Abstract
Background
Although not fully understood, diabetes mellitus (DM) is thought to be associated with cardiac fibrosis and stiffness due to alteration of myocardial extracellular matrix (ECM). Newer CMR techniques may be able to identify ECM expansion by measuring extracellular volume fraction (ECV). We utilized cardiac magnetic resonance imaging (CMR) to evaluate the association of alteration in the extracellular matrix (ECM) with diabetic status and its implications on incident heart failure (HF) events and all-cause mortality.
Methods
We studied 442 patients who underwent comprehensive contrast CMR to assess cardiac morphology and function, left ventricular (LV) replacement fibrosis and pre-post contrast T1 mapping to quantify ECV. The cohort did not have coexisting pathologies associated with ECV alteration. We categorized our final cohort based on diabetic status using criteria from the American Diabetic Association. Subsequent heart failure (HF) hospitalization and all-cause death were ascertained.
Results
Our patients were predominantly white with a median age of 57 with 48% being men. Compared with non-diabetes, diabetes (DM) was significantly associated with elevated ECV after adjusting for clinical and imaging covariates: β coefficient 1.33 [95% CI 0.22 to 2.44]; P = 0.02. Over a median follow-up of 24.5 (interquartile range 14.8–33.4) months, 52 deaths and 24 HF events occurred. Patients with DM and elevated ECV had the worst outcomes compared to patients with DM and normal ECV or non-diabetics. Elevated ECV remained an independent predictor of outcomes (hazard ratio 3.31 [95% CI 1.93 to 5.67]; P < 0.001) after adjusting for covariates.
Conclusions
Elevated ECV is an independent predictor of mortality among patients with DM and may have an additive effect with DM on outcomes. ECV may represent a novel noninvasive biomarker to evaluate severity of diabetic heart disease.
Keywords: diabetes mellitus, cardiovascular magnetic resonance, extracellular volume, diabetic cardiomyopathy
Journal Subject Terms: type 2 diabetes mellitus, cardiomyopathy, mortality/survival, heart failure, magnetic resonance imaging (MRI)
INTRODUCTION
The prevalence of type 2 diabetes mellitus (DM) is rising in the United States and globally [1–3]. Although rates of most complications from diabetes are declining, other less well-known pathologies associated with diabetes are emerging [4]. Cardiac chamber dimensions, function and structure are commonly found to be altered in patients with DM [5, 6]. Heart failure with preserved ejection fraction (HFpEF) has also found to be more prevalent in individuals with DM. There are many postulated pathophysiological mechanisms of HFpEF in diabetic patients, but development of myocardial fibrosis resulting in expansion of the extracellular matrix (ECM) appears to be one of the cardinal features [7, 8]. Histologically, the myocardium of such patients has been shown to have a higher degree of interstitial fibrosis [9, 10]. Novel cardiac magnetic resonance imaging (CMR) techniques have been used to measure the T1 relaxation time of the myocardium [11]. By acquiring the pre-contrast “native” and post-contrast T1 time of myocardium, myocardial extracellular volume fraction (ECV) can be calculated. ECV calculated by CMR has been shown to accurately reflect histologically derived quantification of ECM or interstitial fibrosis [12]. The association of elevated ECV with adverse outcomes has been demonstrated in studies in the diabetic and other patient populations [12–16].
In order to probe the effect of type 2 diabetes mellitus on the myocardium, we had two specific aims: 1) compare the association of clinical and CMR-derived parameters in diabetic, prediabetic, and non-diabetic patients all without overt obstructive CAD, valvular heart disease or systolic left ventricular (LV) dysfunction; and 2) determine the association of ECV with the composite of heart failure and all-cause mortality events in all patients and in sub-cohorts of diabetic, prediabetic, and non-diabetic patients.
METHODS
Patient Cohort
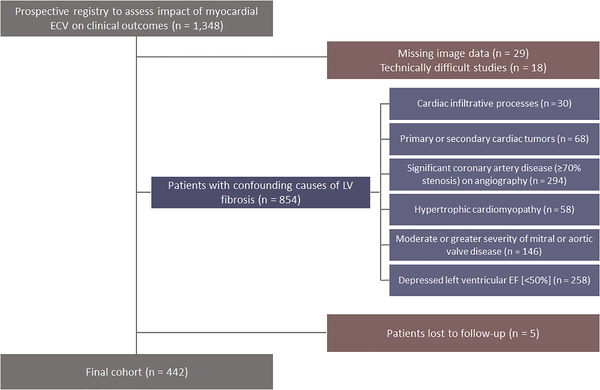
The study was approved by the hospital’s Institutional Review Board and conformed to the Declaration of Helsinki and all participating volunteers and patients provided written informed consent. Because of confidentiality issues, data sets and study materials are safeguarded by the Houston Methodist Research Institute and cannot be made available to outside parties. We enrolled patients into a prospective registry that was formed to assess the impact of ECV on clinical outcomes. Recruitment of 1,348 patients that were referred for a clinically indicated CMR scan occurred from August 2011 to January 2015 at Houston Methodist Hospital (Houston, TX). The inclusion criteria for this study were ability to receive gadolinium-based contrast agent and being 18 years or older. The patient selection process is summarized in Figure 1. Our final cohort consisted of 442 patients. The most common indications for CMR in the study cohort were: congestive heart failure evaluation, assessment for intra-cardiac source of thromboembolism, structural heart disease interrogation and estimation of the severity of valve disorder.
Figure 1. Patient selection process.
ECV = extracellular volume; LV = left ventricular; CAD = coronary artery disease; EF = ejection fraction
Using the American Diabetes Association (ADA) criteria [17], we classified our final cohort based on the severity of impairment of glucose metabolism into the following groups:
Diabetic: Patients with documented hemoglobin A1c > 6.5%, OR having a random blood glucose level > 200 mg/dl with classic symptoms of hyperglycemia, OR patients using medication for hypoglycemia (no patients with metabolic syndrome and polycystic ovarian syndrome)
Prediabetic: Patients with documented hemoglobin A1c between 5.7% to 6.4%, OR having a fasting plasma glucose level between 100 and 125 mg/dl
Non-diabetic: Patients failing to meet any of the above criteria
Clinical Data
Registered nurses performed a thorough patient interview on demographics, medical history, medication use, and cardiovascular risk factors. This data was later verified by accessing patient medical records by a post-doctoral research fellow. Nurses also recorded patient height, weight, heart rate, and blood pressure. Prior to the CMR scan, all patients had blood tests to measure hematocrit and plasma creatinine levels either using i-STAT® analyzer or the hospital laboratory. The estimated glomerular filtration rate (eGFR) was calculated using “The Modification of Diet in Renal Disease” study (MDRD) equation [18]. Patients with eGFR < 30 ml/min could not receive gadolinium-based contrast and were not enrolled in the study. Cardiac rhythm for each patient was assessed during the scan and included in the database.
CMR Imaging Protocol
Patients were scanned on Magnetom Avanto 1.5 Tesla or Magnetom Verio 3.0 Tesla magnetic resonance scanners (Siemens Medical Solutions, Erlangen, Germany). Scans were performed by dedicated CMR technologists. Gadolinium based contrast agents used for this study were gadopentetate dimeglumine (95.7%) and gadoversetamide (4.3%).
Cine images
Balanced steady-state free precession (bSSFP) sequences was used to acquire cine images of the heart in a stack-axis stack (base-apex), and standard 4-, 3-, and 2-chamber LV views. The typical parameters were: in plane spatial resolution 1.7 to 2.0 mm x 1.4 to 1.6; slice thickness 6 mm, with 4 mm interslice gap; and temporal resolution of 35 to 40 msec.
Late gadolinium enhancement (LGE) images
All patients received a bolus of intravenous gadolinium-based contrast agent at a dose of 0.15 to 0.20 mmol/kg. LGE images were obtained 10 minutes after the contrast administration. Images were acquired using a phase-sensitive inversion recovery (PSIR) and magnitude-only (MAG) pulse sequence. Global LGE burden was calculated by detecting LGE within each of the 17-segments of the left ventricle. The extent of LGE within each segment was graded as a percentage which was then cumulatively added to generate a global LV scar burden in percentage [19, 20].
T1 mapping images
An ECG-gated modified Look-Locker inversion recovery sequence (MOLLI) with motion correction was performed at a mid LV short axis level in a matching position for pre and post gadolinium contrast imaging (~ 15 minutes) [21] The pre-contrast MOLLI acquisition was performed using a 5(3)3 sampling scheme and the post contrast acquisition using a 4(1)3(1)2 sampling scheme.
CMR Image Analysis
Cardiac chamber volumes were measured using cine images. Ventricular volumes and EF were determined by manually tracing the endocardial borders in each slice from the short-axis stack in end systole and diastole. LV mass was calculated measuring the area between the endocardial and epicardial tracing in each slice. Left atrial (LA) volume was measured using the biplane-area length method in 4- and 2-chamber LV long axis views. Calculation of LV scar was made by measuring the amount of LGE using images acquired by PSIR and MAG sequences. Matching the location at which cine images were obtained, LGE images were acquired at the exact position. Long-axis and short-axis views confirmed presence of LGE in any segment.
Using the T1 maps, ECV was calculated. The myocardium was divided into 6 segments based off of the AHA 17-segment model. ECV measurement was performed using the CVI42 software. The LGE image corresponding to the T1 maps was reviewed for LGE presence; segments with LGE were excluded from the ECV calculations. After tracing the endocardial and epicardial borders on the T1 maps, 20% of the myocardium on either side was excluded from the measurement of myocardial T1 time for each of the eligible segments. T1 time for the blood pool was measured after careful exclusion of papillary muscles. Extracellular volume fraction was calculated using the following formula:
Hematocrit was measured in the laboratory by the blood sample provided by all patients prior to the scan. Global scar-free ECV value was calculated as the mean of the independently calculated ECV for all the eligible segments.
Extracellular volume fraction measured from our healthy volunteers helped us derive the upper limit of normal for ECV. Patients with ECV greater than 30.0% were classified as having elevated ECV which was the upper 95% bounds derived from the healthy volunteers [22].
Outcome Measures
Follow-up was conducted through phone interviews with participants or review of available electronic health records (EHR). Outcomes were defined as composite heart failure (HF) and all-cause mortality events. HF events were ascertained using ACC/AHA endpoint criteria [23]. This was defined as an urgent, unscheduled clinic/office/emergency department visit or hospital admission with a primary diagnosis of HF with new or worsening symptoms requiring initiation or intensification of specific HF treatment. Deaths were noted from EHR, phone calls to relatives, and infrequently by Social Security Death Index. The median duration of follow-up for our cohort was 24.5 months (interquartile range 14.8 to 33.4 months).
Statistical Analysis
Patient characteristics were reported as frequencies and proportions for categorical variables and as median and interquartile range (IQR) for continuous variables. Differences across groups were determined by Chi-square or Fisher’s exact tests for categorical variables and Kruskal Wallis test for continuous variables as appropriate. Univariate and multiple linear regression analyses were used to identify the characteristics being associated with ECV. Different diagnostic tests were used to evaluate the key assumptions for the linear regression model as part of the modeling such as Stata’s scatter plot (for linearity), pnorm (for the normality of the residuals), and collin (for the multicollinearity) commands. Assumptions were also tested for the Cox proportional hazard model to ensure all the assumptions were met (such as, multicollinearity and proportional-hazards). Univariate survival analysis and multivariable Cox proportional hazards modeling were performed to determine the characteristics associated with composite event. Variables having a P value of <0.2 in the univariate analysis or being considered as clinically important were investigated further by the multivariable modeling. Variable selection for the Cox proportional hazards models was conducted using the Bayesian model averaging (BMA) method [24, 25]. The best model was selected based on the smallest Bayesian information criterion (BIC). The discrimination power of predicting models were assessed using the C-statistic. Event-free survival for the composite events of mortality and heart failure was depicted by the adjusted survival curves based on the Cox models and stratified by diabetes status. All the analyses were performed on Stata version 16.1 (StataCorp LP, College Station, TX, USA). A P value of <0.05 was considered statistically significant.
RESULTS
At baseline, patients with prediabetes and diabetes tended to be older and were more likely to be overweight and be an inpatient at the time of the scan. Similarly, there was an increasing prevalence of history of hypertension, dyslipidemia, and family history of CAD as diabetic status worsened (Table 1). Patient with diabetes had a higher Atherosclerosis Risk in Communities (ARIC) Heart Failure Risk score [26] than patients with prediabetes and no diabetes. Patients with diabetes were more likely to be using renin-angiotensin-aldosterone system (RAAS) inhibitors, beta-blockers, statins, diuretics and digoxin. For cardiac parameters measured by CMR, patients with worsening diabetic status tended to have higher prevalence of LV scar and decreased bi-ventricular end-diastolic, end-systolic, and stroke volumes. Of note, the LV scar pattern within the prediabetic and diabetic population was exclusively non CAD type suggesting that these were unlikely to be unrecognized myocardial infarction. ECV significantly increased between each group in direct correlation with diabetic status. To highlight this relationship, patients with diabetes were grouped into diabetes with non-insulin therapy (DM-NIT) and diabetes with insulin therapy (DM-IT); as a surrogate marker of chronicity and severity of disease. There was an increasing prevalence of patients with elevated ECV with worsening diabetic status (Figure 2).
Table 1.
Baseline characteristics of patient population
| Variable* | All (n = 442) | Non-Diabetic (n = 296) | Prediabetic (n = 76) | Diabetic (n = 70) | P-value† |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), median (IQR) | 57 (43, 66) | 53 (38, 65) | 59 (52, 68) | 61.5 (56, 67) | <0.001 |
| Female | 228 (51.6%) | 152 (51.4%) | 39 (51.3%) | 37 (52.9%) | 0.97 |
| Race/Ethnicity | 0.33 | ||||
| White | 323 (75.6%) | 225 (78.4%) | 53 (71.6%) | 45 (68.2%) | |
| Black | 60 (14.1%) | 35 (12.2%) | 12 (16.2%) | 13 (19.7%) | |
| Asian | 17 (4.0%) | 10 (3.5%) | 3 (4.1%) | 4 (6.1%) | |
| Hispanic | 1 (0.2%) | 0 (0.0%) | 1 (1.4%) | 0 (0.0%) | |
| Other | 26 (6.1%) | 17 (5.9%) | 5 (6.8%) | 4 (6.1%) | |
| Black | 60 (14.1%) | 35 (12.2%) | 12 (16.2%) | 13 (19.7%) | 0.24 |
| Anthropometric Data | |||||
| BSA (m2), median (IQR) | 1.9 (1.7, 2.1) | 1.9 (1.7, 2.1) | 2.0 (1.8, 2.1) | 2.0 (1.8, 2.2) | 0.03 |
| BMI (kg/m2), median (IQR) | 27.3 (23.7, 31.4) | 25.8 (23.2, 30.4) | 27.5 (23.9, 32.3) | 29.7 (25.9, 37.5) | <0.001 |
| Systolic BP (mmHg), median (IQR) | 126.0 (116.0, 138.0) | 126.0 (114.0, 136.0) | 129.5 (121.5, 139.5) | 126.0 (118.0, 139.0) | 0.11 |
| Diastolic BP (mmHg), median (IQR) | 75.0 (65.0, 84.0) | 75.0 (65.0, 84.0) | 74.0 (65.5, 84.0) | 73.0 (65.0, 83.0) | 0.92 |
| Heart Rate (bpm), median (IQR) | 70.5 (62.0, 81.0) | 70.0 (61.0, 79.0) | 70.0 (60.0, 83.0) | 78.0 (67.0, 88.0) | 0.01 |
| Inpatient | 138 (31.2%) | 73 (24.7%) | 33 (43.4%) | 32 (45.7%) | <0.001 |
| Clinical Data | |||||
| Current Smoker | 27 (6.3%) | 20 (6.9%) | 3 (4.2%) | 4 (6.0%) | 0.68 |
| History of atrial fibrillation | 93 (21.0%) | 60 (20.3%) | 20 (26.3%) | 13 (18.6%) | 0.44 |
| History of hypertension | 235 (53.2%) | 141 (47.6%) | 41 (53.9%) | 53 (75.7%) | <0.001 |
| History of dyslipidemia | 175 (39.6%) | 96 (32.4%) | 38 (50.0%) | 41 (58.6%) | <0.001 |
| Family history of CAD | 204 (49.0%) | 128 (46.0%) | 29 (40.8%) | 47 (70.1%) | <0.001 |
| HbA1c, median (IQR) | 5.5 (5.2, 6.1) | 5.3 (5.0, 5.4) | 6.0 (5.8, 6.2) | 6.5 (5.9, 6.9) | <0.001 |
| Hematocrit, median (IQR) | 39.9 (35.6, 43.2) | 40.7 (37.0, 43.7) | 37.4 (34.7, 41.4) | 38.5 (33.3, 42.1) | <0.001 |
| eGFR (ml/min), median (IQR) | 79.7 (65.2, 103.1) | 79.7 (65.4, 100.6) | 79.0 (63.9, 105.9) | 80.1 (64.0, 103.8) | 0.96 |
| ARIC Heart Failure Risk Score | |||||
| ARIC HF risk score (%), median (IQR) | 3.5 (0.8, 10.5) | 2.3 (0.4, 7.9) | 4.4 (1.3, 13.6) | 12.1 (6.0, 24.3) | <0.001 |
| ARIC HF risk score categories | <0.001 | ||||
| <5.0% | 250 (56.6%) | 193 (65.2%) | 43 (56.6%) | 14 (20.0%) | |
| 5.0–9.9% | 78 (17.6%) | 49 (16.6%) | 11 (14.5%) | 18 (25.7%) | |
| 10.0–19.9% | 53 (12.0%) | 27 (9.1%) | 10 (13.2%) | 16 (22.9%) | |
| ≥20.0% | 61 (13.8%) | 27 (9.1%) | 12 (15.8%) | 22 (31.4%) | |
| Medications Use | |||||
| RAAS inhibitors | 157 (35.5%) | 93 (31.4%) | 26 (34.2%) | 38 (54.3%) | 0.002 |
| ACE | 94 (21.3%) | 55 (18.6%) | 18 (23.7%) | 21 (30.0%) | 0.09 |
| ARB | 54 (12.2%) | 36 (12.2%) | 6 (7.9%) | 12 (17.1%) | 0.23 |
| Spironolactone | 20 (4.5%) | 7 (2.4%) | 4 (5.3%) | 9 (12.9%) | <0.001 |
| β-blockers | 192 (43.8%) | 116 (39.3%) | 34 (45.9%) | 42 (60.9%) | 0.01 |
| Calcium channel blockers | 75 (17.2%) | 43 (14.8%) | 16 (21.1%) | 16 (22.9%) | 0.17 |
| HMG reductase inhibitors | 158 (36.0%) | 81 (27.6%) | 38 (50.0%) | 39 (55.7%) | <0.001 |
| Diuretics | 110 (25.1%) | 65 (22.2%) | 17 (22.4%) | 28 (40.6%) | 0.01 |
| Digoxin | 23 (5.3%) | 14 (4.8%) | 1 (1.3%) | 8 (11.6%) | 0.02 |
| Anticoagulants | 86 (19.8%) | 52 (17.9%) | 22 (29.3%) | 12 (17.4%) | 0.08 |
| Warfarin use | 67 (15.2%) | 41 (13.9%) | 17 (22.7%) | 9 (12.9%) | 0.14 |
| Heparin use | 20 (4.6%) | 11 (3.8%) | 5 (6.6%) | 4 (5.8%) | 0.51 |
| Antiplatelets | 140 (32.0%) | 83 (28.1%) | 29 (38.2%) | 28 (41.8%) | 0.04 |
| Aspirin | 133 (30.2%) | 79 (26.7%) | 26 (34.2%) | 28 (40.6%) | 0.054 |
| Thienopyridines | 21 (4.8%) | 11 (3.7%) | 8 (10.5%) | 2 (2.9%) | 0.04 |
| Hypoglycemics | 56 (14.5%) | 0 (0%) | 0 (0%) | 56 (80%) | <0.001 |
| Oral hypoglycemic | 29 (0.07%) | 0 (0%) | 0 (0%) | 29 (41.4%) | <0.001 |
| Insulin | 27 (0.07%) | 0 (0%) | 0 (0%) | 27 (38.6%) | <0.001 |
| Cardiac MRI Findings | |||||
| LA Volume [Index] (ml/m2) | 82.3 (62.4, 108.1) | 83.5 (61.8, 106.3) | 87.1 (61.7, 114.9) | 76.8 (64.9, 109.9) | 0.59 |
| LVEDV [Index] (ml/m2) | 120.0 (99.0, 151.0) | 124.0 (103.0, 151.5) | 112.5 (91.0, 153.5) | 106.0 (90.0, 136.0) | 0.01 |
| LVESV [Index] (ml/m2) | 38.0 (27.0, 52.0) | 40.0 (28.0, 52.0) | 36.0 (26.5, 53.0) | 33.0 (26.0, 51.0) | 0.20 |
| LVSV [Index] (ml/m2) | 82.0 (67.0, 98.0) | 85.0 (69.0, 100.5) | 77.5 (66.5, 96.0) | 69.5 (61.0, 91.0) | 0.003 |
| LVEF (%) | 68.0 (61.0, 74.0) | 67.5 (62.0, 74.0) | 68.0 (61.0, 74.0) | 69.0 (58.0, 74.0) | 0.95 |
| LV CO [Index] (l/min/m2) | 3.0 (2.5, 3.5) | 3.0 (2.6, 3.5) | 2.8 (2.5, 3.3) | 2.8 (2.5, 3.5) | 0.07 |
| LV Mass [Index] (g/m2) | 60.9 (51.5, 70.5) | 60.6 (50.6, 70.5) | 58.3 (51.3, 70.9) | 62.1 (53.9, 71.0) | 0.55 |
| RVEDV [Index] (ml/m2) | 135.0 (104.0, 167.0) | 139.0 (111.5, 168.5) | 126.0 (96.0, 157.0) | 124.0 (95.0, 161.0) | 0.01 |
| RVESV [Index] (ml/m2) | 57.0 (43.0, 76.0) | 57.5 (44.0, 78.0) | 57.0 (36.0, 72.5) | 53.5 (38.0, 76.0) | 0.11 |
| RVSV [Index] (ml/m2) | 76.0 (61.0, 93.0) | 79.5 (63.5, 96.0) | 74.0 (58.5, 86.5) | 65.0 (55.0, 88.0) | 0.002 |
| RVEF (%) | 57.0 (52.0, 62.0) | 57.0 (52.0, 62.0) | 58.0 (52.0, 63.0) | 57.0 (51.0, 62.0) | 0.65 |
| ECV (%) | 28.5 (26.1, 31.3) | 27.9 (25.9, 31.0) | 29.1 (26.5, 31.4) | 30.3 (27.8, 33.0) | 0.001 |
| Native T1 Time (msec) | 1170 (1043, 1252) | 1120 (1047, 1255) | 1100 (1031, 1229) | 1110 (1028, 1280) | 0.027 |
| Scar, median (IQR) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 2.0) | 0.01 |
| Any Scar | 89 (20.1%) | 49 (16.6%) | 16 (21.1%) | 24 (34.3%) | 0.004 |
| Outcomes | |||||
| Death | 52 (11.8%) | 22 (7.4%) | 13 (17.1%) | 17 (24.3%) | <0.001 |
| Heart failure | 23 (5.2%) | 9 (3.0%) | 3 (3.9%) | 11 (15.7%) | <0.001 |
| Composite event | 68 (15.4%) | 26 (8.8%) | 15 (19.7%) | 27 (38.6%) | <0.001 |
| Follow-up time (months) | 24.5 (14.8, 33.4) | 24.2 (15.3, 31.8) | 27.3 (18.0, 35.6) | 21.5 (10.9, 35.7) | 0.14 |
Values are in number (%) unless otherwise indicated
BMI = body mass index; BSA = body surface area; BP = blood pressure; RAAS = renin-angiotensin-aldosterone system; HMG = 3-hydroxy-3-methyl-glutaryl; LA = left atrial; LV = left ventricular; RV = right ventricular; EDV = end diastolic volume; ESV = end systolic volume; SV = stroke volume; EF = ejection fraction; ECV = extracellular volume; ARIC = Atherosclerosis Risk In Communities; HF = heart failure
Figure 2. Association of ECV with diabetic status.
Prevalence of patients with ECV greater than 30% in the non-diabetic, prediabetic and diabetic groups. Patients with diabetes were further divided by hypoglycemic therapy into non-insulin therapy (DM-NIT) and insulin therapy (DM-IT). There is an increasing prevalence of patients with elevated ECV with worsening ADA diabetic classification status.
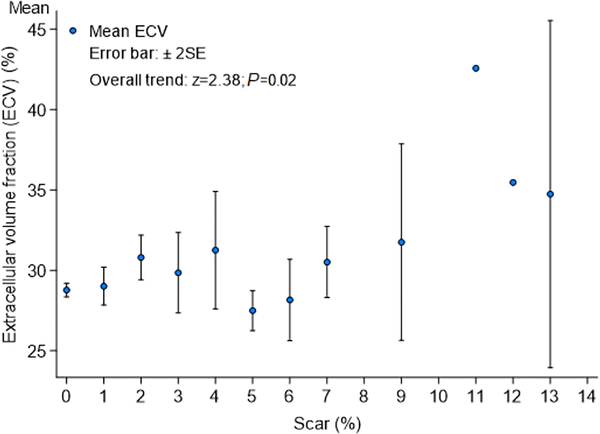
To test whether demographic and clinical variables were associated with elevated ECV, odds ratios were calculated for each variable on a univariate model. Age, female gender, black race, body surface area, blood pressure, heart rate, estimated glomerular filtration rate, increasing ARIC HF risk score, LA volume, LV cardiac output and LV scar showed significant association with elevated ECV. History of hypertension was not associated with elevated ECV (P = 0.88), whereas diabetes mellitus had a strong association with elevated ECV (β coefficient 1.72 [95% confidence interval [CI] 0.67 to 2.78]; P = 0.001). On a multivariable analysis including other relevant predictors of elevated ECV, diabetes mellitus retained its significant association with elevated ECV (β coefficient 1.33 [95% CI 0.22 to 2.44]; P = 0.02) (Table 2). We also measured the correlation of ECV with LV scar using a multiple linear regression model; a visual representation of the distribution of ECV in LV scar strata is shown in Figure 3.
Table 2.
Relationship between ECV and diabetes mellitus
| Variable | Univariate | P-value | Multivariate | P-value |
|---|---|---|---|---|
| β coeficient (95% CI) | β coeficient (95% CI) | |||
| Diabetic Status | ||||
| Non-diabetic | (reference) | (reference) | ||
| Prediabetic | 0.68 (−0.34, 1.70) | 0.19 | 0.59 (−0.41, 1.59) | 0.25 |
| Diabetic | 1.72 (0.67, 2.78) | 0.001 | 1.33 (0.22, 2.44) | 0.02 |
| History of dyslipidemia | −0.38 (−1.16, 0.40) | 0.34 | −0.83 (−1.63, −0.04) | 0.04 |
| eGFR | 0.02 (0.00, 0.03) | 0.01 | 0.02 (0.01, 0.04) | <0.001 |
| ARIC HF risk score | 0.04 (0.01, 0.07) | 0.01 | 0.03 (0.00, 0.06) | 0.04 |
| LA Volume [Index] | 0.01 (0.00, 0.02) | 0.03 | 0.02 (0.01, 0.02) | 0.001 |
| LVEDV [Index] | −0.01 (−0.02, 0.00) | 0.07 | −0.01 (−0.02, 0.01) | 0.35 |
| LVEF | 0.00 (−0.05, 0.04) | 0.97 | 0.00 (−0.05, 0.04) | 0.91 |
| LV CO [Index] | 0.63 (0.20, 1.06) | 0.004 | −0.03 (−0.05, −0.01) | 0.01 |
| Scar | 0.47 (0.26, 0.68) | <0.001 | 0.55 (0.33, 0.78) | <0.001 |
CI = confidence interval; eGFR = estimated glomerular filtration rate; Atherosclerosis Risk in Communities; HF = heart failure; LA = left atrial; LV = left ventricular; EDV = end diastolic volume; EF = ejection fraction; CO = cardiac output
Figure 3. Distribution of ECV in Scar Strata.
A visual representation of the distribution of ECV in patients with increasing amount of LV scar.
There were 52 (11.8%) all-cause mortality events, 23 (5.2%) HF events and 68 (15.4%) composite events. On univariate analysis, older age; black race; lower diastolic blood pressure; faster heart rate; inpatient status; history of hypertension; lower hematocrit; increasing ARIC HF risk; use of RAAS inhibitors, calcium channel blockers, digoxin, or diuretics; decreased LV end-diastolic, end-systolic volume and stroke volume were significant predictors of composite heart failure and mortality events. History of prediabetes (hazard ratio [HR] 2.05, [95% confidence interval (CI) 1.08 to 3.87]; P = 0.03), diabetes mellitus (HR 4.48, [95% CI 2.61 to 7.68]; P < 0.001) and elevated ECV (HR 1.01 [95% CI 1.01 to 1.02]; P < 0.001) were strong predictors of outcomes. Patients with elevated ECV and with a diagnosis of diabetes mellitus had the worst outcomes (Figure 4). Although elevated ECV was significantly associated with worse outcomes regardless of the diabetic status, patients with both elevated ECV and diabetes were likely to have a higher probability of composite outcomes than non-diabetes or prediabetic patients with elevated ECV (Figure 5). After adjusting for variables significant on a univariate model, history of diabetes (HR 2.74 [95% CI 1.49 to 5.04]; P = 0.001) and elevated ECV (HR 3.31 [95% CI 1.93 to 5.67]; P < 0.001) remained significant independent predictors of composite events (Table 3). A similar finding was found in the model using the continuous ECV (per %) with a HR of 1.16 (95% CI 1.10, 1.23) (Supplemental Table S1).
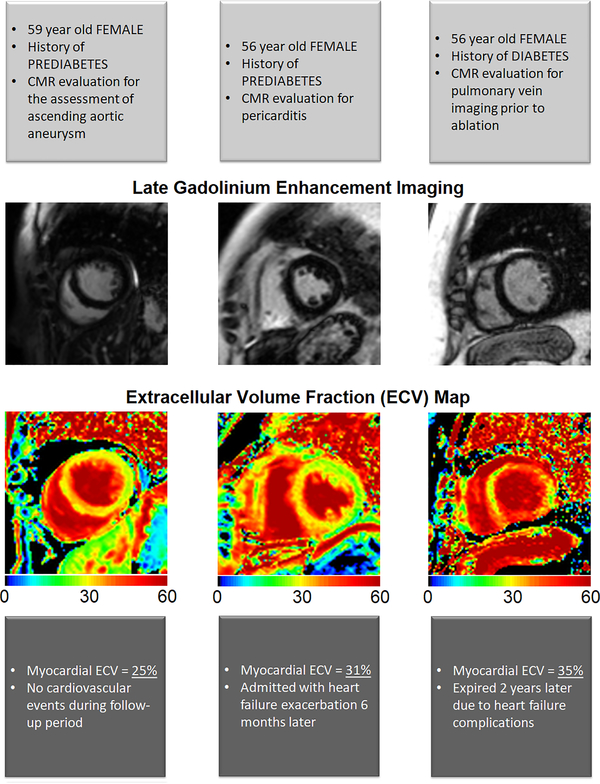
Figure 4. Late gadolinium enhancement images and ECV maps by diabetic status.
Example of two patients, with and without diabetes mellitus. Both patients had no late gadolinium enhancement (indicative of replacement fibrosis). However, the patient with diabetes had significantly higher amount of extracellular volume fraction (indicative of interstitial fibrosis) compared to the patient without diabetes mellitus.
Figure 5.
Adjusted survival curves based on the Cox models for the composite all-cause mortality and heart failure events by ECV and diabetic status. Patients with elevated ECV had worse outcomes in each group based on their diabetic status.
Table 3.
Association of elevated ECV, diabetes mellitus, and composite events
| Variable | Univariate | p-value | Multivariate | p-value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Diabetes Status | ||||
| Non-diabetic | (reference) | (reference) | ||
| Prediabetic | 2.05 (1.08, 3.87) | 0.03 | 2.00 (1.04, 3.85) | 0.04 |
| Diabetic | 4.48 (2.61, 7.68) | <0.001 | 2.74 (1.49, 5.04) | 0.001 |
| History of Dyslipidemia | 1.16 (0.72, 1.87) | 0.55 | 0.88 (0.53, 1.45) | 0.61 |
| ARIC HF risk score* | 1.03 (1.02, 1.04) | <0.001 | 1.02 (1.01, 1.03) | 0.01 |
| LVEF (%)* | 1.01 (0.99, 1.04) | 0.30 | 1.02 (0.99, 1.04) | 0.23 |
| ECV >30% | 3.70 (2.23, 6.15) | <0.001 | 3.31 (1.93, 5.67) | <0.001 |
| Scar (%)* | 1.03 (0.93, 1.15) | 0.56 | 1.03 (0.91, 1.16) | 0.67 |
As a continuous variable (%), median (IQR); Age used as the continuous variable in the ARIC HF risk score
HR = hazard ratio; CI= confidence interval; ARIC = Atherosclerosis Risk in Communities; HF = heart failure; LVEF = left ventricular ejection fraction; ECV = extracellular volume
DISCUSSION
A proposed mechanism for diabetic cardiomyopathy is the deposition of collagen in the extracellular matrix due to increased expression of TGF-β and connective tissue growth factor and decreased expression of matrix metalloproteinases [27, 28]. Prior histological and CMR-based studies have shown an association of diabetes mellitus and ECM expansion [9, 10, 13]. Using ECV as a noninvasive imaging biomarker for ECM expansion, we evaluated the significance of elevated ECV in a cohort of patients without significant CAD, left-sided valve disorders, or impaired LV systolic function (LVEF < 50%). Our study confirms the association of diabetes with increased ECV and shows greater degree of ECM expansion with more advanced ADA diabetic classification status. Even after accounting for confounding factors such as age, gender, race, history of hypertension and medications; diabetes mellitus remained an independent predictor of elevated myocardial ECV. Previous studies have shown the prognostic significance of ECV in patients with diabetes and other patient population. We were able to demonstrate the prognostic significance of ECV in prediabetic as well as diabetic patients.
There is a growing body of evidence that a key mechanism of heart failure includes myocardial fibrosis. Diseases that cause myocyte necrosis usually cause replacement fibrosis, while reactive or infiltrative processes (such as diabetes mellitus) cause interstitial fibrosis evident by increased ECM deposition. Some of the proposed pathogenesis for ECM deposition includes increased ECM synthesis by fibroblasts, decreased ECM degeneration by matrix metaloproteinases or presence of chronic myocardial inflammation. As diabetes advances, there is increased collagen deposition in the extracellular matrix [29]. The effect of increased extracellular volume on the myocardium is not well-understood. In a study [30], the association of elevated ECV and LV stiffness was measured in patients with heart failure with preserved EF (HFpEF). In this patient population, elevated ECV was associated with increased LV stiffness and decreased LV diastolic relaxation.
In addition to showing the significance of elevated ECV in patients with diabetes, elevated ECV was found to be an important prognostic marker in patients with prediabetic status. This association highlights the significance of elevated ECV in the non-diabetic population. Prediabetic individuals with elevated ECV may have a higher risk within the non-diabetic population, who may benefit from early or aggressive medical intervention to prevent heart failure or death.
The process of fibrotic response is complex with the involvement of a network of cytokines, hormones, growth factors and various cell types. Hence, development of medications targeting key components of myocardial fibrosis are available or under investigation. The RAAS inhibitors have been shown to reduce myocardial fibrosis in addition to improving outcomes in patients with heart failure with reduced ejection fraction [31–34]. In diabetic individuals RAAS inhibitors are commonly utilized for their affect in slowing down diabetic nephropathy. However, the beneficial effect of RAAS inhibitors may also include thwarting myocardial fibrosis and preventing diabetic cardiomyopathy. Novel oral hypoglycemic therapy in patients with type 2 diabetes mellitus like sodium-glucose linked transporter type 2 (SGLT2) inhibitors have shown to lower rates of cardiovascular outcomes including heart failure hospitalization [35]. One of the speculated mechanisms of action of SGLT2 inhibitors in reducing heart failure outcomes is believed to be reduction in myocardial fibrosis [36]. Animal models with SGLT2 inhibitors have shown reduction in interstitial fibrosis and improvement in diastolic function [37, 38]. In humans, a small study has shown improvement in diastolic function and reduction in LV mass with short-term Empagliflozin use [39]. Studies like the REFORM trial will shed more light on the effect of SGLT2 on LV remodeling in humans [40]. The use of T1 mapping techniques by CMR may help in monitoring the anti-fibrotic effect of SGLT2 inhibitors. Early recognition of ECM expansion via CMR in diabetic patients may help lower mortality associated with elevated ECV by initiating appropriate anti-fibrotic therapy.
LIMITATIONS
Some limitations of our study exist. Important baseline characteristics such as disease chronicity and lipid profile at the time of scan could not be gathered reliably. Although we excluded most common cardiac pathologies, the patient population we included in our study had some clinical indication to undergo a CMR scan; as such a selection bias exists. Myocardial ischemic workup was conducted only in patients having clinical suspicion of symptomatic CAD. Therefore subclinical CAD could not be ruled out in the final patient population. Within the non-diabetic group, there is the possibility of unrecognized prediabetes or diabetes due to lack of uniformly measured hemoglobin A1c or fasting blood glucose levels. The effect of this misclassification bias may imply that the true magnitude of differences between the non-diabetic and prediabetic/diabetic groups may be understated. Another limitation we had in our study was that we only calculated ECV at the mid-ventricular level. An analysis of cardiac death was not made in our cohort, because unlike all-cause mortality, such analysis can suffer from misclassification bias.
CONCLUSION
Diabetes mellitus is associated with elevated myocardial ECV. The presence of elevated ECV in patients with diabetes is an independent predictor of adverse cardiovascular outcomes. Elevated ECV has an additive effect on all-cause mortality and heart failure outcomes in patients with diabetes. Extracellular volume fraction measurement by CMR may represent a novel noninvasive biomarker to evaluate ECM alterations in diabetes mellitus and to study potential effects of diabetic therapies.
Supplementary Material
Clinical Perspective.
It is well known that patients with diabetes mellitus have a higher prevalence of heart failure with preserved ejection fraction. A postulated mechanism is the development of interstitial myocardial fibrosis, which results in expansion of the extracellular matrix. A novel cardiac magnetic resonance (CMR) technique which measures myocardial extracellular volume fraction (ECV) can be employed to quantify extracellular matrix expansion. In our study we measured ECV and other CMR phenotypic characteristics in a cohort of patient without overt coronary artery disease, valvular heart disease or systolic left ventricular dysfunction. We confirmed the association of diabetes with elevated ECV despite adjustment for confounding factors. We were also able to demonstrate the prognostic significance of ECV in prediabetic as well as diabetic patients. This highlights the prognostic importance of increased ECV in the non-diabetic population and reinforces the fact that diabetes mellitus is a spectrum and that the cardiovascular sequelae may begin earlier in the spectrum. Further study is warranted to determine if at-risk individuals without overt diabetes may be candidates for early or aggressive medical therapy to prevent morbidity and mortality. Using ECV as a marker of interstitial fibrosis, effect of medications targeting key components of myocardial fibrosis can be measured. Renin-angiotensin-aldosterone system inhibitors may halt myocardial fibrosis in patients without overt diabetes mellitus while sodium-glucose linked transporter type 2 inhibitors may exert their heart failure lowering effect by potentially reducing myocardial interstitial fibrosis.
Acknowledgment
We thank the patients at Houston Methodist Hospital for participating in this study. We thank Drs. Mohammed Chamsi-Pasha, Nickalaus Gramze, Mohamad Ghosn, Stephen Pickett, Grant Pickett, Patrick Green, Ms. Laura Gerik and Ms. Isadora Daniels for their grand support. We would also like to thank the technologists and nurses of the CMR laboratory at Houston Methodist Hospital.
Sources of Funding
Dr. Shah receives support from the National Science Foundation (CNS-1931884), the National Institutes of Health (1R01HL137763-01), and the Beverly B. and Daniel C. Arnold Distinguished Centennial Chair Endowment
ABBREVIATIONS LIST
- ADA
American Diabetic Association
- ACC
American College of Cardiology
- AHA
American Heart Association
- ARIC
Atherosclerosis Risk in Communities
- bSSFP
balanced, steady state free precession
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance imaging
- ECM
extracellular matrix
- ECV
extracellular volume fraction
- EF
ejection fraction
- eGFR
estimated glomerular filtration rate
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- LA
left atrial
- LV
left ventricular
- LGE
late gadolinium enhancement
- MOLLI
modified Look-Locker inversion recovery
- RAAS
renin -angiotensin-aldosterone system
Footnotes
Disclosures
None of the authors have potential conflicts to report.
REFERENCES
- 1.Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endo. 2016;4: 537–47. [DOI] [PubMed] [Google Scholar]
- 2.Jaacks LM, Siegel KR, Gujral UP, Narayan KMV. Type 2 diabetes: A 21st century epidemic. Best Pract Res Cl En. 2016;30: 331–43. [DOI] [PubMed] [Google Scholar]
- 3.Shen J, Kondal D, Rubinstein A, Irazola V, Gutierrez L, Miranda JJ, Bernabe-Ortiz A, Lazo-Porras M, Levitt N, Steyn K, et al. A Multiethnic Study of Pre-Diabetes and Diabetes. Glob Heart. 2016;11: 61–70. [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370: 1514–23. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, Butler J, CaseyJr DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128: e240–e327. [DOI] [PubMed] [Google Scholar]
- 6.Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes H, Huikuri H, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34: 3035–87. [DOI] [PubMed] [Google Scholar]
- 7.Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: An Update Of Mechanism Contributing To This Clinical Entity. Circ Res. 2018;122: 624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jellis C, Wright J, Kennedy D, Sacre J, Jenkins C, Haluska B, Martin J, Fenwick J, Marwick TH. Association of Imaging Markers of Myocardial Fibrosis With Metabolic and Functional Disturbances in Early Diabetic Cardiomyopathy. Circ Cardiovasc Imaging. 2011;4: 693–702. [DOI] [PubMed] [Google Scholar]
- 9.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972; 30: 595–602. [DOI] [PubMed] [Google Scholar]
- 10.van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation. 1990;82: 848–55. [DOI] [PubMed] [Google Scholar]
- 11.Teixeira T, Hafyane T, Stikov N, Akdeniz C, Greiser A, Friedrich MG. Comparison of different cardiovascular magnetic resonance sequences for native myocardial T1 mapping at 3T. J Cardiov Magn Reson. 2016;18: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kammerlander AA, Marzluf BA, Zotter-Tufaro C, Aschauer S, Duca F, Bachmann A, Knechtelsdorfer K, Wiesinger M, Pfaffenberger S, Greiser A, et al. T1 Mapping by CMR Imaging: From Histological Validation to Clinical Implication. JACC Cardiovasc Imaging. 2016;9: 14–23. [DOI] [PubMed] [Google Scholar]
- 13.Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, Piechnik SK, Whelan CJ, Herrey AS, Gillmore JD, et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36: 244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maestrini V, Treibel TA, White SK, Fontana M, Moon JC. T1 Mapping for Characterization of Intracellular and Extracellular Myocardial Diseases in Heart Failure. Curr Cardiovasc Imaging Rep. 2014;7: 9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schelbert EB, Piehler KM, Zareba KM, Moon JC, Ugander M, Messroghli DR, Valeti US, Chang CH, Shroff SG, Diez J, Miller CA, et al. Myocardial Fibrosis Quantified by Extracellular Volume Is Associated With Subsequent Hospitalization for Heart Failure, Death, or Both Across the Spectrum of Ejection Fraction and Heart Failure Stage. J Am Heart Assoc. 2015;4: e002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015; 38 Suppl: S8–S16. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, van Lente F, Chronic Kidney Disease Epidemiology Collaboration. Using Standardized Serum Creatinine Values in the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate. Ann Intern Med. 2006;145: 247–254. [DOI] [PubMed] [Google Scholar]
- 19.Klem I, Heiberg E, van Assche L, Parker MA, Kim HW, Grizzard JD, Arheden H, Kim RJ. Sources of variability in quantification of cardiovascular magnetic resonance infarct size - reproducibility among three core laboratories. J Cardiovasc Magn Reson. 2017;19: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine NM, Tandon S, Kim HW, Shah DJ, Thompson T, Drangova M, White JA. Validation of sub‐segmental visual scoring for the quantification of ischemic and nonischemic myocardial fibrosis using late gadolinium enhancement MRI. J Magn Reson Imaging. 2013;38: 1369–76 [DOI] [PubMed] [Google Scholar]
- 21.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52: 141–146. [DOI] [PubMed] [Google Scholar]
- 22.Yang EY, Ghosn MG, Khan MA, Gramze NL, Brunner G, Nabi F, Nambi V, Nagueh SF, Nguyen DT, Graviss EA, et al. Myocardial Extracellular Volume Fraction Adds Prognostic Information Beyond Myocardial Replacement Fibrosis. Circ Cardiovasc Imaging. 2019;12: e009535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation. 2015;132: 302–61. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138: 923–36. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman L Bayesian Model Selection and Model Averaging. J Math Psychol. 2000;44: 92–107. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5: 422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57: 660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westermann D, Rutschow S, Jager S, Linderer A, Anker S, Riad A, Unger T, Schultheiss H, Pauschinger M, Tschope C. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes. 2007;56: 641–6. [DOI] [PubMed] [Google Scholar]
- 29.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47: 693–700. [DOI] [PubMed] [Google Scholar]
- 30.Rommel KP, von Roeder M, Latuscynski K, Oberueck C, Blazek S, Fengler K, Besler C, Sandri M, Lucke C, Gutberlet M, et al. Extracellular Volume Fraction for Characterization of Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2016;67: 1815–1825. [DOI] [PubMed] [Google Scholar]
- 31.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102: 1388–93. [DOI] [PubMed] [Google Scholar]
- 32.Lopez B, Querejeta R, Varo N, Gonzalez A, Larman M, Ubago JLM, Diez J. Usefulness of serum carboxy-terminal propeptide of procollagen type I in assessment of the cardioreparative ability of antihypertensive treatment in hypertensive patients. Circulation. 2001;104: 286–91. [DOI] [PubMed] [Google Scholar]
- 33.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, Marwick TH. Fibrosis and cardiac function in obesity: a randomised controlled trial of aldosterone blockade. Heart. 2013;99: 320–6. [DOI] [PubMed] [Google Scholar]
- 34.Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355: 1582–7. [DOI] [PubMed] [Google Scholar]
- 35.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins BT, Johansen OE, Woerle HJ, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373: 2117–28. [DOI] [PubMed] [Google Scholar]
- 36.Yuliya L, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications and Summary of Clinical Trials. Circulation. 2017;136: 1643–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019; 18: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi L, Zhu D, Wang S, Jiang A, Li F. Dapagliflozin Attenuates Cardiac Remodeling in Mice Model of Cardiac Pressure Overload. Am J Hypertens. 2019;32: 452–459. [DOI] [PubMed] [Google Scholar]
- 39.Verma S, Garg A, Yan AT, Gupta AK, Al-Omran M, Sabongui A, Teoh H, Mazer CD, Connelly KA. Effect of Empagliflozin on Left Ventricular Mass and Diastolic Function in Individuals With Diabetes: An Important Clue to the EMPA-REG OUTCOME Trial?. Diabetes Care. 2016;39: e212–e213. [DOI] [PubMed] [Google Scholar]
- 40.Singh JSS, Fathi A, Vickneson K, Mordi I, Mohan M, Houston JG, Pearson ER, Struthers AD, Lang CC. Research into the effect Of SGLT2 inhibition on left ventricular remodelling in patients with heart failure and diabetes mellitus (REFORM) trial rationale and design. Cardiovasc Diabetol. 2016;15: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.