Abstract
Bovine leukemia virus (BLV) is a virus that infects cattle around the world and is very similar to the human T-cell leukemia virus (HTLV), which causes adult T-cell leukemia/lymphoma (ATL). Recently, presence of BLV DNA and protein was demonstrated in commercial bovine products and in humans. BLV DNA is generally found at higher rates in humans who have or will develop breast cancer, according to research done with subjects from several countries. These findings have led to a hypothesis that BLV transmission plays a role in breast cancer oncogenesis in humans. Here we summarize the current knowledge in the field.
Keywords: breast cancer, bovine leukemia virus, BLV, HTLV
1. Introduction
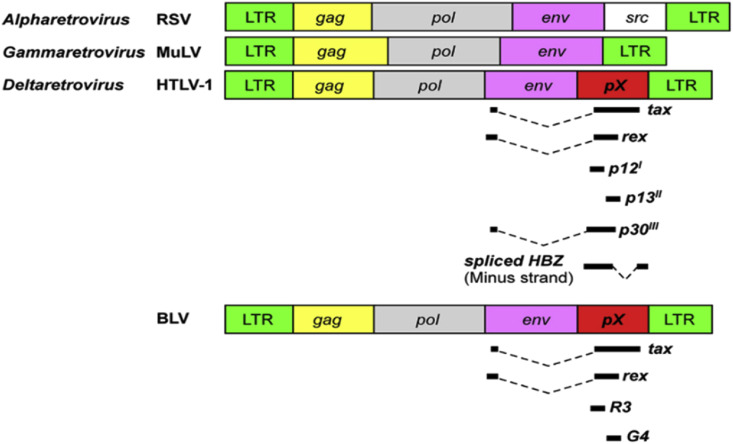
Bovine leukemia virus (BLV) is a delta retrovirus most similar to human T-cell lymphotropic virus 1 (HTLV-1). BLV causes enzootic bovine leukosis in cattle and is extremely common amongst commercial cattle herds around the world. BLV contains an oncogene coding for the Tax protein, which causes deficiency in DNA repair mechanisms, prevents apoptosis, inhibits tumor suppressors. Tax is also notably found in HTLV-1. The Tax gene is found inside the pX region between the env gene and one of the long terminal repeats (LTRs) [1]. (see Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5 )
Fig. 1.
Diagram of genomic organization of several viruses, including HTLV-1, murine leukemia virus, a simple retrovirus in mice (MULV), respiratory syncytial virus (RSV), and BLV. The organization of HTLV-1 and BLV is similar. Tax is present in the pX regions of both HTLV-1 and BLV (reproduced from the open source [1]).
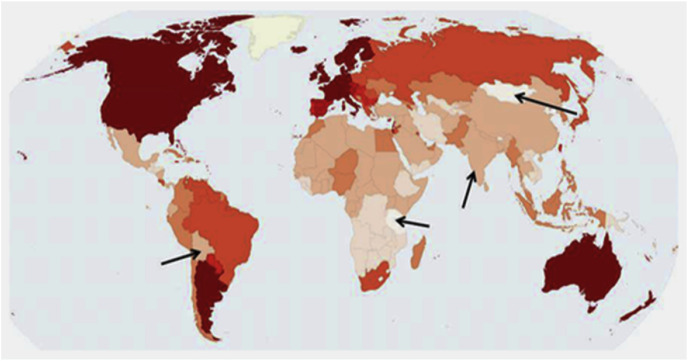
Fig. 2.
A map of global incidence of breast cancer. Bolivia, Mongolia, Tanzania, and sub-Saharan Africa excluding South Africa (note that South Africa is relatively more developed and has greater economy, leading to wider accessibility of beef and dairy, as well as a population of Europeans, which could influence diets) are notable outliers for breast cancer (Reproduced from the open source [30]).
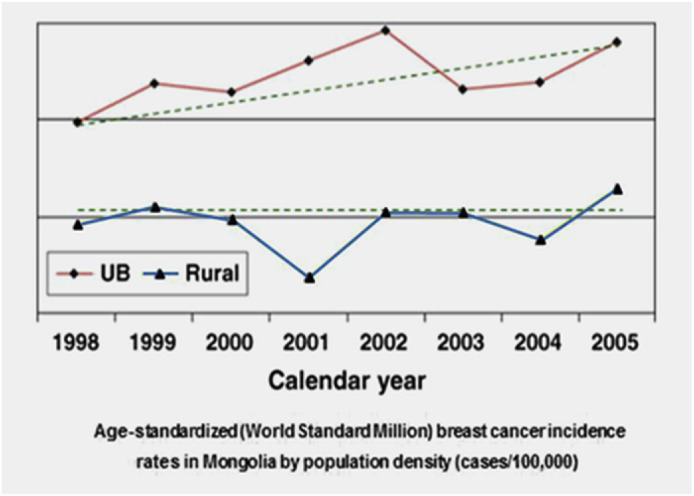
Fig. 3.
Incidence of breast cancer in Mongolia (Reproduced from the open source [29]).
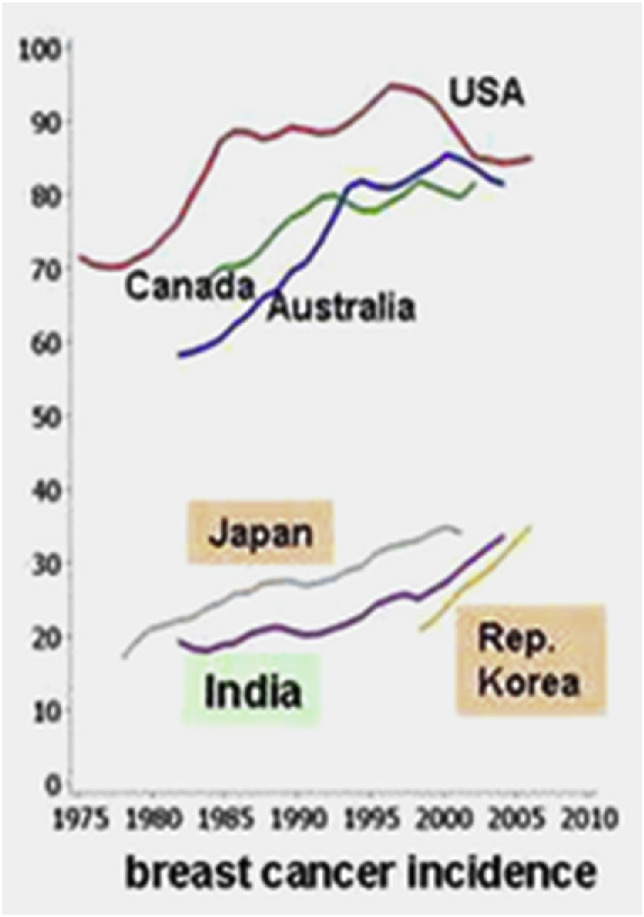
Fig. 4.
Changes in breast cancer incidence in several countries (Reproduced from the open source [30]).
Fig. 5.
The urban areas have more access to imported dairy and beef products from Bos taurus and also have higher consumption of dairy and beef due to higher wealth and location (Reproduced from the open source [30]).
Around 5% of infected cattle develop leukemia. A study by the United States Department of Agriculture (USDA) found that 83.9% of U.S. dairy operations tested positive for BLV [2].
Currently, BLV infection is detected via enzyme-linked immunosorbent assay (ELISA) and variations of PCR. BLV infection induces antibody production to BLV capsid proteins and viral envelope glycoproteins. Usually the capsid proteins are p24 and glycoproteins are gp51. These antibodies can be detected with immunoassays [3].
As infection continues, levels of antibodies usually increase. BLV infection is considered a lifelong infection because the virus integrates into the DNA of cells infected by it. BLV mainly infects B-lymphocytes but T-lymphocytes, monocytes, mammary epithelial cells, and granulocytes have also been shown to be infected.
Immortalized B-lymphocytes end up causing lymphocytosis in many infected cattle.
BLV can integrate itself at several sites in the genome. Many copies of the BLV provirus can thus be integrated in one genome.
Also, BLV infection results in altered immune systems in cattle. Cows with BLV had lower immunoglobin A (IgA) concentrations in milk and saliva [3].
2. Methods
We analyzed the articles on BLV, HTLV-1, BLV-Cancer links, and more using Google Scholar and PubMed. Key search terms included: “BLV”, “BLV causes Breast Cancer”, and “BLV oncogenic mechanisms”. Our primary aim was to identify and summarize the findings of the current research of BLV and the potential links to breast cancer oncogenesis in humans. We were careful to check for article corrections and author comments.
BLV-breast cancer research is relatively new albeit gaining momentum so there is limited original research on the topic and little research on the exact mechanisms of oncogenesis.
3. Results
3.1. BLV and HTLV
It has been established that BLV and HTLV-1 are extremely similar viruses. They share similarities such as: only being transmitted through cell–cell contact, producing few virions, ability to spread through breast feeding, using the Tax protein, and being deltaretroviridae [1]. They do not need to integrate with the host genome to induce malignancies. They inhibit DNA base repair which causes mutation accumulation which in turn can lead to cancer. According to Gillet et al. (2018) “Because their structure and properties differ from any other class of retroviruses, BLV and HTLV-1 viruses were classified into a new group of retroviruses” [4].
This is unique because generally viruses are similar in the same family (primates for example: HTLV, Simian T-cell leukemia virus (STLV)), but BLV affects animals in different families (non-bovine), yet the similarity is present. Furthermore, similar Tax proteins are thought to play a critical role in the leukemogenesis caused by HTLV-1 and BLV Simian T-cell leukemia virus [[5], [6], [7]].
The hypothesis exists that BLV causes breast cancer similarly to how HTLV-1 causes ATL through the Tax protein and other shared characteristics [8]. T-cells infected with HTLV-1 have abnormal transcription, DNA repair impairment, increased genomic instability, and deregulated cell cycle check points, which are symptoms all believed to be influenced heavily by the Tax protein (which analog is also found in BLV). HTLV-1 viruses are able to affect cells in the blood and cerebrospinal fluid. HTLV-1 has also been implicated in the genesis of non-hematological cancers [9]. A Japanese man was reported to have four kinds of cancer, which were accelerated by a HTLV-1 infection in a blood transfusion he received [10]. Zhao and colleagues (2002) also report instances of HTLV-1 causing epithelial cancers in rabbits [11]. Interestingly, the HTLV-1 started by circulating in the bloodstream but was able to infect the vein tissue cells, thus changing from a liquid to a solid infection. It uses a currently unknown surface receptor to enter cells through physical contact with the extracellular matrix, which it passes virions to. Once the epithelia are infected, it could potentially be easier for HTLV-1 to spread to other non-hematological areas.
Olson and colleagues found that when 69 sheep were infected with BLV from bovine lymphosarcoma materials, 24 of them developed lymphosarcoma and died from 13 to 66 (average, 29) months later [12]. Thus, BLV has the potential to initiate oncogenesis in non-bovine animals. Other research has shown that BLV can infect many other species, such as zebus, water buffaloes, goats, pigs, rabbits, rats, and chickens [13].
Furthermore, Altaner and Yokoro found that BLV could be transmitted to human cells of neural origin and some of these cells were highly susceptible to BLV infection [14]. This shows that BLV can enter non-hematological cells, as well as the previously mentioned epithelial cells that line blood vessels.
3.2. Global research on BLV role in breast cancer
Abovementioned findings inspired more researchers in various countries to conduct similar research. The studies in the USA, Brazil, China, Argentina, Australia, Japan, Korea, and Colombia followed. In several studies, there is a clear increase in BLV presence depending on cancer status. For example, in the study of American women, only 29% of controls had BLV, while premalignant samples had 38%, and cancerous samples had 59% [15]. The data of different studies are presented in Table 1, Table 2 with description of the used methods. These results are described in more details below.
Table 1.
Data from several studies on bovine leukemia virus. In some of the studies, there is a clear upward gradient of bovine leukemia virus presence from control to cancerous patients.
Table 2.
Experimental methods in estimation of association of BLV with the breast cancer.
3.2.1. USA
Buehring with coauthors published the first paper reporting the presence of BLV DNA and proteins in breast cancer tissue in 2015 [15]. 114 samples from patients with invasive breast cancer and 104 normal breast tissue samples were used in the study along with 21 premalignant samples were also used. They reported that BLV DNA was found in 59% of mammary epithelium samples from American predominantly Caucasian women with breast cancer, and in only 29% for normal controls. In samples from women with premalignant breast cancer, the frequency was in between, at 38%. It is significant that the premalignant breast cancer frequency is higher than control frequency because it supports the hypothesis that BLV is a factor in cancer genesis. However, there was a difference between races. African descent women showed lower frequencies of BLV, and the relation of BLV and breast cancer was not statistically significant. This could be due to genetic differences and lifestyle and dietary preferences. The researchers noted that the findings were “consistent with the lower breast cancer incidence among African Americans after age 40” [15]. Buehring and colleagues compared closely the possible BLV cancer mechanism with that of HPV, which inhibits p53 to mimic cancerogenic mutations. In a different study they identified p24 antibody to BLV in human blood [16]. The p24 antigen is a structural protein that comprises the majority of the BLV capsid.
Baltzell et al. reported that “Women in Texas diagnosed with breast cancer were significantly more likely to have BLV DNA in their breast tissue compared with women with benign diagnoses and no history of breast cancer [17]. Women with breast pathology classified as premalignant and no history of breast cancer also were found to have an elevated risk of harboring BLV DNA in their breast tissue” [17]. BLV was not found to be associated with tumor stage and severity, although “The attributable risk of 51.82% indicates that BLV could be responsible for at least one‐half of the breast cancer cases in the population studied” [17]. Interestingly, Texas, where the subjects are from, is notable for high beef and dairy consumption and has a burgeoning cattle industry.
BLV is present in buffy coat cells in 38% of subjects from the West Coast of the United States [18]. Authors did not test specifically for breast cancer. However, they analyzed the circumstances of BLV and possible links to leukemia and breast cancer and noted that “In the USA, 84% of US dairy herds and 39% of beef herds are infected” by BLV [18]. The researchers concluded that the primary transmission route of BLV to humans is zoonotic and foodborne, and circulation of BLV infected blood cells could carry BLV to various organs for later cancer genesis. The results are significant because they show that BLV can infect platelets and leukocytes, in addition to CD5+ B lymphocytes, T cells, and mammary epithelium cells.
Robinson LA et al. detected BLV in 80% of squamous cell lung carcinomas [19]. Squamous cell lung carcinomas are closely related to breast cancer despite low evolutionary similarity [9].
3.2.2. Brazil
Schwingel et al. reported that “BLV DNA was found most frequently (30.5%) in breast cancer tissue than in healthy breast (13.9%)” in samples from South Brazilian women using ELISA [20]. The study analyzed tissue samples from 72 healthy and 72 cancerous samples. Also, the researchers amplified two DNA fragments from breast cancer tissue, resulting in 99% homology with BLV's viral capsid protein gene [20]. Schwingel and coauthors noted that there was no association between the BLV DNA and other common tumor prognostic biomarkers, “such as hormonal receptors, HER2 oncoprotein, proliferation index” and more. Additionally, there was no correlation between BLV DNA presence and breast cancer metastasis [20].
The researchers stated that the link of BLV to breast cancer was stronger than that of lifestyle and reproductive history. Additionally, they mentioned that more dairy products are consumed in South Brazil than the rest of Brazil.
3.2.3. China
The results of a similar study in China by Zhang et al. contradicted the other findings [21]. These scientists stated that there was a “lack of association” with breast cancer and BLV. However, this should be taken carefully. The Chinese researchers used a commercial BLV testing kit for cows on the human blood samples, which would affect the results. As Buehring and colleagues commented, “The commercial kits are not intended for testing human sera; some would be negative for all human sera because the final detection step involves a labeled antibody to bovine, not human, immunoglobulin. Kit directions explicitly say, 'for veterinary use only' or 'intended for detection of BLV antibodies in cattle serum and milk'” [22]. It is also quite possible that dietary differences between the Chinese and the rest of the world may influence the findings. For example, Yongfa et al. reported that 92.3% of Han Chinese (the ethnic majority of China) have lactose malabsorption [23]. Thus, the dairy consumption per capita in China is relatively low compared to other countries.
3.2.4. Argentina
According to Ceriani et al., BLV was present in 22.6% of 85 Argentinian breast cancer samples using PCR and the presence of BLV was associated with increases in the cancer biomarkers Ki67 and Her-2 [24]. They report that their results support that BLV could play a role in malignant tissue creation.
3.2.5. Australia
In a population of 96 Australian women, 61.5% showed BLV in the mammary epithelium [25]. 80% of breast cancer samples had BLV present compared to 41% of control samples. The researchers attribute this much higher frequency compared to other studies to the smaller sample size of the research. Of women who were found to be BLV positive and not have breast cancer, 60.4% ended up developing breast cancer and the presence of BLV remained [25]. Meanwhile, only 14.6% of women who were found to be BLV negative developed breast cancer. The authors noted that there was no “conspicuous morphologic difference between individual BLV-infected versus uninfected mammary epithelial cells” of the breast tissues they examined, regardless of whether it was cancerous tissue [25]. They also reported that the BLV positive cells were generally only found together in places like a lobule, instead of randomly dispersed amongst the BLV-negative cells. A case control study design revealed that the “age-adjusted odds ration (OR)” for risk of breast cancer development was 4.72% if the patient had evidence of bovine leukemia virus.
The researchers also analyzed patient data from before they developed breast cancer to see if there was a link to bovine leukemia virus presence before cancer. They found that the breast cancer development probability was significantly higher in women when their breast tissue from before they developed cancer contained BLV. In 74.2% of breast cancer subjects, BLV infection of tissue was present years before the subject was diagnosed. This indicates that BLV may play some role in cancer development or acceleration. The results of this study are in line with that of other studies [15,22]. Australian women had a higher overall frequency of BLV presence in breast tissue in comparison to American women. This is true for both breast cancer patients and control patients. This could be due to the large difference in sample size or another factor entirely such as ethnicity. In Buehring's 2015 US study [15], 26% of subjects had African ancestry while the ethnic composition of the Australian subjects is not known. Authors noted that African Americans appear to have a “lower incidence of BLV-related breast cancer”. Interestingly, in 2012 after several years of work. Australia was declared free of BLV in the dairy herds, taking inspiration from what several European countries had done. Perhaps in a few decades to allow for the latency delay of many oncogenic viruses, we will see a marked decrease in breast cancer incidence in Australia.
3.2.6. Colombia
Giovanna et al. discovered BLV present in 41% of samples total. BLV was present in 36% of breast cancer samples [26]. The researchers noted that “The presence of BLV genes in humans and its location in breast tissue can be confirmed, however, it should be clarified as a possible promoter of malignancy processes on this tissue.” [26]. Researchers in Colombia conducted a case study on 106 tissue samples, half of which were breast cancer and the other half were controls. DNA was extracted, and then sequenced following amplification. Forty-three samples were positive for finding a segment of BLV DNA overall; 35.8% of cancer samples had BLV DNA segments present. Interestingly, a higher percentage of those in the control group had BLV DNA detected – 45.2%. Phylogenetic analysis confirmed that in found BLV there was high homology between the gene sequences taken from the human breast tissues and “those coming from bovine cattle with leukosis”. Giovanna et al. stated that BLV genes should be “clarified as a possible promoter of malignancy processes” [26]. The study authors raised the point that their results seemed to contradict the results of previous studies, like those by Buehring and noted that it “could be considered that …. BLV could be found in healthy breast tissue” as another virus, mouse mammary tumor virus (MMTV) is also able to be present in human breast tissues without causing changes and remain latent. Giovanna et al. also proposed similarities between the potential mechanisms of BLV and those of avian sarcoma leukosis virus (ASLV) [26].
Overall, the results from multiple studies around the world support the hypothesis of BLV affecting breast cancers. The variations in BLV frequency can be attributed to sample size proportions and different ethnicities and diets. Further research should be conducted on the connection between BLV and breast cancer in humans.
3.2.7. Japan
Most recently, the absence of BLV DNA in Japanese human cell lines was reported [27]. Authors examined DNA extracted from 145 cell lines yet did not detect any BLV DNA. This raises the question of whether the protocol was performed optimally as research by others has shown the presence of BLV DNA in at least some proportion, not 0%. Saito et al. [17] acknowledged that a potential flaw was that they used a PCR method designed for sheep cell lines not human cell lines. Buehring et al. pointed out a similar concern for the Chinese study where a bovine ELISA was used on human samples [22]. Additionally, Saito et al. [17] admitted that they only used two breast cancer cell lines.
3.3. Milk and meat consumption global analysis
If the hypothesis of BLV affecting breast cancers is true and the BLV penetrates to the human organism by milk or/and bovine meat, it has to be supported by a correlation between the milk and/or bovine meat consumption with the frequencies of breast cancers. Below we analyze the possibility of such correlation.
In Bolivia milk consumption is among the lowest levels in the world [28]. The map shows a lower breast cancer incidence than the neighboring countries. Another factor could be the different genetic makeup of Bolivia, which has a higher percentage of indigenous population than several neighbors.
In Mongolia, (stats in Fig. 3), breast cancer rates have remained historically low compared to other places. However recently rates have increased, especially in urban areas where the difference gap is increasing. This could be due to wider availability to dairy and meat products from European cattle in urban areas, rather than in rural areas, where the indigenous Mongolian yak is more common than cows for food and consumption. It has been noted that European cattle, Bos taurus, could have a higher propensity for spreading disease due to their association with colon and breast cancer, which does not exist as strongly for different types of cows, i.e., the Mongolian cow [29].
Breast cancer incidence increased slightly in India over the last decades. India is a country with very low beef consumption due to the religions that forbid meat eating or beef eating. However, drinking milk and dairy products is allowed. From 1985 to 2010, the incidence of breast cancer increased in India. At this same time span, milk consumption increased 2.4-fold from 1991 to 2005. This suggests that milk consumption could be linked to breast cancer [30].
Furthermore, examine Japan and Korea, which have high rates of breast cancer, which increased relatively steeply. Both colorectal and breast cancer incidences skyrocketed in the two countries over the last decades. In previous years, dairy was not a stable ingredient or popular in the countries, possibly due to the high rates of lactose intolerance, but recently with globalization, dairy consumption has increased drastically in Japan and Korea [30]. Also note the popularity of raw meat consumption in Japan and Korea, i.e., shabu-shabu dish.
Nearby in China, breast cancer rates are also relatively low. Recently, they have increased, but almost only in urban areas. In China, many people are lactose intolerant and dairy is not so popular. It is known that milk consumption in the Chinese population is four times lower than in Argentina and almost 10 times lower than in industrialized countries like Australia, New Zeeland, or the United States [31].
The Swedish Cancer Registry data and study of Ji et al. (2014) showed that people with lactose intolerance had lower levels of breast cancer incidence than their immediate family who are closely genetically related to them and share similar environments [32]. This is an important finding.
One thing to note is that BLV is not found in all humans with breast cancer, and some humans with breast cancer do not have BLV DNA found. However, it is notable that even in cattle, only about 5% of infected cattle develop symptoms.
Research by Rugierro et al. found that BLV-positive cows had lower concentrations of IgA in milk and saliva, but similar concentrations in serum, when compared to cows without BLV. The research suggested the BLV can disrupt “immunology of mucosal junctions”.
4. Discussion
From the perspective of cattle, living conditions are dense and unsanitary, which propagates and promotes disease. Furthermore, the nature of the farm industry is the presence of multiple species coexisting in the same area, namely chickens and pigs, which with cows, form the quintessential farm animal group. Chickens transfer avian viruses and pigs transfer swine viruses. There is a high chance of infection by avian and swine viruses to bovines, which would provide the necessary coinfection required for antigenic shift and reassortment. Furthermore, it is also likely that a cows may be infected by multiple bovine specific viruses too. Antibiotic treatments of farm animals are commonplace in industry; however, these may be rendered ineffective considering methicillin resistant staphyloccus aureus (MRSA) antibiotic resistance, and new mutated viruses, thus viruses and disease continue to run afoul. However, this is merely an educated guess, as antibiotics work on bacteria and not viruses like BLV. The point about antibiotic resistance is merely to illustrate unsanitary hygiene practices on farms.
The 2009 H1N1 Swine flu was caused by the reassortment and transfer of viruses from avian to swine to humans, partly due to the similarity of swine and human cell surface receptors. In 2019 the COVID-19 virus probably was transferred from bats to human. As aforementioned, the possible swine virus entering the bovine cells and antigenically shifting could result in a new virus that can affect humans. In this case, similarity of BLV to HTLV-1 indicates that BLV already is close to being able to work with human receptors. Combined with swine virus’ similarity may complete the effect.
Another key component of viruses mutating to affect humans is simply mutation rate and replication speed. Retroviruses are notoriously error-prone due to the method of reverse transcription, which does not employ proofreading, leading to more mistakes. Furthermore, retroviruses are structurally very simple compared to other viruses (like influenza) which contributes to their fast replication time. If the swine flu virus was able to replicate and mutate to affect humans, it is possible that the much more volatile and fast-replicating BLV retrovirus would be able to also.
Critics may point out that antigenic shift happens exclusively in influenza. However, research shows that this is false. Antigenic shift has been observed in the visna-maedi virus in sheep. Visna-maedi virus is also a retrovirus like BLV [21].
Buehring et al. reported that the presence of BLV in breast epithelium is a greater risk factor for breast cancer than many others, such as years on hormone replacement and whether one had a mother or sister with breast cancer [33]. The only two risk factors authors ranked higher was the presence of BRCA1 and BRCA2 genes and exposure to ionizing radiation. Meanwhile, another study by Buehring and colleagues found that the overexpression of cell proliferation markers, KI67 and HER2, were significant associated with the presence of bovine leukemia virus in malignant breast tissues from Argentinian patients [34]. They concluded that “DNA damage observed in breast cancer cells, including driver mutations, could theoretically have been initiated due to DNA repair inhibition by BLV infection” [34].
Buehring's group research is especially significant because of the consistency of results obtained. Many other groups confirmed these findings. Although there have been contradictions such as demonstrated by a group of Chinese researchers [21] and a conclusion by the National Animal Health Monitoring System (1969) stating that BLV did not cause any disease in humans, the specific procedure used as well as improvements in technology indicate that it is likely no longer possible to conclude that there is no relation between BLV DNA/proteins and breast cancer [35]. However, in one study that used whole genome sequencing instead of in situ PCR, which was used by almost all other studies, BLV presence was not found [36]. Gillet and coauthors analyzed data from 51 whole genomes of breast cancer samples and 19 normal tissues but did not find evidence of BLV [36]. The samples analyzed came from people in the United States, Mexico, and Vietnam. This directly contradicts the current general findings of researchers who used in situ PCR. Currently it is not clear why there are differences in detection. It seems like groups are either able to detect more BLV in cancer than normal, or unable to detect anything at all. There seems to be a dichotomy. Perhaps the whole genome sequencing is not as sensitive as PCR and did not detect the viral DNA, which constitutes only a miniscule percentage of host DNA. Notably, nearly all the studies using Immunohistochemistry and In Situ PCR had positive results while the studies using alternate methods like sequencing did not find evidence of BLV association with breast cancer.
Immunoblotting methods such as ELISA are highly sensitive and are able to pinpoint previously undetected antibodies and other proteins. Furthermore, the primers used for detection via PCR have very high specificity.
Interesting to note that Buehring reports an especially key finding supporting the fact that BLV is present before cancer develops [17].
BLV has been demonstrated to inhibit cellular repair so it could cause accumulated mutations, eventually leading to cancer.
Regarding a possibility of HTLV and BLV to affect cancer driver genes, van den Broeke [37] noted: “Using genomic approaches, we demonstrated that HTLV-1 and BLV integrate in the vicinity of host cancer driver genes, which they perturb either by provirus-dependent transcription termination or as a result of viral antisense RNA-dependent cis-perturbation via virus–host chimeric transcripts” [37]. In a study by Ashrafi et al., RNA-seq of samples from Adult T cell leukemia/lymphoma positive hosts and samples from enzootic bovine leukosis showed significant similarities between the two diseases, which are caused by HTLV-1 and BLV respectively [38]. The samples of RNA came from infected peripheral blood mononuclear cells and lymph nodes of bovines and humans. Tax was expressed “very low” and HTLV-1–HBZ and BVL–As2 transcripts were “highly expressed” [38]. In both human and bovine samples, hub genes such as IL2, TOP2A, and TP73 were upregulated and were similarly activated. The researchers stated that BLV and HTLV-1 “must induce some common pathways in the oncogenesis of T or B lymphocyte sub-populations” [38]. Thus, the transcriptomics research could help in identifying important genes that are implicated in malignant developments. According to the authors, Tax is not significantly expressed in both Adult T cell leukemia/lymphoma and enzootic bovine leukosis. This supports prior findings that while Tax is heavily involved in the beginning of the disease onset, the levels are decreased later and Tax expression greatly decreases [39]. The authors report that HBZ and As2 transcripts becomes more involved during the malignant stages, while Tax's expression decreases.
Funding
No funding was received.
Declaration of competing interest
Authors declare no conflict of interest.
References
- 1.Aida Y., Murakami H., Takahashi M., Takeshima S.-N. Mechanisms of pathogenesis induced by bovine leukemia virus as a model for human T-cell leukemia virus. Front. Microbiol. 2013;4 doi: 10.3389/fmicb.2013.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Usda . 2008. Veterinary Services Centers for Epidemiology and Animal Health. [Google Scholar]
- 3.Ruggiero V.J., Bartlett P.C. 2019. Field Studies on the Control of Bovine Leukemia Virus in Dairy Cows - ProQuest, search.proquest.Com. [Google Scholar]
- 4.Gillet N., Florins A., Boxus M., Burteau C., Nigro A., Vandermeers F., Balon H., Bouzar A., Defoiche J., Burny A., Reichert M., Kettmann R., Willems L. Mechanisms of leukemogenesis induced by bovine leukemia virus: prospects for novel anti-retroviral therapies in human. Retrovirology. 2007;4:18. doi: 10.1186/1742-4690-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katoh I., Yoshinaka Y., Ikawam Y. Bovine leukemia virus trans-activator p38tax activates heterologous promoters with a common sequence known as a cAMP-responsive element or the binding site of a cellular transcription factor ATF. EMBO J. 1989;8:497–503. doi: 10.1002/j.1460-2075.1989.tb03403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azran I., Schavinsky-Khrapunsky Y., Aboud M. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology. 2004;1:20. doi: 10.1186/1742-4690-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willems L., Grimonpont C., Kerkhofs P., Capiau C., Gheyson D., Conrath K., Roussef R., Mamoun R., Portetelle D., Burny A., Adam E., Lefebvre L., Twizere J., Heremans H., Kettmann R. Phosphorylation of bovine leukemia virus Tax protein is required for in vitro transformation but not for transactivation. Oncogene. 1998;16:2165–2176. doi: 10.1038/sj.onc.1201765. [DOI] [PubMed] [Google Scholar]
- 8.Rosewick N., Durkin K., Artesi M., Marcais A., Hahaut V., Griebel P., Arsic N., Avettand-Fenoel V., Burny A., Charlier C., Hermine O., Georges M., Van den Broeke A. Cis-perturbation of cancer drivers by the HTLV-1/BLV proviruses is an early determinant of leukemogenesis. Nat. Commun. 2017;8 doi: 10.1038/ncomms15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kouznetsova V., Chen S., Tsigelny I. HTLV-1 can be involved in acceleration of different nonhematological cancers. Journal of Cancer Research & Therapy. 2019;7:1–8. [Google Scholar]
- 10.Mori T., Nakase K., Tsuji K., Nagaya S., Ikeda T., Tanigawa M., Tamaki S., Miyanishi E., Kita K., Shirakawa S. vol. 33. Internal medicine; Tokyo, Japan): 1994. pp. 155–157. (Quadruple Cancers in a Human T-Cell Leukemia Virus Type 1 Carrier). [DOI] [PubMed] [Google Scholar]
- 11.Zhao T.M., Bryant M.A., Kindt T.J., Simpson R.M. Short communication: monoclonally integrated HTLV type 1 in epithelial cancers from rabbits infected with an HTLV type 1 molecular clone. AIDS Res. Hum. Retrovir. 2002;18:253–258. doi: 10.1089/088922202753472829. [DOI] [PubMed] [Google Scholar]
- 12.Olson C., Baumgartener L.E. Pathology of lymphosarcoma in sheep induced with bovine leukemia virus. Canc. Res. 1976;36:2365–2373. [PubMed] [Google Scholar]
- 13.Schwartz I., Lévy D. Pathobiology of bovine leukemia virus. Vet. Res. 1994;25:521–536. [PubMed] [Google Scholar]
- 14.Altaner C., Altanerová V., Bán J., Niwa O., Yokoro K. Human cells of neural origin are permissive for bovine leukemia virus. Neoplasma. 1989;36:691–695. [PubMed] [Google Scholar]
- 15.Buehring G.C., Shen H.M., Jensen H.M., Jin D.L., Hudes M., Block G. Exposure to bovine leukemia virus is associated with breast cancer: a case-control study. PloS One. 2015;10 doi: 10.1371/journal.pone.0134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buehring G.C., Philpott S.M., Choi K.Y. Humans have antibodies reactive with Bovine leukemia virus. AIDS Res. Hum. Retrovir. 2003;19:1105–1113. doi: 10.1089/088922203771881202. [DOI] [PubMed] [Google Scholar]
- 17.Baltzell K.A., Shen H.M., Krishnamurthy S., Sison J.D., Nuovo G.J., Buehring G.C. Bovine leukemia virus linked to breast cancer but not coinfection with human papillomavirus: case-control study of women in Texas. Cancer. 2017;124:1342–1349. doi: 10.1002/cncr.31169. [DOI] [PubMed] [Google Scholar]
- 18.Buehring G.C., Delaney A., Shen H., Chu D., Razavian N., Schwartz D.A., Demkovich Z.R., Bates M.N. Bovine leukemia virus discovered in human blood. BMC Infect. Dis. 2019;19 doi: 10.1186/s12879-019-3891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson L.A., Jaing C.J., Pierce Campbell C., Magliocco A., Xiong Y., Magliocco G., Thissen J.B., Antonia S. Molecular evidence of viral DNA in non-small cell lung cancer and non-neoplastic lung. Br. J. Canc. 2016;115:497–504. doi: 10.1038/bjc.2016.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwingel D., Andreolla A.P., Erpen L.M.S., Frandoloso R., Kreutz L.C. Bovine leukemia virus DNA associated with breast cancer in women from South Brazil. Sci. Rep. 2019;9:1–7. doi: 10.1038/s41598-019-39834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang R., Jiang J., Sun W., Zhang J., Huang K., Gu X., Yang Y., Xu X., Shi Y., Wang C. Lack of association between bovine leukemia virus and breast cancer in Chinese patients. Breast Canc. Res. 2016;18 doi: 10.1186/s13058-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buehring G.C. Response to ‘Lack of association between bovine leukemia virus and breast cancer in Chinese patients. Breast Canc. Res. 2017;19 doi: 10.1186/s13058-017-0808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yongfa W., Yongshan Y., Jiujin X., Ruofu D., Flatz S.D., Kuhnau W., Flatz G. Prevalence of primary adult lactose malabsorption in three populations of northern China. Hum. Genet. 1984;67:103–106. doi: 10.1007/BF00270566. [DOI] [PubMed] [Google Scholar]
- 24.Carolina Ceriani M., Anahi Lendez P., Martinez Cuesta L., Victoria Nieto Farias M., Case Buehring G., Laura Dolcini G. Bovine leukemia virus presence in breast tissue of Argentinian women. Its association with cell proliferation and prognosis markers. Multidisciplinary Cancer Investigation. 2018;2:16–24. [Google Scholar]
- 25.Buehring G.C., Shen H., Schwartz D.A., Lawson J.S. Bovine leukemia virus linked to breast cancer in Australian women and identified before breast cancer development. PloS One. 2017;12 doi: 10.1371/journal.pone.0179367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giovanna M., Carlos U.J., María U.A., Gutierrez M.F. Bovine leukemia virus gene segment detected in human breast tissue. Open J. Med. Microbiol. 2013;3:720–726. [Google Scholar]
- 27.Saito S., Kitamura-Muramatsu Y., Komine F., Polat M., Takeshima S., Takei M., Aida Y. Absence of bovine leukemia virus proviral DNA in Japanese human blood cell lines and human cancer cell lines. Arch. Virol. 2020;165:207–214. doi: 10.1007/s00705-019-04474-9. [DOI] [PubMed] [Google Scholar]
- 28.Hudson R.A., Hanratty D.M. Headquarters, Dept. of the Army; 1950. Bolivia, a Country Study. 1991. [Google Scholar]
- 29.Troisi R., Altantsetseg D., Davaasambuu G., Rich-Edwards J., Davaalkham D., Tretli S., Hoover R.N., Frazier A.L. Breast cancer incidence in Mongolia. Cancer Causes Control: CCC (Cancer Causes Control) 2012;23:1047–1053. doi: 10.1007/s10552-012-9973-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.zur Hausen H., de Villiers E.-M. Dairy cattle serum and milk factors contributing to the risk of colon and breast cancers. Int. J. Canc. 2015;137:959–967. doi: 10.1002/ijc.29466. [DOI] [PubMed] [Google Scholar]
- 31.Martinez Cuesta L., Lendez P.A., Nieto Farias M.V., Dolcini G.L., Ceriani M.C. Can bovine leukemia virus Be related to human breast cancer? A review of the evidence. J. Mammary Gland Biol. Neoplasia. 2018;23:101–107. doi: 10.1007/s10911-018-9397-z. [DOI] [PubMed] [Google Scholar]
- 32.Ji J., Sundquist J., Sundquist K. Lactose intolerance and risk of lung, breast and ovarian cancers: aetiological clues from a population-based study in Sweden. Br. J. Canc. 2014;112:149–152. doi: 10.1038/bjc.2014.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buehring G.C., Sans H.M. Breast cancer gone viral? Review of possible role of bovine leukemia virus in breast cancer, and related opportunities for cancer prevention. Int. J. Environ. Res. Publ. Health. 2020;17 doi: 10.3390/ijerph17010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buehring G.C., Shen H., Baltzell K., Sison J., Krishnamurty S., Lendez P., Martinez-Cuesta L., Nieto-Farias M.V., Dolcini G.L., Ceriani M.C. Abstract B30: bovine leukemia virus in breast tissue linked to increased cell proliferation and breast cancer risk. Metabolism. 2018:16. [Google Scholar]
- 35.Nahms Bovine leukosis virus (BLV) on U.S. Dairy Operations. 2007;2008 [Google Scholar]
- 36.Gillet N.A., Willems L. Whole genome sequencing of 51 breast cancers reveals that tumors are devoid of bovine leukemia virus DNA. Retrovirology. 2016;13 doi: 10.1186/s12977-016-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van den Broeke A. A-108 HTLV-1/BLV genomic approaches to understanding ATL. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2019;81:34. [Google Scholar]
- 38.Ashrafi F., Nassiri M., Javadmanesh A., Rahimi H., Rezaee S.A. Epigenetics evaluation of the oncogenic mechanisms of two closely related bovine and human deltaretroviruses: a system biology study. Microb. Pathog. 2020;139 doi: 10.1016/j.micpath.2019.103845. [DOI] [PubMed] [Google Scholar]
- 39.Baratella M., Forlani G., Acolla R.S. HTLV-1 HBZ viral protein: a key player in HTLV-1 mediated diseases. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.02615. [DOI] [PMC free article] [PubMed] [Google Scholar]