Arrhythmogenic cardiomyopathy is an inheritable disease characterized by a fibrofatty infiltration of the heart muscle, often (though not always) of right ventricular predominance, arrhythmias and sudden death in the young.1 Because of the predominant right ventricular phenotype, the disease has been referred to as “arrhythmogenic right ventricular dysplasia” (ARVD) or “arrhythmogenic right ventricular cardiomyopathy” (ARVC).1 Mutations in genes coding for desmosomal proteins (figure 1A) are the most common cause of gene-positive familial cases.1 Among desmosomal genes, PKP2, coding for the protein Plakophilin-2 (PKP2), is the most commonly affected, particularly in the adult population.1 The high propensity to arrhythmias in ARVC has been identified since the early description of the disease by Marcus et al in 1982. Life-threatening ventricular arrhythmias occur mostly in the early (concealed) stage of the disease, often prior to the overt structural phenotype.2 As shown by the analysis of 1001 cases related to ARVC, syncope or sudden cardiac arrest was the first disease manifestation in 11% of the probands.2 Understanding the arrhythmia mechanisms in the early stage of the disease is therefore paramount to lessen the risk, and potentially prevent, lethal arrhythmias in the affected population.
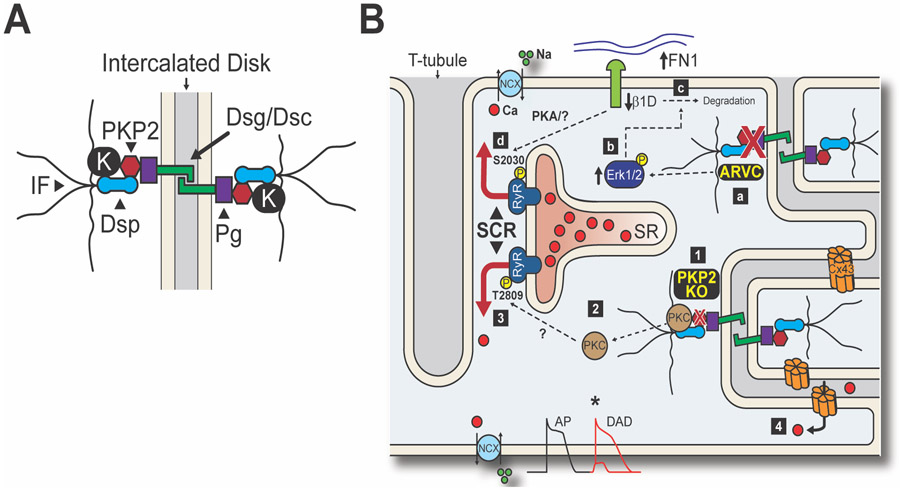
Figure 1. Desmosome-Dyad Axis in ARVC.
A. Schematic representation of a cardiac desmosome depicting the main protein components: desmoglein and desmocollin (Dsg/Dsc), plakoglobin (Pg), plakophilin-2 (PKP2), desmoplakin (Dsp) and the intermediate filaments (IF). In ARVC, loss of desmosomal integrity can dislodge auxiliary proteins such as kinases (K), affecting signaling pathways and Ca2+ regulation. B. Summary of the desmosome-dyad hypotheses in ARVC. Wang et al.8 propose that disruption of the desmosome (a) activates Erk1/2 signaling (b), triggering integrin β1D degradation through a fibronectin (FN1)-ubiquitin/lysosome-dependent mechanism (c). Loss of integrin β1D leads to hyperphosphorylation of RyR2-S2030 through an unknown pathway (d). Kim et al.7 propose that knockout of PKP2 disturbs the desmosome (1) and dislodges other components such as PKC (2). Release of PKC into the cytosol facilitates off-target phosphorylation, including RyR2-T2809 (3). Loss of desmosomal integrity also promotes Ca2+ entry into the cell via Connexin43 (Cx43) hemichannels (4). These mechanisms converge on the notion that RyR2 channels activated by phosphorylation promote SCR events during stress, leading to delayed afterdepolarizations (DADs), triggered activity, and ultimately arrhythmias.
Ventricular arrhythmias in ARVC have a strong catecholaminergic component: they are highly inducible by isoproterenol, are most likely to appear during exercise or heightened sympathetic input, and respond well to sympathectomy.3, 4 Adrenergic stimulation increases cardiac performance in great part by increasing Ca2+ entry and Ca2+i transient amplitude, but these effects also increase arrhythmia vulnerability due to higher propensity for spontaneous Ca2+ release (SCR) and triggered activity.5 Given that most cases of gene-positive familial ARVC associate with desmosomal genes, the existence of a desmosome-Ca2+i (or desmosome-dyad) “axis” as an arrhythmia mechanism is a tantalizing possibility that, if established, may uncover new targets for therapy. Cerrone et al6 and Kim et al.7 examined the cardiac endophenotype of PKP2 in adult murine hearts and showed that cardiomyocyte-specific knockout of PKP2 predisposes the heart to triggered activity and ventricular arrhythmias, and causes aberrant SCR from RyR2 channels “eager” to release Ca2+ from the junctional sarcoplasmic reticulum. These and other results indicate a relation between desmosomal integrity and the control of Ca2+i homeostasis in cardiac myocytes. The data also point to RyR2 channels as key substrates for arrhythmias in ARVC.
The concept of a desmosome-dyad axis and hyperactive RyR2 channels has now been expanded by the elegant study of Wang et al., published in this issue of Circulation.8 These authors analyzed by mass spectrometry four explanted failing hearts with diagnosis of ARVC (three of them with a missense mutation in a desmosomal gene), and determined that integrin β1D was selectively downregulated and that RyR2-Ser2030 was hyper-phosphorylated. These results prompted them to examine the association between integrin β1D, Ca2+i regulation, and RyR2 phosphorylation in ARVC arrhythmogenesis. In vitro studies (single RyR2 channel recordings in lipid bilayers), and analysis of murine hearts deficient in integrin β1D showed a role for this protein in the stabilization of RyR2 function, in Ca2+i homeostasis and in the electrical activity of the ventricles. This convergence of data from experimental models (PKP2 conditional knockout in one case; integrin β1D deficiency in the other) supports the notion that: a) There is an association between desmosomal gene expression/integrity and Ca2+i function/regulation; b) a desmosome-dyad crosstalk is more generalizable and not unique to specific mutations, a single pathway, or specific cases, and c) RyR2 channels are likely to be critical players in the desmosome-dyad interaction.
The presence of hyperactive RyR2 channels in the two models of ARVC is likely related to a change in the phospho-state of the RyR2 protein (figure 1B). The identity of the kinases involved requires further investigation. Wang et al8 reported hyperphosphorylation of RyR2-Ser2030, a phosphosite considered a PKA substrate, relevant for the adrenergic response of cardiac cells.9 Yet, it is worth noting that Zhang et al recently showed that cardiomyocyte ablation of PKA does not affect the basal phospho-state of RyR2-Ser2030;10 the latter suggests that kinases other than PKA can also phosphorylate RyR2-S2030. Separately, Kim et al detected right ventricle-selective phosphorylation of Thr2809, a potential PKC site located within the “phosphorylation hotspot” of RyR2.7 The data converge in indicating that desmosomal deficiency can disrupt the activity of RyR2 by kinase-mediated events, involving more than one kinase. The results further suggest that kinase derangement, likely caused in part by dislocation of intercalated disc-bound kinases after loss of desmosomal integrity,11 can phosphorylate (and affect the function of) multiple off-targets, giving way to the pleiotropic manifestations associated with desmosomal deficiency. Kinases may be the transducers of intercalated disc-transcription coupling reported in various models of ARVC.
The study of Wang et al8 is particularly novel in that it makes a previously unidentified association between the ARVC phenotype and the abundance of integrin β1D. This observation touches on an understudied perspective, namely, the possible association between mechano-sensing and/or mechano-transduction, and ARVC. It is worth noting that desmosomal dysfunction is likely to alter the architecture of intermediate filaments and their coordinated function with the microtubule network.12 The latter would affect not only the stiffness of the cells but also the interaction between cytoskeleton and nucleus, with potential repercussions to the transcriptional program of the cell. Wang et al8 reported no changes in mechanical function in the hearts of mice deficient in integrin β1D. Instead, they found a direct association with RyR2 and consequently, arrhythmias. Whether contractile dysfunction can be observed at a later time point is not mentioned (though some fibrosis is reported 3 months after TAM injection). The results leave open the question of whether reduced abundance of integrin β1D contributes to the cardiomyopathy aspect of the ARVC phenotype. Future studies may address this interesting question.
The findings in this study point to a novel association between integrin β1D and RyR2 in the context of ARVC. However, the absence of changes in the abundance of other proteins in the studied ARVC hearts should not be interpreted as conclusive. Wang et al limited their analysis to four failing, explanted hearts, obtained at the end-stage of the disease (at the time of transplant). ARVC is pleiotropic and progressive, covering multiple stages and different phenotypes. Importantly, the most dangerous arrhythmias actually occur in the early stages, at a time point that is not covered by this study. This limitation impacts on data interpretation, as the molecular events leading to sudden death in the concealed stage may not be those unveiled through analysis of a failing heart. Furthermore, given that the study is statistically under-powered (for understandable reasons), differences between groups may be obscured by intra-group variability. Whether the findings presented in the study of Wang et al8 can be translated to human ARVC during the concealed stage of the disease, when sudden cardiac arrest is more likely to occur, remains to be investigated.
Overall, this study adds to a body of evidence indicating that life-threatening arrhythmias in ARVC are not (only) caused by the anatomical disruption resulting from the fibrofatty infiltration, and the consequent impaired cell-cell electrical coupling. Rather, there are cell-based mechanisms that act as arrhythmia triggers leading to sudden death. The latter is critically important, as it opens the door for novel therapeutic approaches. In particular, the study emphasizes the potential benefit of RyR2 blockers in ARVC. Based on the success of flecainide in patients with CPVT,13 the evidence that flecainide prevents isoproterenol-induced arrhythmias in PKP2-deficient mice,6 and anecdotal evidence of its positive effect in ARVC patients, an NIH-funded pilot effort is underway to evaluate the use of flecainide in patients with ARVC (NIH R34 HL143372; Zareba, PI). Still, it remains to be determined whether more specific RyR2 blockers (see14) can be effective in experimental models and used as a foundation for drug design to help ARVC patients. There is an unmet need for effective therapeutic approaches to manage patients with ARVC. An effort in that direction, following the lead of studies such as those of Chelko et al,15 can hopefully transform into breakthrough approaches that improve quality and life expectancy for patients affected with the disease.
Footnotes
CONFLICT OF INTEREST DISCLOSURE: None
REFERENCES
- 1.Austin KM, Trembley MA, Chandler SF, Sanders SP, Saffitz JE, Abrams DJ, Pu WT. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat Rev Cardiol. 2019; 16:519–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groeneweg JA, Bhonsale A, James CA, te Riele AS, Dooijes D, Tichnell C, Murray B, Wiesfeld AC, Sawant AC, Kassamali B, Atsma DE, Volders PG, de Groot NM, de Boer K, Zimmerman SL, Kamel IR, van der Heijden JF, Russell SD, Jan Cramer M, Tedford RJ, Doevendans PA, van Veen TA, Tandri H, Wilde AA, Judge DP, van Tintelen JP, Hauer RN, Calkins H. Clinical Presentation, Long-Term Follow-Up, and Outcomes of 1001 Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Patients and Family Members. Circ Cardiovasc Genet. 2015; 8:437–446. [DOI] [PubMed] [Google Scholar]
- 3.Denis A, Sacher F, Derval N, Lim HS, Cochet H, Shah AJ, Daly M, Pillois X, Ramoul K, Komatsu Y, Zemmoura A, Amraoui S, Ritter P, Ploux S, Bordachar P, Hocini M, Jais P, Haissaguerre M. Diagnostic value of isoproterenol testing in arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2014; 7:590–597. [DOI] [PubMed] [Google Scholar]
- 4.Assis FR, Krishnan A, Zhou X, James CA, Murray B, Tichnell C, Berger R, Calkins H, Tandri H, Mandal K. Cardiac sympathectomy for refractory ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2019; 16:1003–1010. [DOI] [PubMed] [Google Scholar]
- 5.Eisner D Calcium in the heart: from physiology to disease. Exp Physiol. 2014; 99:1273–1282. [DOI] [PubMed] [Google Scholar]
- 6.Cerrone MMJ, Lin X, Zhao Y-T, Zhang M, Agullo-Pascual E, Alvarado FJ, Dolgalev I, Karathanos TV, Malkani K, van Opbergen CJM, van Bavel JJA, Yang H-Q, Vasquez C, Tester D, Fowler S, Liang F, Rothenberg E, Heguy A, Morley GE, Coetzee WA, Trayanova NA, Ackerman MJ, van Veen TA Valdivia HH, Delmar M. Plakophilin-2 is rquired for transcription of genes that control calcium cycling and cardiac rhythm. Nat Comm. 2017; 8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JC, Perez-Hernandez M, Alvarado FJ, Maurya SR, Montnach J, Yin Y, Zhang M, Lin X, Vasquez C, Heguy A, Liang FX, Woo SH, Morley GE, Rothenberg E, Lundby A, Valdivia HH, Cerrone M, Delmar M. Disruption of Ca(2+)i Homeostasis and Connexin 43 Hemichannel Function in the Right Ventricle Precedes Overt Arrhythmogenic Cardiomyopathy in Plakophilin-2-Deficient Mice. Circulation. 2019; 140:1015–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y LC SL, Chen X, Cui C, Huang J, Chen B, Hall DD, Pan Z, Lu M, Hong J, Song L, Zhao S,. Integrin 1 β1D Deficiency-mediated RyR2 Dysfunction Contributes to Catecholamine-Sensitive Ventricular Tachycardia in ARVC. Circulation. 2020;000:00–00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potenza DM, Janicek R, Fernandez-Tenorio M, Camors E, Ramos-Mondragon R, Valdivia HH, Niggli E. Phosphorylation of the ryanodine receptor 2 at serine 2030 is required for a complete beta-adrenergic response. J Gen Physiol. 2019; 151:131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Wang WE, Zhang X, Li Y, Chen B, Liu C, Ai X, Zhang X, Tian Y, Zhang C, Tang M, Szeto C, Hua X, Xie M, Zeng C, Wu Y, Zhou L, Zhu W, Yu D, Houser SR, Chen X. Cardiomyocyte PKA Ablation Enhances Basal Contractility While Eliminates Cardiac beta-Adrenergic Response Without Adverse Effects on the Heart. Circ Res. 2019; 124:1760–1777. [DOI] [PubMed] [Google Scholar]
- 11.Bass-Zubek AE, Hobbs RP, Amargo EV, Garcia NJ, Hsieh SN, Chen X, Wahl JK 3rd, Denning MF, Green KJ. Plakophilin 2: a critical scaffold for PKC alpha that regulates intercellular junction assembly. J Cell Biol. 2008; 181:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robison P, Caporizzo MA, Ahmadzadeh H, Bogush AI, Chen CY, Margulies KB, Shenoy VB, Prosser BL. Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science. 2016; 352:aaf0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannankeril PJ, Moore JP, Cerrone M, Priori SG, Kertesz NJ, Ro PS, Batra AS, Kaufman ES, Fairbrother DL, Saarel EV, Etheridge SP, Kanter RJ, Carboni MP, Dzurik MV, Fountain D, Chen H, Ely EW, Roden DM, Knollmann BC. Efficacy of Flecainide in the Treatment of Catecholaminergic Polymorphic Ventricular Tachycardia: A Randomized Clinical Trial. JAMA Cardiol. 2017; 2:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batiste SM, Blackwell DJ, Kim K, Kryshtal DO, Gomez-Hurtado N, Rebbeck RT, Cornea RL, Johnston JN, Knollmann BC. Unnatural verticilide enantiomer inhibits type 2 ryanodine receptor-mediated calcium leak and is antiarrhythmic. Proc Natl Acad Sci U S A. 2019; 116:4810–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chelko SP, Asimaki A, Lowenthal J, Bueno-Beti C, Bedja D, Scalco A, Amat-Alarcon N, Andersen P, Judge DP, Tung L, Saffitz JE. Therapeutic Modulation of the Immune Response in Arrhythmogenic Cardiomyopathy. Circulation. 2019;140:1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]