Abstract
Objective
To observe the differentiation of macrophages in lung tissue and alveolar lavage fluid of mice with severe pulmonary infection and the changes after intervention with ceftriaxone and ulinastatin, and to explore the pathogenesis of severe pulmonary infection under immunosuppressive state and the intervention effect of two drugs.
Methods
40 male Balb/c mice are randomly divided into normal group, model group, ulinastatin group, and ceftriaxone group with 10 mice in each group. Mice models of acute lung injury with immunodeficiency are established by methylprednisolone and endotoxin, and then treated with ulinastatin and ceftriaxone. Respiratory frequencies of mice in each group are measured at 3 h and 6 h after drug use through trachea, and then the mice are anaesthetized with uratan and killed 6 h after drug use. The number of alveolar macrophages and neutrophils in alveolar lavage fluid is collected and detected, and the pathological changes are observed. The positive expression of CD163 in lung tissue is detected by IHC (immunohistochemistry), and real-time quantitative PCR (Polymerase Chain Reaction) is used to detect the expression of Ml and M2 markers in bronchoalveolar lavage fluid (BALF).
Result
Compared with the normal group, the mice in the model group breathed shallowly and quickly, occasionally nodded breathing, respiratory distress, and respiratory rate increased. Compared with the model group, the mice in the ulinastatin group and ceftriaxone group breathed slowly, occasionally have shortness of breath, smooth breathing, and slow breathing rate, and the mice in ulinastatin group breathe more smoothly. The number of macrophages and neutrophils in BALF of model group is higher than that of normal group. The number of macrophages and neutrophils in ulinastatin group and ceftriaxone group is lower than that of model group and the difference is statistically significant, and the number of macrophages and neutrophils in ulinastatin group is relatively less than that in model group.
Conclusion
In the early stage of severe pulmonary infection under immunosuppressive state, the organism is in the CARS (Compensatory Anti-inflammatory Response Syndrome) stage; M1 macrophages had immune paralysis and M2 macrophages are abnormally activated. Compared with ceftriaxone, ulinastatin can alleviate lung injury more effectively and protect the lung of mice with acute lung injury. The protective mechanism of ulinastatin on lung of mice infected with immunocompromised endotoxin may be through inhibiting M1 macrophages and regulating non-specific immune function.
Keywords: Ceftriaxone, Ulinastatin, Pulmonary infection, Macrophages
1. Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory disease characterized by progressive airflow limitation and destruction of pulmonary parenchymal function (Liu et al., 2017, Aggeletopoulou et al., 2018). COPD patients with short-term cough, sputum, asthma exacerbated, sputum volume increased, purulent or mucopurulent symptoms known as acute exacerbation of chronic obstructive pulmonary disease (AECOPD) (Yang et al., 2018). The primary cause of acute exacerbation of COPD is bacterial infection. Before the invention of antibiotics, bacterial infection is the main cause of death. With the emergence of anti-infective drug penicillin and its continuous renewal and wide application in clinic, the number of patients who died from infection has dropped dramatically (Li et al., 2017). However, the number of patients who died from infection has increased significantly since then.
Pathophysiological and immunological analysis of COPD patients with excessive inflammation is similar to organ transplantation patients. When neonates and AIDS patients are in low T cell function, when bacteria, viruses, fungi and other serious infections occur, activated natural immune cells (such as macrophages) secrete a large number of immunoinflammatory factors, such as TNF-α, IL-6, IL-12, IL-18 and so on. Over-inflammation is the basis of SIRS (Systemic Inflammatory Response Syndrome) (Cai-Yang et al., 2017, Atal and Atal, 2016). Inflammatory response and specific immune function decrease in sepsis coexist. On the one hand, chemical substances, physical damage and other factors cause cell membrane damage, and then cell necrosis, and inflammation occurs. On the other hand, MO, NK, CD8 and Th1 secrete apoptotic factors, such as TNFa, Granulase and Fas L, which cause non-cellular membrane damage and a large number of immune cell apoptosis, resulting in the decline of human specific immune function. The pathophysiology of CARS means that the body can produce an endogenous anti-inflammatory response that causes a decrease in immune function and an increase in susceptibility to infection to combat the primary pro-inflammatory response when the body releases proinflammatory factors after infection or trauma. Its aim is to reduce the synthesis of pro-inflammatory factors and regulate their effects, thereby restoring homeostasis in vivo (Kim et al., 2017, Wang et al., 2017). Research by Ju et al. (2019) showed that ulinastatin can improve immunosuppression by inhibiting the production of endotoxin-activated monocyte TNF-α (Ju et al., 2019). It is speculated that ulinastatin may affect severe lung infection.
Although a lot of research has been carried out, there has not yet been developed a therapeutic drug that can reduce the mortality rate of acute exacerbation of COPD. In this study, the effects of different treatments on the differentiation of macrophages in patients with COPD with recurrent pulmonary infections are studied to realize the pathogenesis of severe pulmonary infections under immunosuppressive state and the intervention effects of two drugs. This study can provide new directions and guidance for the treatment of COPD patients and patients with recurrent pulmonary infections in the future, which is of great significance.
2. Materials and methods
2.1. Animals
Female and male Balb/c mice with body weight of 23.5 ± 1.5 g and aged 8–10 weeks were selected as experimental subjects. All mice were purchased from XXX animal center. During the experiment, the comments of the Ethics Committee were obtained, and the order of the experiment conformed to the national standards for experimental animals.
2.2. Experimental grouping
Random table method was used to divide the mice into four groups, 10 Balb/c mice in each group, 22.5 ± 2.5 g. Mice were labeled with picric acid, the model group (group A), ulinastatin group (group B), normal group (group C) and ceftriaxone group (group D).
2.3. Modeling and administration
The mice in model group, ulinastatin group and ceftriaxone group were intraperitoneally injected with methylprednisone (30 mg/kg, Xiamen Yanke Biotechnology Co., Ltd., China) at 7p.m. for 3 consecutive days. Mice in the normal group were injected intraperitoneally with the same amount of saline. In the ulinastatin group and the ceftriaxone group, mice were injected with ulinastatin (UTI, 1 * 105 U/kg, Xiamen Yanke Biotechnology Co., Ltd., China) and ceftriaxone (Shanghai Shifeng Biotechnology Co., Ltd., China) respectively before and after lipopolysaccharide (LPS, 10 mg/kg) (Shanghai Yuanye Biological Technology Co., Ltd., China) was administered in the 4th-day tube for 30 min. Mice in the model group and the normal group were injected intraperitoneally with saline. The general signs, mental state and respiratory frequency of the mice were observed at 3 and 6 h after tracheal administration.
2.4. Detection of dry-wet lung specific gravity
The mice were killed by intraperitoneal injection of uratan (Beijing Chreagen Biotechnology Co., Ltd., China) and fixed on the operating board with adhesive tape. Cutting down the midline of the neck to the xiphoid process can expose the chest. After the cervical part of the trachea was removed, line 1 was used to ligate the trachea. After lifting the trachea, the whole lung tissue was separated one by one, the heart was cut off, and the blood stains on the surface of the lung tissue were removed. After cleaning the surface liquid with filter paper, the wet weight of lung tissue was weighed on an electronic balance, and then baked in an oven (Guangzhou Koster Scientific Instrument Co., Ltd., China) at 80 °C for 10 h until constant weight. Wet and dry weights were calculated.
2.5. Collection of bronchoalveolar lavage fluid (BALF)
Six hours later, the mice were anaesthetized and executed. When the righting reflex disappears, the adhesive was used to fix the mice on the operating board. After cutting the neck skin, the trachea was separated. Line 1 was used for sub cricoid ligation. The 18G indwelling needle (Shanghai Hanfei Medical Instrument Co., Ltd., China) was inserted into the trachea along the centripetal direction and fixed with a thread. 2 ml syringe was used to extract 1.5 ml of RPMI (Roswell Park Memorial Institute) 1640 culture medium (Shanghai Genmed Gene Pharmaceutical Technology Co., Ltd., China). 1640 cell culture medium (1.5 ml, 1.5 ml, 1.5 ml) was perfused into the lungs three times, five times each time, to ensure that the amount of recovered BALF was more than 4.0 ml after mixing. After centrifugation for 10 min at 1500 r/min, the supernatant was removed. Precipitation was applied to the smear for Rayleigh staining. Cells were classified and counted under oil microscopy.
2.6. Wright-Giemsa staining and hematoxylin-eosin (HE) staining
Wright-Giemsa staining: After the alveolar lavage fluid was centrifuged at 1000 rpm for 5 min, the supernatant was discarded. Four times the cell volume of the red blood cell lysate was added to the cell pellet and blown evenly. After centrifugation at 800 rpm for 5 min, the supernatant was removed. 10 ml of Phosphate Buffered Saline (PBS, Tianjin Bohua Chemical Reagent Co., Ltd., China) was added and the pellet was resuspended. After centrifugation at 800 rpm for 3 min, the supernatant was discarded and 1 wash was repeated. Then, after the precipitated cell suspension was evenly blown, the micropipette was used to take out 100ul, drip onto the slide, spread evenly, and air dry. In the area where the cells were coated, 4–5 drops of Wright-Gimsa Dye Reagent were added dropwise. The eyeball was quickly blown and evenly covered with cell images, stained for about 2 min. Buffer Reagents 6–10 drops were added and shaken to mix the dyes evenly for 8 min. After washing thoroughly, it needs to be dried. Resin glue was used to seal the film and then observed under a microscope. Cells under oil mirrors were classified. HE staining: After the intact lung tissue was isolated, 1 ml of 10% formaldehyde (Shanghai Jingke Chemical Technology Co., Ltd., China) was used for fixation. After the injection was fixed, the entire lung was placed in a 10% formaldehyde fixative for 10 h, and then washed, dehydrated, embedded, and paraffin-embedded. After the prepared slides, the neutral gel was applied dropwise to the tissue of the slide. The coverslip was gradually covered from one end to prevent air bubbles from occurring.
2.7. Purification of alveolar macrophages in alveolar lavage fluid
Alveolar macrophage collection and purification: Bronchoalveolar lavage fluid was collected and centrifuged at 1500 rpm for 10 min to remove the supernatant. The pellet was suspended in the cell culture medium and placed in a 25 ml culture flask. After mixing, it was placed in a 37 °C, 5% CO2 cell incubator for 12 h to allow the macrophages to adhere sufficiently. After discarding the non-adherent cells, 2 ml of trypsin was added to cause the adherent cells to fall off. The adherent cells were recovered and centrifuged at 1500 r/min for 10 min. It was washed twice with RNase-free, counted and brought the cell concentration to 1.0 × 106 cells/ml to obtain pure alveolar macrophages.
2.8. Statistical methods
Statistical analysis is performed using SPSS 26.0. The results of measurement data are expressed as mean ± standard deviation. The comparison between the two groups is performed by t test. The q test is used to compare the mean of multiple groups. The LSD test is used when the variance is equal. The Dunnett T3 test is used when the variance is not uniform. P < 0.05 is considered statistically significant.
3. Result
3.1. Changes in general signs, mental state, and respiratory rate of mice in each group
According to Table 1, it can be seen that the model group mice are wilting and have poor activity. The oral cavity occasionally secretes reddish secretions, and the breathing is deepened, and breathing difficulties and shortness of breath occur. The mice in the ulinastatin group have a good spirit and a quick breathing. In the control group, the respiratory tract mentality, activity, and breathing are smooth. In the ceftriaxone group, the mental state of the model group is relatively good, but compared with the ulinastatin group, the mental state is relatively poor, and the breathing is relatively deep and fast.
Table 1.
Changes in respiratory rate of mice in each group.
| Group | n | 3 h respiratory rate (times/min) | 6 h respiratory rate (times/min) |
|---|---|---|---|
| Normal group | 10 | 148.6 ± 27.0 | 93 ± 25.5 |
| Model group | 10 | 103.4 ± 22.4ab | 102.4 ± 4.2ab |
| Ulinastatin group | 10 | 97.6 ± 10.7a | 93.9 ± 8.7a |
| Ceftriaxone group | 10 | 100.5 ± 16.5ab | 97.8 ± 10.1ab |
| F | 49.478 | 21.923 |
Compared with the normal group, P < 0.05.
Compared with the ulinastatin group, P < 0.05.
3.2. Lung dry and wet specific gravity changes
After LPS is administered by tracheal tube, the dry weight of lung in the model group is significantly higher than that in the control group (p < 0.05). According to Table 2, the dry weight of the lungs in the model group is slightly higher than that in the ulinastatin group. Lung edema and exudation are slightly improved after administration of ulinastatin. The lung edema state and exudation of the ceftriaxone group are relatively heavy after administration of ulinastatin, and the dry-wet specific gravity is relatively small compared with the model group (P < 0.05).
Table 2.
Changes in lung dry weight of each group of mice.
| Group | n | Mice lung wet weight (g) | Mice lung dry weight (g) |
|---|---|---|---|
| Normal group | 10 | 0.223 ± 0.03 | 0.183 ± 0.25 |
| Model group | 10 | 0.298 ± 0.07ab | 0.183 ± 0.25b |
| Ulinastatin group | 10 | 0.247 ± 0.124a | 0.209 ± 0.32a |
| Ceftriaxone group | 10 | 0.263 ± 0.09ab | 0.185 ± 0.25ab |
| F | 20.399 | 10.361 |
Compared with the normal group, P < 0.05.
Compared with the ulinastatin group, P < 0.05.
3.3. Observation of cell smear morphology in BALF
The morphological observation results of cell smears in BALF are shown in Fig. 1. The cells in mice BALF are mainly neutrophils, macrophages, and lymphocytes. The cytoplasm of neutrophils is lavender. Macrophages are cytoplasmic, with many particles and vacuoles, and the cell membrane is smoother and rounder. In the gray-blue cytoplasm, there are tiny purple-red or blue-violet cytoplasmic granules. The cytoplasm of lymphocytes is light blue.
Fig. 1.
Morphological observation results of cell smears in BALF (A: The normal group; B: The model group; C: The ulinastatin group; D: The ceftriaxone group).
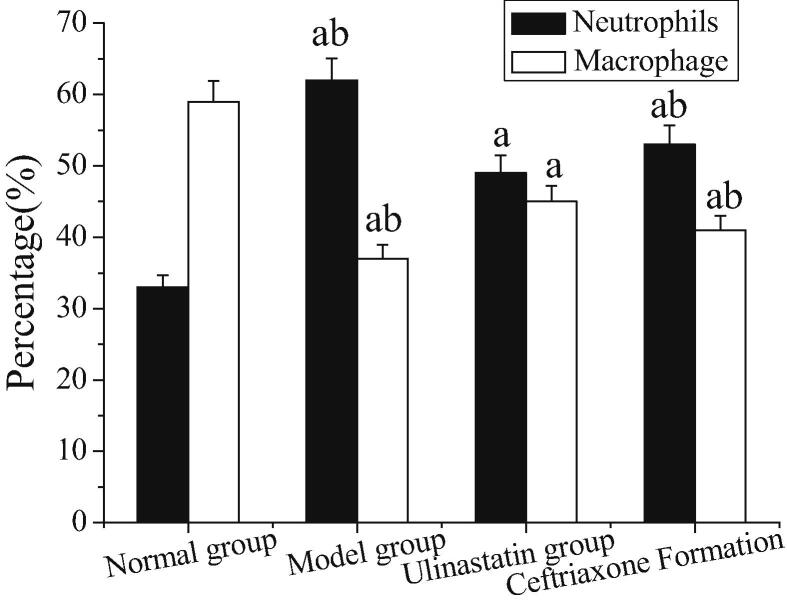
3.4. Total neutrophils and percentage of macrophages in BALF
The total count of neutrophils and the percentage of macrophages in BALF is shown in Fig. 2. The number of neutrophils and the number of macrophages in the model group are higher than those in the normal group (P < 0.05). The neutrophils and the number of macrophages in the model group are lower than those in the ulinastatin group and the ceftriaxone group (P < 0.05). The number of macrophages and neutrophils in the ulinastatin and ceftriaxone groups is lower than that in the model group, and the difference is statistically significant. Moreover, the number of macrophages and neutrophils in the ulinastatin group is relatively small.
Fig. 2.
Total count of neutrophils and percentage of macrophages in BALF (Note: a: compared with the normal group, P < 0.05; b: compared with the ulinastatin group, P < 0.05).
3.5. Pathological changes of lung tissue stained with HE
The HE staining results of lung histopathology of mice in each group are shown in Fig. 3. The naked eye can see the lung tissue of the model group is hyperemia, edema, dark red color, and the texture is harder and expands. In the ulinastatin group, the color of the lung tissue is lighter, and there is flaky congestion. The area of congestion is less than that of the model group. In the normal group, the lung tissue is pink, the texture is soft, the membrane is intact, and the volume is small. HE staining shows a large amount of inflammatory exudation, edema and congestion in the lung tissue of the model group. A large number of neutrophils, macrophage infiltration, alveolar telangiectasia, hyperemia, alveolar exudation, and edema are seen in the pulmonary interstitial alveolar space. The interstitial lung is thickened. The inflammatory cell infiltration is reduced in the ulinastatin group and the collapse of the alveolar space is reduced. In the normal group, the edema of the alveolar and interstitial spaces is significantly reduced, the infiltration of inflammatory cells and inflammatory mediators is reduced, and the congestion of alveolar capillary endothelial cells is significantly improved. It shows that ulinastatin can improve the extent of lung injury and protect the lungs of mice with acute lung injury.
Fig. 3.
Histopathological observation of HE stained lungs of mice in each group (A: The normal group; B: The model group; C: The ulinastatin group; D: The ceftriaxone group).
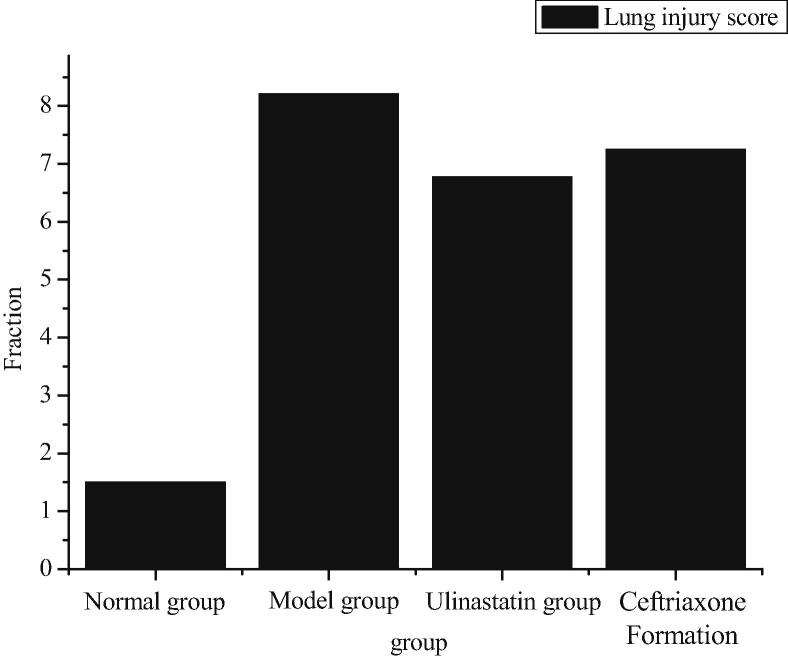
The smith scoring method is used to analyze the lung tissue edema, alveolar interstitial inflammation, alveolar and interstitial exudation, hemorrhage, atelectasis and transparent membrane formation by 0–4 semi-quantitative analysis. No damage is 0 points, lesions <25% is 1 point, lesion range is 25–50% is 2 points, lesion range from 50% to 75% is 3 points, and all lesions are 4 points. All of the above scores are total lung injury scores.
As can be seen from Fig. 4, the mice in the model group have the highest degree of lung injury, followed by the ceftriaxone group and the ulinastatin group. The lowest is the normal group. Compared with the ceftriaxone group and the ulinastatin group, the lung protection of ulinastatin is relatively better.
Fig. 4.
Lung injury score.
3.6. Determination of mean optical density of CD163 positive cells in mouse lung tissue
The results of CD163 immunohistochemistry in lung tissue are shown in Fig. 5. There are different degrees of positive expression of CD163 in lung tissue, and it is mainly located in the cell membrane and is yellowish brown. As can be seen from Fig. 2, the positive expression rate of macrophages (CD163) in the model group is about 9855.0 ± 6489.45. The positive rate of CD163 in the ulinastatin group is approximately 31430.0 ± 2417.97. The positive rate of CD163 in the normal group is about 14477.0 ± 1995.78. The positive rate of CD163 in the ceftriaxone group is about 46790.0 ± 3678.5. Among them, the model group has the highest optical density, followed by the ceftriaxone group, the ulinastatin group and the normal group. The positive expression rate of the macrophage (CD163) in the lung tissue is higher in the model group than in the normal group (p < 0.05).
Fig. 5.
Measurement of mean optical density of CD163 positive cells in mouse lung tissue.
3.7. mRNA expression of M1 (TNF-a, INOS, IL-6) in BALF
According to Fig. 6, the expression of INOS mRNA in BALF is significantly lower in the model group than in the normal group (p < 0.05), which is statistically significant. Compared with the model group, the expression of INOSm RNA is significantly increased in the ulinastatin group (p < 0.05). The ceftriaxone group and the ulinastatin group are compared, and the up-regulation range is basically the same, with little difference. IL-6 mRNA expression is down-regulated in all three groups, with the highest down-regulation in the ulinastatin group, followed by the ceftriaxone group, the model group, and the normal group. There is statistical significance between the model group and the normal group. The ulinastatin group is statistically significant compared with the model group. The expression of TNF-a mRNA is down-regulated, and the amplitude of down-regulation is highest in the ulinastatin group, followed by the ceftriaxone group, the model group and the normal group. Compared with the normal group, the model group has a higher down-regulation and statistical significance. Compared with the model group, the ulinastatin group has a higher degree of reduction, which is statistically significant. Compared with the ceftriaxone group and the ulinastatin group, the down-regulation of the ceftriaxone group is relatively small, but the difference is not significant.
Fig. 6.
mRNA expression of M1 (TNF-a, INOS, IL-6) in BALF.
3.8. mRNA expression of M2 (CD206, Arg-1, IL-10) in BALF
As can be seen from Fig. 7, IL-10 expression in the model group is significantly higher than that in the ulinastatin group, the normal group, and the ceftriaxone group. Among them, the IL-10 expression of IL-10 in the normal group is the lowest, compared with the model group, ulinastatin group and ceftriaxone group, there is significant difference (P < 0.05). The mRNA expression of IL-10 in the model group is higher than that in the normal group, and there is a significant difference (P < 0.05). mRNA expression of Arg-1 in mouse BALF is observed. The model group is significantly higher than the normal group, and there is a significant difference (P < 0.05). The ulinastatin group is compared with the model group and is not statistically significant. The ceftriaxone group is relatively high in expression compared with the ulinastatin group. The expression of CD206 mRNA in BALF of mice shows down-regulated expression, and the normal group has the highest down-regulation, followed by ulinastatin group and ceftriaxone group. The model with the lowest reduction is the model group. Compared with the normal group, the model group shows a decrease in the expression down-regulation, and there is a significant difference (P < 0.05). Compared with the model group, the expression of CD206 mRNA in the ulinastatin group is down-regulated, and the amplitude of the down-regulation is not statistically significant (P < 0.05).
Fig. 7.
mRNA expression of M2 (CD206, Arg-1, IL-10) in BALF.
4. Discussion
Repeated pulmonary infection is a condition that is most common in COPD and is relatively long in treatment (Zheng et al., 2018). Once in an immunosuppressed state, the situation is very complicated and has a high mortality rate (Li et al., 2018). Due to various factors, in and out of the lungs, defense is automatically regulated and it will be transformed into an immunosuppressive state, releasing various inflammatory mediators (Huang, 2017). Among them, the macrophage sub-population plays a very important role in this process, and it secretes a variety of different cytokines and chemokines (Xie et al., 2017). This situation will continue to expand the inflammatory response, and macrophages will directly or indirectly participate in it, causing the body to circulate and inflammatory reactions, and ultimately the body's damage is increasing (Luo et al., 2017).
In this study, the effects of different treatments on the differentiation of macrophages in patients with COPD with recurrent pulmonary infections are studied to realize the pathogenesis of severe pulmonary infections under immunosuppressive state and the intervention effects of two drugs, so as to provide direction and guidance for the treatment of recurrent pulmonary infections in patients with COPD. Firstly, a mouse model is established by taking mice as the research object. Then, the experiment is divided into four groups. After the experiment, general physiological status, lung dry-wet ratio, cell smear morphology in BALF, total number of neutrophils and percentage of macrophages in BALF and expression of M1 and M2 mRNA in BALF are studied and analyzed to explore the changes after intervention with ceftriaxone and ulinastatin.
It is found that in the immunosuppressive state, the early stage of severe pulmonary infection is in the CARS stage. There is immune paralysis of M1 macrophages and abnormal activation of M2 macrophages. Compared with ceftriaxone, ulinastatin can alleviate lung injury more effectively and has a stronger protective effect on lung in mice with acute lung injury. The protective mechanism of ulinastatin on lung of mice infected with immunocompromised endotoxin may be through inhibiting M1 macrophages and regulating non-specific immune function. Research by Yang et al. (2018) shows that the therapeutic effect of ulinastatin on various diseases may be related to the regulation of macrophage polarization (Yang et al., 2018). This is consistent with the above results. This study can provide new directions and guidance for the treatment of COPD patients and patients with recurrent pulmonary infections in the future, which is of great significance.
5. Conclusion
The Balb/c mice were utilized as the research objects to explore the effects of different treatments on the differentiation of chronic obstructive pulmonary disease and recurrent lung infection macrophages. It is found that compared with ceftriaxone, ulinastatin has a stronger protective effect on mice with acute lung injury, and its mechanism may be achieved by inhibiting M1 macrophages and regulating non-specific immune function. This result can provide a reference for the treatment of COPD and recurrent lung infections, which has important significance. However, certain deficiencies were found in the research process; for example, the data collection of samples was relatively less that caused the result being biased to a certain extent. Therefore, the data capacity would be further increased in the subsequent works to make the obtained results more valuable.
Acknowledgement
This work was supported by Research Project of Health and Family Planning Industry in Hainan Province (18A200002) and Scientific Research Projects of Higher Education Institutions in Hainan Province (Hnky 2018-50).
Footnotes
Peer review under responsibility of King Saud University.
References
- Aggeletopoulou I., Assimakopoulos S.F., Konstantakis C. Interleukin 12/interleukin 23 pathway: Biological basis and therapeutic effect in patients with Crohn's disease. World J. Gastroenterol. 2018;24(36):4–14. doi: 10.3748/wjg.v24.i36.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atal S.S., Atal S. Ulinastatin–a newer potential therapeutic option for multiple organ dysfunction syndrome. J. Basic Clin. Physiol. Pharmacol. 2016;27(2):91–99. doi: 10.1515/jbcpp-2015-0003. [DOI] [PubMed] [Google Scholar]
- Cai-Yang C., Zhu M., Wang L. Effects of emulsified sevoflurane and ulinastatin on liver and lung injury induced by bile duct ligation in rats. J. Anesth. Perioper. Med. (JAPM) 2017;4(1):7. [Google Scholar]
- Huang C.L. Effect of ulinastatin on vasoactive substances, oxidative stress and inflammatory response in patients with acute exacerbation of COPD. J. Hainan Med. Univ. 2017;23(16):33–36. [Google Scholar]
- Ju M., He H., Chen S. Ulinastatin ameliorates LPS-induced pulmonary inflammation and injury by blocking the MAPK/NF-κB signaling pathways in rats. Mol. Med. Rep. 2019;20(4):3347–3354. doi: 10.3892/mmr.2019.10561. [DOI] [PubMed] [Google Scholar]
- Kim E.S., Ackermann C., Tóth I. Down-regulation of CD73 on B cells of patients with viremic HIV correlates with B cell activation and disease progression. J. Leukoc. Biol. 2017;101(5):1263–1271. doi: 10.1189/jlb.5A0816-346R. [DOI] [PubMed] [Google Scholar]
- Li D., Ji H., Zhao B. Therapeutic effect of ulinastatin on pulmonary fibrosis via downregulation of TGF-β1, TNF-α and NF-κB. Mol. Med. Rep. 2018;17(1):1717–1723. doi: 10.3892/mmr.2017.8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Yu J., Song H. Squamous differentiation in patients with superficial bladder urothelial carcinoma is associated with high risk of recurrence and poor survival. BMC Cancer. 2017;17(1):530. doi: 10.1186/s12885-017-3520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Pang G., Wang S. Protective effect of ulinastatin on severe pulmonary infection under immunosuppression and its molecular mechanism. Exp. Therapeut. Med. 2017;14(4):3583–3588. doi: 10.3892/etm.2017.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Che W., Zhao M. Ulinastatin post-treatment attenuates lipopolysaccharide-induced acute lung injury in rats and human alveolar epithelial cells. Int. J. Mol. Med. 2017;39(2):297–306. doi: 10.3892/ijmm.2016.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Yang Q., Tang P. Effects of ulinastatin on early postoperative cognitive function after one-lung ventilation surgery in elderly patients receiving neoadjuvant chemotherapy. Metab. Brain Dis. 2017;32(2):427–435. doi: 10.1007/s11011-016-9926-7. [DOI] [PubMed] [Google Scholar]
- Xie F., Min S., Chen J. Ulinastatin inhibited sepsis-induced spinal inflammation to alleviate peripheral neuromuscular dysfunction in an experimental rat model of neuromyopathy. J. Neurochem. 2017;143(2):225–235. doi: 10.1111/jnc.14145. [DOI] [PubMed] [Google Scholar]
- Yang Z., Yan M., Hu C. Ulinastatin reduces LPS-induced THP-1 macrophage M1-like characteristics. Int. J. Clin. Exp. Med. 2018;11(5):4736–4741. [Google Scholar]
- Yang Z., Yan M., Hu C. Ulinastatin reduces LPS-induced THP-1 macrophage M1-like characteristics. Int. J. Clin. Exp. Med. 2018;11(5):4736–4741. [Google Scholar]
- Zheng H., Chen J., Yu X. Ulinastatin combined with magnesium isoglycyrrhizinate suppressed bleomycin-induced acute pulmonary fibrosis by inhibiting MMP-9, ICAM-1 and TGF-β1 expressions. Int. J. Clin. Exp. Med. 2018;11(8):8031–8042. [Google Scholar]