Abstract
Fibrosis is associated with accumulation of excess fibrillar collagen, leading to tissue dysfunction. Numerous processes, including inflammation, myofibroblast activation, and endothelial-to-mesenchymal transition, play a role in the establishment and progression of fibrosis. Relaxin is a peptide hormone with well-known antifibrotic properties that result from its action on numerous cellular targets to reduce fibrosis. Relaxin activates multiple signal transduction pathways as a mechanism to suppress inflammation and myofibroblast activation in fibrosis. In this review, the general mechanisms underlying fibrotic diseases are described, along with the current state of knowledge regarding cellular targets of relaxin. Finally, an overview is presented summarizing the signaling pathways activated by relaxin and other relaxin family peptide receptor agonists to suppress fibrosis.
1. Introduction
Throughout its history, the hormone relaxin has been closely associated with the process of extracellular matrix (ECM) remodeling. Indeed, the first description of the effects of relaxin by Hisaw in 1926, was of the softening and lengthening of the pubic ligament (Hisaw, 1926). This effect involved a dramatic change in the structure of collagen in the pubic ligament and other reproductive organs, including a decreased density of the collagen fibre bundles (Zhao et al., 2000), which in turn reduced the rigidity of the ligament whilst increasing its ability to expand and widen. Other early studies suggested that relaxin reduced fibrillar collagen in nonreproductive tissues, including the skin, leading to clinical studies using relaxin to target scleroderma, a painful disorder characterized by fibrosis of the skin and connective tissue (Casten and Boucek, 1958; Evans, 1959). These studies, using partially purified porcine relaxin, generally involved small numbers of subjects, and reported variable degrees of success. Progress on clinical studies of relaxin in fibrotic diseases was hampered until the availability of recombinant human relaxin (now known as serelaxin), leading to a more recent clinical trial of serelaxin for the treatment of scleroderma (Seibold et al., 2000). While this trial was largely unsuccessful, interest remains in targeting the various signaling pathways that are activated by relaxin as a therapeutic approach for the treatment of fibrotic diseases.
Much of what is known about the antifibrotic effects of relaxin (predominantly involving the use of serelaxin) is the result of cell culture studies. Relaxin was shown to decrease pathological collagen production by inhibiting its synthesis and secretion from myofibroblasts (activated fibroblasts that are key fibrosis-producing cells) derived from numerous organs (Bennett et al., 2003; Samuel et al., 2004a; Unemori and Amento, 1990; Unemori et al., 1996). At the same time, relaxin is able to promote the expression and activity of matrix metalloproteinases (MMPs), and/or decrease the levels of their endogenous inhibitors, the tissue inhibitors of metalloproteinases (TIMPs) to facilitate the degradation of aberrant collagen accumulation (Bennett et al., 2003; Heeg et al., 2005; Masterson et al., 2004; Samuel et al., 2004a; Unemori et al., 1996). Similar effects were observed using rodent models of renal, pulmonary, cardiac, and hepatic fibrosis (Bennett et al., 2014; Fallowfield et al., 2014; Garber et al., 2001; Samuel et al., 2004a; Unemori et al., 1996). Finally, mice deficient in either endogenous relaxin or the relaxin receptor, RXFP1, develop age-related fibrosis in numerous organs (Du et al., 2003; Hewitson et al., 2012; Krajnc-Franken et al., 2004; Samuel et al., 2009; Samuel et al., 2003; Samuel et al., 2005; Samuel et al., 2004b). Therefore, there is considerable in vitro and in vivo evidence that relaxin is an important factor in the progression and treatment of fibrosis. In addition, there is emerging evidence that additional relaxin family peptides, such as relaxin-3, may also play a role in fibrosis (Hossain et al., 2011; Zhang et al., 2018).
Early studies of the signaling pathways activated by relaxin were hampered by the fact that, until 2002, relaxin was an orphan hormone without an identified cognate receptor. However, it was known early that cellular cAMP and nitric oxide levels were increased in response to relaxin administration in some tissues (Braddon, 1978; Masini et al., 1994). Finally, the discovery in 2002 of the first two receptors capable of activation by relaxin family peptide members began the rapid elucidation of signaling pathways activated by relaxin (Hsu et al., 2002). It is now well-established that there are four relaxin family peptide receptors (RXFPs) that are differentially activated by relaxin family peptides, and often have complex signaling mechanisms (Bathgate et al., 2013).
The purpose of this minireview is to present the current state of knowledge regarding the mechanism of the anti-fibrotic effects of relaxin, and the signaling pathways triggered by relaxin family peptides. The review begins with an overview of the general mechanisms common to the development and progression of fibrosis. This is followed by a summary of the mechanisms by which relaxin acts on the cellular and tissue level to impede fibrotic processes. The final section focuses on the established and emerging signaling pathways triggered by relaxin family peptides that may be involved in mediating their antifibrotic effects.
2. General processes underlying fibrosis
2.1. Inflammation and fibrogenesis
Fibrosis is characterized by an accumulation of ECM proteins and increased collagen deposition in various organs, as a result of fibrogenesis, whereby a normal wound healing process is overactive in response to tissue injury in order to preserve the native tissue morphological and functional integrity (Lee and Kalluri, 2010; Wynn, 2008). Fibrosis can affect many tissue and organ systems, where it is most apparent in liver cirrhosis, atherosclerosis, scleroderma, vascular dysfunction, lung, kidney and heart diseases. Initiation of the fibrotic process is highly complex, which involves a vast array of stimulatory and inhibitory factors that are pivotal in modulating cells that are involved in regulating fibrosis. In the initial step, the affected organ is in an inflamed state where there is recruitment and activation of immune cells such as macrophages, eosinophils, CD8+ T cells, lymphocytes CD4+, mast cells and fibroblasts (Gieseck et al., 2018), leading to the secretion of growth factors, proteolytic enzymes and cytokines by these immune cells within the site of injury. These inflammatory products are responsible in the formation of connective tissues that replace normal physiological ones. Major fibrogenic mediators secreted during this process include transforming growth factor-β1 (TGF-β1), connective tissue growth factor (CTGF) and platelet-derived growth factor (PDGF) (Wynn, 2008). TGF-β1 is considered the central mediator of fibrosis to regulate cellular processes and ECM proteins such as collagen, elastin and fibronectin. It is well known that TGF-β1 signaling pathway is upregulated in cardiac (Yue et al., 2017), liver (Katz et al., 2016), kidney (Chen et al., 2018) and lung fibrosis (Eser and Janne, 2018), through both canonical (Smad-dependent) and non-canonical (Smad-independent) pathways. These signaling pathways lead to the activation or recruitment of myofibroblasts and overproduction of ECM proteins, as well as impeding the degradation of ECM components (Meng et al., 2016).
2.2. Myofibroblast activation and matrix remodeling
The primary culprit in all fibrotic diseases is the activation of myofibroblasts (Hinz et al., 2012). Myofibroblasts are the main matrix-producing cells that generate ECM proteins such as fibronectin and collagen type I, III and IV (Kisseleva and Brenner, 2008), and remodel the neo-ECM components by producing contractile forces in response to the surroundings of the cells. Myofibroblasts are thought to be formed from a heterogeneous population derived from resident fibroblasts, as well as endothelial cells, epithelial cells, mesothelial cells, and circulating fibrocytes of bone marrow origin (Davis and Molkentin, 2014). Several cellular mechanisms have been proposed to describe the fibroblast-myofibroblast transdifferentiation process. Upon activation of the process, fibroblasts migrate into the site of injury and attain a myofibroblast phenotype. Activated myofibroblasts are capable of producing substantial amounts of ECM proteins to trigger collagen accumulation. As discussed above, fibrogenic mediators such as TGF-β1 and PDGF produced by activated myofibroblasts and inflammatory cells, as well as angiotensin II (Ang II), which is derived from activation of the renin-angiotensin system (Bataller et al., 2005; Suzuki et al., 2003; Weber et al., 1993), further stimulate the proliferation of myofibroblasts and the production of collagen and fibronectin. The ECM serves as an important scaffold for cells and growth factors, and also provides biomechanical clues that guide myofibroblast activation and activity. The MMPs belong to a multi-gene family of zinc and calcium-dependent endopeptidases secreted by connective tissue cells and inflammatory phagocytes that play pivotal roles in ECM remodeling due to their ability to degrade many matrix components, growth factors and cytokines (Nagase and Woessner, 1999). Under physiological conditions, proteolytic activities of MMPs are tightly controlled by their endogenous protein inhibitors, TIMPs (Gomez et al., 1997). However, under fibrotic conditions, there is an imbalance between MMPs and TIMPs, with suppression of MMP activity and increased TIMP expression, resulting in the protection of ECM and decreased proteolysis. During this period, the synthesis of new collagen from myofibroblasts exceeds its degradation rate following the inflammatory cytokine-driven events at the site of injury (Van Linthout et al., 2014), thus resulting in aberrant collagen deposition over time. This cascade of events will eventually lead to a significant deposition of ECM proteins that outpaces its degradation, resulting in fibrosis and pathological remodeling of target organs.
2.3. Endothelial-to-mesenchymal transition
In addition to directly stimulating the differentiation of fibroblasts into myofibroblasts, TGF-β1 can stimulate epithelial and endothelial cells to acquire a mesenchymal phenotype in which they de-differentiate, migrate and subsequently re-differentiate into myofibroblasts to secrete large amounts of matrix proteins to promote wound healing; commonly known as epithelial-to-mesenchymal cell transition (EMT) and endothelial cell-to-mesenchymal cell transition (EndMT), respectively (Barnes and Gorin, 2011; Du et al., 2012; Klahr and Morrissey, 2002). During an aberrant wound healing process, however, these transitioned epithelial and endothelial cells remain as myofibroblasts and continue to produce ECM components such as collagen and fibronectin. In the murine kidney, it was shown that 10% of endothelial cells undergo EndMT and 5% of epithelial cells undergo EMT, contributing to myofibroblast-induced renal fibrosis (LeBleu et al., 2013). Moreover, in a murine model of cardiac fibrosis, it was revealed that 27–33% of all cardiac fibroblasts were of endothelial origin using a Cre-recombinase genetic marking system to identify cells that express Tie1 (an endothelial marker) (Zeisberg et al., 2007). Furthermore in EndMT, phenotypes of endothelial cells, such as CD31 and vascular endothelial (VE)-cadherin expression are often replaced by specific properties of mesenchymal cells like vimentin and α-smooth muscle actin (α-SMA) expression (Kovacic et al., 2012). These studies collectively demonstrated that EndMT plays a critical role in regulating the pathological processes leading to fibrosis.
3. The antifibrotic effects of relaxin
3.1. Relaxin effects on inflammation and fibrogenesis
Several studies have shown that relaxin inhibits the infiltration of various immune cells into injured/damaged organs, which produce various pro-inflammatory and pro-fibrotic factors to initiate the wound healing response to injury, but which over prolonged periods contribute to the stimulation of excess ECM secretion (Wynn, 2008). Unresolved inflammation, followed by dysregulated wound healing, can lead to fibrosis-induced organ failure. Serelaxin and/or porcine relaxin has been shown to reduce the infiltration of inflammatory cells within several tissues including neutrophils, basophils, mast cells, endothelial cells and macrophages (Bani et al., 1997; Bani et al., 2002; Beiert et al., 2018; Garber et al., 2001; Nistri et al., 2003; Nistri et al., 2008), the latter being a source of TGF-β1 production. Furthermore, relaxin decreases granule exocytosis and mast cell degranulation to reduce pro-inflammatory and allergic cytokines such as histamine, leukotrienes and serotonin (Masini et al., 1997). Relaxin can also inhibit the endothelial adhesiveness to neutrophils and the infiltration of macrophages which are crucial for the recruitment and migration of inflammatory cells to the site of injury (Hewitson et al., 2007; Martin et al., 2018; Nistri et al., 2003), inhibit toll-like receptor-4 signaling, and promote tissue-repairing M2 macrophage polarization (Chen et al., 2017). Moreover, relaxin inhibits NLRP3 inflammasome activity, which is known to increase interleukin (IL)-1β activity (Raleigh et al., 2017), and also plays a role in inhibiting NFkB signaling, a crucial transcription factor that regulates many inflammatory genes (Martin et al., 2018). Aside from its effects on inflammatory cell infiltration, relaxin has been shown to reduce the pro-fibrotic influence of cytokines or mediators such as TGF-β1 (Bennett et al., 2014; Heeg et al., 2005; Kocan et al., 2017; Mookerjee et al., 2009; Samuel et al., 2004a; Unemori and Amento, 1990; Unemori et al., 1996; Wang et al., 2016; Wang et al., 2017), IL-1β (Beiert et al., 2017; Pini et al., 2016; Unemori and Amento, 1990), IL-6 (Beiert et al., 2018; Beiert et al., 2017; Wang et al., 2017; Yoshida et al., 2014), monocyte chemoattractant protein-1 (Brecht et al., 2011; Wang et al., 2017), and tumor necrosis factor-α (Brecht et al., 2011; Yoshida et al., 2014; Yoshida et al., 2013), amongst others.
3.2. Relaxin effects on myofibroblast activation and matrix remodeling
Serelaxin has been found to consistently inhibit TGF-β1, IL-1β and/or angiotensin (Ang) II-mediated fibroblast proliferation and/or differentiation into myofibroblasts, regardless of etiology (Bennett et al., 2003; Fallowfield et al., 2014; Heeg et al., 2005; Hewitson et al., 2010; Huuskes et al., 2015; Lekgabe et al., 2005; Mookerjee et al., 2009; Samuel et al., 2011; Samuel et al., 2004a; Sassoli et al., 2013; Unemori and Amento, 1990; Unemori et al., 1996). Furthermore, serelaxin also inhibited myofibroblast contractility as part of its anti-fibrotic actions (Huang et al., 2011). These combined actions of serelaxin led to reduced myofibroblast-induced ECM (primarily collagen and fibronectin) synthesis and deposition (Bennett et al., 2003; Cernaro et al., 2017; Heeg et al., 2005; Hewitson et al., 2010; Lee et al., 2011; Lekgabe et al., 2005; Royce et al., 2015; Samuel et al., 2011; Samuel et al., 2004a; Sassoli et al., 2013; Unemori and Amento, 1990; Unemori et al., 1996). Furthermore, serelaxin promoted MMP expression and activity and/or inhibited TIMP activity to induce the degradation of aberrant ECM protein accumulation (Heeg et al., 2005; Hewitson et al., 2010; Huuskes et al., 2015; Kang et al., 2017; Lekgabe et al., 2005; Royce et al., 2015; Samuel et al., 2011; Sassoli et al., 2013; Unemori and Amento, 1990; Unemori et al., 1996; Williams et al., 2001).
Mechanistically, serelaxin has been shown to signal through RXFP1 on myofibroblasts to suppress TGF-β1 signal transduction and activity at the level of Smad2 phosphorylation (Chow et al., 2014; Kocan et al., 2017; Mookerjee et al., 2009; Sarwar et al., 2015; Wang et al., 2016). This then resulted in a decrease in TGF-β1-induced myofibroblast differentiation and hence, myofibroblast-mediated ECM/collagen synthesis; a decrease in TGF-β1-induced suppression of MMP (MMP-2, MMP-9, MMP-1/−13) activity (Chow et al., 2012); and a decrease in TGF-β1-induced TIMP activity and inhibition of ECM degradation. In cardiac myofibroblasts, serelaxin signaled through Notch-1 and suppressed Smad3 phosphorylation to inhibit the pro-fibrotic actions of TGF-β1 (Kocan et al., 2017; Sassoli et al., 2013).
3.3. Relaxin effects on endothelial-to-mesenchymal transition
Recent studies showed that serelaxin inhibits EndMT within the heart and kidney of isoprenaline-induced cardiomyopathy in rodents (Cai et al., 2017; Zheng et al., 2017). Zhou et al. found that serelaxin improves cardiac function in rats with myocardial fibrosis, by reducing EndMT and interstitial collagens I and III, while increasing the microvascular density of the heart (Zhou et al., 2015). Similar to cardiac myofibroblasts, serelaxin also inhibited TGF-β1-induced mobility of endothelial cells through a Notch-1-dependent pathway, and increased expression of CD31 while decreasing vimentin content of human umbilical vein endothelial cells (HUVECs) in vitro (Zhou et al., 2015). In a separate study, serelaxin was capable of reducing cardiac fibrosis in vivo via the inhibition of EndMT, and by increasing VE-cadherin and CD31 levels while suppressing vimentin and α-SMA levels in HUVECs in vitro (Cai et al., 2017). While these combined findings suggest that relaxin inhibits myofibroblast-mediated aberrant ECM synthesis and deposition at multiple levels, further studies are required to fully understand the detailed mechanisms underlying its potent antifibrotic actions in various tissues and organs for future therapeutic applications.
4. Relaxin family peptide signaling in fibrosis
4.1. Relaxin family peptides and receptors
The peptides of the relaxin/insulin-like super family exhibit a variety of functions, including antifibrotic activity in diverse organs, e.g. kidney, heart, lung and liver (Samuel et al., 2017). These peptides bind to the G-protein coupled receptors RXFP1–4, which induce diverse signaling cascades (Bathgate et al., 2013). The most well-studied pathways activated by these receptors involve modulation of cAMP production. For example, RXFP1 and RXFP2 activation initially leads to Gs stimulation and thereby enhanced cAMP synthesis. Depending on the cell type, RXFP1 can also couple to Go and Gi to promote a biphasic pattern of cAMP production. In contrast, RXFP3 and 4 are coupled to Gi/o, leading to a reduction in cAMP levels. Of the receptors, only RXFP1 has thus far demonstrated antifibrotic effects. However, as discussed below, most of the signaling pathways that have been tied to antifibrotic effects involve different signaling pathways (summarized in Table 1).
Table 1:
Selected Signaling Pathways Activated by Relaxin Family Peptides and Their Receptors in Fibrosis
| Agonist | Receptor | Cells/Disease Model | Signaling Pathway | Targets | Reference |
|---|---|---|---|---|---|
| Serelaxin | RXFP1 RXFP1/AT2R |
Renal, cardiac, dermal fibroblasts, Renal fibrosis |
pERK1/2-nNOS-NO-sGC-cGMP-PKG1 | TGFβ pSmads SMA Collagen Fibronectin MMPs TIMPs |
(Chow et al., 2012; Chow et al., 2014; Mookerjee et al., 2009; Sarwar et al., 2015; Wang et al., 2016; Wetzl et al., 2017; Wetzl et al., 2016) |
| ML290 | RXFP1 | Cardiac and hepatic myofibroblasts | NOS-NO-sGC-cGMP | TGFβ pSmads SMA MMPs |
(Kocan et al., 2017) |
| B7–33 | RXFP1 | Renal myofibroblasts Cardiac and lung fibrosis | pERK1/2 | pSmads Collagen MMPs |
(Hossain et al., 2016; Praveen et al., 2017) |
| Relaxin-3 | RXFP1, RXFP3? |
Cardiac fibrosis | unknown | NLRP3-inflammasome Collagen MMPs |
(Hossain et al., 2011; Zhang et al., 2018; Zhang et al., 2017) |
| InsL6 | unknown | Cardiac fibrosis | unknown | TGFβ collagen |
(Maruyama et al., 2018) |
4.2. RXFP1 signaling through the nitric oxide-cGMP and ERK pathways
The most important receptor for the suppression of fibrosis in different organs is RXFP1. The physiological ligands that bind to this receptor are relaxin-2, its human recombinant form serelaxin, and relaxin-1 (in other species). In myofibroblasts, (se)relaxin binding to RXFP1 stimulates Gs and GOB proteins (Mookerjee et al., 2009), and through an extracellular signal-regulated kinase phosphorylation (p-ERK1/2) and neuronal NO synthase (nNOS)-mediated NO-soluble guanylate cyclase (sGC)-cyclic guanosine monophosphate (cGMP)-dependent pathway to suppress TGF-β1 signal transduction and activity at the level of Smad2 phosphorylation (Chow et al., 2014; Kocan et al., 2017; Mookerjee et al., 2009; Sarwar et al., 2015; Wang et al., 2016) (Figure 1). Relaxin also directly stimulated cGMP synthesis independent of ERK1/2 activation (Kocan et al., 2017). Furthermore, it was established that signaling via cGMP and the cGMP-activated protein kinase-1 (PKG1) mediates the suppressive effects of serelaxin in unilateral ureter obstruction (UUO)-induced interstitial renal fibrosis (Wetzl et al., 2016). In this model, serelaxin treatment strongly enhanced plasma cGMP. Using PKG1-deficient mice, it was observed that the reduction in pSmad2 and pERK1 in response to serelaxin treatment was dependent on PKG1 (Wetzl et al., 2016). In comparison, the phosphodiesterase-5 (PDE5) inhibitor, zaprinast, acts as an antifibrotic agent via suppression of ERK1/2 signaling, but this effect was independent from PKG1. The combination of serelaxin with this PDE5 inhibitor does not reveal additional benefits against kidney fibrosis (Wetzl et al., 2017).
Figure 1: Scheme for antifibrotic signaling of relaxin-2.
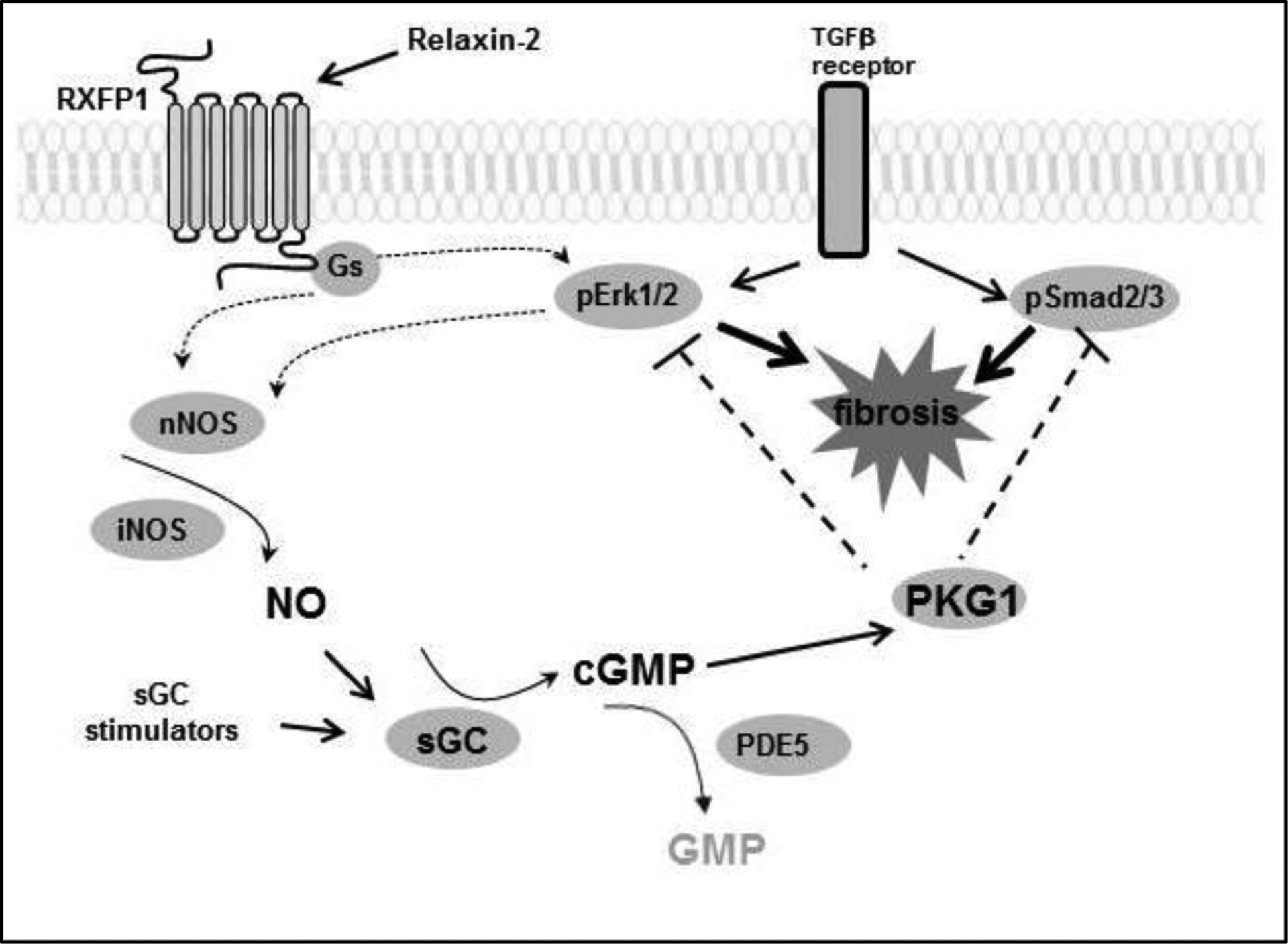
Relaxin-2 activates RXFP1, which stimulates NO/cGMP/PKG1 signaling. Activation of the relaxin-RXFP1 axis subsequently leads to the inhibition of pERK1/2 and pSmad2/3, which ultimately suppresses TGFβ-induced fibrosis.
PKG1 – cGMP-dependent protein kinase 1, sGC – soluble guanylyl cyclase, cGMP – cyclic guanosine monophosphate, ERK – extracellular-signal regulated kinase, GMP – guanosine monophosphate, NOS – nitric oxide synthase, PDE5 – phosphodiesterase 5, RXFP1 – relaxin family peptide receptor 1, Smad – small mothers against decapentaplegic protein, TGFβ – transforming growth factor-β
In summary, the diverse effects of relaxin peptides on signaling systems upon cAMP, cGMP, ERK1/2 and Smad2/3 are important parameters to elucidate their (patho)physiological functions. Interestingly, these effects are also time-dependent. The level of cAMP can be enhanced upon short exposure with relaxin via GS signaling, but it is reduced upon chronic exposure via activation of GOB (Halls et al., 2006). These differential effects can be also observed in the ERK1/2-system which is activated in the short term to enhance NO and thereby cGMP synthesis (Chow et al., 2012). However, ERK1/2 phosphorylation is reduced by serelaxin upon chronic treatment which might be important inter alia for suppression of kidney fibrosis (Schinner et al., 2017).
4.3. Crosstalk and heterodimerization between RXFP1 and AT2R
Many of the signal transduction pathways activated by serelaxin in myofibroblasts require the presence of the Ang II type 2 receptor (AT2R), which appears to occur through an interaction between RXFP1 and the AT2R (Chow et al., 2014). As a result of this interaction, the anti-fibrotic effects of serelaxin can be abrogated by co-administration of an AT2R antagonist (PD123319) or when serelaxin is administered to AT2R knockout mice (Chow et al., 2014). As the AT2R can inhibit both the expression and activation of the AT1R, serelaxin may also signal through the RXFP1:AT2R axis to indirectly inhibit the pro-fibrotic effects of Ang II via the Ang II–AT1R–TGF-β1 interaction (Nakajima et al., 1995; Yang et al., 2012).
4.4. Emerging potential relaxin-based antifibrotic therapies
There is evidence that other relaxin family peptides may also have antifibrotic effects. Human relaxin-3 (also known as H3 relaxin) reduced the fibrotic properties of rat ventricular fibroblasts, acting through RXFP1, and decreased collagen expression in a mouse model of cardiomyopathy (Hossain et al., 2011). Relaxin-3 treatment in cardiac fibroblasts also inhibited reactive oxygen species- and inflammasome-mediated collagen synthesis under high glucose conditions, and there was evidence of both RXFP1 and RXFP3 expression in cardiac tissue in a rat model of diabetic cardiomyopathy (Zhang et al., 2018; Zhang et al., 2017). Hence, relaxin-3 (acting via RXFP1, and possibly via RXFP3) might be therapeutically relevant for the treatment of diabetic cardiomyopathy. A recent study showed that mice lacking insulin-like peptide 6 (Insl6), a member of the relaxin family, exhibit cardiac dysfunction and enhanced cardiac fibrosis, suggesting the role of Insl6 in suppressing cardiac fibrosis and its potential to be used for the treatment of heart failure (Maruyama et al., 2018). Further studies are needed to determine if relaxin-3 or Insl6 can be used to treat established models of tissue fibrosis.
In addition to the endogenous ligands of RXFP1, a recently identified non-peptide agonist of the human relaxin receptor, ML290, shows promising effects in ameliorating fibrosis in cell culture studies. For example, ML290 treatment induces profound antifibrotic effects in human hepatic stellate cell line, LX-2, by down-regulating the gene expression of collagens, α-SMA, PDGF, and up-regulating MMP1 (McBride et al., 2017). In human cardiac fibroblasts, ML290 effectively suppresses TGF-β1-induced myofibroblast activation through a reduction in Smad2/3 phosphorylation and up-regulation of MMP2 (Kocan et al., 2017). The biased signaling of ML290 compared to relaxin might be a powerful tool to evaluate the specific antifibrotic properties of relaxin. Another RXFP1 agonist, B7–33, is a peptide analogue of the B-chain of relaxin which reduces fibrosis in heart and kidney of rodent models (Hossain et al., 2016; Praveen et al., 2017). Its affinity for RXFP1 is lower, but its specificity for RXFP1 is higher than that of (se)relaxin. The peptide mimetic B7–33 acts via preferential activation of pERK1/2 and cGMP in (myo)fibroblast cells compared to cAMP activation (Hossain et al., 2016; Praveen et al., 2017).
One critical requirement for the development of new relaxin analogues/agonists is high throughput bioassay systems for screening potential compounds. One important readout for the activity of candidate compounds is cAMP measurement. Bioluminescence resonance energy transfer (BRET) biosensors provide dynamic real-time measurement of cAMP levels. One such system, the CAMYEL assay (cAMP sensor using YFP-Epac.Rlu) has been validated as an assay for all RXFPs in HEK cells (Valkovic et al., 2018). In addition, cGMP sensor mice might be valuable for cGMP measurements and for screening in vivo (Paolillo et al., 2018); the latter which is more relevant for investigating the anti-fibrotic effects of (se)relaxin in myofibroblasts.
5. Conclusion
Since the development of the relaxin and RXFP1 knockout mice, it has become clear that endogenous relaxin plays a critical role in the protection of organs from age-related fibrosis. As the cellular mechanisms and signaling pathways by which (se)relaxin exerts its antifibrotic effects become clearer, additional therapeutic options are expected to emerge. Furthermore, numerous studies utilizing preclinical in vivo models are underway that support the use of (se)relaxin and relaxin mimetics for the treatment of fibrotic diseases. However, clinical trials using (se)relaxin or mimetics are needed to determine if this pathway can be exploited to treat human fibrotic disease.
Funding:
This work was supported by a National Health & Medical Research Council (NHMRC) of Australia Senior Research Fellowship (C.S.S); Novartis Pharma GmbH, the Bavarian State and Sonderforschungsbereich SFB699 (J.S.); and the U.S. Department of Veterans Affairs Merit Review BX000849 (R.G.B).
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest.
References
- Bani D, Ballati L, Masini E, Bigazzi M and Sacchi TB, 1997. Relaxin counteracts asthma-like reaction induced by inhaled antigen in sensitized guinea pigs, Endocrinology. 138, 1909–15. [DOI] [PubMed] [Google Scholar]
- Bani D, Baronti R, Vannacci A, Bigazzi M, Sacchi TB, Mannaioni PF and Masini E, 2002. Inhibitory effects of relaxin on human basophils activated by stimulation of the Fc epsilon receptor. The role of nitric oxide, Int Immunopharmacol. 2, 1195–204. [DOI] [PubMed] [Google Scholar]
- Barnes JL and Gorin Y, 2011. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases, Kidney Int. 79, 944–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R, Sancho-Bru P, Gines P and Brenner DA, 2005. Liver fibrogenesis: a new role for the renin-angiotensin system, Antioxid Redox Signal. 7, 1346–55. [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M and Summers RJ, 2013. Relaxin family peptides and their receptors, Physiol Rev. 93, 405–80. [DOI] [PubMed] [Google Scholar]
- Beiert T, Knappe V, Tiyerili V, Stockigt F, Effelsberg V, Linhart M, Steinmetz M, Klein S, Schierwagen R, Trebicka J, Roell W, Nickenig G, Schrickel JW and Andrie RP, 2018. Chronic lower-dose relaxin administration protects from arrhythmia in experimental myocardial infarction due to anti-inflammatory and anti-fibrotic properties, Int J Cardiol. 250, 21–28. [DOI] [PubMed] [Google Scholar]
- Beiert T, Tiyerili V, Knappe V, Effelsberg V, Linhart M, Stockigt F, Klein S, Schierwagen R, Trebicka J, Nickenig G, Schrickel JW and Andrie RP, 2017. Relaxin reduces susceptibility to post-infarct atrial fibrillation in mice due to anti-fibrotic and anti-inflammatory properties, Biochem Biophys Res Commun. 490, 643–649. [DOI] [PubMed] [Google Scholar]
- Bennett RG, Heimann DG, Singh S, Simpson RL and Tuma DJ, 2014. Relaxin decreases the severity of established hepatic fibrosis in mice, Liver International. 34, 416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RG, Kharbanda KK and Tuma DJ, 2003. Inhibition of markers of hepatic stellate cell activation by the hormone relaxin, Biochem Pharmacol. 66, 867–874. [DOI] [PubMed] [Google Scholar]
- Braddon SA, 1978. Relaxin-dependent adenosine 6’,5’-monophosphate concentration changes in the mouse pubic symphysis, Endocrinology. 102, 1292–9. [DOI] [PubMed] [Google Scholar]
- Brecht A, Bartsch C, Baumann G, Stangl K and Dschietzig T, 2011. Relaxin inhibits early steps in vascular inflammation, Regulatory Peptides. 166, 76–82. [DOI] [PubMed] [Google Scholar]
- Cai J, Chen X, Chen X, Chen L, Zheng G, Zhou H and Zhou X, 2017. Anti-Fibrosis Effect of Relaxin and Spironolactone Combined on Isoprenaline-Induced Myocardial Fibrosis in Rats via Inhibition of Endothelial-Mesenchymal Transition, Cell Physiol Biochem. 41, 1167–1178. [DOI] [PubMed] [Google Scholar]
- Casten GG and Boucek RJ, 1958. Use of relaxin in the treatment of scleroderma, Journal of the American Medical Association. 166, 319–324. [DOI] [PubMed] [Google Scholar]
- Cernaro V, Medici MA, Bianco F, Santoro D, Lacquaniti A, Romeo A, Lucisano S, Buemi A and Buemi M, 2017. Opposite actions of urotensin II and relaxin-2 on cellular expression of fibronectin in renal fibrosis: A preliminary experimental study, Clin Exp Pharmacol Physiol. 44, 1069–1071. [DOI] [PubMed] [Google Scholar]
- Chen L, Sha ML, Li D, Zhu YP, Wang XJ, Jiang CY, Xia SJ and Shao Y, 2017. Relaxin abrogates renal interstitial fibrosis by regulating macrophage polarization via inhibition of Toll-like receptor 4 signaling, Oncotarget. 8, 21044–21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang T, Lu DW, Zhao H, Feng YL, Chen H, Chen DQ, Vaziri ND and Zhao YY, 2018. Central role of dysregulation of TGF-beta/Smad in CKD progression and potential targets of its treatment, Biomed Pharmacother. 101, 670–681. [DOI] [PubMed] [Google Scholar]
- Chow BS, Chew EG, Zhao C, Bathgate RA, Hewitson TD and Samuel CS, 2012. Relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to up-regulate matrix metalloproteinases: the additional involvement of iNOS, PloS one. 7, e42714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BS, Kocan M, Bosnyak S, Sarwar M, Wigg B, Jones ES, Widdop RE, Summers RJ, Bathgate RA, Hewitson TD and Samuel CS, 2014. Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis, Kidney Int. 86, 75–85. [DOI] [PubMed] [Google Scholar]
- Davis J and Molkentin JD, 2014. Myofibroblasts: trust your heart and let fate decide, J Mol Cell Cardiol. 70, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X-J, Samuel CS, Gao X-M, Zhao L, Parry LJ and Tregear GW, 2003. Increased myocardial collagen and ventricular diastolic dysfunction in relaxin deficient mice: a gender-specific phenotype, Cardiovascular Research. 57, 395–404. [DOI] [PubMed] [Google Scholar]
- Du X, Shimizu A, Masuda Y, Kuwahara N, Arai T, Kataoka M, Uchiyama M, Kaneko T, Akimoto T, Iino Y and Fukuda Y, 2012. Involvement of matrix metalloproteinase-2 in the development of renal interstitial fibrosis in mouse obstructive nephropathy, Lab Invest. 92, 1149–60. [DOI] [PubMed] [Google Scholar]
- Eser PO and Janne PA, 2018. TGFbeta pathway inhibition in the treatment of non-small cell lung cancer, Pharmacol Ther. 184, 112–130. [DOI] [PubMed] [Google Scholar]
- Evans JA, 1959. Relaxin (releasin) therapy in diffuse progressive scleroderma; a preliminary report, AMA Arch Derm. 79, 150–8. [DOI] [PubMed] [Google Scholar]
- Fallowfield JA, Hayden AL, Snowdon VK, Aucott RL, Stutchfield BM, Mole DJ, Pellicoro A, Gordon-Walker TT, Henke A, Schrader J, Trivedi PJ, Princivalle M, Forbes SJ, Collins JE and Iredale JP, 2014. Relaxin modulates human and rat hepatic myofibroblast function and ameliorates portal hypertension in vivo, Hepatology. 59, 1492–504. [DOI] [PubMed] [Google Scholar]
- Garber SL, Mirochnik Y, Brecklin CS, Unemori EN, Singh AK, Slobodskoy L, Grove BH, Arruda JA and Dunea G, 2001. Relaxin decreases renal interstitial fibrosis and slows progression of renal disease, Kidney Int. 59, 876–882. [DOI] [PubMed] [Google Scholar]
- Gieseck RL 3rd, Wilson MS and Wynn TA, 2018. Type 2 immunity in tissue repair and fibrosis, Nat Rev Immunol. 18, 62–76. [DOI] [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H and Thorgeirsson UP, 1997. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions, Eur J Cell Biol. 74, 111–22. [PubMed] [Google Scholar]
- Halls ML, Bathgate RA and Summers RJ, 2006. Relaxin family peptide receptors RXFP1 and RXFP2 modulate cAMP signaling by distinct mechanisms, Mol Pharmacol. 70, 214–26. [DOI] [PubMed] [Google Scholar]
- Heeg MH, Koziolek MJ, Vasko R, Schaefer L, Sharma K, Muller GA and Strutz F, 2005. The antifibrotic effects of relaxin in human renal fibroblasts are mediated in part by inhibition of the Smad2 pathway, Kidney Int. 68, 96–109. [DOI] [PubMed] [Google Scholar]
- Hewitson TD, Ho WY and Samuel CS, 2010. Antifibrotic Properties of Relaxin: In Vivo Mechanism of Action in Experimental Renal Tubulointerstitial Fibrosis, Endocrinology. 151, 4938–4948. [DOI] [PubMed] [Google Scholar]
- Hewitson TD, Mookerjee I, Masterson R, Zhao C, Tregear GW, Becker GJ and Samuel CS, 2007. Endogenous Relaxin Is a Naturally Occurring Modulator of Experimental Renal Tubulointerstitial Fibrosis, Endocrinology. 148, 660–669. [DOI] [PubMed] [Google Scholar]
- Hewitson TD, Zhao C, Wigg B, Lee SW, Simpson ER, Boon WC and Samuel CS, 2012. Relaxin and Castration in Male Mice Protect from, but Testosterone Exacerbates, Age-Related Cardiac and Renal Fibrosis, Whereas Estrogens Are an Independent Determinant of Organ Size, Endocrinology. 153, 188–199. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M and Gabbiani G, 2012. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling, Am J Pathol. 180, 1340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisaw FL, 1926. Experimental relaxation of the pubic ligament of the guinea pig, Proc Soc Exp Biol Med. 23, 661–663. [Google Scholar]
- Hossain MA, Chow Suet Man B, Zhao C, Xu Q, Du X-J, Wade JD and Samuel CS, 2011. H3 Relaxin Demonstrates Antifibrotic Properties via the RXFP1 Receptor, Biochemistry. 50, 1368–1375. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Kocan M, Yao ST, Royce SG, Nair VB, Siwek C, Patil NA, Harrison IP, Rosengren KJ, Selemidis S, Summers RJ, Wade JD, Bathgate RA and Samuel CS, 2016. A single-chain derivative of the relaxin hormone is a functionally selective agonist of the G protein-coupled receptor, RXFP1, Chemical Science. DOI: 10.1039/C5SC04754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD and Hsueh AJW, 2002. Activation of orphan receptors by the hormone relaxin, Science. 295, 671–674. [DOI] [PubMed] [Google Scholar]
- Huang X, Gai Y, Yang N, Lu B, Samuel CS, Thannickal VJ and Zhou Y, 2011. Relaxin Regulates Myofibroblast Contractility and Protects against Lung Fibrosis, The American Journal of Pathology. 179, 2751–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huuskes BM, Wise AF, Cox AJ, Lim EX, Payne NL, Kelly DJ, Samuel CS and Ricardo SD, 2015. Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fibrosis in obstructive nephropathy, FASEB J. 29, 540–53. [DOI] [PubMed] [Google Scholar]
- Kang YM, Lee HM, Moon SH, Kang H and Choi YR, 2017. Relaxin Modulates the Expression of MMPs and TIMPs in Fibroblasts of Patients with Carpal Tunnel Syndrome, Yonsei Med J. 58, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LH, Likhter M, Jogunoori W, Belkin M, Ohshiro K and Mishra L, 2016. TGF-beta signaling in liver and gastrointestinal cancers, Cancer Lett. 379, 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T and Brenner DA, 2008. Mechanisms of fibrogenesis, Exp Biol Med (Maywood). 233, 109–22. [DOI] [PubMed] [Google Scholar]
- Klahr S and Morrissey J, 2002. Obstructive nephropathy and renal fibrosis, Am J Physiol Renal Physiol. 283, F861–75. [DOI] [PubMed] [Google Scholar]
- Kocan M, Sarwar M, Ang SY, Xiao J, Marugan JJ, Hossain MA, Wang C, Hutchinson DS, Samuel CS, Agoulnik AI, Bathgate RAD and Summers RJ, 2017. ML290 is a biased allosteric agonist at the relaxin receptor RXFP1, Sci Rep. 7, 2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic JC, Mercader N, Torres M, Boehm M and Fuster V, 2012. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease, Circulation. 125, 1795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnc-Franken MAM, van Disseldorp AJM, Koenders JE, Mosselman S, van Duin M and Gossen JA, 2004. Impaired Nipple Development and Parturition in LGR7 Knockout Mice, Mol. Cell. Biol 24, 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H and Kalluri R, 2013. Origin and function of myofibroblasts in kidney fibrosis, Nat Med. 19, 1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB and Kalluri R, 2010. Mechanistic connection between inflammation and fibrosis, Kidney Int Suppl. S 22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Kim YO, Choi IK, Rah DK and Yun CO, 2011. Adenovirus-relaxin gene therapy for keloids: implication for reversing pathological fibrosis, British Journal of Dermatology. 165, 673–677. [DOI] [PubMed] [Google Scholar]
- Lekgabe ED, Kiriazis H, Zhao C, Xu Q, Moore XL, Su Y, Bathgate RA, Du XJ and Samuel CS, 2005. Relaxin reverses cardiac and renal fibrosis in spontaneously hypertensive rats, Hypertension. 46, 412–418. [DOI] [PubMed] [Google Scholar]
- Martin B, Gabris-Weber BA, Reddy R, Romero G, Chattopadhyay A and Salama G, 2018. Relaxin reverses inflammatory and immune signals in aged hearts, PLoS One. 13, e0190935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama S, Wu CL, Yoshida S, Zhang D, Li PH, Wu F, Parker Duffen J, Yao R, Jardin B, Adham IM, Law R, Berger J, Di Marchi R and Walsh K, 2018. Relaxin Family Member Insulin-Like Peptide 6 Ameliorates Cardiac Fibrosis and Prevents Cardiac Remodeling in Murine Heart Failure Models, J Am Heart Assoc. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini E, Bani D, Bello MG, Bigazzi M, Mannaioni PF and Sacchi TB, 1997. Relaxin counteracts myocardial damage induced by ischemia-reperfusion in isolated guinea pig hearts: evidence for an involvement of nitric oxide, Endocrinology. 138, 4713–20. [DOI] [PubMed] [Google Scholar]
- Masini E, Bani D, Bigazzi M, Mannaioni PF and Bani-Sacchi T, 1994. Effects of Relaxin on Mast Cells: In vitro and in vivo studies in rats and guinea pigs, J. Clin. Invest 94, 1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson R, Hewitson TD, Kelynack K, Martic M, Parry L, Bathgate R, Darby I and Becker G, 2004. Relaxin down-regulates renal fibroblast function and promotes matrix remodelling in vitro, Nephrol Dial Transplant. 19, 544–552. [DOI] [PubMed] [Google Scholar]
- McBride A, Hoy AM, Bamford MJ, Mossakowska DE, Ruediger MP, Griggs J, Desai S, Simpson K, Caballero-Hernandez I, Iredale JP, Pell T, Aucott RL, Holmes DS, Webster SP and Fallowfield JA, 2017. In search of a small molecule agonist of the relaxin receptor RXFP1 for the treatment of liver fibrosis, Sci Rep. 7, 10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XM, Nikolic-Paterson DJ and Lan HY, 2016. TGF-beta: the master regulator of fibrosis, Nat Rev Nephrol. 12, 325–38. [DOI] [PubMed] [Google Scholar]
- Mookerjee I, Hewitson TD, Halls ML, Summers RJ, Mathai ML, Bathgate RA, Tregear GW and Samuel CS, 2009. Relaxin inhibits renal myofibroblast differentiation via RXFP1, the nitric oxide pathway, and Smad2, FASEB J. 23, 1219–29. [DOI] [PubMed] [Google Scholar]
- Nagase H and Woessner JF Jr., 1999. Matrix metalloproteinases, J Biol Chem. 274, 21491–4. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Hutchinson HG, Fujinaga M, Hayashida W, Morishita R, Zhang L, Horiuchi M, Pratt RE and Dzau VJ, 1995. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer, Proc Natl Acad Sci U S A. 92, 10663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistri S, Chiappini L, Sassoli C and Bani D, 2003. Relaxin inhibits lipopolysaccharide-induced adhesion of neutrophils to coronary endothelial cells by a nitric oxide-mediated mechanism, Faseb J. 17, 2109–11. [DOI] [PubMed] [Google Scholar]
- Nistri S, Cinci L, Perna AM, Masini E and Bani D, 2008. Mast cell inhibition and reduced ventricular arrhythmias in a swine model of acute myocardial infarction upon therapeutic administration of relaxin, Inflamm Res. 57 Suppl 1, S7–8. [DOI] [PubMed] [Google Scholar]
- Paolillo M, Peters S, Schramm A, Schlossmann J and Feil R, 2018. Real-Time Imaging Reveals Augmentation of Glutamate-Induced Ca(2+) Transients by the NO-cGMP Pathway in Cerebellar Granule Neurons, Int J Mol Sci. 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini A, Boccalini G, Lucarini L, Catarinicchia S, Guasti D, Masini E, Bani D and Nistri S, 2016. Protection from Cigarette Smoke-Induced Lung Dysfunction and Damage by H2 Relaxin (Serelaxin), J Pharmacol Exp Ther. 357, 451–8. [DOI] [PubMed] [Google Scholar]
- Praveen P, Bathgate RA, Hossain MA and Patil NA, 2017. A single-chain derivative of the relaxin hormone is a functionally selective agonist of the G protein-coupled receptor, RXFP1, 6th Modern Solid Phase Peptide Synthesis & Its Applications Symposium. Queensland, Australia, pp. 55. [Google Scholar]
- Raleigh JV, Mauro AG, Devarakonda T, Marchetti C, He J, Kim E, Filippone S, Das A, Toldo S, Abbate A and Salloum FN, 2017. Reperfusion therapy with recombinant human relaxin-2 (Serelaxin) attenuates myocardial infarct size and NLRP3 inflammasome following ischemia/reperfusion injury via eNOS-dependent mechanism, Cardiovasc Res. 113, 609–619. [DOI] [PubMed] [Google Scholar]
- Royce SG, Shen M, Patel KP, Huuskes BM, Ricardo SD and Samuel CS, 2015. Mesenchymal stem cells and serelaxin synergistically abrogate established airway fibrosis in an experimental model of chronic allergic airways disease, Stem Cell Res. 15, 495–505. [DOI] [PubMed] [Google Scholar]
- Samuel CS, Cendrawan S, Gao X-M, Ming Z, Zhao C, Kiriazis H, Xu Q, Tregear GW, Bathgate RAD and Du X-J, 2011. Relaxin remodels fibrotic healing following myocardial infarction, Lab Invest. 91, 675–690. [DOI] [PubMed] [Google Scholar]
- Samuel CS, Royce SG, Chen B, Cao H, Gossen JA, Tregear GW and Tang MLK, 2009. Relaxin Family Peptide Receptor-1 Protects against Airway Fibrosis during Homeostasis But Not against Fibrosis Associated with Chronic Allergic Airways Disease, Endocrinology. 150, 1495–1502. [DOI] [PubMed] [Google Scholar]
- Samuel CS, Royce SG, Hewitson TD, Denton KM, Cooney TE and Bennett RG, 2017. Anti-fibrotic actions of relaxin, Br J Pharmacol. 174, 962–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CS, Unemori EN, Mookerjee I, Bathgate RA, Layfield SL, Mak J, Tregear GW and Du XJ, 2004a. Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo, Endocrinology. 145, 4125–4133. [DOI] [PubMed] [Google Scholar]
- Samuel CS, Zhao C, Bathgate RA, Bond CP, Burton MD, Parry LJ, Summers RJ, Tang ML, Amento EP and Tregear GW, 2003. Relaxin deficiency in mice is associated with an age-related progression of pulmonary fibrosis, FASEB J. 17, 121–123. [DOI] [PubMed] [Google Scholar]
- Samuel CS, Zhao C, Bathgate RA, Du XJ, Summers RJ, Amento EP, Walker LL, McBurnie M, Zhao L and Tregear GW, 2005. The relaxin gene-knockout mouse: a model of progressive fibrosis, Ann N Y Acad Sci. 1041, 173–181. [DOI] [PubMed] [Google Scholar]
- Samuel CS, Zhao C, Bond CP, Hewitson TD, Amento EP and Summers RJ, 2004b. Relaxin-1-deficient mice develop an age-related progression of renal fibrosis, Kidney Int. 65, 2054–2064. [DOI] [PubMed] [Google Scholar]
- Sarwar M, Samuel CS, Bathgate RA, Stewart DR and Summers RJ, 2015. Serelaxin-mediated signal transduction in human vascular cells: bell-shaped concentration-response curves reflect differential coupling to G proteins, Br J Pharmacol. 172, 1005–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoli C, Chellini F, Pini A, Tani A, Nistri S, Nosi D, Zecchi-Orlandini S, Bani D and Formigli L, 2013. Relaxin Prevents Cardiac Fibroblast-Myofibroblast Transition via Notch-1-Mediated Inhibition of TGF-Î2/Smad3 Signaling, PLOS ONE. 8, e63896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinner E, Wetzl V, Schramm A, Kees F, Sandner P, Stasch JP, Hofmann F and Schlossmann J, 2017. Inhibition of the TGFbeta signalling pathway by cGMP and cGMP-dependent kinase I in renal fibrosis, FEBS Open Bio. 7, 550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold JR, Korn JH, Simms R, Clements PJ, Moreland LW, Mayes MD, Furst DE, Rothfield N, Steen V, Weisman M, Collier D, Wigley FM, Merkel PA, Csuka ME, Hsu V, Rocco S, Erikson M, Hannigan J, Harkonen WS and Sanders ME, 2000. Recombinant human relaxin in the treatment of scleroderma. A randomized, double-blind, placebo-controlled trial, Ann Intern Med. 132, 871–9. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V and Egido J, 2003. Inflammation and angiotensin II, Int J Biochem Cell Biol. 35, 881–900. [DOI] [PubMed] [Google Scholar]
- Unemori EN and Amento EP, 1990. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts, J Biol Chem. 265, 10681–10685. [PubMed] [Google Scholar]
- Unemori EN, Pickford LB, Salles AL, Piercy CE, Grove BH, Erikson ME and Amento EP, 1996. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo, J Clin Invest. 98, 2739–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkovic AL, Leckey MB, Whitehead AR, Hossain MA, Inoue A, Kocan M and Bathgate RAD, 2018. Real-time examination of cAMP activity at relaxin family peptide receptors using a BRET-based biosensor, Pharmacol Res Perspect. 6, e00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Linthout S, Miteva K and Tschope C, 2014. Crosstalk between fibroblasts and inflammatory cells, Cardiovasc Res. 102, 258–69. [DOI] [PubMed] [Google Scholar]
- Wang C, Kemp-Harper BK, Kocan M, Ang SY, Hewitson TD and Samuel CS, 2016. The Anti-fibrotic Actions of Relaxin Are Mediated Through a NO-sGC-cGMP-Dependent Pathway in Renal Myofibroblasts In Vitro and Enhanced by the NO Donor, Diethylamine NONOate, Front Pharmacol. 7, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Luo Y, Myakala K, Orlicky DJ, Dobrinskikh E, Wang X and Levi M, 2017. Serelaxin improves cardiac and renal function in DOCA-salt hypertensive rats, Sci Rep. 7, 9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KT, Brilla CG, Campbell SE, Guarda E, Zhou G and Sriram K, 1993. Myocardial fibrosis: role of angiotensin II and aldosterone, Basic Res Cardiol. 88 Suppl 1, 107–24. [DOI] [PubMed] [Google Scholar]
- Wetzl V, Schinner E, Kees F, Faerber L and Schlossmann J, 2017. Differences in the renal antifibrotic cGMP/cGKI-dependent signaling of serelaxin, zaprinast, and their combination, Naunyn Schmiedebergs Arch Pharmacol. 390, 939–948. [DOI] [PubMed] [Google Scholar]
- Wetzl V, Schinner E, Kees F, Hofmann F, Faerber L and Schlossmann J, 2016. Involvement of Cyclic Guanosine Monophosphate-Dependent Protein Kinase I in Renal Antifibrotic Effects of Serelaxin, Front Pharmacol. 7, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, Benyon RC, Trim N, Grove BH, Arthur MJ, Unemori EN and Iredale JP, 2001. Relaxin inhibits effective collagen deposition by cultured hepatic stellate cells and decreases rat liver fibrosis in vivo, Gut. 49, 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, 2008. Cellular and molecular mechanisms of fibrosis, J Pathol. 214, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chen C, Ren H, Han Y, He D, Zhou L, Hopfer U, Jose PA and Zeng C, 2012. Angiotensin II AT(2) receptor decreases AT(1) receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats, J Hypertens. 30, 1176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Kumagai H, Kohsaka T and Ikegaya N, 2014. Protective effects of relaxin against cisplatin-induced nephrotoxicity in rats, Nephron Exp Nephrol. 128, 9–20. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Kumagai H, Kohsaka T and Ikegaya N, 2013. Relaxin protects against renal ischemia-reperfusion injury, American journal of physiology.Renal physiology 305, F1169–76. [DOI] [PubMed] [Google Scholar]
- Yue Y, Meng K, Pu Y and Zhang X, 2017. Transforming growth factor beta (TGF-beta) mediates cardiac fibrosis and induces diabetic cardiomyopathy, Diabetes Res Clin Pract. 133, 124–130. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S and Kalluri R, 2007. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis, Nat Med. 13, 952–61. [DOI] [PubMed] [Google Scholar]
- Zhang X, Fu Y, Li H, Shen L, Chang Q, Pan L, Hong S and Yin X, 2018. H3 relaxin inhibits the collagen synthesis via ROS- and P2X7R-mediated NLRP3 inflammasome activation in cardiac fibroblasts under high glucose, J Cell Mol Med. 22, 1816–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Pan L, Yang K, Fu Y, Liu Y, Chen W, Ma X and Yin X, 2017. Alterations of relaxin and its receptor system components in experimental diabetic cardiomyopathy rats, Cell Tissue Res. 370, 297–304. [DOI] [PubMed] [Google Scholar]
- Zhao L, Samuel CS, Tregear GW, Beck F and Wintour EM, 2000. Collagen studies in late pregnant relaxin null mice, Biol Reprod. 63, 697–703. [DOI] [PubMed] [Google Scholar]
- Zheng G, Cai J, Chen X, Chen L, Ge W, Zhou X and Zhou H, 2017. Relaxin Ameliorates Renal Fibrosis and Expression of Endothelial Cell Transition Markers in Rats of Isoproterenol-Induced Heart Failure, Biol Pharm Bull. 40, 960–966. [DOI] [PubMed] [Google Scholar]
- Zhou X, Chen X, Cai JJ, Chen LZ, Gong YS, Wang LX, Gao Z, Zhang HQ, Huang WJ and Zhou H, 2015. Relaxin inhibits cardiac fibrosis and endothelial-mesenchymal transition via the Notch pathway, Drug Des Devel Ther. 9, 4599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]


