Abstract
Aims:
Medicare patients with metastatic or surgically unresectable urothelial carcinoma (mUC) often receive platinum-based chemotherapy as first line of therapy (LOT) but invariably progress, requiring additional LOTs and healthcare resource use (HCRU). To better understand the evolving mUC treatment landscape, we estimated the economic burden of chemotherapy-based mUC treatments among US Medicare patients.
Methods:
Newly diagnosed Medicare patients with mUC were identified from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database. Patients were followed from diagnosis to death, disenrollment, or end of study to characterize LOTs (first [LOT1], second [LOT2], and third+ [LOT3+]). Kaplan-Meier methods were used to estimate overall survival (OS) by LOT. HCRU and mean costs were reported over the follow-up period, LOT duration, or maximum LOT received.
Results:
Among 1,873 eligible patients with mUC (median age, 77 years; median follow-up, 7.5 months), 1,035 (55%) received no chemotherapy. Among chemotherapy-treated patients, 61% had LOT1 only, 25% had LOT1 and LOT2 only, and 14% had ≥3 LOTs. Median OS was 8.1 months, range 4.3 (untreated) to 29.8 (LOT3+) months. HCRU frequency increased with additional LOTs. Mean cumulative per-patient cost was $82,912 for all patients, increasing with additional LOTs (untreated, $57,207; LOT1, $99,213; LOT2, $125,190; LOT3+, $163,884). Mean per-patient per-month cost was $18,827 for all patients, decreasing with increasing number of LOTs received (untreated, $27,211; LOT1, $9,601; LOT2, $7,325; LOT3+, $6,017).
Limitations:
Potential for treatment misclassification when using the algorithm defining LOTs and non-generalizability of results to younger patients.
Conclusions:
Over 50% of Medicare patients with mUC received no chemotherapy. Among chemotherapy-treated patients, most received only one LOT. Additional LOTs led to higher mean costs and HCRU, but as patients were followed longer, monthly costs decreased. As treatments evolve to include immuno-oncology agents, these findings provide a clinically relevant economic benchmark for mUC treatment across different traditional LOTs.
Keywords: Metastatic urothelial carcinoma, SEER-Medicare, healthcare resource use, economic burden, line of therapy
Introduction
Urothelial carcinoma (UC) is the ninth most common cancer worldwide, with 430,000 new cases diagnosed annually, resulting in approximately 145,000 deaths globally each year [1,2]. In 2017, there were an estimated 79,030 new cases of bladder cancers in the United States (US), the majority of which were UC, and 16,870 deaths; 75% of cases occurred in people 65 years or older [3]. Among those diagnosed with UC, about 20% of patients present with de novo metastatic or surgically unresectable UC (mUC). Furthermore, approximately 50% of patients presenting with muscle-invasive UC develop metastatic recurrence after initial therapy for clinically localized disease [4].
Prior to the emergence of immuno-oncology agents, platinum-based chemotherapy was the standard first line of therapy (LOT) for mUC, but some patients may be ineligible for such therapy due to comorbidities, including poor renal function [5]. Although UC is relatively sensitive to chemotherapy, overall survival is poor for patients with mUC as chemotherapy does not result in long-term cure, even among those who initially respond [6,7]. Also, treatment options for patients who do not tolerate or are refractory to chemotherapy remain limited [8]. Before approval of an immuno-oncology agent in 2016 [9], the only approved treatment options for patients who progressed after platinum-based chemotherapy were second-line chemotherapies such as paclitaxel, docetaxel, gemcitabine, pemetrexed, alkylating agents, or possibly anthracyclines [10]. However, response rates and treatment outcomes are even more limited with second-line chemotherapies [10,11].
Since May 2016, the US Food and Drug Administration (FDA) has approved 5 immuno-oncology therapies for mUC: the programmed death 1 (PD-1) targeting monoclonal antibodies nivolumab and pembrolizumab, and the PD-1 ligand 1 (PD-L1) targeting monoclonal antibodies atezolizumab, durvalumab, and avelumab. All 5 agents were approved in the second-line setting, where clinical studies have shown that overall response rates for anti–PD-1 and anti–PD-L1 therapies are between 15% and 31% [12–16]. Responses in many patients have remained durable, and the 12-month overall survival rates were high (36%–51%) [12,13,15,16]. In addition, atezolizumab and pembrolizumab have been approved for the first-line treatment of cisplatin-ineligible patients with PD-L1–positive mUC based on clinical trials where overall response rates among patients with the highest expression of PD-L1 ranged between 28% and 38% [17,18].
Total US medical care expenditures for bladder cancers were $4 billion in 2010 and are expected to rise to $5 billion by 2020 [19]. Of the various cancer types, bladder cancer (including muscle-invasive and non–muscle-invasive bladder cancer) was reported to have the highest lifetime all-treatment costs per patient in the US, ranging from $96,000 to $187,000 in 2001 [19,20]. Post diagnosis, annual continuing care costs per patient ranged from $5,000 to $17,500 depending on stage, being highest for patients with muscle-invasive bladder cancer [21]. Disease progression is a major driver of cost, with muscle-invasive bladder cancer treatment costs reported to be 2 to 3 times greater than non–muscle-invasive bladder cancer treatment costs [22]. While the economic burden of bladder cancer at various stages has been studied, to our knowledge, the economic trajectories of patients with advanced bladder cancers receiving sequential LOTs have yet to be evaluated. This is relevant for patients with mUC, as the majority will require several sequential LOTs due to disease progression. Likewise, the economic burden associated with Medicare patients who may not receive chemotherapy has yet to be evaluated.
This analysis estimated the medical costs and healthcare resource use (HCRU) associated with the current LOTs in Medicare patients in the US to provide a benchmark for treatment-related cost estimates by lines (and number of lines) of chemotherapy received.
Methods
Database
Since the median age at bladder cancer diagnosis is 72 years old and most new cases occur in people age 65 years or older, this analysis was conducted using data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, which links information from the National Cancer Institute’s SEER cancer registries and Medicare claims data from the Centers for Medicare and Medicaid Services (CMS). The SEER program collects cancer incidence and mortality rates from 18 tumor registries across the US that cover 28% of the population [23,24] and provide clinical, demographic, and mortality information for patients diagnosed with cancer. Medicare claims provide information on healthcare services and their costs, including diagnosis and treatment services, which are provided to and covered for Medicare beneficiaries from the time of Medicare eligibility until death.
Study design
This was a retrospective cohort study of patients newly diagnosed with mUC (transitional cell histology in the bladder and upper tract urinary system) between 2004 and 2011. Included patients had TNM) staging of T4b/N0/M0 (i.e., the tumor has grown through the bladder wall into the pelvic or abdominal wall [bladder cancer] or the tumor has spread to nearby organs or into the outer layer of fat on the kidney [cancer of the renal pelvis and ureter], but disease has not spread to the regional lymph nodes and has not metastasized); any T/N1-3/M0 (i.e., regardless of how far the primary tumor has grown through the bladder wall or the lining of the renal pelvis or ureter, the disease has spread to one or more regional lymph nodes and/or the common iliac lymph nodes, but has not metastasized); or any T/any N/M1 (i.e., there are distant metastases, regardless of how far the primary tumor has grown through the bladder wall or the lining of the renal pelvis or ureter, or whether it has spread to the regional lymph nodes). Patients were included if they were 66 years or older at diagnosis and continuously enrolled in Medicare Parts A and B in the 12 months before diagnosis. Patients were excluded if they were enrolled in a health maintenance organization (HMO) in the 12 months before diagnosis, received a postmortem diagnosis, had an unknown diagnosis date, or had another cancer (except non-melanoma skin cancer) in the 5 years before mUC diagnosis. Patients were followed from mUC diagnosis until death or censoring due to the end of the study period (December 31, 2013), HMO enrollment, or Medicare disenrollment (whichever occurred first).
Lines of therapy
Using Medicare claims data, LOTs were defined according to the following algorithm. First line (LOT1) was the first chemotherapy with or without radiation therapy used post diagnosis. A subsequent LOT (i.e., second line [LOT2] and third line [LOT3]) was defined as: (1) new chemotherapy added more than 30 days after the start date of the prior chemotherapy (if an additional chemotherapy was added after 30 or fewer days of prior chemotherapy, it was considered a combination treatment regimen as part of the original treatment), or (2) the same chemotherapy resumed after a more than 90-day gap between treatments [25,26]. Treatment patterns of patients with mUC were analyzed in terms of the maximum number and duration of LOTs (no chemotherapy/LOT1/LOT2/LOT3).
Baseline characteristics
Information on patients’ demographic characteristics was extracted from the SEER registries, including age at diagnosis, sex, race/ethnicity, marital status, and census location. Patients’ comorbidities were examined from Medicare claims in the 12 months before the mUC diagnosis and classified using the Charlson Comorbidity Index (CCI). Medicare claims in the 12 months before diagnosis were also used to create a performance status proxy, defined as having any of the following claims: wheelchair use, oxygen use, walking aid, hospital bed, hospice, skilled nursing facility, or hospitalization.
Overall survival
Time from diagnosis to death was reported using the Kaplan–Meier method and stratified with respect to LOT.
Costs and HCRU
Healthcare resource use (bladder biopsies; cystoscopy; bone scan; computed tomography [CT] scan; magnetic resonance imaging [MRI] scan; positron emission tomography-computed tomography [PET-CT] scan; and hospital, emergency department, and intensive care unit [ICU] admissions) were reported as proportion of patients receiving each HCRU, mean per patient, and mean per patient per month (PPPM) in the full sample and by LOT over the entire follow-up period. Costs obtained from paid amounts within Medicare claims (total, inpatient, outpatient, physician, home health, hospice, durable medical equipment, prescription drug costs, and HCRU-specific costs) were standardized to 2014 US dollars and reported as cumulative mean costs per patient in the full sample and by LOT: (1) over the entire follow-up period, and (2) over the treatment duration of each LOT, wherein the latter costs incurred were attributed to each LOT (Figure 1, top panel). Since costs incurred between the different LOTs may arguably be attributed to the previous LOT (e.g., managing/treating toxicities that may have been due to a previous LOT) or the subsequent LOT (e.g., monitoring for progression that requires a subsequent LOT), we conducted a sensitivity analysis that used a different cost attribution method (Figure 1, bottom panel).
Figure 1.
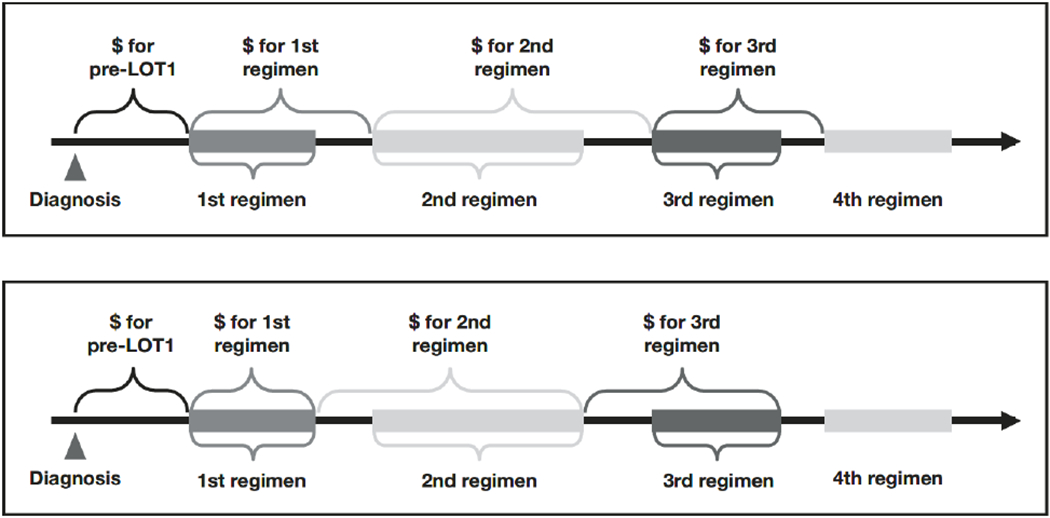
Cost attribution to each line of therapy (LOT) according to main (top panel) and sensitivity analysis (bottom panel).
Statistical analysis
For categorical variables, frequency and percentage distributions were reported. Statistical comparisons were conducted between cohorts using Pearson chi-square tests or Fisher exact tests when any frequencies were less than 5. For continuous variables, median, interquartile range (IQR), mean, and standard deviation were reported. The Kolmogorov–Smirnov test was used to test the distribution of the variables. T-tests were used for normally distributed variables and Mann–Whitney tests for variables not normally distributed. All analyses were conducted using SAS v.9.4 (SAS Institute Inc., Cary, NC).
Results
Study population
A total of 33,379 patients were identified with a histologically confirmed diagnosis of mUC. Of those, 1,873 patients met the study inclusion criteria (Figure 2). Of the included patients, 16% had T4b, 51% had M1, and 53% had N1 to N3 disease (Table 1). The median age at diagnosis was 77 years, 63% of the patients were male, over half were married, and 84% were non-Hispanic whites. Before diagnosis, 71% of the patients had a CCI score of 0 to 1 and 26% had a poor performance proxy indicator. Most patients were in the West or Northeast US census regions. Compared with patients receiving LOT3+, patients who did not receive chemotherapy were older at diagnosis (p < .001), more likely to be female (p = .091), more likely to have a higher CCI score (p = .045), more likely to have a poor performance status (p = .014), more likely to have distant metastasis (M1; p = .006), and less likely to have locoregional (N1-N3) disease (p = .086).
Figure 2.
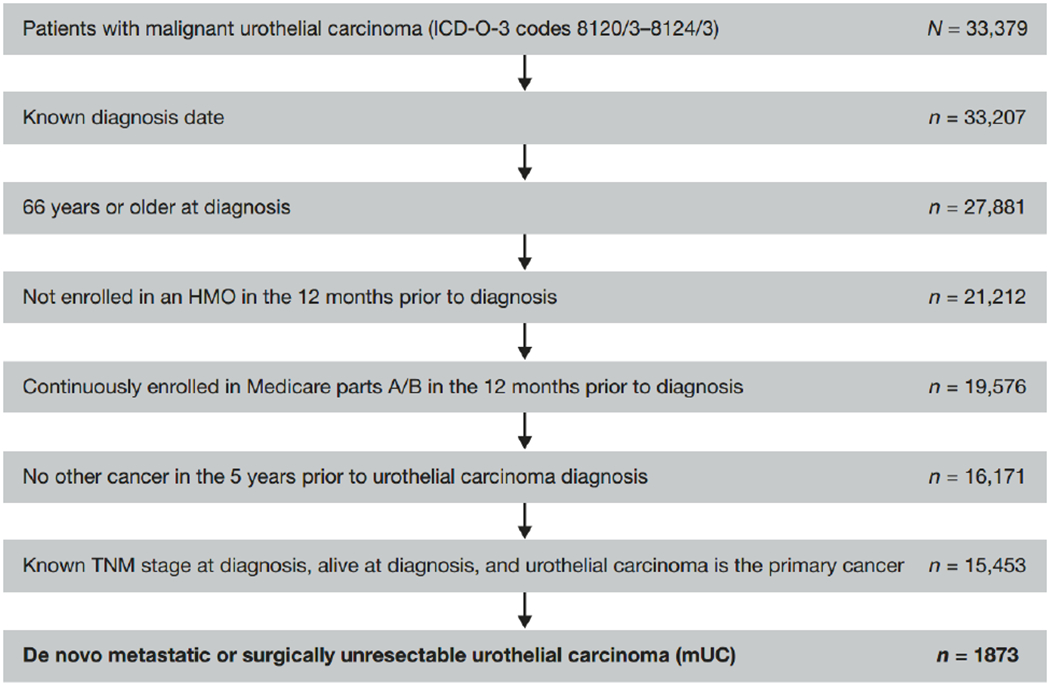
Patient selection flowchart. Abbreviations: dx, diagnosis; HMO, health maintenance organization.
Table 1.
Baseline descriptive characteristics by maximum LOT received.
| Characteristic | No Chemotherapy (n = 1,035) |
LOT1 Only (n = 514) |
LOT1 & LOT2 Only (n = 209) |
LOT3+ (n = 115) |
P-value, LOT3+ vs No Chemotherapy |
|---|---|---|---|---|---|
| Age at diagnosis, median (IQR), years | 80 (74–84) | 74 (70–80) | 73 (70–78) | 73 (69–77) | < .001 |
| Male, n (%) | 618 (59.7) | 332 (64.6) | 154 (73.7) | 78 (67.8) | .091 |
| Race/ethnicity, n (%) | |||||
| Non-Hispanic white | 851 (82.2) | 441 (85.8) | 180 (86.1) | 105 (91.3) | .053 |
| Non-Hispanic black | 99 (9.6) | 33 (6.4) | NR | NR | |
| Hispanic | 59 (5.7) | 19 (3.7) | NR | NR | |
| Other | 26 (2.5) | 21 (4.1) | 15 (7.2) | NR | |
| Marital status at diagnosis, n (%) | |||||
| Single (never married) | 99 (9.6) | 32 (6.2) | NR | NR | < .001 |
| Married | 456 (44.1) | 315 (61.3) | 149 (71.3) | 74 (64.3) | |
| Separated/divorced/widowed | 446 (43.1) | 153 (29.8) | 41 (19.6) | 27 (23.5) | |
| Unknown | 34 (3.3) | 14 (2.7) | NR | NR | |
| Census location, n (%) | |||||
| West | 438 (42.3) | 227 (44.2) | 100 (47.8) | 46 (40.0) | .229 |
| South | 238 (23.0) | 104 (20.2) | 38 (18.2) | 19 (16.5) | |
| Northeast | 229 (22.1) | 116 (22.6) | 42 (20.1) | 32 (27.8) | |
| Midwest | 130 (12.6) | 67 (13.0) | 29 (13.9) | 18 (15.7) | |
| Charlson Comorbidity Index, n (%) | |||||
| 0 | 440 (42.5) | 252 (49.0) | 103 (49.3) | 63 (54.8) | .045 |
| 1 | 261 (25.2) | 125 (24.3) | 58 (27.8) | 27 (23.5) | |
| 2 | 154 (14.9) | 69 (13.4) | 27 (12.9) | NR | |
| 3+ | 180 (17.4) | 68 (13.2) | 21 (10.1) | NR | |
| Poor performance status, n (%) | 311 (30.0) | 109 (21.2) | 36 (17.2) | 22 (19.1) | .014 |
| TNM classification, n (%) | |||||
| M1 | 614 (59.3) | 207 (40.3) | 89 (42.6) | 53 (46.1) | .006 |
| T4b | 183 (17.7) | 71 (13.8) | 24 (11.5) | 23 (20.0) | .538 |
| N1–N3 | 462 (44.6) | 341 (66.3) | 128 (61.2) | 61 (53.0) | .086 |
| Topographic location of tumor, n (%) | |||||
| Lateral wall of bladder | 106 (10.2) | 58 (11.3) | 22 (10.5) | 14 (12.2) | .009 |
| Anterior wall of bladder | 21 (2.0) | 13 (2.5) | NR | NR | |
| Posterior wall of bladder | 56 (5.4) | 22 (4.3) | NR | NR | |
| Trigone of bladder | 64 (6.2) | 34 (6.6) | 20 (9.6) | NR | |
| Dome of bladder | 32 (3.1) | 24 (4.7) | NR | NR | |
| Bladder neck | 20 (1.9) | 22 (4.3) | 11 (5.3) | NR | |
| Ureter | 88 (8.5) | 37 (7.2) | 19 (9.1) | 18 (15.7) | |
| Overlapping lesion of bladder | 200 (19.3) | 107 (20.8) | 31 (14.8) | 14 (12.2) | |
| Bladder, NOS | 411 (39.7) | 176 (34.2) | 84 (40.2) | 35 (30.4) | |
| Ureteric orifice | 17 (1.6) | 13 (2.5) | NR | NR | |
| Urethra | 17 (1.6) | NR | NR | NR | |
| Kidney | NR | NR | NR | NR | |
| Renal pelvis | NR | NR | NR | NR |
Abbreviations. IQR, interquartile range; LOT, line of therapy; NOS, not otherwise specified; NR, not reported per the data use agreement with the National Cancer Institute that numbers less than 11 must be suppressed, and percentages or other mathematical formulas may not be used if they allow for the derivation of counts less than 11
Treatment patterns
More than half of the patients with mUC (55%, n = 1035) received no chemotherapy; of those, 22% received radiation therapy and 24% cystectomy. Of the patients who did receive chemotherapy (n = 838), 45% also received radiation therapy and 43% cystectomy. Among the chemotherapy-treated patients, 61% had LOT1 only, 25% had LOT1 and LOT2 only, and 14% had LOT3+. Patients started LOT1 on average 3.3 months after diagnosis (3 months in T4b, 2.7 months in M1, and 4.4 months in N1-N3 patients). About 63% received gemcitabine with a platinum agent (gemcitabine + carboplatin [32%] or gemcitabine + cisplatin [31%]), while a smaller percentage received gemcitabine monotherapy (9%) or carboplatin + paclitaxel (8%) as part of LOT1. Thirty-eight percent of patients received cisplatin-based chemotherapy. Patients started LOT2 on average 8.9 months after the end of LOT1. The most common LOT2 chemotherapies (41%) were carboplatin combination therapies (carboplatin + gemcitabine [21%], carboplatin + paclitaxel [10%], or carboplatin + gemcitabine + paclitaxel [10%]). Among patients receiving at least two LOTs (LOT2+), 10% received the same chemotherapy regimen for LOT1 and LOT2, with a median interval of 8.4 months. Patients started LOT3 on average 7.9 months after the end of LOT2. Chemotherapy for LOT3 varied, with patients receiving carboplatin + gemcitabine (11%), pemetrexed (11%), or gemcitabine alone (10%). On average, LOT2 began 11.9 months after diagnosis, and LOT3 started 20.9 months after diagnosis.
Overall survival
Median (95% CI) overall survival was 8.1 (7.6-8.7) months for all patients. Among chemotherapy-treated patients, LOT1, LOT2, and LOT3+ patients had median (95% CI) overall survival of 12.4 (11.7-13.4), 16.6 (15.2-19.2), and 29.8 (26.2-33.5) months from diagnosis, respectively. Patients without any chemotherapy treatment had the shortest median survival of 4.3 months (95% CI, 4.0-4.7) from diagnosis. Overall survival was 12.3 (11.4-12.9), 9.1 (8.1-10.2), and 8.3 (6.7-9.8) months from start of LOT1, LOT2, and LOT3, respectively.
Healthcare resource use
During follow-up (median, 7.4 months), all patients had a median (IQR) of 2 (1-4) bladder biopsies, 4 (2-6) CT scans, 0 (0-0) PET-CT scans, 0 (0-1) MRI scans, 1 (0-2) cystoscopy, and 1 (0-1) bone scan. The extent of HCRU increased as patients received subsequent LOTs. Patients who had LOT3+ had higher HCRU compared with those who did not receive chemotherapy, including a greater number of CT, PET-CT, and MRI scans (Table 2).
Table 2.
HCRU per patient during the entire follow-up by maximum LOT received.
| HCRU | All Patients (N = 1,873) |
No Chemotherapy (n = 1,035) |
LOT1 Only (n = 514) |
LOT1 & LOT2 Only (n = 209) |
LOT3+ (nN = 115) |
P-value, LOT3+ vs No Chemotherapy |
|---|---|---|---|---|---|---|
| Bladder biopsy | ||||||
| N (%) of patients | 1,751 (93.5) | 920 (88.9) | 510 (99.2) | 206 (98.6) | 115 (100.0) | < .001 |
| Mean (SD) | 2.85 (2.36) | 2.22 (1.84) | 3.41 (2.62) | 3.83 (2.72) | 4.18 (2.83) | < .001 |
| Median (IQR) | 2 (1–4) | 2 (1–3) | 3 (2–4) | 3 (2–4) | 3 (2–5) | |
| Cystoscopy | ||||||
| N (%) of patients | 1,306 (69.7) | 606 (58.6) | 426 (82.9) | 175 (83.7) | 99 (86.1) | < .001 |
| Mean (SD) | 1.61 (2.11) | 1.11 (1.71) | 2.03 (2.18) | 2.42 (2.72) | 2.77 (2.51) | < .001 |
| Median (IQR) | 1 (0–2) | 1 (0–1) | 1.50 (1–2) | 2 (1–3) | 2 (1–4) | |
| Cystectomy | ||||||
| N (%) of patients | 606 (32.4) | 248 (24.0) | 223 (43.4) | 90 (43.1) | 45 (39.1) | < .001 |
| Mean (SD) | 0.33 (0.49) | 0.25 (0.45) | 0.45 (0.52) | 0.44 (0.53) | 0.42 (0.55) | < .001 |
| Median (IQR) | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0 (0–1) | |
| Radiation | ||||||
| N (%) of patients | 612 (32.7) | 231 (22.3) | 198 (38.5%) | 110 (52.6%) | 73 (63.5%) | < .001 |
| Mean (SD) | 2.16 (3.99) | 1.20 (2.89) | 2.57 (4.15) | 4.17 (5.13) | 5.39 (5.75) | < .001 |
| Median (IQR) | 0 (0–3) | 0 (0–0) | 0 (0–4) | 2 (0–8) | 4 (0–10) | |
| Bone scan | ||||||
| N (%) of patients | 975 (52.1) | 447 (43.2) | 313 (60.9) | 134 (64.1) | 81 (70.4) | < .001 |
| Mean (SD) | 0.75 (1.06) | 0.50 (0.64) | 0.88 (1.03) | 1.13 (1.30) | 1.81 (2.25) | < .001 |
| Median (IQR) | 1 (0–1) | 0 (0–1) | 1 (0–1) | 1 (0–2) | 1 (0–2) | |
| CT scan | ||||||
| N (%) of patients | 1,799 (96.0) | 965 (93.2) | 512 (99.6) | 208 (99.5) | 114 (99.1) | .007 |
| Mean (SD) | 4.82 (4.00) | 3.10 (2.41) | 6.07 (3.81) | 7.40 (4.37) | 10.10 (5.94) | < .001 |
| Median (IQR) | 4 (2–6) | 2 (1–4) | 5 (3–8) | 6 (4–9) | 9 (6–13) | |
| MRI scan | ||||||
| N (%) of patients | 590 (31.5) | 244 (23.6) | 189 (36.8) | 94 (45.0) | 63 (54.8) | < .001 |
| Mean (SD) | 0.54 (1.11) | 0.35 (0.74) | 0.62 (1.06) | 0.91 (1.56) | 1.29 (2.12) | < .001 |
| Median (IQR) | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0 (0–1) | 1 (0–2) | |
| PET-CT scan | ||||||
| N (%) of patients | 414 (22.1) | 105 (10.1) | 157 (30.5) | 88 (42.1) | 64 (55.7) | < .001 |
| Mean (SD) | 0.46 (1.22) | 0.12 (0.44) | 0.56 (1.17) | 0.94 (1.45) | 2.10 (2.91) | < .001 |
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0 (0–2) | 1 (0–4) | |
| Hospital admission | ||||||
| N (%) of patients | 1,759 (93.9) | 949 (91.7) | 497 (96.7) | 200 (95.7) | 113 (98.3) | .009 |
| Mean (SD) | 3.14 (2.43) | 2.52 (1.90) | 3.61 (2.53) | 4.12 (3.01) | 4.83 (3.12) | < .001 |
| Median (IQR) | 3 (1–4) | 2 (1–3) | 3 (2–5) | 4 (2–5) | 4 (3–6) | |
| Length of hospital stay, days† | ||||||
| Mean (SD) | 9.6 (7.0) | 10.9 (8.3) | 8.7 (4.9) | 7.6 (3.8) | 7.1 (4.0) | < .001 |
| Median (IQR) | 8.0 (5.5–11.5) | 8.4 (6.0–13.0) | 7.5 (5.5–10.4) | 6.7 (5.0–9.5) | 6.0 (4.8–8.0) | |
| Emergency department visit | ||||||
| N (%) of patients | 1,690 (90.2) | 923 (89.2) | 461 (89.7) | 195 (93.3) | 111 (96.5) | .009 |
| Mean (SD) | 3.46 (3.22) | 2.97 (2.63) | 3.72 (3.37) | 4.57 (4.49) | 4.75 (3.60) | < .001 |
| Median (IQR) | 3 (1–5) | 2 (1–4) | 3 (2–5) | 3 (2–6) | 4 (2–7) | |
| Intensive care unit visit | ||||||
| N (%) of patients | 994 (53.1) | 490 (47.3) | 311 (60.5) | 125 (59.8) | 68 (59.1) | .016 |
| Mean (SD) | 0.88 (1.19) | 0.74 (1.03) | 1.05 (1.40) | 1.00 (1.20) | 1.07 (1.32) | .004 |
| Median (IQR) | 1 (0–1) | 0 (0–1) | 1 (0–2) | 1 (0–1) | 1 (0–2) | |
| Office visit | ||||||
| N (%) of patients | 1,873 (100.0) | 1,035 (100.0) | 514 (100.0) | 209 (100.0) | 115 (100.0) | > .999 |
| Mean (SD) | 30.5 (37.4) | 13.8 (24.5) | 41.4 (36.7) | 57.2 (36.3) | 84.7 (40.8) | < .001 |
| Median (IQR) | 18 (6–42) | 7 (3–16) | 32 (18–52) | 47 (34–69) | 75 (55–107) |
Abbreviations. CT, computed tomography; HCRU, healthcare resource use; IQR, interquartile range; LOT, line of therapy; MRI, magnetic resonance imaging; PET-CT, positron emission tomography-computed tomography; SD, standard deviation.
Length of stay defined as total number of days hospitalized per patient across all hospital admissions.
Hospitalizations were common, with more than 90% of patients experiencing at least one hospitalization. In our sample there was a median (IQR) of 3 (1-4) hospitalizations per patient, which increased significantly as patients received additional LOTs, from 2 (1-3) hospitalizations in the no-chemotherapy group to 4 (3-6) hospitalizations in the LOT3+ group (Table 2). The median (IQR) time to first hospitalization from diagnosis was 3.7 (1.9-8.9) weeks. The mean duration of hospitalization per patient was 10.9 days in the no-chemotherapy group, decreasing to 8.7, 7.6, and 7.1 days in the LOT1, LOT2, and LOT3+ groups, respectively (Table 2). More than half of the patients with mUC had at least one ICU visit during follow-up, with a higher proportion of patients in the chemotherapy-treated group (60%) versus no-chemotherapy group (47%) visiting the ICU (Table 2); the proportions visiting the ICU were comparable in the LOT2 only and LOT3+ groups (60% and 59%, respectively). Similarly, outpatient office visits also increased as patients received more LOTs: the median (IQR) number of office visits per patient increased from 7 (3-16) visits in the no-chemotherapy group to 32 (18-52), 47 (34-69), and 75 (55-107) visits in the LOT1, LOT2, and LOT3+ groups, respectively (p < .001; Table 2).
Healthcare costs
During the entire follow-up
The mean cumulative cost per patient was $82,912 for all patients, increasing as patients received more LOTs (no chemotherapy, $57,208; LOT1 only, $99,213; LOT1 and LOT2 only, $125,190; LOT2+, $138,924; LOT3+, $163,884). However, the mean cumulative cost PPPM was $18,827 for all patients, decreasing as the number of LOTs increased (no chemotherapy, $27,211; LOT1 only, $9,601; LOT1 and LOT2, $7,325; LOT2+, $6,861; LOT3+, $6,017; Table 3). Most of the PPPM costs were due to inpatient hospitalization and physician visit costs across all groups, with these items’ contribution to the total PPPM costs varying according to the LOT group. For example, inpatient PPPM costs decreased from 68% in the no-chemotherapy group to 34% in the LOT3+ group and physician PPPM costs increased from 19% in the no-chemotherapy group to 39% in the LOT3+ group. Similarly, outpatient PPPM costs also contributed more to the total costs as patients received additional LOTs, increasing from 4% in the no-chemotherapy group to 19% in the LOT3+ group. Across the different LOT groups (including the no-chemotherapy group), combined PPPM costs for home health, hospice, durable medical equipment, and prescription drugs accounted for less than 10% of the total PPPM costs.
Table 3.
Mean healthcare costs (US$) per patient and PPPM during the entire follow-up by maximum LOT received and cost category.
| All Patients (N = 1,873) |
No Chemotherapy (n = 1,035) |
LOT1 Only (n = 514) |
LOT1 & LOT2 Only (n = 209) |
LOT3+ (n = 115) |
P-value, LOT3+ vs No Chemotherapy |
|
|---|---|---|---|---|---|---|
| Median follow-up time, months | 7.4 | 3.7 | 11.8 | 16.1 | 27.0 | – |
| Mean costs, $ (%) | 82,912 (100) | 57,208 (100) | 99,213 (100) | 125,190 (100) | 163,884 (100) | < .001 |
| Inpatient | 43,990 (53) | 36,839 (64) | 51,166 (52) | 54,646 (44) | 56,713 (35) | < .001 |
| Physician | 21,426 (26) | 10,087 (18) | 26,754 (27) | 40,872 (33) | 64,023 (39) | < .001 |
| Outpatient | 9,189 (11) | 3,367 (6) | 12,151 (12) | 19,175 (15) | 30,053 (18) | < .001 |
| Home health | 2,631 (3) | 2,042 (4) | 3,254 (3) | 2,997 (2) | 4,470 (3) | < .001 |
| Hospice | 3,208 (4) | 3,624 (6) | 2,319 (2) | 3,260 (3) | 3,348 (2) | .185 |
| Durable medical equipment | 1,117 (1) | 550 (1) | 1,765 (2) | 1,695 (1) | 2,252 (1) | < .001 |
| Prescription drugs | 1,351 (2) | 698 (1) | 1,803 (2) | 2,545 (2) | 3,025 (2) | < .001 |
| Mean costs PPPM, $ (%) | 18,827 (100) | 27,211 (100) | 9,601 (100) | 7,325 (100) | 6,017 (100) | < .001 |
| Inpatient | 12,275 (65) | 18,626 (68) | 5,497 (57) | 3,323 (45) | 2,057 (34) | < .001 |
| Physician | 3,910 (21) | 5,165 (19) | 2,360 (25) | 2,412 (33) | 2,348 (39) | .611 |
| Outpatient | 1,051 (6) | 1054 (4) | 1,027 (11) | 1,053 (14) | 1,131 (19) | < .001 |
| Home health | 434 (2) | 602 (2) | 266 (3) | 166 (2) | 169 (3) | .341 |
| Hospice | 719 (4) | 1,128 (4) | 245 (3) | 193 (3) | 135 (2) | < .001 |
| Durable medical equipment | 118 (1) | 142 (1) | 99 (1) | 67 (1) | 79 (1) | < .001 |
| Prescription drugs | 320 (2) | 494 (2) | 107 (1) | 111 (2) | 98 (2) | .072 |
Abbreviations. LOT, line of therapy; PPPM, per patient per month.
By duration of each line of therapy
Total unadjusted costs incurred per patient during each LOT were $31,871 ($26,402 in sensitivity analysis) for LOT1; $27,342 ($37,457 in sensitivity analysis) for LOT2; and $27,031 ($12,962 in sensitivity analysis) for LOT3. The costs were primarily driven by physician claims (43% for LOT1, 45% for LOT2, and 44% for LOT3), due to the high number of outpatient office visits per patient during each LOT (median, 13 for LOT1; 11 for LOT2; 10 for LOT3). Among the treated patients, the mean total cost during the pretreatment phase was $23,811, which was driven primarily by inpatient hospitalizations (62% of the pretreatment cost). There was a mean of 1.1 hospitalizations and a median (IQR) of 1 (0-2) hospitalizations in the pretreatment phase, suggesting that the majority of patients are hospitalized before initiating chemotherapy.
Not surprisingly, inpatient hospitalization represented the most expensive component of the reported HCRU: mean costs of hospitalization were $9,045, $7,226, and $6,666 during LOT1, LOT2, and LOT3, respectively (Figure 3). In comparison, the costs of chemotherapy during the different LOTs were $5,416, $3,552, and $4,378 for LOT1, LOT2, and LOT3, respectively. In the no-chemotherapy group, it is worth noting that the costs of hospitalization ($26,429), ICU-related hospitalization ($12,875), and emergency department visits ($328) were higher than the corresponding costs incurred among the treated patients during any LOT.
Figure 3.
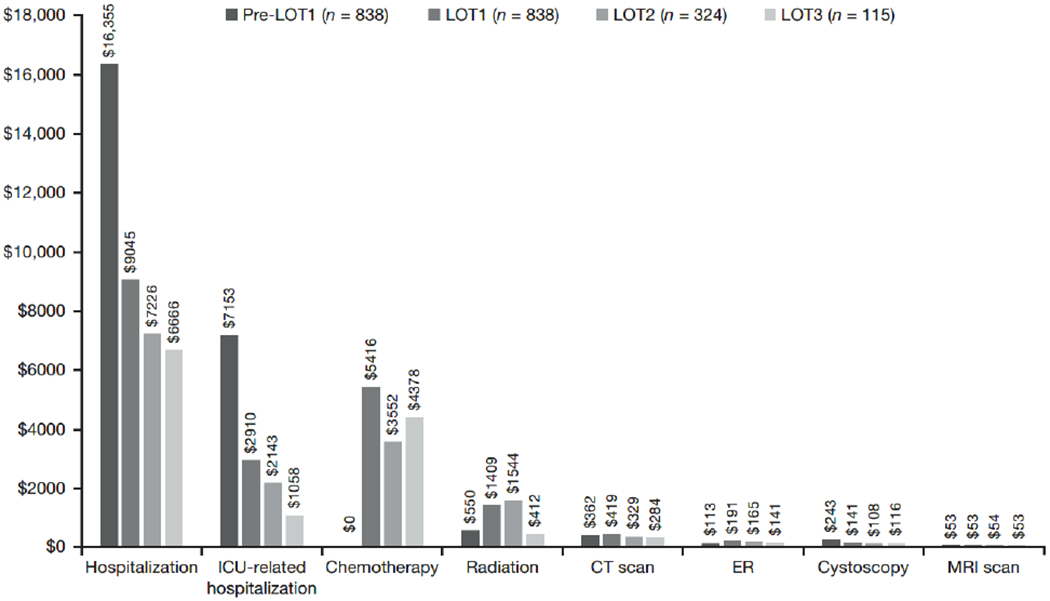
Mean healthcare cost per patient by line of therapy (LOT) over duration of LOT and pretreatment phase. These estimates are not the unit costs of each healthcare resource utilization (HCRU). Rather, they represent the total cost of each HCRU category divided by the number of patients in our sample, regardless of whether or not they have used each resource. Abbreviations: CT, computed tomography; ED, emergency room; ICU, intensive care unit; MRI, magnetic resonance imaging.
Discussion
This study examined the treatment patterns, HCRU, and costs incurred by Medicare patients with mUC in the US. Our findings showed that more than half of the patients with mUC received no chemotherapy, and most patients who did receive chemotherapy had only one LOT. Untreated patients were older, generally sicker, and were more likely to present with distant metastasis compared with treated patients. Patients who continued to receive LOT3+ had higher mean cumulative costs and HCRU, but lower average monthly costs and HCRU compared with patients receiving one or two lines of chemotherapy. Inpatient and physician costs constituted most of the total costs, with the chemotherapy itself comprising a relatively small proportion of the total costs incurred with each LOT (13%-17%, depending on the LOT). Further analysis of cumulative costs over the treatment duration of each LOT revealed that substantial costs are incurred during each LOT.
Analysis of treatment patterns was consistent with a previous study using National Cancer Data Base data that showed that about half of patients (55% of males, 48% of females) with advanced bladder cancer did not receive chemotherapy, per the [27]. Another study using SEER-Medicare data from 2007 to 2013 showed that 66% (804/1,215) of patients with advanced bladder cancer were not treated with chemotherapy, whereas 34% (411/1,215) of patients received LOT1 and 16% (189/1,215) subsequently received LOT2 [28]. Although it is not known why about half of the Medicare patients with UC remain untreated with chemotherapy, we have highlighted that they are likely to be older, sicker, and have worse performance status. As these patients may be untreated because of a reduced capacity for tolerating chemotherapy, they may be candidates for potentially better tolerated immuno-oncology therapies [29]. Indeed, it will be interesting to note whether the proportion of untreated Medicare patients and the patterns of treatments in the real world evolve as immuno-oncology therapies become available and are integrated into the overall management of mUC.
A 2007 to 2013 SEER-Medicare study conducted by Kamat et al. showed that the total costs of advanced bladder cancer care during LOT1 and LOT2 were $36,790 and $26,730, respectively [28]. Our study of 2004 to 2013 SEER-Medicare data analyzed similar clinical and economic outcomes in stage IV bladder cancer patients as the Kamat et al. study, but our LOT1 costs were slightly lower ($31,871). This is likely due to differences in cost attribution methods, years studied, and study approaches: We analyzed HCRU from diagnosis to death and included a more comprehensive list of HCRU costs, while Kamat et al. only looked at HCRU from index chemotherapy date and was limited to inpatient, emergency, outpatient, skilled nursing facility, and hospice costs. Furthermore, our population included patients who did not receive chemotherapy, whereas the Kamat et al study did not, and we did not require a minimum follow-up time (Kamat et al: ≥ 24 months) since a minimum follow-up time can bias cost and overall survival estimates.
Additionally, our analysis underscores that Medicare patients with mUC—especially the untreated—tend to be a sick group, with most patients requiring inpatient hospital stays and more than half also requiring ICU admissions. It remains to be seen whether immuno-oncology therapies can change these dynamics, or at least prolong time to using these resources.
This study has several strengths. First, we used a nationally representative database that includes a US Medicare population with mUC. Second, the results provided here can be used by healthcare payers to estimate the expected monthly and cumulative real-world costs that may be incurred by their enrollees who are diagnosed with mUC and are at various stages of treatment. Third, we estimated the costs of healthcare resources that are commonly used by patients with mUC and quantified the costs incurred, which can help payers identify the major drivers of costs. Most of the literature to date has focused on characterizing HCRU and costs among patients treated with chemotherapy [28,30]. Our study, by contrast, also quantified the economic burden of patients who did not receive chemotherapy, who represent more than half of the Medicare patients presenting with mUC. Finally, we included patients with UC not only of the bladder (as is often reported), but also of the renal pelvis, ureter, ureteric orifice, and urethra (representing 10% of our sample), thus providing cost and HCRU analysis of the entire urinary system.
Limitations of our study include the possibility of treatment misclassification when using the LOT-defining algorithm. Further, systemic treatment for mUC is generally chemotherapy-based, with LOT1 therapies often being combinations of 2 to 4 agents, whereas subsequent LOTs may comprise a single agent. Thus, it can be challenging to define what constitutes a first versus a subsequent LOT, particularly given the complexities inherent to delivering multi-agent treatments as part of a specific regimen, or the effect of treatment gaps or therapy switches that may arise due to drug toxicities. Results may not be generalizable to younger patients with mUC, given that the population studied was aged 66 years or above; however, since the median age at diagnosis of mUC is 72 years [3], the data presented are applicable to the majority of the mUC population seen in clinical practice. Lastly, we did not exclude patients who developed other cancers during the follow-up period to be more representative of ‘real-world’ patients and to preserve sample size.
Conclusions
This real-world analysis provides data on the economic burden of both chemotherapy-treated and untreated patients with mUC. We have shown that more than half of the patients with mUC receive no chemotherapy. The estimated costs for untreated patients were around $57,000 over their lifetime, mostly for inpatient services. The chemotherapy-treated group incurred costs to Medicare of approximately $24,000 before starting treatment, and $32,000 in the LOT1, $27,000 in the LOT2, and $27,000 in the LOT3 settings. As the treatment landscape evolves to include immuno-oncology agents, these findings provide a benchmark for the relative costs associated with mUC treatment across traditional lines of chemotherapy in the US and provide context for comparison with patients treated with newer therapies. It remains to be seen whether immuno-oncology therapies can change the dynamics observed in this analysis. Specifically, immuno-oncology therapies may be expected to shift costs away from the inpatient setting towards the outpatient setting (with increases in drug costs) and lead to longer survival. Based on the pattern observed herein, one might expect that this will result in cumulatively higher cost overall but lower cost per patient per month. The net effect could result in more cost-effective care for patients with mUC.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the SEER program tumor registries in the creation of the SEER-Medicare database.
Professional editorial assistance was provided by Richard Daniel, PhD, and Lawrence Hargett of PPSI (a PAREXEL company), funded by Bristol-Myers Squibb. This study was funded by Bristol-Myers Squibb.
Declaration of funding
This study was funded by Bristol-Myers Squibb.
Declaration of financial/other interests
AA was an employee of Pharmerit International when the work was conducted. CJ and MB are current employees of Pharmerit International. MB is also a shareholder of Pharmerit International. SY and SR are employees of Bristol-Myers Squibb and have stock options. Part of AH’s research time was supported by a Merit Review Award (I01 BX000545), Medical Research Service, Department of Veterans Affairs. No other potential conflicts of interests were reported by the authors.
Footnotes
Previous presentations
An earlier version of this analysis was presented at the European Society for Medical Oncology (ESMO) Annual Congress in 2017. Citation details are as follows: Aly A, Johnson C, Yang S, et al. Medical costs and health care resource use (HCRU) in elderly US patients (pts) with newly diagnosed metastatic or surgically unresectable urothelial carcinoma (mUC) using Surveillance, Epidemiology, and End Results (SEER) Medicare data. Ann Oncol. 2017;28(suppl 5):401 (Abstract 1131P).
Data availability statement
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71:96–108. [DOI] [PubMed] [Google Scholar]
- 2.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 4.Svatek RS, Siefker-Radtke A, Dinney CP. Management of metastatic urothelial cancer: the role of surgery as an adjunct to chemotherapy. Can Urol Assoc J. 2009;3(suppl 4):228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiess PE, Agarwal N, Bangs R, et al. Bladder cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1240–1267. [DOI] [PubMed] [Google Scholar]
- 6.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. [DOI] [PubMed] [Google Scholar]
- 7.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Gill D, Poole A, et al. Systemic immunotherapy for urothelial cancer: Current trends and future directions. Cancers (Basel). 2017;9. doi: 10.3390/cancers9020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich B, Srinivas S. Urothelial carcinoma: the evolving landscape of immunotherapy for patients with advanced disease. Res Rep Urol. 2018;10:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yafi FA, North S, Kassouf W. First- and second-line therapy for metastatic urothelial carcinoma of the bladder. Current Oncol. 2011;18:e25–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldron N, Young T, Enting D. Current management of advanced bladder cancer. Trends Urol Men’s Health. 2017;8:8–12. [Google Scholar]
- 12.Apolo AB, Infante JR, Hamid O. Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase 1b trial: analysis of safety, clinical activity, and PD-L1 expression. J Clin Oncol. 2016;34(suppl):4514. [Google Scholar]
- 13.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–322. [DOI] [PubMed] [Google Scholar]
- 17.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–1492. [DOI] [PubMed] [Google Scholar]
- 18.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeung C, Dinh T, Lee J. The health economics of bladder cancer: an updated review of the published literature. Pharmacoeconomics. 2014;32:1093–1104. [DOI] [PubMed] [Google Scholar]
- 20.Botteman MF, Pashos CL, Redaelli A, et al. The health economics of bladder cancer. Pharmacoeconomics. 2012;21:1315–1330. [DOI] [PubMed] [Google Scholar]
- 21.Cooksley CD, Avritscher EBC, Grossman HB, et al. Clinical model of cost of bladder cancer in the elderly. Urology. 2008;71:519–525. [DOI] [PubMed] [Google Scholar]
- 22.Sievert KD, Amend B, Nagele U, et al. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol. 2009;27:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noone A-M, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with Medicare claims. Medical Care. 2016;54:e55–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Medical Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 25.Bikov KA, Mullins CD, Seal B, et al. Algorithm for identifying chemotherapy/biological regimens for metastatic colon cancer in SEER-Medicare. Medical Care. 2015;53:e58–e64. [DOI] [PubMed] [Google Scholar]
- 26.Liang C, Li L, Fraser CD, et al. The treatment patterns, efficacy, and safety of nab®-paclitaxel for the treatment of metastatic breast cancer in the United States: results from health insurance claims analysis. BMC Cancer. 2015;15:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose TL, Deal AM, Nielsen ME, et al. Sex disparities in use of chemotherapy and survival in patients with advanced bladder cancer. Cancer. 2016;122:2012–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamat A, Cao X, He J, et al. Costs of care for patients receiving chemotherapy for advanced bladder cancer J Clin Pathw. 2017;3:63–70. [Google Scholar]
- 29.Zichi C, Tucci M, Leone G, et al. Immunotherapy for patients with advanced urothelial cancer: current evidence and future perspectives. Biomed Res Int. 2017;2017:5618174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao S, Gooden KM, Landsman-Blumberg P, et al. Treatment patterns and economic burden of illness in patients with advanced bladder cancer receiving second-line therapy. J Clin Oncol. 2017;35(6 suppl):320–320. [Google Scholar]


