Summary
Background
Treatment of multidrug-resistant tuberculosis requires long-term therapy with a combination of multiple second-line drugs. These drugs are associated with numerous adverse events that can cause severe morbidity, such as deafness, and in some instances can lead to death. Our aim was to estimate the absolute and relative frequency of adverse events associated with different tuberculosis drugs to provide useful information for clinicians and tuberculosis programmes in selecting optimal treatment regimens.
Methods
We did a meta-analysis using individual-level patient data that were obtained from studies that reported adverse events that resulted in permanent discontinuation of anti-tuberculosis medications. We used a database created for our previous meta-analysis of multidrug-resistant tuberculosis treatment and outcomes, for which we did a systematic review of literature published between Jan 1, 2009, and Aug 31, 2015 (updated April 15, 2016), and requested individual patient-level information from authors. We also considered for this analysis studies contributing patient-level data in response to a public call made by WHO in 2018. Meta-analysis for proportions and arm-based network meta-analysis were done to estimate the incidence of adverse events for each tuberculosis drug.
Findings
58 studies were identified, including 50 studies from the updated individual patient data meta-analysis for multidrug-resistant tuberculosis treatment. 35 of these studies, with 9178 patients, were included in our analysis. Using meta-analysis of proportions, drugs with low risks of adverse event occurrence leading to permanent discontinuation included levofloxacin (1·3% [95% CI 0·3–5·0]), moxifloxacin (2·9% [1·6–5·0]), bedaquiline (1·7% [0·7–4·2]), and clofazimine (1·6% [0·5–5·3]). Relatively high incidence of adverse events leading to permanent discontinuation was seen with three second-line injectable drugs (amikacin: 10·2% [6·3–16·0]; kanamycin: 7·5% [4·6–11·9]; capreomycin: 8·2% [6·3–10·7]), aminosalicylic acid (11·6% [7·1–18·3]), and linezolid (14·1% [9·9–19·6]). Risk of bias in selection of studies was judged to be low because there were no important differences between included and excluded studies. Variability between studies was significant for most outcomes analysed.
Interpretation
Fluoroquinolones, clofazimine, and bedaquiline had the lowest incidence of adverse events leading to permanent drug discontinuation, whereas second-line injectable drugs, aminosalicylic acid, and linezolid had the highest incidence. These results suggest that close monitoring of adverse events is important for patients being treated for multidrug-resistant tuberculosis. Our results also underscore the urgent need for safer and better-tolerated drugs to reduce morbidity from treatment itself for patients with multidrug-resistant tuberculosis.
Funding
Canadian Institutes of Health Research, Centers for Disease Control and Prevention (USA), American Thoracic Society, European Respiratory Society, and Infectious Diseases Society of America.
Introduction
The treatment of multidrug-resistant tuberculosis and rifampicin-resistant tuberculosis requires long-term therapy with a combination of multiple second-line drugs, which are less effective, more costly, and more toxic than first-line drugs. The WHO report on the global cohort that started multidrug-resistant tuberculosis treatment in 2014 found that only 54% were cured, highlighting the low success rates achieved with regimens of second-line drugs.1 Adverse events are common with existing regimens for multidrug-resistant tuberculosis and can lead to major morbidity, including blindness, deafness, myelosuppression, renal failure, or liver failure, with resultant hospital admission or even death, as well as treatment interruptions or failure.2 A better understanding of the toxicity of all drugs used to treat multidrug-resistant tuberculosis is an important and much-needed first step in improving patient management and treatment outcomes.
Previous reviews of adverse events associated with drugs used to treat multidrug-resistant tuberculosis include a 2016 systematic review that estimated the pooled incidence of specific adverse events; however, this review did not summarise any information regarding the drugs considered to have caused the events, making it difficult to use the findings to inform treatment decisions.3 A systematic review in 2017 analysed severe adverse events occurring during treatment of multidrug-resistant tuberculosis in settings with a high HIV prevalence, but again did not identify the drugs that caused the adverse events; the authors stated that the meta-analysis was limited by heterogeneity in the definitions of adverse events.4 Several other systematic reviews have summarised the adverse event information for specific drugs, such as cycloserine,5 clofazimine,6–8 linezolid,9–11 and carbapenems.12 However, without analysing drug-associated adverse events in the context of all available multidrug-resistant tuberculosis drugs, comparing the relative toxicity of different drugs is difficult.
Individual participant data meta-analysis is based on individual-level data for each participant from all relevant studies. This approach allows consistent inclusion and exclusion criteria, application of standardised exposure or outcome definitions, and adjustment for the same confounders across all studies.13 In 2016, we did an individual patient data meta-analysis for multidrug-resistant tuberculosis treatment (the IPD-MDR), which assessed the association of treatment outcomes with individual drugs.14 Using the same database, the aim of this study was to estimate the absolute and relative frequency of adverse events leading to permanent drug discontinuation associated with different tuberculosis drugs, with an overall goal to provide useful information for clinicians and tuberculosis programmes in selecting optimal treatment regimens.
Methods
Search strategy and selection criteria
To identify eligible studies for the IPD-MDR, in September, 2015, we did a systematic review of the available literature on multidrug-resistant tuberculosis treatment and outcomes published in English, French, Chinese, Portuguese, or Spanish between Jan 1, 2009, and Aug 31, 2015.15 The search was updated using the same search strategy on April 15, 2016, and reference lists of other systematic reviews of multidrug-resistant tuberculosis treatment published since Jan 1, 2009, were also screened. Additionally, we corresponded with authors involved in an individual participant data meta-analysis16 done in 2010 to invite them to contribute any new data. In 2018, WHO announced a public call for data to inform the multidrug-resistant tuberculosis treatment guideline; studies contributing patient-level data as a result of this call were considered potentially eligible for our analysis. A study was considered eligible if it reported treatment regimens and end-of-treatment outcomes for at least 25 patients with culture-confirmed multidrug-resistant tuberculosis (the 25-patient threshold criterion did not apply to studies describing use of bedaquiline, linezolid, or carbapenems), and the authors agreed to share individual patient data.
Reporting of adverse events was not required for inclusion in the IPD-MDR, but more than half of the studies included in this individual patient data meta-analysis did provide participant-level adverse event information. Studies were included in the adverse event analysis if they reported the drug that caused the adverse event and reported adverse events resulting in permanent discontinuation of a drug by the treating physician; or if they graded the severity of adverse events from 1 to 517 and reported that their policy was to permanently discontinue drugs that caused grade 3–4 adverse events (grade 5 is defined as death related to an adverse event). Methods of study selection and data gathering of the IPD-MDR have been described previously, as have the characteristics of centres and patients included.14 To identify eligible studies, MEDLINE (through OVID), EM BASE (through OVID), and The Cochrane Library were searched for literature published between Jan 1, 2009, and Aug 31, 2015 (updated on April 15, 2016), using a combination of Medical Subject Heading terms and free-text words related to multidrug-resistant tuberculosis, tuberculosis medications, and treatment outcomes. The search and data extraction were done by two reviewers (M L Bastos and Z Lan), and disagreements were resolved by consulting a third reviewer (D Menzies).
This study was approved by the ethics committee of McGill University Health Centre (Montreal, QC, Canada; BMB-07-021t). Ethics approvals were obtained at participating centres when necessary.
Data management
The authors of each study were asked to provide the following individual patient-level information: (1) baseline characteristics, including age, sex, HIV status, diabetes diagnosis, smoking, alcohol consumption, history of tuberculosis treatment, acid-fast bacilli smear result, cavitation on chest x-ray, and drug-susceptibility testing results; (2) drugs used during the multidrug-resistant tuberculosis treatment episode, defined as drugs administered for at least 1 month (if a drug was permanently discontinued within 1 month of use because of adverse events, it was considered as used); (3) end-of-treatment outcome, as defined by Laserson and colleagues18 in 2005 or by the WHO Definitions and Reporting Framework for Tuberculosis19 2013; and (4) variables related to adverse events, including severity and type of event, drugs considered to be the cause, and whether drugs were permanently stopped because of the adverse event. The usual dosage of the drugs was obtained as centre-level information.
If more than one drug was stopped because of adverse events in one patient, each drug stopped was counted as having caused an adverse event either if multiple drugs were stopped but all on different days, or if multiple drugs were stopped on the same day because of different types of adverse event. An adverse event was divided equally among all drugs stopped if multiple drugs were stopped on the same day because of the same adverse event type (eg, if both ethionamide and pyrazinamide were stopped at day 300 because of vomiting, each was assigned half an event)20 or if the type of adverse event was unknown; or if multiple drugs were stopped because of the same type of adverse event but the stop day was unknown. If multiple drugs were stopped but there was no information on the type of adverse event or the day on which the drugs were stopped, each drug was assumed to have caused an adverse event independently.
Data analysis
The primary outcome measures were absolute and relative frequency of adverse events leading to permanent discontinuation of each anti-tuberculosis drug. The secondary outcomes were the association between patient characteristics and the occurrence of at least one adverse event leading to permanent drug discontinuation, as well as the most common types of adverse event for each anti-tuberculosis drug. In the descriptive analysis for patient characteristics and adverse event types, simple pooling was done. In all analyses, a study done within a single country was considered as one cohort; a study done in multiple countries was divided into separate cohorts by country because adverse event management might have varied between countries. At a minimum, five patients within each cohort had to receive a specific drug for that cohort to be included in the pooled analysis. If a study or cohort reported adverse events for only specific drugs (such as linezolid), the cohort was used in the meta-analyses for those drugs.
To assess the characteristics of patients in whom at least one drug was stopped because of adverse events (only in the studies that described adverse events for all drugs), we did multivariable logistic regression with cohort-level random intercepts estimated via maximum residual log pseudo-likelihood (PROC GLIMMIX in SAS). The outcome analysed was whether the patient had at least one drug permanently discontinued because of adverse events. The base model contained age, sex, HIV status, previous tuberculosis treatment, and treatment in high-income countries. Additional characteristics were assessed by adding each of them to the base model individually (base model plus one additional variable), including acid-fast bacilli smear positive, cavitary disease, diabetes, smoking, and alcohol consumption. For variables in the base model, missing values were imputed using the means of patients in the same cohort with available information. For the additional variables, only patients with non-missing data were included in the analyses.
We used three approaches to analyse the occurrence of adverse events. First, we did a standard aggregate data meta-analysis for proportions. We estimated the incidence of adverse events leading to permanent discontinuation for each drug within each cohort, calculated as the total number of adverse events leading to permanent drug discontinuation due to that drug, divided by the total number of patients who received the drug. The incidence of adverse events leading to permanent drug discontinuation for each drug from each cohort was then pooled using a generalised linear mixed model with random effects at cohort level using the metaprop function within the R package, meta; a binomial distribution model and logit transformation was implemented to calculate an overall proportion.21,22
Second, we did an arm-based network meta-analysis using the nma.ab.bin function within the R package, pcnetmeta.23 In this approach, drugs not evaluated in a study were considered as missing at random. The use of a multivariate Bayesian mixed model allowed estimation of the population-averaged treatment-specific event rates. This model took into account the correlation between different treatments within each cohort, unlike the first approach, which estimated drug-specific event proportions solely on the basis of cohorts that used the particular drug.24 The absolute risk of adverse events leading to permanent drug discontinuation for each drug was estimated using a random effects model within the Bayesian framework, and the median value with 95% credible interval and the mean value with SD were reported.
The definitions, detection, and management of adverse events varied between cohorts. To account for this variation, we used a ranking-based non-parametric method as the third analytic approach,25 because we felt this would more accurately assess the relative toxicity of different drugs. Within each cohort, the drugs used were ranked in the order of observed incidence of adverse events leading to permanent drug discontinuation, from one to N (representing the total number of distinct drugs prescribed). If two or more drugs had the same adverse event incidence in a study, the drugs were assigned tied ranks. The raw ranks were then adjusted by the maximum distinct number of drugs in all cohorts. The unweighted average rank for each drug was calculated across all cohorts in which the drug was used, with equal weight ascribed to each cohort. The weighted average rank for each drug was obtained in the same way, except a weight was assigned for each cohort on the basis of the number of patients using the drug in that cohort. The unweighted ranking was considered the primary approach to minimise the dominance of cohorts with a large sample size. A permutation test was used to determine the statistical significance of ranks. In this method, the ranks from each cohort were randomly permuted 10 000 times to generate null distributions of average ranks (the distributions that would have been seen if there was no difference in adverse event occurrence among all drugs). Then the observed average rank for each drug was tested against the null distribution to determine the significance level, an indication of whether a drug had a statistically significant low or high average rank.
Data management and logistic regression were done using SAS version 9.4. Meta-analysis of proportions, arm-based network meta-analysis, and drug ranking with permutation tests were done in R (version 3.4.4).26
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
In the IPD-MDR, authors of50 studies agreed to participate and contributed adequate data. Characteristics of these 50 included studies and the included patients were similar to those of the studies that were identified in the 2017 systematic review15 but were not included in the IPD-MDR.14 One additional study that contributed data after the initial data collection phase,27 two studies from a 2010 individual participant data meta-analysis with additional adverse event data provided by the authors,28,29 and five studies with adverse event information identified during WHO’s public call for data in 2018 were also included in this adverse event analysis (Fox G and Chang VWL; Rodrigues D; and Kuksa L, all unpublished).30,31 As shown in the figure, 35 studies (9178 patients) reporting adequate adverse event information were included in this analysis (Jarlsberg L and Nahid P; Brode SK; Barry PM; and Chan ED, all unpublished).27–64 Of these, three studies recorded adverse event severity using standardised grading, and their usual policy was to permanently stop any drug causing a grade 3–4 adverse event. Hence, within these three studies, drugs that caused a grade 3–4 adverse event were considered as permanently stopped.27,45,48 25 of the 35 included studies (8622 patients) had adverse event data available for all drugs used, and 22 of these studies, with 5658 patients, reported adverse event types. Ten studies only reported adverse events for particular drugs; all ten reported adverse events due to linezolid, and three also reported adverse events due to carbapenems or bedaquiline.
Figure: Study selection.
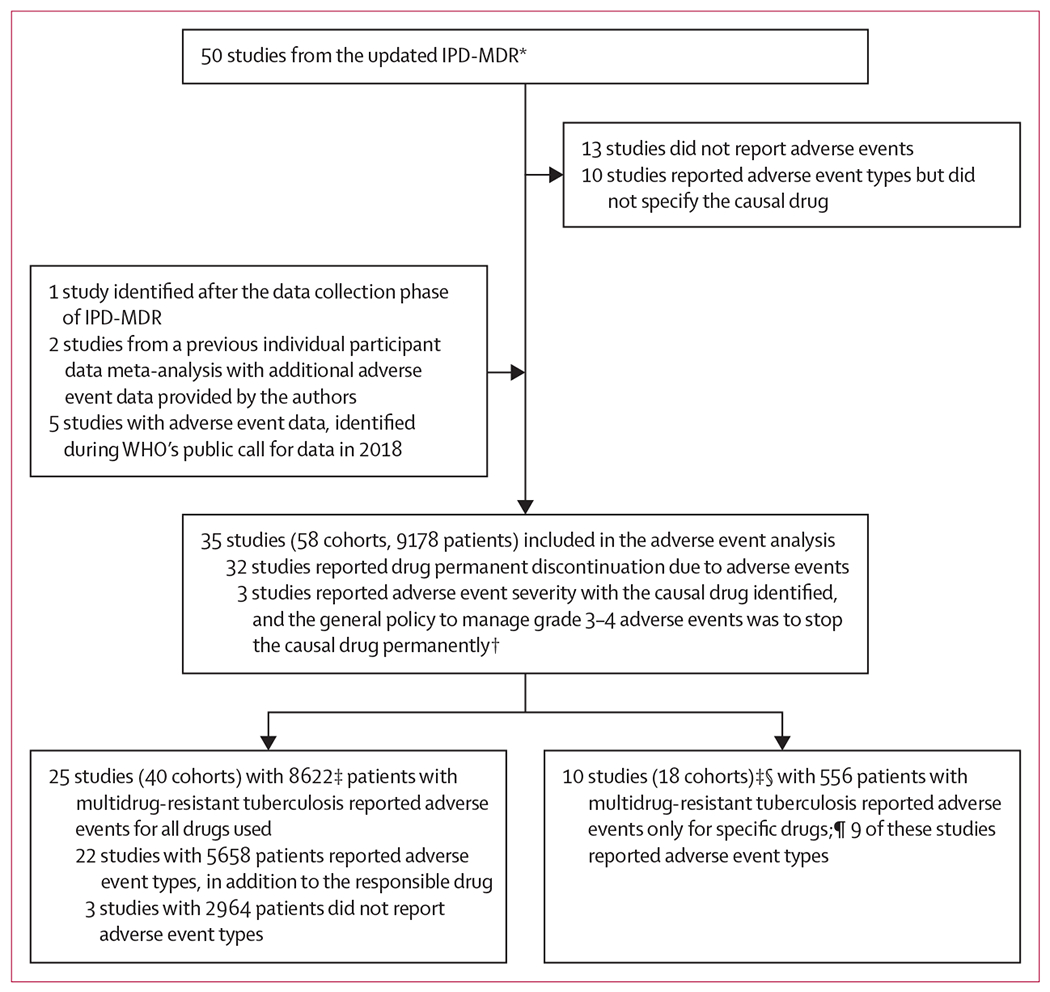
In all analyses, adverse events were defined as those that resulted in permanent discontinuation of a drug. IPD-MDR=individual patient data meta-analysis for multidrug-resistant tuberculosis. *For details of the selection of the 50 studies, refer to Ahmad et al (2018).14 †For these three studies, if the grade of an adverse event was 3–4, the causal drug was considered as permanently stopped. ‡Patients without treatment regimen information and patients who were still on treatment were excluded, patients with extrapulmonary disease were included. §Two studies had adverse event information for more than one drug. ¶dverse events for bedaquiline reported: one study with 130 patients; adverse events for linezolid reported: ten studies with 508 patients; adverse events for carbapenem reported: two studies with 139 patients (each patient number includes only the patients who used the drug for which adverse events were reported).
The characteristics of patients in the 25 studies that reported adverse events for all drugs, the ten studies that reported adverse events for only particular drugs, and the 23 studies that were excluded from this analysis because they either did not report adverse events or did not specify which drug was the cause of the event, were generally similar (appendix p 1). However, the participants in the studies that did not report adverse events, and were therefore excluded from this analysis, were less likely to be from high-income countries or to receive individualised regimens, which might have resulted in an overestimate of adverse events. We were unable to ascertain whether the data from the public call were representative of all potentially available datasets.
In the 25 studies that reported adverse events for all drugs used, the median age was 37 years (IQR 28–47), 5665 (65·7%) of 8622 were men, 821 (10·5%) of 7835 were HIV-positive (271 [51·9%] of 522 received antiretroviral therapy), and the median year of treatment initiation for multidrug-resistant tuberculosis was 2006 (IQR 2003–2008; appendix p 1). By comparison, the 23 studies that were included in the IPD-MDR but were not eligible for adverse event analysis reported similar median age and proportion of men, but a higher HIV prevalence (1317 [21·8%] of 6052) and a later median treatment initiation year (2008; 2005–2011). In the ten studies that only reported adverse events for particular drugs, the HIV prevalence was low (44 [3·3%] of 1316), and most patients were from high-income countries (1223 [87·7%] of 1394; appendix p 1). Risk of bias in selection of studies was judged to be low because there were no important differences between included and excluded studies. Variability between studies was significant for most outcomes analysed.
Of the 8622 patients included in the analysis, 2027 (23·5%) had at least one drug permanently stopped because of an adverse event (table 1), and among these patients, the mean number of adverse events leading to permanent drug discontinuation per patient was 1·4 (SD 0·8). The proportion of patients who had at least one drug stopped because of adverse events varied across different studies, with a median of 29·1% (IQR 16·1–53·3; appendix pp 2–3). Having at least one adverse event leading to permanent drug discontinuation was significantly associated with female sex (adjusted odds ratio 1·3 [95% CI 1·1–1·4]), older age (per 10 years: 1·1 [1·1–1·2]), and treatment in high-income countries (4·0 [2·2–7·6]; appendix p 4). After adjustment for these three factors, HIV infection, previous tuberculosis treatment, acid-fast bacilli smear positive, cavitary disease, diabetes, smoking, or alcohol consumption were not independently associated with the risk of adverse events leading to permanent drug discontinuation. Additionally, after adjustment for the same three factors plus HIV status and previous tuberculosis treatment, the occurrence of adverse events leading to permanent drug discontinuation was significantly lower in individuals who dropped out (odds of adverse event leading to permanent drug discontinuation in patients who were lost to follow-up: 0·56 [0·47–0·66]). After excluding patients who were lost to follow-up from analysis, adverse events leading to permanent drug discontinuation were not associated with failure of the treatment, death, or treatment success (appendix p 4).
Table 1:
Comparison of characteristics among patients with at least one adverse event versus patients with no adverse event
| Patients with at least one adverse event (N=2027) | Patients with no adverse events (N=6595) | |
|---|---|---|
| Baseline clinical characteristics | ||
| Age, years | 39 (29–49)* | 37 (28–47)* |
| Men | 1327/2027 (65·5%)* | 4338/6595 (65·8%)* |
| HIV-positive | 134/1601 (8·4%) | 687/6234 (11·0%) |
| Patients with HIV receiving antiretroviral therapy | 52/107 (48·6%) | 219/415 (52·8%) |
| Diabetes | 199/1671 (11·9%) | 433/5714 (7·6%) |
| Smoking | 425/1367 (31·1%) | 1045/5392 (19·4%) |
| Alcohol | 496/1788 (27·7%) | 1291/5603 (23·0%) |
| Smear positive | 1475/1969 (74·9%) | 5284/6403 (82·5%) |
| Cavitary disease | 1203/1870 (64·3%) | 4581/6161 (74·4%) |
| Previous tuberculosis treatment | 1506/2004 (75·2%) | 5296/6523 (81·2%) |
| Received second-line drugs (among patients with previous treatment) | 291/1102 (26·4%) | 585/2174 (26·9%) |
| Resistance to fluoroquinolone | 249/1711 (14·6%) | 666/3216 (20·7%) |
| Resistance to second-line injectable drug | 536/1738 (30·8%) | 1154/3266 (35·3%) |
| Treatment in high-income countries | 843/2027 (41·6%)* | 1142/6595 (17·3%)* |
| Treatment outcome | ||
| Success | 1412/2027 (69·7%) | 4043/6595 (61·3%) |
| Failure and relapse | 154/2027 (7·6%) | 514/6595 (7·8%) |
| Death | 219/2027 (10·8%) | 995/6595 (15·1%) |
| Lost to follow-up | 242/2027 (11·9%)* | 1043/6595 (15·8%)* |
Data are median (IQR), n/N (%). For all variables, N is the total number of patients with multidrug-resistant tuberculosis with available information. 25 studies reported adverse events for all drugs and were included in this analysis. Adverse events were defined as those that resulted in permanent discontinuation of a drug.
Differences in these characteristics between patients with at least one versus no adverse events were statistically significant in the multivariable analysis adjusted for age (p<0·0001), sex (p=0·0005), HIV-positive (p=0·06), previous tuberculosis treatment (p=0·18), and treatment in high-income countries (p<0·0001).
The effect of drug dosage on adverse event incidence was not assessed in this study because individual-level dosage information was not available and because WHO guidelines were followed in almost all included studies. The usual daily dosage for each drug used in each study is reported in appendix pp 5–7.
23 drugs were analysed. Rifampicin and isoniazid were excluded from the analysis. Fewer than 150 patients used high-dose isoniazid, rifabutin, gatifloxacin, or delamanid, so these four drugs were not analysed. Cycloserine and terizidone were grouped together because they have similar properties, as were ethionamide and protionamide, and imipenem and meropenem.
Using meta-analysis of proportions, low estimates of the occurrence of adverse events leading to permanent drug discontinuation were seen with levofloxacin (1·3% [95% CI 0·3–5·0]), moxifloxacin (2·9% [1·6–5·0]), and clofazimine (1·6% [0·5–5·3]; table 2). Bedaquiline was permanently stopped in nine of 464 patients who received the drug, with a pooled incidence of 1·7% (0·7–4·2). Much higher incidences of adverse events leading to permanent drug discontinuation were observed with the second-line injectable drugs (amikacin: 10·2% [6·3–16·0]; kanamycin: 7·5% [4·6–11·9]; capreomycin: 8·2% [6·3–10·7]), and with aminosalicylic acid (11·6% [7·1–18·3]) and linezolid (14·1% [9·9–19·6]; table 2).
Table 2:
Pooled incidence of adverse events for each drug using generalised linear mixed model
| Cohorts using the drug* | Adverse events†/patients using the drug | Pooled incidence of adverse events, random effect‡ (95% CI) | Pooled incidence of adverse events, fixed effect (95% CI) | Heterogeneity I2 statistics | |
|---|---|---|---|---|---|
| Ciprofloxacin | 8 | 4/723 | 0·6% (0·2–1·5) | 0·6% (0·2–1·5) | 0·0% |
| Ofloxacin | 22 | 71/6062 | 0·9% (0·4·2·1) | 1·2% (0·9–1·5) | 85·9% |
| Levofloxacin | 20 | 22/1012 | 1·3% (0·3–5·0) | 2·2% (1·4–3·3) | 81·6% |
| Clofazimine | 13 | 12/1712 | 1·6% (0·5–5·3) | 0·7% (0·4–1·2) | 69·4% |
| Bedaquiline | 14§ | 9/464 | 1·7% (0·7–4·2) | 1·9% (1·0–3·7) | 25·7% |
| Ethambutol | 33 | 124/6089 | 1·8% (1·0–3·3) | 2·0% (1·7–2·4) | 84·0% |
| Streptomycin | 17 | 34/1208 | 2·9% (1·3–6·2) | 2·8% (2·0–3·9) | 71·1% |
| Moxifloxacin | 27 | 30/904 | 2·9% (1·6–5·0) | 3·3% (2·3–4·7) | 38·0% |
| Amoxicillin-clavulanate | 23 | 21/695 | 2·9% (1·7–4·8) | 3·0% (2·0–4·6) | 11·5% |
| Clarithromycin | 16 | 18/457 | 3·3% (1·5–7·0) | 3·9% (2·5–6·2) | 47·2% |
| Imipenem and meropenem | 7§ | 9/158 | 4·9% (1·0–20·5) | 5·7% (3·0–10·6) | 14·4% |
| Pyrazinamide | 35 | 410/5141 | 5·1% (3·1–8·4) | 8·0% (7·3–8·7) | 93·4% |
| Cycloserine and terizidone | 40 | 337/7547 | 5·7% (4·1–7·8) | 4·5% (4·0–5·0) | 83·8% |
| Ethionamide and protionamide | 39 | 376/4627 | 6·5% (4·1–10·1) | 8·1% (7·4–8·9) | 92·9% |
| Kanamycin | 25 | 268/1995 | 7·5% (4·6–11·9) | 13·4% (12·0–15·0) | 86·8% |
| Capreomycin | 29 | 161/1932 | 8·2% (6·3–10·7) | 8·3% (7·2–9·7) | 45·1% |
| Amikacin | 23 | 235/4106 | 10·2% (6·3–16·0) | 5·7% (5·1–6·5) | 86·9% |
| Aminosalicylic acid | 35 | 532/2929 | 11·6% (7·1–18·3) | 18·2% (16·8–19·6) | 94·9% |
| Linezolid | 35§ | 140/783 | 14·1% (9·9–19·6) | 17·9% (15·4–20·7) | 67·6% |
| Thioacetazone | 3 | 103/719 | 14·3% (12·0–17·1) | 14·3% (12·0–17·1) | 0·0% |
A study done in a single country was considered as one cohort; a study done in multiple countries was divided into separate cohorts by country.
Adverse events were defined as those that resulted in permanent discontinuation of a drug.
Generalised linear mixed model was used to pool the incidence of adverse events.
If a study or cohort only reported adverse events for specific drugs, the cohort was used in the meta-analyses for those drugs.
The arm-based network meta-analysis estimated slightly higher absolute risks of adverse events leading to permanent drug discontinuation for each drug, but the pooled risk estimates were low for bedaquiline, moxifloxacin, and clofazimine, and high for amikacin, kanamycin, aminosalicylic acid, and linezolid (table 3).
Table 3:
Pooled absolute risk of adverse events for each drug using arm-based network meta-analysis
| Cohorts using the drug* | Adverse events†/patients using the drug | Pooled absolute risk of adverse events, median‡ (95% credible interval) | Pooled absolute risk of adverse events, mean (SD) | |
|---|---|---|---|---|
| Ciprofloxacin | 8 | 4/723 | 1·0% (0·2–3·9) | 1·2% (1·0) |
| Bedaquiline | 10 | 7/348 | 2·8% (0·9–7·3) | 3·1% (1·7) |
| Moxifloxacin | 27 | 30/904 | 3·1% (1·6–5·8) | 3·2% (1·1) |
| Amoxicillin-clavulanate | 23 | 21/695 | 3·3% (1·8–6·0) | 3·5% (1·1) |
| Clofazimine | 13 | 12/1712 | 3·5% (1·3–8·3) | 3·8% (1·8) |
| Ofloxacin | 22 | 71/6062 | 3·8% (1·6–9·2) | 4·2% (2·0) |
| Ethambutol | 33 | 124/6089 | 4·1% (2·5–6·9) | 4·2% (1·1) |
| Levofloxacin | 20 | 22/1012 | 4·2% (2·1–8·0) | 4·5% (1·5) |
| Streptomycin | 17 | 34/1208 | 5·1% (2·7–9·8) | 5·4% (1·8) |
| Clarithromycin | 16 | 18/457 | 5·2% (2·6–10·3) | 5·6% (2·0) |
| Imipenem and meropenem | 3 | 3/44 | 5·5% (0·6–27·0) | 7·7% (7·1) |
| Cycloserine and terizidone | 40 | 337/7547 | 7·9% (5·8–11·0) | 8·0% (1·3) |
| Capreomycin | 29 | 161/1932 | 9·4% (6·6–13·4) | 9·5% (1·7) |
| Pyrazinamide | 35 | 410/5141 | 9·5% (6·5–14·5) | 9·8% (2·1) |
| Ethionamide and protionamide | 39 | 376/4627 | 10·7% (7·7–15·3) | 10·9% (2·0) |
| Kanamycin | 25 | 268/1995 | 12·0% (7·9–17·8) | 12·2% (2·5) |
| Amikacin | 23 | 235/4106 | 13·6% (9·3–19·6) | 13·8% (2·6) |
| Thioacetazone | 3 | 103/719 | 14·4% (4·8–31·8) | 15·5% (7·0) |
| Linezolid | 17 | 58/292 | 16·6% (10·9–23·9) | 16·8% (3·3) |
| Aminosalicylic acid | 35 | 532/2929 | 17·6% (13·0–24·1) | 17·9% (2·8) |
Studies that only reported adverse events of specific drugs were excluded from this analysis.
A study done in a single country was considered as one cohort; a study done in multiple countries was divided into separate cohorts by country.
Adverse events were defined as those that resulted in permanent discontinuation of a drug.
Absolute risk of adverse events for each drug was estimated using a random effects model within the Bayesian framework.
Using the non-parametric unweighted ranking approach to assess the relative safety of the 20 different drugs (table 4), the fluoroquinolones had the lowest incidence of adverse events leading to permanent drug discontinuation, followed by bedaquiline, clofazimine, and ethambutol. Cycloserine and terizidone, ethionamide and protionamide, second-line injectable drugs, aminosalicylic acid, and linezolid had the highest incidence of adverse events leading to permanent drug discontinuation. When comparing the three analytic approaches (appendix p 8), fluoroquinolones, clofazimine, and bedaquiline consistently had the lowest incidence of adverse events leading to permanent drug discontinuation, and second-line injectable drugs, aminosalicylic acid, and linezolid had the highest incidence of adverse events leading to permanent drug discontinuation.
Table 4:
Ranking of drugs on the basis of their associated adverse events
| Unweighted average ranking* | Weighted average ranking† | |||||
|---|---|---|---|---|---|---|
| Drug name | Cohorts using the drug‡ | Average ranking§ | Drug name | Cohorts using the drug‡ | Weighted average ranking§ | |
| 1 | Ciprofloxacin | 8 | 4·2¶ | Ciprofloxacin | 8 | 3·4¶ |
| 2 | Moxifloxacin | 27 | 5·0¶ | Ofloxacin | 22 | 4·2‖ |
| 3 | Imipenem and meropenem | 3 | 5·6 | Moxifloxacin | 27 | 4·5¶ |
| 4 | Amoxicillin-clavulanate | 23 | 5·7¶ | Clofazimine | 13 | 5·0 |
| 5 | Bedaquiline | 10 | 6·1‖ | Bedaquiline | 10 | 5·5‖ |
| 6 | Ofloxacin | 22 | 6·1¶ | Amoxicillin-clavulanate | 23 | 6·4‖ |
| 7 | Clofazimine | 13 | 6·3‖ | Imipenem and meropenem | 3 | 6·5 |
| 8 | Levofloxacin | 20 | 6·4¶ | Clarithromycin | 16 | 6·8 |
| 9 | Ethambutol | 33 | 6·8¶ | Streptomycin | 17 | 6·8 |
| 10 | Streptomycin | 17 | 7·2 | Levofloxacin | 20 | 7·4 |
| 11 | Clarithromycin | 16 | 7·4 | Ethambutol | 33 | 8·3 |
| 12 | Thioacetazone | 3 | 8·0 | Pyrazinamide | 35 | 10·3 |
| 13 | Capreomycin | 29 | 9·3 | Ethionamide and protionamide | 39 | 10·5 |
| 14 | Pyrazinamide | 35 | 9·7 | Capreomycin | 29 | 10·7 |
| 15 | Cycloserine and terizidone | 40 | 10·3** | Cycloserine and terizidone | 40 | 11·1 |
| 16 | Kanamycin | 25 | 10·4** | Linezolid | 17 | 12·9†† |
| 17 | Ethionamide and protionamide | 39 | 10·9†† | Thioacetazone | 3 | 12·9 |
| 18 | Aminosalicylic acid | 35 | 11·9†† | Kanamycin | 25 | 13†† |
| 19 | Linezolid | 17 | 12·2†† | Aminosalicylic acid | 35 | 13·4†† |
| 20 | Amikacin | 23 | 12·3†† | Amikacin | 23 | 13·7 |
Unweighted average ranking assigns equal weight to all cohorts, regardless of the sample size of the cohort.
Each cohort was weighted on the basis of the number of patients using the drug in that cohort.
25 studies (40 cohorts) that reported adverse events for all drugs used were included in this analysis; studies that only reported adverse events of specific drugs were excluded.
Drugs were first ranked on the basis of their adverse event incidences within each study, then average ranks or weighted average ranks were calculated; permutation tests were done to determine the statistical significance of ranks.
Significant (p<0·1) for low average rank.
Significant (p<0·5) for low average rank.
Significant (p<0·5) for high average rank.
Significant (p<0·1) for high average rank.
Subgroup analyses to investigate heterogeneity were based on age, sex, and country income level, because these factors were significantly associated with the occurrence of adverse events leading to permanent drug discontinuation in multivariable analyses (appendix p 4). The rank orders of drugs from least to most toxic was not different between these different subgroups (appendix pp 9–11).
Information on the types of adverse events leading to permanent drug discontinuation was available in 31 studies (22 studies that reported adverse events for all drugs used and nine studies that reported adverse events for only specific drugs) for 1145 events (table 5). Among the patients who had linezolid-associated adverse events, the three most common adverse event types leading to permanent drug discontinuation were peripheral neuropathy (87 [64%] of 137), myelosuppression (30 [22%] of 137), and optic neuritis (7 [5%] of 137). Among injectable drugs, types of adverse events leading to permanent drug discontinuation for amikacin and kanamycin were most likely to be ototoxicity (183 [87%] of 211 for amikacin and 42 [75%] of 56 for kanamycin), whereas nephrotoxicity accounted for 36 (51%) of 71 capreomycin-associated adverse events leading to permanent drug discontinuation. Gastrointestinal disorders were the most common type of adverse event leading to permanent drug discontinuation due to aminosalicylic acid (95 [79%] of 120) and ethionamide and protionamide (52 [48%] of 108), whereas psychiatric disorders were the most common type of adverse event leading to permanent drug discontinuation due to cycloserine and terizidone (92 [66%] of 140). Clofazimine was stopped in only 12 (1·6%) of 1712 patients who were taking it, but skin hyperpigmentation and rash accounted for more than half of these episodes.
Table 5:
Type of adverse events for each drug
| Adverse events*/patients using the drug | Pooled incidence of adverse events, random effect† (95% CI) | Adverse events type reported‡ | Type 1§ | Type 2 | Type 3 | Type 4 | Type 5 | |
|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin¶ | 4/723 | 0·6% (0·2–1·5) | 1 | Gynaecomastia (1) | .. | .. | .. | .. |
| Ofloxacin | 71/6062 | 0·9% (0·4–2·1) | 12 | Musculoskeletal (5, 42%) | Psychiatric (2, 17%) | Gastrointestinal (1, 8%) | Hepatotoxicity (1, 8%) | Rash (1, 8%) |
| Levofloxacin | 22/1012 | 1·3% (0·3–5·0) | 14 | Musculoskeletal (9, 64%) | Peripheral neuropathy (2, 14%) | Rash (2, 14%) | Hypoglycaemia (1, 7%) | .. |
| Clofazimine | 12/1712 | 1·6% (0·5–5·3) | 12 | Cardiovascular (4, 33%) | Hyperpigmentation (5, 42%) | Rash (2, 17%) | Gastrointestinal (1, 8%) | .. |
| Bedaquiline | 9/464 | 1·7% (0·7–4·2) | 9 | Cardiovascular (5, 56%) | Hepatotoxicity (2, 22%) | CNS toxicity (1, 11%) | Musculoskeletal (1, 11%) | .. |
| Ethambutol | 124/6089 | 1·8% (1·0–3·3) | 59 | Visual impairment (41, 70%) | Gastrointestinal (10, 17%) | Musculoskeletal (2, 3%) | Rash (2, 3%) | Hepatotoxicity (1, 2%) |
| Streptomycin | 34/1208 | 2·9% (1·3–6·2) | 6 | Ototoxicity (5, 83%) | Peripheral neuropathy (1, 17%) | .. | .. | .. |
| Moxifloxacin | 30/904 | 2·9% (1·6–5·0) | 24 | Cardiovascular (5, 21%) | Hepatotoxicity (4, 17%) | Gastrointestinal (3, 13%) | Peripheral neuropathy (3, 13%) | Musculoskeletal (2, 8%) |
| Amoxicillin–clavulanate | 21/695 | 2·9% (1·7–4·8) | 9 | Gastrointestinal (6, 67%) | Rash (1, 11%) | Musculoskeletal (1, 11%) | Peripheral neuropathy (1, 11%) | .. |
| Clarithromycin | 18/457 | 3·3% (1·5–7·0) | 7 | Gastrointestinal (4, 57%) | Hepatotoxicity (1, 14%) | Peripheral neuropathy (1, 14%) | Fatigue (1, 14%) | .. |
| Imipenem and meropenem | 9/158 | 4·9% (1·0–20·5) | 6 | Hepatotoxicity (3, 50%) | Rash (1, 17%) | Fatigue (1, 17%) | Pneumonia (1, 7%) | .. |
| Pyrazinamide | 410/5141 | 5·1% (3·1–8·4) | 142 | Musculoskeletal (47, 33%) | Gastrointestinal (33, 23%) | Hepatotoxicity (29, 20%) | Rash (18, 13%) | Hyperuricaemia (8, 6%) |
| Cycloserine and terizidone | 337/7547 | 5·7% (4·1–7·8) | 140 | Psychiatric (92, 66%) | CNS toxicity (35, 25%) | Gastrointestinal (5, 4%) | Peripheral neuropathy (2, 1%) | Rash (1, 1%) |
| Ethionamide protionamide | 376/4627 | 6·5% (4·1–10·1) | 108 | Gastrointestinal (52, 48%) | Hepatotoxicity (24, 22%) | Psychiatric (6, 6%) | Gynaecomastia (5, 5%) | Musculoskeletal (5, 5%) |
| Kanamycin | 268/1995 | 7·5% (4·6–11·9) | 56 | Ototoxicity (42, 75%) | Musculoskeletal (3, 5%) | CNS toxicity (2, 4%) | Gastrointestinal (2, 4%) | Hypotension (2, 4%) |
| Capreomycin | 161/1932 | 8·2% (6·3–10·7) | 71 | Nephrotoxicity (36, 51%) | Ototoxicity (12, 17%) | Rash (8, 11%) | Gastrointestinal (5, 7%) | Hypotension (2, 3%) |
| Amikacin | 235/4106 | 10·2% (6·3–16·0) | 211 | Ototoxicity (183, 87%) | Nephrotoxicity (22, 10%) | Gastrointestinal (2, 1%) | Intolerance (2, 1%) | Musculoskeletal (1, 1%) |
| Aminosalicylic | 532/2929 | 11·6% (7·1–18·3) | 120 | Gastrointestinal (95, 79%) | Hypothyroidism (6, 5%) | Hepatotoxicity 5, 4%) | Rash (5, 4%) | Nephrotoxicity (4, 3%) |
| Linezolid | 140/783 | 14·1% (9·9–19·6) | 137 | Peripheral neuropathy (87, 64%) | Myelosuppression (30, 22%) | Optic neuritis (7, 5%) | Gastrointestinal (3, 2%) | Rash (3, 2%) |
| Thioacetazone¶ | 103/719 | 14·3% (12·0–17·1) | 1 | Rash (1) | .. | .. | .. | .. |
Adverse events were defined as those that resulted in permanent discontinuation of a drug.
Pooled incidence of adverse events was estimated through meta-analysis of proportions (table 2).
This analysis included only studies that reported adverse event types.
For each drug, simple pooling was done to calculate the number of each type of adverse event; the five most common adverse event types with the corresponding proportions were presented.
Adverse event types were reported for only one patient.
Discussion
In this study, we showed that fluoroquinolones, bedaquiline, and clofazimine were the drugs associated with the lowest incidences of adverse events leading to permanent drug discontinuation, whereas second-line injectable drugs, aminosalicylic acid, and linezolid had the highest incidences of adverse events leading to permanent drug discontinuation. Our findings provide valuable information to guide clinicians and tuberculosis programmes in selecting regimens, and support treatment guidelines for injectable-free regimens.65
To our knowledge, the individual participant database we assembled is the largest cohort with detailed individual-level data including information on adverse events that led to permanent discontinuation of anti-tuberculosis medications. The 9178 patients originated from 28 countries; this diversity of populations should enhance the generalisability of the results. Another major strength of our study was the consistency of findings between three different analytical methods to estimate absolute and relative frequency of adverse events leading to permanent discontinuation of the different second-line tuberculosis drugs.
Our study had several limitations. Despite the large number of patients analysed, all but two of the studies included in this individual patient data meta-analysis had observational designs, which might result in biases. In particular, some drugs were already known to cause specific types of adverse event and might, therefore, have been more likely to be identified by unmasked clinicians as being the cause of specific adverse events in the analysed studies, confirming previous results. We only considered adverse events that led to permanent discontinuation of the drug because this definition was used in most studies reporting adverse events. However, information on the severity or seriousness of adverse events, and information on adverse events that led to temporary discontinuation or dose reduction was not available. This restriction to adverse events that resulted in permanent discontinuation meant that we restricted this analysis to the most serious events, with important clinical consequences. This definition is relevant and well understood by clinicians, but this approach excludes consideration of milder toxicity, which still can be important from a patient perspective. We did not consider drug duration for each individual, because this information was not available in more than half of the studies. The effect of drug dosage on adverse event incidence could not be assessed because most patients received standard dosing of drugs according to WHO guidelines. Linezolid had the most diverse dosing regimens, with 513 (71%) of 719 patients receiving 600 mg per day, 86 (12%) patients receiving 300 mg per day, and 92 (13%) patients receiving 1200 mg per day (data not shown). We did not include delamanid in our analysis because individual-level data from recent trials66,67 were not available, and only 41 patients took delamanid in the studies included in this analysis.
The much higher incidence of adverse events leading to permanent drug discontinuation among patients treated in high-income countries than in low, lower-middle, and upper-middle income countries might have reflected true differences due to older age of the patients in high-income countries, but might also have reflected differences in management policies, diagnostic resources, or availability of alternative regimens in different settings. Compared with resource-limited settings, higher-resource settings might have greater availability of monitoring tools to detect adverse events, greater resources to monitor patients more frequently, better reporting, or lower clinical threshold for discontinuation of drugs suspected of causing an adverse event because of better access to alternative tuberculosis drugs. There might also have been other differences in ascertainment and reporting between sites. However, this problem of between-site differences should not have affected the ranking of the relative safety of drugs generated by the non-parametric approach, because the relative toxicities of each drug to all the other drugs used should have been similar in different settings.
The findings for some drugs, such as bedaquiline, should be interpreted with caution because of the relatively few studies and small number of participants. The occurrence of permanent discontinuation of bedaquiline because of adverse events might be underestimated because several studies had strict criteria for the use of this drug; these criteria excluded patients with specific comorbidities, such as HIV infection with CD4 cell count less than 250 cells per μL or cardiac arrhythmia.32,36
This study has several implications for the clinical care of patients with multidrug-resistant tuberculosis. Using three different analytical approaches, later-generation fluoroquinolones, bedaquiline, and clofazimine were consistently shown to have the lowest incidences of discontinuation due to adverse events. In other analyses using the same data, the use of these three drugs was associated with significantly improved treatment success and reduced mortality compared with not using each of these three drugs.14 These findings together suggest that the use of these three drugs could improve the effectiveness and tolerability of multidrug-resistant tuberculosis treatment regimens. Among second-line injectable drugs, amikacin showed modest benefits in multidrug-resistant tuberculosis treatment, whereas kanamycin and capreomycin were associated with worse outcomes in efficacy analyses. The relatively high incidence of adverse events leading to permanent discontinuation of all three second-line injectable drugs shown in this study support the need for evaluation of all-oral multidrug-resistant tuberculosis regimens using new and repurposed drugs.68 Linezolid has shown a substantial benefit for patients with multidrug-resistant and extensively drug-resistant tuberculosis,14 but this drug had the highest incidence of discontinuation due to adverse events in this study, similar to the Nix-TB study,69 which also reported a high incidence of permanent discontinuation of linezolid due to adverse events. These results suggest that in switching from regimens with injectable drugs to all-oral regimens with linezolid, tuberculosis programmes might be trading a familiar problem for a new problem with less well understood consequences. The hyperpigmentation associated with prolonged use of clofazimine is an adverse event that is important to many patients. However, hyperpigmentation did not seem to be a common cause of permanent drug discontinuation, either because there were few alternatives, or perhaps because only patients who accepted this predictable adverse event started taking the drug.
Despite the importance of adverse events to patients, and the frequency of these events in the treatment of multidrug-resistant tuberculosis, diagnosis and reporting of adverse events are poorly done in many studies. We found marked differences in the diagnosis and management algorithms between the 35 studies included, and another 23 studies could not be included because they reported adverse events inadequately, or not at all. These findings emphasise the urgent need for improved reporting of adverse events;15 the WHO guidelines for pharmacovigilance could serve as a framework to develop a more robust adverse event reporting system.70 The use of this system by a large collaborative group was reported in 2019,71 although the incidence of adverse events reported in that system for most drugs was much lower than in this analysis, suggesting that these reports might account for just a small proportion of the total adverse events. Innovations are also needed to improve adverse event monitoring, including the use of patient-friendly technology.72 This improvement will be particularly important as new drugs and new regimens are developed and tested.
This individual patient data meta-analysis of adverse events highlights the relatively low frequency of adverse events leading to permanent discontinuation of later-generation fluoroquinolones, bedaquiline, and clofazimine, but also highlights the high incidence of adverse events leading to permanent discontinuation of linezolid and the second-line injectable drugs. These results suggest that close monitoring of adverse events is important for patients being treated for multidrug-resistant tuberculosis. Our results also underscore the urgent need for safer and better-tolerated drugs to reduce the morbidity from treatment itself for patients with multidrug-resistant tuberculosis.
Supplementary Material
Research in context.
Evidence before this study
Treatment of rifampicin-resistant or multidrug-resistant tuberculosis has low cure rates, despite the use of multiple second-line tuberculosis drugs; these drugs are used second-line because of lower efficacy or greater toxicity, or both, compared with first-line treatments. Guidelines for rifampicin-resistant or multidrug-resistant tuberculosis treatment suggest use of at least five effective drugs initially for a total of 20 months or more. Use of these toxic drugs for such long periods can cause major morbidity, including permanent neuropathies, deafness, and blindness, or death from haematological, renal, or hepatic failure. As many as half of all patients have at least one adverse event that requires discontinuation of one of these second-line drugs. The resultant changes in the regimen will further reduce the already low likelihood of cure. Fortunately, after decades of neglect, there has been renewed interest in development of drugs for multidrug-resistant tuberculosis treatment. In the past 10 years, new drugs, such as bedaquiline, have been introduced and other drugs, such as the fluoroquinolones, carbapenems, clofazimine, and linezolid have been repurposed. These drugs have superior efficacy to many of the older second-line drugs, as shown in a 2019 individual patient data meta-analysis of more than 13 000 patients. However, there is little evidence of the toxicity of these new agents, particularly in comparison with the second-line drugs traditionally used. MEDLINE (through OVID), EMBASE (through OVID), and The Cochrane Library were searched for literature published between Jan 1, 2009, and Aug 31, 2015 (updated on April 15, 2016), using a combination of Medical Subject Heading terms and free-text words related to multidrug-resistant tuberculosis, tuberculosis medications, and treatment outcomes. Studies were included if they reported treatment information and clinical characteristics for at least 25 patients with microbiologically confirmed pulmonary multidrug-resistant tuberculosis, and either end-of-treatment outcomes, 6-month culture conversion, or severe adverse events.
Added value of this study
We assembled a dataset of more than 13 000 patients with rifampicin-resistant or multidrug-resistant tuberculosis treated at 53 centres from more than 30 countries. In a subset of 35 studies with 9178 patients, adverse events were reported in a sufficiently standardised way to allow pooling of results and comparison across these studies. We used three different approaches to estimate the rates of adverse events causing permanent drug discontinuation, and the relative toxicity of the different drugs used. In the 35 studies included in this analysis, the rates of adverse events leading to drug discontinuation were lowest with the fluoroquinolones and bedaquiline, and highest with the use of injectable second-line drugs (amikacin, kanamycin, and capreomycin) as well as aminosalicylic acid and linezolid. The relative order of drugs from least to most toxic was consistent using the three different methods of analysis.
Implications of all the available evidence
The fluoroquinolones and bedaquiline appear to have the ideal combination of good efficacy and low toxicity. At the other end of the spectrum are the second-line injectable drugs and aminosalicylic acid, which combine modest efficacy with relatively high toxicity. The use of these drugs should be reconsidered and, if possible, avoided. Other commonly used second-line drugs such as cycloserine or ethionamide and protionamide have moderate efficacy and toxicity. Linezolid is notable among the drugs introduced in the past decade for evidence of very good efficacy but also relatively high toxicity. Given the high efficacy, the toxicity of this drug requires further evaluation to define the optimal dose that is efficacious while minimising risk of toxicity. It would also be helpful to identify patient characteristics that are associated with increased or reduced risk of toxicity, to optimise patient selection for use of this drug. Overall, we conclude that the search for efficacious and safe, well tolerated drugs for the treatment of rifampicin-resistant and multidrug-resistant tuberculosis must continue.
Acknowledgments
Funding was from Canadian Institutes of Health Research (grant number FDN-143350), Centers for Disease Control and Prevention (USA), American Thoracic Society, European Respiratory Society, and Infectious Diseases Society of America.
Footnotes
Declaration of interests
LB has received personal fees from Ewopharma, and personal fees from Otsuka, outside of the submitted work. CL has received personal fees from Chiesi, and personal fees from Gilead, Janssen, Lucane, Novartis, Oxoid, Berlin Chemie, and Thermofisher, outside of the submitted work. All other authors declare no competing interests.
See Online for appendix
References
- 1.WHO. Global tuberculosis report 2017. Geneva: World Health Organization, 2017. [Google Scholar]
- 2.WHO. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization, 2014. [PubMed] [Google Scholar]
- 3.Wu S, Zhang Y, Sun F, et al. Adverse events associated with the treatment of multidrug-resistant tuberculosis: a systematic review and meta-analysis. Am J Ther 2016; 23: e521–30. [DOI] [PubMed] [Google Scholar]
- 4.Schnippel K, Firnhaber C, Berhanu R, Page-Shipp L, Sinanovic E. Adverse drug reactions during drug-resistant TB treatment in high HIV prevalence settings: a systematic review and meta-analysis. J Antimicrob Chemother 2017; 72: 1871–79. [DOI] [PubMed] [Google Scholar]
- 5.Hwang TJ, Wares DF, Jafarov A, Jakubowiak W, Nunn P, Keshavjee S. Safety of cycloserine and terizidone for the treatment of drug-resistant tuberculosis: a meta-analysis. Int J Tuberc Lung Dis 2013; 17: 1257–66. [DOI] [PubMed] [Google Scholar]
- 6.Dey T, Brigden G, Cox H, Shubber Z, Cooke G, Ford N. Outcomes of clofazimine for the treatment of drug-resistant tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother 2013; 68: 284–93. [DOI] [PubMed] [Google Scholar]
- 7.Gopal M, Padayatchi N, Metcalfe JZ, O’Donnell MR. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis. Int J Tuberc Lung Dis 2013; 17: 1001–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang TJ, Dotsenko S, Jafarov A, et al. Safety and availability of clofazimine in the treatment of multidrug and extensively drug-resistant tuberculosis: analysis of published guidance and meta-analysis of cohort studies. BMJ Open 2014; 4: e004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotgiu G, Centis R, D’Ambrosio L, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J 2012; 40: 1430–42. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Falagas ME, Vardakas KZ, et al. Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. J Thorac Dis 2015; 7: 603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agyeman AA, Ofori-Asenso R. Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 2016; 15: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotgiu G, D’Ambrosio L, Centis R, et al. Carbapenems to treat multidrug and extensively drug-resistant tuberculosis: a systematic review. Int J Mol Sci 2016; 17: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010; 340: c221. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad N, Ahuja SD, Akkerman OW, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018; 392: 821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastos ML, Lan Z, Menzies D. An updated systematic review and meta-analysis for treatment of multidrug-resistant tuberculosis. Eur Respir J 2017; 49: 1600803. [DOI] [PubMed] [Google Scholar]
- 16.Ahuja SD, Ashkin D, Avendano M, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 2012; 9: e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Common terminology criteria for adverse events: version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf (accessed Jan 1, 2020). [DOI] [PMC free article] [PubMed]
- 18.Laserson KF, Thorpe LE, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005; 9: 640–45. [PubMed] [Google Scholar]
- 19.WHO. Definitions and reporting framework for tuberculosis: 2013 revision (updated December 2014). Geneva: World Health Organization, 2014. [Google Scholar]
- 20.Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med 2003; 167: 1472–77. [DOI] [PubMed] [Google Scholar]
- 21.Schwarzer G Meta: an R package for meta-analysis. R News 2007; 7: 40–45. [Google Scholar]
- 22.Hamza TH, van Houwelingen HC, Stijnen T. The binomial distribution of meta-analysis was preferred to model within-study variability. J Clin Epidemiol 2008; 61: 41–51. [DOI] [PubMed] [Google Scholar]
- 23.Lin L, Zhang J, Hodges JS, Chu H. Performing arm-based network meta-analysis in R with the pcnetmeta package. J Stat Softw 2017; 80: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Carlin BP, Neaton JD, et al. Network meta-analysis of randomized clinical trials: reporting the proper summaries. Clin Trials 2014; 11: 246–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zintzaras E, Ioannidis JP. Meta-analysis for ranked discovery datasets: theoretical framework and empirical demonstration for microarrays. Comput Biol Chem 2008; 32: 38–46. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2018. [Google Scholar]
- 27.Dalcolmo M, Gayoso R, Sotgiu G, et al. Effectiveness and safety of clofazimine in multidrug-resistant tuberculosis: a nationwide report from Brazil. Eur Respir J 2017; 49: 1602445. [DOI] [PubMed] [Google Scholar]
- 28.Shin SS, Pasechnikov AD, Gelmanova IY, et al. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis 2007; 11: 1314–20. [PubMed] [Google Scholar]
- 29.Bloss E, Kuksa L, Holtz TH, et al. Adverse events related to multidrug-resistant tuberculosis treatment, Latvia, 2000–2004. Int J Tuberc Lung Dis 2010; 14: 275–81. [PubMed] [Google Scholar]
- 30.Kuksa L, Barkane L, Hittel N, Gupta R. Final treatment outcomes of multidrug- and extensively drug-resistant tuberculosis patients in Latvia receiving delamanid-containing regimens. Eur Respir J 2017; 50: 1701105. [DOI] [PubMed] [Google Scholar]
- 31.Guglielmetti L, Barkane L, Le Dû D, et al. Safety and efficacy of exposure to bedaquiline-delamanid in multidrug-resistant tuberculosis: a case series from France and Latvia. Eur Respir J 2018; 51: 1702550. [DOI] [PubMed] [Google Scholar]
- 32.Pym AS, Diacon AH, Tang SJ, et al. Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J 2016; 47: 564–74. [DOI] [PubMed] [Google Scholar]
- 33.Guglielmetti L, Jaspard M, Le Dû D, et al. Long-term outcome and safety of prolonged bedaquiline treatment for multidrug-resistant tuberculosis. Eur Respir J 2017; 49: 1601799. [DOI] [PubMed] [Google Scholar]
- 34.Cegielski JP, Kurbatova E, van der Walt M, et al. Multidrug-resistant tuberculosis treatment outcomes in relation to treatment and initial versus acquired second-line drug resistance. Clin Infect Dis 2016; 62: 418–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012; 367: 1508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diacon AH, Pym A, Grobusch MP, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 2014; 371: 723–32. [DOI] [PubMed] [Google Scholar]
- 37.van Altena R, de Vries G, Haar CH, et al. Highly successful treatment outcome of multidrug-resistant tuberculosis in the Netherlands, 2000–2009. Int J Tuberc Lung Dis 2015; 19: 406–12. [DOI] [PubMed] [Google Scholar]
- 38.Udwadia ZF, Sen T, Moharil G. Assessment of linezolid efficacy and safety in MDR- and XDR-TB: an Indian perspective. Eur Respir J 2010; 35: 936–38. [DOI] [PubMed] [Google Scholar]
- 39.Tiberi S, Payen MC, Sotgiu G, et al. Effectiveness and safety of meropenem/clavulanate-containing regimens in the treatment of MDR- and XDR-TB. Eur Respir J 2016; 47: 1235–43. [DOI] [PubMed] [Google Scholar]
- 40.Tiberi S, Sotgiu G, D’Ambrosio L, et al. Comparison of effectiveness and safety of imipenem/clavulanate- versus meropenem/clavulanate-containing regimens in the treatment of MDR- and XDR-TB. Eur Respir J 2016; 47: 1758–66. [DOI] [PubMed] [Google Scholar]
- 41.Tabarsi P, Chitsaz E, Baghaei P, et al. Impact of extensively drug-resistant tuberculosis on treatment outcome of multidrug-resistant tuberculosis patients with standardized regimen: report from Iran. Microb Drug Resist 2010; 16: 81–86. [DOI] [PubMed] [Google Scholar]
- 42.Tabarsi P, Chitsaz E, Tabatabaei V, et al. Revised category II regimen as an alternative strategy for retreatment of category I regimen failure and irregular treatment cases. Am J Ther 2011; 18: 343–49. [DOI] [PubMed] [Google Scholar]
- 43.Smith SE, Ershova J, Vlasova N, et al. Risk factors for acquisition of drug resistance during multidrug-resistant tuberculosis treatment, Arkhangelsk Oblast, Russia, 2005–2010. Emerg Infect Dis 2015; 21: 1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singla R, Caminero JA, Jaiswal A, et al. Linezolid: an effective, safe and cheap drug for patients failing multidrug-resistant tuberculosis treatment in India. Eur Respir J 2012; 39: 956–62. [DOI] [PubMed] [Google Scholar]
- 45.Shean K, Streicher E, Pieterson E, et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLoS One 2013; 8: e63057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pietersen E, Ignatius E, Streicher EM, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet 2014; 383: 1230–39. [DOI] [PubMed] [Google Scholar]
- 47.Palmero D, González Montaner P, Cufré M, García A, Vescovo M, Poggi S. First series of patients with XDR and pre-XDR TB treated with regimens that included meropenen-clavulanate in Argentina. Arch Bronconeumol 2015; 51: e49–52. [DOI] [PubMed] [Google Scholar]
- 48.O’Donnell MR, Padayatchi N, Kvasnovsky C, Werner L, Master I, Horsburgh CR Jr. Treatment outcomes for extensively drug-resistant tuberculosis and HIV co-infection. Emerg Infect Dis 2013; 19: 416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller AC, Gelmanova IY, Keshavjee S, et al. Alcohol use and the management of multidrug-resistant tuberculosis in Tomsk, Russian Federation. Int J Tuberc Lung Dis 2012; 16: 891–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leimane V, Dravniece G, Riekstina V, et al. Treatment outcome of multidrug/extensively drug-resistant tuberculosis in Latvia, 2000–2004. Eur Respir J 2010; 36: 584–93. [DOI] [PubMed] [Google Scholar]
- 51.Kwak N, Kim HR, Yoo CG, Kim YW, Han SK, Yim JJ. Changes in treatment outcomes of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2015; 19: 525–30. [DOI] [PubMed] [Google Scholar]
- 52.Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 2009; 360: 2397–405. [DOI] [PubMed] [Google Scholar]
- 53.Baghaei P, Tabarsi P, Dorriz D, et al. Adverse effects of multidrug-resistant tuberculosis treatment with a standardized regimen: a report from Iran. Am J Ther 2011; 18: e29–34. [DOI] [PubMed] [Google Scholar]
- 54.Koh WJ, Kang YR, Jeon K, et al. Daily 300 mg dose of linezolid for multidrug-resistant and extensively drug-resistant tuberculosis: updated analysis of 51 patients. J Antimicrob Chemother 2012; 67: 1503–07. [DOI] [PubMed] [Google Scholar]
- 55.Jo KW, Lee SD, Kim WS, Kim DS, Shim TS. Treatment outcomes and moxifloxacin susceptibility in ofloxacin-resistant multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2014; 18: 39–43. [DOI] [PubMed] [Google Scholar]
- 56.Jeong BH, Jeon K, Park HY, et al. Outcomes of pulmonary MDR-TB: impacts of fluoroquinolone resistance and linezolid treatment. J Antimicrob Chemother 2015; 70: 3127–33. [DOI] [PubMed] [Google Scholar]
- 57.Guglielmetti L, Le Dû D, Jachym M, et al. Compassionate use of bedaquiline for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis: interim analysis of a French cohort. Clin Infect Dis 2015; 60: 188–94. [DOI] [PubMed] [Google Scholar]
- 58.Chang KC, Yew WW, Cheung SW, et al. Can intermittent dosing optimize prolonged linezolid treatment of difficult multidrug-resistant tuberculosis? Antimicrob Agents Chemother 2013; 57: 3445–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuen CM, Kurbatova EV, Tupasi T, et al. Association between regimen composition and treatment response in patients with multidrug-resistant tuberculosis: a prospective cohort study. PLoS Med 2015; 12: e1001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson LF, Tamne S, Watson JP, et al. Treatment outcome of multi-drug resistant tuberculosis in the United Kingdom: retrospective-prospective cohort study from 2004 to 2007. Euro Surveill 2013; 18: 20601. [DOI] [PubMed] [Google Scholar]
- 61.Ahmad N, Javaid A, Basit A, et al. Management and treatment outcomes of MDR-TB: results from a setting with high rates of drug resistance. Int J Tuberc Lung Dis 2015; 19: 1109–14. [DOI] [PubMed] [Google Scholar]
- 62.Anger HA, Dworkin F, Sharma S, Munsiff SS, Nilsen DM, Ahuja SD. Linezolid use for treatment of multidrug-resistant and extensively drug-resistant tuberculosis, New York City, 2000–06. J Antimicrob Chemother 2010; 65: 775–83. [DOI] [PubMed] [Google Scholar]
- 63.Eker B, Ortmann J, Migliori GB, et al. Multidrug- and extensively drug-resistant tuberculosis, Germany. Emerg Infect Dis 2008; 14: 1700–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borisov SE, Dheda K, Enwerem M, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J 2017; 49: 1700387. [DOI] [PubMed] [Google Scholar]
- 65.WHO. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization, 2019. [PubMed] [Google Scholar]
- 66.Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 2012; 366: 2151–60. [DOI] [PubMed] [Google Scholar]
- 67.Skripconoka V, Danilovits M, Pehme L, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J 2013; 41: 1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reuter A, Tisile P, von Delft D, et al. The devil we know: is the use of injectable agents for the treatment of MDR-TB justified? Int J Tuberc Lung Dis 2017; 21: 1114–26. [DOI] [PubMed] [Google Scholar]
- 69.Conradie F, Diacon A, Howell P, et al. Sustained high rate of successful treatment outcomes: interim results of 75 patients in the Nix-TB clinical study of pretomanid, bedaquiline and linezolid. Netherlands: TB Alliance, 2018. [Google Scholar]
- 70.WHO. A practical handbook on the pharmacovigilance of medicines used in the treatment of tuberculosis. Geneva: World Health Organization, 2012. [Google Scholar]
- 71.Borisov S, Danila E, Maryandyshev A, et al. Surveillance of adverse events in the treatment of drug-resistant tuberculosis: first global report. Eur Respir J 2019; 54: 1901522. [DOI] [PubMed] [Google Scholar]
- 72.Doshi R, Falzon D, Thomas BV, et al. Tuberculosis control, and the where and why of artificial intelligence. ERJ Open Res 2017; 3: 00056–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


