Abstract
OBJECTIVE
To evaluate whether African American (AA) men are at higher risk of reclassification in a large, prospective multi-institutional active surveillance (AS) cohort.
METHODS
The Canary Prostate Active Surveillance Study (PASS) is a protocol-driven active surveillance cohort with pre-specified prostate-specific antigen (PSA) and surveillance biopsies regimen. Men included in this study had Gleason Grade Group 1 or 2 at diagnosis, < 5 years between diagnosis and enrollment, and had undergone ≥ 1 surveillance biopsy. Risk of reclassification, defined as an increase in Gleason score on subsequent biopsy, was compared between AA and CA using cox proportional hazards models. For the subset of men undergoing delayed prostatectomy, rates of adverse pathology, defined as pT3a or greater or Gleason Grade Group 3 or greater, were compared for AA and CA.
RESULTS
Of 1,315 men, there were 89 (7%) AA and 1,226 (93%) CA. There were no differences in the rate of treatment for AA and CA. In multivariate models, AA race was not associated with the risk of reclassification (HR=1.16, 95% CI: 0.78 – 1.72). Among 441 men who had a prostatectomy after a period of AS, rate of adverse pathology was similar for AA and CA (46% vs 47%, p=0.99).
CONCLUSIONS
Among men on AS who follow a standardized protocol of regular PSA and biopsy, AA men were not at increased risk of pathologic reclassification on AS or adverse pathology at prostatectomy. AS appears to be an appropriate management strategy for AA men with favorable risk prostate cancer.
Keywords: prostate cancer, reclassification, active surveillance, race, African-American
INTRODUCTION
Over the last decade, the utilization of active surveillance (AS) has increased among men with low-risk prostate cancer (PCa) in the US.1, 2 There is strong evidence from many studies supporting the use of AS as an alternative to immediate radiation or radical prostatectomy for favorable-risk PCa3–6; However, there remains uncertainty about the utilization of AS among some high-risk groups, and although rates of AS among AA have increased over time, they remain far lower than rates among Caucasian (CA) men.7 African-American (AA) men have a higher incidence of PCa and increased potential for underlying aggressive disease compared to whites.8 Recent studies also suggest that the underlying biology of PCa among AA men may be distinct.9 Given this knowledge and the underrepresentation AA men in patient cohorts demonstrating favorable active surveillance outcomes for men with low-risk PCa, the appropriateness of AS for AA men is unclear.10
Several studies have evaluated the influence of AA race on PCa outcomes among AS-eligible, low-risk men who underwent radical prostatectomy (RP). The results of these studies are inconsistent, with the majority reporting a significant positive association between AA race and adverse outcomes after prostatectomy, and several reporting no differences in outcomes between AA and Caucasian (CA) men.10 The comparability of these studies to AS patient populations is limited as all men underwent surgery and there may be racial disparities in referral patterns, patient selection, and treatment preferences and recommendations.7, 11, 12 Few studies to date have evaluated the association of AA race with risk of progression among AS patients13–17, many of which are based on single-institution practices with small numbers of AA men. Here we evaluate the association of AA race with risk of disease reclassification and adverse pathology among men managed by AS in the multicenter Canary Prostate Active Surveillance Study (PASS).18
METHODS
The Canary PASS cohort utilizes a standardized active surveillance protocol (clinicaltrials.gov NCT000756665). The PASS protocol includes PSA measured every 3 months and clinic visits occurring every 6 months. The protocol also includes ultrasound-guided prostate biopsies with the first surveillance (confirmatory) biopsy between 6 and 12 months after diagnosis followed by a biopsy at 24 months after diagnosis and at 2-year intervals thereafter. Other tests, including genomic testing and multiparametric magnetic resonance imaging (mpMRI), may be performed at the discretion of participating clinicians. Data collected include Gleason score, number of cores at each biopsy, number of cores positive for cancer at each biopsy, prostate volume, and the corresponding PSA values for diagnostic and any follow-up biopsies prior to enrollment. At enrollment in PASS, patients provided self-reported race/ethnicity, family history of PCa, and smoking status, and clinic staff measured height and weight to calculate body mass index (BMI). All men provided written informed consent prior to enrollment in PASS, and study procedures were approved by the local institutional review board for each study site.
Men included in this analysis were enrolled as of February 2018, had a prostate cancer diagnosis within 5 years of enrollment, had Gleason Grade Group ≤ 2 (≤ 3+4) cancer, and had at least one surveillance biopsy after diagnostic biopsy (1,434 participants). For this analysis, the cohort was limited to AA and CA men, leaving 1,351 participants for analysis.
Outcomes
The primary outcome for these analyses was time to disease reclassification while on AS. Reclassification was defined as any increase in primary or secondary Gleason grade at re-biopsy. As a secondary outcome, rates of adverse pathology at RP, defined as Gleason Grade Group ≥ 3, ≥ pT3a disease, or pN1, were evaluated between AA and CA men.
Statistical Methods
Descriptive statistics were used to characterize the study sample. Differences between AA and CA were evaluated using Wilcoxon sign rank tests for continuous variables and Fisher’s tests for categorical variables.
Kaplan Meier curves were plotted to examine differences in reclassification- and treatment-free probability by race (AA vs CA). Participants without reclassification were censored at date of last study contact, treatment, or 2 years after their last biopsy, whichever came first. For treatment-free survival, participants without treatment were censored at date of last study contact. Cox proportional hazards models (PH) were used to estimate the unadjusted and covariate-adjusted hazards ratios for the association between AA race and risk of reclassification. Covariate adjusted models considered the following variables: diagnostic Gleason Grade Group (1 vs 2), BMI, percentage of cores positive for cancer (calculated as the number of cores positive for cancer divided by the total number of cores collected), prostate size, diagnostic age, diagnostic PSA, family history of PCa, and smoking status (current/former vs never). The baseline hazard for each Cox PH model was stratified by study site to account for site-by-site differences in reclassification rates. Tests of proportionality confirmed that PH assumptions were met. Sensitivity analyses were performed among the subset of men with Gleason Grade Group 1 PCa.
To evaluate whether bias related to compliance with the surveillance protocol may have impacted results, exploratory analyses compared the rates of loss to follow up, number of surveillance biopsies, annual number of PSA values, timing and rates of treatment, and compliance to the protocol-directed biopsy schedule between AA and CA participants. All analyses were 2-tailed with alpha set at 0.05 and were performed using SAS version 9.4 and R version 3.3.0.
RESULTS
Of the 1,315 men in this study, 89 (7%) self-identified as AA. The median age of men overall was 63 years (95% CI: 58 – 67). AA and CA men were comparable with respect to Gleason Grade Group at diagnosis, the percentage of total cores positive for prostate cancer, prostate size, family history of prostate cancer and smoking status. Compared to CA, AA had significantly higher median PSA and PSA density at diagnosis and body mass index at study entry (Table 1).
Table 1.
PASS participant characteristics by race
| Variable | AA only (n=89) | CA only (n=1226) | P-value4 |
|---|---|---|---|
| n (%) or median [IQR] | n (%) or median [IQR] | ||
| Age (years)1 | 63 [55, 67] | 63 [58, 67] | 0.30 |
| Gleason1 | 0.42 | ||
| Grade group 1 | 80 (90) | 1129 (92) | |
| Grade group 2 | 9 (10) | 97 (8) | |
| Very low Risk | 35 (39.3) | 558 (45.5) | |
| % Positive cores1,2 | 8 [8, 17] | 10 [8, 17] | 0.26 |
| Prostate size (cm3)3 | 42 [32, 64] | 42 [31, 57] | 0.14 |
| PSA (ng/ml)1 | 5.6 [4.4, 7.8] | 4.9 [3.8, 6.4] | <.001 |
| PSA density1 | 0.14 [0.09, 0.18] | 0.11 [0.08, 0.16] | 0.01 |
| BMI (kg/m2)3 | 28 [26, 32] | 27 [25, 30] | 0.04 |
| Family history of PCa3 | 28 (32) | 342 (28) | 0.47 |
| Smoking Status3 | 0.10 | ||
| Current/Former | 45 (51) | 507 (41) | |
| Never | 44 (49) | 719 (59) |
Corresponds to the value at prostate cancer diagnosis
% Positive cores is defined as # cores positive for cancer/total # cores collected
Corresponds to the value at enrollment in PASS
P-value from Wilcoxon rank sum test for continuous variables, and Fisher’s exact test for categorical variables.
Median length of follow-up among men with no reclassification was 3.9 years (IQR 2.2 to 5.9) overall, with AA having approximately 1 year less follow-up compared to CA (Table 2), which is consistent with the relatively recent addition of PASS clinical sites serving racially diverse populations. The median number of surveillance biopsies on study, number of PSA per year and use of mpMRI were similar for AA and CA men. Overall treatment rates were comparable for AA and CA men, though AA men were more likely to undergo radiation and less likely to undergo radical prostatectomy than CA men (Table 2).
Table 2.
Clinical, surveillance and treatment characteristics of AA and CA men in PASS.
| Variable | AA only (n=89) | CA only (n=1226) |
|---|---|---|
| n (%) or median [IQR] | n (%) or median [IQR] | |
| Length of Follow-up, years1 | 2.9 [2.0, 4.6] | 3.9 [2.2, 6.0] |
| Number surveillance biopsies | 2 [1, 2] | 2 [1,3] |
| Number PSA/year | 3 [2, 4] | 3 [3, 4] |
| mpMRI use | 23 (26) | 365 (30) |
| Grade reclassification | 30 (34) | 419 (34) |
| Treatment (any) | 30 (34) | 411 (34) |
| RP | 13 (43) | 233 (57) |
| RAD | 17 (57) | 155 (38) |
| ADT/Other | 0 | 23 (5) |
| Adverse Pathology at RP* | 6/13 (46) | 109/233 (47) |
Among men who did not reclassify
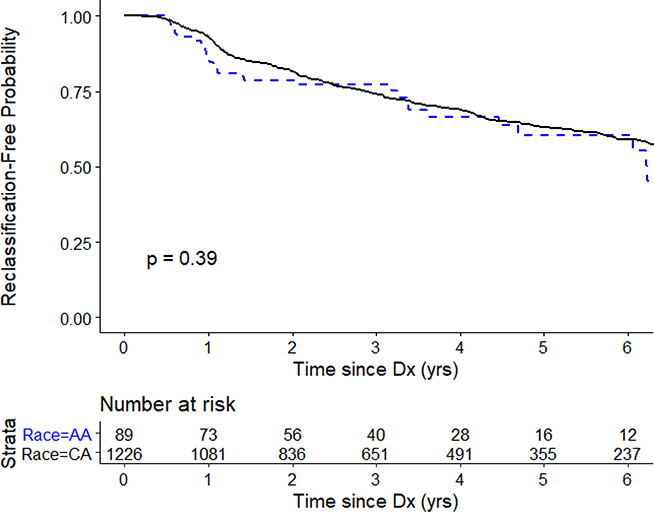
Overall, there was no difference in reclassification-free survival between AA and CA men (p=0.39; Figure 1). Table 3 gives the unadjusted and multivariable adjusted hazards of pathologic reclassification at biopsy among AA men relative to CA men on AS. On unadjusted analysis, AA men had a similar risk of reclassification as CA men. In multivariate models adjusted for diagnostic biopsy and clinical variables, AA race was not significantly associated with the risk of reclassification (HR=1.16, 95% CI: 0.78 – 1.72). Results were nearly identical for a sensitivity analysis limited to men with Grade Group 1 PCa at diagnosis (data not shown). Among the subset of men treated via RP (n=13 AA, n=223 CA), rates of adverse pathology at RP were similar for AA and CA men (46% vs 47%; p=0.99).
Figure 1.
Kaplan-Meier curve of reclassification-free survival by race.
Table 3.
Predictors of grade reclassification on surveillance biopsy in PASS.
| Variable | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR (95% CI)^ | P-value^ | HR (95% CI)^ | P-value^ | |
| AA race | 1.17 (0.79 – 1.72) | 0.44 | 1.16 (0.78 – 1.72) | 0.46 |
| Age1 | 1.02 (1.00 – 1.03) | 0.01 | 1.03 (1.01 – 1.04) | <0.001 |
| Gleason Grade Group1 | 0.97 (0.66 – 1.41) | 0.86 | 0.66 (0.45 – 0.98) | 0.04 |
| % Positive cores1 | 1.37 (1.26 – 1.47) | <0.001 | 1.34 (1.24 – 1.45) | <0.001 |
| Prostate size | 0.56 (0.46 – 0.69) | <0.001 | 0.40 (0.32 – 0.50) | <0.001 |
| PSA1 | 1.37 (1.17 – 1.61) | <0.001 | 1.67 (1.41 – 1.97) | <0.001 |
| BMI2 | 1.03 (1.01 – 1.05) | 0.01 | 1.05 (1.02 – 1.07) | <0.001 |
| Family history2 | 0.94 (0.85 – 1.25) | 0.56 | 0.96 (0.77 – 1.19) | 0.71 |
At diagnosis
At enrollment
Hazard ratios, 95% confidence intervals and p-values from Cox proportional hazard models
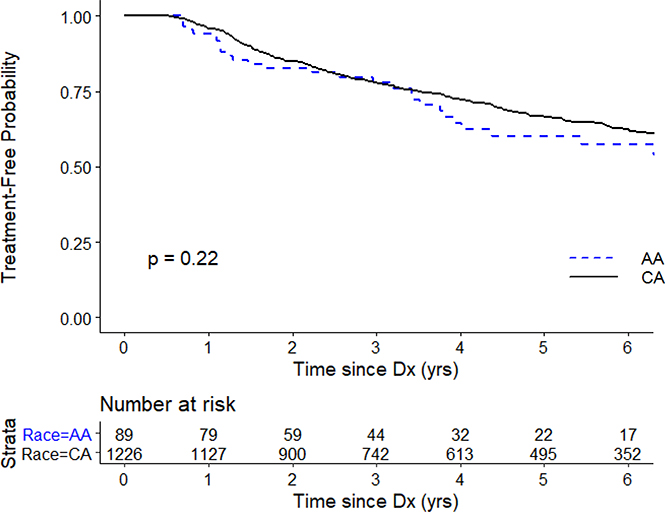
We conducted several analyses to explore possible bias related to biopsy timing and compliance with the active surveillance protocol. There were no differences between AA and CA men in the number of surveillance biopsies or annual number of PSA screenings. Overall treatment rates (35% vs 34%; p=0.99) and timing of treatment were similar for AA and CA men (Figure 2). In addition, the proportions of surveillance biopsies conducted according to the protocol-directed schedule was similar between AA and CA men (data not shown).
Figure 2.
Kaplan-Meier curve of treatment-free survival by race.
DISCUSSION
Limited data exist on the safety and efficacy of AS as a management strategy for AA men with favorable risk PCa at diagnosis. Concerns about the use of AS for AA men are driven by the significant disparities in PCa incidence and mortality, which have remained consistent even in the era of PSA screening.19 In this analysis from a large, prospective multi-institutional cohort, we found similar rates of disease reclassification among AA and CA men with favorable-risk prostate cancer managed by a standardized AS protocol. In addition, among the small subset of men who ultimately underwent surgical treatment after a period of AS, rates of adverse pathology at prostatectomy were similar between AA and CA men.
Numerous studies have reported conflicting outcomes for AA men diagnosed with low-risk prostate cancer, the majority of which are based on populations of AS-eligible men who underwent RP. Many of these studies report significant increases in risks of adverse pathologic findings, recurrence, and cancer-specific mortality among AA compared to men of other racial groups.10 In contrast several studies reported no differences in stage-for stage PCa outcomes by race20–23, which is consistent with our finding of similar rates of adverse pathology at RP between AA and CA men in PASS. Studies limited to men who were surgically treated may be biased by factors associated with the selection of treatment. AA men are less likely to undergo primary RP and are therefore underrepresented in in these studies.7, 11, 12 In addition, reported racial disparities in prostate cancer outcomes among RP patients may be related to differences in access to care.24 In fact, studies conducted in populations with similar access to care have reported no differences in stage-for-stage prostate cancer outcomes for AA and CA.20–22
Few studies to date have evaluated the association of race with PCa outcomes among AS populations.13–17 Of the five prior studies, four reported a significant positive association between AA ethnicity and risk of reclassification13, or disease progression on AS14, 15, 17. Most studies were based on retrospectively collected data from a single-institution with smaller numbers of AA men and are limited by short follow-up. Comparisons between our study and those reported in the literature are further complicated by the use of different definitions of disease progression, the majority of which used an aggregate of changes in disease volume/grade or an increase in PSA.14, 15, 17 Only one prior study among men with very low-risk PCa assessed reclassification by grade, and found a 3-fold increased risk of grade reclassification among AA compared to CA.13 Of note, this cohort had a substantially lower rate of grade reclassification among CA patients at three years (16%) compared to PASS and other AS cohorts.25 To evaluate whether variations in the underlying risk of upgrading could account for the difference between our results and this prior study, we conducted an additional analysis restricted to PASS patients diagnosed with very low risk PCa (T1c, Gleason score ≤6/grade group 1, PSA <10 ng/mL, < 3 prostate biopsy cores positive, ≤50% cancer in each core, and PSA density <0.15 ng/mL/g). Within the subset of men who met the criteria for very low risk PCa at diagnosis (n=593), there remained no association of AA race with risk of grade reclassification (multivariate HR=0.75, 95% CI: 0.32 – 1.75; p=0.51).
It is important to note several potential sources of bias that may be impacting associations of AA race and outcomes in studies of active surveillance. Racial disparity in the intensity of surveillance (i.e., the frequency of PSA/digital rectal exam (DRE) or biopsy) or conduct of surveillance (e.g. biopsy compliance, the method of biopsy (e.g. use of mpMRI), or differences in pathologic review 13), could impact the likelihood of detecting progression events on AS. In particular, retrospective studies of active surveillance, in which the conduct of surveillance activities was not protocol-directed, may be subject to these biases, although few studies present data to allow evaluation of their potential presence.
There are several elements of the PASS cohort that serve as strengths for this study. First is the use of a standardized active surveillance protocol across all clinical sites. PSA/DRE tests and surveillance biopsies are collected at pre-specified, protocol-directed time-points in PASS18, allowing for a similar opportunity to detect reclassification among all men on PASS and minimizing the potential for screening-related ascertainment bias. Additional strengths for this study include the intentionally broad eligibility criteria of the PASS cohort, which includes diagnostic Gleason Grade Group 1 and 2 with no limitations on PSA/PSA density or tumor burden, increases the generalizability of these results to the current population of men eligible for and electing to undergo AS. In addition, the large multicenter design of PASS includes 10 clinical sites throughout North America; thus, these results are less likely to be impacted by the judgement of an individual or small group of providers.
Some limitations also deserve mention. Although the number of African Americans in this study is the largest to date (n=89), it is still modest, and the number of AA men who underwent surgery is small; thus, the findings reported here should be interpreted cautiously. Recent efforts to improve diversity in PASS include the addition clinical sites serving more diverse populations. It is also notable that the length of overall follow-up in PASS is modest (median = 3.9 years), although longer than previous studies14–17, which limits the evaluation of intermediate/long-term oncologic outcomes of interest. In addition, the length of follow-up time is somewhat shorter for AA than for CA men (2.9 vs. 3.9 years). Given that drop-out rates and the time to treatment and reclassification were similar between AA and CA men in PASS, this difference is most likely due to the relatively recent addition of PASS clinical sites serving racially diverse populations, which have accrued less follow-up time. Continued enrollment of patients from these sites is ongoing in PASS, and it will be important to confirm these findings once more follow-up time has accrued.
In conclusion, in this prospective cohort of men on AS who follow a standardized protocol of regular PSA and biopsy, AA men did not demonstrate a higher risk of adverse pathologic reclassification while on AS. Furthermore, those who eventually underwent RP did not have a higher risk of adverse pathology by delaying their therapy. The results from Canary PASS support the use of a standardized AS protocol among AA men with favorable risk PCa.
Funding:
Supported by the Canary Foundation, Department of Defense Prostate Cancer Research Program W81XWH1410595, Institute for Prostate Cancer Research
Key of Definitions: African American race and risk of reclassification in PASS
- AA
African American
- AS
Active Surveillance
- CA
Caucasian
- DRE
Digital Rectal Exam
- mpMR
multiparametric magnetic resonance
- PASS
Prostate Cancer Active Surveillance Study
- PCa
Prostate Cancer
- PH
Proportional Hazards
- PSA
Prostate Specific Antigen
- RP
Radical Prostatectomy
REFERENCES
- 1.Mahal BA, Butler S, Franco I et al. : Use of Active Surveillance or Watchful Waiting for Low-Risk Prostate Cancer and Management Trends Across Risk Groups in the United States, 2010–2015. JAMA, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Carroll PR: Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA, 314: 80, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Tosoian JJ, Mamawala M, Epstein JI et al. : Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol, 33: 3379, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klotz L, Vesprini D, Sethukavalan P et al. : Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol, 33: 272, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Newcomb LF, Thompson IM Jr., Boyer HD et al. : Outcomes of Active Surveillance for Clinically Localized Prostate Cancer in the Prospective, Multi-Institutional Canary PASS Cohort. J Urol, 195: 313, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welty CJ, Cowan JE, Nguyen H et al. : Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol, 193: 807, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Butler S, Muralidhar V, Chavez J et al. : Active Surveillance for Low-Risk Prostate Cancer in Black Patients. N Engl J Med, 380: 2070, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSantis CE, Miller KD, Goding Sauer A et al. : Cancer statistics for African Americans, 2019. CA Cancer J Clin, 69: 211, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Faisal FA, Sundi D, Tosoian JJ et al. : Racial Variations in Prostate Cancer Molecular Subtypes and Androgen Receptor Signaling Reflect Anatomic Tumor Location. Eur Urol, 70: 14, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gokce MI, Sundi D, Schaeffer E et al. : Is active surveillance a suitable option for African American men with prostate cancer? A systemic literature review. Prostate Cancer Prostatic Dis, 20: 127, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Krishna S, Fan Y, Jarosek S et al. : Racial Disparities in Active Surveillance for Prostate Cancer. J Urol, 197: 342, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Gray PJ, Lin CC, Cooperberg MR et al. : Temporal Trends and the Impact of Race, Insurance, and Socioeconomic Status in the Management of Localized Prostate Cancer. Eur Urol, 71: 729, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Sundi D, Faisal FA, Trock BJ et al. : Reclassification rates are higher among African American men than Caucasians on active surveillance. Urology, 85: 155, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odom BD, Mir MC, Hughes S et al. : Active surveillance for low-risk prostate cancer in African American men: a multi-institutional experience. Urology, 83: 364, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Iremashvili V, Soloway MS, Rosenberg DL et al. : Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. J Urol, 187: 1594, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Davis JW, Ward JF 3rd, Pettaway CA et al. : Disease reclassification risk with stringent criteria and frequent monitoring in men with favourable-risk prostate cancer undergoing active surveillance. BJU Int, 118: 68, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abern MR, Bassett MR, Tsivian M et al. : Race is associated with discontinuation of active surveillance of low-risk prostate cancer: results from the Duke Prostate Center. Prostate Cancer Prostatic Dis, 16: 85, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Newcomb LF, Brooks JD, Carroll PR et al. : Canary Prostate Active Surveillance Study: design of a multi-institutional active surveillance cohort and biorepository. Urology, 75: 407, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly SP, Rosenberg PS, Anderson WF et al. : Trends in the Incidence of Fatal Prostate Cancer in the United States by Race. Eur Urol, 71: 195, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dess RT, Hartman HE, Mahal BA et al. : Association of Black Race With Prostate Cancer-Specific and Other-Cause Mortality. JAMA Oncol, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leapman MS, Freedland SJ, Aronson WJ et al. : Pathological and Biochemical Outcomes among African-American and Caucasian Men with Low Risk Prostate Cancer in the SEARCH Database: Implications for Active Surveillance Candidacy. J Urol, 196: 1408, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jalloh M, Myers F, Cowan JE et al. : Racial variation in prostate cancer upgrading and upstaging among men with low-risk clinical characteristics. Eur Urol, 67: 451, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Resnick MJ, Canter DJ, Guzzo TJ et al. : Does race affect postoperative outcomes in patients with low-risk prostate cancer who undergo radical prostatectomy? Urology, 73: 620, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Barocas DA, Penson DF: Racial variation in the pattern and quality of care for prostate cancer in the USA: mind the gap. BJU Int, 106: 322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue LYT, Lin DW, Newcomb LF et al. : Comparative Analysis of Biopsy Upgrading in Four Prostate Cancer Active Surveillance Cohorts. Ann Intern Med, 168: 1, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]