Abstract
The efficacy of photodynamic therapy (PDT) using aminolevulinic acid (ALA), which is preferentially taken up by cancerous cells and converted to protoporphyrin IX (PpIX), can be substantially improved by pretreating the tumor cells with Vitamin D (Vit D). Vit D is one of several “differentiation-promoting agents” that can promote the preferential accumulation of PpIX within the mitochondria of neoplastic cells, making them better targets for PDT. This article provides a historical overview of how the concept of using combination agents (“neoadjuvants”) for PDT evolved, from initial discoveries about neoadjuvant effects of methotrexate and fluorouracil, to later studies to determine how Vitamin D and other agents actually work to augment PDT efficacy. While this review focuses mainly on skin cancer, it includes a discussion about how these concepts may be applied more broadly toward improving PDT outcomes in other types of cancer.
Keywords: Vitamin D, calcitriol, calcipotriol, skin cancer, phototherapy, photodynamic therapy, oncology
Graphical Abstract
This review describes how certain drugs and hormones can be used to augment aminolevulinate-based photodynamic therapy (PDT). These agents (methotrexate, fluorouracil, and Vitamin D) can serve as neoadjuvants for PDT of cancer. The article describes the original research that uncovered mechanisms responsible for the neoadjuvants’ effects, and then explains the rationale for using Vitamin D as a particularly promising and safe form of PDT combination therapy. While the majority of the research described was performed in skin cancer models, the review also looks prospectively at opportunities for employing neoadjuvants in other types of cancer both for PDT and for photodiagnosis.
Introduction
Photodynamic therapy (PDT) is a treatment modality in which a photosensitizing molecule (dye or drug) is used to target and kill bacteria, parasites, or cancer cells (1–3). For human cancer, most PDT photosensitizers in use at the current time are porphyrins or porphryin-related molecules that absorb visible light, undergo photochemical breakdown, and release free radicals; these highly reactive species cause cell membrane damage and trigger cell death (4). A unique variation on this theme, proposed and developed in the 1990’s (5, 6), is the use of 5-aminolevulinic acid (ALA) which is a precursor for the synthesis of porphyrins within mitochondria. ALA is taken up and converted into protoporphyrin IX (PpIX) within carcinoma cells to a relatively greater extent than in the normal surrounding tissues, thereby providing a high degree of cancer selectivity and reducing off-target phototoxicity (7). Use of ALA (Levulan, Ameluz) or ALA methyl-ester (Metvix) as pro-drugs to drive PpIX generation is now the most prevalent form of PDT used in dermatology for treating skin cancer (8–10). ALA and its esters have also been explored for a number of internal malignancies including cancers of the gynecologic tract (11–13), oral cavity (14, 15), head and neck (16–18) and brain (19–21). Besides PDT, ALA is proving valuable as a tool for photodiagnosis. Thus, after preferential accumulation of PpIX within cancer cells, low-intensity blue light can stimulate a red fluorescent signal that can help the physician to identify residual tumor nests within a surgical field when attempting to excise difficult-to-visualize cancers such as bladder wall carcinoma (22, 23) or glioblastoma of the brain (24, 25). A recent example was the approval in 2017 of ALA as a photodiagnostic agent (Gleolan) for image-guided surgical resection of high-grade gliomas (26).
Effective PDT and photodiagnostic visualization both depend upon achieving high levels of PpIX accumulation preferentially within target cells. For skin cancer, and indeed for most cancers, ALA diffusion through the skin and penetration into thick neoplastic lesions is limited by depth. This makes the problem of how to enhance intratumoral PpIX production from low starting amounts of ALA even more important. It is no wonder then that many researchers have sought ways to induce higher PpIX levels within cancer cells using a variety of approaches, such as increasing ALA uptake into the cells, decreasing ALA efflux, or using iron chelators to block the final enzymatic step in heme synthesis so as to increase PpIX accumulation within mitochondria (3). However, most of these approaches require drugs or chemicals that are investigational and/or not widely approved for human use. An alternative approach (and one that we focus on here) is to find already-available, licensed agents or drugs that have an ability to boost PpIX accumulation, and to re-purpose them for use in combination with PDT. Almost 2 decades ago we began to investigate drugs that modulate the differentiation status of cancer cells and simultaneously alter cellular PpIX metabolism. This idea was greeted with some skepticism at first, but subsequent studies have ultimately proven the validity of this approach, and mechanisms of action have become apparent over time. This review provides a historical perspective on the discovery of how differentiating-promoting agents can be used as neoadjuvants (combination agents) together with ALA-based PDT to improve therapeutic outcomes. The latter part of the review focuses more specifically on Vitamin D.
The concept of differentiation therapy
The first hint that PpIX production in epithelial tissues might be related to the differentiation state of the cells came from studies in epidermal keratinocytes (27). Ortel et al investigated the effect of terminal differentiation upon ALA-induced synthesis in primary mouse keratinocytes and subsequent PDT phototoxicity. Differentiation of the keratinocytes, triggered by elevating the calcium concentration in the medium, led to greater intracellular PpIX accumulation in ALA-treated cells. It also enhanced photodynamic sensitization and cell lethality. Further experiments showed that the differentiation-dependent increase in PpIX levels resulted from a combination of effects, including increased ALA uptake, enhanced PpIX synthesis, and decreased PpIX export into the culture media. This paper also provided the first evidence that heme-synthetic enzymes might be part of the connection between cellular differentiation and enhanced PpIX accumulation, since the mRNA level of coproporphyrinogen oxidase (CPO) was greater in differentiated cells. Furthermore, the stimulation of heme synthesis was seen in primary keratinocytes but not in PAM 212 cells that fail to undergo differentiation.
In a subsequent paper by Ortel et al, it was shown that the effects of combining differentiation-promoting agents with PDT was generalizable to other epithelial cell types, such as human prostate cancer cells (28). LNCaP prostate cancer cells are hormonally responsive, so in this study hormonal agents were applied for a short course of differentiation therapy (3–4 days) prior to giving ALA. Three classes of hormones were tried, namely androgens, isomers of retinoic acid (Vitamin A), and analogues of vitamin D. After each of these pretreatments, it was demonstrated that ALA-dependent PpIX production was increased, in parallel with markers for growth arrest and for differentiation. Due to the elevated PpIX production, cytotoxicity after visible light exposure was also enhanced, indicating the possibility of using these agents to boost PDT efficacy.
Methotrexate, the first differentiation-promoting drug for ALA-PDT
The first non-hormonal drug to be investigated for its effects on cellular differentiation and ALA-PDT was methotrexate (MTX). MTX is an old drug that is still widely used for the treatment of psoriasis and other hyperproliferative conditions in which excessive cellular proliferation is linked to failure of terminal differentiation. Sinha et al pretreated LNCaP prostate cancer cells with MTX for 3 days, and showed that MTX alone promoted growth arrest and differentiation. Furthermore, MTX increased intracellular PpIX levels by 3-fold, leading to significant enhancement of phototoxic killing in the MTX-preconditioned cells (29). The reverse order of treatments, ALA-PDT followed by MTX, yielded no enhancement. Following MTX pretreatment, similar increases in PpIX were observed regardless of whether the cells were incubated with ALA, methyl-ALA, or hexyl-ALA, arguing against a major effect upon ALA transport. In an examination of porphyrin-synthetic enzymes, coproporphyrinogen oxidase (CPO) was found to be increased 3-fold after MTX pretreatment. Transfection of the cells with a CPO-expressing vector stimulated PpIX accumulation, arguing that differentiation-related increases in CPO might be driving the observed increases in PpIX levels.
To investigate the link between MTX and heightened PDT responsiveness in a different system, Anand et al examined the effects of combining MTX and ALA-PDT in skin carcinoma cell lines in vitro and skin tumor models in vivo (30). Human SCC13 cells, HEK1 cells, and normal keratinocytes were preconditioned with MTX for 72 h, followed by incubation with ALA for 4 h. In the monolayer cultures, MTX preconditioning increased intracellular PpIX levels by 2- to 4-fold in carcinoma cells, relative to normal keratinocytes. PpIX enhancement correlated with changes in protein expression of two heme pathway enzymes, coproporphyrinogen oxidase (CPO; increased) and ferrochelatase (FC; slightly decreased). These enzymes are located near the end of the heme synthesis pathway, described in Fig. 1. Differentiation markers E-cadherin, involucrin, and filaggrin were also increased by MTX (30). Combination therapy led to synergistically enhanced photodynamic killing compared to PDT alone. Clinical relevance was established by studies performed in vivo, showing that MTX preconditioning enhanced PpIX accumulation in three models: (a) organotypic cultures of immortalized keratinocytes, (b) chemically induced skin tumors in mice; and (c) human A431 squamous cell tumors implanted subcutaneously in mice.
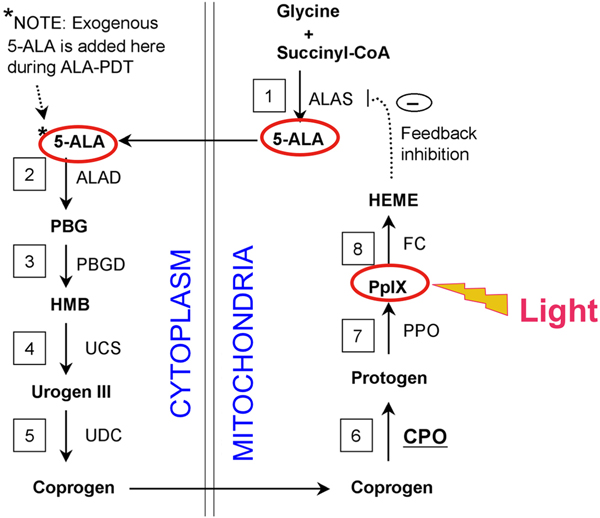
Fig. 1.
The heme synthetic pathway. Heme is a 4-ring porphyrin molecule that is a used as a cofactor in many kinds of enzymes, including hemoglobin. Its synthesis requires 8 enzymatic conversion steps (numbered 1–8). The necessary enzymes are ALAS, aminolevulinic acid synthase; ALAD, aminolevulinic acid dehydratase; PBGD, porphobilinogen deaminase; UCS, uroporphyrinogen III cosynthase; UDC, uroporphyrinogen decarboxylase; CPO, coproporphyrinogen oxidase; PPO, protoporphyrinogen oxidase; FC, ferrochelatase. In the final reaction step, catalyzed by ferrochelatase, an atom of iron is inserted into the protoporphyrin IX (PpIX) molecule. The system is normally shut off by negative feedback inhibition at the first step, but this can be bypassed by adding large amounts of exogenous 5-ALA, which leads to accumulation of high levels of PpIX. Adapted from ref. (34).
5-Fluorouracil as a topical alternative to oral methotrexate
Although systemic MTX was clearly an effective neoadjuvant for PDT in mouse models, the idea of using oral MTX as a PDT neoadjuvant for skin cancer was problematic because MTX is associated with a long-term risk of liver toxicity. In the dermatologic arena, use of oral MTX and PDT in large populations of patients with actinic pre-cancers or basal cell carcinoma (diseases that in themselves are not life-threatening) could present an unfavorable risk-to-benefit ratio. Looking for alternatives, we considered 5-Fluorouracil (5-FU). MTX and 5-FU each inhibit the same metabolic pathway (involving pyrimidine salvage) but unlike MTX, 5-FU can be administered as a topical cream that is minimally absorbed. Importantly, topical 5-FU is approved by the FDA and is widely used for the treatment of skin precancers (so-called “actinic keratoses”). Therefore, there are no regulatory barriers when using topical 5-FU (31).
To test the idea of using 5-FU as a neoadjuvant for ALA-based PDT, Anand et al pursued a mechanism-based approach (32). In several mouse models of squamous cell carcinoma, including skin lesions induced by UV light or subcutaneously-implanted carcinoma cells (A431 and 4T1), a 5-FU pretreatment was administered for 3 days, followed by ALA for 4 hours. This led to enhanced PpIX levels within tumor cells and to greater cell death upon illumination. Several mechanisms for these observations were identified. First, changes in the expression of two critical enzymes in the heme synthesis pathway (CPO and FC) were observed. Second, a large induction of p53, a protein known to induce apoptosis, was observed in the 5-FU-pretreated tumors. Interestingly, A431 cells (which contain a mutant form of p53) still showed a strong induction of PpIX after neoadjuvant 5-FU. Even 4T1 tumors (which lack p53 altogether), showed significant beneficial inductions of PpIX accumulation and PDT-induced cell death (2.5-fold) after 5-FU pretreatment. These findings are significant because they indicate that a 5-FU neoadjuvant/PDT combination approach may be useful for p53-mutant and p53-null tumors that typically do not respond to traditional chemotherapy.
Armed with these preclinical data in mice, Maytin et al undertook a clinical trial to test the concept of using 5-FU cream as a neoadjuvant for PDT of actinic keratosis (AK); AK are highly prevalent, precancerous lesions that occur most often on the face and scalp (33). In a bilaterally controlled trial of 17 patients, AK lesions were counted, and then one side of the body (face and scalp) was pretreated with 5-FU cream daily for 6 days. The contralateral side served as the no-pretreatment control. Topical methylaminolevulinate (MAL) cream was applied for 3 hours, and then PpIX levels were measured by surface fluorimetry. A skin biopsy was done of one representative lesion on each side. Red light illumination was then performed, and AK lesion counts were monitored at repeat visits at 3, 6, 9, and 12 months post-PDT. PpIX fluorescent measurements done at the first visit revealed a 2- to 3-fold increase in 5-FU pretreated AK lesions, relative to non-pretreated controls. Lesion clearance at 3 months post PDT were 75% with neoadjuvant 5-FU versus 45% without 5-FU, a difference that was not only statistically significant but also clinically meaningful. The conclusion is that a simple combination treatment in which the patient applies 5-FU cream daily for one week prior to PDT treatment offers significant improvement in patient care, and this approach is immediately actionable because the necessary drugs are already licensed and approved in both Europe and the U.S.A.
What about Vitamin D as a possible neoadjuvant for PDT?
In the mechanistic context of Figure 1, the apparent similarity of PpIX-elevating mechanisms after MTX and 5-FU, along with the previous demonstration that Vit D can promote terminal differentiation and PpIX production in normal keratinocytes and prostate cancer cells (28), prompted us to consider Vit D more seriously as a potential neoadjuvant for PDT. For an initial look, Sato et al asked what effect Vit D might have in 3-D organotypic cultures of rat keratinocytes, which mimic the physiology of the skin much better than do monolayer keratinocyte cultures (35). In the organotypic system, a 4 day exposure to 10−10 M of calcitriol (the active form of Vitamin D; more on this later) stimulated the expression of the terminal differentiation markers K10 and loricrin. After addition of ALA (1 mM for 4 hours), PpIX levels were significantly increased in the Vit D-preconditioned cultures. Phototoxic cell killing after exposure to 635 nm light was significantly higher as well. A truly remarkable finding here was the sensitivity of the hormonally-mediated response; maximal PpIX inductions (2-fold) were observed with calcitriol concentrations in the picomolar range (35). In squamous cell carcinoma A431 cells grown in monolayers, calcitriol pretreatment also induced PpIX levels, but to lesser degree and requiring higher (nanomolar) concentrations of calcitriol (36).
To investigate neoadjuvant Vit D and PDT in animals in vivo, Anand et al studied mice with squamous cell skin cancers generated by DMBA/TPA treatment, or by subcutaneous injection of A431 cells, for a preclinical proof of concept (37). Calcitriol was delivered topically or intraperitoneally, and increased intratumoral accumulation of PpIX up to 10-fold was observed. To investigate potential reasons for these PpIX increases, the mitochondrial enzymes most commonly involved in regulation of heme synthesis were investigated, and significant changes in expression of two enzymes were observed, i.e., CPO was increased and FC was decreased (Fig. 2). Calcitriol-pretreated tumors exhibited enhanced apoptotic cell death after PDT. Tumors that had been preconditioned by calcitriol prior to receiving ALA-PDT showed greater activation of the extrinsic apoptotic pathway (greater cleavage of caspase-8, and increased production of TNFα). Very low doses of calcitriol (0.1 – 1.0 μg/kg body weight) were sufficient to elicit preferential enhancement of ALA-PDT efficacy in tumors, suggesting minimal toxicity with this approach (37).
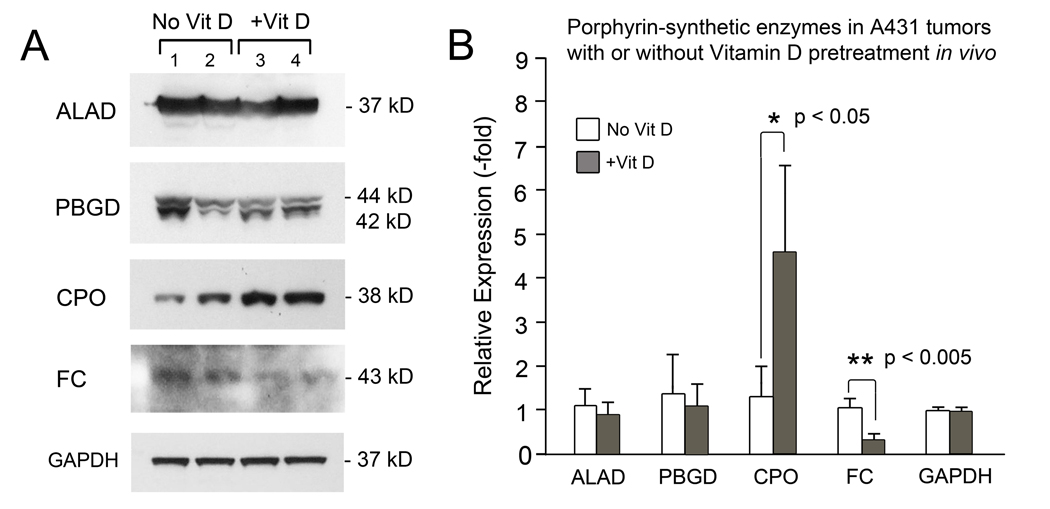
Fig. 2.
Analysis of the expression of mitochondrial enzymes most commonly involved in regulation of heme synthesis. Subcutaneous tumors (A431 squamous cell carcinoma line) in mice pretreated with calcitriol (Vit D) or with vehicle alone, were analyzed by Western blotting (A). Densitometric quantification of 3 tumors per condition is shown (B). ALAD, aminolevulinic acid dehydratase; PBGD, porphobilinogen deaminase; CPO, coproporphyrinogen oxidase; FC, ferrochelatase. GAPDH, glyceraldehyde 3-phosphate dehydrogenase (loading control). Reproduced with permission from ref (37).
Systemic delivery of calcitriol is easily performed in mice, but in humans, high calcitriol levels can be associated with a risk of developing hypercalcemia (described more fully under “Safety of systemic Vitamin D treatment”; see below). To mitigate that risk, it was important to ask whether topical calcitriol (instead of systemic) is effective as a neoadjuvant for skin cancer, given that systemic absorption of topical calcitriol should be low. In a study by Rollakanti et al, hairless mice were subjected to repeated UVB exposure in 24-week carcinogenesis protocol to induce Squamous cell carcinoma (SCC) on their backs (38). SCC tumor-bearing mice were pretreated with topical calcitriol, or vehicle (Aquaphor), prior to application of ALA. Using noninvasive fluorescence optical techniques, the authors confirmed that preconditioning with topical Vit D induces a preferential increase of PpIX levels in tumors (38).
As a second skin cancer model in which to investigate topical calcitriol as a neoadjuvant, Basal cell carcinoma (BCC)-bearing mice were preconditioned for 3 days with topical calcitriol ointment (3 μg/g) prior to PDT (39). After ALA application, PpIX accumulation in the BCC lesions was increased by up to 6-fold. The pretreatment also led to increased expression of differentiation marker E-cadherin (145 fold), and proliferation maker Ki-67 (42 fold), associated with enhanced tumor destruction upon PDT illumination (18-fold higher staining with TUNEL, a marker of apoptotic cell death).
In humans, ointments containing calcitriol, or calcipotriol (a synthetic Vit D analog), are widely used as topical treatment for psoriasis, a human skin disease that features scaly plaques of the elbows and knees. In psoriasis, abnormal cellular proliferation (too much) and differentiation (too little) play a major etiological role. As a first-in-human trial to ask whether a topical Vit D formulation can work as a PDT neoadjuvant to ameliorate an epithelial disease with abnormal differentiation, Maytin et al. conducted a pilot study of 7 patients with bilaterally-matched plaques of psoriasis (40). Calcipotriol 0.005% ointment was applied to a test plaque on one elbow daily for 3 days prior to blue light ALA-PDT; a control plaque on the contralateral elbow received Aquaphor only. Calcipotriol-pretreated plaques showed preferential increases in PpIX (~130%), and reduced thickness, redness, scaling, and itching. Although ALA-PDT has now fallen out of favor as a viable treatment for psoriasis, partly due to the pain experienced during illumination, the results of this study supported the principle of differentiation as an important determinant of PDT response in a hyperproliferative skin disease in humans.
Summary of known mechanisms for differentiating agents acting as PpIX-elevating agents for ALA-PDT
At this point, we will pause to summarize what is known about the mechanisms of the differentiation-promoting agents identified in the studies discussed up to now. The main focus of action appears to be the heme synthetic pathway, shown in Fig. 1. While many different enzymes were surveyed in previous studies, only two (CPO and FC) appear to be consistently altered by differentiation-promoting pretreatments (Table 1). In other words, for all three neoadjuvant agents that were examined (MTX, 5-FU, and Vit D), the most consistent changes were observed in levels of CPO (increased in all cases) and FC (decreased in 2 of 3 cases). The net effect of these changes is to increase the size of the PpIX pool within mitochondria.
Table 1.
Changes in expression of two critical heme enzymes after pretreatment with neoadjuvants.
An interesting question is how these 3 different agents might be acting to exert a similar overall effect upon the mitochondrial enzyme levels. The answer, at least for CPO, indicates that the explanation may lie at the gene transcriptional level (Fig. 3) (41). A well-known transcriptional regulator in epithelial cell differentiation is the C/EBP family of transcription factors, considered “master regulators” of differentiation-related genes in keratinocytes and other stratifying epithelial systems (42, 43). C/EBP factors may also play a regulatory role in squamous cell carcinoma (44). To ask whether C/EBP factors might be involved in differentiation-related regulation of the CPO gene, Anand et al examined 1,300 DNA base pairs of the upstream promoter region of mouse CPO and human CPO genes in great detail (41). Administration of the differentiating agents was able to increase different members of the C/EBP family; for example, Vit D induced expression of C/EBPβ. Transfecting the cancer cells with various C/EBP expression vectors led to increases in PpIX, C/EBP, and CPO levels in parallel. Transfection experiments, employing different C/EBP family members (recombinant proteins C/EBPα, C/EBPβ, C/EBPδ, and C/EBPζ), revealed the existence of multiple C/EBP consensus binding sites in the CPO upstream regulator regions (15 for mouse, 18 for human). When binding sites were inactivated by site-directed mutagenesis in the context of the native promoter, transcriptional activity was reduced regardless of whether the individual site was a high-affinity or a low-affinity binding site, indicating that cooperative interactions between regularly spaced C/EBP sites are critical for CPO transcriptional regulation by differentiation therapy (Fig. 3). These results provide a mechanistic rationale for how different agents may be able to enhance CPO expression and therefore, enhance combination PDT therapy in cancer cells.
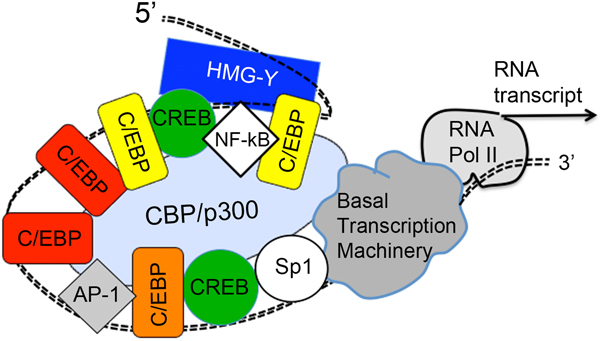
Fig. 3.
Effect of Vit D combination pretreatment upon the transcription of the CPO gene. Vit D may act indirectly by increasing the expression of C/EBP transcription factors; these C/EBP factors bind to the upstream CPO gene promoter region and cooperate with other factors to stimulate gene transcription. Adapted from ref. (41).
A more detailed look at Vitamin D as a clinical neoadjuvant for PDT of cancer
The Vitamin D system is actually a family of signaling molecules within a regulatory pathway present in all mammals (Fig. 4). The most upstream molecule in this system is cholecalciferol, a vitamin made in the skin through the action of sunlight. Cholecalciferol is then modified in the liver and the kidney to produce calcitriol; (more about this pathway below). Calcitriol is a hormone that binds to a steroid hormone receptor (the Vit D receptor, or VDR. The VDR translocates to the nucleus, where it binds to DNA-regulatory regions of many genes whose functions are to regulate serum calcium levels, facilitate proper growth of bones and muscles, and regulate a wide variety of other important physiological functions that are beyond the scope of this minireview (45). What about Vit D and cancer? An extensive literature on this topic suggests that Vit D plays a role in cancer etiology (46–50). In fact, many lines of evidence suggest that Vit D deficiency may favor the development of skin cancer, although this is far from proven and mechanisms remain controversial (47, 50). Here however we are only interested in Vit D as a therapeutic neoadjuvant (a short-term preparatory agent for PDT). Below we will only focus upon possible effects of Vit D in an acute clinical setting.
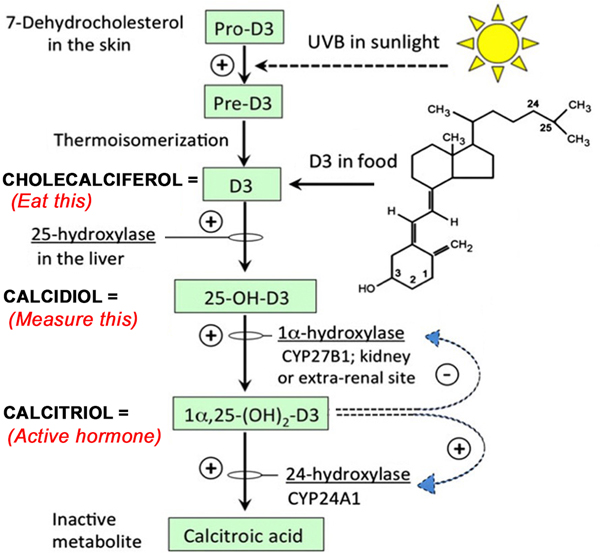
Fig. 4.
Vitamin D is both an oral vitamin and a steroid-like hormone. Adapted from ref. (51).
As alluded to earlier, it would be highly problematic to use calcitriol (the active hormonal form of Vit D) as a combination PDT treatment in patients, because of the potential risk of inducing hypercalcemia. Before explaining this further, and to explore possible alternatives, we will now describe some basics of Vit D metabolism in more detail (see Fig. 4). Calcitriol is a steroid hormone that represents the final result of modifications of cholecalciferol (also called Vitamin D3, or simply “D3”). D3 is normally synthesized from a cholesterol-based precursor in the skin, via a photoconversion reaction that requires (solar) ultraviolet radiation. D3 is the substance that people normally take as a Vitamin D supplement. Conversion to the active hormone requires that D3 must be modified by addition of two hydroxyl (-OH) groups. D3 gains the first hydroxyl in the liver, becoming 25-hydroxy-D3 (“calcidiol”). The second hydroxyl is added in the kidney, to create 1,25-dihydroxy-D3 (“calcitriol”), the active hormone. Calcidiol is the form of Vitamin D that is normally measured in blood tests, because it is present at relatively high (nanomolar) concentrations and is quite good as an indicator of the overall Vit D status of the patient. In contrast, calcitriol levels in serum are typically 1000-fold lower (i.e., picomolar concentrations). Therefore calcitriol is not routinely assayed in clinical practice, although it is certainly possible to do so (45).
To avoid the hypercalcemic risk of calcitriol, an obvious-sounding alternative might be to use cholecalciferol (D3, the natural vitamin) as the PDT neoadjuvant. However, this can only work if D3 is converted to calcitriol in exactly the right amount to boost PpIX levels in tumor cells while not raising serum calcium to dangerous levels. To test the safety and potential efficacy of oral cholecalciferol as a PDT neoadjuvant, a preclinical study was undertaken in mice (51). Mice with subcutaneous A431 tumors received D3 supplementation by ingesting a diet with D3 amounts 10-fold higher than normal, for a period of 10 days. This pretreatment led to a 3- to 4-fold enhancement of PpIX levels, and a 20-fold enhancement of PDT-mediated cell death. PpIX levels and cell viability in normal tissues were not affected. The hydroxylated forms of D3 (calcidiol and calcitriol) were only modestly elevated in serum (51), indicating minimal hypercalcemic risk and suggesting that a brief oral administration of cholecalciferol can serve as a safe neoadjuvant to ALA-PDT.
Safety of systemic Vitamin D treatment
Vitamin D toxicity, a clinical syndrome with both high serum Vit D levels (hypervitaminosis D) and high blood calcium levels (hypercalcemia), is extremely rare (52, 53). Patients with clinical Vit D toxicity can have symptoms and signs associated with hypercalcemia (nausea, dehydration, constipation) and with high urinary calcium levels (polyuria, kidney stones). A high serum Vit D level in the absence of hypercalcemia is not considered a medical emergency, although the cause should be investigated. The most common reason for hypervitaminosis D is ingestion of excess Vit D supplements, but because of the highly-regulated nature of Vit D conversion to calcitriol (see Fig. 4), clinical symptoms are rare. Thus, the minimum calcidiol (25-OH-D) level that was ever reported to be associated with clinical toxicity was 80 ng/mL (with the ‘normal range’ being 30–75 ng/mL, with varying slight variations between reference laboratories), whereas most patients with signs of toxicity have calcidiol levels greater than 150 ng/mL (52). Overall, administration of Vit D supplements (cholecalciferiol) is very safe, and problems only occur after ingesting large doses (>10,000 IU/d) for prolonged periods. Even administration of 50,000 IU/month of Vit D supplements was not associated with any laboratory parameters of toxicity (53).
Clinical trials of Vitamin D as a neoadjuvant for AK/squamous precancer
The preclinical studies described earlier showed that topical Vitamin D analogs are effextive as PDT neoadjuvants for skin cancer in mice. Moving forward from there, Torezan et al performed a clinical study in 2018 in which topical calcipotriol pretreatment was combined with methyl aminolevulinate (MAL)-PDT in patients with actinic keratoses (AKs) of the scalp (54). Twenty patients were randomized to receive conventional MAL-PDT (which included surgical curettage prior to MAL) on one side of the scalp, and Vit D-assisted PDT (calcipotriol application daily for 15 days prior to MAL-PDT) on the other side. After 3 months, overall AK lesion clearance rates were 92.1% for Vit-D assisted PDT versus 82.0% for conventional PDT (P < 0.001). PpIX fluorescence intensity was higher in lesions on the Vit D-assisted side. The authors concluded, in this first human trial, that pretreatment with calcipotriol can increase PpIX accumulation within the skin and may enhance the efficacy.
The above-mentioned clinical trial on topical Vit D and PDT (54) was conducted in Europe. Physicians in the Unites States, unfortunately, cannot legally prescribe topical Vit D analogs for use in the treatment of skin cancer because this considered an “off-label” use, and is not approved by the US Food and Drug Administration. To address this problem, an alternative approach using the natural vitamin (cholecalciferol) as a neoadjuvant for ALA-PDT is now being tested. In a clinical trial initiated in 2017 (NCT03319251), patients with AK of the face or scalp are being treated with ALA and blue light PDT to test correlations between AK lesion response rate and serum Vit D (calcidiol) level. The hypothesis is that patients with Vit D deficiency will have a worse (lower) AK lesion response rate than patients with normal Vit D levels. In another arm of this study, a cohort of patients will receive a bolus pretreatment (5 or 10 days of Vit D supplementation, depending upon their initial Vit D status) prior to ALA-PDT, to ask whether this pretreatment will enhance clinical responses to ALA-PDT.
One additional rationale for developing novel combination approaches for PDT treatment, such as oral Vitamin D, is that such measures might help to boost PDT efficacy in a regimen that reduces pain during treatment. This idea requires a little more background explanation. Conventional PDT protocols are notorious for the stinging pain that many patients experience during illumination (55, 56). This unpleasant side effect occurs when patients undergo ALA preincubation for >1 h, probably due to diffusion of PpIX out of cancer cells and into nearby nerve endings (57). We recently described a new “painless” ALA-PDT regimen for AK lesions of the face, in which pain is drastically reduced by eliminating the ALA pre-incubation step and instead administering topical ALA and illumination simultaneously (58). In the new regimen ALA, is applied to the skin and then the light is immediately switched on and left on for double the usual illumination period (i.e., for 30 min rather than for 16 min 40 sec). With this modified regimen patients experience essentially no pain, yet cutaneous inflammation still develops (by ~24 h) and AK lesions are effectively cleared by 3 months post-treatment (58). Recognizing that AK clearance is never 100% effective after a single treatment, for any ALA-based PDT techniques whether “painless” or conventional, it would seem that adding a neoadjuvant such as 5-FU or topical Vit D might be a reasonable way to improve AK clearance. However, conventional MAL-PDT preceded by topical Vit D reportedly led to worse pain during illumination (pain score of 5.4 on the Vit D-PDT side, versus 4.0 on the conventional PDT side) (54). It will be interesting in the future to see whether combining neoadjuvant Vit D with our newly-described “painless” PDT regimen might boost PDT efficacy while still maintaining a relatively pain-free experience.
Clinical trials of Vitamin D as a PDT neoadjuvant for BCC
Basal cell carcinoma is the most common skin cancer, with over 3 million new cases per year in the United States alone (59). Although BCC are curable with surgery, the scars that result can cause considerable morbidity, especially for patients with the hereditary condition called Gorlin Syndrome in which dozens-to-hundreds of BCC tumors develop over a lifetime (60). PDT, a non-scarring treatment, has been approved in Europe for treatment of BCC for many years, but is not yet approved in the USA. This is partly because PDT is not as effective as surgery for deep (nodular) variants of PDT. Combination PDT regimens might be a solution for this problem.
Translation of the European PDT experience to the United States is difficult, due to the fact that different light sources are used in each location (red light in Europe, blue light in the U.S.). This means that European clinical trial data cannot be easily extrapolated to support a drug application to the FDA. As a first step toward reconciling these differences, a clinical trial was recently conducted to compare the efficacy of blue light versus red light PDT in Gorlin syndrome patients with multiple BCC tumors (61). A total of 141 BCC lesions were studied. Patients received ALA (preincubation for 4 hours), after which half of the tumors were illuminated with blue light and the remainder with red light. Using a dose-escalation scheme, light delivery was increased gradually during three biweekly sessions over 4 months. Clearance rates at 2 months after the final PDT session were 98% for blue light and 93% for red light; these values were deemed statistically indistinguishable (61). Despite certain caveats (i.e., a longer follow-up is needed), the study established that blue light PDT is a promising modality and is worth pursuing in further studies to optimize BCC treatment.
Since red light and blue light PDT were shown to be equally effective for BCC tumors, a study to examine the neoadjuvant potential of Vit D could now be designed that is directly applicable to the American medical landscape. This study, now underway, is testing whether oral Vit D supplements administered prior to blue light PDT can improve BCC treatment outcomes (ClinicalTrials.gov NCT#03483441). Fifty patients with multiple BCC (Gorlin syndrome patients, or other patients with at least 3 BCC tumors simultaneously present) will be enrolled in a randomized, placebo-controlled, double-blinded study in which patients receive 3 blue light PDT treatments. Each patient serves as his/her own control. Prior to the first PDT treatment visit, serum levels of Vit D (calcidiol) are tested to determine the patient’s Vitamin D status (> 30 ng/mL normal; < 30 ng/ml deficient). To boost PpIX levels within tumors, all patients receive a pretreatment regimen of cholecalciferol (10,000 units/day), with the overall dosage adjusted according to the initial Vit D status (14 days for Vit D deficient patients, 5 days for Vit D normal patients). Prior to each PDT visit, the Research Pharmacy provides the patient with either Vit D pills or placebo pills; both the patients and the study physicians remain unaware of which pills are assigned. The primary study endpoint is BCC tumor volume, which is measured at each visit using a digital 3-D camera and computer image-processing software to facilitate exact measurement of tumor volumes (LifeViz Micro, Quantificare, Inc). The primary hypothesis of the study is that the rate of tumor shrinkage after a PDT session preceded by neoadjuvant Vit D will be greater than after PDT alone.
Vitamin D as a PDT neoadjuvant: Other mechanisms and other cancers
Up to this point, our discussion of Vit D as a PDT neoadjuvant has focused on mechanisms of action that occur within cancer cells, (e.g., heme-synthetic enzyme levels and mitochondrial PpIX accumulation). However, other mechanistic effects of Vit D might be useful to exploit, such as manipulation of the mesenchymal cells (fibroblasts) that surround most tumor nests. Cancer associated fibroblasts (CAFs) have been shown in numerous reports to contribute to poor treatment outcomes by promoting fibrosis, altering tumor metabolism, increasing metastasis, and suppressing immunogenicity (62–64). An example of the powerful influence of CAFs in the tumor microenvironment can be found in pancreatic ductal adenocarcinoma (PDAC), a type of cancer known for its dense, tumor-associated stromal components that comprise a majority of the total tumor mass in PDAC. The desmoplastic reaction driven by these tumor-associated CAFs leads to extensive fibrosis, high interstitial fluid pressures, and low blood perfusion, precluding an effective response to chemotherapy at reasonable doses due to poor drug delivery and intrinsic tumor resistance (65). The Vitamin D analog calcipotriol was shown to effectively modulate the PDAC-associated microenvironment by inducing epigenetic and transcriptomic changes in CAF precursors called cancer-associated pancreatic stellate cells (CAPSCs), consistent with their “reprogramming” to a non-cancer associated, or quiescent, state (66, 67). Vitamin D has also been shown to modulate chemokines, such as CXCL12 (also known as stromal derived factor 1, or SDF1) that are implicated in PDAC progression and whose secretion by CAFs triggers aggressive behavior of the PDAC cells (68).
In a paper by Anbil et al (69), a type of PDT regimen called “photodynamic priming” (PDP) was used together with chemotherapy and Vitamin D to produce a triple therapy that yielded an improved therapeutic outcome in PDAC orthotopic murine models. The chemotherapeutic agent was irinotecan in a nanoliposomal formulation (nal-IRI). The PDT treatment used liposomal verteporfin (Visudyne) as the photosensitizer, which was activated after a 1-hour drug-light interval via a 690 nm laser. Vit D combination treatment was performed using calcipotriol (100 μg/kg, i.p.), given daily for 4 days prior to the PDP treatment, and again for another 4 days following PDP. Tumor growth was monitored for 90 days. Many experimental conditions and associated controls were necessary in this extensive study. Two major findings with important implications were reported. First, the data showed that combining photodynamic (PDP) and biochemical manipulation (calcipotriol) to modulate the tumor microenvironment allowed a 75% dose reduction of the chemotherapeutic drug (nal-IRI), while still maintaining long-term treatment efficacy. Second, the combined PDP and calcipotriol treatment led to reduced CXCL12 secretion by CAFs, thereby suppressing the pro-tumorigenic signaling axis. In summary, modifying the tumor microenvironment with Vit D and PDP can allow for better tolerability by lowering the effective dose of a toxic chemotherapeutic drug. This approach represents a promising and relatively underexplored strategy to enable dose de-escalation and improve tolerability.
Although most of the hard data in our review on exogenous Vit D as a neoadjuvant have come from studies on skin cancer, there is no obvious reason why the neoadjuvant effects of Vit D should be limited to the skin. In fact, evidence exists that Vit D can modulate PpIX accumulation in other cancers of epithelial origin. For example, Rollakanti et al demonstrated that experimental breast carcinoma in mice (MDA-MB-231 cells implanted in the subcutaneous breast fat pad) can be effectively treated by preconditioning with calcitriol (1 μg/kg) for 3 days prior to ALA-PDT (70). In that study, neoadjuvant Vit D led to 3.3-fold enhancement of PpIX levels in the breast tumors relative to mice without preconditioning; cancer cell-specific death was increased as well. Clearly, ALA-PDT will need major technical improvements to become clinically useful for human breast cancer, given the challenge of delivering sufficient light to these deep tumors. However, a variety of other epithelial cancers occur in superficial surface tissues (mucosa) that line various body cavities. Tumors in these squamous epithelial tissues begin as superficial pre-carcinomas, before undergoing further neoplastic progression and deep invasion. Situations in which ALA-based photodiagnosis and PDT are showing great promise for early detection and treatment include dysplasia and carcinoma in-situ of the oral cavity (15, 14), cervix (12, 13, 11), and bladder (22, 23). For all of these conditions, the use of Vitamin D in combination with ALA-PDT make sense conceptually. Malignant brain cancers such as glioblastoma multiforme (GBM) are another good candidate for this approach. In the operating room, neurosurgeons find it very difficult to distinguish GBM from surrounding normal tissues because everything looks essentially the same. In this situation, ALA has proven quite useful and is now an FDA-approved technique, to help the surgeon visualize the malignant tissues to be excised (26, 71–73). Subsequent illumination (ALA-PDT) of the surgical bed might clean up any residual GBM cells, and a combination approach with Vit D might prove useful for both intial photodiagnosis (PDD) and for subsequent PDT. As evidence of feasibility, a promising in vitro study by Chen et al in 2014 showed that pretreatment of cultured glioma cells with calcitriol increased ALA-driven PpIX levels and subsequent photodynamic cell killing (74). This clearly suggests a need for further studies on neoadjuvant Vit D and ALA as a way to improve detection and treatment of GBM.
Conclusion
In this review, we have described in detail the mechanisms and clinical rationale for the use of differentiation-modifying agents (e.g., fluorouracil and Vitamin D) as neoadjuvants for ALA-based PDT of epithelial skin cancers. Vitamin D in particular is proving to be very safe and effective for this application. We suggest that the potential use of Vit D as a neoadjuvant for PDT should be explored and extended to a wider variety of human cancers.
Acknowledgments
Funding sources:
Grant P01CA084203 (T. Hasan and E. Maytin), and Grants 1 R01 CA204158 (E. Maytin), R21 CA187805 (E. Maytin), and R21 CA156227 (E. Maytin), all from National Cancer Institute, National Institutes of Health
Footnotes
This article is part of a Special Issue dedicated to Dr. Thomas Dougherty.
REFERENCES
- 1.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, et al. (2011) Photodynamic therapy of cancer: an update. CA Cancer J Clin 61, 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW and Hasan T (2010) Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev 110, 2795–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juzeniene A, Peng Q and Moan J (2007) Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci 6, 1234–1245. [DOI] [PubMed] [Google Scholar]
- 4.Abrahamse H and Hamblin MR (2016) New photosensitizers for photodynamic therapy. Biochem J 473, 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy JC, Pottier RH and Pross DC (1990) Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B 6, 143–148. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy JC and Pottier RH (1992) Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B 14, 275–292. [DOI] [PubMed] [Google Scholar]
- 7.Anand S, Ortel BJ, Pereira SP, Hasan T and Maytin EV (2012) Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Lett 326, 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braathen LR, Morton CA, Basset-Seguin N, Bissonnette R, Gerritsen MJ, Gilaberte Y, Calzavara-Pinton P, Sidoroff A, Wulf HC and Szeimies RM (2012) Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol 26, 1063–1066. [DOI] [PubMed] [Google Scholar]
- 9.Collier NJ, Haylett AK, Wong TH, Morton CA, Ibbotson SH, McKenna KE, Mallipeddi R, Moseley H, Seukeran D, Ward KA, et al. (2018) Conventional and combination topical photodynamic therapy for basal cell carcinoma: systematic review and meta-analysis. Br J Dermatol 179, 1277–1296. [DOI] [PubMed] [Google Scholar]
- 10.O’Connell KA, Okhovat JP and Zeitouni NC (2018) Photodynamic therapy for Bowen’s Disease (squamous cell carcinoma in situ) current review and update. Photodiagnosis Photodyn Ther 24, 109–114. [DOI] [PubMed] [Google Scholar]
- 11.Matoba Y, Banno K, Kisu I and Aoki D (2018) Clinical application of photodynamic diagnosis and photodynamic therapy for gynecologic malignant diseases: A review. Photodiagnosis Photodyn Ther 24, 52–57. [DOI] [PubMed] [Google Scholar]
- 12.Tao XH, Guan Y, Shao D, Xue W, Ye FS, Wang M and He MH (2014) Efficacy and safety of photodynamic therapy for cervical intraepithelial neoplasia: a systemic review. Photodiagnosis Photodyn Ther 11, 104–112. [DOI] [PubMed] [Google Scholar]
- 13.Maldonado Alvarado E, Osorio Peralta MO, Moreno Vazquez A, Martinez Guzman LA, Melo Petrone ME, Enriquez Mar ZI, Jovel Galdamez DE, Carrion Solana B, Balderas Martinez G, Parra E, et al. (2017) Effectiveness of Photodynamic Therapy in Elimination of HPV-16 and HPV-18 Associated with CIN I in Mexican Women. Photochem Photobiol 93, 1269–1275. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Daly L, Rudd G, Khan AP, Mallidi S, Liu Y, Cuckov F, Hasan T and Celli JP (2019) Development and evaluation of a low-cost, portable, LED-based device for PDT treatment of early-stage oral cancer in resource-limited settings. Lasers Surg Med 51, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vohra F, Al-Kheraif AA, Qadri T, Hassan MI, Ahmed A, Warnakulasuriya S and Javed F (2015) Efficacy of photodynamic therapy in the management of oral premalignant lesions. A systematic review. Photodiagnosis Photodyn Ther 12, 150–159. [DOI] [PubMed] [Google Scholar]
- 16.Allison RR, Cuenca RE, Downie GH, Camnitz P, Brodish B and Sibata CH (2005) Clinical photodynamic therapy of head and neck cancers-A review of applications and outcomes. Photodiagnosis Photodyn Ther 2, 205–222. [DOI] [PubMed] [Google Scholar]
- 17.Ahn PH, Finlay JC, Gallagher-Colombo SM, Quon H, O’Malley BW Jr., Weinstein GS, Chalian A, Malloy K, Sollecito T, Greenberg M, et al. (2018) Lesion oxygenation associates with clinical outcomes in premalignant and early stage head and neck tumors treated on a phase 1 trial of photodynamic therapy. Photodiagnosis Photodyn Ther 21, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Civantos FJ, Karakullukcu B, Biel M, Silver CE, Rinaldo A, Saba NF, Takes RP, Vander Poorten V and Ferlito A (2018) A Review of Photodynamic Therapy for Neoplasms of the Head and Neck. Adv Ther 35, 324–340. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoudi K, Garvey KL, Bouras A, Cramer G, Stepp H, Jesu Raj JG, Bozec D, Busch TM and Hadjipanayis CG (2019) 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J Neurooncol 141, 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermandel M, Quidet M, Vignion-Dewalle AS, Leroy HA, Leroux B, Mordon S and Reyns N (2019) Comparison of different treatment schemes in 5-ALA interstitial photodynamic therapy for high-grade glioma in a preclinical model: An MRI study. Photodiagnosis Photodyn Ther 25, 166–176. [DOI] [PubMed] [Google Scholar]
- 21.Dupont C, Vermandel M, Leroy HA, Quidet M, Lecomte F, Delhem N, Mordon S and Reyns N (2019) INtraoperative photoDYnamic Therapy for GliOblastomas (INDYGO): Study Protocol for a Phase I Clinical Trial. Neurosurgery 84, E414–E419. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Matsuyama H, Fujimoto K, Hirao Y, Watanabe H, Ozono S, Oyama M, Ueno M, Sugimura Y, Shiina H, et al. (2016) The clinical trial on the safety and effectiveness of the photodynamic diagnosis of non-muscle-invasive bladder cancer using fluorescent light-guided cystoscopy after oral administration of 5-aminolevulinic acid (5-ALA). Photodiagnosis Photodyn Ther 13, 91–96. [DOI] [PubMed] [Google Scholar]
- 23.Nakai Y, Inoue K, Tsuzuki T, Shimamoto T, Shuin T, Nagao K, Matsuyama H, Oyama M, Furuse H, Ozono S, et al. (2018) Oral 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: A multicenter phase III study. Int J Urol 25, 723–729. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko S and Kaneko S (2016) Fluorescence-Guided Resection of Malignant Glioma with 5-ALA. Int J Biomed Imaging 2016, 6135293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakomkin N and Hadjipanayis CG (2018) Fluorescence-guided surgery for high-grade gliomas. J Surg Oncol 118, 356–361. [DOI] [PubMed] [Google Scholar]
- 26.Hadjipanayis CG and Stummer W (2019) 5-ALA and FDA approval for glioma surgery. J Neurooncol 141, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortel B, Chen N, Brissette J, Dotto GP, Maytin E and Hasan T (1998) Differentiation-specific increase in ALA-induced protoporphyrin IX accumulation in primary mouse keratinocytes. Br J Cancer 77, 1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortel B, Sharlin D, O’Donnell D, Sinha AK, Maytin EV and Hasan T (2002) Differentiation enhances aminolevulinic acid-dependent photodynamic treatment of LNCaP prostate cancer cells. Br J Cancer 87, 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha AK, Anand S, Ortel BJ, Chang Y, Mai Z, Hasan T and Maytin EV (2006) Methotrexate used in combination with aminolaevulinic acid for photodynamic killing of prostate cancer cells. Br J Cancer 95, 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand S, Honari G, Hasan T, Elson P and Maytin EV (2009) Low-dose methotrexate enhances aminolevulinate-based photodynamic therapy in skin carcinoma cells in vitro and in vivo. Clin Cancer Res 15, 3333–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maytin EV, Anand S, Wilson C and Iyer K (2011) 5-Fluorouracil as an enhancer of aminolevulinate-based photodynamic therapy for skin cancer: New use for a venerable agent. In Proc SPIE Int Soc Opt Eng, Vol. 7886 (Edited by Kessel D), pp. 7886K7881–7888; doi:7810.1117/7812.874188. [Google Scholar]
- 32.Anand S, Rollakanti KR, Brankov N, Brash DE, Hasan T and Maytin EV (2017) Fluorouracil Enhances Photodynamic Therapy of Squamous Cell Carcinoma via a p53-Independent Mechanism that Increases Protoporphyrin IX levels and Tumor Cell Death. Mol Cancer Ther 16, 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maytin EV, Anand S, Riha M, Lohser S, Tellez A, Ishak R, Karpinski L, Sot J, Hu B, Denisyuk A, et al. (2018) 5-Fluorouracil Enhances Protoporphyrin IX Accumulation and Lesion Clearance during Photodynamic Therapy of Actinic Keratoses: A Mechanism-Based Clinical Trial. Clin Cancer Res 24, 3026–3035. [DOI] [PubMed] [Google Scholar]
- 34.Bickers DR, Pathak MA and Lim HW (2005) The Porphyrias. In Fitzpatrick’s Dermatology in General Medicine, 5th edition (Edited by Freedberg IM et al. ), pp. 1766–1803, McGraw Hill, New York. [Google Scholar]
- 35.Sato N, Moore BW, Keevey S, Drazba JA, Hasan T and Maytin EV (2007) Vitamin D enhances ALA-induced protoporphyrin IX production and photodynamic cell death in 3-D organotypic cultures of keratinocytes. J Invest Dermatol 127, 925–934. [DOI] [PubMed] [Google Scholar]
- 36.Cicarma E, Tuorkey M, Juzeniene A, Ma LW and Moan J (2009) Calcitriol treatment improves methyl aminolaevulinate-based photodynamic therapy in human squamous cell carcinoma A431 cells. Br J Dermatol 161, 413–418. [DOI] [PubMed] [Google Scholar]
- 37.Anand S, Wilson C, Hasan T and Maytin EV (2011) Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res 71, 6040–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rollakanti KR, Anand S, Davis SC, Pogue BW and Maytin EV (2015) Noninvasive Optical Imaging of UV-Induced Squamous Cell Carcinoma in Murine Skin: Studies of Early Tumor Development and Vitamin D Enhancement of Protoporphyrin IX Production. Photochem Photobiol 91, 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rollakanti K, Anand S and Maytin EV (2015) Topical calcitriol prior to photodynamic therapy enhances treatment efficacy in non-melanoma skin cancer mouse models. Proc SPIE Int Soc Opt Eng 9308, 93080Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maytin EV, Honari G, Khachemoune A, Taylor CR, Ortel B, Pogue BW, Sznycer-Taub N and Hasan T (2012) Vitamin D Combined with Aminolevulinate (ALA)-Mediated Photodynamic Therapy (PDT) for Human Psoriasis: A Proof-of-Principle Study. Isr J Chem 52, 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anand S, Hasan T and Maytin EV (2013) Mechanism of differentiation-enhanced photodynamic therapy for cancer: upregulation of coproporphyrinogen oxidase by C/EBP transcription factors. Mol Cancer Ther 12, 1638–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maytin EV and Habener JF (1998) Transcription factors C/EBP alpha, C/EBP beta, and CHOP (Gadd153) expressed during the differentiation program of keratinocytes in vitro and in vivo. J Invest Dermatol 110, 238–246. [DOI] [PubMed] [Google Scholar]
- 43.Maytin EV, Lin JC, Krishnamurthy R, Batchvarova N, Ron D, Mitchell PJ and Habener JF (1999) Keratin 10 gene expression during differentiation of mouse epidermis requires transcription factors C/EBP and AP-2. Dev Biol 216, 164–181. [DOI] [PubMed] [Google Scholar]
- 44.Anand S, Ebner J, Warren CB, Raam MS, Piliang M, Billings SD and Maytin EV (2014) C/EBP transcription factors in human squamous cell carcinoma: selective changes in expression of isoforms correlate with the neoplastic state. PLoS One 9, e112073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitamin D Edited by Feldman D, Pike JW and Glorieux FH (2005) Elsevier Academic Press, San Diego, 2 volumes, 1892 pages. [Google Scholar]
- 46.Tang JY, Fu T, Lau C, Oh DH, Bikle DD and Asgari MM (2012) Vitamin D in cutaneous carcinogenesis: part II. J Am Acad Dermatol 67, 817.e811–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang JY, Fu T, Lau C, Oh DH, Bikle DD and Asgari MM (2012) Vitamin D in cutaneous carcinogenesis: part I. J Am Acad Dermatol 67, 803.e801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burns EM, Elmets CA and Yusuf N (2015) Vitamin D and skin cancer. Photochem Photobiol 91, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reichrath J, Saternus R and Vogt T (2017) Endocrine actions of vitamin D in skin: Relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol Cell Endocrinol 453, 96–102. [DOI] [PubMed] [Google Scholar]
- 50.Slominski AT, Brozyna AA, Zmijewski MA, Jozwicki W, Jetten AM, Mason RS, Tuckey RC and Elmets CA (2017) Vitamin D signaling and melanoma: role of vitamin D and its receptors in melanoma progression and management. Lab Invest 97, 706–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anand S, Rollakanti KR, Horst RL, Hasan T and Maytin EV (2014) Combination of oral vitamin D3 with photodynamic therapy enhances tumor cell death in a murine model of cutaneous squamous cell carcinoma. Photochem Photobiol 90, 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin MR, Thys-Jacobs S, Chan FKW, Koberle LMC and Bilezikian JP (2005) Hypercalcemia due to Vitamin D toxicity In Vitamin D, Vol. II (Edited by Feldman D, Pike JW and Glorieux FH), pp. 1355–1377. Elsevier Academic Press, San Diego. [Google Scholar]
- 53.Kennel KA, Drake MT and Hurley DL (2010) Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc 85, 752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torezan L, Grinblat B, Haedersdal M, Valente N, Festa-Neto C and Szeimies RM (2018) A randomized split-scalp study comparing calcipotriol-assisted methyl aminolaevulinate photodynamic therapy (MAL-PDT) with conventional MAL-PDT for the treatment of actinic keratosis. Br J Dermatol 179, 829–835. [DOI] [PubMed] [Google Scholar]
- 55.Warren CB, Karai LJ, Vidimos A and Maytin EV (2009) Pain associated with aminolevulinic acid-photodynamic therapy of skin disease. J Am Acad Dermatol 61, 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ang JM, Riaz IB, Kamal MU, Paragh G and Zeitouni NC (2017) Photodynamic therapy and pain: A systematic review. Photodiagnosis Photodyn Ther 19, 308–344. [DOI] [PubMed] [Google Scholar]
- 57.Babes A, Sauer SK, Moparthi L, Kichko TI, Neacsu C, Namer B, Filipovic M, Zygmunt PM, Reeh PW and Fischer MJ (2016) Photosensitization in Porphyrias and Photodynamic Therapy Involves TRPA1 and TRPV1. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 5264–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaw U, Ilyas M, Bullock T, Rittwage L, Riha M, Vidimos A, Hu B, Warren CB and Maytin EV (2019) A regimen to minimize pain during blue light photodynamic therapy of actinic keratoses: Bilaterally controlled, randomized trial of simultaneous versus conventional illumination. J Am Acad Dermatol. 2019 September 13. doi: 10.1016/j.jaad.2019.09.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donaldson MR and Coldiron BM (2011) No end in sight: the skin cancer epidemic continues. Semin Cutan Med Surg 30, 3–5. [DOI] [PubMed] [Google Scholar]
- 60.Tom WL, Hurley MY, Oliver DS, Shah MR and Bree AF (2011) Features of basal cell carcinomas in basal cell nevus syndrome. Am J Med Genet A 155A, 2098–2104. [DOI] [PubMed] [Google Scholar]
- 61.Maytin EV, Kaw U, Ilyas M, Mack JA and Hu B (2018) Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: A bilaterally controlled comparison study. Photodiagnosis Photodyn Ther 22, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16, 582–598. [DOI] [PubMed] [Google Scholar]
- 63.McCarthy JB, El-Ashry D and Turley EA (2018) Hyaluronan, Cancer-Associated Fibroblasts and the Tumor Microenvironment in Malignant Progression. Front Cell Dev Biol 6, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu T, Zhou L, Li D, Andl T and Zhang Y (2019) Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment. Front Cell Dev Biol 7, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD and Hingorani SR (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, et al. (2014) Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sherman MH, Yu RT, Tseng TW, Sousa CM, Liu S, Truitt ML, He N, Ding N, Liddle C, Atkins AR, et al. (2017) Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc Natl Acad Sci U S A 114, 1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhan HX, Zhou B, Cheng YG, Xu JW, Wang L, Zhang GY and Hu SY (2017) Crosstalk between stromal cells and cancer cells in pancreatic cancer: New insights into stromal biology. Cancer Lett 392, 83–93. [DOI] [PubMed] [Google Scholar]
- 69.Anbil S, Pigula M, Huang H-C, Mallidi S, Broekgaarden M, Baglo Y, Simeone D, Mino-Kenudson M, Maytin EV, Rizvi I, et al. (2020) VDR activation and photodynamic priming enable durable low-dose chemotherapy for improved tolerability without compromising efficacy. Mol Cancer Ther, in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rollakanti KR, Anand S and Maytin EV (2015) Vitamin D enhances the efficacy of photodynamic therapy in a murine model of breast cancer. Cancer Med 4, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stummer W, Beck T, Beyer W, Mehrkens JH, Obermeier A, Etminan N, Stepp H, Tonn JC, Baumgartner R, Herms J, et al. (2008) Long-sustaining response in a patient with non-resectable, distant recurrence of glioblastoma multiforme treated by interstitial photodynamic therapy using 5-ALA: case report. J Neurooncol 87, 103–109. [DOI] [PubMed] [Google Scholar]
- 72.Yano H, Nakayama N, Ohe N, Miwa K, Shinoda J and Iwama T (2017) Pathological analysis of the surgical margins of resected glioblastomas excised using photodynamic visualization with both 5-aminolevulinic acid and fluorescein sodium. J Neurooncol 133, 389–397. [DOI] [PubMed] [Google Scholar]
- 73.Yamaguchi F, Takahashi H and Teramoto A (2007) Photodiagnosis for frameless stereotactic biopsy of brain tumor. Photodiagnosis Photodyn Ther 4, 71–75. [DOI] [PubMed] [Google Scholar]
- 74.Chen X, Wang C, Teng L, Liu Y, Chen X, Yang G, Wang L, Liu H, Liu Z, Zhang D, et al. (2014) Calcitriol enhances 5-aminolevulinic acid-induced fluorescence and the effect of photodynamic therapy in human glioma. Acta Oncol 53, 405–413. [DOI] [PubMed] [Google Scholar]