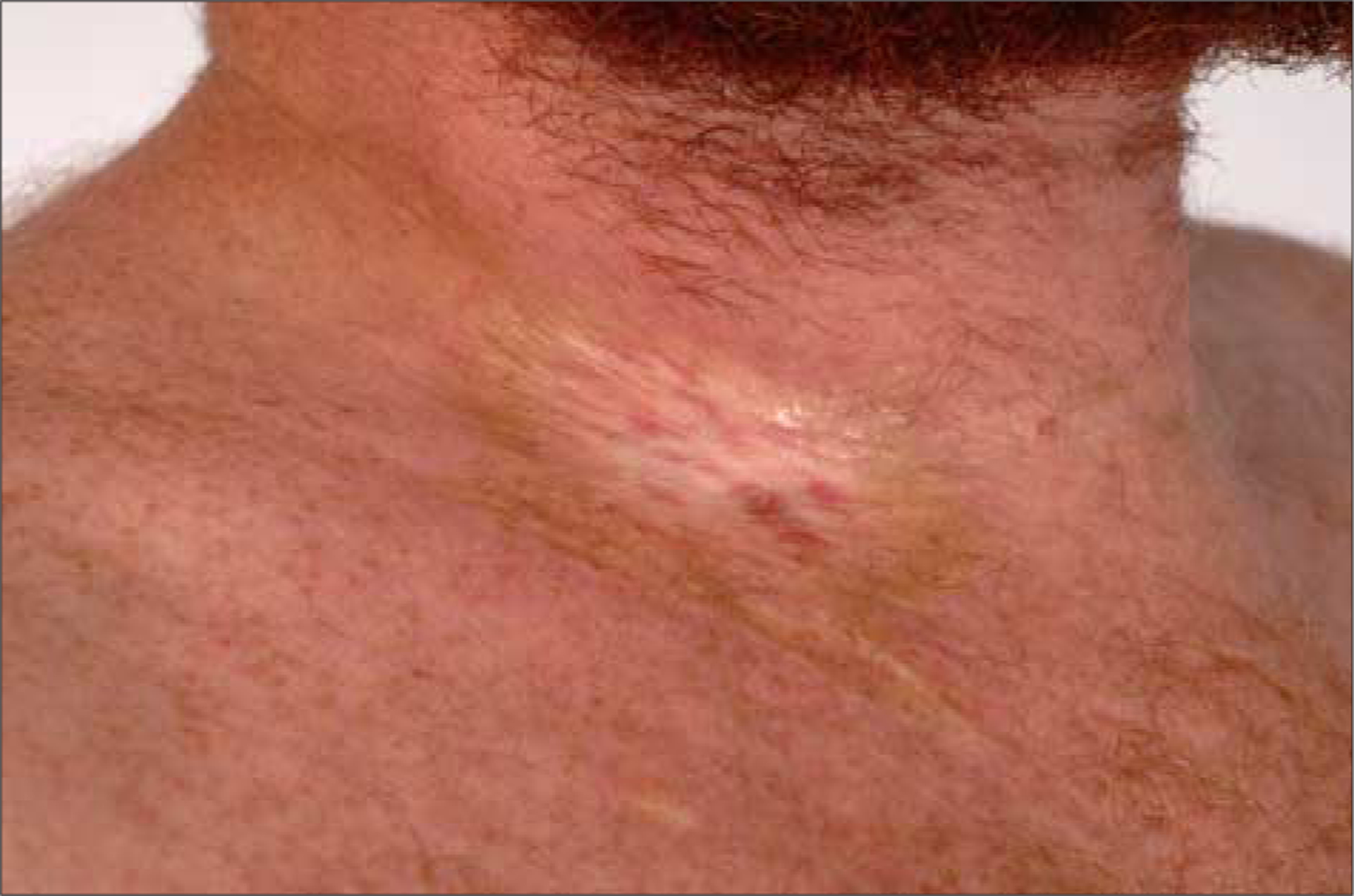
Sclerotic plaque localized to right side of the neck at the site of a previous subclavian venous catheter placement.
CASE 2
A 52-year-old man with a history of chronic myelogenous leukemia and a bone marrow HCT, followed 2 years later by treatment with imatinib mesylate for relapse, presented for evaluation of progressive skin thickening approximately 8 years after HCT. Skin tightening of the abdomen, arms, and legs had begun 2 years before presentation. He had a history of multiple peripheral blood draws, most frequently from the right antecubital fossa.
Examination revealed a raised sclerotic band of the right antecubital fossa, limiting full extension at the elbow (Figure 2). The skin surrounding the band was also sclerotic. The left antecubital fossa demonstrated similar hyperpigmentation and sclerosis, without the prominent raised band. There were several areas of hyperpigmentation on the chest, lichen planus–like cGVHD changes of the axillae and neck, and diffuse subcutaneous fibrosis of the arms, lower abdomen, and lower extremities bilaterally.
Figure 2.
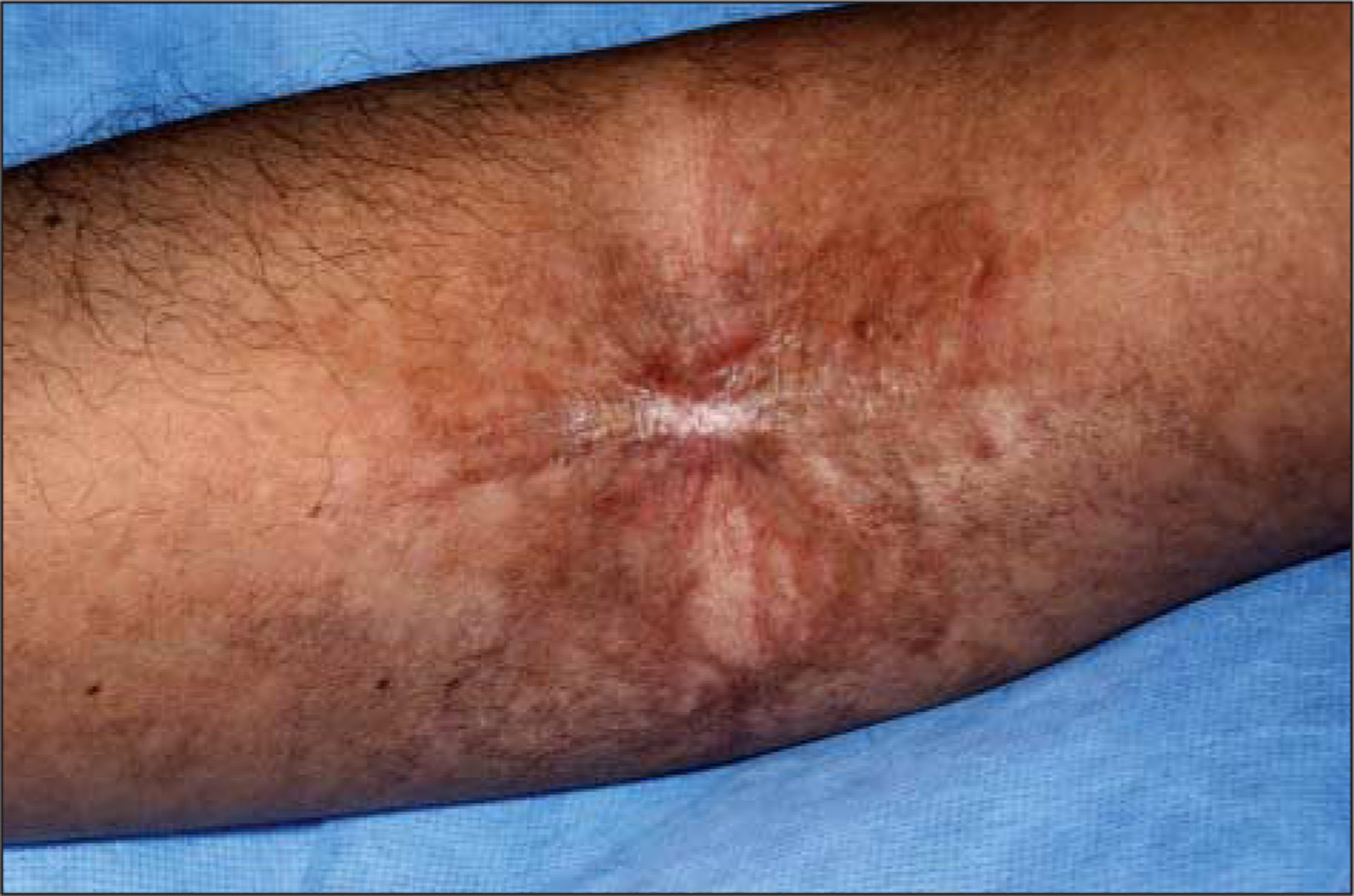
Morphealike chronic cutaneous graft-vs-host disease with a fibrous band at site of multiple previous needle sticks in the right antecubital fossa.
CASE 3
A 60-year-old man with a history of B-cell lymphoma treated by allogeneic peripheral blood HCT presented for evaluation of skin thickening. Eight months after HCT, the patient developed VZV reactivation of the left flank, corresponding to the T9-T10 dermatomes. He was treated with oral valacyclovir hydrochloride. After resolution, postherpetic neuralgia symptoms persisted. Eighteen months after HCT, the patient developed erythema and patchy hyperpigmentation of his chest and back. Skin fibrosis at the flanks and waistband was first reported 3 years after HCT.
At the time of examination 3½ years after HCT, multiple centrally hypopigmented sclerotic plaques of varying sizes with hyperpigmented borders corresponded to the distribution of previous VZV eruption (Figure 3). There was also diffuse firmness and rippling of the waistband area. Multiple superficial sclerotic plaques were noted on the patient’s upper back and midabdomen. Subcutaneous rippling of the skin was seen on both upper arms. Skin biopsy findings of the plaque on the right abdomen demonstrated hyperkeratosis with necrotic keratinocytes in the lower epidermis, as well as collagen thickening of the dermis and thickening of the fat septae consistent with epidermal involvement of cGVHD with underlying cGVHD-related skin fibrosis.
Figure 3.
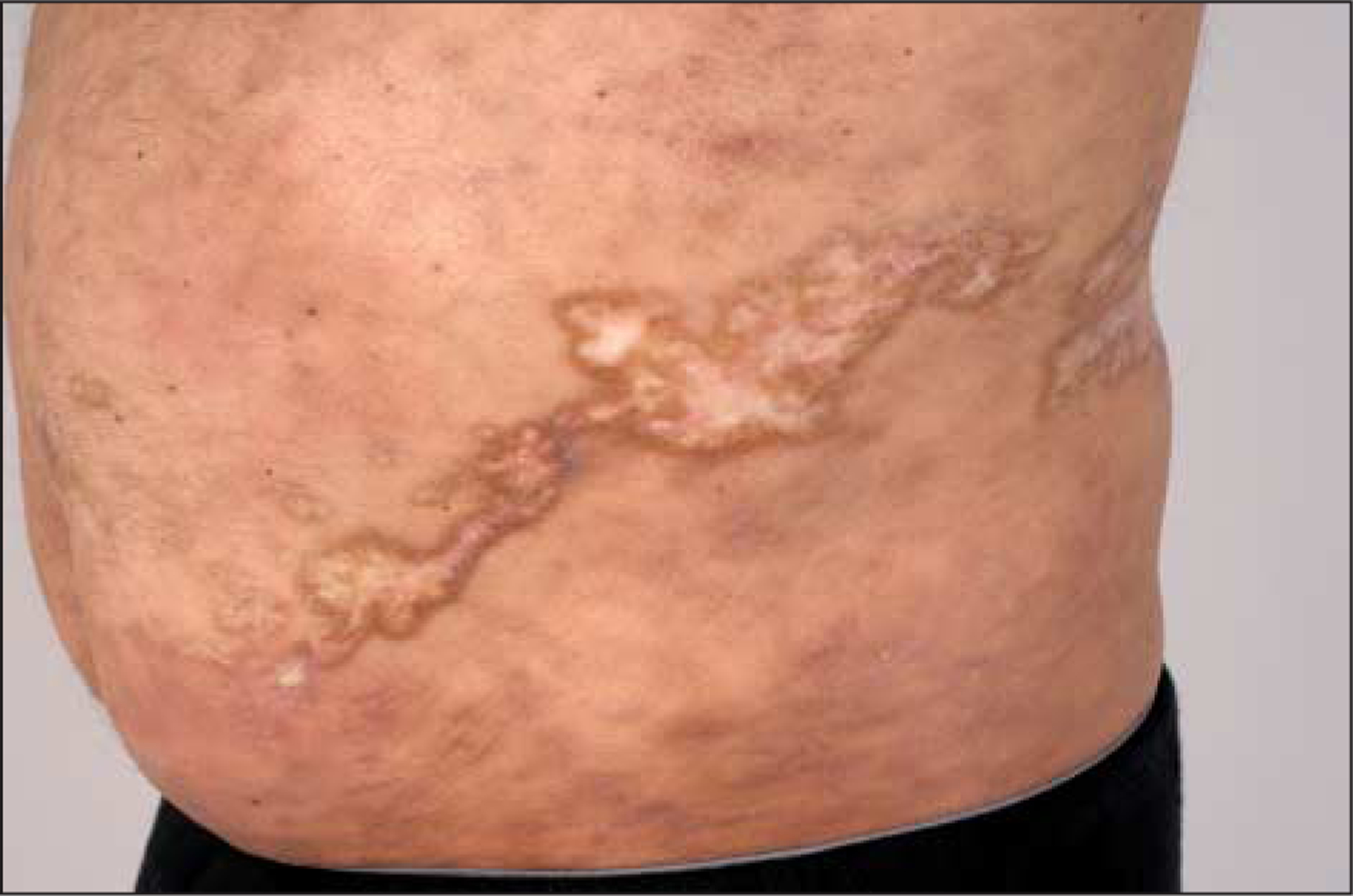
Localization of morphealike chronic cutaneous graft-vs-host disease to the distribution of a previous varicella-zoster virus infection.
CASE 4
A 54-year-old man with a history of B-cell lymphoma presented for evaluation of a large sclerotic plaque involving his left thigh. His lymphoma treatment had consisted of rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone (the R-CHOP regimen), followed by induction chemotherapy consisting of etoposide, vincristine, doxorubicin, cyclophosphamide, prednisone, and fludarabine phosphate before allogeneic peripheral blood HCT in October 2009. Four months before HCT, he received external beam radiotherapy consisting of 35 Gy in 15 fractions to the left thigh and 30 Gy in 10 fractions to the left humerus during the course of 1 month for palliation of bony metastasis. He began experiencing swelling and tightening of the left thigh area approximately 7 months after HCT.
At the time of examination 1 year after HCT, a large, well-circumscribed, hyperpigmented sclerotic plaque extended circumferentially from the left midthigh to 5 cm inferior to the knee, corresponding precisely to the field of previous radiotherapy for the femoral lesion, as determined by review of the patient’s radiation treatment records (Figure 4). The area was diffusely indurated without overlying surface textural changes or erythema. There was marked limitation in his range of motion at the left knee joint. There was no firmness or epidermal changes overlying the radiation treatment site of the left upper extremity. A biopsy specimen of the plaque on the left thigh demonstrated dermal and subcutaneous sclerosis, as well as necrotic keratinocytes in the basal epidermis, consistent with cGVHD. Findings suggestive of radiation fibrosis, including radiation fibroblasts, intimal thickening, or vessel dropout, were not observed. A magnetic resonance imaging study of the left thigh confirmed dermal thickening with subcutaneous edema.
Figure 4.
Isoradiotopic response in chronic cutaneous graft-vs-host disease presenting as a well-circumscribed plaque confined to the field of previous radiotherapy of the left distal femur.
COMMENT
The geographic distribution of skin lesions on the body is a diagnostic clue to many dermatologic conditions, such as psoriasis, lichen planus, and pityriasis rosea. By the same token, recognition of diseases that demonstrate the isomorphic and isotopic phenomena in response to skin injury may be an important diagnostic clue. The Koebner isomorphic response, the appearance of a skin lesion at a site of injury that is morphologically similar to an existing skin disease, has been associated with many diseases, most frequently psoriasis.4 Boyd and Neldner10 proposed that, for a disease to be considered isomorphic, it must be reproducible in all patients by a variety of insults (excluding external infective or allergic elements), and it must duplicate the pathogenesis of the disease as it occurs naturally. In cases 1 and 2, minor external skin injury (subclavian venous catheter insertion with cellulitis on the neck and repeated blood draws in the antecubital fossa, respectively) was a localizing factor for the development of sclerotic-type cGVHD.
In 1995, Wolf et al9 defined the term isotopic as the occurrence of a new, unrelated disease in the same location as a previously healed disease. The interval between the primary trauma and appearance of disease may be extremely variable, ranging from weeks to years, although most occur in the setting of active disease.11 Most commonly, VZV infection has been reported as the initial skin insult; however, additional reports have described isotopic responses after herpes simplex virus infection and thrombophlebitis.9,12 Although lichenoid-type cGVHD has been described at healed VZV sites,5–8 (and in a dermatomal distribution on previously unaffected skin),13,14 an isotopic presentation of sclerotic-type cGVHD occurring at the site of a previous VZV infection has not been frequently reported15 (Table). In case 3, sclerosis developed in the dermatomal distribution of previous VZV reactivation; however, the patient exhibited other sites of skin involvement, including prominent sclerosis in the area of the waistband, demonstrating overlap between isomorphic and isotopic disease. Proposed mechanisms of the isotopic phenomenon include residual hypersensitivity to viral or tissue antigens, collagen rearrangement due to scarring, and damage to cutaneous nerves despite the presence of clinically normal-appearing skin.9,11
Table.
Reported Cases of GVHD Localized to Sites of Injury
| Source | No. of Patients |
Sex/Age, y |
Primary Disease |
Type of Tx |
Type of Localized GVHD |
Antecedent Injury |
Latencya | Other Skin GVHD Involvement |
Other Organ GVHD Involvementb |
Reaction Type |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 1 | M/34 | AML | PBSC | Sc | Subclavian venous atheter cellulitis |
3 y | Yes | Yes | Isomorphic/ isotopic |
| Case 2 | 1 | M/52 | CML | BM | Sc | Repeated blood draws |
Unknown | Yes | Yes | Isomorphic |
| Case 3 | 1 | M/60 | NHL | PBSC | Sc | VZV | 2.5 y | Yes | Yes | Isotopic |
| Case 4 | 1 | M/54 | MHL | PBSC | Sc | External beam radiotherapy |
7 mo | No | No | Isoradiotopic |
| Patel et al,2 2008 | 11 | 5M; 6 F/20–57 |
MM (n=3), NHL(n=3), ALL(n=2), MDS(n = 1), CML(n = 1), CLL(n=1) |
PBSC (n=8), BM(n=3) | Sc | Waistband and brassiere band pressure/ friction | Variable | Yes (n=9), no(n=2) |
Yes(n=10), no (n=1) |
Isomorphic |
| Baselga et al,51996 | 1 | F/16 | MLD | BM | Non-Sc | 2 Episodes VZV (48 days apart) |
55 db | Yes | NR | Isotopic |
| Lacour et al,61999 | 1 | M/4 | ALL | BM | IMon-Sc | 2 Episodes VZV (2 months apart) |
6–9 mo (est)b |
NR | Yes | Isotopic |
| Cordoba et al,7 2000 | 1 | F/34 | ALL | BM | Mon-Sc | 2 Episodes VZV (8 days apart) |
1 mob | No | No | Isotopic |
| Sanli et al,8 2003 | 2 | F/23, 47 | CML | BM | Non-Sc | VZV | 3 mo | Yes | Yes(n=1), NR (n = 1) |
Isotopic |
| Socie et al,16 1989 | 4 | M/9–20 | AA | BM | Sc | Thoracoab-dominal radiotherapy | 5 mo to 3.5 yc |
No | Yes(n=1), NR(n=3) |
Isoradiotopic |
| Zwaan et al,171980 | 1 | M/35 | AA | BM | Non-Sc | Total nodal irradiation therapy | 1–2 mo (est) |
Yes | Yes | Isoradiotopic |
| Fenyk et al,181978 | 1 | F/14 | AA | BM | Sc | Measles exanthem | 2 mo | No | No | Isotopic |
| Vasallo et al,19 2009 | 4 | 2M; 2 F/5–57 | ALL (n = 1), Diamond-Blackfan anemia (n=1), MM(n=2) |
BM | Sc(n=1), Non-Sc (n=3) |
Narrow band UVB(n=1), sun exposure (n=3) | Immediate | Yes | Yes(n=1), NR(n=3) |
Isomorphic |
Abbreviations: AA, aplastic anemia; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; BM, bone marrow; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; est, estimated; GVHD, graft-vs-host disease; MDS, myelodysplastic syndrome; MLD, metachromatic leukodystrophy; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; Non-Sc, nonsclerotic (includes acute, erythematous, and lichenoid-types GVHD); NR, not reported; PBSC, peripheral blood stem cell; Sc, sclerotic (includes morphealike cGVHD, sclerodermalike cGVHD, and cGVHD-related fasciitis); Tx, transplant; VZV, varicella-zoster virus.
Indicates from injury to GVHD.
Indicates after first outbreak of VZV.
One patient in this series had 2 courses of transcatheter arterial infusion (TAI) before development of chronic GVHD due to recurrence of the underlying disease; the latency to chronic GVHD is reported as the time from first TAI conditioning.
In case 4, the occurrence of sclerotic-type cGVHD was entirely limited to the site of a radiation portal, a phenomenon that has been termed an isoradiotopic response. Other diseases, including acne, lichen planus, and pemphigus vulgaris, have similarly been described at sites of ionizing radiotherapy, conventional external beam radiotherapy, and UV radiation.4,20–30
In 1989, Socie et al15 described 4 patients with sclerotic-type cGVHD occurring within radiation portals. This phenomenon has been also observed with acute GVHD and lichenoid-type cGVHD.15,16 Although ionizing radiotherapy may lead to radiation fibrosis,31 the dose and fractionation of radiation given to this patient are unlikely to have led to fibrosis within 7 months.32 Furthermore, skin biopsy findings did not demonstrate radiation fibroblasts (atypical stellate cells with large nuclei containing clumped chromatin). Radiation-induced morphea may also be considered in the differential diagnosis for this case. It is characterized by focal dermal and subcutaneous sclerosis indistinguishable from morphea and may occur within weeks to months of radiotherapy exposure.33 However, radiation-induced morphea has been reported almost exclusively in the setting of radiotherapy for breast cancer,34,35 and the presence of necrotic keratinocytes in the biopsy specimen favors a diagnosis of GVHD rather than radiation-induced morphea.33 The other site of focal irradiation in this patient (left arm) did not develop sclerotic-type cGVHD despite receiving a comparable dose of radiation, suggesting an additional unknown localizing factor.
Although the present cases and those reported previously in the setting of GVHD demonstrate the challenge of differentiating isomorphic and isotopic phenomena based on their current definitions, the phenomena may offer avenues to explore underlying disease mechanisms. The isotopic mechanism may be related to dysregulation of the local immune response in an area of healing that renders it susceptible to the development of a second injury. This concept is similar to locus minoris resistentiae, that is, a localized area with diminished resistance to disease.36 The 2 events need not share the same mechanism; rather, the first disease predisposes the area to development of the second. Ruocco et al37 proposed that a vulnerable district of immune alteration occurs, resulting in a reduction (eg, tumor development) or induction (eg, autoimmune conditions) of effective immunity. Desbarats et al38 theorized that irradiation nonspecifically depletes local immune regulatory factors, allowing a permissive environment for the development of GVHD.
The propensity of sclerotic-type cGVHD to develop at sites of skin injury, a phenomenon also observed in morphea, may also reflect shared disease pathways between cGVHD and other skin conditions. Recent analyses in cutaneous systemic lupus and systemic sclerosis implicate type I interferon (IFN) as a central factor in the initiation and maintenance of inflammatory and fibrotic autoimmune processes.39,40 Immunohistochemical studies by our group and others suggest that type I IFN plays a similarly significant role in oral and cutaneous lichen planus–like cGVHD.41–43 Signaling by IFN can be activated by pathogen- and damage-associated molecular patterns through toll-like receptor molecules.44,45 Damage to skin produces an influx of plasmacytoid dendritic cells that produce type I IFN in response to toll-like receptor 7– and toll-like receptor 9–dependent recognition of nucleic acids from damaged cells; the inflammatory process is further exacerbated in hosts with ongoing autoimmune reactions.38,46,47 Similarly, viral infection can have systemic and local effects on IFN production. Cytomegalovirus infection is a well-recognized risk factor for the development of GVHD.48 Locally, VZV has been shown to produce a massive recruitment of plasmacytoid dendritic cells into affected skin.49 Therefore, if the overall levels of IFN production are elevated systemically owing to the ongoing inflammatory processes of cGVHD, as in systemic lupus erythematosus and systemic sclerosis, then local augmentation of the process may result in highly localized exacerbations of the inflammatory and fibrotic processes.
Much remains to be learned about the protean manifestations of cGVHD in the skin. The tendency of cGVHD to localize to areas of skin injury may prove a useful clinical clue to begin elucidating these intricacies. In addition, an awareness of isotopic and isomorphic presentations of cGVHD-mediated skin disease will allow for early disease detection in patients with sclerotic-type GVHD, prompting appropriate intervention before significant functional disability occurs.
Funding/Support:
This study was supported by the Intramural Research Program of the National Institutes of Health (NIH), Center for Cancer Research, National Cancer Institute, and by the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for the NIH from Pfizer Inc) (Ms Martires).
Contributor Information
Kathryn J. Martires, Dermatology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland..
Kristin Baird, Pediatric Oncology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland..
Deborah E. Citrin, Radiation Oncology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland..
Fran T. Hakim, Experimental Transplantation and Immunology Branches, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland..
Steven Z. Pavletic, Experimental Transplantation and Immunology Branches, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland..
Edward W. Cowen, Dermatology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland..
REFERENCES
- 1.Filipovich AH. Diagnosis and manifestations of chronic graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21(2):251–257. [DOI] [PubMed] [Google Scholar]
- 2.Patel AR, Pavletic SZ, Turner ML, Cowen EW. The isomorphic response in morphealike chronic graft-vs-host disease. Arch Dermatol. 2008;144(9):1229–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehara M, Oono T, Yamasaki O, Matsuura H, Iwatsuki K. Generalized morphealike lesions arising in mechanically-compressed areas by underclothes. Eur J Dermatol. 2006;16(3):307–309. [PubMed] [Google Scholar]
- 4.Rubin AI, Stiller MJ. A listing of skin conditions exhibiting the Koebner and pseudo-Koebner phenomena with eliciting stimuli. J Cutan Med Surg. 2002;6(1):29–34. [DOI] [PubMed] [Google Scholar]
- 5.Baselga E, Drolet BA, Segura AD, Leonardi CL, Esterly NB. Dermatomal lichenoid chronic graft-vs-host disease following varicella-zoster infection despite absence of viral genome. J Cutan Pathol. 1996;23(6):576–581. [DOI] [PubMed] [Google Scholar]
- 6.Lacour JP, Sirvent N, Monpoux F, et al. Dermatomal chronic cutaneous graft-versus-host disease at the site of prior herpes zoster. Br J Dermatol. 1999; 141(3):587–589. [DOI] [PubMed] [Google Scholar]
- 7.Córdoba S, Fraga J, Bartolomé B, García-Díez A, Fernández-Herrera J. Giant cell lichenoid dermatitis within herpes zoster scars in a bone marrow recipient. J Cutan Pathol. 2000;27(5):255–257. [DOI] [PubMed] [Google Scholar]
- 8.Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42(7):562–564. [DOI] [PubMed] [Google Scholar]
- 9.Wolf R, Brenner S, Ruocco V, Filioli FG. Isotopic response. Int J Dermatol. 1995; 34(5):341–348. [DOI] [PubMed] [Google Scholar]
- 10.Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 199;29(6):401–410. [DOI] [PubMed] [Google Scholar]
- 11.Ghorpade A Wolf’s isotopic response: lichen planus at the site of healed herpes zoster in an Indian woman. Int J Dermatol. 2010;49(2):234–235. [DOI] [PubMed] [Google Scholar]
- 12.Claudy AL, Chignol MC, Chardonnet Y. Detection of herpes simplex virus DNA in a cutaneous squamous cell carcinoma by in situ hybridization. Arch Dermatol Res. 1989;281(5):333–535. [DOI] [PubMed] [Google Scholar]
- 13.Freemer CS, Farmer ER, Corio RL, et al. Lichenoid chronic graft-vs-host disease occurring in a dermatomal distribution. Arch Dermatol. 1994;130(1):70–72. [PubMed] [Google Scholar]
- 14.Cohen PR, Hymes SR. Linear and dermatomal cutaneous graft-versus-host disease. South Med J. 1994;87(7):758–761. [DOI] [PubMed] [Google Scholar]
- 15.Hymes SR, Turner ML, Champlin RE, Couriel DR. Cutaneous manifestations of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2006;12(11):1101–1113. [DOI] [PubMed] [Google Scholar]
- 16.Socie G, Gluckman E, Cosset JM, et al. Unusual localization of cutaneous chronic graft-versus-host disease in the radiation fields in four cases. Bone Marrow Transplant. 1989;4(1):133–135. [PubMed] [Google Scholar]
- 17.Zwaan FE, Jansen J, Noordijk EM. Graft-versus-host disease limited to area of irradiated skin. Lancet. 1980;1(8177):1081–1082. [DOI] [PubMed] [Google Scholar]
- 18.Fenyk JR Jr, Smith CM, Warkentin PI, et al. Sclerodermatous graft-versus-host disease limited to an area of measles exanthem. Lancet. 1978;1(8062):472–473. [DOI] [PubMed] [Google Scholar]
- 19.Vassallo C, Brazzelli V, Zecca M, Locatelli F, Alessandrino PE, Borroni G. Isomorphic cutaneous graft-versus-host disease reaction after ultraviolet exposure: clinical, histological and direct immunofluorescence studies of four allotransplanted patients. J Eur Acad Dermatol Venereol. 2009;23(8):913–918. [DOI] [PubMed] [Google Scholar]
- 20.Shurman D, Reich HL, James WD. Lichen planus confined to a radiation field: the “isoradiotopic” response. J Am Acad Dermatol. 2004;50(3):482–483. [DOI] [PubMed] [Google Scholar]
- 21.Adriaans B, du Vivier A. Acne in an irradiated area. Arch Dermatol. 1989;125(7): 1005. [PubMed] [Google Scholar]
- 22.Crovato F, Desirello G, Nazzari G, De Marchi R. Linear pemphigus vulgaris after x-ray irradiation. Dermatologica. 1989;179(3):135–136. [DOI] [PubMed] [Google Scholar]
- 23.Kemmett D, Gawkrodger DJ, Mclaren KM, Hunter JA. Two atypical fibroxanthomas arising separately in X-irradiated skin. Clin Exp Dermatol. 1988;13(6): 382–384. [DOI] [PubMed] [Google Scholar]
- 24.Yamamura T, Takada A, Higashiyama M, Yoshikawa K. Subcutaneous leiomyosarcoma developing in a radiation dermatitis. Dermatologica. 1991;183(2):154–156. [DOI] [PubMed] [Google Scholar]
- 25.Del Guidice SM, Gerstley JK. Sunlight-induced radiation recall. Int J Dermatol. 1988;27(6):415–416. [DOI] [PubMed] [Google Scholar]
- 26.Wolf R, Tamir A, Weinberg M, Mitrani-Rosenbaum S, Brenner S. Eczema herpeticum induced by sun exposure. Int J Dermatol. 1992;31(4):298–299. [DOI] [PubMed] [Google Scholar]
- 27.Brodkin RH, Bleiberg J. Grenz rays and lichen planus: report of a case of isomorphic phenomenon following grenz ray therapy. Arch Dermatol. 1965;91:149–150. [DOI] [PubMed] [Google Scholar]
- 28.Yates VM, King CM, Dave VK. Lichen sclerosus et atrophicus following radiation therapy. Arch Dermatol. 1985;121(8):1044–1047. [PubMed] [Google Scholar]
- 29.Robbins AC, Lazarova Z, Janson MM, Fairley JA. Pemphigus vulgaris presenting in a radiation portal. J Am Acad Dermatol. 2007;56(5)(suppl):S82–S85. [DOI] [PubMed] [Google Scholar]
- 30.Shelley WB, Shelley ED, Campbell AC, Weigensberg IJ. Drug eruptions presenting at sites of prior radiation damage (sunlight and electron beam). J Am Acad Dermatol. 1984;11(1):53–57. [DOI] [PubMed] [Google Scholar]
- 31.Robinson DW. Surgical problems in the excision and repair of radiated tissue. Plast Reconstr Surg. 1975;55(1):41–49. [DOI] [PubMed] [Google Scholar]
- 32.De Santis M, Luzi S, Errico A, et al. Impact of dose and volume on subcutaneous fibrosis. Rays. 2005;30(2):169–173. [PubMed] [Google Scholar]
- 33.Morganroth PA, Dehoratius D, Curry H, Elenitsas R. Postirradiation morphea: a case report with a review of the literature and summary of the clinicopathologic differential diagnosis [published online May 24, 2010]. Am J Dermatopathol. 2010. doi: 10.1097/DAD.0b013e3181cb3fdd. [DOI] [PubMed] [Google Scholar]
- 34.Smith KJ, Yeager J, Skelton HG. Localized scleroderma in breast cancer patients treated with supervoltage external beam radiation: radiation port scleroderma. J Am Acad Dermatol. 1997;37(5, pt 1):806–808. [DOI] [PubMed] [Google Scholar]
- 35.Bleasel NR, Stapleton KM, Commens C, Ahern VA. Radiation-induced localized scleroderma in breast cancer patients. Australas J Dermatol. 1999;40(2):99–102. [DOI] [PubMed] [Google Scholar]
- 36.Jackson R The lines of Blaschko: a review and reconsideration: observations of the cause of certain unusual linear conditions of the skin. Br J Dermatol. 1976; 95(4):349–360. [DOI] [PubMed] [Google Scholar]
- 37.Ruocco V, Brunetti G, Puca RV, Ruocco E. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23(12):1364–1373. [DOI] [PubMed] [Google Scholar]
- 38.Desbarats J, Seemayer TA, Lapp WS. Irradiation of the skin and systemic graft-versus-host disease synergize to produce cutaneous lesions. Am J Pathol. 1994; 144(5):883–888. [PMC free article] [PubMed] [Google Scholar]
- 39.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25(3):383–392. [DOI] [PubMed] [Google Scholar]
- 40.Assassi S, Mayes MD, Arnett FC, et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. 2010;62(2):589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wenzel J, Tüting T. An IFN-associated cytotoxic cellular immune response against viral, self-, or tumor antigens is a common pathogenetic feature in “interface dermatitis”. J Invest Dermatol. 2008;128(10):2392–2402. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel J, Lucas S, Zahn S, et al. CXCR3 <-> ligand-mediated skin inflammation in cutaneous lichenoid graft-versus-host disease. J Am Acad Dermatol. 2008; 58(3):437–442. [DOI] [PubMed] [Google Scholar]
- 43.Imanguli MM, Swaim WD, League SC, Gress RE, Pavletic SZ, Hakim FT. Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood. 2009;113(15): 3620–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penack O, Holler E, van den Brink MRM. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood. 2010; 115(10):1865–1872. [DOI] [PubMed] [Google Scholar]
- 46.Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010; 207(13):2921–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guiducci C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207(13):2931–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pavletic SZ, Smith LM, Bishop MR, et al. Prognostic factors of chronic graft-versus-host disease after allogeneic blood stem-cell transplantation. Am J Hematol. 2005;78(4):265–274. [DOI] [PubMed] [Google Scholar]
- 49.Gerlini G, Mariotti G, Bianchi B, Pimpinelli N. Massive recruitment of type I interferon producing plasmacytoid dendritic cells in varicella skin lesions. J Invest Dermatol. 2006;126(2):507–509. [DOI] [PubMed] [Google Scholar]


