Abstract
Previous studies demonstrated that the pathophysiological changes after temporal lobe epilepsy (TLE) such as oxidative stress, inflammatory reaction contribute to cognitive defect and neuronal damage. The present study was conducted to evaluate the anticonvulsant effect of wogonin ameliorates kainate-induced TLE, and to investigate the mechanism underlying these effects. Rats were divided into control, wogonin, kainate, and wogonin-pretreated kainate groups. The rat model of TLE was induced by unilateral intrahippocampal injection of 0.4 ug/ul of kainate. The results showed that the cognitive function in TLE rats was significantly impaired, and wogonin treatment improved cognitive function in the Morris water maze (MWM). H & E staining and TUNEL staining showed obvious damage in the hippocampus of TLE rats, and wogonin alleviated the damage. To evaluate the oxidative stress, the expression of MDA and GSH in plasma were detected. Nrf-2 and HO-1 mRNA expression in the hippocampus were detected. The levels of MDA in plasma increased in TLE rats, and the levels of GSH in plasma and Nrf-2, HO-1 in the brain decreased. Treatment with wogonin alleviated these changes. We also detected the mRNA expression of inflammatory mediators like IL-1β, TNF-α, and NF kB in the brain. The inflammatory reaction was significantly activated in the brain of TLE rats, and wogonin alleviated neuroinflammation. We detected the mRNA expression of Bcl-2, Bax, caspase-3, in the hippocampus. The levels of Bcl-2 decreased in TLE rats, Bax and caspase-3 increased, while wogonin alleviated these changes. The present study indicated that wogonin exerted a noticeable neuroprotective effect in kainate-induced TLE rats.
Keywords: Kainate, Temporal lobe epilepsy, Wogonin, Neurodegeneration, Antioxidant, TUNEL
1. Introduction
Epilepsy is a common chronic neurological disorder affecting more than 68 million individuals worldwide. It is associated with both physical distress and psychological stress. Up to 50% of patients with epilepsy suffer from psychiatric or cognitive comorbidities. Moreover, the burden of comorbidities severely affects the quality of life (Ali et al., 2019). Temporal lobe epilepsy (TLE) is the most prevalent form of epilepsy, accounts for 60% of all epilepsy cases. It is originating primarily from the hippocampus and amygdala. Since the hippocampus is the main structure involved in learning and memory, damage to this structure often results in cognitive impairment (Ji et al., 2020, Wang et al., 2020b). Neuroimaging studies show that hippocampal atrophy, neuronal cell loss in the hippocampus, and decreased neuronal density in the dentate gyrus positively correlate with memory impairment in patients with TLE. Inhibition of hippocampal neuronal death and oxidative injury improves cognitive dysfunction in temporal lobe epilepsy in rats. Although damage to the hippocampal structures is a common cause of seizures and cognitive dysfunction, a growing body of literature suggests that the anesis of cognitive comorbidities of epilepsy is associated with neuroprotection but not with a concomitant decrease in seizure burden. Further, there are few targeted therapies for the management of cognitive comorbidities of epilepsy (Y. Li et al., 2020). Besides, our understanding of the mechanisms of the current drugs is not sufficient (Bajorat et al., 2018).
Intrahippocampal administration of kainic acid (KA) is particularly useful in studying the behavior and neuropathological characteristics of TLE. Activation of the KA receptor results in the release of excess glutamate caused neuronal death and sustained epileptic activity in the hippocampus (Bumanglag and Sloviter, 2018). Central KA injections result in a progressive increase in iron concentration in the rat hippocampus, leading to accumulation of a large number of lipid peroxides, accompanied by the depletion of glutathione (GSH), which could promote free radical damage in the lesioned areas (Wang et al., 2020a).
Scutellaria baicalensis is one of the most important medicinal herbs in traditional Chinese medicine. Wogonin, the primary chemical constituents of this herb, is flavone derivatives containing a phenylbenzopyrone nucleus. As part of the screening of conventional herbal extracts for benzodiazepine-like activity, it was reported that several flavonoids isolated from this herb exhibited moderate affinities for the GABA (γ-aminobutyric acid) A receptor (Afzal et al., 2012, Gupta et al., 2017, Hong et al., 2018). There is increasing evidence that wogonin could afford neuroprotection against oxidative stress-induced brain insult. Wogonin protects neurons from Ab-induced oxidative injury and preserves nigrostriatal dopaminergic neurons in a 6-hydroxydopamine hemiparkinsonian model (Zhang et al., 2018). This study was undertaken to investigate whether wogonin could attenuate hippocampal kainate-induced seizures, hippocampal neurodegeneration, inflammation, and cognitive defects.
2. Material and methods
2.1. Animals
All tests on adult Wistar male rats (200–220 g; n = 48) were carried out in The first affiliated hospital of Air Force Military Medical University, Shaanxi, China. They were housed three to four per cage in a temperature-controlled colony room under a light/dark cycle with food and water available ad libitum. Procedures involving animals were conducted in conformity with the National Institutes of Health guidelines for the care and use of laboratory animals. In this study, all efforts were made to minimize the number of animals used and their suffering.
2.2. Experimental procedure
Rats were divided into an equal-sized group: group 1-control, group 2-wogonin, group 3-kainite, and group 4- wogonin-treated kainate (wogonin + kainate) groups. For intrahippocampal injections, rats were anesthetized with chloral hydrate (350 mg/kg) and placed into the stereotaxic frame (Stoelting Co., Wood Dale, IL) with the incisor bar set at 3.3 mm below the interaural line. The dorsal surface of the skull was exposed, and a burr hole was drilled in the skull using the following stereotaxic coordinates according to the atlas of anteroposterior, 4.1 mm caudal to bregma; 4 mm lateral to the midline (right side), and 4–4.2 mm ventral to the surface of the skull. A 5 ml microsyringe filled with normal saline containing 0.4 ug/ul of kainate was placed over the burr hole, and kainate solution was injected at a rate of 1 ml/min to induce the experimental model of TLE. Kainic acid (kainate; Sigma-Aldrich, St. Louis, MO) was dissolved in normal cold saline just before surgery. The control group received an equivalent volume of normal saline at the same stereotaxic coordinates. The microsyringe was slowly withdrawn after 5 min, and the rat scalp was sutured. The wogonin group received wogonin (Sigma-Aldrich, St. Louis, MO) p.o. using a gavage needle at a dose of 100 mg/ kg/d starting one week before the surgery, and the last treatment was one h before surgery. Wogonin was dissolved in a 10% cremophor (Sigma-Aldrich, St. Louis, MO). The dose of wogonin was chosen according to previous reports on its antiepileptic activity (Hirsch et al., 1992, Lees, 1992).
The progression of kainate-induced seizures was scored according to Racine’s standard classification: stage 0, no reaction; stage 1, stereotype mounting, eye blinking, and/or mild facial clonus; stage 2, head nodding and/ or several facial clonus; stage 3, myoclonic jerks in the forelimbs; stage 4, clonic convulsions in the forelimbs with rearing; and stage 5, generalized clonic seizures associated with loss of balance. In the first 24 h post-surgery, all animals were evaluated for status epileptics. At 21 days of post-surgery, the animals were also assessed for the behavioral progression of kainate-induced seizures to record the chronic phases of seizures.
2.3. Assessment of cognitive function
The cognitive function of all rats was assessed by a Y-maze test. If a rat experienced a seizure before testing, they were tested at least one h after the seizure. If a rat experienced a seizure during the trial, the rat data were excluded at the time of analysis. On day 22, after intrahippocampal injection, the morris water-maze (MWM) test was performed as previously described. A circular water tank (diameter, 140 cm; depth, 60 cm) was fled with water (23 ± 1 °C) and made opaque by the addition of white, non-toxic paint (Dulux; AkzoNobel, Amsterdam, Netherlands). The rats were placed into the tank from different quadrants for 120 sec of training. Subsequently, the rats were tested for their ability to locate the hidden platform (10 × 10 cm) and given two trials/day. When the rat escaped onto the platform, the trial was terminated. Each test was started and terminated manually by the experimenter, and the experiment duration was five days (Van Nieuwenhuyse et al., 2015).
2.4. Measurement hippocampal lipid peroxidation and GSH
Rats were anesthetized with diethyl ether and decapitated. Hippocampi were isolated and blotted dry and then weighed and prepared as a 5% tissue homogenate in ice-cold 0.9% saline solution. After centrifugation (1000 g, 4C, 10 min), the supernatant was stored aliquots as at 70C until assayed. Concentrations of MDA, used as a marker of lipid peroxidation, were measured with the thiobarbituric acid (TBA) method spectrophotometric assay kit (Nanjing Jiancheng Bioengineering Institute, China) as previously described. Briefly, this assay was based on the spectrophotometric measurement of the color generated during the reaction to TBA with MDA. MDA concentrations were calculated by reading the absorbance of TBA reactive substances in the supernatant at 532 nm. GSH was determined by a GSH assay kit (Nanjing Jiancheng Bioengineering Institute, China) as commercially recommended by the manufacturer’s protocol. The absorbance of samples was read at 420 nm by spectrophotometer, and the values represented the final concentration according to the plotted standard curves (Ourdev et al., 2019).
2.5. Histopathological analysis
The brain tissues of rats were fixed with 4% paraformaldehyde (Shenggong, Shanghai, China), washed, dehydrated, transparentized, immersed in wax, and cut into slices. The dried sections were immersed in a dyeing vessel containing xylene I and xylene II for dewaxing. Parts were successively immersed in different concentrations of alcohol, double-distilled water to hydrate, stained in HE, dehydrated, and sealed. The prepared tissue sections were observed under an optical microscope and photographed using a microimaging system (Christiaen et al., 2020).
2.6. TUNEL staining
The brain tissues of rats were fixed with 4% paraformaldehyde (Shenggong, Shanghai, China), washed, dehydrated, transparentized, immersed in wax, and cut into slices. The dried sections were immersed in a dyeing vessel containing xylene I and xylene II for dewaxing. The sections were hydrated, digested with trypsin, and stained in the TUNEL mix. The sections were dehydrated, transparentized, and sealed with neutral gum. The CA1 region of the hippocampus was observed and analyzed using an optical microscope to calculate the apoptosis rate (Twele et al., 2017).
2.7. Real-time polymerase chain reaction (RT-PCR)
The total RNA of hippocampal brain tissue was extracted using the Trizol Reagent kit (Nanjing Jiancheng Bioengineering Institute, China). All procedures were performed under the guidance of the manufacturer’s instructions. cDNA of the mRNA of target genes was synthesized using the PrimeScriptTM reagent kit (Nanjing Jiancheng Bioengineering Institute, China). Primer Express software v3.0.1 was used to design the RT-PCR primers. SYBR Premix Ex Taq (Tli RNaseH Plus; TaKaRa) was used to perform RT-PCR in the Applied Biosystem 7300 (Applied Biosystems, Foster City, CA, USA), to detect the levels of b-actin and target genes. The 2-44Ct method was used to assess the levels of relative mRNA normalized to b-actin. The primer sequences were shown in Table 1 (Canas et al., 2018).
Table 1.
Primers for RT-PCR assay, 5′-3′.
| Primer | Sequence |
|---|---|
| Nrf2 | GGCGATCCTCCTGTAAACCC CCGAAGGATCCGTCTTCGGT |
| HO-1 | GAGCCTGGGGTTGCTAAGTT GTCACATTTATGCTCGGCGG |
| IL-1β | GGCCAGTGATGGTGGGTCAG TCAACAACGCCACCTTGTGTA |
| TNF-α | CACAGTGAAGTGCTGGCAAC ACATTGGGTCCCCCAGGATA |
| NF-kB | CCGCCCGTATAGTTGTCTCC TGGGAAGCTTGCTGTCATGT |
| Bcl-2 | GAACTGGGGGAGGATTGTGG CATCCCAGCCTCCGTTATCC |
| Bax | AAGATGGCATCACACCAGGG TTTGCTGTCTCCATGTCGCT |
| Caspase-3 | GGGGAGCTTGGAACGCTAAG CCGTACCAGAGCGAGATGAC |
| β-actin | CTACAACGAGCTGAGGGTGG TCCAGAGAGGAAGAGGAGGC |
2.8. Statistical analysis
The results were expressed as the mean value ± SD. All data analyses were performed using GraphPad Prism 7. The paired t-test was used to compare the differences between the two groups. The escape latency of the MWM was analyzed using repeated-measures ANOVA, with factors of strain and days. P < 0.05 was used to indicate significant differences. All experiments were repeated at least three times.
3. Results
Control and wogonin groups exhibited no signs of seizure behavior during the first 24 h or 21 days after the intrahippocampal injection of kainic acid. But, in the kainate group, 88.33%, rats showed the class 5 seizures (status epilepticus), and 58.33% of them had spontaneous seizures. The pretreatment of kainite rats with wogonin caused the scores of seizure activity to be lower as compared to the kainite group. So, 25 and 8.33% of pretreated rats showed signs of SE and spontaneous seizures, respectively (Table 2).
Table 2.
Numbers and rates of status epilepticus (SE) and spontaneous seizures (SS) in each group
| Group (n = 12) | Number (SE) | Rate(%) | Number (SS) | Rate(%) |
|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 |
| Wogonin | 0 | 0 | 0 | 0 |
| Kainate | 10 | 83.33 | 7 | 58.33 |
| Wogonin + Kainate | 3 | 25** | 1 | 8.33*** |
χ2 test, ** P < 0.01 and *** P < 0.001 compared to Kainate group.
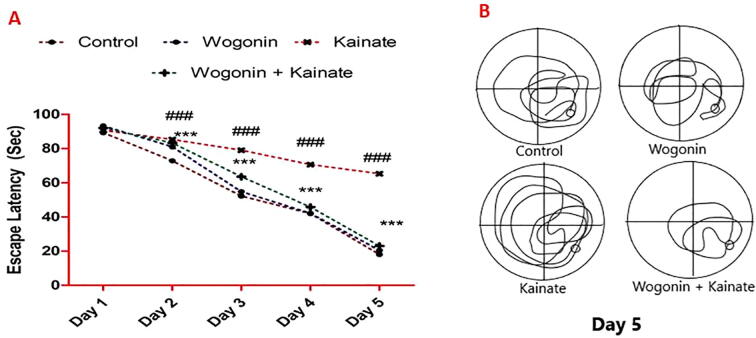
The induction of epilepsy using kainate significantly increased the escape latency in status epilepticus rats compared with the control rats. However, wogonin significantly decreased the escape latency compared with rats with kainate-induced epilepsy (P < 0.001; Fig. 1).
Fig. 1.
Anticonvulsant effects of wogonin prevent cognitive deficit in rats with kainate–induced epilepsy. (A) Escape latency. Where ### P < 0.001, vs. the control group; ***P < 0.001, vs. the kainate group. (B) Representative navigation path recording of the behavioral test.
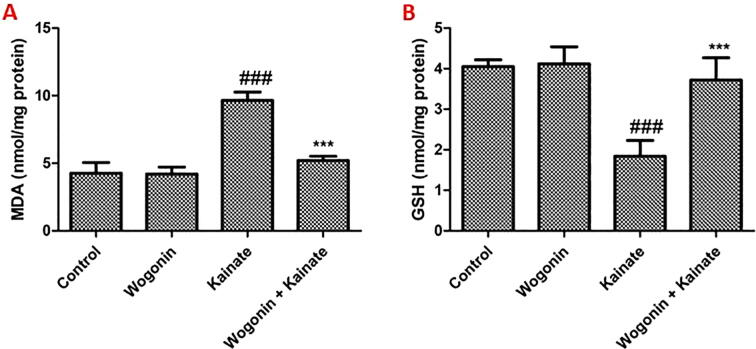
Compared to the control group, the levels of MDA in the hippocampus increased significantly in the kainate group (P < 0.001). Treatment with wogonin attenuated the increment of MDA in the hippocampus of kainate-treated rats significantly (P < 0.001) (Fig. 2A). While the levels of GSH in the hippocampus decreased substantially in the kainite group (P < 0.001). Treatment with wogonin attenuated the reduction of GSH in the hippocampus of kainate-treated rats significantly (P < 0.001) (Fig. 2B).
Fig. 2.
Wogonin reduces a MDA level and increases in GSH level in the hippocampus of kainite treated rats. Where ### P < 0.001, vs. the control group; ***P < 0.001, vs. the kainate group.
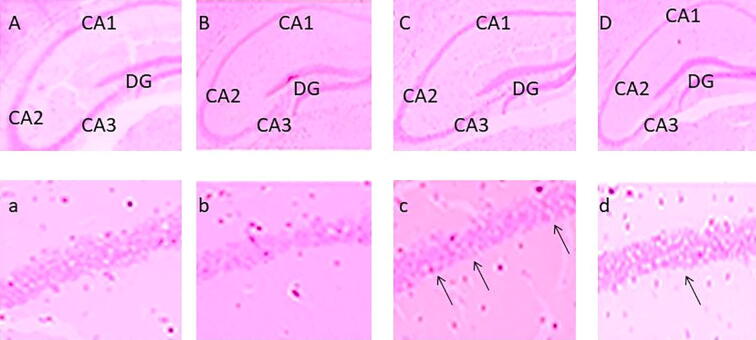
We utilized H&E staining to evaluate morphological changes in the rat hippocampus. Neurons in the hippocampus of the kainate group (Fig. 3C) were decreased, swollen, and loosely arranged compared to the control group, and karyopyknosis was observed in the CA1 region of the hippocampus in the kainate group. These morphological dysfunctions were ameliorated in the wogonin + kainite group (Fig. 3D) compared to the kainate group (Fig. 3).
Fig. 3.
A photomicrograph of rat hippocampus cornu ammonis areas CA1, CA2, CA3 and DG: (A, a) Control; (B, b) Wogonin; (C, c) Kainate; and (D, d) Wogonin + Kainate.
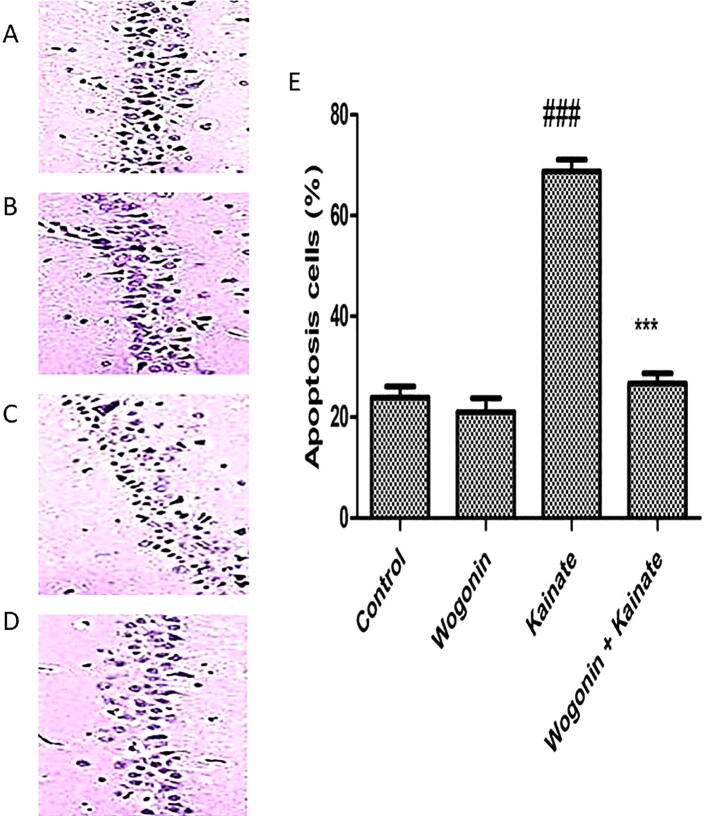
TUNEL staining showed that wogonin intervention ameliorated kainate-induced apoptosis of hippocampal neurons, and wogonin intervention ameliorated the apoptosis of neurons in the CA1 region of the hippocampus. As shown in Fig. 4, the apoptosis rate of neurons in hippocampus CA1 significantly increased in the kainate group compared to the control group. In contrast, wogonin reduced the apoptosis rate.
Fig. 4.
The apoptosis levels in the hippocampal CA1 region via TUNEL staining. (A) Control group. (B) Wogonin group. (C) Kainate group. (D) Wogonin + Kainate group. (E) The bar chart shows an apoptosis cells in the CA1 region in the hippocampus. Where ### P < 0.001, vs. the control group; ***P < 0.001, vs. the kainate group.
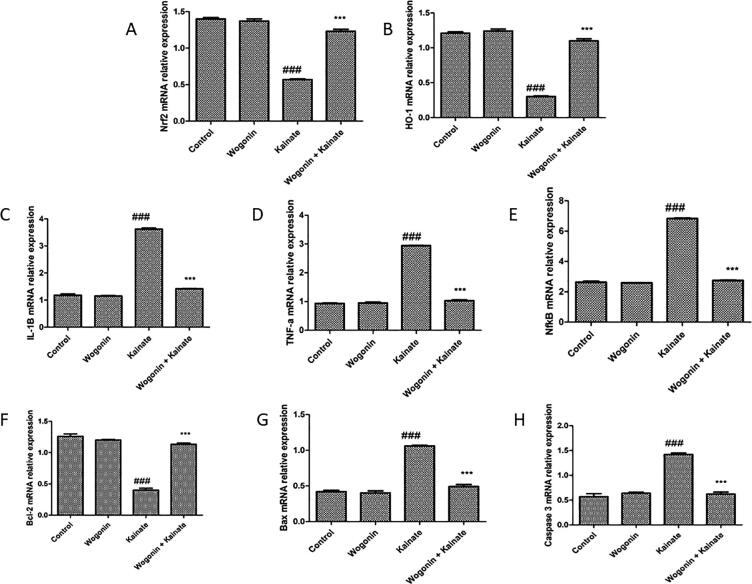
As per Fig. 5, compared to the control group, mRNA relative expression of antioxidant marker Nrf2, HO-1, and apoptotic marker bcl-2 were reduced (P < 0.001) in the kainate group of the rat. Whereas inflammatory marker IL-1β, TNF-α, and NF-kB, as well as apoptotic marker Bax and caspase 3 mRNA relative expression, were increased in the kainate group of animals as compared to the control group of rats. Treatment with wogonin in the kainate group of animals has attenuated the reduction of mRNA expression of Nrf2, HO-1, and bcl-2 (P < 0.001) and increment of mRNA expression of IL-1β, TNF-α, NF-kB, Bax and caspase 3 as compared to kainate group of animals.
Fig. 5.
Effect of wogonin on relative mRNA expression of oxidation, inflammation and apoptosis related genes in kainite induced epileptic rats. (A and B), Nrf2 and HO-1; (C, D and E), IL-1β, TNF-α and NF-kB; (F, G and H), Bcl-2, Bax and caspase 3. Where ### P < 0.001, vs. the control group; ***P < 0.001, vs. the kainate group.
4. Discussion
In rodents, the early pathophysiological damage of TLE involves severe inflammatory reactions in neurons, disturbed levels of oxidative stress, degeneration of neurons, and brain-blood barrier, contributing to eventual cognitive dysfunction. The longer epilepsy lasts, the higher the risk of neurological injury and cognitive impairment after TLE becomes. TLE's essential clinical approach is to terminate epileptic activity as early as possible through first and second-line treatment. Certain researchers focused on intravenous doses of propofol, midazolam, and other anesthetics as soon as possible to stop status epileptics rather than several second-line medications if the first-line therapy does not stop the seizure (Han et al., 2019, Sunkara et al., 2018, Tiwari et al., 2018).
Such medications have clear effects on seizure cessation but do not significantly alter pathophysiological improvements after epilepsy or prevent TLE growth. They aggravate the synaptic injury or cognitive disability. Therefore, novel therapeutic approaches to reduce pathophysiological threats in the post-TLE brain are critically required. We speculated that wogonin would have neuroprotection in rats with TLE based on anti-oxidation, anti-inflammation, and anti-apoptosis response. Long-term epileptic seizures ultimately impair TLE patients 'executive capacity and undermine their quality of life. Prior research indicated that kainate model TLE rats displayed cognitive deficiency relative to normal rats. TLE can cause long-term spatial memory deficits due to progressive TLE-induced hippocampal neuron loss. Our results confirmed these predictions (Gupta et al., 2014; T. R. Li et al., 2018). Interestingly, Wogonin therapy enhanced TLE rat learning and memory capacity. Increasing preclinical research over the past decade has shown wogonin's neuroprotective role in various neurological disorders.
Oxidative stress caused by prolonged free radicals is implicated in the pathogenesis of various neurodegenerative diseases. The association between oxidative stress and TLE was only recently recognized. Accruing data shows that oxidative stress is not only a result of the development of epilepsy but can also cause epilepsy. Antioxidants that reduce oxidative stress have recently attracted interest in epilepsy therapy (Zhu et al., 2018). Oxidative stress injury, however, has been seen in all epilepsy seizure models. The findings revealed an improvement in MDA levels in the present investigation and a decrease in GSH expression levels in rats with kainate-induced TLE. There is a close relationship between neuroglobin (NGB) and oxidative stress in the brain in terms of NGB ability to preserve cell survival of neuron and astrocytes, in vitro and in vivo, in the presence of high levels of ROS.
Additionally, findings indicated that wogonin therapy reversed these effects. Compared to these findings, wogonin suppresses oxidative stress and inflammation in collagen-induced arthritis in earlier reported studies. Some medicines have enhanced neuronal damage after epilepsy by improving the body's antioxidant function Nrf2/ARE pathway (Gupta et al., 2012, Kazmi et al., 2012). The level of plasma MDA increased in the TLE group relative to the control group in this study, the level of plasma GSH decreased, and the expression of Nrf-2 and Ho-1 in the hippocampus decreased. Wogonin, however, decreased these changes.
The central role of the inflammatory reaction in TLE pathogenesis was emphasized in research studies. Promoting inflammatory reactions develops TLE. The brain tissues of TLE patients suffering from surgical resections display substantial inflammatory responses including a reactive proliferation of astrocytes, invasion of active microglia and upregulation of specific variables in pro-inflammatory such as IL-6, IL-1b, and TNF-a (Das et al., 2017, Dua et al., 2018, Kodam et al., 2019). Inflammatory cells, including mononuclear and neutrophil granulocytes, are elevated in the brain, and inflammatory cells invade the periphery and may cause inflammatory brain reactions and exacerbate the local neural injury (Mehta et al., 2019, Samuel et al., 2019). Different studies with TLE also demonstrated strong, sustained forebrain neuroinflammatory responses, mainly caused by microglia, mononuclear infiltrated cells, and astrocytes. Cytokines and their receptors are the inflammatory mediators induced by epileptic seizures. Inflammatory factors, including IL-1b and TNF-a, are quickly triggered by large anterior brain neurons, including granulose dentate cells and pyramidal hippocampal cells (Singh et al., 2017, Twele et al., 2017). Such elevated pro-inflammatory factors, which may lead to neuronal death, are shown to intensify neuroglial cell proliferation, blood–brain injury, neuronal excitation, and seized strength.
In this phase, the activation of the NF-kB pathway has demonstrated an important role. In vitro studies and animal models are providing an increasing amount of indications that the suppression of the inflammatory response has a primary neuroprotective function and lowers epilepsy and mortality (Kumar Chellappan et al., 2017, Liu et al., 2019). Expression of mRNA IL-1b, TNF-α, NF-kB increased considerably in TLE rats in the present test, and wogonin decreased these levels. Several studies have shown that the application of wogonin inhibits the expression of inflammatory factors like IL-1b and TNF-a, and wogonin can suppress inflammatory response across many pathways. Our results show that wogonin reduces inflammatory reaction in kainate-induced TLE through the NF-kB pathway (Gupta et al., 2018, Hatware et al., 2018). Reduction in a level of TNF-a and IL-1b also related to the NGB. In earlier studies NGB protected a LPS induced inflammation in brain through reducing a TNF-a and IL-1b mRNA expression in brain. So our findings suggest that wogonin might be also increase an expression of NGB protein in brain which would be involve in an anti-inflammatory effect. Further study need to perform for finding a exact relation between wogonin and NGB.
Our study showed that neuronal apoptosis in the hippocampal CA1 region was evident in TLE rats using TUNEL staining, which is consistent with previous studies. TUNEL staining directly detects apoptotic cells, and the Bcl-2 protein family regulates apoptosis by controlling the release of mitochondrial apoptosis factors, cytochrome c, and apoptosis induction factors, which activate downstream executive reactions, including bax and caspase 3. Previous studies demonstrated that bax and caspase-3 were significantly activated in the brain of rats with epilepsy, cleaved caspase-3 significantly increased, the pro-apoptotic protein Bax increased, and the anti-apoptotic protein Bcl-2 decreased. Our results confirmed these changes, and wogonin reversed these changes.
5. Conclusion
In brief, the present study demonstrated that wogonin exerted significant neuroprotective effects on neurological damage after kainate-induced TLE. The data highlighted that wogonin afforded neuroprotection after a delayed post-injury intervention. Wogonin blocked the rapid onset of oxidative stress, inflammatory pathway, and neuronal death during TLE. Wogonin is likely to be a promising strategy to improve treatment efficacy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Afzal M., Gupta G., Kazmi I., Rahman M., Afzal O., Alam J.…Anwar F. Anti-inflammatory and analgesic potential of a novel steroidal derivative from Bryophyllum pinnatum. Fitoterapia. 2012;83(5):853–858. doi: 10.1016/j.fitote.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Ali I., Van Eetveldt A., Van Elzen R., Kalathil Raju T., Van Der Veken P., Lambeir A.M., Dedeurwaerdere S. Spatiotemporal expression and inhibition of prolyl oligopeptidase contradict its involvement in key pathologic mechanisms of kainic acid-induced temporal lobe epilepsy in rats. Epilepsia Open. 2019;4(1):92–101. doi: 10.1002/epi4.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajorat R., Porath K., Kuhn J., Gossla E., Goerss D., Sellmann T.…Kirschstein T. Oral administration of the casein kinase 2 inhibitor TBB leads to persistent KCa2.2 channel up-regulation in the epileptic CA1 area and cortex, but lacks anti-seizure efficacy in the pilocarpine epilepsy model. Epilepsy Res. 2018;147:42–50. doi: 10.1016/j.eplepsyres.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Bumanglag A.V., Sloviter R.S. No latency to dentate granule cell epileptogenesis in experimental temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2018;59(11):2019–2034. doi: 10.1111/epi.14580. [DOI] [PubMed] [Google Scholar]
- Canas P.M., Porciuncula L.O., Simoes A.P., Augusto E., Silva H.B., Machado N.J.…Cunha R.A. Neuronal adenosine A2A receptors are critical mediators of neurodegeneration triggered by convulsions. eNeuro. 2018:5(6). doi: 10.1523/eneuro.0385-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaen E., Goossens M.-G., Descamps B., Larsen L.E., Boon P., Raedt R., Vanhove C. Dynamic functional connectivity and graph theory metrics in a rat model of temporal lobe epilepsy reveal a preference for brain states with a lower functional connectivity, segregation and integration. Neurobiol. Disease. 2020;139 doi: 10.1016/j.nbd.2020.104808. [DOI] [PubMed] [Google Scholar]
- Das P., Gupta G., Velu V., Awasthi R., Dua K., Malipeddi H. Formation of struvite urinary stones and approaches towards the inhibition—A review. Biomed. Pharmacother. 2017;96:361–370. doi: 10.1016/j.biopha.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Dua K., Rapalli V.K., Shukla S.D., Singhvi G., Shastri M.D., Chellappan D.K., Pinto T.D.J.A. Multi-drug resistant Mycobacterium tuberculosis & oxidative stress complexity: Emerging need for novel drug delivery approaches. Biomed. Pharmacother. 2018;107:1218–1229. doi: 10.1016/j.biopha.2018.08.101. [DOI] [PubMed] [Google Scholar]
- Gupta, G., Chellappan, D.K., Kikuchi, I.S., Pinto, T.d.J.A., Pabreja, K., Agrawal, M., . . . Dua, K., 2017. Nephrotoxicity in rats exposed to paracetamol: the protective role of moralbosteroid, a steroidal glycoside. J. Environ. Pathology, Toxicol. Oncol., 36(2). [DOI] [PubMed]
- Gupta G., Kazmi I., Afzal M., Rahman M., Saleem S., Ashraf M.S., Mujeeb M. Sedative, antiepileptic and antipsychotic effects of Viscum album L. (Loranthaceae) in mice and rats. J. Ethnopharmacol. 2012;141(3):810–816. doi: 10.1016/j.jep.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Gupta G., Singhvi G., Chellappan D.K., Sharma S., Mishra A., Dahiya R., Dua K. Peroxisome proliferator-activated receptor gamma: promising target in glioblastoma. Panminerva Med. 2018;60(3):109–116. doi: 10.23736/S0031-0808.18.03462-6. [DOI] [PubMed] [Google Scholar]
- Gupta G., Verma R., David S.R., Chellappan D.K., Anwar F., Dua K. Hepatoprotective activity of moralbosteroid, a steroidal glycoside isolated from Morus alba. Oriental Pharmacy Exp. Med. 2014;14(3):285–289. [Google Scholar]
- Han C.L., Zhao X.M., Liu Y.P., Wang K.L., Chen N., Hu W.…Meng F.G. Gene expression profiling of two epilepsy models reveals the ECM/integrin signaling pathway is involved in epiletogenesis. Neuroscience. 2019;396:187–199. doi: 10.1016/j.neuroscience.2018.10.021. [DOI] [PubMed] [Google Scholar]
- Hatware K.V., Sharma S., Patil K., Shete M., Karri S., Gupta G. Evidence for gastroprotective, anti-inflammatory and antioxidant potential of methanolic extract of Cordia dichotoma leaves on indomethacin and stress induced gastric lesions in Wistar rats. Biomed. Pharmacother. 2018;103:317–325. doi: 10.1016/j.biopha.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Hirsch E., Snead O.C., Gomez I., Baram T.Z., Vergnes M. Section of the corpus callosum in kainic acid induced seizures in rats: behavioral, electroencephalographic and neuropathological study. Epilepsy Res. 1992;11(3):173–182. doi: 10.1016/0920-1211(92)90096-C. [DOI] [PubMed] [Google Scholar]
- Hong K.B., Han S.H., Park Y., Suh H.J., Choi H.S. Romaine lettuce/skullcap mixture improves sleep behavior in vertebrate models. Biol. Pharm. Bull. 2018;41(8):1269–1276. doi: 10.1248/bpb.b18-00267. [DOI] [PubMed] [Google Scholar]
- Ji G., Sun R., Hu H., Xu F., Yu X., Priya Veeraraghavan V.…Chi X. Vannilic acid ameliorates hyperglycemia-induced oxidative stress and inflammation in streptozotocin-induced diabetic rats. J. King Saud Univ. – Sci. 2020 doi: 10.1016/j.jksus.2020.04.010. [DOI] [Google Scholar]
- Kazmi I., Afzal M., Rahman M., Gupta G., Anwar F. Aphrodisiac properties of Polygonatum verticillatum leaf extract. Asian Pacific J. Tropical Dis. 2012;2:S841–S845. [Google Scholar]
- Kodam A., Ourdev D., Maulik M., Hariharakrishnan J., Banerjee M., Wang Y., Kar S. A role for astrocyte-derived amyloid beta peptides in the degeneration of neurons in an animal model of temporal lobe epilepsy. Brain Pathol. 2019;29(1):28–44. doi: 10.1111/bpa.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Chellappan D., Yenese Y., Chian Wei C., Gupta G. Nanotechnology and diabetic wound healing: a review. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) 2017;17(2):87–95. doi: 10.2174/1871530317666170421121202. [DOI] [PubMed] [Google Scholar]
- Lees G.J. Effects of anaesthetics, anticonvulsants and glutamate antagonists on kainic acid-induced local and distal neuronal loss. J. Neurol. Sci. 1992;108(2):221–228. doi: 10.1016/0022-510X(92)90055-P. [DOI] [PubMed] [Google Scholar]
- Li T.R., Jia Y.J., Ma C., Qiu W.Y., Wang Q., Shao X.Q., Lv R.J. The role of the microRNA-146a/complement factor H/interleukin-1beta-mediated inflammatory loop circuit in the perpetuate inflammation of chronic temporal lobe epilepsy. Dis. Model Mech. 2018;11(3) doi: 10.1242/dmm.031708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Clark C., Abdulazeeme H.M., Salehisahlabadi A., Rahmani J., Zhang Y. The effect of Brazil nuts on selenium levels, Glutathione peroxidase, and thyroid hormones: A systematic review and meta-analysis of randomized controlled trials. J. King Saud Univ. – Sci. 2020;32(3):1845–1852. doi: 10.1016/j.jksus.2020.01.019. [DOI] [Google Scholar]
- Liu X., Sharma R.K., Mishra A., Chinnaboina G.K., Gupta G., Singh M. Role of aqueous extract of the wood ear mushroom, Auricularia polytricha (Agaricomycetes), in avoidance of haloperidol-lnduced catalepsy via oxidative stress in rats. Int. J. Med. Mushrooms. 2019;21(4) doi: 10.1615/IntJMedMushrooms.2019030351. [DOI] [PubMed] [Google Scholar]
- Mehta M., Sharma N., Vyas M., Khurana N., Maurya P.K., Singh H., Gupta G. Chemico-Biological Interactions; 2019. Interactions with the macrophages: An emerging targeted approach using novel drug delivery systems in respiratory diseases. [DOI] [PubMed] [Google Scholar]
- Ourdev D., Schmaus A., Kar S. Kainate receptor activation enhances amyloidogenic processing of APP in astrocytes. Mol. Neurobiol. 2019;56(7):5095–5110. doi: 10.1007/s12035-018-1427-8. [DOI] [PubMed] [Google Scholar]
- Samuel V.P., Dahiya R., Singh Y., Gupta G., Sah S.K., Gubbiyappa S.K., Dua K. Metformin: a salutary candidate for colorectal cancer treatment in patients with diabetes. J. Environ. Pathol. Toxicol. Oncol. 2019;38(2) doi: 10.1615/JEnvironPatholToxicolOncol.2019029388. [DOI] [PubMed] [Google Scholar]
- Singh Y., Gupta G., Shrivastava B., Dahiya R., Tiwari J., Ashwathanarayana M., Dua K. Calcitonin gene-related peptide (CGRP): A novel target for Alzheimer's disease. CNS Neurosci. Ther. 2017;23(6):457–461. doi: 10.1111/cns.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara K.P., Gupta G., Hansbro P.M., Dua K., Bebawy M. Functional relevance of SATB1 in immune regulation and tumorigenesis. Biomed. Pharmacother. 2018;104:87–93. doi: 10.1016/j.biopha.2018.05.045. [DOI] [PubMed] [Google Scholar]
- Tiwari, J., Gupta, G., de Jesus Andreoli Pinto, T., Sharma, R., Pabreja, K., Matta, Y., . . . Dua, K., 2018. Role of microRNAs (miRNAs) in the pathophysiology of diabetes mellitus. Panminerva Medica. [DOI] [PubMed]
- Twele F., Schidlitzki A., Tollner K., Loscher W. The intrahippocampal kainate mouse model of mesial temporal lobe epilepsy: Lack of electrographic seizure-like events in sham controls. Epilepsia Open. 2017;2(2):180–187. doi: 10.1002/epi4.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nieuwenhuyse B., Raedt R., Sprengers M., Dauwe I., Gadeyne S., Carrette E., Vonck K. The systemic kainic acid rat model of temporal lobe epilepsy: Long-term EEG monitoring. Brain Res. 2015;1627:1–11. doi: 10.1016/j.brainres.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Wang R., Younis E.M., Priya Veeraraghavan V., Tian C. Antiurolithiatic effect of Fucoxanthin on ethylene glycol-induced renal calculus in experimental rats. J. King Saud Univ. – Sci. 2020;32(3):1896–1901. doi: 10.1016/j.jksus.2020.01.027. [DOI] [Google Scholar]
- Wang Y., Cui S., Zheng J., Li Y., Li P., Hou H. Berberine ameliorates intestinal mucosal barrier dysfunction in nonalcoholic fatty liver disease (NAFLD) rats. J. King Saud Univ. – Sci. 2020 doi: 10.1016/j.jksus.2020.03.019. [DOI] [Google Scholar]
- Zhang R., Guo L., Ji Z., Li X., Zhang C., Ma Z., Ma S. Radix scutellariae attenuates CUMS-induced depressive-like behavior by promoting neurogenesis via cAMP/PKA pathway. Neurochem. Res. 2018;43(11):2111–2120. doi: 10.1007/s11064-018-2635-3. [DOI] [PubMed] [Google Scholar]
- Zhu G., Meng D., Chen Y., Du T., Liu Y., Liu D.…Zhang J. Anterior nucleus of thalamus stimulation inhibited abnormal mossy fiber sprouting in kainic acid-induced epileptic rats. Brain Res. 2018;1701:28–35. doi: 10.1016/j.brainres.2018.07.014. [DOI] [PubMed] [Google Scholar]