Clinical Implications.
-
•
This is the first study describing the effects of the COVID-19 pandemic in a selected group of children presenting allergic asthma.
During the SARS-CoV-2 pandemic, Spain has been one of the most severely affected countries, with 217,573 confirmed cases as of the time of writing. Our institution, which serves a population of 300,000 (50,500 children), is located in one of the hardest-hit areas in the region of Madrid, an epicenter of the disease.
The incidence and severity of COVID-19 have been milder in children, who account for 0.6% of all cases nationwide.1 Among Spanish children, 1122 polymerase chain reaction (PCR)-confirmed cases have been detected between ages 0 and 14 years (53.4% male). Of these, 29.6% required hospitalization (55% male) and 4% intensive care (67.4% male).1 However, the actual incidence could be significantly higher, as no diagnostic tests were performed in this group because of the lower frequency of infection and severity.
In Spain, asthma affects 10% of children,2 making it the most common chronic disease. SARS-CoV-2 is a respiratory virus that, like others, could exacerbate asthma. Interestingly, the risk of asthma among children infected with the SARS-CoV-2 virus is unclear.3 This study aims to determine the impact of SARS-CoV-2 infection among children with asthma in our allergy department during the pandemic.
We included children aged ≤14 years with allergic asthma classified according to the 2019 Global Initiative for Asthma (GINA) guidelines.4 Of these, we analyzed probable cases of COVID-19 according to the World Health Organization recommendations.5
Demographic data, clinical characteristics (allergic comorbidities including atopic dermatitis, food allergy, rhinoconjunctivitis, aeroallergen sensitization assessed by skin prick test and/or specific IgE, asthma severity), lung function (forced spirometry in children ≥5 years), asthma treatment, and asthma exacerbations (oral corticosteroids and/or emergency care assistance) were recorded by consulting electronic medical records for the previous year.
During the pandemic (February-April 2020), the following data were obtained by 2 allergists via telephone interview: family size, presence of SARS-CoV-2 infection (adult cohabitants affected, signs and symptoms, date of onset, treatment, and severity) as well as an asthma-control assessment based on a validated Spanish child asthma-control questionnaire6 and asthma-control measures required in the previous 4 weeks.
Statistical analysis was performed using SPSS version 25 (SPSS Inc., Chicago, Ill). Quantitative variables were compared using the Student t test, and for nonparametric quantitative variables, we used the Mann-Whitney test; qualitative data were compared via the χ2 test or Fisher's exact test for categorical variables. The relation between the presence of infected adult cohabitants and probable child cases was assessed using the Pearson correlation coefficient.
A total of 212 children diagnosed with allergic asthma in our allergy unit were included. Cases were randomly selected from consecutive children with a scheduled medical appointment between April and May 2020. The median age was 10 years (range: 2-14 years); 63% were male and 76% had a family history of atopy. Regarding allergic comorbidities, 90% presented rhinitis, 85% conjunctivitis, 50% atopic dermatitis, and 36% food allergy. All were sensitized to at least 1 aeroallergen (Table I ).
Table I.
Total cases (n = 212): description of epidemiological and allergic features
| Epidemiological features | |
| Age (y), median (IQR) | 10 (7-11) |
| Gender M/T | 63% |
| Family atopy | 76% |
| Allergic comorbidities | |
| Rhinitis | 90% |
| Conjunctivitis | 85% |
| Atopic dermatitis | 50% |
| Food allergy | 36% |
| Sensitization to aeroallergens | |
| Pollen | 90% |
| Animal dander | 49% |
| Molds | 23% |
| House dust mite | 19% |
| Type of allergic asthma | |
| Seasonal | 49% |
| Perennial | 51% |
| Asthma treatment∗ | |
| Step 1 | 40% |
| Step 2 | 21% |
| Steps 3 and 4 | 35% |
| Step 5 | 4% |
GINA, Global Initiative for Asthma; IQR, interquartile range.
According to respiratory symptoms during the preceding year following GINA personalized management.
The median time between onset of asthma symptoms and evaluation was 4 years (range: 0.5-11 years; interquartile range = 2-6), including perennial (51%) and seasonal (49%) asthma.
The last spirometry performed in our unit showed normal lung function in 82% of patients. In accordance with the GINA guidelines,4 40% of children were included in step 1, 21% in step 2, 35% in steps 3 and 4, and 4% in step 5.
Sixty-six children (31%) had been living with an adult who presented compatible symptoms, 9 of these adults being severe cases confirmed by PCR. Nearly half of these children (44%) developed symptoms suggestive of infection (r = 0.569, P < .001).
We therefore identified 29 children (14%) as probable cases. Their median age was 10 years (range: 2-14 years), 79% were male, and 83% had a family history of atopy.
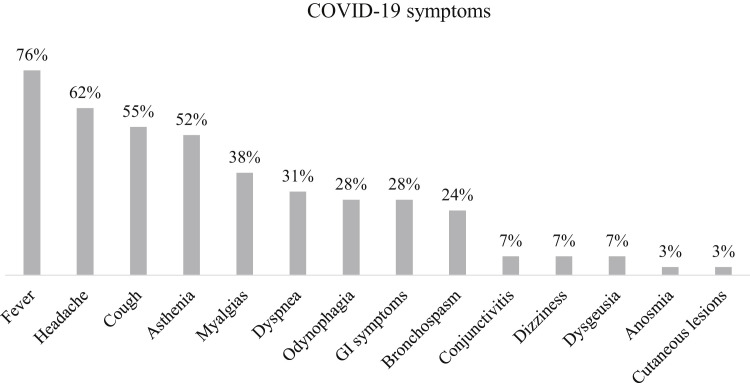
All of these patients developed mild symptoms related to COVID-19, mainly fever (76%), headache (62%), cough (55%), asthenia (52%), myalgia (38%), dyspnea (31%), odynophagia (28%), gastrointestinal symptoms (28%), and mild bronchospasm (24%) (Figure E1, available in this article's Online Repository at www.jaci-inpractice.org).
Figure E1.
Symptoms related to COVID-19 in allergic asthmatic children (n = 29). GI, Gastrointestinal.
Symptomatic treatment included paracetamol (86%), β2-agonist rescue inhaler (34%), or increased long-term asthma-control medications (14%). None required blood test, chest X-ray, or hospital admission, and only 1 child required treatment with oral corticosteroids. These results show a low prevalence of severe cases compared with other series published.1
An analysis of probable COVID-19 versus non-COVID-19 allergic asthmatic children revealed no statistically significant differences in age, gender, or family history of atopy. Regarding allergic comorbidities, we found significant differences in food allergy: 59% versus 33% (P = .007). No differences were observed with respect to atopic dermatitis, rhinitis, or conjunctivitis (Table II ).
Table II.
Comparison between COVID-19 and non-COVID-19 allergic asthmatic children
| Probable COVID-19 cases 29 (14%) | Non-COVID-19 cases 183 (86%) | Statistical differences | |
|---|---|---|---|
| Epidemiological features | |||
| Age (y), median (IQR) | 10 (7-11) | 10 (7-11) | P = .72 |
| Gender M/T | 79% | 61% | P = .06 |
| Family atopy | 83% | 75% | P = .35 |
| Allergic comorbidities | |||
| Rhinitis | 83% | 91% | P = .19 |
| Conjunctivitis | 76% | 87% | P = .12 |
| Atopic dermatitis | 62% | 47% | P = .15 |
| Food allergy | 59% | 33% | P = .007 |
| Asthma treatment∗ | |||
| Step 1 | 45% | 39% | P = .537 |
| Step 2 | 31% | 20% | P = .585 |
| Steps 3 and 4 | 24% | 37% | P = .487 |
| Step 5 | 0% | 4% | P = .378 |
| Lung function† | |||
| FVC, FEV1, and FEV1/FVC ≥ 80% | 72% | 75% | P = .682 |
| Asthma control‡ | |||
| OCS | 10% | 17% | P = .328 |
| Emergency care | 27% | 22% | P = .652 |
| Hospital admission | 3% | 6% | P = .505 |
| COVID-19 period (February-April 2020) | |||
| Asthma control questionnaire CAN < 8§ | 93% | 97% | P = .301 |
| Rhinoconjunctivitis | 38% | 42% | P = .714 |
| Increase in reliever treatment (SABA) | 34% | 8% | P < .001 |
| Increase in asthma controller treatment | 14% | 3% | P < .01 |
CAN, validated Spanish child asthma-control questionnaire; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GINA, Global Initiative for Asthma; IQR, interquartile range; M, male; OCS, oral corticosteroid; SABA, short-acting β-agonists; T, total cases in each group.
Bold indicates statistical significance (P < .05).
According to respiratory symptoms during the preceding year following GINA personalized management.
Forced basal spirometry performed on the last visit.
Control of asthma during the preceding year.
CAN questionnaire <8 indicates well-controlled asthma.
In addition, we found no differences in terms of lung function or the need for oral corticosteroids, emergency care, or hospital admission over the previous year.
During the pandemic, 38% of patients with COVID-19 complained of allergic rhinoconjunctivitis versus 42% of noninfected patients, with no differences observed between groups. However, we did find significant differences in asthma treatment: children with probable COVID-19 needed more β2-agonist inhaler treatment (34% vs 8%; P < .001) and increased controller treatment (14% vs 3%; P < .01).
Data showed no differences in asthma control or severity. In fact, during the phone call, parents did not report worsening of symptoms compared with the same period in the previous year.
This study, performed in a child allergic asthmatic cohort living in an area of Madrid with high exposure to SARS-CoV-2, found no demographic differences between asthmatic children with probable COVID-19 and those without infection.
As described in other reports,7 we observed familial clustering in SARS-CoV-2 infection, likely related to difficulties in self-isolation as a result of family living conditions.
Unexpectedly, asthmatic children did not develop an aggressive form of COVID-19 infection independently of asthma severity and control over the previous year. The symptoms related to COVID-19 were mild, the most frequent being fever, as described in another pediatric series.8 However, the cases studied required significantly more rescue and asthma-control treatment than patients without COVID-19.
Although this study was conducted during a period of seasonal allergy, no differences in the frequency of rhinoconjunctivitis were found between groups. This finding suggests that the asthma exacerbation observed in this cohort was due to SARS-CoV-2 infection and not to pollen exposure, as respiratory viruses such as this one can worsen symptoms.4
Interestingly, food allergy was more frequent in children with probable COVID-19, and no differences were found related to other allergic comorbidities. However, the relevance of this finding should be analyzed further.
This report describes a group of allergic asthmatic children during the COVID-19 pandemic. All presented mild symptoms, although SARS-CoV-2 infection elicited asthma exacerbation. Asthma severity and control were not associated with a worse clinical course, so we can conclude that allergic asthmatic children are not more vulnerable to suffer from COVID-19.
Footnotes
No funding was received for this work.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository
References
- 1.Informe n° 29 sobre la situacion de COVID-19 en España. Red Nacional de Vigilancia Epidemiológica. Instituto de Salud Carlos III. Ministerio de Ciencia e Innovacion. May 7, 2020. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/-COVID-19.-Informes-previos.aspx Available from: Accessed May 7, 2020.
- 2.Carvajal-Urueña I., García-Marcos L., Busquets-Monge R., Morales Suárez-Varela M., García de Andoin N., Batlles-Garrido J. Geographic variation in the prevalence of asthma symptoms in Spanish children and adolescents. International Study of Asthma and Allergies in Childhood (ISAAC) Phase 3, Spain. Arch Bronconeumol. 2005;41:659–666. doi: 10.1016/s1579-2129(06)60333-9. [DOI] [PubMed] [Google Scholar]
- 3.Abrams E.M., Szefler S.J. Managing asthma during COVID-19: an example for other chronic conditions in children and adolescents. J Pediatr. 2020;222:221–226. doi: 10.1016/j.jpeds.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma Global Strategy for Asthma Management and Prevention. 2020. www.ginasthma.org/ Available from: Accessed May 5, 2020.
- 5.World Health Organization Global surveillance for COVID-19 caused by human infection with COVID-19 virus. March 20, 2020. https://www.who.int/docs/default-source/coronaviruse/global-surveillance-for-covid-v-19-final200321-rev.pdf Available from: Accessed May 5, 2020.
- 6.Pérez-Yarza E.G., Badía X., Badiola C., Cobos N., Garde J., Ibero M. Development and validation of a questionnaire to assess asthma control in pediatrics. Pediatr Pulmonol. 2009;44:54–63. doi: 10.1002/ppul.20929. [DOI] [PubMed] [Google Scholar]
- 7.Chan J.F., Yuan S., Kok K.H., Kai-Wang To K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X., Xiang Y., Du H., Wing-Kin Wong G. SARS-CoV 2 infection in children—understanding the immune responses and controlling the pandemic. Pediatr Allergy Immunol. 2020;31:449–453. doi: 10.1111/pai.13267. [DOI] [PubMed] [Google Scholar]