Abstract
Ethnopharmacological relevance
Viral respiratory infections are amongst the most common infections globally, with most of the world's population contracting at least one infection annually. Numerous plant species are used in traditional southern African healing systems to treat these diseases and to alleviate the symptoms. Despite this, the therapeutic potential of these plants against viral respiratory diseases remains poorly explored.
Aim of the study
The aim of this study was to document the southern African plant species used in traditional medicine to treat viral respiratory infections. We also examined the extent of scientific evaluations of southern African plant species against the respiratory-infective viruses, with the aim of stimulating interest in this area and focusing on future studies.
Materials and methods
We undertook an extensive review of ethnobotanical books, reviews and primary scientific studies to identify southern African plants which are used in traditional southern African medicine to treat viral respiratory diseases. This information was used to identify gaps in the current research that require further study.
Results
Two hundred and fifty-seven southern African plant species were identified as traditional therapies for viral respiratory diseases. Surprisingly, only one of those species (as well as twenty-one other species not recorded for these purposes) has been evaluated for the ability to block respiratory virus production. Furthermore, most of these studies screened against a single viral strain and none of those studies examined the mechanism of action of the plant preparations.
Conclusions
Despite well documented records of the use of southern African plants to treat respiratory viral diseases, the field is poorly explored. Nearly all of the plant species used in traditional healing systems to treat these diseases are yet to be tested. Substantial further work is required to verify the efficacy of these traditional medicines.
Keywords: South African plants, Traditional medicine, Influenza, Rhinovirus, Parainfluenza, Bronchitis
1. Introduction
Respiratory diseases are amongst the most prevalent diseases globally and impose an immense burden on the healthcare system. Several of these diseases are common causes of severe illness and mortality (Wang et al., 2015). The greatest burden of respiratory diseases is due to pathogenic infections. Acute lower respiratory tract infections are one of the top causes of mortality and disability globally, causing approximately four million deaths annually (Unicef et al., 2006). These conditions are particularly concerning in children and are the leading cause of mortality in children under five years of age. Influenza virus infections account for many acute lower respiratory tract infections and it is estimated that 250,000–500,000 people die from severe influenza virus infections annually. While Semenya and Maroyi (2018) reported on 306 medicinal plant species used to treat and manage respiratory infections, a concerted effort to date has not been undertaken in catagorising these medicinal plants specifically to respiratory viral and bacterial sources. This review focusing on southern African medicinal plants for viral infections forms part one of a two part review whereby the focus on bacterial pathogens is reviewed as Part 2 in a separate manuscript (Cock and Van Vuuren, 2020).
1.1. Viral respiratory infections
Several viruses can infect respiratory tissue and induce respiratory illnesses. Of these, the most common viral causes of respiratory disease are influenza virus, rhinoviruses, respiratory syncytial virus (RSV), parainfluenza virus, adenovirus, human metapneumovirus, and enterovirus.
1.1.1. Influenza
Influenza (often abbreviated to flu) is a highly infectious airborne disease caused by the influenza virus. There are four genus's of influenza virus, of which three genus's (A, B and C) can infect humans (Paules and Subbarao, 2017). Influenza virus D genus has never been reported as a human infection and instead only infects pigs and cattle. However, it is believed that this genus has the potential to infect humans and cross species infections may be reported in the future (Asha and Kumar, 2019). Of the other three classes of influenza virus, influenza virus A is the most important to human health and is the most virulent of the human influenza pathogens (Paules and Subbarao, 2017). Whilst this genus consists of only a single species, numerous serotypes have been described (listed in descending order of deaths caused):
-
•
H1N1. This serotype has several viral sub-serotypes which have caused considerable mortality. The H1N1 serotypes were responsible for the Spanish influenza outbreak of 1918 (which infected approximately 500 million people worldwide and caused an estimated 50–100 million deaths) (Taubenberger and Morens, 2006), and the swine influenza outbreak of 2009 (which infected approximately 20% of the world's population and caused thousands of deaths) (Reddy et al., 2018).
-
•
H2N2. This serotype was the cause of the Asian influenza outbreak of 1957. This serotype may have resulted from a mutation in ducks that subsequently crossed species to humans (Scholtissek et al., 1978). The death toll of this virus has been estimated to be between one and four million.
-
•
H3N2 serotype caused the Hong Kong influenza outbreak of 1968, which is estimated to have killed at least a million people between 1968 and 1969. This serotype is believed to have descended from H2N2 via an antigenic shift mutation (Scholtissek et al., 1978).
-
•
H5N1, also known as avian influenza or bird flu. Whilst this virus can infect humans and several other animal species, its virulence in humans is relatively benign compared to the previously described serotypes. This virus is highly pathogenic in many bird species and an outbreak of bird-adapted strain of H5N1 in 2004 in southeast Asia directly resulted in the deaths of tens of millions of birds. Hundreds of millions of more birds (particularly domestic fowl) were culled to inhibit the spread of the disease, causing serious economic hardship in the region. Airborne spread of this sub-serotype virus from birds to humans is not yet possible, although it is believed from genetic analyses that only a few mutations are required for this to occur (Sorrell et al., 2011), raising concerns of a future pandemic.
-
•
H7N7 is a zoonotic serotype that can infect humans, pigs, horses, seals and birds by natural transmission routes in the wild, and has been used to infect mice in laboratory studies (Paules and Subbarao, 2017). The zoonotic nature of the virus indicates that is poses a future pandemic threat.
-
•
H1N2 is also a zoonotic serotype, which infects humans, pigs and birds (Paules and Subbarao, 2017). Due to its cross-species infectivity, it is also believed to be a future pandemic threat.
-
•
H9N2, H7N2, H7N3 and H10N7 are other bird-infective serotypes, although they can also infect humans, especially children (Paules and Subbarao, 2017).
A common feature of the influenza virus A serotypes, whether they infect humans or not, is their high rate of mutation and therefore their potential for major pandemics. If such pandemics occur, lack of immunity, ease of transmission and increased virulence may cause major losses of life, as previously seen for the Spanish influenza outbreak over a 100 years ago. It is therefore important that therapies to treat these infections be developed to guard against future pandemics.
Influenza virus genus's B and C are less common than influenza virus A and are generally considered to be less of a concern due to their lower virulence and slower mutation rates (Paules and Subbarao, 2017). Indeed, influenza virus B mutates at a third to a half of the rate of influenza A. Furthermore, this virus only infects humans. The reduced rate of antigenic change, combined with the single species host infectivity (thereby inhibiting cross-species antigenic shifts), substantially decreases the chances of pandemic shifts occurring in these influenza genus's.
There are currently relatively few effective cures for any influenza virus species. The exception are the neuraminidase inhibitors which prevent influenza virus reproduction by inhibiting viral budding from the cell. Most other therapies target the symptoms of the illness to make the infected person feel better whilst their immune system fights the infection. However, influenza vaccines are now available against most influenza serotypes, although the vaccine effectiveness ranges from 10 to 60% (depending on the year and the serotypes targeted by the vaccine) (Osterholm et al., 2012). Therefore, many people will still contract influenza, despite receiving a vaccine and an effective therapeutic alternative is urgently required.
1.1.2. Enterovirus
Enterovirus is a particularly diverse genus of viruses, consisting of a number of medically relevant pathogens including polioviruses and rhinoviruses. Due to their particular relevance to respiratory disease, the rhinoviruses will be discussed separately in detail. Enteroviruses most commonly infect the gastrointestinal tract and are most readily transmitted via the intestine (accounting for their name) (Stalkup and Chilukuri, 2002). However, they frequently also infect the respiratory system. Most commonly, when enteroviruses infect respiratory tissue, they induce symptoms similar to the common cold. However, these infections can also cause substantially more serious symptoms including inflammation of the heart and surrounding tissue, inflammation of the membranes surrounding the brain and inflammation of the pancreas. There are currently no cures for any enterovirus-induced diseases. Instead, treatment is supportive and aims to alleviate the symptoms (e.g. providing analgesics for pain relief) and more effective therapies are required. For the polioviruses, effective vaccines already exist, which allow for the prevention of poliomyelitis. However, these vaccines are ineffective if a poliovirus infection is already established. To date, there are no effective vaccines for the other respiratory infective enteroviruses.
1.1.2.1. Rhinovirus
Rhinovirus is the predominant cause of the common cold and is the most infectious viral pathogen in humans (Baille et al., 2018). Indeed, it has been estimated that the average adult may contract two or three colds per year, whilst children may contract as many as eight colds annually. Like influenza viruses, rhinoviruses are prone to mutations, resulting in a large genetic diversity. Indeed, three species consisting of 160 main subtypes have been reported. Each of these viruses has different complements of surface antigens. Therefore, rhinovirus may evade the immune response and a cold will develop, even when the infected person has been exposed to several other rhinovirus subtypes. Similar to influenza virus, rhinovirus is largely spread via airborne transmission, although it may also be spread by direct contact.
Rhinovirus infections progress very rapidly. Indeed, the virus adheres to cell surface receptors within 15 min of entering the respiratory tract and the disease has an incubation period of approximately two days (although this can be as short as 20 h) (Baille et al., 2018). As well as being substantially less severe than influenza, the course of rhinovirus infections is also much shorter, with recovery generally in seven to ten days. For these reasons, rhinovirus infections (and colds due to other viruses described below) are considered to be of less concern than influenza and substantially less research has aimed at finding a cure to the common cold. Despite this, rhinovirus infections can still cause substantial distress and even death in children, the elderly and immunocompromised individuals and research to develop a cure is still required, even if it is not considered as high a priority as the development of new influenza therapies. Unlike influenza, no effective rhinovirus vaccine is currently available.
1.1.3. Adenovirus
Adenoviruses can infect a broad range of vertebrate hosts and can induce a wide range of illnesses and symptoms, dependent on the tissue infected, the adenovirus species and serotype, and the severity of infection. They may infect the gastrointestinal tract (causing gastritis, the eye (causing conjunctivitis) or the bladder (causing cystitis), although they most frequently infect the respiratory tract and are the second most frequent cause of colds (after rhinoviruses) (Rubin, 1993). Adenoviruses are spread in a similar manner to the previously described viruses i.e. airborne respiratory droplets. However, they may also be spread by contact with faecal contaminated surfaces. Adenoviruses are stable for a particularly long time outside the body, which substantially enhances their transmission rates. Furthermore, they are stable in a variety of environments and are relatively resistant to chemical decontaminants. They can also infect a variety of animal hosts apart from humans, allowing for major antigenic shifts and the potential for pandemics.
Given their resistance to decontamination and ease of transmission, it is fortunate that adenovirus infections are generally relatively benign compared to other viral respiratory infections. Humans infected with adenoviruses are often asymptomatic, although when they do display symptoms, these may vary widely. For most adenovirus serotypes, these generally present with the same symptoms as the common cold. However, some serotypes can induce substantially more severe symptoms and may require medical intervention. Adenovirus serotype 14 is particularly virulent and can cause severe respiratory distress, which may be fatal in some people (Louie et al., 2008). Of concern, this serotype has mutated substantially since it was first detected and increased spread of the new virus has been noted in recent years. Despite this, very little research has targeted this virus to develop new therapies and further research is urgently required.
1.1.4. Parainfluenza
Despite their name similarity, parainfluenza viruses are not closely related to influenza viruses, although they share several symptom similarities. Human parainfluenza virus (HPIV) infections are particularly prevalent in children. It has been estimated that of the five million childhood lower respiratory tract infections reported in the United States each year, approximately a third of these are due to HPIV infections (Henrickson et al., 1994). HPIV can also infect the upper respiratory tract, although this is substantially less common. Four main HPIV serotypes have been described (Branche and Falsey, 2016):
-
•
HPIV-1 is the most common serotype and is the major cause of croup.
-
•
HPIV-2 also causes croup, as well as several other upper and lower respiratory tract diseases.
-
•
HPIV-3 and HPIV-4 both cause bronchitis and viral pneumonia.
Despite the prevalence of HPIV infections, they are generally considered to be of low concern for most people. Infections in children last at least seven days and symptoms similar to colds are most common. Most children >10 years age are asymptomatic with HPIV infections. However, HPIV infections can be much more serious in younger children. HPIV-1 and HPIV-2 infections can induce severe upper respiratory tract distress in children up to four years of age. HPIV-3 can cause bronchitis and viral pneumonia in infants (<1 year of age). Similarly, HPIV can cause severe pneumonia that can be fatal in immunocompromised people. Therefore, although HPIV research is relatively neglected, effective anti-HPIV therapies are required.
1.1.5. Human respiratory syncytial virus (HRSV)
Human respiratory syncytial virus (HRSV) is another common cause of respiratory virus infections, particularly in infants and children. Infections are very common and it is estimated that 60% of infants are infected in their first year and nearly all children are infected by 3 years of age (Glezen et al., 1986). In most infected people, HRSV infections are asymptomatic, or produce relatively benign symptoms, similar to a common cold. However, HRSV infections may induce severe bronchitis requiring hospitalisation in approximately 3% of the population and prior HRSV infections do not confer lifelong immunity (Hall et al., 2009). Instead, people may acquire repeated infections as early as 1 year after a prior infection. There is no cure for HRSV infections and treatment is supportive and aims to alleviate the disease symptoms and allow the infected persons immune response to combat the disease. In older people with a decreased immune response and other immunocompromised individuals, HRSV infections may be substantially more severe and in rare cases may result in death.
HRSV is very readily spread and avoiding infection is almost impossible (Glezen et al., 1986). Unlike other respiratory viruses where the route is mostly airborne, HRSV transmission is most frequently via direct contact. The virus is particularly hardy and remains viable on surfaces for up to 5 h. The virus is also relatively resistant to chemical decontaminants, making its prevention particularly difficult. For this reason, HRSV spreads rapidly through childcare centres and treatment facilities for the elderly. HRSV infections are most frequent in the colder months for most regions of the world, when people congregate together indoors, with closer contact and thus more efficient transmission.
1.1.6. Human metapneumovirus (HMPV)
HMPV infections are common causes of respiratory illness and are associated with 6–40% of viral respiratory infections in children seeking medical intervention. Indeed, serological studies indicate that nearly all children worldwide have been exposed to HMPV by 5 years of age (Kahn, 2006). However, as for HRSV, prior infections with HMPV do not provide life-long immunity and repeated infections are common in older children and in adults. Generally, HPMV infections result in mild upper respiratory tract infections and present with similar symptoms to the common cold. However, the symptoms may be substantially more severe in older people and immunocompromised individuals and whilst relatively rare, HMPV-associated fatalities may occur. HPMV infections may also produce substantially more severe symptoms in individuals with asthma or chronic obstructive pulmonary disease (COPD).
The method of transmission of HMPV is less understood than for the other respiratory-infective viruses although it is likely to spread via similar pathways (i.e. airborne transmission, direct contact) (Chow et al., 2016). There is currently no known cure for HMPV infections and there is relatively little research into developing a treatment as the disease is relatively benign in most people and is self-limiting, usually resolving in similar time as a common cold. However, ribavirin has shown efficacy in animal models and may be useful in severe HPMV infections (Kitanovski et al., 2013). A number of studies have also investigated the potential for a HPMV vaccine (Karron et al., 2017) although it is likely that this is still at least several years from development.
1.1.7. Coronaviruses
Coronaviruses also cause respiratory tract infection that are usually mild compared to many of the previously listed viruses. However, rarer coronaviruses such as the viruses those that cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) induce substantially more severe symptoms and can even be lethal. Lastly and more topically and important is the SARS-CoV-2 coronavirus serotype which has caused recent global panic. The identified 2019-nCoV genome has been sequenced to some beta-coronaviruses detected in bats, with initial zoonotic mutations from snake expected (Ji et al., 2020; She et al., 2020). This remains at present the number one global viral respiratory threat. To date, neither SARS nor MERS have yet had a significant impact on southern Africa and therefore are not a focus of this study. However, recent SARS-CoV-2 infection and mortality rates in South Africa have highlighted the global nature of COVID 19. Indeed, as at 13 May 2020, the number of infections in South Africa was in excess of 12,000 and the mortality rate was above 200 (worldometersiinfo/coronavirus/). This figure is highly dynamic and is likely to be substantially higher by the time this review is published. Furthermore, due to the relatively high number of asymptomatic individuals with SARS-CoV-2 infections, these figures are likely to be substantially underestimated. The ease of transmission of this virus highlights the need to test southern African plants against this serotype in future studies.
It is noteworthy that all the respiratory viruses discussed here (with the exception of adenoviruses) are RNA viruses and this may impact on the efficacy of any prevention or therapy in the future. In general, RNA viruses incorporate mutations at a substantially faster rate than for DNA viruses. Indeed, the mutation rates in influenza A virus has been estimated to be approximately one mutation per genome per replication (Drake, 1993). This mutation rate is up to 300 times faster than reported for DNA viruses. It is likely that this high mutation rate may induce changes to viral surface proteins. As antibodies are produced against these proteins following prior exposure (either via exposure to the virus or through vaccination), this may impact on an individual's immunity to these viruses. This is particularly apparent for influenza viruses and annual influenza vaccinations include updated antigens to account for this and to provide for immunity to new and emerging strains. The mutability of the other RNA respiratory viruses also affects an individual's ability to respond to future reinfections. Indeed, it was noted in the sections discussing the individual respiratory viruses that prior exposure generally does not confer life-long immunity. Similarly, the mutability of the RNA viruses may provide them with resistance to antiviral drugs and the new strains should be screened against these drugs as those strains. This also highlights the need to develop new antiviral therapies.
Also noteworthy, influenza viruses, parainfluenza viruses, HRSV, HMPV and coronaviruses are enveloped viruses, whereas the viruses that cause colds (enteroviruses, rhinoviruses, adenoviruses) are non-enveloped viruses. Non-enveloped viruses have substantially greater stability than enveloped viruses outside of the host cell as lipid envelopes are relatively fragile and sensitive to denaturation by environmental factors (e.g. temperature and pH) and well as by detergents and other chemicals (Lucas, 2001). For this reason, using detergents and soaps to wash hands is considered an effective preventative measure for enveloped viruses including influenza virus and is recommended for the current SARS-CoV-2 pandemic. In contrast, the viruses that cause colds are non-enveloped and therefore more stable outside host cells. For this reason, they last for longer times, which accounts for the greater rate of spread of colds.
1.2. Spread of respiratory viruses
Viral respiratory infections are very common, largely due to their ease of transmission. Viral respiratory infections may be spread by inhalation when an infected individual coughs or sneezes, or even when they talk. Small droplets of saliva containing large numbers of the viral pathogen are released and dispersed into the air. If other individuals in the vicinity of the sick person breathe in these droplets, and they may become infected. Unless that person has already acquired immunity to the virus via prior exposure, it is likely that they will develop the illness. Thus, one of the most effective ways to retard the spread of respiratory viruses is via behaviour modification. If an infected person covers their mouth when coughing or sneezing, the incidence of spread of the viral respiratory disease is dramatically reduced. Respiratory viruses may also be spread via direct contact with an infected person, or via indirect contact with items and surfaces onto which an infected person has coughed or sneezed. Frequent and effective use of hand washes and surface disinfectants may therefore also substantially reduce the risks of infection. As adenoviruses and enteroviruses may also infect the gastrointestinal tract, contact with objects and surfaces contaminated with faeces from an infected individual (particularly via the hands) may also result in respiratory infections. Disinfection of hands and surfaces also reduces the spread of these viruses.
1.3. Signs and symptoms of viral respiratory diseases
Whilst a number of different viral pathogens may cause respiratory disease, there is similarity between the symptoms of many of those infections. Coughing, sneezing, runny noses, sore throats, blocked airways and laboured breathing are common symptoms of viral respiratory infections. Fever may also be associated with some infections (e.g. influenza). Infected individuals may also suffer from headaches and/or muscle aches and pains, and lethargy is common. The onset of the symptoms begins within a day or two of contracting the virus and last for up to 14 days (or longer for influenza).
In addition to differences related to the viral pathogen, the site of the infection (i.e. upper or lower respiratory tract infections) also significantly affects the disease symptoms. Upper respiratory tract infections affect the upper respiratory passages (larynx, nasal cavity and nasal passages, as well as the pharynx). The common cold is an example of an upper respiratory infection. In addition to the symptoms listed above that are common for all viral respiratory infections, upper respiratory infections are also characterised by discomfort of the nasal passages, excess mucus production, nasal congestion, runny nose and a sore throat. Headache, muscular pain and fever may also occur.
Lower respiratory tract infections occur when the virus infects the bronchial tubes (causing bronchitis) or the alveoli (causing pneumonia). The severity of the symptoms is dependent on the infective virus and the severity of the infection. Less severe infections may present with similar symptoms to the common cold (described above). In more severe infections, a phlegm-producing cough, fever, difficulty breathing, chest pain and wheezing may also be evident. Many of the symptoms are common between upper and lower respiratory tract infections. However, the nature of the symptoms can help differentiate between the sites of infection. People with lower respiratory infections generally present with consistent coughing with high phlegm production as the most apparent symptom. For upper respiratory tract infections, sneezing, sore throats and headaches are more apparent.
1.4. Current treatment options for viral respiratory diseases
There are few effective treatments for viral respiratory diseases and medical intervention is generally not required for most individuals. Viral respiratory diseases are generally self-limiting and most people will recover with rest and the consumption of adequate volumes of fluids. In most cases, therapy aims to alleviate the symptoms rather than to treat the cause of the disease. Acetaminophen (paracetamol) is useful for relieving much of the pain and discomfort associated with viral respiratory infections. Antiviral medicines are usually not used except in severe and/or prolonged cases of influenza. As of 2020, six prescription influenza antiviral medications had been approved by the United States Food and Drug Administration (FDA):
-
•
Three chemically and functionally related drugs: oseltamivir phosphate (known as Tamiflu®), zanamivir (marketed as Relenza®) and intravenous peramivir (Rapivab®), are used as pharmaceutical treatments of influenza (FDA, 2019). All of these medications block the viral neuraminidase enzymes, thereby inhibiting viral release from infected cells. These neuraminidase inhibitors are effective against both influenza A and B viruses. They are relatively effective and generally are safe for most people to use, although side effects including nausea and vomiting have been reported in some people. The cost of these drugs can be prohibitive in some regions of the world (particularly in developing countries) and they are often not available in rural and isolated regions. Furthermore, influenza virus resistance is increasingly being reported to these drugs (Sheu et al., 2008) and new therapies are urgently required.
-
•
Baloxavir marboxil (marketed as Xofluza®), is also effective for the treatment of influenza A and B infections (FDA, 2019). This drug functions by inhibiting the cap-dependent endonuclease activity of influenza viruses, thereby blocking influenza virus RNA synthesis. However, several influenza strains have rapidly developed resistance to this drug since its clinical introduction in 2018, already limiting its efficacy (Hayden et al., 2018). Furthermore, adverse side effects including bronchitis and diarrhoea are reported in >20% of people who take this drug.
-
•
The adamantine drugs amantadine (Symmetrel®) and rimantadine (Flumadine®) target the M2 ion channel protein of influenza A viruses (FDA, 2019). Therefore, they are indicated for influenza A but are ineffective against influenza B viruses. However, several influenza A serotypes have developed high levels of resistance (>99%) to these drugs and they are no longer considered effective against H1N1 and H3N2 serotypes (Deyde et al., 2007).
Whilst influenza virus resistance to the neuraminidase and cap-dependent endonuclease inhibitors classes of drugs are still relatively low, this is likely to change in the future with continued overuse of these drugs. These drugs should be used sparingly and reserved for high risk individuals (children, the elderly and immunocompromised individuals). Furthermore, medical practitioners will also often prescribe antibiotics for viral respiratory infections. Antibiotics have no effects against viruses and they should not be used for viral respiratory diseases unless a secondary bacterial infection is suspected. The incorrect use of antibiotics for these purposes may result in antibiotic resistant bacteria and it is recommended that this practice should cease.
Plant-based and traditional medicines are also used by many cultures to treat viral respiratory infections and their symptoms. This is especially true in developing countries. In many areas (particularly in isolated and rural communities), consultation with traditional healers is prevalent and is often the primary healthcare modality. Furthermore, allopathic drugs are often perceived in these areas as being relatively ineffective and are generally expensive compared to traditional medicines. Isolated rural communities may have limited access to allopathic medical practitioners and conventional pharmaceuticals are often not readily available. An examination of the antiviral properties of traditional medicines against viral respiratory pathogens may highlight new leads for drug discovery and may provide effective new therapies to treat viral respiratory diseases.
2. Viral respiratory diseases: A South African context
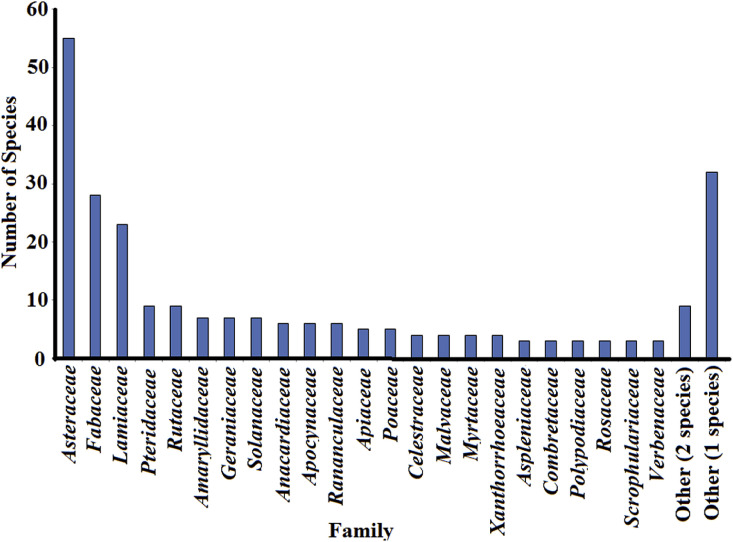
Southern Africa has similar viral respiratory disease epidemiology trends to other regions with similar population densities. The incidence of viral respiratory diseases substantially increases in the colder winter months (commonly referred to as cold and flu season) (Gessner et al., 2011). Geographical differences for viral respiratory diseases are also evident in various parts of southern Africa. Viral respiratory diseases are substantially more frequent in cold mountainous regions. These regions tend to have lower temperatures and therefore people tend to congregate indoors for longer periods, facilitating viral transmission. Related ethnic correlations also occur. For example, the southern Sotho tend to have higher prevalence's of the viral respiratory diseases than other ethnic groups in South Africa. The increased prevalence is due to the living environments of this group, rather than genetic factors. As the Southern Sotho often live in cold mountainous regions, they spend more time indoors in groups than many other ethnic groups. Thus, viral transmission rates are higher. Incidentally, the higher prevalence of the viral respiratory diseases in the southern Sotho correlates with the development and usage of substantially more therapies for these diseases than have been reported for other ethnic groups. Indeed, nearly three times the amount of plant species have been recorded for the treatment of respiratory virus diseases by the southern Sotho compared to the Zulu (as discussed later and summarised in Table 1 ). This is despite the population size differences between these groups and is in contrast to the substantially higher volume of studies reported for the Zulu ethnobotany. Indeed, Hutchings et al.(1996) and Ngwenya et al. (2003) are concerned only with Zulu ethnobotany, whereas many of the other texts used in this study examine the ethnobotany of multiple groups (Smith, 1888; Watt and Breyer-Brandwijk, 1962; Von Koenen, 2001; Van Wyk et al., 2009). Similarly, a large proportion of the ethnobotanical surveys cited in our study also concentrate on Zulus and regions in Zulu areas. In contrast, a single book (Moffett, 2010) and limited ethnobotanical surveys (Kose et al., 2015; Moteetee et al., 2019) have focused specifically on southern Sotho ethnobotany.
Table 1.
Southern African plants used traditionally to treat viral respiratory illnesses.
| Plant species | Family | Common name(s) | Plant part used | Used for | References |
|---|---|---|---|---|---|
| Acacia arenaria Schinz | Fabaceae | Sand acacia (English), sanddoring (Afrikaans) | Root | A decoction is consumed to treat colds. | Von Koenen (2001) |
| Acacia decurrens Willd. | Fabaceae | Black wattle, early green wattle (English) | Trunk exudate/gum | Used to treat bronchitis. | Watt and Breyer-Brandwijk (1962) |
| Acacia hebeclada DC. | Fabaceae | Cattlepod acacia (English), kersdoringboom (Afrikaans) | Root | A decoction is consumed to treat colds. | Von Koenen (2001) |
| Acacia karoo Hayne | Fabaceae | Sweet thorn (English), soetdoring (Afrikaans), mooka (Tswana), umuNga (Zulu, Xhosa) | Leaves and bark | Used to treat colds. Preparation and application are not specified. | Watt and Breyer-Brandwijk (1962); Hutchings et al.(1996); Van Wyk et al. (2009) |
| Acacia mearnsii De Wild. | Fabaceae | Uwatela (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Acacia mellifera (Vahl.) Benth. | Fabaceae | Hook thorn, black hook (English), swarthaak (Afrikaans) | Roots | Chewed to treat colds. | Von Koenen (2001) |
| Acacia nilotica (L.) Delile | Fabaceae | Redheart, scented thorn (English), lekkerreulpeul (Afrikaans) | Bark and leaves | Used to treat colds. | Hutchings et al.(1996); Von Koenen (2001) |
| Acacia senegal (L.) Willd. | Fabaceae | Three thorn tree (English), driedoringakasia (Afrikaans) | Gum (trunk exudate) | Used as an expectorant in people with colds and influenza. | Von Koenen (2001) |
| Acacia sieberana var. woodii (Burtt Davy) Keayb & Brenan | Fabaceae | Paperbark thorn (English), papierbasdoring (Afrikaans) | Leaf and bark | Decoctions are used as an expectorant in people with colds and influenza. | Von Koenen (2001) |
| Achyranthes aspera L. | Amaranthaceae | Devil's horsewhip (English), langplitskafblom (Afrikaans) | Roots | The Zulus use a decoction to treat bronchitis. Also used in Namibia to treat colds. | Von Koenen (2001); Watt and Breyer-Brandwijk (1962) |
| Acokanthera oppositifolia (Lam.) Codd. | Apocynaceae | Bushman's poison (English), boesmansgif (Afrikaans), inhlungunyembe (Zulu), intlungunyembe (Xhosa) | Leaves | Leaf decoctions are a Xhosa remedy for colds. However, this species is highly toxic and caution is required. | Watt and Breyer-Brandwijk (1962); Van Wyk et al. (2009); Philander (2011). |
| Acorus calamus L. | Acoraceae | Indaluqwatha, indawolucwatha, uzulucwatha (Zulu) | Not specified | Used by the Zulu to treat colds and influenza. | Mhlongo and Van Wyk (2019) |
| Adenopodia spicata (E.Mey.) C.Presl | Fabaceae | Spiny splinter bean (English), stekelsplinterboontjie (Afrikaans), ibobo, ubobo, umbambangwe (Zulu) | Bark | The Zulus use a decoction to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Adiantum chilense var. sulphureum (Kaulf.) Kuntze ex Hicken | Pteridaceae | Common maidenhair fern (English) | Leaves | The southern Sotho smoke the leaves to treat colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Adiantum capillus-veneris L. | Pteridaceae | Maidenhair fern (English), venushaar (Afrikaans) | Leaves | The southern Sotho smoke the leaves to treat colds. | Watt and Breyer-Brandwijk (1962); Hutchings et al. (1996); Von Koenen (2001); Moffett (2010) |
| Adansonia digitata L. | Malvaceae | Baobab (English), kremetartboom (afrikaans) | Fruit | Boiled and consumed to treat colds. | Von Koenen (2001) |
| Aeollanthus buchnerianus Briq. | Lamiaceae | Rock sage (English), klipsalie (Afrikaans) | Leaves | The Sotho smoke the leaves to treat colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Agathosma betulina (P.J.Bergius) Pillans | Rutaceae | Bucgh (English), boegoe, letuling (Afrikaans) | Leaves | An infusion is drunk to treat colds. | De Beer and Van Wyk (2011) |
| Albizia antunesiana Harms | Fabaceae | Purple-leaved false thorn (English), persblaarvalsdoring (Afrikaans) | Bark | Chewed to treat colds. | Von Koenen (2001) |
| Alepidea amatymbica Eckl. & Zeyh. | Apiaceae | Kalmoes (Afrikaans), lesoko (Sotho), iqwili (Xhosa), ikhathazo (Zulu) | Roots and stem | Zulus burn the stems and inhale the smoke to treat colds. Zulu and Sotho use a root decoction is drunk to treat colds and influenza. | ; Watt and Breyer-Brandwijk (1962); Hutchings et al. (1996); Van Wyk et al. (2009); Moffett (2010); Philander (2011); |
| Allium cepa L. | Amaryllidaceae | Onion (English) | Bulb | Used to treat influenza. | Watt and Breyer-Brandwijk (1962) |
| Allium sativum L. | Amaryllidaceae | Garlic (English) | Bulb | Used to treat influenza. | Watt and Breyer-Brandwijk (1962) |
| Aloe arborescens Mill. | Xanthorrhoeaceae | Krantz aloe (English), kransaalwyn (Afrikaans), ikalene (Xhosa), inkalane, umhlabana (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Aloe maculata All. | Xanthorrhoeaceae | Soap aloe, zebra aloe (English) | Leaves | The Zulu use a decoction to treat colds and influenza. | Watt and Breyer-Brandwijk (1962); Kose et al. (2015) |
| Anemone caffra Harv. | Rananculaceae | Unknown | Roots | Zulus used the powdered roots as a snuff to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Anemone vesicatoria (L.f.) Prantl | Rananculaceae | Blister leaf (English), brandblaar, katjiedrieblaar (Afrikaans) | Leaves and roots | Root decoctions are used to treat colds and influenza. No application is specified for the leaves | Van Wyk et al. (2009); Watt and Breyer-Brandwijk (1962) |
| Anisodontea triloba (Thunb.) D.M.Bates | Malvaceae | Wildsalie (Afrikaans) | Leaves | A leaf infusion is consumed to treat colds. | De Beer and Van Wyk (2011) |
| Antiphiona pinnatisecta (S.Moore) Merxm. | Asteraceae | Unknown | Roots | The San use an infusion to treat influenza. | Von Koenen (2001) |
| Aptosimum indivisum Burch. ex Benth. | Scrophulariaceae | Kinkhoesbos, agtdaegeneesbos (Afrikaans) | Not specified | Used to treat colds. | Hulley and Van Wyk (2019) |
| Artemisia afra Jacq. Ex Willd. | Asteraceae | African wormwood (English), als, alsem, wildeals (Afrikaans), lengana (Sotho, Tswana), umhlonyane (Xhosa, Zulu) | Leaves | Infusions and decoctions are used to treat coughs, colds, influenza and bronchitis. Fresh leaves may also be inserted directly into the nose. | Smith (1888); Watt and Breyer-Brandwijk (1962); Van Wyk et al. (2009); Moffett (2010); Kose et al. (2015). |
| Asplenium cordatum (Thunb.) Sw. | Aspleniaceae | Resurrection fern (English), skubcaring (Afrikaans) | Leaves and rhizome | Leaves are smoked by Sotho to treat colds. A rhizome decoction is drunk to treat colds and relieve sore throats. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Asplenium monanthes L. | Aspleniaceae | Unknown | Leaves | Smoked by southern Sotho to treat colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010). |
| Asplenium trichomanes L. | Aspleniaceae | Maidenhair spleenwort (English) | Leaves | Smoked by southern Sotho to treat colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Baccharoides adoensis var. mossambiquensis (Steetz) "Isawumi, El-Ghazaly & B.Nord." | Asteraceae | Unknown | Not specified | The Zulu use infusions to treat influenza. | Smith (1888); Watt and Breyer-Brandwijk (1962) |
| Ballota africana (L.) Benth. | Lamiaceae | kattekruid, kattekruie (Afrikaans) | Whole plant | In infusion is used to treat colds and influenza. | Watt and Breyer-Brandwijk (1962); Van Wyk et al. (2009); Hulley and Van Wyk (2019) |
| Bauhinia petersiana Bolle | Fabaceae | Camel's foot (English), koffiebos (Afrikaans) | Leaves | Boiled and the steam inhaled to treat colds and influenza. | Von Koenen (2001) |
| Berkheya setifera DC. | Asteraceae | Leleme la khomo (southern Sotho) | Roots and leaves | Used to treat colds. Preparation and application not specified. | Kose et al. (2015) |
| Bidens pilosa L. | Asteraceae | Black jack, beggars ticks, cobblerspegs, sticky beaks (English) | Not specified | Used to treat colds and influenza. Preparationa and application not specified. | Mhlongo and Van Wyk (2019) |
| Blepharis espinosa Phillips | Acanthaceae | Unknown | Not specified | Used by the Sotho to treat colds. | Moffett (2010). |
| Buddleja saligna Willd. | Scrophulariaceae | False olive (English), witolien (Afrikaans), lelothwane (southern Sotho), ungqeba (Xhosa), igqeba-elimhlope (Zulu) | Leaves | The Tswana us a leaf decoction to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Berkheya setifera DC. | Asteraceae | Buffalo-tongue thistle (English), rasperdissel (Afrikaans), ikhakhasi (Zulu), indlebe-lenkomo (Xhosa), lelelemia-khomo, ntsoantsane (southern Sotho) | Roots | A decoction prepared and consumed for coughs and colds. | Watt and Breyer-Brandwijk (1962) |
| Brunsvigia grandiflora Lindl. | Amaryllidaceae | Giant candellabria (English), reusekandelaarblom (Afrikaans) | Bulb | A decoction is prepared from the crushed bulb and consumed for coughs and colds. | Watt and Breyer-Brandwijk (1962) |
| Bulbine frutescens Willd. | Xanthorrhoeaceae | Unknown | Leaves | The dried leaves are smoked by the Sotho to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Bulbine narcissifolia Salm-Dyck | Xanthorrhoeaceae | Khomo ea balisa | Bulbs/roots | Used to treat colds. Preparation and application not specified. | Kose et al. (2015) |
| Carpobrotus edulis (L.) N.E.Br. | Aizoaceae | Sour fig, Cape fig, Hottentot's fig (English), vyerank, ghaukum, ghoenavy, hotnotsvye, Kaapvy, perdevy, rankvy (Afrikaans), ikhambi-lamabulawo, umgongozi (Zulu) | Leaves | Juice from squeezed leaves is diluted in water and used to relieve sore throats associated with colds. | Hutchings et al.(1996); Felhaber and Mayeng (1997) |
| Carissa bispinosa (L.) Defs. ex Brenan. | Apocynaceae | Amathungulu (Zulu) | Not specified | Used to treat colds and influenza. Preparationa and application not specified. | Mhlongo and Van Wyk (2019) |
| Cassine transvaalensis (Burtt Davy) Codd. | Celestraceae | Transvaal saffron (English), lepel, lepelhout, waterboom (Afrikaans) | Leaves | Adults chew the leaves and swallow the sap to treat influenza. An infusion is given to children for the same purpose. | Hutchings et al. (1996); Von Koenen (2001); |
| Catha edulis (Vahl) Endl. | Celestraceae | Bushman's tea (English), boesmanstee (Afrikaans), khat (Arabic) | Leaves | The Xhosa use the leaves to treat influenza. | Watt and Breyer-Brandwijk (1962); Van Wyk et al. (2009) |
| Chamarea capensis (Thunb.) Eckl. & Zeyh. | Apiaceae | Vinkelbol, vinkelwortel (Afrikaans) | Root | The infusions are drunk to treat colds. | Hulley and Van Wyk (2019) |
| Cheilanthes eckloniana Met. | Pteridaceae | Ecklon's lip fern, resurrection fern (English) | Root, leaves | A decoction is consumed by the Sotho to treat colds. The leaves are also smoked to treat colds. | Moffett (2010); Watt and Breyer-Brandwijk (1962) |
| Cheilanthes hirta Sw. | Pteridaceae | Mamauoaneng (southern Sotho) | Root | A decoction is consumed by the southern Sotho to treat colds. | Watt and Breyer-Brandwijk (1962); Hutchings et al. (1996); Kose et al. (2015) |
| Cheilanthes involuta var. obscura (N.C.Anthony) N.C. Anthony | Pteridaceae | Unknown | Root | A decoction is consumed by the Sotho to treat colds. | Moffett (2010) |
| Chaenostoma floribundum Benth. | Scrophulariaceae | Unknown | Root | The Southern Sotho use a decoction to treat colds in children. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Chloris virgata Sw | Poaceae | Feather finger grass (English) | Not specified | Used by the Sotho to treat colds. | Moffett (2010) |
| Chrysocoma ciliata L. | Asteraceae | Beesbos (Afrikaans) | Leaves | A decoction is drunk to treat colds. | De Beer and Van Wyk (2011) |
| Cinnamomum camphora (L.) J.Presl. | Lauriaceae | Camphor tree (English), kanferboom (Afrikaans), uroselina (Zulu) | Essential oil (distilled from the wood) | Used to treat colds. | Van Wyk et al. (2009); Philander (2011). |
| Clematis brachiata Thunb. | Rananculaceae | Morara oa thaba (southern Sotho) | Not specified | Used by the Sotho to treat colds. | Hutchings et al.(1996); Moffett (2010); Kose et al. (2015). |
| Cleome angustifolia Forssk. | Cleomaceae | Unknown | Roots | The San inhale the steam from boiling roots to treat influenza. | Von Koenen (2001) |
| Cliffortia ilicifolia L. | Rosaceae | Unknown | Not specified | Used by Boers as an expectorant to relieve the symptoms of colds. | Watt and Breyer-Brandwijk (1962) |
| Cliffortia odorata L.f. | Rosaceae | Wildewingerd (Afrikaans) | Not specified | An infusion is drunk to treat colds. | Watt and Breyer-Brandwijk (1962); Philander (2011); |
| Conyza scabrida DC. | Asteraceae | Bakhos, oondbos (Afrikaans), isavu (Xhosa) | Leaves | Infusions are consumed and powdered. Used as a snuff to treat colds and influenza. | Hutchings et al. (1996); Van Wyk et al. (2009); De Beer and Van Wyk (2011); Philander (2011); Hulley and Van Wyk (2019) |
| Chloris virgata Sw. | Poaceae | Feather-finger grass, Rhodes grass (English) | Roots | A decoction is added to the bath by Xhosa to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Chrysanthemum morifolium Ramat. | Asteraceae | Unknown | Leaves and flowers | People with colds sleep on a pillow filled with leaves and flowers to relieve the symptoms. | Watt and Breyer-Brandwijk (1962) |
| Clematis brachiata Thunb. | Rananculaceae | Ihlonzo leziduli, inhlongo, umdloza, umfufuna (Zulu) | Stem | The Xhosa sniff the bruised stem to treat colds. | Smith (1888); Watt and Breyer-Brandwijk (1962); York et al. (2011) |
| Combretum molle R.Br. ex G.Don | Combretaceae | Umbondo, umbondwe (Zulu) | Leaves | An infusion is drunk to treat colds. | York et al. (2011) |
| Corymbia gummifera (Gaertn.) K.D.Hill & L.A.S.Johnson | Myrtaceae | Red bloodwood (English) | Leaves | Essential oils are inhaled to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Citrus limon (L.) Osbeck | Rutaceae | Lemon (English) | Fruit juice | Consumed to treat colds. | Watt and Breyer-Brandwijk (1962); York et al. (2011) |
| Crassula alba Forssk. | Crassulaceae | Unknown | Juice | The Zulu and Swati mix the juice with water as a nasal cleanser for influenza sufferers. | Watt and Breyer-Brandwijk (1962); Hutchings et al.(1996) |
| Crinum bulbispermum (Burm.f.) Milne-Redh. & Schweik. | Amaryllidaceae | Orange River lily, Vaal River lily (English), Oranjierivierlelie (Afrikaans), umnduze (Zulu) | Bulb | A decoction is prepared from the crushed bulb and consumed by the southern Sotho to treat colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010); |
| Crinum macowanii Baker | Amaryllidaceae | umduze (Zulu) | Bulbs | A decoction is prepared from the crushed bulb and consumed by the southern Sotho to treat colds. | Van Wyk et al. (2009) |
| Cymbopogon nardus (L.) Rendle | Poaceae | Citronella grass (English) | Entire plant | Used to treat colds. Treatment and application is not specified. | Watt and Breyer-Brandwijk (1962); Hutchings et al. (1996) |
| Cyperus longus L. | Cyperaceae | Galingale (English) | Tuber | The Zulu inhale the powdered tuber to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Datura spp. | Solanaceae | Various common names for different species. | Not specified | Used by the Sotho to treat colds. | Moffett (2010) |
| Dicerothamnus rhinocerotis (L.f.) Koek. | Asteraceae | Rhinoceros bush (English) | Not specified | Used to treat influenza. Preparation and application methods are not specified. | Watt and Breyer-Brandwijk (1962) |
| Dicoma anomala Sond. | Asteraceae | Fever bush, stomach bush (English), maagbitterwortel, kalwerbossie, koorsbossie, gryshout, maagbossie (Afrikaans), inyongana (Xhosa), isihlabamakhondlwane, umuna (Zulu) | Roots | Powdered root bark is inhaled by the Sotho as a snuff to treat colds. A decoction may also be consumed for the same purpose. | Watt and Breyer-Brandwijk (1962); Hutchings et al. (1996); Von Koenen (2001); Moffett (2010); Kose et al. (2015) |
| Dicoma capensis Less. | Asteraceae | Wilde karmedik, koorsbossie (Afrikaans) | Roots | Powdered root bark is inhaled by the Sotho as a snuff to treat colds. A decoction may also be consumed for the same purpose. | Hutchings et al. (1996); Van Wyk et al. (2009); De Beer and Van Wyk (2011) |
| Digitaria sanguinalis (L.) Scop. | Poaceae | Crab grass (English) | Whole plant | A decoction is consumed to treat colds. | Smith (1888) |
| Dioscorea dregeana (Kunth.) D. Durand & Schinz. | Dioscoreaceae | Ingevu, intana ebovu, udakawa (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Dodonaea viscosa (L.) Jacq. | Sapindaceae | Sand olive (English), sandolien, ysterbos (Afrikaans), mutata-vhana (Venda) | Leaves, twigs | Used to treat colds and influenza. | Nortje and Van Wyk (2015); Van Wyk et al. (2009); De Beer and Van Wyk (2011); Nortje and Van Wyk (2015); Hulley and Van Wyk (2019). |
| Dolichothrix ericoides (Lam.) Hilliard & B.L.Burtt. | Asteraceae | Klipanoster, berganoster (Afrikaans) | Not specified | Infusions are drunk to treat colds. | Hulley and Van Wyk (2019) |
| Drimia altissima (L.f.) Ker Gawl. | Asparagaceae | Tall white squill (English) | Bulb | The powdered bulb is ingested to treat influenza and bronchitis. | Watt and Breyer-Brandwijk (1962) |
| Dysphania ambrosoides (L.) Mosyakin & Clemants | Amaranthaceae | Wormseed, Jesuit's tea (English), Ikhambi leslumo, isinuka, isinukamasimba, umanxiweni (Zulu) | Not specified | Used by the Zulu to treat colds and influenza. | ; Hulley and Van Wyk (2019); Mhlongo and Van Wyk (2019); Watt and Breyer-Brandwijk (1962); Hutchings et al. (1996) |
| Elaphoglossum conforme (Sw.) Schott | Pteridaceae | Unknown | Rhizome | A decoction is consumed by the southern Sotho to treat colds. | Moffett (2010) |
| Elaphoglossum petiolatum (Sw.) Urb. | Pteridaceae | Graceful tongue fern (English) | Rhizome | A decoction is consumed by the southern Sotho to treat colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Empleurum unicapsulare (L.f.) Skeels | Rutaceae | Bergboegoe, bokboegoe, langblaarboegoe (Afrikaans) | Not specified | Used to treat colds and influenza, Preparation and application are not specified. | Hulley and Van Wyk (2019) |
| Equisetum ramosissimum Desf. | Equisetaceae | Branched horsetail (English) | Rhizome | Zulu and Sotho drink a decoction of the rhizome to treat colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Eriocephalus africanus L. | Asteraceae | Kapokbos, skaapkaroo (Afrikaans) | Not specified | Infusions are used to treat colds. | Hulley and Van Wyk (2019) |
| Eriocephalus punctalatis DC. | Asteraceae | Wild rosemary, Cape snowbush (English), kapokbos (Afrikaans) | Unspecified | Used to fumigate huts of people with colds. | Watt and Breyer-Brandwijk (1962) |
| Erigeron bonariensis L. | Asteraceae | Unknown | Leaves | A leaf infusion is consumed by Zulus to treat colds. | Watt and Breyer-Brandwijk (1962); Hutchings et al. (1996) |
| Eriosema cordatum E.Mey. | Fabaceae | Ugwayana, umuthi wamadoda, umvusandoda (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Eriosema distinctum N.E.Br. | Fabaceae | Ugqomfane, umvusandoda, uqonsi (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Erythrophleum suaveolens (Guill. & Perr.) Brenan | Fabaceae | Woodland waterberry, water pear (English), waterpeer (Afrikaans) | Bark | The Zulu use the powdered bark as a snuff to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Eucalyptus camaldulensis Dehnh. | Myrtaceae | Umgamthrini (Zulu) | Gum (trunk exudate) | The gum is dissolved in water and used to treat colds. | Watt and Breyer-Brandwijk (1962); Mhlongo and Van Wyk (2019) |
| Eucalyptus globulis Labill. | Myrtaceae | Blue gum (English) | Leaves | Decoctions and infusions are used to treat colds and influenza. | Smith (1888); Watt and Breyer-Brandwijk (1962) |
| Eucomis autumnalis (Mill.) Chitt. | Asparagaceae | Autumn pineapple lily, pineapple flower (English), wildepynappel, krulkoppie (Afrikaans), ubuhlungu becanti, isithithibala esimathunzi (Xhosa), umathunga, ukhokho, umakhandakantsele (Zulu) | Not specified | Used to treat colds and influenza. Preparationa and application not specified. | Mhlongo and Van Wyk (2019) |
| Euryops spp. | Asteraceae | Different names for different species | Not specified | Ingested to treat influenza. | Watt and Breyer-Brandwijk (1962) |
| Foeniculum vulgare Mill. | Apiaceae | Vinkel, makvinkel (Afrikaans) | Not specified | Infusions are drunk to treat colds. | Hulley and Van Wyk (2019) |
| Geranium incanum Burm.f. | Geraniaceae | vrouebossie, bergtee, amarabossie (Afrikaans), ngope-sethsoha, tlaka (Sotho) | Leaves | Leaf infusions are used to treat bronchitis. | Van Wyk et al. (2009) |
| Geranium ornithopodon Eckl. & Zeyh. | Geraniaceae | Unknown | Leaves | A leaf decoction is consumed to treat colds. | Moffett (2010) |
| Gerbera ambigua (Cass.) Sch. Bip. | Asteraceae | Seboka (southern Sotho) | Roots | A root infusion is drunk to treat severe colds. | Kose et al. (2015) |
| Gerbera piloselloides (L.) Cass. | Asteraceae | Tsebe ea pela (southern Sotho) | Unspecified | The southern Sotho use it to fumigate huts of people with colds. | Watt and Breyer-Brandwijk (1962); Hutchings et al. (1996); Moffett (2010); Kose et al. (2015); |
| Gerbera viridifolia (DC.) Sch. Bip. | Asteraceae | Blushing baberton daisy (English), griquateebossie (Afrikaans), lyeza lamazi (Xhosa) | Unspecified | The southern Sotho inhale smoke from the burning plant to treat colds. An infusion is consumed for the same purpose. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Gladiolus dalenii Van Geel | Iridaceae | Parrot gladiolus, Natal lily (English), papegaai-gladiolus, wildeswaardlelie (Afrikaans), umnunge (Xhosa), udwendweni, uhlakahle (Zulu), khahla-e-kholo (Sotho) | Corm | A decoction is used by the southern Sotho to treat colds. Smoke from burning corms is inhaled for the same purpose. | Hutchings et al. (1996); Von Koenen (2001); Moffett (2010); Watt and Breyer-Brandwijk (1962) |
| Glycyrrhiza glabra L. | Fabaceae | Liquorice, licorice (English) | Rhizome | Decoctions and infusions are used to treat colds, influenza and bronchitis. | Watt and Breyer-Brandwijk (1962) |
| Gnidia anthylloides (L.f.) Gilg. | Thymelaeaceae | Brandbossie (Afrikaans), indolo, intozwane (Zulu) | Roots | Used by the southern Sotho to treat influenza. | Hutchings et al.(1996); Moffett (2010); Watt and Breyer-Brandwijk (1962) |
| Gnidia kraussiana Meisn. | Thymelaeaceae | Impevu, umsila wengwe, umahedeni (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Gomphocarpus fruticosus (L.) W.T.Aiton | Apocynaceae | Milkweed (English), melkbos, tontelbos (Afrikaans), lebegana, lereke-la-ntja (Sotho), modimolo (southern Sotho), umsinga-lwesalukazi (Zulu) | Not specified | Used by the southern Sotho to treat colds. | Von Koenen (2001); Moffett (2010) |
| Grangea maderaspatana (L.) Poir. | Asteraceae | Unknown | Unspecified | A decoction is used by the Xhosa to treat colds and influenza. The crushed leaves may also be directly inserted into the nostrils for the same purpose. | Watt and Breyer-Brandwijk (1962) |
| Gunnera perpensa L. | Gunneraceae | Wild rhubarb, river pumpkin (English), wilde ramenas, ravierpampoen (Afrikaans), qobo (Sotho), rambola-vhadzimu (Venda), iphuzi, ighobo (Xhosa), ugobhe (Zulu) | Leaves and roots | Used to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Gymnosporia buxifolia Szysyzl. | Celestraceae | Lemoendoring, wondedoring, pendoringbos (Afrikaans) | Roots | Root decoctions are consumed to treat colds. | Hulley and Van Wyk (2019); Watt and Breyer-Brandwijk (1962) |
| Gymnosporia heterophylla (Eckl. & Zeyh.) Loes. | Celestraceae | Gewone pendoring (Afrikaans) | Leaves | An infusion is consumed to treat colds and influenza. | Von Koenen (2001); Hutchings et al. (1996) |
| Haplocarpha scaposa Harv. | Asteraceae | Papetloana (southern Sotho) | Root | A decoction is consumed by the Xhosa to treat colds. | Kose et al. (2015); Watt and Breyer-Brandwijk (1962) |
| Helichrysum appendiculatum (L.f.) Less. | Asteraceae | Sheep's ears everlasting (English), skaapoorbossie (Afrikaans), senkotoana (southern Sotho), ibode, indlebeyemvu (Zulu) | Leaves | The leaves are eaten raw to treat colds. | Watt and Breyer-Brandwijk (1962); Smith (1888) |
| Helichrysum caespititium (DC.) Sond. Ex Harv. | Asteraceae | Phate ea ngaka (southern Sotho) | Leaves | Smoke from burning leaves is inhaled by the southern Sotho to treat colds. | Kose et al. (2015); Moffett (2010); Watt and Breyer-Brandwijk (1962) |
| Helichrysum cymosum (L.) D.Don. | Asteraceae | Kooibos (Afrikaans) | Not specified | Used to treat colds. Preparation and application not specified. | Hulley and Van Wyk (2019) |
| Helichrysum dregeanum Sond. & Harv. | Asteraceae | Bergankeerkaroo, vaalberganker (Afrikaans) | Leaves | The southern Sotho smoke the leaves to relieve colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Helichrysum cochleariforme DC. | Asteraceae | Unknown | Not specified | Used to treat bronchitis. Preparation and application not specified. | Watt and Breyer-Brandwijk (1962) |
| Helichrysum luteoalbum (L.) Rchb. | Asteraceae | Impepho, inkoldlwane (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Helichrysum nudifolium Less. | Asteraceae | Everlastings (English), hottentotsteebossie, kooigoed (Afrikaans), isicwe, indlebe zebhokwe, undleni (Xhosa), icholocholo, imphepho (Zulu) | Leaves and roots | Khoikhoi and Xhosa consume the leaves as a remedy for colds. Root decoctions are used for the same purpose. | Watt and Breyer-Brandwijk (1962); Hutchings et al. (1996); Van Wyk et al. (2009) |
| Helichrysum odoratissimum (L.) Sweet | Asteraceae | Everlastings (English), kooigoed (Afrikaans) imphepho (Zulu) | Roots | Decoctions are consumed to treat colds. | Hulley and Van Wyk (2019); Kose et al. (2015); Moffett (2010); Van Wyk et al. (2009); Hutchings et al. (1996); Watt and Breyer-Brandwijk (1962) |
| Helichrysum pedunculare Hilliard & B.L.Burtt. | Asteraceae | Unknown | Roots | Root decoctions are consumed to treat coughs and colds. | Watt and Breyer-Brandwijk (1962) |
| Helichrysum rugulosum Less. | Asteraceae | Marotole, motlosa-ngaka, motoantoanyane (southern Sotho) | Unspecified | Used by the southern Sotho to fumigate huts of people with colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Hermannia cuneifolia Jacq. | Malvaceae | Wilde heuning, geneesbossie, pleisterbossie (Afrikaans) | Leaves | A leaf infusion is used to treat colds. | De Beer and Van Wyk (2011) |
| Hermannia salviifolia L.f. | Sterculiaceae | Katjiedrieblaar (Afrikaans) | Not specified | Used to treat colds. Preparation and application not specified. | Hulley and Van Wyk, 2019 |
| Herniaria hirsuta L. | Caryophyllaceae | Hairy rupture wort (English) | Root | A root decoction is used to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Hoodia gordonii (Masson) Sweet ex Decne. | Apocynaceae | Hoodia, ghaap, kakimas (Afrikaans) | Fleshy stems | Used to treat colds. Preparation and application not specified. | Philander (2011) |
| Hypoxis hemerocallidea Fisch., C.A.Mey. & Ave-Lall. | Hypoxidaceae | Yellow star, star lily, star flower (English), sterblom, geelsterretjie, gifbol (Afrikaans), moli kharatsa, lotsane (southern Sotho); inkomfe, inkomfe enkulu (Zulu), inongwe, ilabatheka, ixhalanxa, ikhubalo lezithunzela (Xhosa), tshuka (Tswana) | Not specified | Used to treat colds and influenza. Preparationa and application not specified. | Mhlongo and Van Wyk (2019) |
| Ilex mitis (L.) Radlk. | Aquifoliaceae | Cape holly, African holly, water tree (English), waterboom, waterhout (Afrikaans), nonaname (Northern Sotho), ipuphuma (Zulu), unduma (Xhosa), phukgu, phukgile (Sotho), mutanzwa-khameol (Venda) | Leaves and bark | The Zulu pound the leaves and bark to produce a lather which is used to wash the bodies of influenza sufferers. | Watt and Breyer-Brandwijk (1962) |
| Imperata cylindrica (L.) Raeusch | Poaceae | Kunai grass, cogongrass (English) | Roots | The southern Sotho used the roots to treat colds. | Moffett (2010); Watt and Breyer-Brandwijk (1962) |
| Kalanchoe paniculata Harv. | Crassulaceae | Hasieoor (Afrikaans), indabulaluvalo (Zulu), sehlakwahlakwane (southern Sotho) | Roots | The southern Sotho chew the fresh root or use the powdered root as a snuff to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Laggera decurrens (Vahl.) Merxm. | Asteraceae | Wolbos (Afrikaans) | Leaves and roots | Boiled and the stem is inhaled to treat colds. | Von Koenen (2001) |
| Lantana camara L. | Verbenaceae | Tickberry (English) | Leaves | Used to treat colds. Preparation and application are not specified. | Watt and Breyer-Brandwijk (1962); Von Koenen (2001) |
| Lantana rugosa Thunb. | Verbenaceae | Bird's brandy (English) | Leaves | The Xhosa use leaf extracts to treat colds. | Von Koenen (2001) |
| Lebeckia sericea Thunb. | Fabaceae | Silverpea (English), bloufluitjiesbos, vaalertjiebos (Afrikaans) | Not specified | The Nama use the plant as a remedy for colds. | Watt and Breyer-Brandwijk (1962) |
| Leonitis ocymifolia (Burm.f) Iwarsson | Lamiaceae | Wild dagga (English), wildedagga (Afrikaans) | Leaves and stems | Used as a treatment for colds. Preparation and application not specified. | Hulley and Van Wyk (2019); Watt and Breyer-Brandwijk (1962) |
| Leonotis ocymifolia var. schinzii (Gürke) Iwarsson | Lamiaceae | Unknown | Leaves and stems | The Tswana use a decoction to treat coughs and colds. | Watt and Breyer-Brandwijk (1962) |
| Leonotis leonurus (L.) R.Br. | Lamiaceae | Wild dagga (English), wildedagga, duiwelstabak (Afrikaans), mvovo (Xhosa), uyshwala-bezinyoni (Zulu) | Leaves and stems | The Sotho, Zulu and Tswana use a decoction of the leaves and stems to treat coughs, colds and influenza. | Moffett (2010); Van Wyk et al. (2009); Smith (1888); Watt and Breyer-Brandwijk (1962); Hutchings et al.(1996); Van Wyk et al. (2009); Moffett (2010); Hulley and Van Wyk (2019) |
| Lessertia frutescens subsp. frutescens Goldblatt & J,C.Manning | Fabaceae | Keurtjie, beeskeurtiebos, kankerbos (Afrikaans) | Not specified | An infusion is used to treat colds. | Hulley and Van Wyk (2019) |
| Leucas martinicensis (Jacq.) R.Br. | Lamiaceae | Tumbleweed (English), tolbossie (Afrikaans) | Leaves | An infusion is used to treat colds and influenza. | Von Koenen (2001) |
| Leucas lavandulifolia (Sm.) Raf. | Lamiaceae | Umagumede (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Leucas pechuelii (Kuntze) Baker | Lamiaceae | Unknown | Entire plant | A decoction is made from the entire plant and sniffed to treat influenza. | Von Koenen (2001) |
| Lichtensteinia interrupta E. Mey. | Apiaceae | Umkhalaphanga (Zulu) | Roots | A root decoction is consumed by the Zulu, Xhosa and Sothern Sotho to treat colds. | Smith (1888); Watt and Breyer-Brandwijk (1962); Hutchings et al. (1996); Moffett (2010) |
| Lippia javanica (Burm F.) Spreng | Verbenaceae | Fever tea, lemon bush (English), koorbossie, beukesbossie, lemoenbossie (Afrikaans), inzinzinba (Xhosa), umsuzwane, umswazi (Zulu) | Leaves and stems | The Xhosa, Zulu and Tswana drink a decoction to treat colds, influenza and bronchitis. | Watt and Breyer-Brandwijk (1962); York et al. (2011); Mhlongo and Van Wyk (2019) |
| Lonchocarpus nelsii (Schinz) Heering & Grimme | Fabaceae | Apple leaf (English), appleblaar (Afrikaans) | Roots | Smoke from the burning roots is inhaled to treat colds. | Von Koenen (2001) |
| Lycopodium clavatum L. | Lycopodiaceae | Clubmoss, stag-horn clubmoss, running clubmoss, ground pine (English) | Whole plant | Used by the southern Sotho to treat colds | Moffett (2010) |
| Melolobium candicans (E.Mey.) Eckl. & Zeyh. | Fabaceae | Wild dagga (English), wildedagga (Afrikaans) | Leaves and stems | A decoction of the leaves and stems is drunk to treat colds. | De Beer and Van Wyk (2011) |
| Mentha aquatica L. | Lamiaceae | Water mint (English) | Bark | A decoction is used by Xhosa, Tswana and southern Sotho to treat colds. | Watt and Breyer-Brandwijk (1962); Hutchings et al.(1996) |
| Mentha longifolia (L.) L. | Lamiaceae | Wild mint (English), kruisement, balderjan (Afrikaans), koena-ya-thabo (Sotho), inixina, inzinziniba (Xhosa), ufuthana, lomhlanga (Zulu) | Leaves, roots and stems | The Xhosa, Zulu and Sotho take a decoction or add the leaves to milk and consume to treat colds, influenza and bronchitis. | Watt and Breyer-Brandwijk (1962); Hutchings et al.(1996); Van Wyk et al. (2009); Moffett (2010); De Beer and Van Wyk (2011); Hulley and Van Wyk (2019) |
| Mentha spicata L. | Lamiaceae | Spearmint (English), imboza (Xhosa) | Leaves | A decoction is used to treat colds. | Hulley and Van Wyk (2019) |
| Metalasia densa (Lam.) P.O.Karis | Asteraceae | Tee (southern Sotho) | Unspecified | Used by the southern Sotho to fumigate huts of people with colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Microglossa mespilifolia (Less.) B.L. Rob. | Asteraceae | Inkhambi elimhlophe, umazambezi (Zulu) | Not specified | Used to treat colds and influenza. Preparationa and application not specified. | Mhlongo and Van Wyk (2019) |
| Millettia grandis (E.Mey.) Skeels | Fabaceae | Ubobolwehlathi (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Mohria caffrorum (L.) Desv. | Anemiaceae | Carrot fern, scented fern (English), brandbossie (Afrikaans) | Leaves | The southern Sotho smoke the leaves to treat colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Mikania natalensis DC. | Asteraceae | Ihlozi (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Monsonia burkeana Planch. ex Harv. | Geraniaceae | Crane's bill (English), angelbossie, keitabossie, naaldbossie, teebos (Afrikaans) | Leaves and roots | A decoction is used by the Xhosa to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Monsonia emarginata L'Hér. | Geraniaceae | Dysentry herb (English), geita, geitabossie, keitabossie, naaldbossie (Afrikaans) | Leaves and roots | A decoction is used by the Xhosa to treat colds. | Smith (1888); Watt and Breyer-Brandwijk (1962); |
| Montinia caryophyllaceae Thunb. | Montiniaceae | Pepperbush, wild colve-bush (English), bergklapper, peperbos (Afrikaans) | Leaves | Pulverised leaves are used as a snuff to treat colds. | Von Koenen (2001) |
| Morella serrata (Lam.) Killick | Myricaceae | Mountain waxberry, lance-leaved waxberry (English), smalblar-wasbessie, berg wasbessie, waterolier (Afrikaans), isibhara, umaluleka (Xhosa), lyethi, ulethi, umakhuthula (Zulu) | Not specified | Used by the Sotho to treat colds. | Moffett (2010) |
| Myrothamnus flabellifolia Welw. | Myrothamnaceae | Resurrection plant (English), bergboegoe (Afrikaans), uvukwabafile (Zulu) | Leaves | Leaf infusions are used to treat colds. | Watt and Breyer-Brandwijk (1962); Von Koenen (2001); Van Wyk et al. (2009) |
| Nicotiana glauca Graham | Solanaceae | Mustard tree, tree tobacco (English), tabakboom, wildetabak, volstruisgifboom (Afrikaans), mohlafotha (Sotho) | Leaves | Powdered leaves are used by the Sotho as a snuff to treat colds. | Moffett (2010) |
| Nicotiana rustica L. | Solanaceae | Strong tobacco, Aztec tobacco (English) | Leaves | Powdered leaves are used by the southern Sotho as a snuff to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Notobubon tenuifolium (Thunb.) Magee | Apiaceae | Wildekoelsaad, wilde vinkel (Afrikaans) | Not specified | Used to treat colds. Preparation and application not specified. | Hulley and Van Wyk (2019) |
| Ocimum americanum L. | Lamiaceae | Hoary basil (English), wilde basielkruid (Afrikaans) | Leaves | The leaves are burnt and the smoke inhaled to treat colds. | Von Koenen (2001) |
| Oncosiphon piluliferum L.f. Källersjö | Asteraceae | Stinkkruid (Afrikaans) | Not specified | Used to treat colds and bronchitis. Preparation and application not specified. | Hulley and Van Wyk (2019) |
| Oncosiphon suffruticosum L. Källersjö | Asteraceae | Stinkkruid, wirmkruid (Afrikaans) | Whole plant | Used to treat influenza. | Van Wyk et al. (2009); Nortje and Van Wyk (2015); Hulley and Van Wyk (2019). |
| Osmitopsis asteriscoides Less. | Asteraceae | Bels, belskruie (Afrikaans) | Leaves | Used to treat influenza. | Van Wyk et al. (2009); Philander (2011) |
| Otholobium polystictum (Harv.) C.H.Stirt. | Fabaceae | Kite hook-leaved pea (English), vlieebos (Afrikaans), mohlonecha, mohlonepshoa (southern Sotho) | Root | Used by the southern Sotho to treat colds. | Moffett (2010); Watt and Breyer-Brandwijk (1962) |
| Pachycarpus rigidus E. Mey. ex Eckl. & Zeyh. | Apocynaceae | Ishongwe (Zulu) | Not specified | Used by the southern Sotho to treat colds. | Moffett (2010) |
| Packera heterophylla (Fisch.) E.Wiebe | Asteraceae | Unknown | Leaves | Smoke from burning leaves is inhaled by the Sotho to treat colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Pechuel-Loeschea leubnitziae (Kuntze) O.Hoffm. | Asteraceae | Bitterbos (mbiguous) | Leaves | Smoke from burning is inhaled to treat colds. | Von Koenen (2001) |
| Pegolettia baccharidifolia Less. | Asteraceae | Ghwarrieson, heuningdou (Afrikaans) | Not specified | Used to treat colds. Preparation and application not specified. | Hulley and Van Wyk (2019) |
| Pellaea calomelanos (Sw.) Link. | Pteridaceae | Hard fern (English), lehorometso (Sotho), inkomankomo (Zulu) | Leaves | The Xhosa and Sothern Sotho smoke the leaves to treat colds. | Watt and Breyer-Brandwijk (1962); Van Wyk et al. (2009); Moffett (2010); Philander (2011); Smith (1888) |
| Pellaea ambiguou (Sw.) Baker | Pteridaceae | Unknown | Leaves | The Sothern Sotho smoke the leaves to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Pentzia incana (Thunb.) Kuntze | Asteraceae | Skaapkaroobos, ankerkaroo, kleinskaapkaroobos (Afrikaans) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Hulley and Van Wyk (2019) |
| Passiflora suberosa L. | Passifloriaceae | Unyawo lenkukhu (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Pelargonium abrotanifolium Jacq. | Geraniaceae | Bergsalie (Afrikaans) | Leaves | A leaf infusion is used to treat colds and influenza. | De Beer and Van Wyk (2011) |
| Pelargonium graveolens L'Her. | Geraniaceae | Rose geranium (English), wildemalva (Afrikaans) | Leaves | Leaves are steamed and vapours are inhaled to treat colds. | Van Wyk et al. (2009) |
| Pelargonium ramosissimum Willd. | Geraniaceae | Dassieboegoe, dassiebos (Afrikaans) | Leaves | the Xhosa consume decoctions or tinctures (preferred) to treat colds. | Smith (1888); Watt and Breyer-Brandwijk (1962); De Beer and Van Wyk (2011) |
| Pentanisia prunelloides (Koltzsch) Walp. | Rubiaceae | Wild verbena (English), sooibrandbossie (Afrikaans), setimamollo (Sotho), icimamlilo (Zulu) | Roots | Used to treat colds and influenza. Preparation and application were not specified | Van Wyk et al. (2009); Watt and Breyer-Brandwijk (1962); Hutchings et al.(1996) ; Kose et al., 2015 |
| Persicaria lapathifolia (L.) Delarbre | Polygalaceae | Uxhaphoxana, uxhaphozi (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Phyla scaberrima (Juss. Ex Pers.) Moldenke | Verbenaceae | Unknown | Leaves | A decoction of the leaves is used to treat colds. | Smith (1888) |
| Plectranthus ambiguous (Bolus) Codd. | Lamiaceae | Iboza, imbatatane (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Plectranthus laxiflorus Benth. | Lamiaceae | Citronella spur flower (English), sitrinella spoorsalie (Afrikaans), ubebebe (Xhosa) | Leaves | A decoction made from powdered leaves was used by the Zulu as an enema to treat influenza. | Watt and Breyer-Brandwijk (1962); Hutchings et al.(1996); Ngwenya et al. (2003) |
| Polygala schinziana Chodat | Polygalaceae | Kanjengena (Afrikaans) | Roots | A decoction is consumed to treat coughs and colds. | Hutchings et al.(1996); Von Koenen (2001) |
| Pleopeltis macrocarpa (Bory ex Willd.) Kaulf. | Polypodiaceae | Lance leaf polypody (English) | Not specified | A decoction is consumed by the southern Sotho to treat colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010); |
| Protea repens L. | Proteaceae | Sugarbush (English), suikerbos (Afrikaans) | Flowers | Syrup prepared from the flower nectar is used by people with colds and influenza as a cough mixture. | Van Wyk et al. (2009) |
| Pteronia incana (Brum.) DC. | Asteraceae | Skieterbos, keurtjiebos, kraakbos (Afrikaans) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Hulley and Van Wyk (2019) |
| Pulicaria scabra (Thunb.) Druce | Asteraceae | Fleabane (English), aambeibos (Afrikaans), isithaphuka (Zulu) | Leaves | The powdered leaves are used by Zulus to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Pycreus nitidus (Lam.) J.Raynal | Cyperaceae | Unknown | Rhizome | Used by the southern Sotho to treat colds. Preparation and application is not specified. | Watt and Breyer-Brandwijk (1962) |
| Ranunculus capensis Thunb. | Rananculaceae | Blistering leaves (English), blandblare, katjiedrieblaar (Afrikaans) | Roots | A root infusion is consumed to treat colds. | Smith (1888) |
| Ranunculus multifidus Forssk. | Rananculaceae | Buttercup flower (English), botterblom, brandblaar, geelbotterbom, kankerblaar (Afrikaans), hlapi (southern Sotho), uxhaphozi, ishashakazane (Zulu) | Leaf | The Zulu use bruised leaves to treat colds. The southern Sotho also use the leaves to treat colds. | Smith (1888); Watt and Breyer-Brandwijk (1962); Hutchings et al.(1996); Moffett (2010) |
| Rhigozum trichotomum Burch. | Bignoniaceae | Driedoring (Afrikaans) | Stems | The Nama chew the stems to treat colds. | Von Koenen (2001) |
| Rhoicissus tridentata (L.f.) Willd. & R.B.Drumm. | Vitaceae | Northern Bushman's grape, bitter grape (English), noordelike boesmandruif, bitterdruif, droog-my-keel (Afrikaans), isaqoni, umnxeba, ulatile (Xhosa), isinwazi, umthwazi (Zulu), morara-oa-thaba (southern Sotho), murumbula-mbudzana (Venda) | Not specified | Used by the southern Sotho to treat colds. | Moffett (2010). |
| Rhus divaricata Eckl. & Zeyh. | Anacardiaceae | Fire thorn Karee, rusty leaved currant, mountain kuni-bush (English) | Roots | A root decoction is used to treat colds, influenza and bronchitis. | Watt and Breyer-Brandwijk (1962) |
| Rubus ludwigii Eckl. & Zeyh. | Rosaceae | Wild raspberry, silver bramble (English), braambos, wildebraam (Afrikaans), itshalo, unomhloshane (Zulu), monoko-metsi (southern Sotho) | Roots | The southern Sotho use a root decoction to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Rumex lanceolatus Thunb. | Polygalaceae | Tongblaar (Afrikaans) | Leaves | Fresh leaves are ground and used as a snuff to treat colds. | Von Koenen (2001) |
| Ruta graveolens L. | Rutaceae | Wynruit (Afrikaans) | Leaves | Leaf infusions are drunk to treats colds and influenza. | De Beer and Van Wyk (2011); Nortje and Van Wyk (2015); Hulley and Van Wyk (2019) |
| Salix hirsute Thunb. | Salicaceae | Cape willow (English), vaalwilger, wilgerboom (Afrikaans) | Bark | Used to treat colds and influenza. | Von Koenen (2001); Hulley and Van Wyk (2019) |
| Salvia africana-lutea L. | Lamiaceae | Bloebloomsalie (Afrikaans) | Not specified | A decoction is used by the Nama to treat colds. | Watt and Breyer-Brandwijk (1962); Philander (2011) |
| Salvia chameleagnea Berg. | Lamiaceae | Bloublomsalie (Afrikaans) | Leaves and flowers | A decoction is used by the Nama to treat colds. | Watt and Breyer-Brandwijk (1962); Hulley and Van Wyk (2019) |
| Salvia dentata Aiton | Lamiaceae | Bergsalie (Afrikaans) | Leaves | Leaf decoctions are drunk to treat colds. | De Beer and Van Wyk (2011); Nortje and Van Wyk (2015) |
| Salvia microphylla Kunth. | Lamiaceae | Rooisalie, rooiblomsalie (Afrikaans) | Not specified | Infusions are drunk to treat colds. | Hulley and Van Wyk (2019) |
| Salvia stenophylla Burch. ex Benth. | Lamiaceae | Maagpynbos, fynblaarsalie (Afrikaans) | Leaves | A leaf infusion is consumed to treat could and influenza. | Von Koenen (2001) |
| Schistostephium flabelliforme Less. | Asteraceae | Unknown | Not specified | An infusion is consumed to treat colds. | Smith (1888) |
| Schkuhria pinnata (Lam.) Kuntze. ex. Thell. | Asteraceae | Kleinkakiebos, klein-gousblom (Afrikaans) | Leaves | Powdered leaves are swallowed with water to treat colds and influenza. | Watt and Breyer-Brandwijk (1962); Von Koenen (2001) |
| Schotia brachypetala Sond. | Fabaceae | Weeping boer bean, tree fuchsia, African walnut (English), hullboerboon (Afrikaans), umfofofo, umgxam (Xhosa), ihluze, umgxamu, uvovovo (Zulu) | Not specified | Used to treat colds and influenza. Preparationa and application not specified. | Mhlongo and Van Wyk (2019) |
| Securidaca longipedunculata Fresen. | Polygalaceae | Violet tree (English), krinkhout (Afrikaans) | Roots | A root infusion is used by the San to treat colds. | Von Koenen (2001) |
| Searsia divaricata (Eckl. & Zeyh.) Moffett | Anacardiaceae | Mountain kuni-bush (English) | Not specified | Used by the southern Sotho to treat colds. | Moffett (2010) |
| Searsia erosa (Thunb.) Moffett | Anacardiaceae | Broom karee, besembos (English) | Not specified | Used by the southern Sotho to treat colds. | Moffett (2010) |
| Searsia lancea (L.f.) F.A.Barkley | Anacardiaceae | African sumuc, willow rhus (English) | Leaves | Used to treat colds. Preparation and application is not specified. | Mulaudzi et al. (2012) |
| Searsia natalensis (Bernh. ex C.Krauss) F.A.Barkley | Anacardiaceae | Natal rhus (English) | Roots | A decoction is used to treat influenza. | Watt and Breyer-Brandwijk (1962) |
| Sersia undulata (Jacq.) T.S.Yi, A.J.Mill. & J.Wen. | Anacardiaceae | Kuni-bush (English), koeniebos, garrabos (Afrikaans), t'kuni (Khoi) | Leaves, bark and roots | The Nama chew the leaves to relieve colds. A leaf decoction is consumed to treat colds. Alternatively, fresh leaves are chewed for the same purpose. No preparation is given for the bark and roots. | Van Wyk et al. (2009); Hulley and Van Wyk (2019); Watt and Breyer-Brandwijk (1962) |
| Selaginella caffrorum (Milde) Hieron | Selaginellaceae | Resurrection plant (English) | Not specified | Used by the southern Sotho to treat colds. | Moffett (2010); Kose et al. (2015) |
| Senecio asperulus DC. | Asteraceae | Moferefere (southern Sotho) | Unspecified | The southern Sotho use a decoction to treat colds and influenza. | Watt and Breyer-Brandwijk (1962); Moffett (2010); Kose et al. (2015) |
| Senecio brachypodus DC. | Asteraceae | Forest senecio (English) | Roots | An infusion is consumed by the southern Sotho to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Senecio dregeanus DC. | Asteraceae | Unknown | Roots | A root decoction is consumed by southern Sotho to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Senecio rhyncholaenus DC. | Asteraceae | Unknown | Leaves | The southern Sotho inhale the smoke from burning leaves to treat colds. | Watt and Breyer-Brandwijk (1962); Moffett (2010) |
| Senna italica Mill. | Fabaceae | Italian senna (English), elandsertjie (Afrikaans) | Root | The root is pounded, soaked in milk and consumed to treat influenza. | Watt and Breyer-Brandwijk (1962); Hutchings et al.(1996); Von Koenen (2001) |
| Seriphium plumosum L. | Asteraceae | Khoi-kooigoed, slangbos, slangbossie (Afrikaans) | Leaves | The Zulu boil the leaves and inhaled the steam to treat influenza. | Ngwenya et al. (2003) |
| Siphonochilus aethiopicus (Schweif.) B.L. Burt | Zingiberacaee | African ginger (English), isiphephetho, indungulo (Zulu) | Roots and rhizomes | Chewed to treat influenza. | Hutchings et al. (1996); Van Wyk et al. (2009); Philander (2011) |
| Solanum capense L. | Solanaceae | Nightshade (English) | Bark and roots | A decoction is drunk to treat colds. | Smith (1888); Hutchings et al.(1996) |
| Solanum americanum Mill. | Solanaceae | Black nightshade (English) | Fruit | Ripe fruit is taken with honey to treat colds. | Von Koenen (2001) |
| Solanum refractum Hook. & Arn. | Solanaceae | Nastergal, nasgal (Afrikaans) | Not specified | Infusions are drunk to treat colds. | Hulley and Van Wyk (2019) |
| Spilanthes mauritiana (A.Rich exPres.) DC. | Asteraceae | Isishoshokazane, isisinini (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Spirostachys africana Sond. | Euphorbiaceae | African mahogany, African sandalwood, headache tree, jumping bean tree, tamboti (English) agelhout, angelhout, gifboom, melkhout, dandaleenhout, tambootie (Afrikaans), muonze (Venda), morekuri (Northern Sotho), umtamboti (Zulu), umthombothi (Xhosa) | Bark | Used to treat influenza. The preparation and application were not specified. | Mulaudzi et al. (2012) |
| Stachys hyssopoides Burch. ex Benth. | Lamiaceae | Pinksalie (Afrikaans), selaoane (southern Sotho) | Unspecified | Used by the southern Sotho to treat colds. | Moffett (2010) |
| Sutherlandia frutescens (L.) R.Br. | Fabaceae | Cancer bush (English), kankerbos (Afrikaans), 'musa-pelo, motlepelo (Sotho), insiswa, unwele (Xhosa, Zulu) | Leaves | A decoction is used to treat influenza. | Van Wyk et al. (2009); De Beer and Van Wyk (2011); Nortje and Van Wyk (2015) |
| Sutherlandia humilis E.Phillips & R.A.Dyer | Fabaceae | Unknown | Leaves | A decoction is used to treat influenza. | Watt and Breyer-Brandwijk (1962) |
| Sutherlandia microphylla Burch. | Fabaceae | Unknown | Leaves | Infusions or decoctions are used to treat influenza. | Watt and Breyer-Brandwijk (1962) |
| Syzygium cordatum Hochst. ex Krauss | Myrtaceae | Waterberry (English), waterbessie, waterboom (Afrikaans), undoni (Zulu), umswi, umjomi (Xhosa), mawthoo (southern Sotho), motlho (Northern Sotho), mutu (Venda) | Leaves | Used to treat colds. Preparation and application not specified. | York et al. (2011); Mulaudzi et al. (2012); Mhlongo and Van Wyk (2019) |
| Tagetes minuta L. | Asteraceae | Ikhambi lempaka, usangwana (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Tarchonanthus camphoratus L. | Asteraceae | Wild camphor bush(English), wildekanferbos, vaalbos (Afrikaans), sefahla (Sotho), mofahlana (southern Sotho), mathola (Xhosa), igqeba-elimhlophe (Zulu) | Leaves and twigs | Infusions or tinctures are taken to treat bronchitis. | Von Koenen (2001); Van Wyk et al. (2009) |
| Tecoma capensis (Thunb.) Lindl. | Bignoniaceae | Umunyane, uthswala benyoni (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Terminalia prunioides M.A.Lawson | Combretaceae | Purple-pod Terminalia (English), deurmekaar, sterkbos (Afrikaans) | Root | A root infusion is consumed to treat colds. | Von Koenen (2001) |
| Terminalia sericea Burch. ex DC. | Combretaceae | Silver cluster leaf (English), vaalboom (Afrikaans), mususu (Venda) | Roots and leaves | Infusions are consumed to treat colds. | Hutchings et al. (1996) |
| Tetradenia riparia (Hochst.) Codd. | Lamiaceae | Misty plume bush, ginger bush (English), gemerbos, watersalie (Afrikaans), iboza, ibozane (Zulu) | Leaves | An infusion is used to treat stuffy and runny noses of people with colds and influenza. | Felhaber and Mayeng (1997); Van Wyk et al. (2009); York et al. (2011); Mhlongo and Van Wyk (2019); Van; |
| Teucrium africanum Thunb. | Lamiaceae | Drievingertee (Afrikaans) | Not specified | A decoction is used by Xhosa and Afrikaners to treat influenza. | Watt and Breyer-Brandwijk (1962); Hulley and Van Wyk (2019) |
| Thamnosma africana Engl. | Rutaceae | Flea bush (English) | Leaves and twigs | An infusion is consumed to treat colds and influenza. | Von Koenen (2001) |
| Thesium costatum A.W.Hill | Santalaceae | Kleinswartstorm (Afrikaans), marakalle (southern Sotho) | Not specified | The southern Sotho use multiple Thesium spp. to treat colds. | Watt and Breyer-Brandwijk (1962); Kose et al. (2015) |
| Toddalia asiatica (L.) Lam. | Rutaceae | Cockspur orange, climbing orange (English) | Root | The Venda use a root decoction to treat influenza. | Watt and Breyer-Brandwijk (1962) |
| Trephrosia semiglabra Sond. | Fabaceae | Unknown | Roots | The southern Sotho use a root decoction to treat colds. | Watt and Breyer-Brandwijk (1962) |
| Tropaeolum majus L. | Tropaeolaceae | Kappertjie (Afrikaans) | Not specified | Used to treat colds. Preparation and application not specified. | Hulley and Van Wyk (2019) |
| Tulbaghia acutiloba Harv. | Amaryllidaceae | Wild garlic (English), wilde knoffel (Afrikaans), konofolo, setotha-fotha (southern Sotho) | Whole plant | Used to treat colds. Preparation and application not specified. | Kose et al. (2015). |
| Tulbaghia violacea Harv. | Amaryllidaceae | Wild garlic (English), wilde knoffel (Afrikaans), isihaqa (Zulu) | Bulbs and leaves | Used to treat colds. | Van Wyk et al. (2009); Hulley and Van Wyk (2019); Mhlongo and Van Wyk (2019) |
| Valeriana capensis Thunb. | Caprifoliaceae | Cape valerian (English), wildebalderjan (Afrikaans) | Rhizome | Used to treat bronchitis. | Van Wyk et al. (2009) |
| Vangueria infausta Burch. | Rubiaceae | Wild medlar (English), wilde mispel (Afrikaans) | Leaves and roots | Infusions are consumed to treat colds. | Hutchings et al.(1996); Von Koenen (2001) |
| Vepris lanceolata G. Don. | Rutaceae | White ironwood (English), witysterhout (Afrikaans), muhondwa (Venda), umzane (Xhosa), umozana (Zulu) | Root | The Zulu use the powdered root as a treatment for influenza. | Smith (1888); Watt and Breyer-Brandwijk (1962) |
| Viscum capense L.f. | Santalaceae | Cape mistletoe (English), lidjiestee, voelent (Afrikaans) | Whole plant | An infusion is used to treat bronchitis. | Van Wyk et al. (2009); Nortje and Van Wyk (2015) |
| Volkameria glabra (E.Mey.) Mabb. & Y.W.Yuan | Lamiaceae | Umqoqongo (Zulu) | Not specified | Used to treat colds and influenza. Preparation and application not specified. | Mhlongo and Van Wyk (2019) |
| Waltheria indica L. | Malvaceae | Unknown | Roots | The San chew the roots to treat colds and influenza. | Von Koenen (2001) |
| Warburgia salutaris (G.Bertol.) Chiov. | Canellaceae | Pepper-bark tree (English), peperbasboom (Afrikaans), mulanga, manaka (Venda), isibhaha (Zulu) | Bark | Used to treat coughs and colds. | Hutchings et al. (1996); Van Wyk et al. (2009) |
| Withania somnifera (L.) Dunal | Solanaceae | Indian ginseng, poison gooseberry, winter cherry (English), bitterappelliefie, koorshout (Afrikaans), ubuvuma (Xhosa), ubuvimbha (Zulu) | Root | The southern Sotho consume a decoction to treat colds and influenza. | Smith (1888); Watt and Breyer-Brandwijk (1962); Moffett (2010); Kose et al. (2015) |
| Xysmalobium undulatum (L.) W.T.Aiton. | Apocynaceae | Wild cotton (English), melkbos (Afrikaans) | Root | Decoctions prepared are applied to the chest to treat colds. | Von Koenen (2001); Watt and Breyer-Brandwijk (1962); Von Koenen (2001) |
| Zanthoxylum capense (Thunb.) Harv. | Rutaceae | Knobwood (English) | Bark | Used to treat colds. Preparation and application not specified. | Philander (2011) |
| Zanthoxylum davyi Waterm. | Rutaceae | Forest knobwood (English) | Bark | The Zulu eat the powdered bark to treat colds and severe coughs. | Watt and Breyer-Brandwijk (1962) |
| Zingiber officinale Roscoe | Zingiberacaee | Ginger (English), gemmer (Afrikaans) | Root | Used to treat colds, influenza and sinusitis. | Hulley and Van Wyk (2019) |
High density lower socio-economic urban communities often have higher prevalence's and rates of transmission of viral respiratory infections than other populations. Living in high density conditions facilitates viral transmission rates. Rural communities may also have different disease profiles, with higher incidences of zoonotic viral strains than in urban populations. Some respiratory viruses can infect domestic animals and livestock, as well as humans. For example, then H1N1 swine influenza strain, as well as H7N7 and H1N2 serotypes infect both humans and pigs. The latter two serotypes also infect birds and other farm livestock. All of these viruses are readily infective by airborne transmission between species. The H5N1 serotype (avian flu) can also infect both humans and birds, but is not yet readily transmissible to humans by airborne routes. People living in rural communities may have close contact with livestock, providing an extra avenue for infection. Therefore, these serotypes are often more common in rural communities than in urban environments. Several other attributes are shared between lower socio-economic urban communities and rural communities. For example:
-
•
both communities often have limited access to effective clinical treatment. Medical care is often not affordable to poor and/or rural people. Instead, these communities tend to be more reliant on traditional medicine. Alternatively, given the comparatively low concern about many of the viral respiratory diseases compared to other infectious pathogens, and their self-limiting nature, many people suffering from these illnesses forgo medical treatment and allow the infection to run their course.
-
•
Economically disadvantaged people and those living in rural communities are often less educated and have less understanding of simple preventative measures (e.g. frequent hand washing, disinfecting surfaces etc.) that limit the transmission of viral respiratory diseases.
The prevalence and severity of symptoms of viral respiratory diseases often correlates with age. Indeed, several of these diseases are generally considered to be childhood diseases only. A very common childhood disease is HRSV and nearly 100% of children have contracted HRSV by the age of three (Glezen et al., 1986). Whilst prior contact does not provide lifelong immunity to this disease, it does provide some protection and adult HRSV infections are far less common. Parainfluenza in also substantially more common in children than in adults as most children contract a HPIV infection before the age of 10 years (Henrickson et al., 1994), conferring them a degree of immunity. In most children, this is a relatively innocuous disease, although the symptoms may be substantially more serious in infants and can be life threatening. Children are also more prone to influenza and colds than adults because of their relative lack of immunity to these diseases. Throughout the course of their life, humans encounter multiple respiratory viral pathogens. In response, we develop antibodies against these viruses which confer protection against future infections with the same virus. Thus, adults often only contract influenza infections every few years as they encounter influenza strains they have previously not contracted. The large number of viral pathogens that cause colds allows adults to potentially contract multiple colds within a single year. However, once a particular strain has been encountered, the immune system confers protection against future infections with that specific virus. In contrast, children will usually have encountered relatively few respiratory viruses and therefore have limited immunological protection so they may be infected far more frequently than adults.
The incidence and severity of viral respiratory infections is also higher and the symptoms more severe in older people. The immune systems of the elderly are less efficient than in the younger generation, allowing these diseases to last for longer periods and allowing the symptoms to be substantially more severe. Indeed, influenza and colds are of considerable concern in elderly people and the risk of mortality is substantially increased. Similarly, immunocompromised individuals are also at far greater risk of contracting a viral respiratory disease than the general population, and when they contract these diseases, the symptoms are often substantially more severe and may be life threatening (Nunes et al., 2014; Annamalay et al., 2016). This is particularly concerning for southern Africa. Sub-Saharan Africa, including South Africa, has a substantially higher prevalence of HIV infected people than many other regions of the world. Indeed, as of 2018, it was estimated that 7.52 million South Africans (equating to >13% of the entire population and 19% of the adult population) are living with HIV (Statistics South Africa, 2018). This is particularly concerning in a southern African context as these individuals with untreated HIV would have be expected to have greatly increased rates of respiratory virus infections, and the infections have far greater consequences. Timely diagnosis and the usage of highly active antiretroviral therapies (HAART) is necessary to overcome these more profound effects in individuals with HIV.
Vaccinations to some of the respiratory infective viruses are readily available. In particular, influenza vaccinations are updated annually to provide protection against the latest influenza strains. Their efficacy has been improving in recent years, now consistently providing protection rates of 50–60%. These vaccinations are made readily available. Despite this, the uptake of these services is particularly low in South Africa. Indeed, it was recently estimated that <5% of South Africans received the influenza vaccination in 2015 (Solanki et al., 2018). As vaccination is currently the best preventative option for influenza, the rate of uptake is low and higher risk (children, the elderly and immunocompromised) people in particular would benefit from annual vaccinations.
3. Materials and methods
This study aimed to identify southern African plants which are used in traditional healing systems for the treatment of viral respiratory diseases in humans. This information was sourced from a variety ethnobotanical books (Smith, 1888; Watt and Breyer-Brandwijk, 1962; Hutchings et al., 1996; Von Koenen, 2001; Ngwenya et al., 2003; Van Wyk et al., 2009), as well as ethnobotanical reviews (Hulley and Van Wyk, 2019; De Beer and Van Wyk, 2011; Nortje and Van Wyk, 2015; Philander, 2011; Van Wyk et al., 2009). Google-Scholar, Science-Direct, PubMed and Scopus electronic databases were searched to identify original ethnobotanical research papers using the following terms as filters, and were searched both alone and as combinations: “South African”, “medicinal plant”, “traditional medicine”, “ethnobotany”, “respiratory infection” “colds”, “influenza”, “bronchitis”, “respiratory syncytial virus”, “RSV”, “parainfluenza”, “rhinovirus”, “human metapneumonovirus”. These terms were searched alone and as combinations. Only English language publications were used in the preparation of this review. This study is non-biased and does not have taxonomic preference (although several of the studies we review targeted specific families or genera). All species names were checked using the Plant List website (http://www.theplantlist.org/). Whilst most of the plant species included herein are native to southern Africa, introduced plant species were also included where there is evidence of their usage in at least one southern African traditional healing system. Our initial literature search identified two hundred and sixty-eight plant species that have been used as traditional remedies for viral respiratory diseases in southern Africa. The vast majority of these species are native to southern Africa, although, several introduced species were also included. For introduced species to be included in this report, they must either be naturalised or widely cultivated, and there must be documented evidence that they are commonly used by at least one southern African ethnic group to treat viral respiratory disease.
Each plant species identified by this initial search were subjected to a further literature review to establish the extent (if any) of the scientific research into the efficacy of that species, and to scientifically validate their traditional use. Specific criteria to filter studies included the terms ethnomedicine, southern African medicinal plants and other key words related to viral respiratory infections. Only plants used to treat viral respiratory infections in humans are included in this study. Several studies documented the use of some southern African plants to treat viral respiratory diseases in livestock and domestic animals. However, these studies were excluded from this review unless they specifically mention the use of the plant species to also treat human respiratory viral infections. We aimed through this review to summarise traditional southern African ethnopharmacological knowledge on the treatment of viral respiratory diseases, and to discuss the plant species used traditionally to treat these infections. Through this study, we aim to foster further research into southern African plants as treatments for viral respiratory diseases by highlighting plant species traditionally used to treat those diseases.
4. Southern African medicinal plants used traditionally to treat viral respiratory diseases
Viral respiratory diseases exhibit several symptoms that are relatively generic and also occur with multiple other diseases. Traditional medicine practitioners often use plant preparations to target disease symptoms rather than specifically targeting the disease. Indeed, many ethnobotanical texts and reviews record the use of plants to treat symptoms such as coughs and runny noses. Many other diseases produce similar symptoms. For example, other bacterial respiratory diseases such as tuberculosis elicits similar symptoms. Likewise, the early phases of acquired immunodeficiency syndrome (AIDS), anthrax, bubonic plague, cytomeglavirus, Lyme disease, malaria, measles, rabies, severe acute respiratory syndrome (SARS) and smallpox (to list just a few) present with similar symptoms to the viral respiratory diseases and may be mistaken for influenza. Other ethnobotanical texts may list plants used to treat “chest complaints”, ‘breathing disorders” or other similar symptoms. In this study, only plant species that have specifically been reported to be used to treat viral respiratory infections are included. Where ambiguity occurs regarding a plants specific usage, that plant has been excluded from this study.
An extensive literature search identified 257 southern African plants used in at least one southern African traditional healing system to treat viral respiratory infections (Table 1). The high number of species used to treat these diseases is hardly surprising given the prevalence of these infections. Indeed, it is likely that this list is incomplete and has underestimated the number of species used for this purpose as we have excluded plant species from this record where there is ambiguity about their use. Whilst many of the ethnobotanical texts (Van Wyk et al., 2009; Von Koenen, 2001; Smith, 1888 etc.) examine plant use independent of ethnic groups, several studies examined the ethnobotanical usage by specific ethnic groups. Hutchings et al. (1996) and Ngwenya et al. (2003) itemise plant species used by the Zulu to treat viral respiratory diseases. Similarly, Moffett (2010) concentrates on southern Sotho plant usage, Adeniji et al. (2000) summarises Swazi medicinal plants and Gelfand et al. (1985) itemises the medicinal plants used by ethnic groups in Zimbabwe. Numerous ethnobotanical surveys used in the preparation of Table 1 also reports on medicinal plant use by ethnic groups in specific locations (Hulley and Van Wyk, 2019; De Beer and Van Wyk, 2011; Nortje and Van Wyk, 2015; Philander, 2011; Van Wyk et al, 2009). Together, these studies provide an evaluation of the usage of southern African plants for the treatment of viral respiratory disease and allow for the detection of specific trends.
Interestingly, a high proportion of the studies which mention the usage of plants by specific ethnic groups to treat viral respiratory diseases relate to southern Sotho traditional medicine. Indeed, of the plant species that were listed as being used by specific ethnic groups, 70 species were listed as used by the southern Sotho, 26 species are listed as used by the Zulu, 16 species by the Xhosa, five species each by the Tswana and Nama, four species by the San, and one species each by the Venda and Khoikhoi. This is hardly surprising given the environments that each of these groups live in. The southern Sotho live at higher altitudes and in substantially cooler environments than the other ethnic groupings. These conditions require the Sothern Sotho to spend greater periods of time indoors, living in close proximity, especially during the colder periods. Such conditions are ideal for the transmission of respiratory viruses and it is likely that the southern Sotho would contract these infections at higher rates than the groups living in warmer environments.
It is also noteworthy that the Southern Sotho has substantially more treatments for the common cold than they did for influenza. This is also likely to correspond to the transmission of these viruses. More than 200 viral serotypes (including over 160 rhinovirus serotypes) have been identified as causes of colds. Due to the antigenic variability of these viruses, otherwise healthy adults may catch up to two or three colds a year. In contrast, there are less influenza virus serotypes, although frequent mutations provide new antigenic variants. However, on average, otherwise healthy adults will only contract an influenza infection every five years. Thus, although influenza infections are substantially more severe than colds, the frequency that the southern Sotho contracted each illness would make cold treatments of particular importance to them.
A further trend regarding the usage of the medicinal plants was also evident. Previous studies have reported that decoctions and infusions are the most common methods for treating most pathogenic diseases (Cock et al., 2018, 2019). Whilst these methods of preparation and usage were also the most common for the treatment of respiratory viruses, burning the plant part and inhaling the smoke was substantially more common for this purpose than previously reported for the treatment of other diseases. This is not unexpected as burning of plant volatiles for pulmonary treatment regimens is common practice from a global perspective (Mohagheghzadeh et al., 2006) Indeed, of the 70 plant species specifically listed as used by the southern Sotho for the treatment of viral respiratory diseases, 21 of these were burned and the smoke inhaled. A further four of the southern Sotho medicinal plant species were burned and used to fumigate the huts of sick people. Inhaling the smoke from burning plant materials was less frequently used by other ethnic groups, although this treatment method was also used by the Zulu and Xhosa for one species each. A further four plant species were also smoked to treat viral respiratory disease, although the ethnic group was not specified for those species. Boiling the plant material and inhaling the steam was used for a further four plant species, although the ethnic group(s) using these species was only stipulated for one of these species (by the San). Several plant species were also powdered and used as a snuff. Five species used by the southern Sotho were administered in this way. The Zulu also used four plant species as snuffs, and a further two plant species (for which the ethnic group was not specified) were also used this way.
In some instances (five plant species) the fruit was indicated for treatment. It is possible that the vitamin C content was targeted here and hence symptomatic treatment was considered rather than a cure sought.
Two hundred and fifty-seven southern African plant species that are used in traditional medicine to treat viral respiratory infections are listed in Table 1. These plant species come from seventy-one families including Amaryllidaceae, Anaradriaceae, Apiaceae, Apocynaceae, Asphodelaceae, Aspleniaceae, Asteraceae, Celestraceae, Combretaceae, Fabaceae, Geraniaceae, Lamiaceae, Malvaceae, Myrtaceae, Poaceae, Polypodiaceae, Pteridaceae, Rananculaceae, Rosaceae, Rutaceae, Scrophulariaceae, Solanaceae, Verbenaceae and Xanthorrhoeaceae (Fig. 1 ).
Fig. 1.
The number of plant species per families related to medicinal plants for the treatment of viral respiratory infections. Others refers to the number of other genuses (not named individually) that are represented by the indicated number of species.
Few of these species have had their inhibitory activity validated against viral respiratory pathogens via in vitro testing (Table 2 ). Most species have only had their use to treat viral respiratory diseases documented in ethnobotanical surveys and laboratory-based studies are required to validate their traditional use. The Asteraceae, Fabaceae and Laminaceae families were particularly well represented for the treatment of fungal skin infections, with 55, 28 and 23 species identified from these families respectively (Fig. 1). The higher representation of these families compared to other families may indicate that they have better potential against respiratory viruses. However, these are large families, each consisting of many species. Indeed, Asteraceae consists of nearly 33,000 individual species and has a wide global distribution (Bremer et al., 1992). Therefore, the greater representation of Asteraceae, Fabaceae and Laminaceae is consistent with the higher number of species in these families.
Table 2.
Scientific evaluations of the inhibitory activity of southern African plants against viral respiratory pathogens.
| Plant Species | Common Name | Family | Plant Part Used | Formulation | Results | Reference |
|---|---|---|---|---|---|---|
| Acokanthera schimperi (A.DC.) Schweinf. | Unknown | Apocynaceae | Leaves | Multiple solvent extracts were tested. | Inhibited 50% parainfluenza production at a 1 in 10 dilution. | Bagla et al. (2012) |
| Aspalathus linearis (Burm.f.) R.Dahlgren | Red bush, bush tea (English), Rooibos (Afrikaans) | Fabaceae | Leaves | Leaf infusions | An IC50 value was not reported. Instead, the authors state that the extract inhibited influenza virus A and B production by 50% at 0.2% of the extract concentration (not stated in that study). | Rahmasaria et al. (2017) |
| Barleria prionitis L. subsp. delagoensis (Oberm.) Brummitt & J.R.I.Wood | Delagoa Bay barleria, porcupine flower (English) | Acanthaceae | Whole plant | DCM extract | Compounds isolated from the extract (6-O-transp-coumaroyl-8-O-acetylshanzhiside methyl ester and its cis isomer) inhibited syncytial virus (RSV) production (EC50 = 2.5 μg/mL) | Chen et al. (1998) |
| Burkea africana Hook. | Wild syringa (English) | Fabaceae | Bark | Purified saponins | Bark extracts displayed apparent antiviral activity in a cytopathic effect (CPE) assay. Eight individual saponins purified from the bark were potent inhibitors of H3N2 influenza Hong Kong strain, with IC50 values 0.05–0.27 μM. | Mair et al. (2018) |
| Carissa spinarum L. | Climbing num-num, small num-num (English), enkeldoringnoemnoem, ranknoemnoem, kleinnoemnoem (Afrikaans), mothokolo (Sotho) | Apocynaceae | Leaves | Multiple solvent extracts were tested. | Inhibited 25% parainfluenza production at a 1 in 10 dilution. | Bagla et al. (2012) |
| Clerodendrum glabrum E.Mey. var. glabrum | Tinderwood (English), tontelhout (Afrikaans), moswaapeba (Sotho), munukha-tshilongwe (Venda), umqwaqwanam (Xhosa), umQoqonga (Zulu) | Lamiaceae | Leaves | Methanol extract | Tested against influenza virus A. EC50 values of 110 μg/mL against both pre- and post-penetration phases of viral replication. Solvent extracts were substantially more potent than aqueous extracts. No direct interaction with viral hemagglutuinin glycoprotein. | Mehrbod et al. (2018) |
| Crassula globularioides subsp. argyrophylla (Diels ex Schönland & Baker f.) Toelken | Unknown | Crassulaceae | Aerial parts | Methanol and DCM extracts | DCM extract strongly inhibited rhinovirus type 2 production in HeLa cells at concentrations >6 μg/mL. | Beuscher et al. (1994) |
| Cussonia spicata Thunb. | Cabbage-tree (English), kiepersol (Afrikaans), umsenge(Zulu and Xhosa), motshetshe (Sotho) | Araliaceae | Leaves | Methanol extract | Tested against influenza virus A. EC50 values of 5–15 μg/mL against both pre- and post-penetration phases of viral replication. Solvent extracts were substantially more potent than aqueous extracts. No direct interaction with viral hemagglutuinin glycoprotein. | Mehrbod et al. (2018) |
| Ekebergia capenses Sparm. | Cape ash (English), essenhout (Afrikaans), mmidibidibi (Northern Sotho), umnyamathi (Zulu) | Meliaceae | Bark | Extracted with multiple solvents of varying polarity | Inhibited 75% parainfluenza production at a 1 in 10 dilution. | Bagla et al. (2012) |
| Helichrysum armenium DC | Unknown | Asteraceae | Leaves | Aqueous and ethanolic extracts | Inhibited parainfluenza virus production. MIC = 4 μg/mL | Kutluk et al. (2018) |
| Helichrysum melanacme DC. | Hotnotskooigoed (Afrikaans) | Asteraceae | Leaves | Ethanol extract | Inhibited influenza virus A production. IC50 = 10 μg/mL | Lall et al. (2006) |
| Heteromorpha arborescens (Spreng.) Cham. & Schltdl. | Parsley tree (English), wildepietersielie (Afrikaans) | Apiaceae | Root bark | Methanol and DCM extracts | DCM extract strongly inhibited rhinovirus type 2 production in HeLa cells at concentrations >25 μg/mL. | Beuscher et al. (1994) |
| Holarrhena pubescens Wall. ex G. Don | Fever-pod (English) Jasmine-tree (English) | Apocynaceae | Bark | Methanol and DCM extracts | DCM extract strongly inhibited rhinovirus type 2 production in HeLa cells at concentrations >10 μg/mL. | Beuscher et al. (1994) |
| Jasminum fluminense Vell. | River jasmine (English), rivier jasmyn (Afikaans) | Olacaceae | Stems | Methanol and DCM extracts | DCM extract strongly inhibited rhinovirus type 2 production in HeLa cells at concentrations >50 μg/mL. | Beuscher et al. (1994) |
| Pelargonium sidoides DC. | Black pelargonium, South African geranium (English), kalwerbossie, rabassam (Afrikaans), ikubalo, iyeza lesikhali (Xhosa), khoara-e-nyenyane (Southern Sotho | Geraniaceae | Unspecified | A proprietary preparation named EPs 7630 was tested. | EPs 7360 at a concentration of 100 μg/mL inhibited the replication of H1N1and H3N2 influenza virus, parainfluenza virus, HRSV and human coronavirus strain 229E, but did not affect H5N1 influenza strain, or several adenoviruses and rhinoviruses. | Michaelis et al. (2011) |
| Pittosporum viridiflorum Sm. | Cheesewood (English), kasuur (Afrikaans), kgalagangwe (Northern Sotho), umkhwenkhwe (Zhosa, Zulu), umfusamvu (Zulu) | Pittosporaceae | Leaf | Aqueous, methanol, ethanol and acetone extracts | Tested against influenza virus A. EC50 values of 3–82 μg/mL against both pre- and post-penetration phases of viral replication. Solvent extracts were substantially more potent than aqueous extracts. No direct interaction with viral hemagglutuinin glycoprotein. | Mehrbod et al. (2018) |
| Polygala lancifolia A. St. -Hil. & Moq. | Purple broom (English), persboom, bloukappie (Afrikaans), hlokoa leleue (Sotho), ithethe (Zulu) | Polygalaceae | Aerial parts | Methanol and DCM extracts | DCM extract strongly inhibited rhinovirus type 2 production in HeLa cells at concentrations >12 μg/mL. | Beuscher et al. (1994) |
| Pterocarpus angolensis DC. | Bloodwood, paddle-wood, sealing-wax tree, wild teak, Transvaal teak (English), bloedhout, dolfhout, greinhout, kajatenhout, lakhout, wilde-kiaat (Afrikaans), morôtô (Sotho), umvangazi, umbilo (Zulu) | Fabaceae | Bark | Methanol and DCM extracts | DCM extract strongly inhibited rhinovirus type 2 production in HeLa cells at concentrations >12 μg/mL. | Beuscher et al. (1994) |
| Rapanea melanophloeos (L.) Mez. | Cape beech (English); boekenhout, beukehout (Afrikaans), isiCalabi, umaPhipha, iKhubalwane, isiQalaba sehlati (Zulu), isiQwane sehlati (Xhosa) | Primulaceae | Leaves and twigs | Aqueous, methanol, ethanol and acetone extracts | Tested against influenza virus A. EC50 values of 113 μg/mL against both pre- and post-penetration phases of viral replication. No direct interaction with viral hemagglutuinin glycoprotein. | Mehrbod et al. (2018) |
| Steganotaenia araliacea Hochest. | Carrot tree (English) | Apiaceae | Root | Methanol and DCM extracts | DCM extract strongly inhibited rhinovirus type 2 production in HeLa cells at concentrations >5 μg/mL. | Beuscher et al. (1994) |
| Tabernaemontana ventricosa Hochst. ex. A.DC. | Forest toad tree (English), bospaddaboom (Afrikaans), uKhamamasane (Zulu) | Apocynaceae | Leaves | Extracted with multiple solvents of varying polarity | Tested against influenza virus A. EC50 values of 0.05 μg/mL against both pre- and post-penetration phases of viral replication. Solvent extracts were substantially more potent than aqueous extracts. No direct interaction with viral hemagglutuinin glycoprotein. | Mehrbod et al. (2018) |
| Xanthocercis zambesiaca (Baker) Dumaz-le-Grand | Nyala tree (English), njalaboom, hoenderspoor (Afrikaans), mutshato (Venda) | Fabaceae | Leaves | Methanol and DCM extracts | DCM extract strongly inhibited rhinovirus type 2 production in HeLa cells at concentrations >60 μg/mL. | Beuscher et al. (1994) |
Leaves were the most frequently used plant part for the treatment of viral respiratory infections across the traditional southern African healing systems (Fig. 2 ). Notably, several other studies have reported that leaves are the most frequently used plant part for the treatment of other pathogenic diseases (De Beer and Van Wyk, 2011; Nortje and Van Wyk, 2015; Philander, 2011; Hulley and Van Wyk, 2019; Cock et al., 2018, 2019; Cock et al., 2019). Roots, bulbs, rhizomes and corms, are also commonly used to treat viral respiratory diseases (80 species). Bark is also relatively commonly used for this purpose (15 species). However, wild harvesting bark can damage trees and may even kill the plant. Therefore, tree bark should be used sparingly to avoid damage to the tree and allow for sustainable production. Further studies similar to those conducted by Zschocke et al. (2000) and Jena et al. (2017) can be undertaken to determine if plant part substitution can yield similar efficacies and therefore provide an alternate medicinal source that would require sustainable harvesting.
Fig. 2.
Frequency of use of different plant parts to treat viral respiratory infections.
Many of the plant species traditionally used for the treatment of viral respiratory infections are herbaceous. This is advantageous for sustainable production of these medicines as herbaceous plants grow rapidly. The usage of plant parts that does not extensively damage the plant is preferred, particularly for endangered and threatened plant species. Unfortunately, the ethnobotanical records were incomplete for many plant species identified as treatments for viral respiratory diseases and we were unable to identify which plant part is used. Further ethnobotanical studies are required to clarify this.
5. Scientific studies into the therapeutic properties of southern African plants against viral respiratory diseases
Surprisingly few studies have examined the effects of southern African plants against viral respiratory infections (Table 2). Indeed, we were only able to find nine studies that have reported activity for southern African plants for activity against respiratory viruses. Not surprisingly given its prevalence and more serious nature, the influenza virus has been the most extensively studied. However, we were only able to find five studies that reported anti-influenza virus activity for some southern African plants (Lall et al., 2006; Michaelis et al., 2011; Rahmasaria et al., 2017; Mair et al., 2018; Mehrbod et al., 2018) The most extensive of these studies reported that five of the screened plants inhibited influenza virus A viral production against a H1N1 reference serotype (Mehrbod et al., 2018). Similarly, a preparation called EPs 7630 prepared from P. sidoides inhibited the replication of H1N1and H3N2 influenza virus, parainfluenza virus, HRSV and human coronavirus strain 229E at 100 μg/mL (Michaelis et al., 2011), but did not affect H5N1 influenza strain or several adenoviruses and rhinoviruses. The susceptibility of the 229E coronavirus to this preparation is particularly interesting given the current SARS-CoV-2 pandemic and highlights this preparation as a potential treatment for COV-19. Whilst we were unable to find publications screening EPs 7630 against that virus, this would be a priority for future studies.
T. ventricosa was also particularly strong inhibitor of influenza virus replication, with an EC50 of 0.05 μg/mL. This was notable inhibition and was similar to the inhibition recorded for the control antivirals amantadine hydrochloride and oseltamivir carboxylate. C. spicata and P. viridiflorum were also strong inhibitors of influenza virus A production, with EC50 values as low as 5 and 3 μg/mL respectively. Noteworthy activity was also recorded for C. glabrum and R. melanophloeos extracts, albeit with substantially higher EC50 values (110 and 113 μg/mL respectively). However, T. ventricosa also displayed some toxicity, with a selectivity index (SI) of 2 reported. This may limit the use of T. ventricosa extracts, as only relatively low concentrations would be considered safe to use to treat influenza A. The lowest toxicity and highest SI (8) was determined for C. spicata. This plant may therefore be the preferred therapeutic option for the treatment of influenza A H1N1 serotypes.
Two other studies also reported influenza virus inhibitory activity for A. linearis (Rahmasaria et al., 2017) and H. melanacme (Lall et al., 2006). The Lall et al. study (2006), screened H. melanacme extract (and purified compounds) against a single clinical strain of the virus designated strain Panama, which is a H3N2 influenza A serotype virus. Noteworthy inhibition of viral replication was reported in that study (IC50 = 10 μg/mL). This was the only study that we found that screened against a H3N2 serotype, making it difficult to compare these results with those obtained from other studies. The Rahmasaria et al. (2017) study screened A. linearis extracts against a much more extensive panel of influenza viruses. Indeed, that study screened against five oseltamivir sensitive and one resistant influenza H1N1 serotype strains. Good activity was noted against all influenza strains, with IC50 values < 5% of the extract concentration against all strains, and as low as 0.5% against one H1N1 strain. Interestingly, the inhibition of viral replication by the A. linearis extracts was not substantially different between the oseltamivir sensitive and resistant viral strains, further highlighting the potential of A. linearis extracts to treat influenza virus infections. Of further note, the A. linearis extracts were non-toxic and SI's were between 12 and 140.
Three studies also screened southern African plant extracts against parainfluenza virus (Michaelis et al., 2011; Bagla et al., 2012; Kutluk et al., 2018). The Kutluk et al. (2018) study screened H. armenium against parainfluenza and reported strong inhibition (MIC = 4 μg/mL). The Bagla et al. (2012) study screened a more extensive panel of plant extracts and reported noteworthy activity for A. schimperi, C. edulis and E. capenses (Bagla et al., 2012). However, that study screened extracts of varying concentrations at fixed dilutions, and measured the % inhibition of viral replication. IC50 values were not determined, making it impossible to compare the efficacy of these extracts with other studies. However, notable inhibition was reported for all three plants and future studies are required to better quantify the anti-parainfluenza activity of these extracts and allow them to be benchmarked against other plant species. The Michaelis et al. (2011) study tested a proprietary preparation prepared from P. sidoides against parainfluenza virus at 100 μg/mL and reported inhibition of viral replication. However, a single concentration was tested so IC50 valuse were not determined.
It was perhaps surprising given the prevalence of colds, that we were only able to locate two study that screened southern African plant extracts against rhinovirus (Beuscher et al., 1994; Michaelis et al., 2011), and only one study that screened against adenovirus and enterovirus strains, although the P. sidoides preparation tested in that study had no effects on adenovirus and enterovirus replication. Strong inhibition of rhinovirus production were reported for C. swaziensis (at concentrations >6 μg/mL), H. arborescens (at concentrations >25 μg/mL), H. pubescens (at concentrations >10 μg/mL), J. fluminense (at concentrations >50 μg/mL), P. virgata (at concentrations >12 μg/mL), P. angolensis (at concentrations >12 μg/mL), S. araliacea (at concentrations >5 μg/mL) and X. zambesiaca (at concentrations >60 μg/mL) (Beuscher et al., 1994). A single study also tested iridoid glycosides isolated from B. prionitis against RSV and reported EC50 values as low as 2.5 μg/mL for the pure compounds (Chen et al., 1998). Unfortunately, that study did not test the crude extract against RSV and it is not known whether it also has noteworthy anti-RSV activity.
Interestingly, only a single species of plant (C. glabra) tested for the ability to inhibit the production of respiratory viruses corresponded to plants used in traditional healing systems to treat those infections (Mhlongo and Van Wyk, 2019). A further four species were not recorded as being used traditionally to treat these infections, although are closely related to other species used for this purpose. The inhibitory activity of H. armenium (Kutluk et al., 2018) and H. melanacme (Lall et al., 2006) were screened against parainfluenza and influenza virus A respectively. Whilst there is no record of these plant species being used as traditional medicines to treat respiratory viral infections, ten other Helichrysum species are recorded as being used for these purposes (Table 1). Similarly, we found no evidence that Carissa edulis was used traditionally to treat parainfluenza despite it being tested for this activity (Bagla et al., 2012). However, the related species C. bispinosa is used to treat viral respiratory disease, although it is recorded for use against influenza, rather than parainfluenza infections (Mhlongo and Van Wyk, 2019). Similarly, we were unable to find evidence that P. virgata was used to treat colds, despite it being screened against rhinovirus (Beuscher et al., 1994). However, the related species P. schinziana was used traditionally to treat colds (Von Koenen, 2001; Hutchings et al., 1997). The inclusion of those species in the antiviral studies may have been on the basis of these taxonomic relationships, although this is not explained by the authors of those studies. It is likely that all other species were selected randomly for screening.
More recently, mechanisistic computational modeling of the anti-influenza effects of quercetin-3-O-α-L-rhamnopyranoside isolated from the medicinal plant R.melanophloeos with known antiviral activity against H1N1 was undertaken. Results demonstrated that the compound was effective against influenza infection by regulating immunomodulatory properties, inhibiting the binding ability to viral receptors M2 transmembrane and neuraminidase of 2009 pandemic H1N1 and human RhoA cellular protein (Mehrbod et al., 2019).
6. Discussion
The development of viral resistance to the current complement of antiviral chemotherapies has necessitated the development of new drugs with novel targets. Two of the six drugs currently in clinical usage to treat influenza (the adamantine drugs amantadine and rimantadine) are no longer considered effective against H1N1 and H3N2 serotypes as they have developed almost complete resistance to these drugs (Deyde et al., 2007). Similarly, several influenza strains have developed resistance to baloxavir marboxil (which functions via a different mechanism to the adamantine drugs) (Hayden et al., 2018), effectively limiting its efficacy. The speed with which influenza A and B strains have developed resistance to this drug (it has been in clinical use for less than 2 years) indicates that it is likely to be of little therapeutic value in a short time. Only oseltamivir phosphate, zanamivir and peramivir are still effective against most influenza virus strains (FDA, 2019), although resistance to these drugs is also increasingly being reported (Sheu et al., 2008). The development of new influenza therapies are urgently required.
Ideally, new anti-influenza drugs should have different therapeutic mechanisms to the existing drugs to allow them to inhibit influenza strains that have acquired resistance to the other medications. New chemotherapies should also be effective at low concentrations and have low toxicity. Traditional plant-based medicines are attractive targets to combat respiratory viruses. Many traditional medicines have been used for hundreds of years and their efficacy has been demonstrated over long periods. Furthermore, due to their extensive usage, their toxicity and side effects are often known, and their safety verified. Additionally, traditional medicines are used as crude preparations that containing multiple components. These components may target different aspects of viral replication, which may not only increase the efficacy of the mixture, but also decrease the possibility of inducing further resistance in respiratory-infective viruses.
This study aimed to identify southern African plant species that had been specifically recorded as being used for the treatment of viral respiratory diseases. In order to maximise the number of plants used for these purposes and highlight plants for future studies, we searched all available English language published records, including older literature sources dating back to the later part of the nineteenth century (Smith, 1888). Whilst, this has maximised the number of species that we have recorded, it is noteworthy that some older literature is prone to generalisations, without definitive evidence. However, with few exceptions, we were able to confirm the use of the plant species named in the older literature with similar reports in more recent reports. Indeed, of the species listed in Smith (1888) as used for the treatment of colds and influenza, all except four species (L. asperifolia, P. sanguinale, R. capensis, S. flabelloforme) were also listed in more recent studies as being used for the same purposes. Similarly, relatively few plant species were named in Watt and Breyer-Brandwijk (1962) without also being listed for the same uses in one or more other more recent publications. However, the use of older literature also presented further complications related to classification, with numerous taxonomic revisions occurring in the intervening period.
A further limitation was highlighted when using ethnobotanical texts and published ethnobotanical literature. Many of these publications discuss the southern African ethnic groups as homogenous entities under broad groupings such as Zulu, Xhosa, Sotho etc. This particularly applies to the older literature, although it is also true of many newer publications. Unfortunately, this implies that there is uniform traditional knowledge regarding the usage of medicinal plants throughout these groups, independent of geographic and other factors. This is a simplistic view and individual cohorts within these larger ethnic groups have a diversity of traditional knowledge arising from differing experiences and differing knowledge transfer pathways. As many publications summarise the traditional knowledge of entire ethnic groupings, our review has also listed the use of these plants in this way out of necessity and for reasons of brevity. However, several recent ethnobotanical studies not only record the usage of medicinal plants within broad ethnic groups, but also compare the usage between different regions within the same ethnic groupings. For example, Hulley and Van Wyk (2019) not only record the traditional knowledge of an ethnic group, but in individual villages within a region, thereby highlighting the heterogeneity of knowledge within that broader group. A discussion of the specific differences within different sub-populations of the various ethnic groups is beyond the scope of this review and the reader is referred to the individual studies cited in Table 1 for more detailed understanding of the heterogeneity of traditional knowledge.
Our study identified 257 plant species that are traditionally used to treat respiratory viral infections. Several previous studies have also examined the use of southern African plants to treat respiratory symptoms. However, those symptoms are common to other diseases as well as those caused by respiratory viruses. As the aim of our study was to categorise southern African medicinal plants based on their reported use to treat specific viral respiratory diseases, plant species listed to treat symptoms associated with colds and influenza without specifying their use against those diseases were excluded from our study. Therefore, we are confident that all species used in our study are used in southern African traditional medicine to treat these diseases. However, excluding species because of a lack of definitive ethnobotanical records may have substantially underestimated the number of species traditionally used against these diseases. Several otherwise promising species have not been included for this reason. For example, P. sidoides and Cyclopia sp. are reported to treat symptoms consistent with acute bronchitis and colds, as well as numerous other diseases (Michaelis et al., 2011). As these symptoms are generic, these species have not been included in our study. However, as they can alleviate some symptoms consistent with viral respiratory diseases, they should not be ignored and future studies to test their activity against respiratory viruses is required.
It is noteable that most of plant species listed in our study were used traditionally to treat influenza (generally considered to be the most serious viral respiratory infection) and colds (the most prevalent respiratory disease). Given the number of plants traditionally used to treat viral respiratory diseases, the low number of studies screening southern African plants for the ability to these viruses is surprising. Indeed, only one of the traditionally used species (C. glabrum) has been screened for the ability to block respiratory virus production to date (Mehrbod et al., 2018). That study tested the plant extracts against a single H1N1 influenza strain (Mehrbod et al., 2018) and the possible activity against other influenza strains (and against other respiratory-infective viruses) remains to be studied. Therefore, the remaining 256 plant species that have been used for hundreds of years to treat viral respiratory diseases are yet to be tested and much work is required.
There was a lack of studies investigating most of the other viral respiratory diseases discussed in this review other than colds and influenza. Distinguishing between the other respiratory viruses can be difficult. This is evident from the complete lack of ethnobotanical records of plant species used to treat parainfluenza, HRSV, HMPV and coronavirus induced diseases. Traditional healers would have been able to prescribe therapies to alleviate the symptoms of these diseases, although they are unlikely to have been able to distinguish them from a suite of respiratory and other diseases. Thus, researchers seeking herbal targets to test against those diseases may benefit from screening using plants recorded as remedies to coughs, sore throats, fevers and other generic respiratory symptoms. Many of these plants will also have been used to treat colds and influenza, so screening against the plants identified in this study is likely to yield promising leads against the other respiratory diseases.
It is likely that several factors may contribute to the relative neglect of studies screening southern African plants against respiratory viruses. As previously discussed, viral respiratory diseases are generally (and in some cases, erroneously) thought to be of low concern compared to other pathogenic diseases. However, even for those diseases for which this is true (e.g. colds), the prevalence of the disease makes the development of an effective therapy an attractive prospect. Viral assays are often more complex than many other bioassays (e.g. antibacterial assays), requiring specialised training and equipment, as well as expensive reagents. This may deter some researchers from the field. Furthermore, potential researchers may not be sure of which plants to target. It is hoped that this review may address this final point by providing a comprehensive listing of the southern African plant species known to be used to treat these diseases.
Whilst only one plant species traditionally used to treat viral respiratory diseases has had its antiviral activity verified, twenty-one other species have also been screened. The inclusions of these species may have been on the basis of taxonomic similarity to traditionally used species, or due to the author's bias towards a particular family or genus. Alternatively, inclusion in the studies may have been random. All of these are valid reasons for inclusion in screening studies. However, using a targeted approach that aims to screen the species used traditionally for these purposes is more likely to identify effective new therapeutic targets.
When a plant preparation displays good activity against one strain of respiratory virus, it is necessary to screen it against other strains of the same viral species. Different serotypes within a species, and different strains of those serotypes, often have very different susceptibility and resistance profiles. This is particularly apparent for the influenza viruses. Indeed, rapid and continual mutation resulting in antigenic shifts is the way that influenza virus is able to evade the immune response (and often, antiviral chemotherapies). Thus, newly developed influenza therapies (as well as therapies against other respiratory-infective viruses) should not only be tested against common viral strains. They should also be tested at regular intervals against new and emerging strains to evaluate their continued efficacy against viral respiratory diseases. This is particularly important for influenza virus due to the high probability of future pandemics for which medical science may have limited treatment options.
Notably, most of the plant species already screened for anti-respiratory virus activity were also tested concurrently for toxicity. This is particularly true for the recent studies. Evaluation of toxicity is imperative to quantify the therapeutic potential of a plant-derived medicine as even the most promising preparation will be of limited value if it possesses substantial toxicity. Even when a plant preparation has previously been tested for toxicity in studies evaluating other therapeutic properties, it should also be tested in parallel with the antiviral studies to allow quantification of a selectivity index (SI) or therapeutic index (TI). Ideally, toxicity evaluation studies should also incorporate more than one assay model to provide confidence in the safety of the therapy, and to allow for comparisons between studies. Much work is required to screen southern African plants against respiratory viruses. This review provides a reference to aid in plant species selection to focus future studies.
7. Conclusions
Our study has highlighted 257 southern African plant species used traditionally to treat viral respiratory diseases (particularly influenza and colds). Despite extensive ethnobotanical records and the relatively large number of southern African plant species identified, only a single species has yet been tested for the ability to inhibit the growth of respiratory viruses on the basis of its ethnobotanical usage. Substantial further work is need to test the other 256 species against the viral pathogens that they were traditionally used against. Furthermore, with some notable exceptions, the southern African plant species that have been tested against viral respiratory pathogens have generally only been tested against one, or at most, a few viral respiratory pathogens. Other viruses (particularly those whose diagnosis is more complicated), have been relatively neglected. Testing against other respiratory viruses is warranted. Given its prevalence and relatively serious nature, screening against influenza strains should be considered a priority. Similarly, given the high level of infections and mortality associated with the current COVID-19 pandemic, screening against the SARS-CoV-2 virus is also an attractive option which may highlight plant species for drug development. It is hoped that this review may highlight the more promising species for screening.
Acknowledgements
The authors are grateful to the Environmental Futures Research Institute, Griffith University, Australia, and the Department of Pharmacy and Pharmacology, University of the Witwatersrand, South Africa for the support provided to undertake these studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jep.2020.113194.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adeniji K.O., Amusan O.O.G., Dlamini P.S., Enow-Orock E.G., Gamedze S.T., Gbile Z.O., Langa A.D., Makhubu L.P., Mahunnah R.L.A., Mshana R.N., Sofowora A., Vilane M.J. 2000. Traditional Medicine and Pharmacopeia. Contribution to Ethnobotanical and Floristic Studies in Swaziland. Published by Organisation of African Unity, Ethiopia. [Google Scholar]
- Annamalay A.A., Abbottc S., Sikazwed C., Khooa S.-K., Bizzintinoa J., Zhanga G., Lainga I., Chidlow G.R., Smith D.W., Gern J., Goldblatta J., Lehmannb D., Green R.J., Le Souëf P.N. Respiratory viruses in young South African children with acute lower respiratory infections and interactions with HIV. J. Clin. Virol. 2016;81:58–63. doi: 10.1016/j.jcv.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha K., Kumar B. Emerging influenza D virus threat: what we know so far! J. Clin. Med. 2019;8(2):E192. doi: 10.3390/jcm8020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagla V.P., McGaw L.J., Eloff J.N. The antiviral activity of six South African plants traditionally used against infections in ethnoveterinary medicine. Vet. Microbiol. 2012;155:198–206. doi: 10.1016/j.vetmic.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Baille V.L., Olwagen C.P., Madhi S.A. Review on clinical and molecular epidemiology of humah rhinovirus-associated lower respiratory tract infections in African and Southeast Asian children. Pediatr. Infect. Dis. J. 2018;37(7):e185–e194. doi: 10.1097/INF.0000000000001897. [DOI] [PubMed] [Google Scholar]
- Beuscher N., Bodinet C., Neumann-Haefelin D., Marston A., Hostettmann K. Antiviral activity of African medicinal plants. J. Ethnopharmacol. 1994;42:101–109. doi: 10.1016/0378-8741(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Branche A.R., Falsey A.R. Parainfluenza virus infection. Semin. Respir. Crit. Care Med. 2016;37(4):538–554. doi: 10.1055/s-0036-1584798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer K., Jansen R.K., Karis P.O., Källersjö M., Keeley S.C., Kim K.J., Michaels H.J., Palmer J.D., Wallace R.S. A review of the phylogeny and classification of the Asteraceae. Nord. J. Bot. 1992;12(2):141–148. [Google Scholar]
- Chen J.L., Blanc P., Stoddart C.A., Bogan M., Rozhon E.J., Parkinson N., Ye Z., Cooper R., Balick M., Nanakorn W., Kernan M.R. New iridoids from the medicinal plant Barleria prionitis with potent activity against respiratory syncytial virus. J. Nat. Prod. 1998;61:1295–1297. doi: 10.1021/np980086y. [DOI] [PubMed] [Google Scholar]
- Chow W.Z., Chan Y.F., Oong X.Y., Ng L.J., Nor’E S.S., Ng K.T., Chan K.G., Hanafi N.S., Pang Y.K., Kamarulzaman A., Tee K.K. Genetic diversity, seasonality and transmission network of human metapneumovirus: identification of a unique sub-lineage of the fusion and attachment genes. Sci. Rep. 2016;6:27730. doi: 10.1038/srep27730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cock I.E., Selesho I., Van Vuuren S.F. A review of the traditional use of South African medicinal plants for the treatment of malaria. J. Ethnopharmacol. 2019 doi: 10.1016/j.jep.2019.112176. [DOI] [PubMed] [Google Scholar]
- Cock I.E., Selesho M.I., Van Vuuren S.F. A review of the traditional use of southern African medicinal plants for the treatment of selected parasite infections affecting humans. J. Ethnopharmacol. 2018;220:250–264. doi: 10.1016/j.jep.2018.04.001. [DOI] [PubMed] [Google Scholar]
- Cock I.E., Van Vuuren S.F. A review of the traditional use of southern African medicinal plants to treat bacterial respiratory infections. J. Ethnopharmacol. 2020 doi: 10.1016/j.jep.2020.113204. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beer J.J.J., Van Wyk B.-E. An ethnobotanical survey of the Agter-Hantam, Northern Cape Province, South Africa. South Afr. J. Bot. 2011;77:741–754. [Google Scholar]
- Deyde V.M., Xu X., Bright R.A., Shaw M., Smith C.B., Zhang Y., Shu Y., Gubareva L.V., Cox N.J., Klimov A.I. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 2007;196:249–257. doi: 10.1086/518936. [DOI] [PubMed] [Google Scholar]
- Drake J.W. Rates of spontaneous mutation amongst RNA viruses. Proc. Natl. Acad. Sci. Unit. States Am. 1993;90:4171–4175. doi: 10.1073/pnas.90.9.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felhaber T., Mayeng I. Kagiso Publishers; Cape Town, South Africa: 1997. South African Traditional Healers' Primary Health Care Handbook. [Google Scholar]
- FDA Influenza (flu) antiviral drugs and related information. 2019. https://www.fda.gov/drugs/information-drug-class/influenza-flu-antiviral-drugs-and-related-information Accessed 31 October 2019.
- Gelfand M., Mavi S., Drummond R.B., Ndemera E.B. Mambo Press; Zimbabwe: 1985. The Traditional Medical Practitioner in Zimbabwe. [Google Scholar]
- Gessner B.D., Shindo N., Briand S. Seasonal influenza epidemiology in sub-Saharan Africa: a systematic review. Lancet. 2011;11:223–235. doi: 10.1016/S1473-3099(11)70008-1. [DOI] [PubMed] [Google Scholar]
- Glezen W.P., Taber L.H., Frank A.L., Kasel J.A. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 1986;140(6):543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- Hall C.B., Weinberg G.A., Iwane M.K., Blumkin A.K., Edwards K.M., Staat M.A., Auinger P., Griffin M.R., Poehling K.A., Erdman D., Grijalva C.G., Zhu Y., Szilagyi P. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F.G., Sugaya N., Hirotsu N., Lee N., de Jong M.D., Hurt A.C., Ishida T., Sekino H., Yamada K., Portsmouth S., Kawaguchi K. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N. Engl. J. Med. 2018;379(10):913–923. doi: 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- Henrickson K.J., Kuhn S.M., Savatski L.L. Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Clin. Infect. Dis. 1994;18(5):770–779. doi: 10.1093/clinids/18.5.770. [DOI] [PubMed] [Google Scholar]
- Hulley I.M., Van Wyk B.-E. Quantitative medicinal ethnobotany of Kannaland (western Little Karoo, South Africa): non-homogeneity amongst villages. South Afr. J. Bot. 2019;122:225–265. [Google Scholar]
- Hutchings A., Scott A.H., Lewis G., Cunningham B. first ed. University of Natal Press; Pietermaritzburg: 1996. Zulu Medicinal Plants: an Inventory. [Google Scholar]
- Jena A.K., Karan M., Vasisht K. Plant parts substitution based approach as a viable conservation strategy for medicinal plants: a case study of Premna latifolia Roxb. J. Ayurveda Integr. Med. 2017;8(2):68–72. doi: 10.1016/j.jaim.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Wang W., Zhao X., Zai J., Li X. Cross‐species transmission of the newly identified coronavirus 2019‐nCoV. J. Med. Virol. 2020;92(4):433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J.S. Epidemiology of human metapneumovirus. Clin. Microbiol. Rev. 2006;19(3):546–557. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron R.A., San Mateo J., Wanionek K., Collins P.L., Buchholz U.J. Evaluation of a live attenuated human metapneumovirus vaccine in adults and children. J. Pediatr. Infect. Dis. Soc. 2017;7(1):86–89. doi: 10.1093/jpids/pix006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanovski L., Kopriva S., Pokorn M., Dolnicar M.B., Rajic V., Stefanovic M., Jazbec J. Treatment of severe human metapneumovirus (hMPV) pneumonia in an immunocompromised child with oral ribavirin and IVIG. J. Pediatr. Hematol. Oncol. 2013;35(7):e311–e313. doi: 10.1097/MPH.0b013e3182915d2d. [DOI] [PubMed] [Google Scholar]
- Kose L.S., Moteetee A., Van Vuuren S. Ethnobotanical survey of medicinal plants used in the Maseru district of Lesotho. J. Ethnopharmacol. 2015;170:184–200. doi: 10.1016/j.jep.2015.04.047. [DOI] [PubMed] [Google Scholar]
- Kutluk I., Aslan M., Orhan I.E., Özçelik B. Antibacterial, antifungal and antiviral bioactivities of selected Helichrysum species. South Afr. J. Bot. 2018;119:252–257. [Google Scholar]
- Lall N., Hussein A.A., Meyer J.J.M. Antiviral and antituberculosis activity of Helichrysum melanacme constituents. Fitoterapia. 2006;77:230–232. doi: 10.1016/j.fitote.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Louie J.K., Kajon A.E., Holodniy M., Guardia-LaBar L., Lee B., Petru A.M., Hacker J.K., Schnurr D.P. Severe pneumonia due to adenovirus serotype 14: a new respiratory threat? Clin. Infect. Dis. 2008;46(3):421–425. doi: 10.1086/525261. [DOI] [PubMed] [Google Scholar]
- Lucas W. eLS: John Wiley & Sons, Ltd; 2001. Viral Caspids and Envelopes: Structure and Function. 2001. [Google Scholar]
- Mohagheghzadeh A., Faridi P., Shams-Ardakani M., Ghasemi Y. Medicinal smokes. J. Ethnopharmacol. 2006;108(2):161–184. doi: 10.1016/j.jep.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Mair C.E., Grienke U., Wilhelm A., Urban E., Zehl M., Schmidtke M., Rollinger J.M. Anti-influenza triterpene saponins from the bark of Burkea africana. J. Nat. Prod. 2018;81(3):15–23. doi: 10.1021/acs.jnatprod.7b00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrbod P., Abdalla M.A., Njoya E.M., Ahmed A.S., Fotouhi F., Farahmand B., Gado D.A., Tabatabaian M., Fasanmi O.G., Eloff J.N., McGaw L.J., Fasina F.O. South African medicinal plant extracts active against influenza A virus. BMC Compl. Alternative Med. 2018;18:12. doi: 10.1186/s12906-018-2184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrbod P., Ebrahimi S.N., Fotouhi F., Eskandari F., Eloff J.N., McGaw L.J., Fasina F.O. Experimental validation and computational modeling of anti-influenza effects of quercetin-3-O-α-L-rhamnopyranoside from indigenous South African medicinal plant Rapanea melanophloeos. BMC Compl. Alternative Med. 2019;19:346. doi: 10.1186/s12906-019-2774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhlongo L.S., Van Wyk B.E. Zulu medicinal ethnobotany: new records from the Amandawe area of KwaZulu-Natal. South Afr. J. Bot. 2019;122:266–290. [Google Scholar]
- Michaelis M., Doerr H.W., Cinatl J. Investigation of the influence of EPs 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine. 2011;18:384–386. doi: 10.1016/j.phymed.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett R. Sun Press; Free State, South Africa: 2010. Sesotho Plant and Animal Names and Plants Used by the Basotho. [Google Scholar]
- Moteetee A., Moffett R.O., Seleteng-Kose L. A review of the ethnobotany of the Basotho of Lesotho and free state Province of South Africa (South Sotho) South Afr. J. Bot. 2019;122:21–56. [Google Scholar]
- Mulaudzi R.B., Ndhlala A.R., Kulkarni M.G., Van Staden J. Pharmacological properties and protein binding capacity of phenolic extracts of some Venda medicinal plants used against cough and fever. J. Ethnopharmacol. 2012;143:185–193. doi: 10.1016/j.jep.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Ngwenya M.A., Koopman A., Williams R. National Botanical Institute; Durban, South Africa: 2003. Zulu Botanical Knowledge. [Google Scholar]
- Nortje J.M., Van Wyk B.-E. Medicinal plants of the Kamiesberg, Namaqualand, South Africa. J. Ethnopharmacol. 2015;171:205–222. doi: 10.1016/j.jep.2015.04.049. [DOI] [PubMed] [Google Scholar]
- Nunes M.C., Kuschner Z., Rabede Z., Madimabe R., Van Niekerk N., Moloi J., Kuwunda L., Rossen J.W., Klugman K.P., Adrian P.V., Madhi S.A. Clinical epidemiology of bocavirus, rhinovirus, two polyomaviruses and four coronaviruses in HIV-infected and HIV-uninfected South African children. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0086448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholm M.T., Kelley N.S., Sommer A., Belongia E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- Paules C., Subbarao K. Influenza. Lancet. 2017;390(10095):697–708. doi: 10.1016/S0140-6736(17)30129-0. [DOI] [PubMed] [Google Scholar]
- Philander L.A. An ethnobotany of Western Cape Rasta bush medicine. J. Ethnopharmacol. 2011;138:578–594. doi: 10.1016/j.jep.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Rahmasaria R., Haruyamaa T., Charyasriwonga S., Nishidaa T., Kobayashia N. Antiviral activity of Aspalathus linearis against human influenza virus. Nat. Prod. Commun. 2017;12(4):599–602. [PubMed] [Google Scholar]
- Reddy J.G.M., Prathyusha K., Venkataswarmy M., Ramesh A. Spreading of swine flu disease: past and present. Res. J. Pharm. Dosage Forms Technol. 2018;10(2):70–78. [Google Scholar]
- Rubin B.A. Clinical picture and epidemiology of adenovirus infections (a review) Acta Microbiol. Hung. 1993;40(4):303–323. [PubMed] [Google Scholar]
- Semenya S.S., Maroyi A. Data on medicinal plants used to treat respiratory infections and related symptoms in South Africa. Data Brief. 2018;21:419–423. doi: 10.1016/j.dib.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Rohde W.V., Von Hoyningen V., Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978;87(1):13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]
- She J., Jiang J., Ye L., Hu L., Bai C., Song Y. Novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin. Transl. Med. 2020;9:19. doi: 10.1186/s40169-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu T.G., Deyde V.M., Okomo-Adhiambo M., Garten R.J., Xu X., Bright R.A., Butler E.N., Wallis T.R., Klimov A.I., Gubareva L.V. Surveillance for Neuraminidase Inhibitor resistance among Human Influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob. Agents Chemother. 2008;52(9):3284–3292. doi: 10.1128/AAC.00555-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. second ed. Lovedale Institution Press; South Africa: 1888. A Contribution to South African Materia Medica. [Google Scholar]
- Solanki G., Cornell M., Lalloo R. Uptake and costs of influenza vaccines in a private health insured South African population. S. Afr. J. Infect. Dis. 2018:1–4. doi: 10.1080/23120053.2018.1504532. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell E.M., Schrauwen E.J., Linster M., De Graaf M., Herfst S., Fouchier R.A. Predicting ‘airborne’influenza viruses:(trans-) mission impossible? Curr. Opin. Virol. 2011;1(6):635–642. doi: 10.1016/j.coviro.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalkup J.R., Chilukuri S. Enterovirus infections: a review of clinical presentation, diagnosis, and treatment. Dermatol. Clin. 2002;20(2):217–223. doi: 10.1016/s0733-8635(01)00009-2. [DOI] [PubMed] [Google Scholar]
- Statistics South Africa . Statistics South Africa Government Publications; 2018. Midyear Population Estimates 2018.https://www.statssa.gov.za/publications/P0302/P03022018.pdf Available from: Cited 31 October 2019. [Google Scholar]
- Taubenberger J.K., Morens D.M. 1918 Influenza: the mother of all pandemics. Rev. Biomed. 2006;17:69–79. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unicef, WHO, WHO Unicef . UNICEF/WHO; 2006. Pneumonia: the Forgotten Killer of Children.http://www.who.int/maternal_child_adolescent/documents/9280640489/en/ 2006; 140. Available from: Cited 28 October 2019. [Google Scholar]
- Van Wyk B.-E., Van Oudtshoorn B., Gericke N. second ed. Briza Publications; Pretoria, South Africa: 2009. Medicinal Plants of South Africa. [Google Scholar]
- Von Koenen E. fourth ed. Klaus Hess Publishers; Windhoek, Namibia: 2001. Medicinal, Poisonous and Edible Plants in Namibia. [Google Scholar]
- Wang H., Naghavi M., Allen C., Barber R.M., Bhutta Z.A., Carter A., Casey D.C., Charlson F.J., Chen A.Z., Coates M.M., Coggeshall M. Mortality and causes of death collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2015;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt J.M., Breyer-Brandwijk M.G. second ed. Livingstone; Edinburg and London: 1962. The Medicinal and Poisonous Plants of Southern and Eastern Africa. [Google Scholar]
- York T., De Wet H., Van Vuuren S.F. Plants used for treating respiratory infections in rural Maputaland, KwaZulu-Natal, South Africa. J. Ethnopharmacol. 2011;135:696–710. doi: 10.1016/j.jep.2011.03.072. [DOI] [PubMed] [Google Scholar]
- Zschocke S., Rabe T., Taylor J.L.S., Jäger A.K., van Staden J. Plant part substitution – a way to conserve endangered medicinal plants? J. Ethnopharmacol. 2000;71(1-2):281–292. doi: 10.1016/s0378-8741(00)00186-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.