Abstract
Introduction
Epicardial exit sites of ventricular tachycardia (VT) are frequently encountered during VT ablation requiring an epicardial ablation approach for successful elimination of VT. We sought to assess the utility of repolarization markers in identifying individuals requiring an epicardial ablation approach in addition to an endocardial approach.
Methods
32 patients who underwent successful ablation for scar mediated VT were included in the study. Fourteen patients who required a combined endocardial and epicardial VT ablation were defined as epicardial VT group (Epi) whereas 18 patients who were successfully ablated from the endocardium alone constituted the endocardial VT group (Endo). Repolarization markers during sinus rhythm were compared between the two groups.
Results
A higher QTc max and QTc dispersion were seen in the Epi group compared to Endo group (479 ± 34 vs 449 ± 20, p = 0.008 and 63 ± 13 vs 38 ± 8, p = 0.001, respectively). Ts-p and Ts-p/Tp-e were higher in the Epi group (166 ± 23 vs 143 ± 23, p = 0.008 and 1.55 ± 0.26 vs 1.3 ± 0.21, p < 0.005). On multivariate regression, QTc dispersion was an independent predictor of the need for an epicardial approach to ablation. A QTc dispersion more than 51.5 msec identified individuals requiring a combined epicardial and endocardial approach to ablation with a sensitivity of 92.9% and a specificity of 100%.
Conclusions
Patients requiring an epicardial ablation have a higher QTc dispersion. A value greater than 51.5 msec reliably differentiates between the two groups with high sensitivity and specificity.
Keywords: Ventricular tachycardia, Epicardial, QTc dispersion
1. Introduction
A percutaneous epicardial approach is frequently employed to successfully ablate ventricular tachycardias (VT) with a re-entrant circuit in the epicardium. While relatively safe in the hands of experienced operators, identifying the need for an epicardial approach pre-procedurally helps improve procedural preparedness which significantly improves outcomes.
Several criteria utilizing depolarization events on the electrocardiographic (ECG) during ventricular tachycardia (VT) have been proposed to differentiate between VTs of epicardial (Epi) and endocardial origin (Endo) including Q waves in leads that reflect local ventricular activation, delayed intrinsicoid deflection and pseudo-delta waves. However, the usefulness of these criteria in patients with scar mediated VT is suboptimal [1,2]. Further, merging of the T wave with the QRS during rapid rates seen during VT limit the utilization of most of these criteria which are based on ECG during VT. Also delayed depolarization due to slow conduction through scar and the effect of sodium channel blocking drugs, may increase false positivity of plausible epicardial exit with currently available criteria in scar mediated VT. Repolarization parameters are increasingly being used to identify risk of arrhythmias and sudden cardiac death in the general population as well as in individuals with structural heart disease. Corrected QT (QTc) interval, QTc dispersion, interval from peak of T wave to end of T wave (Tp-e), Tp-e/QTc ratio and interval from onset of T wave to peak of T wave (Ts-p) during sinus rhythm are some of the electrocardiographic ventricular repolarization (VRP) markers that have been used [[3], [4], [5]]. These parameters have also been shown to be useful for risk stratification in patients with Chagas’ Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) and NICM which often present an epicardial substrate for ventricular arrhythmias [6].
In this study, we sought to identify differences in repolarization markers, during sinus rhythm, between individuals undergoing successful endocardial ablation of VT (Endo group) and those requiring a combined epicardial and endocardial approach (Epi group). We hypothesized that the presence of an epicardial or midmyocardial anatomic substrate for VT, by altering regional epicardial depolarization and subsequent repolarization would result in a regional prolongation of QTc, which by altering QRS and T-wave vector loops would result in an increased QTc dispersion [7].
2. Methods
2.1. Study population
All patients with structural heart disease undergoing successful endocardial or combined epicardial and endocardial VT ablation from January 2009 to May 2014 at our institute were considered for analysis. Patients who were paced or with ECGs in which VRP markers could not be analyzed in all 12 leads were excluded. Patients in whom antiarrhythmic drugs could not be discontinued were also excluded from the study.
2.2. Electrocardiography (ECG)
The baseline digitized 12 lead ECG of the study patients was recorded using the Prucka Cardiolab recording system (Houston, TX, USA) at an amplification of 10 mm/mV and a sweep speed of 100 mm/s and was analyzed by two independent electrophysiologists who were blinded to the results of the electrophysiology study. Sinus rhythm ECG was recorded in the basal state prior to anesthesia or any intervention.
The various VRP intervals measured include: maximum QT interval (QTmax) defined as the longest QT interval in the 12 lead ECG, minimum QT interval defined as the shortest QT interval in the 12 lead ECG (QTmin), QT dispersion (QTd) defined as the difference between QTmax and QTmin. Similarly, the maximum and minimum corrected QT intervals (QTc max and QTc min respectively) were calculated using Bazett’s formula and the corrected QT dispersion (QTc-d) was calculated as the difference between QTc max and QTc min. Ts-p was defined as the interval from the onset to the peak of T wave. The Tp-e interval was defined as the interval from the peak of the T wave to the end of the T wave [8].
2.3. Electrophysiology study and ablation
All electrophysiology studies and ablation were performed with the patient under general anesthesia. Antiarrhythmic drugs were stopped at least five half-lives prior to the procedure. Activation, substrate and pace mapping was done in all patients with hemodynamically stable VT while substrate and pace mapping was done in all patients with hemodynamically unstable VT. RV endocardial ablation was done using the standard approach through the femoral vein. LV endocardial ablation was done using a trans-septal or a retrograde trans-aortic approach, and was left to the discretion of the electrophysiologist.
Endocardial mapping was done in all patients initially. Epicardial mapping and ablation was considered in the presence of multiple factors including, VT 12-lead EKG criteria suggestive of an epicardial exit, voltage maps suggestive of an epicardial substrate and persistence or inducibility of VT following endocardial ablation [1,9]. Epicardial mapping and ablation was done through the pericardial space using previously described techniques [10]. Electroanatomic mapping was done using Carto 3D mapping system (Biosense Webster, Diamond Bar, CA, USA. A 3.5 mm irrigated tip catheter (Thermocool, Biosense Webster, Diamond Bar, CA, USA) was used for mapping and ablation of target sites.
Successful RFA sites were defined as sites showing entrainment with concealed fusion, with a return cycle within 20 msec of the tachycardia cycle length or pace-mapping with >95% match to induced VT using the commercially available PASO® software [11,12]. The end point for ablation was non-inducibility of any VT (clinical and non-clinical). The Morady’s protocol with two drive trains and quadruple extra-stimuli was used for re-induction of VT following ablation [13].
2.4. Statistical analysis
Institutional Review Board approved the data collection and data was systematically gathered for analysis. All statistical analysis was done using the SPSS version 23 (SPSS Inc. version 23.0™, IBM Corporation, Chicago, USA). Continuous variables were expressed as mean ± SD. Categorical variables were described as proportions and frequencies (%). Continuous variables were compared using the Student’s t-test. Categorical variables were compared using the Fisher’s exact test.
To identify factors that independently predicted the need for a combined epicardial and endocardial approach to ablation, multivariate logistic regression was done. All covariates were initially assessed in a univariate fashion, and variables with a p-value < 0.1 were included in the multivariate analysis model. All variables from the univariate analysis were added in a stepwise fashion, and interaction variables were introduced to identify effect modification. The influence of each variable on the model was assessed by using a likelihood ratio test. Model accuracy was assessed by calculating the area under the receiver-operating characteristic curve.
3. Results
3.1. Clinical characteristics
A total of 139 patients underwent VT ablation at our institute between January 2009 and May 2014. Patients who underwent ablation for idiopathic VT/premature ventricular contraction (PVC) (n = 89), patients with failed ablation (n = 8) and patients with ECGs uninterpretable for Tp-e, Ts-p and QT intervals (n = 10) were excluded. A total of 32 patients who had successful scar related VT ablation, via either an endocardial or combined epicardial and endocardial approach were included in the study. In 14 of the 32 patients, endocardial ablation alone was not sufficient with successful ablation requiring an epicardial approach (non-inducibility of VT) - these patients constituted the Epi group. 18 patients had a successful ablation from the endocardial approach (Endo group) alone with non-inducibility of any VT (clinical and non-clinical) at the end of the endocardial ablation and they constituted the control group.
All patients were males with the mean age of the study population being 64 ± 10.5 years. There was an equal distribution of comorbidities between the two groups. Half the patients in the Endo group had ischemic cardiomyopathy while 9 patients in the Epi group had ischemic cardiomyopathy [9 (50%) vs 9 (64.3%); p = 0.53]. Left ventricular ejection fraction (LVEF) (34 ± 14% vs 32 ± 13%, p = 0.65), left ventricular end diastolic diameter (LVEDD) (60 ± 8 mm vs 58 ± 8 mm, p = 0.47) and left ventricular end systolic diameter (LVESD) (44 ± 12 mm vs 43 ± 11, p = 0.81) were similar between the two groups. The QRS duration (111 ± 28 msec vs 94 ± 27 msec, p = 0.13), PR interval (186 ± 53 msec vs 171 ± 49 msec, p = 0.29) and heart rate (73 ± 21 beats per minute vs 68 ± 14 beats per minute, p = 0.43) during sinus rhythm were similar between the two groups. Baseline characteristics of the two groups are shown in Table 1. All patients were receiving amiodarone and betablockers; amiodarone was stopped five half-lives prior to the procedure.
Table 1.
Baseline characteristics of study population.
| Endo group (n = 18) | Epi group (n = 14) | P value | ||
|---|---|---|---|---|
| Age (years), Mean ± SD | 61 ± 12 | 67 ± 9 | 0.14 | |
| Gender | Male, n (%) | 18 (100) | 14 (100) | NA |
| Female, n (%) | 0 (0) | 0 (0) | ||
| HTN, n (%) | 8 (44.4) | 9 (64.3) | 0.26 | |
| DM n(%) | 5 (27.8) | 2 (14.3) | 0.36 | |
| Ischemic cardiomyopathy, n (%) | 9(50) | 9(64.3) | 0.53 | |
| Echocardiographic parameters | ||||
| LVEF (%), Mean ± SD | 34 ± 14 | 32 ± 13 | 0.65 | |
| LVEDD (mm), Mean ± SD | 60 ± 8 | 58 ± 8 | 0.47 | |
| LVESD (mm), Mean ± SD | 44 ± 12 | 43 ± 11 | 0.81 | |
| Electrocardiographic parameters in sinus rhythm | ||||
| Heart rate (bpm), Mean ± SD | 73 ± 21 | 68 ± 14 | 0.43 | |
| PR interval (msec), Mean ± SD | 186 ± 53 | 171 ± 49 | 0.29 | |
| QRS duration (msec), Mean ± SD | 111 ± 28 | 94 ± 27 | 0.13 | |
SD – Standard Deviation.
HTN – Hypertension, DM – Diabetes mellitus.
LVEF – Left ventricular ejection fraction.
LVEDD – Left ventricular end-diastolic diameter.
LVESD – Left ventricular end-systolic diameter.
Bpm – Beats per minute, msec - milliseconds.
3.2. Ventricular repolarization markers
There was no significant difference in QT max (447 ± 48 vs 416 ± 36, p = 0.053), QT min (388 ± 41 vs 381 ± 36, p = 0.606) and QTc min (416 ± 31 vs 405 ± 26, p = 0.309) measurements between 2 groups. QTc max (479 ± 34 vs 449 ± 20, p = 0.008) and QTc dispersion (63 ± 13 vs 38 ± 8, p = 0.001) measurements were significantly higher in the Epi group compared to the Endo group. Another VRP marker, Ts-p which reflects epicardial repolarization time was significantly higher in Epi group compared to the Endo group (166 ± 23 vs 143 ± 23, p = 0.008, respectively). There was a significant difference in Ts-p/Tp-e ratio between the two groups (1.55 ± 0.26 vs 1.3 ± 0.21, p = 0.001, respectively). Measures of VRP in patients of both groups are given in Table 2.
Table 2.
Ventricular repolarization markers in the two groups.
| Endo group (n = 18) | Epi group (n = 14) | p value | |
|---|---|---|---|
| QT Maximum (QT max) | 416 ± 36 | 447 ± 48 | 0.053 |
| QT Minimum (QT min) | 381 ± 36 | 388 ± 41 | 0.606 |
| QTc Maximum (QTc max) | 449 ± 20 | 479 ± 34 | 0.008 |
| QTc minimum (QTc min) | 405 ± 26 | 416 ± 31 | 0.309 |
| QT dispersion (QT dispersion) | 35 ± 8 | 59 ± 14 | 0.001 |
| QTc dispersion (QTc dispersion) | 38 ± 8 | 63 ± 13 | 0.001 |
| Tstart-Tpeak (Ts-p) | 143 ± 23 | 166 ± 23 | 0.008 |
| Tpeak-Tend (Tp-e) | 112 ± 18 | 108 ± 14 | 0.538 |
| Ts-p/QT max ratio | 0.35 ± 0.06 | 0.38 ± 0.06 | 0.158 |
| Tp-e/QT max ratio | 0.27 ± 0.04 | 0.25 ± 0.05 | 0.110 |
| Ts-p/Tp-e ratio | 1.3 ± 0.21 | 1.55 ± 0.26 | 0.005 |
All values expressed as Mean ± Standard deviation.
Tstart-Tpeak – Time interval from start of T wave to peak of T wave.
Tpeak-Tend – Time interval from peak of T wave to end of T wave.
Endo group – Patients requiring endocardial ablation only.
Epi group – Patients requiring both endocardial and epicardial ablation.
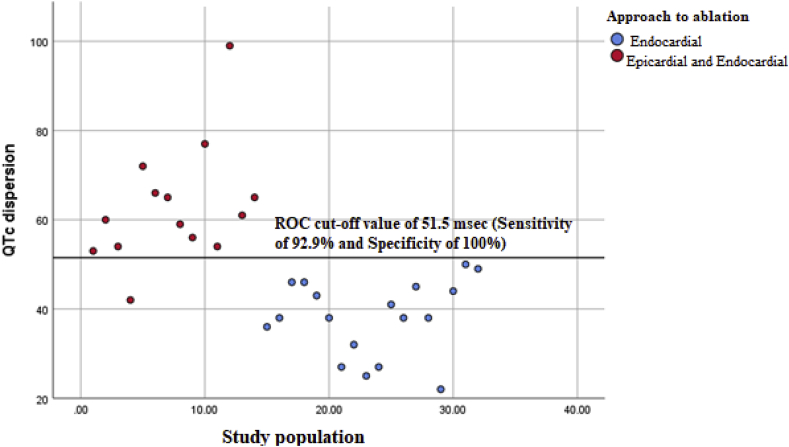
A binomial stepwise logistic regression was performed to determine the effects of the various VRP markers on the approach needed for ablation (endocardial only vs endocardial and epicardial). Linearity of the continuous variables with respect to the logit of the dependent variable was analyzed using the Box-Tidwell procedure [14]. A Bonferroni correction was applied using all 11 elements in the model resulting in statistical significance being accepted when p < 0.005. Based on this assessment, all continuous independent variables were found to be linearly related to the logit of the dependent variable. The logistic regression model was statistically significant, χ2(4) = 32.007, p < 0.0005. The model explained 87.4% (Nagelkerke R2) of the variance in approach to ablation (endocardial only vs epicardial and endocardial) and correctly classified 93.8% of cases. Sensitivity was 92.9%, specificity was 94.4%, positive predictive value was 92.9% and negative predictive value was 94.4%. Of the five predictor variables only one were statistically significant: QTc dispersion. Increasing QTc dispersion was associated with an increased likelihood of requiring a combined epicardial and endocardial approach to ablation [OR – 1.47, (95% CI, 1.09–2)] (Fig. 1).
Fig. 1.
A scatter plot showing the QTc dispersion in the two groups. Patients requiring endocardial ablation only (blue dots) had a significantly shorter QTc dispersion compared to patients requiring a combined epicardial and endocardial ablation (red dots).
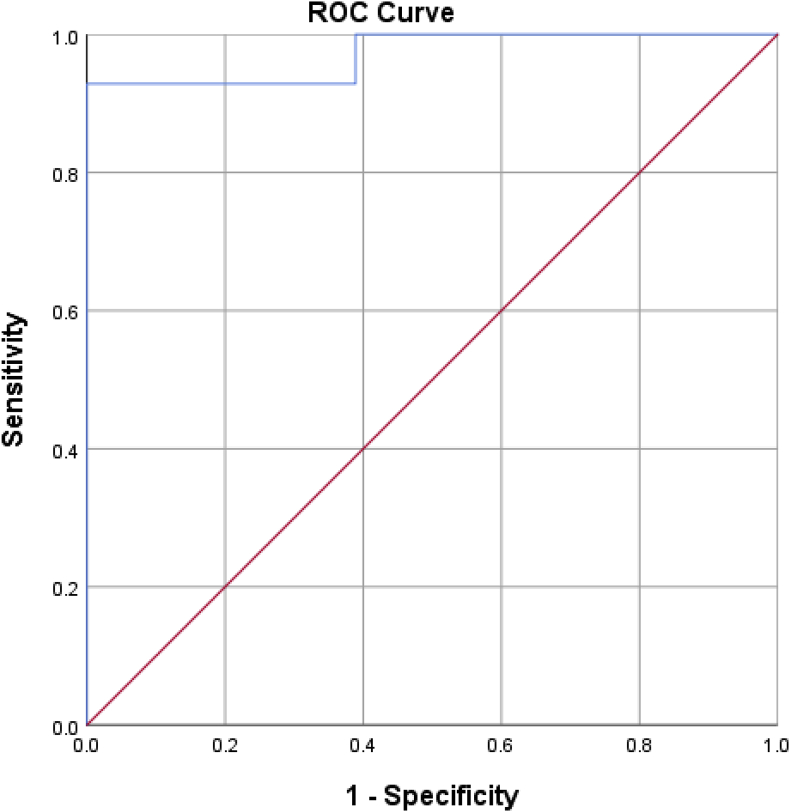
A receiver under operation curve (ROC) analysis was performed to identify the QTc dispersion value that differentiated individuals requiring only an endocardial approach from those requiring both an endocardial and epicardial approach. The area under curve was 0.97 (95% CI, 0.92–1), which is an outstanding level of discrimination [15]. (Fig. 2) A QTc dispersion of 51.5 msec had a sensitivity of 92.9% and a specificity of 100% in differentiating between individuals who required both an epicardial and an endocardial approach to ablation from individuals requiring only an endocardial approach.
Fig. 2.
Receiver under operator curve (ROC) analysis. The area under curve was equal to 0.97 (95% CI, 0.92–1) which is an outstanding level of discrimination. A QTc dispersion of 51.5 msec had a 92.9% sensitivity and 100% specificity for differentiating between the two groups.
4. Discussion
Scar mediated re-entry is the leading mechanism of ventricular tachycardia (VT) accounting for the majority of cases. While coronary artery disease is the leading cause for such scars, myocardial scar and scar mediated VT is also seen in individuals with non-ischemic cardiomyopathy (NICM). An epicardial origin/exit site of ventricular tachycardia (VT) is present in 10–25% of all patients undergoing VT ablation [16,17]. Ablation of these VTs from the endocardial aspect is less rewarding, with remarkable success while ablating from the epicardial aspect. Accurate identification of an epicardial origin/exit site of VTs is therefore important to improve success rates while at the same time reducing the number of ablation lesions and procedural time.
VT in individuals with coronary artery disease is most commonly mediated by scar tissue that is predominantly sub-endocardial in location congruent with the sub-endocardium being most susceptible to ischemia. However, in individuals with transmural scars, epicardial exit sites are likely to be present. In contrast, a higher number of individuals with NICM have epicardial scar and epicardial VT exit sites. The presence of regional epicardial scar would lead to differences in epicardial depolarization with subsequent heterogeneity in repolarization. This increased dispersion of repolarization is likely to provide an electrophysiologic substrate for re-entrant arrhythmias.
QTc dispersion is one of the markers of heterogeneity of repolarization [18]. Considerable debate exists regarding the mechanism of QTc dispersion on the surface EKG. Initially, it was believed that regional pathological processes would lead to prolongation of QT intervals in overlying leads and this was responsible for QTc dispersion [18,19]. Mirvis further showed increased QT dispersion in patients with myocardial infarction with increased QT max and relatively similar QT min compared to the general population [20].
This theory was subsequently questioned by several investigators with the underlying premise being that all EKG leads are affected by cardiac electrical activity and hence the duration of electrical activity on the surface EKG should be the same despite regional differences. According to the electrocardiographic lead theory, information of all ventricular electrical activity is contained in the QRS and T loops and differences in the projection of these loops onto the different leads contribute to differences in QT duration. This was further substantiated by Kors et al. who showed differences in QTc dispersion with different T-wave loop morphologies. Pathological states result in smaller and wider T wave loops which result in greater QT dispersion [21]. Further, increased QTc dispersion has consistently been shown to be associated with increased mortality [4]. Thus while the mechanism of QT dispersion may be controversial, its association with pathological states is consistent.
In our study patients requiring an epicardial and endocardial approach to VT ablation had a significantly higher QTc dispersion when compared to patients requiring an endocardial approach only. In the normal heart the epicardial myocytes have the shortest action potential duration (APD) while the endocardial myocytes have longer action potential durations giving rise to a transmural repolarization gradient [22]. The presence of scar tissue in the epicardium would lead to prolongation of action potential duration with delayed repolarization [23]. This regional delayed repolarization in the epicardium leads to a reduction in the normal transmural gradient and an altered T-wave vector loop making it smaller and wider which might explain the greater QTc dispersion seen on the surface electrocardiogram [21].
Repolarization in the normal heart proceeds from the epicardium to the endocardium. Yan and Antzelevitch showed that epicardial repolarization is responsible for the initial part of the T-wave while repolarization of the endocardial and midmyocardial M cells are responsible for the terminal portions of the T-wave [7]. The Ts-p interval and Ts-p/Tp-e ratio were significantly higher in the Epi group compared to the Endo group on univariate analysis, however these values were not statistically significant on multivariate analysis.
Substrate evaluation can help determine possible epicardial exit sites which can enable one to be prepared for possible epicardial mapping and ablation. Imaging studies such as CE-MRI are useful for such substrate evaluation. Similarly, unipolar voltage mapping in patients without significant endocardial scar on bipolar voltage maps has been proposed to identify epicardial scar (<5.5 mV RV, <8.27 mV LV) in patients with NICM and ARVC [24]. The limitation of this approach is that it provides information about a possible epicardial substrate/origin of VT only during the procedure and thus does not improve pre-procedural planning. Using ECG criteria either during sinus rhythm (like in our study) or VT is cheaper and more readily available when compared to CE-CMR, and it can provide clues to an epicardial origin/exit site of VT and the need for epicardial access pre-procedurally.
The main limitation of our study is the small sample size involved. A multi-center study involving a higher number of patients would help confirm this novel finding which would enhance pre-procedural planning in patients with VT. Another limitation with the use of QTc dispersion is the high inter-observer variation seen. In our study we only included those patients with EKGs which were clearly interpretable with well-defined T-waves with identifiable T-wave end points. Further, two electrophysiologists who were blinded to the results of the procedure independently reviewed the EKGs and were involved in making measurements, with good agreement. While this may have helped reduce some of the inter-observer variation we appreciate that this is an inherent limitation of the use of QTc dispersion as a marker.
5. Conclusion
To conclude, individuals requiring a combined epicardial and endocardial ablation approach have a higher QTc dispersion compared to individuals requiring only an endocardial ablation with a QTc of greater than 51.5 msec identifying the former group of individuals with a sensitivity of 92.9% and a specificity of 100%.
Funding sources
None.
Disclosures
None.
Declaration of competing interest
None of the authors report potential conflicts of interest pertinent to this report.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Bazan V., Gerstenfeld E.P., Garcia F.C., Bala R., Rivas N., Dixit S. Site-specific twelve-lead ECG features to identify an epicardial origin for left ventricular tachycardia in the absence of myocardial infarction. Heart Rhythm. 2007;4:1403–1410. doi: 10.1016/j.hrthm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Martinek M., Stevenson W.G., Inada K., Tokuda M., Tedrow U.B. QRS characteristics fail to reliably identify ventricular tachycardias that require epicardial ablation in ischemic heart disease. J Cardiovasc Electrophysiol. 2012;23:188–193. doi: 10.1111/j.1540-8167.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 3.Perkiömäki J.S., Koistinen M.J., Yli-Mäyry S., Huikuri H.V. Dispersion of QT interval in patients with and without susceptibility to ventricular tachyarrhythmias after previous myocardial infarction. J Am Coll Cardiol. 1995;26:174–179. doi: 10.1016/0735-1097(95)00122-g. [DOI] [PubMed] [Google Scholar]
- 4.de Bruyne M.C., Hoes A.W., Kors J.A., Hofman A., van Bemmel J.H., Grobbee D.E. QTc dispersion predicts cardiac mortality in the elderly: the Rotterdam Study. Circulation. 1998;97:467–472. doi: 10.1161/01.cir.97.5.467. [DOI] [PubMed] [Google Scholar]
- 5.Okin P.M., Devereux R.B., Fabsitz R.R., Lee E.T., Galloway J.M., Howard B.V. Principal component analysis of the T wave and prediction of cardiovascular mortality in American Indians: the Strong Heart Study. Circulation. 2002;105:714–719. doi: 10.1161/hc0602.103585. [DOI] [PubMed] [Google Scholar]
- 6.Vallès E., Bazan V., Marchlinski F.E. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2010;3(1):63–71. doi: 10.1161/CIRCEP.109.859942. [DOI] [PubMed] [Google Scholar]
- 7.Yan G.X., Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 8.Monitillo F., Leone M., Rizzo C., Passantino A., Iacoviello M. Ventricular repolarization measures for arrhythmic risk stratification. World J Cardiol. 2016;8:57–73. doi: 10.4330/wjc.v8.i1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berruezo A., Mont L., Nava S., Chueca E., Bartholomay E., Brugada J. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation. 2004;109:1842–1847. doi: 10.1161/01.CIR.0000125525.04081.4B. [DOI] [PubMed] [Google Scholar]
- 10.Sosa E., Scanavacca M. Epicardial mapping and ablation techniques to control ventricular tachycardia. J Cardiovasc Electrophysiol. 2005;16:449–452. doi: 10.1046/j.1540-8167.2005.40710.x. [DOI] [PubMed] [Google Scholar]
- 11.de Chillou C., Groben L., Magnin-Poull I., Andronache M., MagdiAbbas M., Zhang N. Localizing the critical isthmus of postinfarct ventricular tachycardia: the value of pace-mapping during sinus rhythm. Heart Rhythm. 2014;11:175–181. doi: 10.1016/j.hrthm.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S., Tedrow U.B., Stevenson W.G. Entrainment mapping. Card Electrophysiol Clin. 2017;9:55–69. doi: 10.1016/j.ccep.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Morady F., Kadish A., de Buitleir M., Kou W.H., Calkins H., Schmaltz S. Prospective comparison of a conventional and an accelerated protocol for programmed ventricular stimulation in patients with coronary artery disease. Circulation. 1991;83:764–773. doi: 10.1161/01.cir.83.3.764. [DOI] [PubMed] [Google Scholar]
- 14.Box G.E.P., Tidwell P.W. Transformation of the independent variables. Technometrics. 1962;4:531–550. doi: 10.1080/00401706.1962.10490038. [DOI] [Google Scholar]
- 15.Hosmer D., Lemeshow S. second ed. John Wiley and Sons; 2000. Applied logistic regression. [Google Scholar]
- 16.Della Bella P., Brugada J., Zeppenfeld K., Merino J., Neuzil P., Maury P. Epicardial ablation for ventricular tachycardia: a European multicenter study. Circ Arrhythm Electrophysiol. 2011;4:653–659. doi: 10.1161/CIRCEP.111.962217. [DOI] [PubMed] [Google Scholar]
- 17.Sacher F., Roberts-Thomson K., Maury P., Tedrow U., Nault I., Steven D. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010;55:2366–2372. doi: 10.1016/j.jacc.2009.10.084. [DOI] [PubMed] [Google Scholar]
- 18.Zabel M., Portnoy S., Franz M.R. Electrocardiographic indexes of dispersion of ventricular repolarization: an isolated heart validation study. J Am Coll Cardiol. 1995;25:746–752. doi: 10.1016/0735-1097(94)00446-W. [DOI] [PubMed] [Google Scholar]
- 19.Zabel M., Lichtlen P.R., Haverich A., Franz M.R. Comparison of ECG variables of dispersion of ventricular repolarization with direct myocardial repolarization measurements in the human heart. J Cardiovasc Electrophysiol. 1998;9:1279–1284. doi: 10.1111/j.1540-8167.1998.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 20.Mirvis D.M. Spatial variation of QT intervals in normal persons and patients with acute myocardial infarction. J Am Coll Cardiol. 1985;5:625–631. doi: 10.1016/S0735-1097(85)80387-9. [DOI] [PubMed] [Google Scholar]
- 21.Kors J.A., van Herpen G., van Bemmel J.H. QT dispersion as an attribute of T-loop morphology. Circulation. 1999;99:1458–1463. doi: 10.1161/01.cir.99.11.1458. [DOI] [PubMed] [Google Scholar]
- 22.Antzelevitch C. Cardiac repolarization. The long and short of it. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2005;7:3–9. doi: 10.1016/j.eupc.2005.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan N.T., Orini M., Providencia R., Dhinoja M.B., Lowe M.D., Ahsan S.Y. Prolonged action potential duration and dynamic transmural action potential duration heterogeneity underlie vulnerability to ventricular tachycardia in patients undergoing ventricular tachycardia ablation. Europace. 2019;21:616–625. doi: 10.1093/europace/euy260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polin G.M., Haqqani H., Tzou W., Hutchinson M.D., Garcia F.C., Callans D.J. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:76–83. doi: 10.1016/j.hrthm.2010.09.088. [DOI] [PubMed] [Google Scholar]