Abstract
Background
Little is known on sleep quality of children with atopic dermtitis (AD) during flares and how treatment impacts their sleep. The purpose of this study is to evaluate variations in sleep quality of children with AD during flares and its response to intensified treatment.
Material and Methods
Prospective case-crossover study in 10 children with moderate-severe AD. At baseline, AD severity was assessed using SCORAD and patients were prescribed intensified AD therapy. All subjects were monitored by actigraphy during 14 days and returned for SCORAD assessment.
Results
Subjects' age was 5.6 ± 5.3 years; 50% were female. Sleep duration was decreased in all subjects and awakenings were increased in 90%. Parental perception of sleep significantly differed from actigraphy results: parents estimated less sleep duration and less awakenings. Nocturnal sleep efficiency at baseline was reduced in 50%. After intensified treatment, median SCORAD decreased from 58.5 to 31.3 (p=0.005), with significant improvement in sleep loss and pruritus visual analogue scales. Despite improvement of SCORAD and parental perception of sleep loss and pruritus, objective sleep duration and efficiency measured by actigraphy did not vary significantly after intensified treatment. Change in SCORAD, sleep loss and pruritus scales did not correlate significantly with change in sleep duration, efficiency or other actigraphic sleep quality measurements.
Conclusions
Children with moderate-severe AD have sleep quality abnormalities, with decreased sleep duration, low sleep efficiency and increased awakenings. Improvement in AD severity upon intensified AD treatment was associated with improved parental perception of sleep loss, but not of objective sleep quality assessed by actigraphy.
Keywords: Atopic Dermatitis, Sleep, Sleep Efficiency, Awakenings, Actigraphy
INTRODUCTION
Atopic dermatitis is a chronic relapsing inflammatory skin disease characterized by pruritic eczematous lesions of the skin1. It is one of the most common skin diseases of childhood, with a prevalence ranging between 5 and 20%. High rates of sleep alterations have been described in this population2,3. The consequences of sleep loss are multiple, including daytime fatigue, headaches, neurocognitive and mood disorders, behavioral problems, as well as decreased school and work performance. Indeed, all of these have been well documented to have higher prevalence in AD patients2-6. For patients with AD, sleep loss is considered one of the most relevant symptoms, probably only secondary to itch7.
It is commonly thought that the main trigger of sleep disturbance in AD is the vicious itch-scratch cycle, however, some studies show that this may only partially explain sleep problems in AD8. Research has shown that sleep loss in childhood influences future development and has a negative impact over neurocognitive function in this population9,10. Thus, understanding how sleep alterations relate to AD severity and flares in children is of crucial importance to improve quality of life of patients and families, and to reduce the negative impact of the disease. However, relatively few studies have examined how sleep quality varies with changes in AD severity. The purpose of this study is to evaluate temporal variations in sleep quality of children with moderate to severe AD during flares and after 2 weeks of intensified AD treatment. We hypothesized that change in AD severity assessed by SCORAD index would correlate with change in sleep duration and efficiency.
MATERIAL AND METHODS
A prospective case-crossover pilot study was performed in children with AD between 2018 and 2019. Patients were recruited at Pediatric and Dermatology Clinics of the UC Christus Health Network. Inclusion criteria were: children aged between 6 months and 15 years old with AD diagnosed by Hanifin and Rajka criteria, moderate or severe AD defined by SCOring AD (SCORAD) index ≥ 25, and acute disease exacerbation (flare) defined as an episode requiring escalation of treatment or seeking additional medical advice11. All patients were treated with topical medicine during the trial, as patients were not receiving either topical or systemic treatment at recruitment. In addition, systemic corticosteroids that could disrupt sleep patterns were purposefully avoided. Non-inclusion criteria were: use of topical corticosteroids or calcineurin inhibitors in past 7 days, use of antibiotics in past 30 days, use of immunosuppression in past 30 days, physician-diagnosed neurologic disease, obstructive sleep apnea or another previously diagnosed sleep disorder, use of benzodiazepines or neuroleptic drugs. The Scientific Ethics Committee at the Pontificia Universidad Católica de Chile approved the study (approval number: 161206004, date of approval: 10/03/2017).
At baseline visit all subjects and their parents completed sociodemographic and clinical questionnaires regarding AD and sleep. The Brief Infant Sleep Questionnaire (BISQ) was applied to all subjects using its Spanish-adapted version. This is a widely used multidimensional questionnaire for infants and children that evaluated hours of night and daytime sleep, number and duration of nocturnal awakenings, among other sleep habit variables. The Patient-Oriented Eczema Measure, the preferred patient-reported outcome for assessing AD symptoms, and the Spanish-version of the Children's Dermatology Life Quality Index (CDLQI) were applied to all subjects. All subjects underwent a skin exam to evaluate AD severity with the SCORAD index by trained evaluators.
An actigraph (ActTrust®, Condor Instruments, Sao Paulo, SP, Brazil) was given to all subjects at baseline visit, and attached to the subject´s wrist. In infants that did not tolerate the wrist actigraph, it was placed around the ankle. Children and parents were asked to maintain a normal regular sleep-wake schedule for 14 days after this visit, following their usual sleep habits. All patients were prescribed intensified topical therapy to treat their AD flare. This included education on skin irritant prevention, use of emollient creams twice daily and mild and/or moderate topical corticosteroids twice daily. Patients were instructed to maintain therapy until their next visit 14 days later. At this time, all patients returned for re-evaluation with SCORAD and ending actigraphy monitoring.
Statistical analyses
Data analyses were performed using STATA software version 14 (College Station, TX). Wilcoxon signed-rank test was used to compare continuous variables at start and end of treatment. Average of actigraphic measurements during first and last 3 days were used to compare effects of treatment on sleep parameters. Correlations between numerical variables were performed using Spearman's rank correlation coefficient. All values are expressed as mean ± standard deviation unless stated otherwise. A two-tailed P value < 0.05 was considered statistically significant.
RESULTS
Ten children were included in the study. The subjects' age was 5.6 ± 5.3 years and 50% were female. At baseline, median SCORAD index was 58.5 (range 38-72), with 90% being severe (SCORAD > 50). Patient-oriented eczema severity assessed by POEM revealed that 70% of parents considered their children had severe eczema, 20% had moderate eczema, and 10% mild eczema. On CDLQI, 40% reported very or extremely important effect of AD on quality of life. Baseline demographic, clinical and actigraphic summary results are presented in Table 1.
Table 1.
Demographic, clinical and actigraphic characteristics of children with moderate to severe atopic dermatitis.
| Patient characteristics | (n = 10) | |
|---|---|---|
| Age (years) | 5.87 ± 5.34 | |
| Gender, male | 5 (50%) | |
| SCORAD severity | Severe | 9 (90%) |
| Moderate | 1 (10%) | |
| POEM | ||
| Mild eczema | 1 (10.0%) | |
| Moderate eczema | 2 (20.0%) | |
| Severe eczema | 7 (70.0%) | |
| CDLQI | ||
| Small effect | 3 (30.0%) | |
| Moderate effect | 3 (30.0%) | |
| Very large effect | 1 (10.0%) | |
| Extremely large effect | 3 (30.0%) | |
| Allergic comorbidity | 5 (41.7%) | |
| n (%) | Food allergy | |
| Allergic rhinitis | 5 (41.7%) | |
| Asthma | 4 (33.3%) | |
| Brief Infant Sleep Questionnaire | ||
| Time to bed, mean ± SD | 21:29 ± 01:30 | |
| Nocturnal sleep hours, mean ± SD | 6.85 ± 1.33 | |
| Daytime sleep hours, mean ± SD | 1.40 ± 1.17 | |
| Night awakenings, mean ± SD | 3.60 ± 4.38 | |
| Nocturnal wakefulness (hours), mean ± SD | 1.89 ± 1.65 | |
| Sleep-onset time, mean ± SD | 0.45 ± 0.35 | |
| Actigraphy | ||
| Nocturnal sleep hours, mean ± SD | 8.89 ± 1.07 | |
| Nocturnal sleep diagnosis | Decreased | 8 (88.9%) |
| n (%) | Mildly decreased | 1 (11.1%) |
| Nocturnal sleep efficiency (%), mean ± SD | 86 ± 6 | |
| Nocturnal sleep efficiency diagnosis (%) | Normal range | 5 (50%) |
| Low | 5 (50%) | |
| Night awakenings, mean ± SD | 11.28 ± 2.83 | |
| Night awakenings diagnosis, n (%) | Increased | 9 (100%) |
| Normal | 1 (10%) |
Actigraphic evaluation revealed that, at baseline, average sleep hours/day was 8.6 ± 1.4, in all cases below the recommended sleep duration for their age, while average number of awakenings was 14 ± 3.8, increased in 90% of subjects. In contrast, parental perception of sleep duration was 6.9 ± 1.3 hours/day, significantly less than duration evaluated by objective actigraphic measurement (p=0.007). In turn, parents responded that their children in average had 3.6 ± 4.4 awakenings/night, significantly less than the average number of awakenings measured by actigraphy (p=0.008).
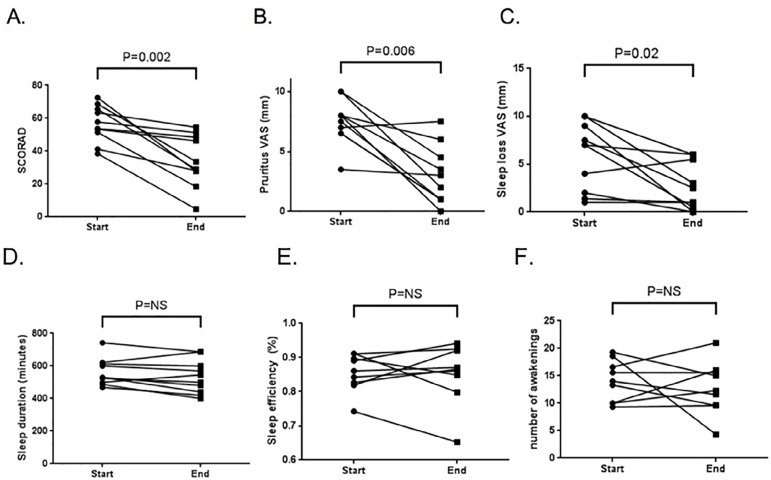
Mean nocturnal sleep efficiency at baseline was 86 ± 6 percent, being reduced in 50% of subjects. When comparing start and end of intensified treatment, median SCORAD decreased from 58.5 to 31.3 [range 4.7-54.6], p=0.005), with significant improvement in sleep loss and pruritus visual analogue scales (Figure 1). However, despite these improvements, objective sleep duration, efficiency, latency, number of awakenings, or wake after sleep onset measured by actigraphy did not vary significantly after intensified treatment.
Figure 1.
Change in severity of atopic dermatitis and sleep quality parameters assessed by actigraphy at start and end of intensified AD treatment. A. SCORAD; B. Pruritus visual analogue scale; C. sleep loss visual analogue scale; D. Sleep duration; E. sleep efficiency; F. nocturnal awakenings. All figures show actigraphic data.
Baseline SCORAD, POEM, and CDLQI scores did not correlate with actigraphic sleep quality parameters. Likewise, change in SCORAD, sleep loss and pruritus scales after intensified treatment did not correlate significantly with change in sleep duration, efficiency or other actigraphic sleep quality measurements.
DISCUSSION
The results of the present study confirm the association of moderate-severe AD during flares with sleep alterations, revealing decreased sleep duration, low efficiency and increased awakenings. These results are consistent with evaluations performed in AD studies from different populations in which sleep abnormalities have been evaluated12,13. Interestingly, our study shows that parents underestimate both sleep duration and number of awakenings in their children. This suggests that parental questionnaires may not accurately reflect these parameters in children with AD. Possible reasons for this fact are various. One possible cause is parental tiredness and underrecognition of child's sleep characteristics. Sleep habits may also vary from one household to another, e.g., if the child does sleep in his/her own bedroom or with the parents. Hence, observation of awakenings and sleep duration may be underestimated in those parents who do not sleep with their child near to them.
Although multiple studies have evaluated sleep in AD patients, most of these have assessed sleep by subjective questionnaires or within eczema severity scores (e.g. SCORAD). Several studies have applied sleep questionnaires to the parents of children with AD, generally observing that about 60% of AD patients have sleep alterations, which increases to 83% during flares14. Many of these studies report prolonged nocturnal wakefulness, sleep fragmentation, increased number and duration of arousals, and decreased sleep efficiency compared to healthy controls10,15,16. Reid et al.16 also noted that sleep disturbance was far greater during AD flares than when AD was controlled.
In our study, parental evaluation of sleep loss at baseline (i.e. during flare) correlated with AD severity evaluated by SCORAD, but not with actigraphy-measured sleep quality. This is in agreement with Bringhurst et al.17 who found that activity measured by actigraphy was not associated with severity. Two studies have concomitantly used actigraphy and infrared video to evaluate scratching, showing that AD patients have greater restlessness and wrist activity due to scratching throughout the night and this correlated with AD severity18,19. It is possible that subjective assessment of sleep may be influenced by AD severity per se, or that actigraphy may be missing other signs related to sleep loss such as scratching, daytime fatigue and irritability, that parents could be using to evaluate their child's sleep.
It is noteworthy that intensified AD treatment significantly improved SCORAD, pruritus and sleep loss visual analogue scales as expected, but was not associated with improvement in sleep duration, efficiency, latency, number of awakenings or wake after sleep onset. A large recent study of sleep quality in children with AD from the UK showed that children with active AD had sleep duration similar to children without AD, but worse sleep quality20. Interestingly, even children with inactive AD had significantly increased odds of impaired sleep quality. This suggests that sleep abnormalities may remain even after AD improves, in agreement with our results.
As a pilot study, the small number of subjects limits our research. In addition, actigraphy is not the gold standard of sleep quality evaluation, and as discussed previously, may not detect some signs of impaired sleep quality.
In conclusion, children with moderate and severe AD have sleep quality abnormalities, with decreased sleep duration, low sleep efficiency and increased awakenings. Improvement in AD severity upon intensified AD treatment was associated with improved parental perception of sleep loss, but not of objective sleep quality assessed by actigraphy.
ACKNOWLEGDEMENT
This work was supported by the Fondo Nacional de Desarrollo Científico y Tecnológico [grant numbers 1180397, 1160858] and Resident Research Grant 2015 of the School of Medicine, Pontificia Universidad Católica de Chile. PEB was supported by FONDECYT number 1180397.
REFERENCES
- 1.Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361(9352):151–160. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- 2.Camfferman D, Kennedy JD, Gold M, Simpson C, Lushington K. Sleep and neurocognitive functioning in children with eczema. Int J Psychophysiol. 2013;89:265–272. doi: 10.1016/j.ijpsycho.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Lewis-Jones S. Quality of life and childhood atopic dermatites: the misery of living with childhood eczema. Int J Clin Pract. 2006;60(8):984–992. doi: 10.1111/j.1742-1241.2006.01047.x. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg JI. Association between childhood eczema and headaches: an analysis of 19 US population-based studies. J Allergy Clin Immunol. 2016;137(2):492–499. doi: 10.1016/j.jaci.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatites: summary of a report for the national eczema association. J Invest Dermatol. 2016;137(1):26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135(1):56–66. doi: 10.1038/jid.2014.325. [DOI] [PubMed] [Google Scholar]
- 7.Simpson EL, Bieber T, Eckert L, Wu R, Ardeleanu M, Graham NM, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–498. doi: 10.1016/j.jaad.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Reuveni H, Chapnick G, Tal A, Tarasiuk A. Sleep fragmentation in children with atopic dermatitis. Arch Pediatr Adolesc Med. 1999;153:249–253. doi: 10.1001/archpedi.153.3.249. [DOI] [PubMed] [Google Scholar]
- 9.Camfferman D, Kennedy JD, Gold M, Martin AJ, Winwood P, Lushington K. Eczema, sleep, and behavior in children. J Clin Sleep Med. 2010;6(6):581–588. [PMC free article] [PubMed] [Google Scholar]
- 10.Ricci G, Bendandi B, Bellini F, Patrizi A, Masi M. Atopic dermatites: quality of life of young Italian children and their families and correlation with severity score. Pediatr Allergy Immunol. 2007;18(3):245–249. doi: 10.1111/j.1399-3038.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 11.Langan SM, Thomas KS, Williams HC. What is meant by a "flare" in atopic dermatites: A systematic review and proposal. Arch Dermatol. 2006;142:1190–1196. doi: 10.1001/archderm.142.9.1190. [DOI] [PubMed] [Google Scholar]
- 12.Dogan DG, Canaloglu SK, Kivilcim M, Kum YE, Topal E, Catal F. Sleep patterns of young children with newly diagnosed atopic dermatitis. Postepy Dermatol Alergol. 2017;34(2):143–147. doi: 10.5114/ada.2017.67080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishbein AB, Vitaterna O, Haugh IM, Bavishi AA, Zee PC, Turek FW, et al. Nocturnal eczema: review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. J Allergy Clin Immunol. 2015;136(5):1170–1177. doi: 10.1016/j.jaci.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Camfferman D, Kennedy JD, Gold M, Martin AJ, Lushington K. Eczema and sleep and its relationship to daytime functioning in children. Sleep Med Rev. 2010;14:359–369. doi: 10.1016/j.smrv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Bartlet LB, Westbroek R, White JE. Sleep patterns in children with atopic eczema. Acta Derm Venereol. 1997;77(6):446–448. doi: 10.2340/0001555577446448. [DOI] [PubMed] [Google Scholar]
- 16.Reid P, Lewis-Jones MS. Sleep difficulties and their management in preschoolers with atopic eczema. Clin Exp Dermatol. 1995;20(1):38–41. doi: 10.1111/j.1365-2230.1995.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 17.Bringhurst C, Waterston K, Schofield O, Benjamin K, Rees JL. Measurement of itch using actigraphy in pediatric and adult populations. J Am Acad Dermatol. 2004;51(6):893–898. doi: 10.1016/j.jaad.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin K, Waterston K, Russell M, Schofield O, Diffey B, Rees JL. The development of an objective method for measuring scratch in children with atopic dermatitis suitable for clinical use. J Am Acad Dermatol. 2004;50(1):33–40. doi: 10.1016/s0190-9622(03)02480-0. [DOI] [PubMed] [Google Scholar]
- 19.Ebata T, Iwasaki S, Kamide R, Niimura M. Use of a wrist activity monitor for the measurement of nocturnal scratching in patients with atopic dermatitis. Br J Dermatol. 2001;144(2):305–309. doi: 10.1046/j.1365-2133.2001.04019.x. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez FD, Chen S, Langan SM, Prather AA, McCulloch CE, Kidd SA, et al. Association of atopic dermatitis with sleep quality in children. JAMA Pediatr. 2019;173(5):e190025. doi: 10.1001/jamapediatrics.2019.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]