Abstract
Metabotropic glutamate (mGlu) receptors are family C G protein-coupled receptors (GPCRs) that modulate neuronal excitability and synaptic transmission throughout the nervous system. Owing to recent advances in development of subtype-selective allosteric modulators of mGlu receptors, individual members of the mGlu receptor family have been proposed as targets for treating a variety of neurological and psychiatric disorders, including substance use disorders. In this chapter, we highlight preclinical evidence that allosteric modulators of mGlu receptors could be useful for reducing alcohol consumption and preventing relapse in alcohol use disorder (AUD). We begin with an overview of the preclinical models that are used to study mGlu receptor involvement in alcohol-related behaviors. Alcohol exposure causes adaptations in both expression and function of various mGlu receptor subtypes, and pharmacotherapies aimed at reversing these adaptations have the potential to reduce alcohol consumption and seeking. Positive allosteric modulators (PAMs) of mGlu2 and negative allosteric modulators of mGlu5 show particular promise for reducing alcohol intake and/or preventing relapse. Finally, this chapter discusses important considerations for translating preclinical findings towards the development of clinically useful drugs, including the potential for PAMs to avoid tolerance issues that are frequently observed with repeated administration of GPCR agonists.
Keywords: alcohol use disorder, metabotropic glutamate receptor, positive allosteric modulator, negative allosteric modulator, self-administration, chronic intermittent ethanol
1. Introduction
Chronic alcohol exposure is associated with widespread adaptations in brain function from the cellular to circuit level. Alcohol use disorder (AUD) affects millions of individuals worldwide, and is implicated in 3.3. million deaths per year along with substantial economic and medical consequences (World Health Organization, 2014; Sacks, Gonzales, Bouchery, Tomedi and Brewer, 2015). A variety of therapeutic strategies have been developed to treat AUD including counselling, community-based and pharmacological approaches (Jonas, Amick, Feltner, Bobashev, Thomas, Wines et al., 2014; Koob, Kenneth Lloyd and Mason, 2009; Miller and Wilbourne, 2002). While some of these treatments are efficacious in reducing alcohol drinking and relapse in individuals with AUD, the success rates, while encouraging, are less than ideal for any given therapy (Jonas et al., 2014; Maisel, Blodgett, Wilbourne, Humphreys and Finney, 2013; Miller and Wilbourne, 2002). Indeed, the most effective pharmacotherapeutics, acamprosate and naltrexone, appear to be somewhat less effective than other medications used for other mental health indications (Maisel et al., 2013). Furthermore, information about therapeutic options is not disseminated as widely as it could be (Carvalho, Heilig, Perez, Probst and Rehm, 2019), although the NIAAA Alcohol Treatment Navigator provides a new web-based tool to address this need (https://alcoholtreatment.niaaa.nih.gov/). Thus, there is an ongoing need for more effective AUD-targeted therapies and better information about potential treatments.
To effectively develop new pharmacological treatments AUD, an improved understanding of the neurotransmitter systems and circuits involved in alcohol-related behaviors is required. In recent years, alcohol-induced adaptations in glutamatergic circuits of the cerebral cortex, basal ganglia, and extended amygdala have been the subject of increased investigations (reviewed in Bell, Hauser, McClintick, Rahman, Edenberg, Szumlinski et al., 2016; Gremel and Lovinger, 2017; Hwa, Besheer and Kash, 2017). Adaptations in glutamatergic systems in these regions are associated with many maladaptive aspects of behavior that likely contribute to problematic alcohol use, including impaired behavioral flexibility, enhanced habit formation, emergence of negative affect during withdrawal, and susceptibility to relapse. Thus, targeting glutamatergic systems involved in alcohol-related maladaptive behaviors represents an important strategy for identifying novel therapeutic mechanisms.
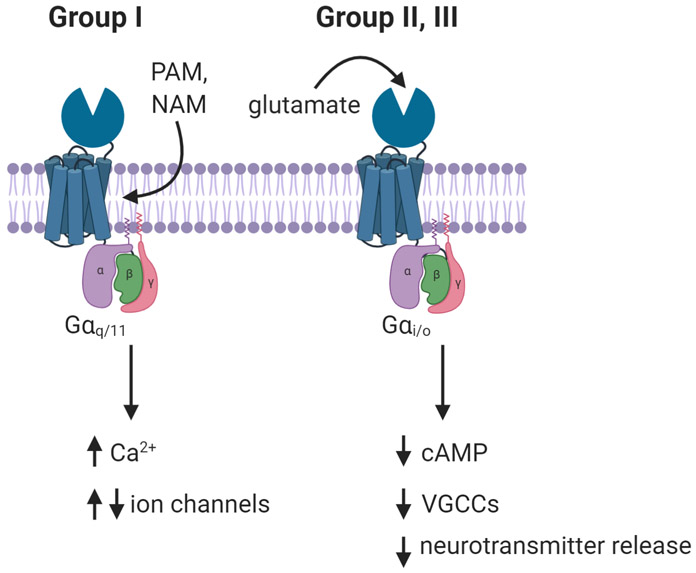
The metabotropic glutamate (mGlu) receptor family of G protein-coupled receptors (GPCRs) modulate glutamatergic transmission throughout the CNS and have received substantial attention as therapeutic targets in a wide variety of CNS disorders. The eight subtypes of mGlu receptors, which are class C GPCRs, are classified into three subgroups based on sequence homology, ligand-binding profiles, and shared downstream signaling pathways (Figure 1) (Niswender and Conn, 2010). Group I mGlu receptors include mGlu1 and mGlu5. These receptors typically couple to Gq/11 family G proteins and are typically found postsynaptically, where they generate second messenger signals, modulate neuronal excitability and synaptic transmission via effects on ion channels, and play important roles in synaptic plasticity. Group II mGlu receptors (mGlu2 and mGlu3) typically signal through Gi/o family G proteins, and are often found to inhibit glutamatergic transmission by acting at presynaptic sites. The group III mGlu receptors include mGlu4, mGlu6, mGlu7, and mGlu8, all of which typically signal through Gi/o family G proteins. Of these receptors, mGlu4, mGlu7, and mGlu8 are known to presynaptically regulate glutamatergic transmission, but also modulate release of other neurotransmitters as well. Conversely, expression of mGlu6 is historically considered to be restricted to the retina, and this receptor has not been of interest in the context of AUD. Each mGlu receptor subtype has a unique distribution in the CNS, and the physiological effects of activating each receptor subtype can vary widely depending on the specific neuron population in which it is expressed. In addition, mGlu receptors form obligatory dimers that can be either homo- or heterodimers, further expanding the diversity of CNS functions (Doumazane, Scholler, Zwier, Trinquet, Rondard and Pin, 2011; Ferre, Casado, Devi, Filizola, Jockers, Lohse et al., 2014).
Figure 1. Ligand binding sites and signaling downstream of mGlu receptors.
mGlu receptors have a large N-terminal venus fly trap domain that contains the glutamate binding site, whereas known PAM and NAM binding sites are typically in the transmembrane domain. Group I mGlu receptors (mGlu1 and mGlu5) couple to Gq/11 family G proteins and activate phospholipase C pathways to increase intracellular calcium levels. These receptors can also modulate activity of ion channels to impact neuronal excitability and synaptic transmission (i.e. via effects on ionotropic glutamate receptors). Groups II (mGlu2, mGlu3) and III (mGlu4, mGlu7, and mGlu8) couple to Gi/o family G proteins, which leads to inhibition of adenylyl cyclase and decreases in cyclic AMP production, but also impacts neurotransmitter release by inhibiting voltage-gated calcium channels (VGCCs) and directly modulating vesicle fusion machinery.
The mGlu receptor family has proven highly amenable to drug discovery efforts geared towards identification of allosteric ligands, as selective positive and/or negative allosteric modulators (PAMs, NAMs) suitable for use in vivo have been identified for most mGlu receptor subtypes (Hellyer, Leach and Gregory, 2017; Nickols and Conn, 2014). Notably, a number of recently developed drugs targeting mGlu receptors (particularly mGlu5 and mGlu2) have had properties suitable for advancement to clinical trials, allowing critical assessments of the translational validity of targets identified in preclinical studies. Allosteric ligands of mGlu receptors bind to sites on the receptor that are distinct from the N-terminal glutamate binding domain, often in the transmembrane domain (Dore, Okrasa, Patel, Serrano-Vega, Bennett, Cooke et al., 2014; Koehl, Hu, Feng, Sun, Zhang, Robertson et al., 2019; Wu, Wang, Gregory, Han, Cho, Xia et al., 2014). Because these allosteric sites are less conserved among receptor subtypes, targeting these sites has proven a fruitful approach to developing drugs selective for individual mGlu receptor subtypes (Conn, Lindsley, Meiler and Niswender, 2014; Foster and Conn, 2017; Leach and Gregory, 2017). The success of mGlu receptor drug discovery efforts has provided preclinical tools that are indispensable for studying the roles of mGlu receptors in a variety of CNS disorders, and this progress has been accompanied by extensive efforts to develop mGlu receptor allosteric modulators that have suitable pharmacodynamic, pharmacokinetic, and safety profiles for use in human neurological and psychiatric disorders (e.g., mavoglurant, basimglurant, AZD8529).
Substantial research efforts have focused on identifying roles for mGlu receptors in the etiology and treatment of AUD. On the genetic level, variants of at least one mGlu receptor are associated with high levels of drinking, and deletion of several mGlu receptor subtypes in rats or mice alters levels of alcohol reward and consumption. Chronic alcohol exposure can change both expression and function of various mGlu receptor subtypes in ways that support the emergence of maladaptive alcohol self-administration. This chapter will review evidence that mGlu receptors influence alcohol reward, consumption, and seeking in preclinical experimental systems, and that allosteric modulators of mGlu receptors, particularly mGlu5 and mGlu2, have effects on alcohol seeking and taking in animals that could translate to treatments for AUD. A list of allosteric modulators used in preclinical investigations of AUD and other substance use disorders (SUDs) can be found in Table 1. Although it is outside the scope of this chapter, there is extensive literature detailing preclinical investigations of mGlu receptor orthosteric and allosteric ligands on reward, consumption, and seeking of other psychoactive drugs, and we refer readers to several recent reviews that address these topics (Barnes, Sheffler, Semenova, Cosford and Bespalov, 2018; Caprioli, Justinova, Venniro and Shaham, 2018; Cross, Anthenelli and Li, 2018; Joffe, Centanni, Jaramillo, Winder and Conn, 2018; Johnson and Lovinger, 2016; Li and Markou, 2015; Scofield, Heinsbroek, Gipson, Kupchik, Spencer, Smith et al., 2016).
Table 1.
mGlu receptor PAMs and NAMs used in alcohol-related experiments.
| Receptor | PAM | NAM | Selected References |
|---|---|---|---|
| Group I | |||
| mGlu1 | CPCCOEt | Schroeder et al., 2005; Hodge et al., 2006; Lominac et al., 2006 | |
| JNJ16259685 | Besheer et al., 2008; Cozzoli et al., 2014; Lum et al., 2014 | ||
| mGlu5 | CDPPB | Gass et al., 2014; Gass et al., 2017 | |
| MPEP | McGeehan and Olive, 2003; Backstrom et al., 2004; McMillen et al., 2005; Hodge et al., 2006; Lominac et al., 2006; Besheer et al., 2008; Gupta et al., 2008; Schroeder et al., 2008; Lee et al., 2016 | ||
| MTEP | Cowen et al., 2005; Cowen et al., 2007; Adams et al., 2008; Adams et al., 2010; Cozzoli et al., 2014; Lee et al., 2018; Gobin and Schwendt, 2019 | ||
| Group II | |||
| mGlu2 | AZD8529 | Augier et al., 2016; Li et al., 2016; Sidhpura et al., 2010 | |
| BINA | Windisch and Czachowski, 2018 | ||
| JNJ-42153605 | Ahnaou et al., 2015, 2016 | ||
| AZD8418 | Li et al., 2016 | ||
| LY487379 | Nikiforuk et al., 2010; | ||
| Group III | |||
| mGlu7 | AMN082 (agonist) | Bahi, 2012; Kotlinska et al., 2019 |
2. Experimental systems for studying mGlu receptor relevance to AUD
A variety of organisms and behavioral paradigms have been used to examine the actions of acute and chronic ethanol that are thought to contribute to drinking. The majority of studies have used rodent models (e.g. rat and mouse), and these models have been used in almost all experiments examining mGlu roles in ethanol-induced neuroadaptations and drinking. Thus, we will focus on rodent models in the current discussion. We will not describe all the techniques used for these purposes, but will instead focus on those that have been used to examine mGlu roles in alcohol actions and alcohol drinking.
2.1. Evaluation of ethanol-mediated reward
To assess if ethanol has rewarding effects (i.e. if intoxication is a desired outcome), researchers often use the conditioned place preference (CPP) test (Huston, Silva, Topic and Muller, 2013; Valyear, Villaruel and Chaudhri, 2017). This involves ethanol exposure in one compartment of a chamber containing two or more total compartments. In other compartments the animal normally receives vehicle. Following training with the treatment/compartment combinations, the animal is given a test trial in which it can freely choose which compartment it prefers, and the percentage of time spent in the ethanol-paired compartment signals the animal’s ethanol preference. This paradigm has been used extensively in mice, and C57BL/6J mice (as well as transgenic mice with this genetic background) show preference for a compartment in which they received low-moderate ethanol doses. Interestingly, DBA/2J mice show stronger CPP for ethanol (Cunningham, Niehus, Malott and Prather, 1992), perhaps indicating more rewarding subjective ethanol effects in this strain. The ethanol CPP test is not administered often in rat for the odd reason that commonly-used rat strains do not exhibit CPP, but rather conditioned place aversion, after ethanol injection or intragastric infusion (Cunningham, 1981; Fidler, Bakner and Cunningham, 2004; Stewart and Grupp, 1981). While CPP is useful for assessing subjective reward, this is not the only factor that controls ethanol intake, and thus more direct measures of drinking or operant self-administration often show outcomes that appear to differ from CPP in the same animal or treatment conditions.
Locomotor activation and sensitization (i.e. increased locomotion following repeated drug administration) were originally touted as measures of rewarding drug actions (Wise and Bozarth, 1987). Sensitization to ethanol-induced locomotor activation can be seen in some rodent strains, but is highly dependent on dose, as some strains show little locomotor activation following acute ethanol administration. Several studies have now revealed discrepancies between findings with sensitization and CPP or other measures of drug reward, raising concerns as to whether this behavioral change provides information about reward.
2.2. Models of voluntary ethanol drinking
Investigators have also employed a variety of more direct measures of ethanol intake to determine animals’ willingness to consume the drug. The most straightforward technique is to provide the animal with a choice between two or more bottles (i.e. two-bottle choice), one containing ethanol dissolved in water and the other water alone (Griffin, 2014). The amount of ethanol consumed and the preference for ethanol compared to water are measured. Ethanol content is often increased from low (e.g. 5%) to high (20%) levels as the animals become more familiar with the solution. More sophisticated versions include “lickometers” or other devices to measure consumption rate and patterns. Different rat and mouse strains show varying ethanol intake and preference in two-bottle choice experiments, with C57BL/6J mice consistently observed to prefer alcohol (Belknap, Crabbe and Young, 1993; Yoneyama, Crabbe, Ford, Murillo and Finn, 2008). In addition to strain differences, sex also influences levels of voluntary ethanol consumption, and female mice are routinely reported to consume larger amounts of ethanol and show higher ethanol preference than male mice (Hilderbrand and Lasek, 2018). While this approach provides a ready index of how much ethanol an animal is willing to drink, all factors that control drinking, such as taste preference, contribute to the experimental outcome. Thus, two-bottle choice drinking and preference does not always provide a good index of how much a particular rodent likes the subjective effects of ethanol.
Researchers have also developed other relatively simple ethanol drinking paradigms based on more naturalistic patterns of activity and feeding/drinking in rodents. The drinking-in-the-dark paradigm is the most popular of these paradigms, as it is designed to assay drinking during the circadian period when mice, a nocturnal species, are most active (Crabbe, Phillips and Belknap, 2010; Thiele and Navarro, 2014). Mice are given access to ethanol in water for 2-4 hours starting a few hours into the dark period. They will generally drink more alcohol and achieve higher blood alcohol concentrations during this period in comparison to all-day drinking or drinking in the light period (Thiele and Navarro, 2014). It must be noted that placement of the ethanol-containing bottle in the cage is one trigger for the enhanced drinking (Wilcox, Cuzon Carlson, Sherazee, Sprow, Bock, Thiele et al., 2014). Two-bottle choice variants of the paradigm have also been developed, but drinking is generally not as robust as in the original single-bottle version.
Early studies from the Wise laboratory found that rats would consume more ethanol in a two-bottle choice paradigm if the ethanol was only available every other day, as opposed to every day (Carnicella, Ron and Barak, 2014; Wise, 1973). This “intermittent access” paradigm has now been used in mice as well (Hwa, Chu, Levinson, Kayyali, DeBold and Miczek, 2011; Melendez, 2011). In both species, ethanol intake and preference increases across access sessions, and this escalation can lead to consumption of levels generally associated with intoxication. The factors that drive increased consumption in this paradigm are not clear, but may involve unique neural effects of cycles of intoxication and sobriety as well as the general finding that rodents increase rate of intake of commodities when access is tightly scheduled. The intermittent access paradigm affords a good test of how much animals are willing to escalate ethanol intake and effects of treatments on this intake. In some strains the schedule results in what might be considered binge or excessive drinking. However, it should be noted that drinking and preference levels usually fall back to the original level if continuous access is provided following intermittent access. This indicates that the animals do not show permanent enhancement of their desire to consume ethanol, and thus consume excessively only under conditions of intermittent access.
Rodents do not generally prefer the taste of ethanol, and thus paradigms have been developed to foster ethanol drinking by adding desired tastants during the initial phases of drinking. The most common procedure is “sucrose fading”, in which the sweetened ethanol solution is available when the animal first starts drinking, and then the sucrose is gradually removed, and ethanol content often increased, as the animal become more familiar with the ethanol taste (Tolliver, Sadeghi and Samson, 1988). This procedure generally increases ethanol intake relative to unadulterated ethanol solution once the fading is complete. A similar paradigm has also been developed with monosodium glutamate (an umami tastant) (McCool and Chappell, 2012). This procedure is especially effective in DBA mice who have a strong aversion to ethanol taste, a poor preference of sweet, but a strong preference for umami. The major concern with tastant fading procedures is that neural associations will develop to the tastant or tastant-alcohol combination that are different from those produced by ethanol alone. However, humans often consume ethanol with tastants, so this procedure has some naturalistic appeal.
2.3. Operant self-administration of ethanol
Different paradigms for operant ethanol self-administration have been developed to determine what actions rodents will perform to obtain the drug. This can be viewed as a measure of alcohol seeking or wanting (Samson and Czachowski, 2003). Operant ethanol self-administration models have been used extensively to preclinically evaluate interventions that reduce ethanol seeking or taking. Indeed, there is some predictive validity observed using these models, as some drugs used to treat human AUD (e.g. acamprosate, naltrexone, nalmefene) reduce ethanol self-administration in rodents (Bachteler, Economidou, Danysz, Ciccocioppo and Spanagel, 2005; Czachowski, Legg and Samson, 2001; Herz, 1997; Spanagel, 2017). The most commonly used self-administration technique involves rodents pressing a lever to receive access to an ethanol drinking opportunity. The ethanol is usually delivered to a receptacle or a “dipper” that becomes accessible following the correct number of presses. Versions of ethanol self-administration involving intragastric, intravenous and intracerebral injection have also been developed. These approaches are understandably more difficult than the drinking-based design due to the need for surgery, patency of delivery lines, and irritation of blood vessels or stomach by ethanol. Nonetheless, useful information has been obtained with these techniques, as they eliminate taste effects on preference. In the case of intracerebral injection investigators can also locate brain regions involved in alcohol consumption (McBride, Murphy and Ikemoto, 1999).
As with all instrumental conditioning paradigms, different schedules of reinforcement can be used to control operant SI. Simple fixed ratio (FR) schedules are most often employed to maximize pressing rates. The progressive ratio (PR) schedule in which increased numbers of presses must be made for each consecutive reinforcer is a favorite paradigm for assessing how hard animals will work for the drug. Random interval schedules have also been used to produce lever pressing that is independent of outcome (so-called stimulus-response behavior) (Corbit and Janak, 2016). Ethanol self-administration paradigms designed to separate “seeking’ and taking have also been devised (Samson and Czachowski, 2003). In these paradigms animals are usually taught to perform one action (e.g. lever press) to obtain access to perform a second action (e.g. chain pull) that results in ethanol delivery.
Investigators often using predictive stimuli or conditioned reinforcers (e.g. brief light or tone presentations) in their self-administration training and testing paradigms. The predictive “cues” are thought to mimic environmental stimuli that lead to enhanced ethanol seeking or reinstatement of behavior (Venniro, Caprioli and Shaham, 2016). Indeed, cue-induced reinstatement is readily measured in most ethanol self-administration paradigms. Operant responding for ethanol might also be invigorated by prior presentation of the predictive stimulus through Pavlovian-to-Instrumental transfer (PIT) conditioning, although whether this translates to a meaningful increase in ethanol intake has been called into question (Lamb, Schindler and Pinkston, 2016). Conditioned reinforcers can support operant actions in the absence of the primary reinforcer. It should be noted that there is evidence for differential engagement of different neural circuits in self-paced (e.g. cue-free) versus PIT-driven operant responding (Cartoni, Balleine and Baldassarre, 2016; Yin and Knowlton, 2006). Thus, it is important to consider the choice of experimental design when evaluating neurobiological underpinnings of ethanol self-administration. The general features of the environmental context also alter ethanol self-administration (Valyear et al., 2017), and context-induced reinstatement has also been demonstrated for this drug. Experimenter administration of low doses of ethanol can also induce relapse to ethanol self-administration, perhaps mimicking the first drink in relapse (Venniro et al., 2016).
There is extensive literature from both humans and laboratory animal studies indicating interactions between stress and drug use disorders (Blaine and Sinha, 2017; Koob, 2015). A prominent hypothesis is that stressful life events can drive individuals toward relapse to drug seeking and taking that provides temporary relief from the effects of stress. This has been modeled in rodents using approaches for stress-induced reinstatement of drinking and self-administration (Becker, 2017; Cozzoli, Tanchuck-Nipper, Kaufman, Horowitz and Finn, 2014b; Spanagel, Noori and Heilig, 2014). With respect to ethanol, the majority of studies have been performed in rat, although there is increasing evidence that mice will also show stress-induced reinstatement (Mantsch, Baker, Funk, Le and Shaham, 2016).
2.4. Compulsive ethanol seeking
Various measures of persistent drug seeking and taking in the absence of drug or the presence of adverse events have been developed with the aim of assessing if seeking/taking are compulsive (Hopf and Lesscher, 2014). These include measuring responses when the drug is not delivered and examining effects of foot shock or other painful and stress inducing stimuli on seeking/taking. With ethanol drinking, investigators have also developed techniques for taste adulteration, particularly by adding quinine to the ethanol solution, to discourage drinking. Animals that continue to drink in spite of the addition of quinine are considered to show compulsive drinking. In this paradigm it is important to demonstrate that experience with the ethanol/quinine mixture does not alter the aversion to quinine taste.
2.5. Ethanol effects on cognition
Other experimental approaches have been used to determine consequences of chronic ethanol exposure or drinking that could affect decision-making. Performance in set-shifting tasks is disrupted following chronic ethanol exposure in rats and mice (Gass, Glen, McGonigal, Trantham-Davidson, Lopez, Randall et al., 2014a; Spear, 2018). This cognitive impairment may be related to ethanol effects that impair prefrontal cortex and its connections to the hippocampus. Spatial learning that involves hippocampus is also disrupted by ethanol (Van Skike, Goodlett and Matthews, 2019). Chronic ethanol exposure and drinking also promote stimulus-response (aka habit) learning in mice and rats (Barker, Corbit, Robinson, Gremel, Gonzales and Chandler, 2015; Corbit and Janak, 2016; Everitt and Robbins, 2016; Renteria, Baltz and Gremel, 2018). This is generally thought to involve effects on cortico-basal ganglia circuitry. Indeed, there is evidence that chronic ethanol exposure enhances behaviors that involve the sensorimotor striatum that is part of this circuitry (DePoy, Daut, Brigman, MacPherson, Crowley, Gunduz-Cinar et al., 2013). The combination of disrupted cognition and increased habitual responding following chronic ethanol exposure may foster poor decision making, especially in the presence of the drug.
2.6. Passive ethanol exposure models
It should be noted that effects of chronic ethanol in rodents are examined using a variety of drug exposure methods. In addition to the drinking and self-administration paradigms mentioned above, several methods for involuntary ethanol administration have been used over the years. These include intragastric gavage, intraperitoneal injection, liquid diet consumption in which no alternative liquid is provided, and vapor inhalation-based exposure (Crabbe, Harris and Koob, 2011). These “forced administration” paradigms are very useful, as rodents generally will not voluntarily consume doses of ethanol that produce blood and brain levels commonly observed in humans. These procedures also ensure predictable blood and brain concentrations on a regular schedule, free from the variability observed with drinking or ethanol self-administration. Passive ethanol administration is often employed in conjunction with voluntary drinking or self-administration paradigms to produce escalation of drinking and to allow investigators to study volitional alcohol consumption during dependence (Griffin, 2014; Lopez and Becker, 2014; Vendruscolo and Roberts, 2014). As we will see, some effects of mGlu receptor ligands on alcohol-related behaviors are particularly robust in animals that have been exposed to both passive and voluntary forms of alcohol exposure. Thus, experimenter-administered ethanol provides information that complements results obtained from drinking and self-administration paradigms.
3. Group I mGlu receptors in preclinical models of alcohol exposure
The group I mGlu receptors, particularly mGlu5, have received considerable attention as therapeutic targets for the treatment of alcohol and other substance use disorders due to their ability to modulate glutamatergic transmission in brain regions associated with psychoactive drug misuse (Caprioli et al., 2018; Joffe et al., 2018; Kasten, Holmgren and Wills, 2019). Alcohol exposure affects expression and function of mGlu1 and mGlu5, and pharmacological reversal of adaptations that support alcohol taking and seeking could therefore modify maladaptive behaviors. Alcohol exposure causes upregulation of mGlu1 mRNA in several brain regions, whereas mGlu5 regulation by alcohol appears to be more complex. Voluntary alcohol drinking or passive alcohol exposure can increase mGlu1 expression in the nucleus accumbens, central amygdala, and hippocampus depending on the model used (Alasmari, Bell, Rao, Hammad and Sari, 2018; Cozzoli, Courson, Wroten, Greentree, Lum, Campbell et al., 2014a; Cozzoli, Goulding, Zhang, Xiao, Hu, Ary et al., 2009; Goulding, Obara, Lominac, Gould, Miller, Klugmann et al., 2011; Lee, Coelho, McGregor, Solton, Cohen and Szumlinski, 2016b; Obara, Bell, Goulding, Reyes, Larson, Ary et al., 2009; Szumlinski, Ary, Lominac, Klugmann and Kippin, 2008), and mice selectively bred for high levels of binge-like drinking show higher expression levels of mGlu1 and the associated scaffolding protein Homer2 in the nucleus accumbens (Cozzoli, Courson, Caruana, Miller, Greentree, Thompson et al., 2012; Cozzoli et al., 2009). Alcohol effects on mGlu5 expression are more complex and depend on the alcohol exposure paradigm, species, and time since last alcohol exposure. Voluntary drinking and passive forms of alcohol exposure are associated with increased mGlu5 mRNA in rodents (Lee et al., 2016b) although a longitudinal study of mGlu5 availability measured using positron emission tomography (PET) showed that voluntary alcohol consumption decreases mGlu5 availability in the amygdala and hippocampus (de Laat, Weerasekera, Leurquin-Sterk, Gsell, Bormans, Himmelreich et al., 2019). In humans, lower mGlu5 receptor availability in cortical and striatal regions is associated with alcohol dependence (Ceccarini, Leurquin-Sterk, Crunelle, de Laat, Bormans, Peuskens et al., 2019; Leurquin-Sterk, Ceccarini, Crunelle, de Laat, Verbeek, Deman et al., 2018), whereas in moderate social drinkers, mGlu5 availability in cortical, striatal, and thalamic regions is positively correlated with euphoric effects of alcohol (Leurquin-Sterk, Ceccarini, Crunelle, Weerasekera, de Laat, Himmelreich et al., 2018). Higher mGlu5 availability in the striatum at onset of alcohol abstinence is associated with risk of relapse (Ceccarini et al., 2019). Conversely, after ~1 month of alcohol abstinence, higher mGlu5 levels in the amygdala are associated with lower temptation to drink (Akkus, Mihov, Treyer, Ametamey, Johayem, Senn et al., 2018), indicating that there are complex, region-specific dynamics of mGlu5 expression in humans with a history of heavy alcohol use.
In addition to effects on expression, alcohol impacts the modulatory effects of mGlu1 and mGlu5 in multiple brain regions. For example, acute application of alcohol to nucleus accumbens slices disrupts group I mGlu receptor-mediated potentiation of NMDA receptors (Mishra, Zhang and Chergui, 2012). Group I mGlu receptor-mediated mobilization of retrograde endocannabinoid signaling in the nucleus accumbens and cerebellum are also disrupted by acute alcohol exposure (Carta, Mameli and Valenzuela, 2006; Renteria, Jeanes and Morrisett, 2014; Su, Sun and Shen, 2010), and CIE disrupts mGlu1/5-mediated long-term depression in the CA1 region of the hippocampus (Wills, Baucum, Holleran, Chen, Pasek, Delpire et al., 2017).
Although NAMs of mGlu1 have been evaluated in preclinical models of AUDs, the vast majority of studies have focused on mGlu5. Thus, this section will primarily explore the effects of mGlu5 NAMs in models of alcohol reward, drinking, seeking, and will briefly consider the potential for mGlu5 NAMs to impact negative affective states induced by alcohol withdrawal.
3.1. mGlu5 NAM effects on alcohol reward
Blockade of mGlu5 reduces expression of ethanol-induced CPP in both rats and mice (Kotlinska, Bochenski and Danysz, 2011; Lominac, Kapasova, Hannun, Patterson, Middaugh and Szumlinski, 2006). In CPP studies that were designed to dissociate effects of mGlu5 blockade on acquisition, expression, and reinstatement following extinction training in mice, the mGlu5 NAM MPEP (2-Methyl-6-(phenylethynyl)pyridine) was found to differentially modulate discrete phases involved in reward conditioning in mice. Specifically, when MPEP was administered during conditioning, it did not affect place preference post-conditioning (Lee, Choe, Yang, Choi, Cheong, Jang et al., 2016a; McGeehan and Olive, 2003). This finding aligns with intact low-dose ethanol CPP observed in mice with global mGlu5 deletion (Bird, Kirchhoff, Djouma and Lawrence, 2008). Conversely, MPEP administration prior to a post-conditioning test or a reinstatement challenge reduces both expression and reinstatement of ethanol CPP (Lee et al., 2016a). Effects of mGlu5 NAMs on ethanol-induced changes in neurotransmission could contribute to the observation that these drugs reduce ethanol reward. Notably, MPEP pretreatment prevents ethanol-induced changes in dopamine, glutamate and GABA levels in the nucleus accumbens of mice (Lominac et al., 2006).
3.2. mGlu5 NAM effects on alcohol consumption and seeking
The findings that mGlu5 is involved in alcohol reward suggest that mGlu5 blockade could interfere with the primary reinforcing effects of alcohol, and thus reduce voluntary alcohol consumption. mGlu5 NAM effects have been evaluated in a variety of models of volitional alcohol consumption, including two-bottle choice and DID tasks as well as operant ethanol self-administration models. Across rat and mouse strains and models, there has been remarkable consistency in the reported ability of mGlu5 NAMs to reduce alcohol consumption. Chronic administration of the mGlu5 NAM MTEP (3-((2-Methyl-1,3-thiazol-4-yl)ethynyl)pyridine) reduces voluntary drinking in Fawn-Hooded and alcohol-preferring rats (Cowen, Djouma and Lawrence, 2005), and MPEP administration reduces alcohol intake in a mouse DID model (Gupta, Syed, Revis, Miller, Martinez, Cohn et al., 2008) and in Myers high ethanol-preferring rats (McMillen, Crawford, Kulers and Williams, 2005). Similarly, MTEP administration prior to binge drinking sessions reduces drinking in adolescent and adult mice of both sexes (Cozzoli et al., 2014a). Interestingly, adolescent female mice that are treated with MTEP during binge drinking show reduced alcohol consumption when re-introduced to alcohol after a period of abstinence. Conversely, MTEP treatment during binge drinking leads to higher levels of alcohol consumption upon re-exposure in adult female mice (Cozzoli et al., 2014a). Despite decreasing alcohol consumption during binge drinking sessions, MTEP does not significantly alter subsequent alcohol consumption in adolescent or adult male mice (Cozzoli et al., 2014a). Studies in mice lacking mGlu5 expression specifically in D1 dopamine receptor-expressing neurons suggest that mGlu5 activity in D1-expressing neurons is critical for escalation of alcohol drinking following forced abstinence (Parkitna, Sikora, Golda, Golembiowska, Bystrowska, Engblom et al., 2013). MPEP fails to reduce ethanol consumption or preference in mice with genetic deletion of the ε isoform of protein kinase C (PKCε) (Olive, McGeehan, Kinder, McMahon, Hodge, Janak et al., 2005). Further, PKCε-null mice show lower levels of voluntary alcohol consumption, suggesting that signaling through this pathway, perhaps downstream of mGlu5, supports alcohol consumption under normal conditions (Olive et al., 2005).
In addition to effects in voluntary drinking models, mGlu5 NAMs also reduce alcohol consumption in operant ethanol self-administration models. For example, in an operant ethanol self-administration paradigm designed to distinguish between appetitive and consummatory phases of self-administration in mice, MTEP reduced both appetitive responding and consumption (Cowen, Krstew and Lawrence, 2007), and this finding is supported by earlier studies showing reduced operant ethanol self-administration in mice after MPEP administration (Hodge, Miles, Sharko, Stevenson, Hillmann, Lepoutre et al., 2006; Lominac et al., 2006) and in alcohol-preferring rats following MPEP or MTEP administration (Cowen et al., 2005; Schroeder, Overstreet and Hodge, 2005). These effects likely involve mGlu5 in the nucleus accumbens, as direct infusion of MPEP or MTEP into the accumbens (but not into the dorsomedial striatum or medial prefrontal cortex) of alcohol-preferring rats also reduces alcohol self-administration (Besheer, Grondin, Cannady, Sharko, Faccidomo and Hodge, 2010; Gass and Olive, 2009). In addition to reduced alcohol self-administration, MPEP also reduces the breakpoint in alcohol-preferring rats during a progressive ratio task, suggesting a specific modulation of motivation to take ethanol (Besheer, Faccidomo, Grondin and Hodge, 2008b). Moreover, mGlu5 NAM administration reduces the subjective (discriminative stimulus) effects of ethanol in rats, which could contribute to reduced alcohol self-administration (Besheer and Hodge, 2005; Besheer, Stevenson and Hodge, 2006).
Reducing mGlu5 activity is also associated with inhibition of cue-induced reinstatement of ethanol seeking following extinction training. Systemic administration of MPEP reduces cue-induced reinstatement in Long Evans rats (Backstrom, Bachteler, Koch, Hyytia and Spanagel, 2004), and MPEP also attenuates cue-induced reinstatement in selectively-bred alcohol-preferring rats (Schroeder, Spanos, Stevenson, Besheer, Salling and Hodge, 2008). Interestingly, robust reductions of cue-induced reinstatement of ethanol seeking have been observed when MTEP is co-administered with either an antagonist of the CB1 cannabinoid receptors (Adams, Short and Lawrence, 2010) or an adenosine A2A receptor antagonist (Adams, Cowen, Short and Lawrence, 2008) in alcohol-preferring rats, suggesting that simultaneous targeting of mGlu5 and other receptor systems could be an effective strategy for prevention of relapse in AUD. Studies evaluating the effects of direct infusion of MTEP into the basolateral amygdala or nucleus accumbens prior to cue-induced reinstatement of ethanol seeking suggest that both regions contribute to effects on reinstatement following systemic MTEP administration (Sinclair, Cleva, Hood, Olive and Gass, 2012). In addition, systemic administration of MPEP attenuates reinstatement-induced increases in ERK phosphorylation in the basolateral amygdala and nucleus accumbens (Schroeder et al., 2008). The ability of mGlu5 NAMs to reduce relapse-like behavior is particularly interesting in light of recent findings in humans diagnosed with AUD that high striatal mGlu5 availability at the onset of alcohol abstinence is associated with a higher risk of relapse (Ceccarini et al., 2019). Although we have focused on findings specifically related to alcohol here, mGlu5 NAMs reduce taking and seeking of other psychoactive drugs as well (Caprioli et al., 2018), suggesting that these drugs affect circuitry that is commonly recruited during self-administration of many drugs.
Although preclinical investigations have focused on using mGlu5 NAMs to reduce ethanol taking and seeking, enhancement of mGlu5 activity using PAMs has also been explored for effects on drug seeking behavior. Interestingly, the mGlu5 PAM CDPPB (3-Cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide), which (along with other mGlu5 PAMs) has been shown to enhance learning in a variety of models, facilitates extinction of ethanol seeking in rat self-administration studies (Gass, Trantham-Davidson, Kassab, Glen, Olive and Chandler, 2014b). Further, CDPBB administration during extinction reduces subsequent sensitivity to cue-induced reinstatement. Rats exhibit impaired extinction of ethanol seeking following CIE, and this resistance to extinction can be alleviated by CDPPB administration (Gass, McGonigal and Chandler, 2017). Thus, while preclinical studies have largely focused on mGlu5 blockade for treatment of AUD, both positive and negative modulation of mGlu5 impact drug taking and seeking by modulating unique aspects of behavior.
3.3. mGlu5 NAM effects on negative affective states following alcohol withdrawal
Alcohol withdrawal after voluntary drinking or passive forms of exposure is associated with behaviors characteristic of negative affect including increased measures of anhedonia, avoidance, and anxiety-like behavior (Centanni, Bedse, Patel and Winder, 2019; Cover, Kerkhoff and Mathur, 2018; Holleran, Wilson, Fetterly, Bluett, Centanni, Gilfarb et al., 2016; Holleran and Winder, 2017; Koob, 2009; Lee, Coehlo, McGregor, Waltermire and Szumlinski, 2015; Lee, Coehlo, Solton and Szumlinski, 2017; Metten, Schlumbohm, Huang, Greenberg, Hack, Spence et al., 2018; Vranjkovic, Winkler and Winder, 2018), and negative affective states are viewed as a critical component of the addition cycle in humans (Koob and Volkow, 2016). There is extensive preclinical evidence that mGlu5 NAMs reduce behavioral despair and avoidance behavior in rodents (Barnes et al., 2018; Nickols and Conn, 2014; Terbeck, Akkus, Chesterman and Hasler, 2015), and the mGlu5 NAM fenobam (N-(3-Chlorophenyl)-N’-(4,5-dihydro-1-methyl-4-oxo-1H-imidazol-2-yl)urea) reduced anxiety (or anxiety-like behavior) in both preclinical assessments and in a clinical trial (Pecknold, McClure, Appeltauer, Wrzesinski and Allan, 1982; Porter, Jaeschke, Spooren, Ballard, Buttelmann, Kolczewski et al., 2005). These findings spurred investigation of mGlu5 NAM effects in rodent models of negative affect associated with alcohol withdrawal (reviewed in Kasten et al., 2019). In rats and mice with a history of alcohol exposure, mGlu5 NAMs (MPEP and MTEP) reverse avoidance/anxiety-like measures in elevated plus maze, light-dark box, and marble burying tests (Kotlinska and Bochenski, 2008; Kumar, Hapidin, Bee and Ismail, 2013; Lee, Coelho, Class, Sern, Bocz and Szumlinski, 2018a; Lee, Coelho, Class and Szumlinski, 2018b; Lee, Coelho, Sern, Class, Bocz and Szumlinski, 2017). Direct infusion of MTEP into the nucleus accumbens shell in mice replicates some, but not all, effects of systemic MTEP administration on alcohol withdrawal-induced negative affective behaviors (Lee et al., 2018a), suggesting that mGlu5 modulation in other circuitry is also involved.
3.4. Additional considerations regarding exploration of mGlu5 as a therapeutic target in AUD
Despite the extensive preclinical evidence that mGlu5 NAMs have potential for treating SUDs, to date there has been little progress translating these findings in clinical trials. Trials for smoking cessation conducted with the mGlu5 NAM mavoglurant (AFQ-056; Methyl (3aR,4S,7aR)-4-hydroxy-4-[(3-methylphenyl)ethynyl]octahydro-1H-indole-1-carboxylate) were completed (ClinicalTrials.gov ID: NCT00414752), but results of this trial have not been disclosed. A clinical trial evaluating the efficacy of mavoglurant in cocaine use disorder is also in progress (NCT03242928), and disclosure of results from this trial will be eagerly anticipated.
Concerns related to on- or off-target adverse effects of mGlu5 NAMs could hinder translation of this class of compounds to clinical use (Barnes et al., 2018). For example, mGlu5 NAMs including fenobam and AZD9272 (3-Fluoro-5-[3-(5-fluoro-2-pyridinyl)-1,2,4-oxadiazol-5-yl]benzonitrile) have produced pro-psychotic effects in clinical trials for other indications (Pecknold et al., 1982; Stahle, 2012), although recent evidence of off-target binding of these drugs suggests that the psychotic-like effects of these drugs could be independent of mGlu5 inhibition (Varnas, Cselenyi, Arakawa, Nag, Stepanov, Moein et al., 2019). Additional concerns stem from findings that enhancing mGlu5 activity with PAMs has pro-cognitive effects, raising the possibility that mGlu5 NAM administration could cause undesired cognitive impairments when used to decrease drug taking or relapse. Supporting this concern, when rats that have been trained to self-administer cocaine are assessed in a working memory task (delayed match-to-sample) during abstinence, they show markedly impaired performance with repeated MTEP administration (Gobin and Schwendt, 2019). As mentioned previously, the mGlu5 PAM CDPPB facilitates extinction of ethanol self-administration (Gass et al., 2014b). In addition, CDPPB reduces impairments in behavioral flexibility during adulthood in rats exposed to alcohol during adolescence (Gass et al., 2014a). These findings raise the possibility that mGlu5 activation rather than inhibition might be useful as a therapeutic strategy in conjunction with behavioral interventions for AUD treatment. If mGlu5-selective compounds advance toward evaluation for AUD, It will be important to consider how these pharmacotherapies might interact with behavioral interventions that reduce drug use or relapse in humans.
A final consideration is that many people diagnosed with AUD also use other psychoactive drugs. Thus, consideration of mGlu5 effects on alcohol taking and seeking must be considered in the context of polysubstance use. This concern is particularly relevant to nicotine, as roughly three quarters of individuals diagnosed with AUD also smoke cigarettes (Guydish, Passalacqua, Pagano, Martinez, Le, Chun et al., 2016). Notably, nicotine use is associated with reduced mGlu5 availability (Akkus, Ametamey, Treyer, Burger, Johayem, Umbricht et al., 2013; Hulka, Treyer, Scheidegger, Preller, Vonmoos, Baumgartner et al., 2014; Muller Herde, Mihov, Kramer, Mu, Adamantidis, Ametamey et al., 2019), which is likely to alter the efficacy of mGlu5 NAM treatment. As pharmacotherapies for AUDs progress to clinical trials, it will be critical to consider additional factors such as concurrent or former use of other drugs to identify subpopulations that are most amenable to the treatment in question.
3.5. Effects of mGlu1 modulation in models of alcohol exposure
Relative to mGlu5, mGlu1 modulation has received far less attention as a therapeutic strategy in AUD. Among the few reports that exist, there are several conflicting results. Multiple studies reported that the mGlu1 NAM CPCCOEt (7-(Hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester) does not significantly reduce alcohol self-administration in male P rats or male C57Bl/6J mice (Hodge et al., 2006; Schroeder et al., 2005). However, in another study, CPCCOEt decreased alcohol self-administration, voluntary drinking, and CPP in male C57Bl/6J mice (Lominac et al., 2006). Interestingly, CPCCOEt facilitates reduction of spontaneous locomotor activity following ethanol administration, which suggests that mGlu1 blockade increases sensitivity to sedating effects of alcohol (Lominac et al., 2006). However, another study failed to observe this effect on locomotor activity (Sharko and Hodge, 2008). Another mGlu1 NAM, JNJ16259685 ((3,4-Dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone), decreases operant responding for alcohol in male P rats under both a fixed ratio 1 (FR1) schedule and in a progressive ratio task, but also decreases locomotor activity and responding for sucrose, indicating a possible nonspecific motor effect (Besheer, Faccidomo, Grondin and Hodge, 2008a; Besheer et al., 2008b). Direct infusion of JNJ16259685 into the nucleus accumbens shell or central amygdala of C57Bl/6J mice reduces alcohol drinking in a DID paradigm at doses that do not decrease sucrose intake (Cozzoli et al., 2014a; Lum, Campbell, Rostock and Szumlinski, 2014), suggestive of a specific effect of mGlu1 blockade when this circuitry is targeted. However, intra-accumbens infusion of CPCCOEt does not significantly reduce voluntary drinking, which could reflect differences in the models used or the pharmacodyanamic properties of different mGlu1 NAMs (Cozzoli et al., 2012; Cozzoli et al., 2009). Effects of mGlu1 NAMs on reinstatement of alcohol seeking after extinction or abstinence have not been reported. Although the preclinical data are inconclusive with regards to the potential for targeting mGlu1 in AUDs, a recent finding that genetic variants of the gene encoding mGlu1 (GRM1) predict higher levels of alcohol consumption and are associated adverse events in a human population suggests that further investigation of this target may be warranted (Meyers, Salling, Almli, Ratanatharathorn, Uddin, Galea et al., 2015).
4. Group II mGlu receptors in preclinical models of alcohol exposure
A number of recent studies evaluating the relationship between group II mGlu receptor expression and function and alcohol consumption have provided substantial support for the involvement of group II mGlu receptors, particularly mGlu2, in AUD. Selectively-bred alcohol-preferring (P) rats are homozygous for a Grm2 variant containing a premature stop codon (Grm2 *407), and mice with global mGlu2 deletion show higher alcohol consumption and preference in a two-bottle choice test (Zhou, Karlsson, Liang, Xiong, Kimura, Tapocik et al., 2013). Genetic analysis of other alcohol-preferring rat strains has identified a high prevalence of the Grm *407 allele in other selectively-bred lines as well (Wood, Nicolas, Choi, Roman, Nylander, Fernandez-Teruel et al., 2016). Rats lacking mGlu2 also self-administer cocaine and heroin at higher rates (Gao, Jordan, Bi, He, Yang, Gardner et al., 2018; Yang, Zhang, Bi, He, Gao and Xi, 2017), suggesting a broad tendency for loss of mGlu2 function to predict high levels of drug consumption
In addition to the discovery that mGlu2 deletion predispose rats to high rates of drug and alcohol self-administration, several studies have considered the effects of alcohol exposure on mGlu2 expression and function. Rats trained to self-administer alcohol and made alcohol-“dependent” by CIE show lower mGlu2 mRNA levels in the infralimbic cortex, and this correlates with impaired ability of the mGlu2/3 agonist LY379268 to reduce extracellular glutamate levels in the nucleus accumbens (Meinhardt, Hansson, Perreau-Lenz, Bauder-Wenz, Stahlin, Heilig et al., 2013). Notably, the same study reported downregulation of mGlu2 mRNA in the anterior cingulate cortex in postmortem brain samples obtained from humans diagnosed with AUD (Meinhardt et al., 2013), supporting the translational relevance of these findings. Our recent work demonstrated that CIE also disrupts mGlu2-mediated inhibition of glutamatergic transmission in the dorsolateral and dorsomedial striatum, but this effect is limited to alcohol exposure during adolescence (Johnson, Liput, Homanics and Lovinger, 2019). Importantly, mGlu2 function could be rescued with an mGlu2 PAM, suggesting that pharmacological targeting of mGlu2 could be a viable strategy for reversing alcohol-induced behavioral adaptations associated with altered synaptic modulation. Upregulation of mGlu3 mRNA has also been reported in postmortem brain tissue from humans with AUD (Enoch, Rosser, Zhou, Mash, Yuan and Goldman, 2014), but because preclinical studies have not identified a specific role for allosteric modulation of mGlu3 in relation to alcohol, the section will primarily focus on mGlu2. We will then briefly consider evidence that mGlu3 may also warrant attention as a target for AUD.
4.1. mGlu2 PAM effects on alcohol consumption and seeking
Preclinical studies using nonselective group II mGlu receptor agonists such as LY379268 ((1R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid) have identified these receptors as targets for reducing alcohol consumption and seeking. mGlu2/3 agonist administration reduces alcohol self-administration (Backstrom and Hyytia, 2005; Sidhpura, Weiss and Martin-Fardon, 2010; Windisch and Czachowski, 2018). This regulation of alcohol self-administration is bidirectional, as mGlu2/3 blockade with the orthosteric antagonist LY341495 ((2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid) increases alcohol self-administration in rats (Zhou et al., 2013). Systemic or intra-amygdala administration of the agonist LY379268 disrupts the discriminative stimulus properties of alcohol in rats (Cannady, Grondin, Fisher, Hodge and Besheer, 2011), which could contribute to the ability of this drug to reduce alcohol self-administration. In a limited access two-bottle choice model, intra-accumbal infusion of LY379268 reduces voluntary drinking (Griffin, Haun, Hazelbaker, Ramachandra and Becker, 2014), suggesting that regulation of glutamatergic transmission in the nucleus accumbens could also support agonist effects on alcohol self-administration. Activation of mGlu2/3 is also effective at reducing both cue-induced and stress-induced reinstatement of alcohol seeking following extinction training (Backstrom and Hyytia, 2005; Kufahl, Martin-Fardon and Weiss, 2011; Sidhpura et al., 2010; Zhao, Dayas, Aujla, Baptista, Martin-Fardon and Weiss, 2006). The effect of LY379268 on stress-induced reinstatement is enhanced in “dependent” rats that had a history of investigator-administered alcohol exposure (Sidhpura et al., 2010). These effects of mGlu2/3 activation extend to other psychoactive drugs including nicotine, methamphetamine, and cocaine (Johnson and Lovinger, 2016). Moreover, pharmacological inhibition of reinstatement is consistent with findings that viral overexpression of mGlu2 in the medial prefrontal cortex prevents reinstatement of alcohol seeking in dependent rats (Meinhardt et al., 2013).
Development of mGlu2 PAMs suitable for in vivo behavioral testing has allowed more specific assessment of mGlu2 as a target for treating AUD. For example, the mGlu2-selective PAM AZD8529 (7-methyl-5-[3-(piperazin-1-ylmethyl)-1,2,4-oxadiazol-5-yl]-2-[[4-(trifluoromethoxy)phenyl]methyl]-3H-isoindol-1-one), which has advanced to clinical trials for smoking cessation in female subjects (ClinicalTrials.gov ID: NCT02401022), modestly reduces alcohol self-administration in rats (Augier, Dulman, Rauffenbart, Augier, Cross and Heilig, 2016). The ability of AZD8529 to modify relapse-like behavior is more robust, as cue-induced reinstatement of alcohol seeking was prevented by this drug (Augier et al., 2016). Interestingly, AZD8529 failed to prevent stress-induced reinstatement, despite earlier findings that mGlu2/3 agonists effectively reduce stress-induced reinstatement (Sidhpura et al., 2010). Conversely, a recent study using the mGlu2-selective PAM BINA (biphenyl-indanone A) failed to replicate the effects of AZD8529 on alcohol taking and seeking (Windisch and Czachowski, 2018), suggesting that these effects may be dependent on the specific PAM used. Differences in self-administration paradigms between groups could also contribute to discordant findings. Given the effects of agonists and AZD8529 on alcohol self-administration and seeking, as well as promising effects in preclinical models of other substance use disorders (Caprioli, Venniro, Zeric, Li, Adhikary, Madangopal et al., 2015; Dhanya, Sheffler, Dahl, Davis, Lee, Yang et al., 2014; Justinova, Panlilio, Secci, Redhi, Schindler, Cross et al., 2015; Li, D’Souza, Nino, Doherty, Cross and Markou, 2016) and substantial progress in developing drug candidates that have suitable properties and promising safety profiles in humans, further assessment of mGlu2 as a target for reducing alcohol consumption and relapse is warranted.
4.2. Avoiding tolerance with mGlu2 PAMs
Chronic dosing of GPCR agonists is commonly associated with development of tolerance to therapeutic effects. Because PAMs enhance the effects of endogenous agonists when they are naturally released rather than persistently activating the receptor, one potential advantage of PAMs is that tolerance to the therapeutic effects of these drugs due to receptor desensitization could be less likely (Conn et al., 2014). Although tolerance to the effects of mGlu2 ligands has not been explicitly assessed in alcohol self-administration paradigms, important insight comes from preclinical assessments of mGlu2 ligands in other behavioral measures, including nicotine self-administration and effects on sleep architecture. For example, in rats trained to self-administer nicotine, treatment with the mGlu2/3 agonist LY379268 for 14 days reduces nicotine infusions earned early in the treatment course, but nicotine infusion rates gradually return to baseline levels by the end of the treatment period, an observation which is suggestive of tolerance to LY379268 effects (Liechti, Lhuillier, Kaupmann and Markou, 2007). Similarly, repeated dosing of LY379268 produces tolerance to analgesic effects (Jones, Eberle, Peters, Monn and Shannon, 2005; Zammataro, Chiechio, Montana, Traficante, Copani, Nicoletti et al., 2011), and repeated dosing of the mGlu2/3 agonist LY354740 ((1S,2S,5R,6S)-2-Aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid) causes tolerance to effects on sleep architecture (Ahnaou, Lavreysen, Tresadern, Cid and Drinkenburg, 2015). Promisingly, several preclinical studies employing mGlu2 PAMs lend support to the idea that use of PAMs could circumvent the problem of tolerance. Notably, the mGlu2 PAM AZD8529 retains its ability to reduce nicotine self-administration in both rats and squirrel monkeys following repeated dosing (Justinova et al., 2015; Li et al., 2016). Another mGlu2 PAM, JNJ-42153605 (3-(cyclopropylmethyl)-7-(4-phenyl-1-piperidinyl)-8-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyridine), shows similar resistance to development of tolerance when measuring effects on sleep architecture in rats (Ahnaou, de Boer, Lavreysen, Huysmans, Sinha, Raeymaekers et al., 2016; Ahnaou et al., 2015). Interestingly, tolerance does develop to inhibition of nicotine self-administration by another mGlu2 PAM, AZD8418 (5-{7-chloro-2-[(1S)-1-cyclopropylethyl]-1-oxo-2,3-dihydro-1H-isoindol-5-yl}-N,N-dimethyl-1,2-oxazole-3-carboxamide) (Li et al., 2016), indicating that resistance to tolerance is not common to all mGlu2 PAMs, and needs to be evaluated for each drug. Some PAMs possess intrinsic efficacy and thus could be more likely to desensitize the receptor than PAMs that exclusively act to enhance the affinity or coupling of a GPCR when it is activated by its endogenous agonist. Thus, attention to the unique properties of individual PAMs will be critical as efforts to develop mGlu2 PAMs for clinical use continue. Further, careful attention should be paid to drug efficacy over time when evaluating results of clinical trials.
4.3. Additional considerations regarding exploration of mGlu2 as a therapeutic target in AUD
Identification of Grm2 mutations that lead to global loss of mGlu2 protein in Wistar rats and selectively-bred lines derived from this strain provided a serendipitous opportunity to identify a role for mGlu2 in psychoactive drug consumption (Ceolin, Kantamneni, Barker, Hanna, Murray, Warburton et al., 2011; Wood et al., 2016; Zhou et al., 2013). However, it is important to note that some commercial suppliers of rats commonly used in SUD research still maintain colonies in which some rats are heterozygous or homozygous for the mutant allele, and thus it is important to consider the source and strain of rats used and their Grm2 genotype when studying the effects of mGlu2 in preclinical models of SUDs. Rats lacking mGlu2 have proven useful, however, for verifying the specificity of pharmacological manipulations targeting mGlu2. For example, Augier et al. employed mGlu2-lacking P rats to verify that the inhibition of cue-induced reinstatement of alcohol seeking by AZD8529 was indeed dependent on mGlu2 (Augier et al., 2016).
While most studies have focused on roles for mGlu2 in seeking and consumption of alcohol and other psychoactive drugs, it will be interesting to consider what other behavioral effects of mGlu2 PAMs could be advantageous in the context of AUD. For example, enhancement of mGlu2 activity using the PAM LY487379 (2,2,2-Trifluoro-N-[4-(2-methoxyphenoxy)phenyl]-N-(3-pyridinylmethyl)ethanesulfonamide) has been shown to improve behavioral flexibility in rats (Nikiforuk, Popik, Drescher, van Gaalen, Relo, Mezler et al., 2010). This finding is of particular interest because history of alcohol exposure is commonly associated with impaired behavioral flexibility, which could contribute to maladaptive decision-making related to alcohol use. To date, the effects of mGlu2 PAMs in tasks requiring cognitive flexibility (e.g., reversal learning, attentional set-shifting) have not been evaluated in alcohol-exposed animals, but this question merits future investigation.
Progress in establishing safety profiles of multiple mGlu2 PAMs that have entered clinical trials provides hope that efforts to target this receptor will not be hampered by safety or tolerability issues. Multiple PAMs that have undergone Phase I or II clinical trials have been reported to be well-tolerated (Kent, Daly, Kezic, Lane, Lim, De Smedt et al., 2016; Litman, Smith, Doherty, Cross, Raines, Gertsik et al., 2016; Salih, Anghelescu, Kezic, Sinha, Hoeben, Van Nueten et al., 2015). Results of clinical trials of mGlu2 PAMs for AUD and other SUDs are eagerly anticipated, and development of new clinical candidates with improved profiles for use in humans continues.
4.4. Potential involvement of mGlu3 AUD
In addition to changes in mGlu3 expression associated with AUD (Enoch et al., 2014), several additional lines of evidence suggest that mGlu3 involvement in alcohol-related behaviors warrants future investigation. For example, variants of Grm3 have been associated with alcohol use in humans (Levey, Le-Niculescu, Frank, Ayalew, Jain, Kirlin et al., 2014; O’Brien, Way, Kandaswamy, Fiorentino, Sharp, Quadri et al., 2014; Xia, Ma, Hu, Tang, Wu, Liu et al., 2012; Xia, Wu, Ma, Tang, Liu, Xin et al., 2014). Further, global deletion of mGlu3 in mice abolishes ethanol CPP, suggesting a role for mGlu3 in contextual reward (Lainiola, Hietala, Linden and Aitta-Aho, 2019). Although studies of mGlu3-selective allosteric modulators in animal models of alcohol-related behaviors have not been reported, other known roles of mGlu3 in behavior could provide clues regarding the potential involvement of mGlu3 in AUD. Notably, mGlu3 NAM administration decreases passive coping and measures of anhedonia in models of chronic stress (Joffe, Santiago, Oliver, Maksymetz, Harris, Engers et al., 2020); these findings suggest that inhibition of mGlu3 could also be evaluated as a strategy to alleviate negative affect associated with alcohol withdrawal.
5. Group III mGlu receptors in preclinical models of alcohol exposure
Group III mGlu receptors modulate neurotransmission in many regions associated with alcohol reward, consumption, and seeking. Alcohol exposure has been associated with changes in expression of various group III mGlu receptor subtypes, suggesting that evaluation of each of these receptors for effects on alcohol-related behavioral measures is warranted. In rats fed an ethanol-containing diet, lower levels of mGlu7 expression in the hippocampus have been reported (Simonyi, Christian, Sun and Sun, 2004). Conversely, in human postmortem hippocampal tissue, upregulation of mGlu4 expression is observed in association with AUD diagnosis (Enoch et al., 2014).
On the behavioral level, there is evidence that mGlu4 regulates stimulatory effects of ethanol, as mice lacking mGlu4 are resistant to ethanol-induced increases in locomotion (Blednov, Walker, Osterndorf-Kahanek and Harris, 2004); interestingly, the same study failed to observe mGlu4-mediated differences in ethanol consumption or sedation. Intracerebroventricular infusion of the dual orthosteric mGlu4/mGlu7 agonist LSP2-9166 (((2S)-2-amino-4-(((4-(carboxymethoxy)-3-(trifluoromethoxy)phenyl)(hydroxy)methyl)(hydroxy)phosphoryl)butanoic acid) reduces alcohol self-administration as well as motivation to obtain alcohol in a progressive ratio test in rats (Lebourgeois, Vilpoux, Jeanblanc, Acher, Marie, Noble et al., 2018). Despite recent advances in identification of subtype-selective orthosteric and allosteric ligands targeting these receptors, few results of studies employing these compounds have been reported. This is partially owing to a relative paucity of subtype-selective allosteric ligands that target these receptors and are well-suited for in vivo studies. However, the somewhat surprising lack of reports of effects of known drugs that selectively target these receptors might also be due to negative results remaining unpublished.
The mGlu7-selective allosteric agonist AMN082 (N,N’-Bis(diphenylmethyl)-1,2-ethanediamine) has been evaluated for modulation of ethanol reward and self-administration. Further studies evaluating the effects of mGlu7 activation on alcohol withdrawal-induced negative affect found that AMN082 decreases alcohol-enhanced avoidance of open arms in the elevated plus maze test in rats (Kotlinska, Lopatynska-Mazurek, Gawel, Gabka, Jenda-Wojtanowska, Kruk-Slomka et al., 2019). AMN082 administered during CPP extinction training does not modify the rate at which ethanol CPP is extinguished (Bahi, 2012). However, following extinction, AMN082 attenuates ethanol priming-induced reinstatement of CPP (Bahi, 2012). In operant ethanol self-administration experiments in mice, AMN082 reduces levels of responding for ethanol reinforcement, but also reduces responding for sucrose reinforcement and decreases locomotor activity in an open field test, suggestive of non-specific suppression of behavior (Salling, Faccidomo and Hodge, 2008). We note that significant off-target effects of AMN082 on monoamine transporters and physiological measures have been reported (Ahnaou, Raeyemaekers, Huysmans and Drinkenburg, 2016; Sukoff Rizzo, Leonard, Gilbert, Dollings, Smith, Zhang et al., 2011), highlighting the need for control experiments in mGlu7 knockout mice or attenuation of AMN082 effects with an mGlu7 NAM. In addition, AMN082 promotes rapid internalization of mGlu7 in dissociated hippocampal neurons (Pelkey, Yuan, Lavezzari, Roche and McBain, 2007), thus whether the effects of this drug can be attributed to enhanced receptor activation or reduced receptor availability is unclear, and may be context-dependent.
Although selective allosteric modulators of mGlu8 suitable for in vivo investigations have not been reported, studies using the mGlu8-selective agonist DCPG ((S)-3,4-Dicarboxyphenylglycine) suggest that mGlu8 activation can modulate ethanol reward and consumption. Systemic injection of DCPG in mice reduces alcohol consumption and preference in a two-bottle choice test and attenuates acquisition of ethanol CPP (Bahi, 2017). DCPG also attenuates alcohol self-administration and cue-induced reinstatement of alcohol seeking, but concerns about nonspecific effects on locomotor activity limit the impact of these findings (Backstrom and Hyytia, 2005). As more selective allosteric modulators of group III mGlu receptors that are suitable for long-term in vivo studies become available, evaluation of these receptors in preclinical models of AUD will likely continue to be of interest.
6. Concluding Remarks
The ability of mGlu receptor allosteric modulators to modify maladaptive behaviors in experimental systems used to identify potential AUD treatments has produced substantial interest in targeting these receptors in the clinic. Although clinical evaluation of an mGlu2 PAM and an mGlu5 NAM for nicotine use disorder have been performed, no data resulting from these trials has been disclosed. mGlu receptor allosteric modulators have not yet entered trials specifically for treatment of AUD, but recent success in development of clinical candidates suggests that such trials could commence in the near future. As novel pharmacotherapies for AUD and other SUDs continue to be developed, attention to complex details will be required to ensure that clinical trials are designed optimally and that analyses take into account all relevant factors. High rates of comorbidity between AUD and other SUDs highlight the need for more preclinical investigations of therapeutic target evaluation in animals exposed to multiple psychoactive drugs. Interestingly, a recent study demonstrated that sequential self-administration of cocaine followed by alcohol altered glutamate homeostasis in the nucleus accumbens core, and these changes were accompanied by impaired anti-reinstatement effects of a drug known to target glutamate dysregulation (Stennett, Padovan-Hernandez and Knackstedt, 2019). As noted above, current or recent nicotine exposure alters mGlu5 expression, which will likely impact how drugs targeting this receptor modulate behavior. Currently, the majority of potential interactions between use of multiple drugs and mGlu receptor expression and function remain untested. Looking forward, polysubstance use will be an important consideration in both preclinical and clinical investigations of mGlu receptor allosteric modulators.
There is still much to be learned about the circuitry affected by mGlu receptor ligands that reduce alcohol consumption and seeking, and an increased understanding of how these receptors modulate circuitry to drive behavioral effects should improve our ability to predict the translational potential of these effects. Direct-site infusion of mGlu5 NAMs into the nucleus accumbens and basolateral amygdala have demonstrated roles for these regions in the behavioral effects of mGlu5 NAMs, but most cellular and/or circuit-level mechanisms by which these drugs modify maladaptive behaviors have not been identified. Even less is known about the circuitry modulated by mGlu2 PAMs to inhibit reinstatement of alcohol seeking. The increased availability of PET tracers and other imaging techniques that allow evaluation of localization of mGlu receptors in relevant circuitry in the human brain (Xu and Li, 2019), as well as occupancy of these receptors by drug candidates, will likely improve translation of these drugs to success in clinical trials.
Acknowledgments:
This work was supported by NIAAA Division of Intramural Clinical and Biological Research ZIA AA000416 (D.M.L.) and NIH grant R00 AA025403 (K.A.J.). The authors are employees of the U.S. Government, and this work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C §101 defined a U.S. Government work as a work prepared by a military service member or employees of the U.S. Government as part of that person’s official duties. The views in this article are those of the authors and do not necessarily reflect the views, official policy, or position of the Uniformed Services University of the Health Sciences, the Armed Forces Radiobiology Research Institute, Department of the Navy, Department of Defense or the U.S. Federal Government. Figure 1 was created using Biorender.com.
Nonstandard abbreviations:
- AUD
alcohol use disorder
- CIE
chronic intermittent ethanol exposure
- CPP
conditioned place preference
- DID
drinking in the dark
- GPCR
G protein-coupled receptor
- mGlu
metabotropic glutamate receptor
- NAM
negative allosteric modulator
- PAM
positive allosteric modulator
- PET
positron emission tomography
- SUD
substance use disorder, drug tolerance
Footnotes
Conflicts of interest statement: The authors declare no conflicts of interest.
References
- Adams CL, Cowen MS, Short JL, Lawrence AJ (2008). Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol 11, 229–241. [DOI] [PubMed] [Google Scholar]
- Adams CL, Short JL, Lawrence AJ (2010). Cue-conditioned alcohol seeking in rats following abstinence: involvement of metabotropic glutamate 5 receptors. Br J Pharmacol 159, 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnaou A, de Boer P, Lavreysen H, Huysmans H, Sinha V, Raeymaekers L, et al. (2016). Translational neurophysiological markers for activity of the metabotropic glutamate receptor (mGluR2) modulator JNJ-40411813: Sleep EEG correlates in rodents and healthy men. Neuropharmacology 103, 290–305. [DOI] [PubMed] [Google Scholar]
- Ahnaou A, Lavreysen H, Tresadern G, Cid JM, Drinkenburg WH (2015). mGlu2 Receptor Agonism, but Not Positive Allosteric Modulation, Elicits Rapid Tolerance towards Their Primary Efficacy on Sleep Measures in Rats. PLoS One 10, e0144017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnaou A, Raeyemaekers L, Huysmans H, Drinkenburg W (2016). Off-target potential of AMN082 on sleep EEG and related physiological variables: Evidence from mGluR7 (−/−) mice. Behav Brain Res 311, 287–297. [DOI] [PubMed] [Google Scholar]
- Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D, et al. (2013). Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc Natl Acad Sci U S A 110, 737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkus F, Mihov Y, Treyer V, Ametamey SM, Johayem A, Senn S, et al. (2018). Metabotropic glutamate receptor 5 binding in male patients with alcohol use disorder. Transl Psychiatry 8, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Bell RL, Rao PSS, Hammad AM, Sari Y (2018). Peri-adolescent drinking of ethanol and/or nicotine modulates astroglial glutamate transporters and metabotropic glutamate receptor-1 in female alcohol-preferring rats. Pharmacol Biochem Behav 170, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augier E, Dulman RS, Rauffenbart C, Augier G, Cross AJ, Heilig M (2016). The mGluR2 Positive Allosteric Modulator, AZD8529, and Cue-Induced Relapse to Alcohol Seeking in Rats. Neuropsychopharmacology 41, 2932–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R (2005). The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology 30, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R (2004). mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology 29, 921–928. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P (2005). Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol 528, 110–118. [DOI] [PubMed] [Google Scholar]
- Bahi A (2012). The selective metabotropic glutamate receptor 7 allosteric agonist AMN082 prevents reinstatement of extinguished ethanol-induced conditioned place preference in mice. Pharmacol Biochem Behav 101, 193–200. [DOI] [PubMed] [Google Scholar]
- Bahi A (2017). Decreased anxiety, voluntary ethanol intake and ethanol-induced CPP acquisition following activation of the metabotropic glutamate receptor 8 "mGluR8". Pharmacol Biochem Behav 155, 32–42. [DOI] [PubMed] [Google Scholar]
- Barker JM, Corbit LH, Robinson DL, Gremel CM, Gonzales RA, Chandler LJ (2015). Corticostriatal circuitry and habitual ethanol seeking. Alcohol 49, 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Sheffler DJ, Semenova S, Cosford NDP, Bespalov A (2018). Metabotropic Glutamate Receptor 5 as a Target for the Treatment of Depression and Smoking: Robust Preclinical Data but Inconclusive Clinical Efficacy. Biol Psychiatry 83, 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC (2017). Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology 122, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER (1993). Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 112, 503–510. [DOI] [PubMed] [Google Scholar]
- Bell RL, Hauser SR, McClintick J, Rahman S, Edenberg HJ, Szumlinski KK, et al. (2016). Ethanol-Associated Changes in Glutamate Reward Neurocircuitry: A Minireview of Clinical and Preclinical Genetic Findings. Prog Mol Biol Transl Sci 137, 41–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW (2008a). Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol 42, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW (2008b). Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res 32, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW (2010). Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry 67, 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Hodge CW (2005). Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology 30, 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Stevenson RA, Hodge CW (2006). mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur J Pharmacol 551, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Kirchhoff J, Djouma E, Lawrence AJ (2008). Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int J Neuropsychopharmacol 11, 765–774. [DOI] [PubMed] [Google Scholar]
- Blaine SK, Sinha R (2017). Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology 122, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Osterndorf-Kahanek E, Harris RA (2004). Mice lacking metabotropic glutamate receptor 4 do not show the motor stimulatory effect of ethanol. Alcohol 34, 251–259. [DOI] [PubMed] [Google Scholar]
- Cannady R, Grondin JJ, Fisher KR, Hodge CW, Besheer J (2011). Activation of group II metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology 36, 2328–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Justinova Z, Venniro M, Shaham Y (2018). Effect of Novel Allosteric Modulators of Metabotropic Glutamate Receptors on Drug Self-administration and Relapse: A Review of Preclinical Studies and Their Clinical Implications. Biol Psychiatry 84, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. (2015). Effect of the Novel Positive Allosteric Modulator of Metabotropic Glutamate Receptor 2 AZD8529 on Incubation of Methamphetamine Craving After Prolonged Voluntary Abstinence in a Rat Model. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S (2014). Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 48, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF (2006). Alcohol potently modulates climbing fiber-->Purkinje neuron synapses: role of metabotropic glutamate receptors. J Neurosci 26, 1906–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni E, Balleine B, Baldassarre G (2016). Appetitive Pavlovian-instrumental Transfer: A review. Neurosci Biobehav Rev 71, 829–848. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Heilig M, Perez A, Probst C, Rehm J (2019). Alcohol use disorders. Lancet 394, 781–792. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Leurquin-Sterk G, Crunelle C, de Laat B, Bormans G, Peuskens H, et al. (2019). Recovery of decreased metabotropic glutamate receptor 5 availability in abstinent alcohol-dependent patients. J Nucl Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni SW, Bedse G, Patel S, Winder DG (2019). Driving the Downward Spiral: Alcohol-Induced Dysregulation of Extended Amygdala Circuits and Negative Affect. Alcohol Clin Exp Res 43, 2000–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceolin L, Kantamneni S, Barker GR, Hanna L, Murray L, Warburton EC, et al. (2011). Study of novel selective mGlu2 agonist in the temporo-ammonic input to CA1 neurons reveals reduced mGlu2 receptor expression in a Wistar substrain with an anxiety-like phenotype. J Neurosci 31, 6721–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Meiler J, Niswender CM (2014). Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nat Rev Drug Discov 13, 692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH (2016). Habitual Alcohol Seeking: Neural Bases and Possible Relations to Alcohol Use Disorders. Alcohol Clin Exp Res 40, 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover KK, Kerkhoff WG, Mathur BN (2018). The best defense is a strong offense: preventing alcohol abstinence-induced depression. Neuropsychopharmacology 43, 2331–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen MS, Djouma E, Lawrence AJ (2005). The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther 315, 590–600. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Krstew E, Lawrence AJ (2007). Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacology (Berl) 190, 21–29. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, et al. (2012). Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol Clin Exp Res 36, 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, et al. (2014a). Binge alcohol drinking by mice requires intact group 1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology 39, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, et al. (2009). Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci 29, 8655–8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Tanchuck-Nipper MA, Kaufman MN, Horowitz CB, Finn DA (2014b). Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner. Alcohol 48, 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF (2011). Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci 1216, 24–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Belknap JK (2010). The complexity of alcohol drinking: studies in rodent genetic models. Behav Genet 40, 737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AJ, Anthenelli R, Li X (2018). Metabotropic Glutamate Receptors 2 and 3 as Targets for Treating Nicotine Addiction. Biol Psychiatry 83, 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL (1981). Spatial aversion conditioning with ethanol. Pharmacol Biochem Behav 14, 263–264. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK (1992). Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 107, 385–393. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH (2001). Effects of acamprosate on ethanol-seeking and self-administration in the rat. Alcohol Clin Exp Res 25, 344–350. [PubMed] [Google Scholar]
- de Laat B, Weerasekera A, Leurquin-Sterk G, Gsell W, Bormans G, Himmelreich U, et al. (2019). Effects of alcohol exposure on the glutamatergic system: a combined longitudinal (18) F-FPEB and (1) H-MRS study in rats. Addict Biol 24, 696–706. [DOI] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, et al. (2013). Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci U S A 110, 14783–14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanya RP, Sheffler DJ, Dahl R, Davis M, Lee PS, Yang L, et al. (2014). Design and synthesis of systemically active metabotropic glutamate subtype-2 and −3 (mGlu2/3) receptor positive allosteric modulators (PAMs): pharmacological characterization and assessment in a rat model of cocaine dependence. J Med Chem 57, 4154–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore AS, Okrasa K, Patel JC, Serrano-Vega M, Bennett K, Cooke RM, et al. (2014). Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature 511, 557–562. [DOI] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP (2011). A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J 25, 66–77. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Rosser AA, Zhou Z, Mash DC, Yuan Q, Goldman D (2014). Expression of glutamatergic genes in healthy humans across 16 brain regions; altered expression in the hippocampus after chronic exposure to alcohol or cocaine. Genes Brain Behav 13, 758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2016). Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol 67, 23–50. [DOI] [PubMed] [Google Scholar]
- Ferre S, Casado V, Devi LA, Filizola M, Jockers R, Lohse MJ, et al. (2014). G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 66, 413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler TL, Bakner L, Cunningham CL (2004). Conditioned place aversion induced by intragastric administration of ethanol in rats. Pharmacol Biochem Behav 77, 731–743. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Conn PJ (2017). Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron 94, 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JT, Jordan CJ, Bi GH, He Y, Yang HJ, Gardner EL, et al. (2018). Deletion of the type 2 metabotropic glutamate receptor increases heroin abuse vulnerability in transgenic rats. Neuropsychopharmacology 43, 2615–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB Jr., McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, et al. (2014a). Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology 39, 2570–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, McGonigal JT, Chandler LJ (2017). Deficits in the extinction of ethanol-seeking behavior following chronic intermittent ethanol exposure are attenuated with positive allosteric modulation of mGlu5. Neuropharmacology 113, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF (2009). Role of protein kinase C epsilon (PKCvarepsilon) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl) 204, 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Trantham-Davidson H, Kassab AS, Glen WB Jr., Olive MF, Chandler LJ (2014b). Enhancement of extinction learning attenuates ethanol-seeking behavior and alters plasticity in the prefrontal cortex. J Neurosci 34, 7562–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin C, Schwendt M (2019). The cognitive cost of reducing relapse to cocaine-seeking with mGlu5 allosteric modulators. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- Goulding SP, Obara I, Lominac KD, Gould AT, Miller BW, Klugmann M, et al. (2011). Accumbens Homer2-mediated signaling: a factor contributing to mouse strain differences in alcohol drinking? Genes Brain Behav 10, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Lovinger DM (2017). Associative and sensorimotor cortico-basal ganglia circuit roles in effects of abused drugs. Genes Brain Behav 16, 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd (2014). Alcohol dependence and free-choice drinking in mice. Alcohol 48, 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC (2014). Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 39, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, et al. (2008). Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcohol Clin Exp Res 32, 1992–1998. [DOI] [PubMed] [Google Scholar]
- Guydish J, Passalacqua E, Pagano A, Martinez C, Le T, Chun J, et al. (2016). An international systematic review of smoking prevalence in addiction treatment. Addiction 111, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer S, Leach K, Gregory KJ (2017). Neurobiological insights and novel therapeutic opportunities for CNS disorders from mGlu receptor allosteric and biased modulation. Curr Opin Pharmacol 32, 49–55. [DOI] [PubMed] [Google Scholar]
- Herz A (1997). Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 129, 99–111. [DOI] [PubMed] [Google Scholar]
- Hilderbrand ER, Lasek AW (2018). Studying Sex Differences in Animal Models of Addiction: An Emphasis on Alcohol-Related Behaviors. ACS Chem Neurosci 9, 1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, et al. (2006). The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 183, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM, Wilson HH, Fetterly TL, Bluett RJ, Centanni SW, Gilfarb RA, et al. (2016). Ketamine and MAG Lipase Inhibitor-Dependent Reversal of Evolving Depressive-Like Behavior During Forced Abstinence From Alcohol Drinking. Neuropsychopharmacology 41, 2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM, Winder DG (2017). Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes Brain Behav 16, 8–14. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HM (2014). Rodent models for compulsive alcohol intake. Alcohol 48, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulka LM, Treyer V, Scheidegger M, Preller KH, Vonmoos M, Baumgartner MR, et al. (2014). Smoking but not cocaine use is associated with lower cerebral metabotropic glutamate receptor 5 density in humans. Mol Psychiatry 19, 625–632. [DOI] [PubMed] [Google Scholar]
- Huston JP, Silva MA, Topic B, Muller CP (2013). What's conditioned in conditioned place preference? Trends Pharmacol Sci 34, 162–166. [DOI] [PubMed] [Google Scholar]
- Hwa L, Besheer J, Kash T (2017). Glutamate plasticity woven through the progression to alcohol use disorder: a multi-circuit perspective. F1000Res 6, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA (2011). Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res 35, 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Centanni SW, Jaramillo AA, Winder DG, Conn PJ (2018). Metabotropic Glutamate Receptors in Alcohol Use Disorder: Physiology, Plasticity, and Promising Pharmacotherapies. ACS Chem Neurosci 9, 2188–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Santiago CI, Oliver KH, Maksymetz J, Harris NA, Engers JL, et al. (2020). mGlu2 and mGlu3 Negative Allosteric Modulators Divergently Enhance Thalamocortical Transmission and Exert Rapid Antidepressant-like Effects. Neuron 105, 46–59 e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Liput DJ, Homanics GE, Lovinger DM (2019). Age-dependent impairment of metabotropic glutamate receptor 2-dependent long-term depression in the mouse striatum by chronic ethanol exposure. Alcohol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Lovinger DM (2016). Presynaptic G Protein-Coupled Receptors: Gatekeepers of Addiction? Front Cell Neurosci 10, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. (2014). Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 311, 1889–1900. [DOI] [PubMed] [Google Scholar]
- Jones CK, Eberle EL, Peters SC, Monn JA, Shannon HE (2005). Analgesic effects of the selective group II (mGlu2/3) metabotropic glutamate receptor agonists LY379268 and LY389795 in persistent and inflammatory pain models after acute and repeated dosing. Neuropharmacology 49 Suppl 1, 206–218. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Panlilio LV, Secci ME, Redhi GH, Schindler CW, Cross AJ, et al. (2015). The Novel Metabotropic Glutamate Receptor 2 Positive Allosteric Modulator, AZD8529, Decreases Nicotine Self-Administration and Relapse in Squirrel Monkeys. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten CR, Holmgren EB, Wills TA (2019). Metabotropic Glutamate Receptor Subtype 5 in Alcohol-Induced Negative Affect. Brain Sci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent JM, Daly E, Kezic I, Lane R, Lim P, De Smedt H, et al. (2016). Efficacy and safety of an adjunctive mGlu2 receptor positive allosteric modulator to a SSRI/SNRI in anxious depression. Prog Neuropsychopharmacol Biol Psychiatry 67, 66–73. [DOI] [PubMed] [Google Scholar]
- Koehl A, Hu H, Feng D, Sun B, Zhang Y, Robertson MJ, et al. (2019). Structural insights into the activation of metabotropic glutamate receptors. Nature 566, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2009). Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56 Suppl 1, 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2015). The dark side of emotion: the addiction perspective. Eur J Pharmacol 753, 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Kenneth Lloyd G, Mason BJ (2009). Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nat Rev Drug Discov 8, 500–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016). Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlinska J, Bochenski M (2008). The influence of various glutamate receptors antagonists on anxiety-like effect of ethanol withdrawal in a plus-maze test in rats. Eur J Pharmacol 598, 57–63. [DOI] [PubMed] [Google Scholar]
- Kotlinska JH, Bochenski M, Danysz W (2011). The role of group I mGlu receptors in the expression of ethanol-induced conditioned place preference and ethanol withdrawal seizures in rats. Eur J Pharmacol 670, 154–161. [DOI] [PubMed] [Google Scholar]
- Kotlinska JH, Lopatynska-Mazurek M, Gawel K, Gabka P, Jenda-Wojtanowska M, Kruk-Slomka M, et al. (2019). Impact of the metabotropic glutamate receptor7 (mGlu7) allosteric agonist, AMN082, on fear learning and memory and anxiety-like behavior. Eur J Pharmacol 858, 172512. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, Weiss F (2011). Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology 36, 2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Hapidin H, Bee YT, Ismail Z (2013). Effects of the mGluR5 antagonist MPEP on ethanol withdrawal induced anxiety-like syndrome in rats. Behav Brain Funct 9, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainiola M, Hietala L, Linden AM, Aitta-Aho T (2019). The lack of conditioned place preference, but unaltered stimulatory and ataxic effects of alcohol in mGluR3-KO mice. J Psychopharmacol 33, 855–864. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Schindler CW, Pinkston JW (2016). Conditioned stimuli's role in relapse: preclinical research on Pavlovian-Instrumental-Transfer. Psychopharmacology (Berl) 233, 1933–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K, Gregory KJ (2017). Molecular insights into allosteric modulation of Class C G protein-coupled receptors. Pharmacol Res 116, 105–118. [DOI] [PubMed] [Google Scholar]
- Lebourgeois S, Vilpoux C, Jeanblanc J, Acher F, Marie N, Noble F, et al. (2018). Pharmacological activation of mGlu4 and mGlu7 receptors, by LSP2-9166, reduces ethanol consumption and relapse in rat. Neuropharmacology 133, 163–170. [DOI] [PubMed] [Google Scholar]
- Lee JY, Choe ES, Yang CH, Choi KH, Cheong JH, Jang CG, et al. (2016a). The mGluR5 antagonist MPEP suppresses the expression and reinstatement, but not the acquisition, of the ethanol-conditioned place preference in mice. Pharmacol Biochem Behav 140, 33–38. [DOI] [PubMed] [Google Scholar]
- Lee KM, Coehlo M, McGregor HA, Waltermire RS, Szumlinski KK (2015). Binge alcohol drinking elicits persistent negative affect in mice. Behav Brain Res 291, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coehlo MA, Solton NR, Szumlinski KK (2017). Negative Affect and Excessive Alcohol Intake Incubate during Protracted Withdrawal from Binge-Drinking in Adolescent, But Not Adult, Mice. Front Psychol 8, 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Class MA, Sern KR, Bocz MD, Szumlinski KK (2018a). mGlu5 Receptor Blockade Within the Nucleus Accumbens Shell Reduces Behavioral Indices of Alcohol Withdrawal-Induced Anxiety in Mice. Front Pharmacol 9, 1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Class MA, Szumlinski KK (2018b). mGlu5-dependent modulation of anxiety during early withdrawal from binge-drinking in adult and adolescent male mice. Drug Alcohol Depend 184, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK (2016b). Adolescent Mice Are Resilient to Alcohol Withdrawal-Induced Anxiety and Changes in Indices of Glutamate Function within the Nucleus Accumbens. Front Cell Neurosci 10, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Sern KR, Class MA, Bocz MD, Szumlinski KK (2017). Anxiolytic effects of buspirone and MTEP in the Porsolt Forced Swim Test. Chronic Stress (Thousand Oaks) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leurquin-Sterk G, Ceccarini J, Crunelle CL, de Laat B, Verbeek J, Deman S, et al. (2018). Lower Limbic Metabotropic Glutamate Receptor 5 Availability in Alcohol Dependence. J Nucl Med 59, 682–690. [DOI] [PubMed] [Google Scholar]
- Leurquin-Sterk G, Ceccarini J, Crunelle CL, Weerasekera A, de Laat B, Himmelreich U, et al. (2018). Cerebral dopaminergic and glutamatergic transmission relate to different subjective responses of acute alcohol intake: an in vivo multimodal imaging study. Addict Biol 23, 931–944. [DOI] [PubMed] [Google Scholar]
- Levey DF, Le-Niculescu H, Frank J, Ayalew M, Jain N, Kirlin B, et al. (2014). Genetic risk prediction and neurobiological understanding of alcoholism. Transl Psychiatry 4, e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, D'Souza MS, Nino AM, Doherty J, Cross A, Markou A (2016). Attenuation of nicotine-taking and nicotine-seeking behavior by the mGlu2 receptor positive allosteric modulators AZD8418 and AZD8529 in rats. Psychopharmacology (Berl) 233, 1801–1814. [DOI] [PubMed] [Google Scholar]
- Li X, Markou A (2015). Metabotropic Glutamate Receptor 7 (mGluR7) as a Target for the Treatment of Psychostimulant Dependence. CNS Neurol Disord Drug Targets 14, 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A (2007). Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci 27, 9077–9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman RE, Smith MA, Doherty JJ, Cross A, Raines S, Gertsik L, et al. (2016). AZD8529, a positive allosteric modulator at the mGluR2 receptor, does not improve symptoms in schizophrenia: A proof of principle study. Schizophr Res 172, 152–157. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK (2006). Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend 85, 142–156. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC (2014). Operant ethanol self-administration in ethanol dependent mice. Alcohol 48, 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum EN, Campbell RR, Rostock C, Szumlinski KK (2014). mGluR1 within the nucleus accumbens regulates alcohol intake in mice under limited-access conditions. Neuropharmacology 79, 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW (2013). Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 108, 275–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y (2016). Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology 41, 335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S (1999). Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res 101, 129–152. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM (2012). Using monosodium glutamate to initiate ethanol self-administration in inbred mouse strains. Addict Biol 17, 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF (2003). The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse 47, 240–242. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Crawford MS, Kulers CM, Williams HL (2005). Effects of a metabotropic, mglu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol Alcohol 40, 494–497. [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, et al. (2013). Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci 33, 2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI (2011). Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res 35, 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Schlumbohm JP, Huang LC, Greenberg GD, Hack WR, Spence SE, et al. (2018). An alcohol withdrawal test battery measuring multiple behavioral symptoms in mice. Alcohol 68, 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JL, Salling MC, Almli LM, Ratanatharathorn A, Uddin M, Galea S, et al. (2015). Frequency of alcohol consumption in humans; the role of metabotropic glutamate receptors and downstream signaling pathways. Transl Psychiatry 5, e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Wilbourne PL (2002). Mesa Grande: a methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction 97, 265–277. [DOI] [PubMed] [Google Scholar]
- Mishra D, Zhang X, Chergui K (2012). Ethanol disrupts the mechanisms of induction of long-term potentiation in the mouse nucleus accumbens. Alcohol Clin Exp Res 36, 2117–2125. [DOI] [PubMed] [Google Scholar]
- Muller Herde A, Mihov Y, Kramer SD, Mu L, Adamantidis A, Ametamey SM, et al. (2019). Chronic Nicotine Exposure Alters Metabotropic Glutamate Receptor 5: Longitudinal PET Study and Behavioural Assessment in Rats. Neurotox Res 36, 806–816. [DOI] [PubMed] [Google Scholar]
- Nickols HH, Conn PJ (2014). Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol Dis 61, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P, Drescher KU, van Gaalen M, Relo AL, Mezler M, et al. (2010). Effects of a positive allosteric modulator of group II metabotropic glutamate receptors, LY487379, on cognitive flexibility and impulsive-like responding in rats. J Pharmacol Exp Ther 335, 665–673. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ (2010). Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50, 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien NL, Way MJ, Kandaswamy R, Fiorentino A, Sharp SI, Quadri G, et al. (2014). The functional GRM3 Kozak sequence variant rs148754219 affects the risk of schizophrenia and alcohol dependence as well as bipolar disorder. Psychiatr Genet 24, 277–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, et al. (2009). Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res 33, 1924–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, et al. (2005). The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol 67, 349–355. [DOI] [PubMed] [Google Scholar]
- Organization WH, 2014. Global Status Report on Alcohol and Health. [Google Scholar]
- Parkitna JR, Sikora M, Golda S, Golembiowska K, Bystrowska B, Engblom D, et al. (2013). Novelty-seeking behaviors and the escalation of alcohol drinking after abstinence in mice are controlled by metabotropic glutamate receptor 5 on neurons expressing dopamine d1 receptors. Biol Psychiatry 73, 263–270. [DOI] [PubMed] [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T (1982). Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J Clin Psychopharmacol 2, 129–133. [PubMed] [Google Scholar]
- Pelkey KA, Yuan X, Lavezzari G, Roche KW, McBain CJ (2007). mGluR7 undergoes rapid internalization in response to activation by the allosteric agonist AMN082. Neuropharmacology 52, 108–117. [DOI] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard TM, Buttelmann B, Kolczewski S, et al. (2005). Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther 315, 711–721. [DOI] [PubMed] [Google Scholar]
- Renteria R, Baltz ET, Gremel CM (2018). Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat Commun 9, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Jeanes ZM, Morrisett RA (2014). Ethanol attenuation of long-term depression in the nucleus accumbens can be overcome by activation of TRPV1 receptors. Alcohol Clin Exp Res 38, 2763–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD (2015). 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med 49, e73–e79. [DOI] [PubMed] [Google Scholar]
- Salih H, Anghelescu I, Kezic I, Sinha V, Hoeben E, Van Nueten L, et al. (2015). Pharmacokinetic and pharmacodynamic characterisation of JNJ-40411813, a positive allosteric modulator of mGluR2, in two randomised, double-blind phase-I studies. J Psychopharmacol 29, 414–425. [DOI] [PubMed] [Google Scholar]
- Salling MC, Faccidomo S, Hodge CW (2008). Nonselective suppression of operant ethanol and sucrose self-administration by the mGluR7 positive allosteric modulator AMN082. Pharmacol Biochem Behav 91, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL (2003). Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol 54, 107–143. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW (2005). The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 179, 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW (2008). Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology 55, 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. (2016). The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev 68, 816–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharko AC, Hodge CW (2008). Differential modulation of ethanol-induced sedation and hypnosis by metabotropic glutamate receptor antagonists in C57BL/6J mice. Alcohol Clin Exp Res 32, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R (2010). Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry 67, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Christian MR, Sun AY, Sun GY (2004). Chronic ethanol-induced subtype- and subregion-specific decrease in the mRNA expression of metabotropic glutamate receptors in rat hippocampus. Alcohol Clin Exp Res 28, 1419–1423. [DOI] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT (2012). mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol Biochem Behav 101, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R (2017). Animal models of addiction. Dialogues Clin Neurosci 19, 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, Heilig M (2014). Stress and alcohol interactions: animal studies and clinical significance. Trends Neurosci 37, 219–227. [DOI] [PubMed] [Google Scholar]
- Spear LP (2018). Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci 19, 197–214. [DOI] [PubMed] [Google Scholar]
- Stahle LKR; Jonzon B; Eriksson B.; Kagedal M (2012). Safety Evaluation of the mGluR5 Antagonists AZD9272, AZD2066 and AZD2516 in Healthy Volunteers and Patients with Neuropathic Pain or Major Deppresive Disorder 14th World Congress on Pain, August 27-31, Milan, Italy. [Google Scholar]
- Stennett BA, Padovan-Hernandez Y, Knackstedt LA (2019). Sequential cocaine-alcohol self-administration produces adaptations in rat nucleus accumbens core glutamate homeostasis that are distinct from those produced by cocaine self-administration alone. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RB, Grupp LA (1981). An investigation of the interaction between the reinforcing properties of food and ethanol using the place preference paradigm. Prog Neuropsychopharmacol 5, 609–613. [DOI] [PubMed] [Google Scholar]
- Su LD, Sun CL, Shen Y (2010). Ethanol acutely modulates mGluR1-dependent long-term depression in cerebellum. Alcohol Clin Exp Res 34, 1140–1145. [DOI] [PubMed] [Google Scholar]
- Sukoff Rizzo SJ, Leonard SK, Gilbert A, Dollings P, Smith DL, Zhang MY, et al. (2011). The metabotropic glutamate receptor 7 allosteric modulator AMN082: a monoaminergic agent in disguise? J Pharmacol Exp Ther 338, 345–352. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE (2008). Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology 33, 1365–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terbeck S, Akkus F, Chesterman LP, Hasler G (2015). The role of metabotropic glutamate receptor 5 in the pathogenesis of mood disorders and addiction: combining preclinical evidence with human Positron Emission Tomography (PET) studies. Front Neurosci 9, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M (2014). "Drinking in the dark" (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol 48, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver GA, Sadeghi KG, Samson HH (1988). Ethanol preference following the sucrose-fading initiation procedure. Alcohol 5, 9–13. [DOI] [PubMed] [Google Scholar]
- Valyear MD, Villaruel FR, Chaudhri N (2017). Alcohol-seeking and relapse: A focus on incentive salience and contextual conditioning. Behav Processes 141, 26–32. [DOI] [PubMed] [Google Scholar]
- Van Skike CE, Goodlett C, Matthews DB (2019). Acute alcohol and cognition: Remembering what it causes us to forget. Alcohol 79, 105–125. [DOI] [PubMed] [Google Scholar]
- Varnas K, Cselenyi Z, Arakawa R, Nag S, Stepanov V, Moein MM, et al. (2019). The pro-psychotic metabotropic glutamate receptor compounds fenobam and AZD9272 share binding sites with monoamine oxidase-B inhibitors in humans. Neuropharmacology 162, 107809. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ (2014). Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol 48, 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y (2016). Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res 224, 25–52. [DOI] [PubMed] [Google Scholar]
- Vranjkovic O, Winkler G, Winder DG (2018). Ketamine administration during a critical period after forced ethanol abstinence inhibits the development of time-dependent affective disturbances. Neuropsychopharmacology 43, 1915–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE, et al. (2014). Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology 39, 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Baucum AJ 2nd, Holleran KM, Chen Y, Pasek JG, Delpire E, et al. (2017). Chronic intermittent alcohol disrupts the GluN2B-associated proteome and specifically regulates group I mGlu receptor-dependent long-term depression. Addict Biol 22, 275–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch KA, Czachowski CL (2018). Effects of group II metabotropic glutamate receptor modulation on ethanol- and sucrose-seeking and consumption in the rat. Alcohol 66, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1973). Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29, 203–210. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA (1987). A psychomotor stimulant theory of addiction. Psychol Rev 94, 469–492. [PubMed] [Google Scholar]
- Wood CM, Nicolas CS, Choi SL, Roman E, Nylander I, Fernandez-Teruel A, et al. (2016). Prevalence and influence of cys407* Grm2 mutation in Hannover-derived Wistar rats: mGlu2 receptor loss links to alcohol intake, risk taking and emotional behaviour. Neuropharmacology. [DOI] [PubMed] [Google Scholar]
- Wu H, Wang C, Gregory KJ, Han GW, Cho HP, Xia Y, et al. (2014). Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science 344, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Ma D, Hu J, Tang C, Wu Z, Liu L, et al. (2012). Effect of metabotropic glutamate receptor 3 genotype on N-acetylaspartate levels and neurocognition in non-smoking, active alcoholics. Behav Brain Funct 8, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Wu Z, Ma D, Tang C, Liu L, Xin F, et al. (2014). Association of single-nucleotide polymorphisms in a metabotropic glutamate receptor GRM3 gene subunit to alcohol-dependent male subjects. Alcohol Alcohol 49, 256–260. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li Z (2019). Imaging metabotropic glutamate receptor system: Application of positron emission tomography technology in drug development. Med Res Rev 39, 1892–1922. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Zhang HY, Bi GH, He Y, Gao JT, Xi ZX (2017). Deletion of Type 2 Metabotropic Glutamate Receptor Decreases Sensitivity to Cocaine Reward in Rats. Cell Rep 20, 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ (2006). The role of the basal ganglia in habit formation. Nat Rev Neurosci 7, 464–476. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA (2008). Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammataro M, Chiechio S, Montana MC, Traficante A, Copani A, Nicoletti F, et al. (2011). mGlu2 metabotropic glutamate receptors restrain inflammatory pain and mediate the analgesic activity of dual mGlu2/mGlu3 receptor agonists. Mol Pain 7, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F (2006). Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci 26, 9967–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Karlsson C, Liang T, Xiong W, Kimura M, Tapocik JD, et al. (2013). Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc Natl Acad Sci U S A 110, 16963–16968. [DOI] [PMC free article] [PubMed] [Google Scholar]