Abstract
Introduction
Abnormal coagulation function has been demonstrated to be involved in the disease progression of COVID-19. However, the association between D-dimer levels and the severity of COVID-19 is not clear. The study was aimed to investigate the association between D-dimer levels and the severity of COVID-19 based on a cohort study and meta-analysis.
Materials and methods
Demographic and clinical data of all confirmed cases with COVID-19 on admission to Tongji Hospital from January 27 to March 5, 2020, were collected and analyzed, and coagulation function parameters were described and compared between patients with severe infection and those with non-severe infection. Cohort studies reporting risk estimates for the D-dimer and severity of COVID-19 association were searched and included to perform a meta-analysis.
Results
In our cohort study, patients with severe disease were more likely to exhibit dysregulated coagulation function, and a significantly higher D-dimer level (median 1.8 μg/ml [interquartile range 0.9–4.6] vs 0.5 [0.3–1.1], p < 0.001) was found in severe cases than the mild ones, on admission. In the meta-analysis of 13 cohort studies (including the current study), patients with severe disease had an increase in mean D-dimer value by 0.91 (95% confidence interval, 0.51–1.31, p < 0.001) μg/ml compared to those with non-severe disease, and odds of severe infection was associated with D-dimer greater than 0.5 μg/ml (odds ratio = 5.78, 95% confidence interval, 2.16–15.44, p < 0.001) on admission.
Conclusions
Patients with severe COVID-19 have a higher level of D-dimer than those with non-severe disease, and D-dimer greater than 0.5 μg/ml is associated with severe infection in patients with COVID-19.
Abbreviations: COVID-19, coronavirus disease 2019; CI, confidence interval; OR, odds ratio; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; SD, standard deviation; WMD, weighted mean difference; IQR, interquartile range; SARS-CoV, severe acute respiratory syndrome coronavirus
Keywords: COVID-19, D-dimer, Severity
Highlights
-
•
Severe infection of COVID-19 exhibit more obvious dysregulated coagulation function compared with mild cases.
-
•
Patients with severe COVID-19 have a higher level of D-dimer than mild patients.
-
•
D-dimer greater than 0.5 μg/ml is associated with severity of COVID-19.
1. Introduction
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) and the disease it caused, now known as coronavirus disease 2019 (COVID-19), were initially reported in Wuhan, China and rapidly spread throughout the world [1]. As of June 25, 2020, more than 9 million laboratory-confirmed cases have been identified in 208 countries and areas, with more than 480,000 fatal cases, according to the data from World Health Organization (WHO) reports [2].
.In recent studies documenting the clinical features of confirmed patients with COVID-19, it has been reported that most of them would present a type of mild infection of the COVID-19 disease [3,4]. However, a number of patients were observed to present with severe infection on admission with high mortality. Therefore, it is crucial to discriminate accurately among subjects with COVID-19 who have a high risk of severe infection and guide the use of different therapies at an early stage. Abnormal coagulation function, including elevated D-dimer, has been demonstrated to be more common in deceased patients with COVID-19, and increasing odds of in-hospital death was associated with D-dimer greater than 1 μg/ml [5,6]. However, the association between D-dimer and the severity of COVID-19 is not clear.
In our cohort study, epidemiological, clinical characteristics, and coagulation function parameters of patients with confirmed COVID-19 on admission were collected and compared, between patients with severe infection and those with non-severe infection. Further, a meta-analysis including our cohort study was then conducted to evaluate the association between D-dimer levels and severe COVID-19.
2. Materials and methods
2.1. Study design and participants recruited
Totally 1561 patients with COVID-19 were recruited retrospectively from January 27th to March 5th, 2020, at Tongji hospital, the largest comprehensive medical center in Wuhan and the specific hospital for the treatment of patients with severe COVID-19 in Wuhan designated by the Chinese government. Laboratory confirmation of COVID-19 was carried out by real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) using method described previously [4]. This study was approved by Tongji Hospital Ethics Committee (IRB ID: TJ-C20200121). Written informed consent was waived by the Ethics Commission of Tongji hospital for emerging infectious diseases.
2.2. Data collection from electronic medical records
Epidemiological characteristics, comorbidities, coagulation function markers on admission and disease severity, were obtained from patients' medical records. D-dimer were detected using a STA-R MAX coagulation analyzer and original reagents (Diagnostica Stago, Saint-Denis, France). The severity of COVID-19 was defined according to WHO clinical management guidance of COVID-19 [7]. Severe type was defined as followed: fever or suspected respiratory infection, plus one of respiratory rate > 30 breaths/min, severe respiratory distress, or SpO2 < 90% on room air.
2.3. Search strategy of meta-analysis
This work has been performed according to PRISMA guidelines (http://www.prisma-statement.org). PubMed, Web of Science, Embase, bioRxiv and medRxiv were searched using the following search terms: (2019 novel coronavirus OR 2019-nCoV OR coronavirus disease 2019 OR COVID-19 OR SARS-CoV-2) AND (laboratory OR D-dimer). The search was limited between the December 1, 2019 and March 17, 2020, without language restriction. The studies that reported the value of D-dimer on admission in patients with severe disease and those with non-severe disease, were included in a meta-analysis. Studies from our center (Tongji hospital) were excluded. Two of the authors scanned and selected the studies independently, and any controversies were resolved by discussion, with a kappa of 0.78 for inter-rater agreement.
2.4. Data extraction of meta-analysis
The identified name of the study (first author, year of publication), sample size, the value of D-dimer, the number of patients with abnormal D-dimer (≥0.5 μg/ml), were retrieved from the articles directly or by calculation. Two of the authors extracted the corresponding data independently, and any controversies were resolved by discussion. The mean and standard deviation (SD) were imputed when a study only provided median and interquartile ranges, as described in other studies [8,9].
2.5. Efficacy measures of meta-analysis
Differences of mean value of D-dimer between patients with severe disease and patients with non-severe disease, and odds of severe COVID-19 associated with D-dimer greater than 0.5 μg/ml, on admission, were defined as the outcome of the meta-analysis.
2.6. Statistical analysis
For our cohort study, continuous variables were described as medians (IQR), and compared using Mann-Whitney U test. Categorical variables were delineated as n (%) and compared by χ2 test and Fisher's exact test. The association between clinical characteristics and the presence of severe infection were determined by multivariable logistic regression analysis. The results were expressed as odds ratio (OR), 95% confidence intervals (CI), and p values. For individual statistical tests on the coagulation function, we calculated a corrected critical P value of 0.0063 (0.05/8 where 8 = the eight tested parameters of coagulation function) using the Bonferroni correction method. For multivariable logistic regression analysis, Bonferroni-Holm (B-H) correction was used to adjust for multiple testing, and results were defined as significant if the p-value of the regression analysis was lower or equal as the B-H corrected p-value. For all other analyses, the statistical significance was set at 0.05. All tests were two-sided.
For meta-analysis, weighted mean difference (WMD) with 95% confidence interval (95% CI) and odds ratio (OR) with 95% CI, were pooled for differences of D-dimer value and odds of severe COVID-19, respectively. The heterogeneity was evaluated using I2 and p value based on Chi-square test. I2 ≤ 50% or p ≥ 0.1 demonstrated no significant heterogeneity, and a fixed-effects model was used. I2 > 50% or p < 0.1 indicated a significant heterogeneity, and a random-effects model was applied. Funnel plot and the Egger's test were performed to evaluate publication bias. STATA statistical version 12.0 (Stata Corporation, College Station, Texas, USA) was used for the data analyses.
3. Results
3.1. Clinical characteristics and coagulation function of patients with COVID-19 in our cohort
Comparison of characteristics between patients with severe disease and mild cases in our cohort study is shown in Table 1 . Among the 1561 patients with confirmed COVID-19, 365 (23.4%) patients were clinically diagnosed with severe infection, and 1196 (76.6%) were diagnosed with mild infection. Older (median 67 years [interquartile range, IQR 58–73] vs 60 [47–68], p < 0.001) and male (204/365 [56%] vs 576/1196 [48%], p = 0.006) patients were more common in the severe group when compared with those with non-severe disease. More comorbidities, including hypertension (162/365[44%] vs 349/1196[29%], p < 0.001), diabetes (74/365[20%] vs 159/1196[13%], p = 0.001), chronic obstructive pulmonary disease (11/365[3%] vs 11/1196[1%], p = 0.006), and cardiovascular disease (47/365[13%] vs 108/1196[9%], p = 0.022), were found in patients with severe disease as compared to those with mild COVID-19. It should be interpreted with caution as the p-values were not adjusted for multiple testing.
Table 1.
Clinical characteristics and coagulation function of patients with COVID-19.
| No. (%) |
p value | |||
|---|---|---|---|---|
| All patients (n = 1561) | Mild (n = 1196) | Severe (n = 365) | ||
| Characteristics | ||||
| Age, median (IQR), range, years | 62 (50–70) | 60 (47–68) | 67 (58–73) | <0.001 |
| Sex | 0.006 | |||
| Male | 780 (50) | 576 (48) | 204 (56) | |
| Female | 781 (50) | 620 (52) | 161 (44) | |
| Smoking | 103 (7) | 75 (6) | 28 (8) | 0.204 |
| Comorbidities | ||||
| Chronic obstructive pulmonary disease | 22 (1) | 11 (1) | 11 (3) | 0.006 |
| Hypertension | 511 (33) | 349 (29) | 162 (44) | <0.001 |
| Cardiovascular disease | 155 (10) | 108 (9) | 47 (13) | 0.022 |
| Cerebrovascular disease | 40 (3) | 29 (2) | 11 (3) | 0.323 |
| Chronic liver disease | 22 (1) | 18 (2) | 4 (1) | 0.388 |
| Diabetes | 233 (15) | 159 (13) | 74 (20) | 0.001 |
| Tuberculosis | 20 (1) | 18 (2) | 2 (1) | 0.119 |
| Malignant tumor | 46 (3) | 30 (3) | 16 (4) | 0.051 |
| Chronic kidney disease | 35 (2) | 25 (2) | 10 (3) | 0.289 |
| Coagulation function, median (IQR) | ||||
| Prothrombin time (s; normal range 11.5–14.5) | 13.8 (13.2–14.5) | 13.6 (13.1–14.2) | 14.4 (13.7–15.4) | <0.001a |
| Activated partial thromboplastin time (s; normal range 29.0–42.0) | 38.7 (35.7–42.4) | 38.5 (35.7–42) | 39.4 (35.8–43.8) | 0.009a |
| Thrombin time (s; normal range 14.0–19.0) | 16.5 (15.7–17.5) | 16.4 (15.7–17.3) | 16.9 (15.8–18.2) | <0.001a |
| Fibrinogen (g/L; normal range 2.00–4.00) | 4.6 (3.6–5.7) | 4.3 (3.4–5.5) | 5.3 (4.0–6.5) | <0.001a |
| Fibrin(ogen) degradation products (μg/ml; normal range < 5.0) | 4.0 (4.0–6.0) | 4.0 (4.0–4.5) | 6.5 (4.0–21.8) | <0.001a |
| Prothrombin activity (%; normal range 75.0–125.0) | 91.0 (84.0–99.0) | 93.0 (86.0–101.0) | 84.0 (74.0–92.5) | <0.001a |
| International normalized ratio (normal range 0.80–1.20) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.1 (1.1–1.2) | <0.001a |
| D-dimer (μg/ml; normal range 0.0–0.5) | 0.7 (0.3–1.6) | 0.5 (0.3–1.1) | 1.8 (0.9–4.6) | <0.001a |
Data are median (IQR), and n (%). p values were calculated from the comparison between patients with mild infection and patients with severe infection using χ2 test, Fisher's exact-test (for categorical variables), t-test or Mann-Whitney U test (for continuous variables).
IQR = interquartile range. COVID-19 = coronavirus disease 2019.
Statistically significant when p values were lower or equal as the Bonferroni corrected p value of 0.0063.
Moreover, patients with severe disease were significantly more likely to exhibit dysregulated coagulation function. Elevated levels of prothrombin time (median 14.4 [IQR 13.7–15.4] vs 13.6 [13.1–14.2] s, p < 0.001), thrombin time (16.9 [15.8–18.2] vs 16.4 [15.7–17.3] s, p < 0.001), fibrinogen (5.3 [4.0–6.5] vs 4.3 [3.4–5.5] g/L, p < 0.001), fibrin(ogen) degradation products (6.5 [4.0–21.8] vs 4.0 [4.0–4.5] μg/ml, p < 0.001), and international normalized ratio (1.1 [1.1–1.2] vs 1.0 [1.0–1.1], p < 0.001), and decreased prothrombin activity (84.0 [74.0–92.5] vs 93.0 [86.0–101.0] %, p < 0.001) were observed in patients with severe disease when compared with non-severe group. Particularly, a significantly increased level of D-dimer (1.8 [0.9–4.6] vs 0.5 [0.3–1.1] μg/ml, p < 0.001) was found in patients with severe disease (Table 1, Fig. 1 ). Multivariate logistic regression analysis showed presence of severe COVID-19 was associated with prothrombin time longer than 14.5 s (2.71, 2.00–3.67; p < 0.001, B-H corrected significance level: p = 0.0029), fibrin(ogen) degradation products greater than 5.0 μg/ml (2.33, 1.74–3.12, p < 0.001, B-H corrected significance level: p = 0.0031), and D-dimer greater than 0.5 μg/ml (3.44, 2.29–5.17; p < 0.001, B-H corrected significance level: p = 0.0028), on admission, as shown in Table 2 . These results suggested that the severity of COVID-19 was associated with coagulation dysfunction in Tongji Hospital.
Fig. 1.
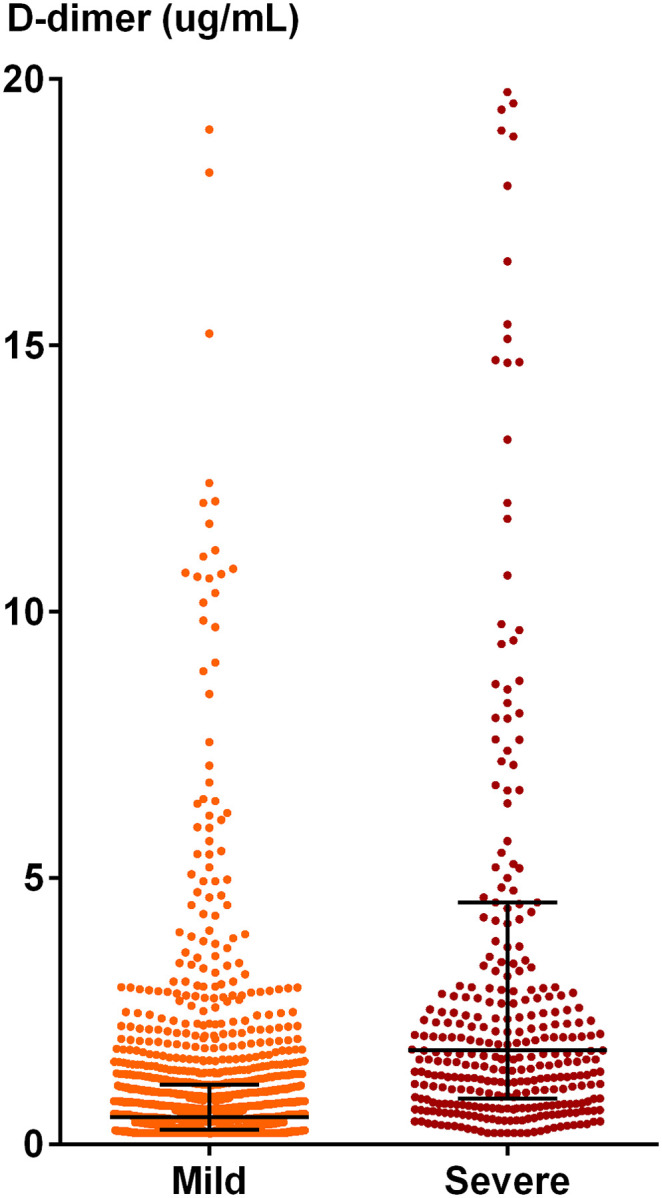
Scatter plots of D-dimer levels in the severe and mild groups in our cohort study. 58 data points are outside the axis limit as 13 patients in mild group and 45 patients in severe group have D-dimer values over the upper limit of measuring range (>21 μg/ml). Data are shown in median (interquartile range).
Table 2.
Logistics regression of factors associated with severity of COVID-19.
| Multivariate analysis |
Bonferroni-Holm corrected p-value | |||
|---|---|---|---|---|
| OR | 95% CI | p value | ||
| Characteristics | ||||
| Age, years | 1.01 | (0.99, 1.02) | 0.07 | 0.0045 |
| Sex (male vs female) | 1.04 | (0.78, 1.37) | 0.80 | 0.025 |
| Smoking history (yes vs no) | 1.15 | (0.68, 1.96) | 0.61 | 0.01 |
| Comorbidities (yes vs no) | ||||
| Chronic obstructive pulmonary disease | 2.17 | (0.82, 5.74) | 0.12 | 0.005 |
| Hypertension | 1.48 | (1.09, 1.99) | 0.01 | 0.0033 |
| Cardiovascular disease | 0.87 | (0.57, 1.33) | 0.51 | 0.0083 |
| Cerebrovascular disease | 0.61 | (0.27, 1.35) | 0.22 | 0.0063 |
| Chronic liver disease | 0.75 | (0.23, 2.44) | 0.63 | 0.013 |
| Diabetes | 1.24 | (0.86, 1.78) | 0.24 | 0.0071 |
| Tuberculosis | 0.23 | (0.05, 1.09) | 0.06 | 0.0038 |
| Malignant tumor | 1.73 | (0.85, 3.49) | 0.13 | 0.0056 |
| Chronic kidney disease | 0.90 | (0.39, 2.06) | 0.80 | 0.05 |
| Coagulation function | ||||
| Prothrombin timea, s | ||||
| ≤14.5 | Reference | |||
| >14.5 | 2.71 | (2.00, 3.67) | <0.001 | 0.0029 |
| Activated partial thromboplastin time, s | ||||
| ≤42.0 | Reference | |||
| >42.0 | 1.06 | (0.79, 1.44) | 0.69 | 0.017 |
| Thrombin time, s | ||||
| ≤19.0 | Reference | |||
| >19.0 | 1.51 | (0.97, 2.35) | 0.07 | 0.0042 |
| Fibrinogen, g/L | ||||
| ≤4.00 | Reference | |||
| >4.00 | 1.48 | (1.08, 2.03) | 0.02 | 0.0036 |
| Fibrin(ogen) degradation products, μg/ml | ||||
| <5.0 | Reference | |||
| ≥5.0 | 2.33 | (1.74, 3.12) | <0.001 | 0.0031 |
| D-dimer, μg/ml | ||||
| <0.5 | Reference | |||
| ≥0.5 | 3.44 | (2.29, 5.17) | <0.001 | 0.0028 |
p values were calculated from logistic regression analysis to estimate the odds ratio of presence of severe infection in patients with COVID-19.
COVID-19 = coronavirus disease 2019; OR = odds ratio; CI = confidence interval.
Prothrombin time, international normalized ratio and prothrombin activity are three different expressions of the same laboratory parameter, and only prothrombin time was included in the multivariable logistic regression model.
3.2. Study selection for meta-analysis in patients with COVID-19
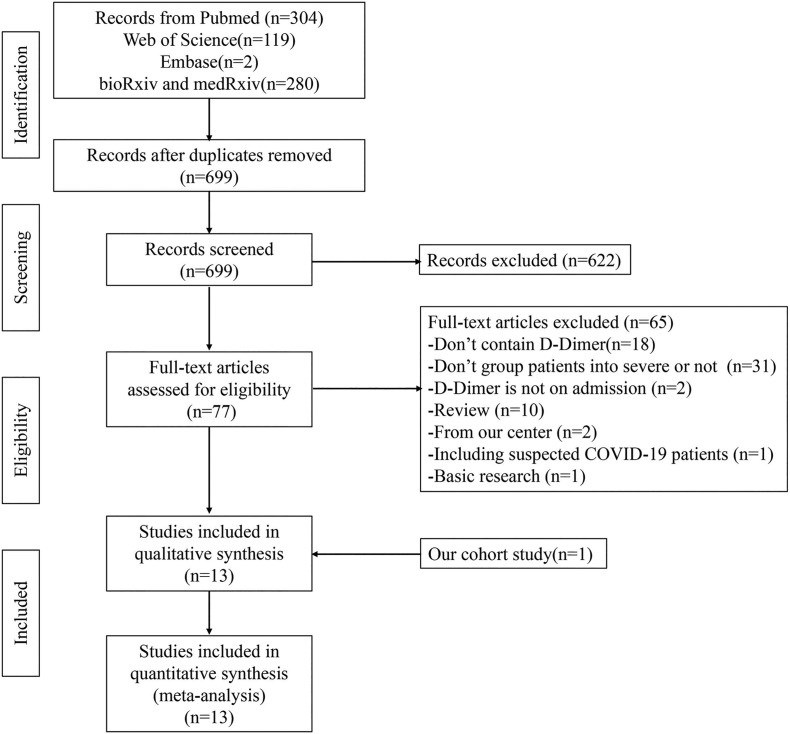
As shown in Fig. 2 , a total of 699 articles were identified from electronic database after removal of duplicate studies. Finally, 13 studies (including our cohort study) were included for this meta-analysis. The characteristics of these 13 studies were shown in Table 3 . Of the 12 studies other than our cohort study, 8 studies reported the absolute value of D-dimer in patients with severe disease and those with non-severe disease [[10], [11], [12], [13], [14], [15], [16], [17]]. The odds ratio (OR) of severe infection in patients with D-dimer greater than 0.5 μg/ml were obtained directly or by calculation from our cohort study and 4 other studies [3,[18], [19], [20]].
Fig. 2.
Flow chart presenting the process of literature search for this meta-analysis.
Table 3.
Characteristics of patients from 12 published studies and our cohort study included in the meta-analysis.
| Study | All patients |
Non-severe |
Severe |
|||||
|---|---|---|---|---|---|---|---|---|
| Sample size | D-dimer (μg/ml) | Sample size | D-dimer (μg/ml) | No. of abnormal D-dimer | Sample size | D-dimer (μg/ml) | No. of abnormal D-dimer | |
| Cao M et al. [10] | 195 | 0.39(0.28–0.67) | 176 | 0.37(0.26–0.56) | NA | 19 | 0.77(0.43–1.23) | NA |
| Liu J et al. [11] | 40 | 0.6(0.3–0.9) | 27 | 0.4(0.2–0.8) | NA | 13 | 0.9(0.7–1.5) | NA |
| Liu L et al. [12] | 51 | 0.28(0.19–0.51) | 44 | 0.28(0.18–0.46) | NA | 7 | 0.6(0.28–1.4) | NA |
| Lu HZ et al. [13] | 265 | 0.42(0.29–0.8) | 243 | 0.39(0.28–0.72) | NA | 22 | 0.8(0.5–3.5) | NA |
| Qian GQ et al. [14] | 91 | 0.3(0.11–0.45) | 82 | 0.3(0.11–0.4) | NA | 9 | 0.45(0.16–0.49) | NA |
| Xu Y et al. [15] | 69 | 0.5(0.3–1.2) | 44 | 0.5(0.3–0.9) | NA | 25 | 2.3(0.6–14.1) | NA |
| Zhang GQ et al. [16] | 221 | 0.23(0.13–0.49) | 166 | 0.18(0.12–0.32) | NA | 55 | 0.44(0.21–1.30) | NA |
| Zhang JJ et al. [17] | 81 | 0.2(0.1–0.5) | 43 | 0.2(0.1–0.3) | NA | 38 | 0.4(0.2–2.4) | NA |
| Chen X et al. [18] | 254 | NA | 209 | NA | 199 | 45 | NA | 44 |
| Guan W et al. [3] | 560 | NA | 451 | NA | 195 | 109 | NA | 65 |
| Liu T et al. [19] | 80 | NA | 11 | NA | 0 | 69 | NA | 45 |
| Qi D et al. [20] | 267 | NA | 217 | NA | 6 | 50 | NA | 13 |
| Our study | 1561 | 0.7(0.33–1.61) | 1196 | 0.52(0.28–1.13) | 621 | 365 | 1.77(0.86–4.55) | 327 |
Data of D-dimer value were shown in median (IQR). IQR = interquartile range.
3.3. Higher level of D-dimer in patients with severe COVID-19
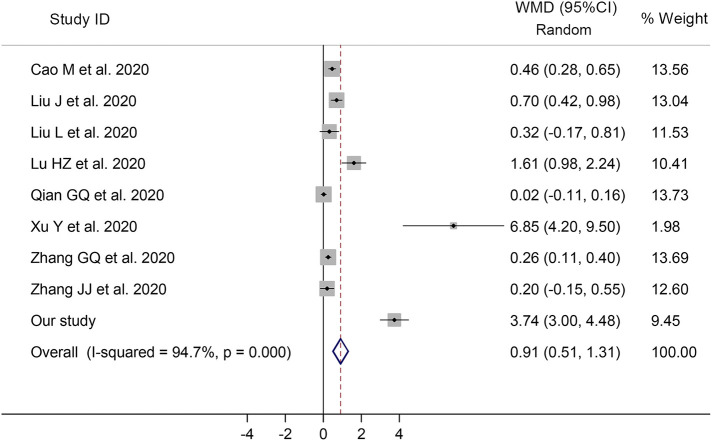
For meta-analysis concerning the mean value of D-dimer, 9 studies (including our cohort study) were included, with 2574 patients in total. The mean value of D-dimer of all patients was 0.34 (standard deviation SD, 0.14) μg/ml, 0.30 (0.12) for patients with non-severe disease, and 0.89 (0.34) for severe patients. Differences of mean value of D-dimer between severe and non-severe group was 0.91 μg/ml (95% confidence interval CI, 0.51–1.31, p < 0.001, I2 = 94.7%), after pooling WMD for these studies, as shown in Fig. 3 , suggesting a significant increase of D-dimer level in patients with severe disease than those with non-severe disease, on admission.
Fig. 3.
Forest plot showing the weighted mean difference (WMD) of D-dimer values between patients with severe disease and those with non-severe disease.
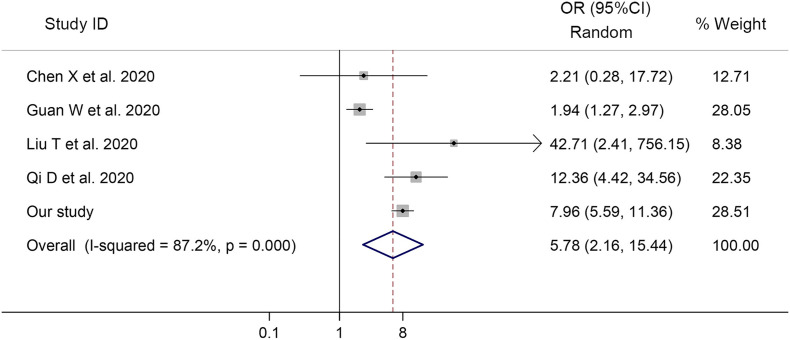
3.4. Odds of severe COVID-19 in patients with abnormal D-dimer
For meta-analysis concerning the odds of severe COVID-19, 4 studies and our cohort study were included. The odds ratio of severe COVID-19 associated with D-dimer greater than 0.5 μg/ml was 5.78 (95% CI, 2.16–15.44, p < 0.001, I2 = 87.2%, Fig. 4 ), indicating that the severe COVID-19 was associated with increased level of D-dimer on admission.
Fig. 4.
Forest plot showing the odds ratios (OR) with 95% confidence interval (95% CI) for severe COVID-19 in patients with D-dimer greater than 0.5 μg/ml compared to patients with normal D-dimer values.
3.5. Publication bias
The funnel plots and the Egger's test (p = 0.01) for the studies reporting the absolute value of D-dimer showed that a publication bias existed in these studies (Supplementary Fig. 1A). And no significant publication bias was detected among studies evaluating the odds of severe COVID-19 in patients with abnormal D-dimer, with Egger's test showing p = 0.794 (Supplementary Fig. 1B).
4. Discussion
Abnormal coagulation function, including elevated D-dimer, has been demonstrated to be involved in the disease progression of COVID-19 [5,21]. In this study, we analyzed the association between elevated D-dimer levels and the disease severity of COVID-19 based on the evidence from our cohort study and meta-analysis. In our retrospective cohort study, the level of D-dimer was markedly increased in patients with severe COVID-19, and the meta-analysis further confirmed that odds of severe COVID-19 was associated with D-dimer greater than 0.5 μg/ml.
D-dimer assays are commonly used in clinical practice to exclude a diagnosis of deep vein thrombosis or pulmonary embolism, and elevated D-dimer indicates increased risk of abnormal blood clotting. Elevated levels of D-dimer were also found to be related with higher mortality rate of community-acquired pneumonia [22]. Patients with severe community-acquired pneumonia had significantly higher D-dimer levels, and D-dimer within normal range indicated low risk for complications [23]. Augmented activity of urokinase could cause hyperfibrinolysis, by increasing cleavage of plasminogen into the active plasmin, and finally led to diffuse alveolar damage and acute lung injury, in a mouse model of SARS-CoV disease [24]. In our cohort study, the level of coagulation function parameters, including prothrombin time, fibrinogen, fibrin(ogen) degradation products, and D-dimer, were found elevated in patients with severe COVID-19. Presumably, the severity of COVID-19 might also be associated with coagulation dysfunction.
Recent studies documenting the laboratory changes of patients with confirmed COVID-19 have noted that elevated D-dimer might be associated with the disease progression of COVID-19. The level of D-dimer in patients with COVID-19 admitted to the ICU was reported significantly increased [25]. Clinical attention to venous thromboembolism risk should particularly be paid to those patients with severe COVID-19, who were often bedridden and presented with abnormal coagulation function [25,26]. Rapid deterioration was observed in cases with significantly increased D-dimer during the disease progression. In this regard, pulmonary embolism after deep vein thrombosis detachment should be considered and immediately on the alert, especially when patients presented clinical manifestations such as a rapid drop in blood pressure, sudden deterioration of oxygenation, and respiratory distress.
In addition to thrombosis and pulmonary embolism, D-dimer might be a manifestation of severe virus infection. A virus infection may develop into sepsis and induce coagulation dysfunction, which was common in serious disease progression. Moreover, the increase of D-dimer may be an indirect manifestation of inflammatory reaction, as inflammatory cytokines could cause the imbalance of coagulation and fibrinolysis in the alveoli, which may activate the fibrinolysis system, and then increase the level of D-dimer [27,28]. And D-dimer greater than 1 μg/ml was found a risk factor of poor prognosis for patients with COVID-19 [6]. Abnormal levels of D-dimer were also associated with 28-day mortality in patients with COVID-19, and low molecular weight heparin treatment might be beneficial to COVID-19 patients with markedly elevated D-dimer (i.e. over 3 μg/ml) with reduced mortality rate [27].
There were several limitations in our study. Firstly, a significant degree of heterogeneity and a publication bias were detected in the meta-analysis, because most of included studies were retrospective and non-randomized controlled trial. Secondly, converting non-normally distributed statistics (median and range) to normally distributed statistics (mean and SD) may cause a bias, when evaluating the changes of D-dimer value between severe patients and non-severe patients. Thirdly, the methods of D-dimer assay were not clear in studies included in this meta-analysis. Finally, the odds of severe COVID-19 associated with abnormal level of D-dimer, was based on univariable analysis or obtained by calculation in some studies [3,[18], [19], [20]]. Therefore, the bias may be inevitable.
Nevertheless, our study demonstrated that the D-dimer level in patients with severe COVID-19 was higher than that in mild cases. Thus, the evidence that patients with elevated D-dimer levels might have a higher risk of severe infection from our cohort study and the meta-analysis, provided a timely reminder to physicians that those COVID-19 patients with higher D-dimer should attract more attention in early time.
The following are the supplementary data related to this article.
Funnel plots of weighted mean difference (WMD) of D-dimer values (A) and univariate odds ratios (OR) for severe COVID-19 (B).
Authorship contributions
Dai-Shi Tian and Wei Wang conceived and designed study. Hai-Han Yu, Chuan Qin and Man Chen collected data. Chuan Qin performed statistical analyses about the cohort study, and Hai-Han Yu for the meta-analysis. Dai-Shi Tian, Hai-Han Yu and Chuan Qin wrote the initial draft of the manuscript. Final approval was required by all authors.
Funding
This work was supported by the National Natural Science Foundation of China (81873743 to D.S. Tian, 81801223 to C. Qin).
Declaration of competing interest
None.
Acknowledgments
None.
References
- 1.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. New Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Coronavirus disease 2019 (COVID-19) situation report – 51. March 11 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10
- 3.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. New Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Li D., Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO 2019-nCoV/clinical/2020.4. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 8.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T., Li G., Li L. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J. Am. Coll. Cardiol. 2007;49(15):1642–1648. doi: 10.1016/j.jacc.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 10.Cao M., Zhang D., Wang Y. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv. 2020 doi: 10.1101/2020.03.04.20030395. 2020.03.04.20030395. [DOI] [Google Scholar]
- 11.Liu J., Li S., Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. medRxiv. 2020 doi: 10.1101/2020.02.16.20023671. 2020.02.16.20023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L., Gao J.-Y. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxiv. 2020 doi: 10.1101/2020.02.20.20025536. 2020.02.20.20025536. [DOI] [Google Scholar]
- 13.Lu H., Ai J., Shen Y. A descriptive study of the impact of diseases control and prevention on the epidemics dynamics and clinical features of SARS-CoV-2 outbreak in Shanghai, lessons learned for metropolis epidemics prevention. medRxiv. 2020 doi: 10.1101/2020.02.19.20025031. 2020.02.19.20025031. [DOI] [Google Scholar]
- 14.Qian G.-Q., Yang N.-B., Ding F. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. medRxiv. 2020 doi: 10.1101/2020.02.23.20026856. 2020.02.23.20026856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y., Li Y.-r., Zeng Q. Clinical characteristics of SARS-CoV-2 pneumonia compared to controls in Chinese Han population. medRxiv. 2020 doi: 10.1101/2020.03.08.20031658. 2020.03.08.20031658. [DOI] [Google Scholar]
- 16.Zhang G., Hu C., Luo L. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.03.02.20030452. 2020.03.02.20030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 18.Chen X., Zheng F., Qing Y. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. medRxiv. 2020 doi: 10.1101/2020.03.03.20030353. 2020.03.03.20030353. [DOI] [Google Scholar]
- 19.Liu T., Zhang J., Yang Y. The potential role of IL-6 in monitoring severe case of coronavirus disease 2019. medRxiv. 2020 doi: 10.1101/2020.03.01.20029769. 2020.03.01.20029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi D., Yan X., Tang X. Epidemiological and clinical features of 2019-nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple-center study. medRxiv. 2020 doi: 10.1101/2020.03.01.20029397. 2020.03.01.20029397. [DOI] [Google Scholar]
- 21.Han H., Yang L., Liu R. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 22.Querol-Ribelles J.M., Tenias J.M., Grau E. Plasma d-dimer levels correlate with outcomes in patients with community-acquired pneumonia. Chest. 2004;126(4):1087–1092. doi: 10.1378/chest.126.4.1087. [DOI] [PubMed] [Google Scholar]
- 23.Snijders D., Schoorl M., Schoorl M. D-dimer levels in assessing severity and clinical outcome in patients with community-acquired pneumonia. A secondary analysis of a randomised clinical trial. Eur J Intern Med. 2012;23(5):436–441. doi: 10.1016/j.ejim.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Gralinski L.E., Bankhead A., 3rd, Jeng S. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. mBio. 2013;4(4) doi: 10.1128/mBio.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang N., Bai H., Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X.Y., Du B., Wang Y.S. The keypoints in treatment of the critical coronavirus disease 2019 patient. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E026. doi: 10.3760/cma.j.cn112147-20200224-00159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plots of weighted mean difference (WMD) of D-dimer values (A) and univariate odds ratios (OR) for severe COVID-19 (B).