Abstract
Tea (Camellia sinensis) flower saponins (TFS) have various biological properties. However, the anti-cancer effects of TFS have not been investigated in any detail. Here, we evaluated the anti-cancer effects of TFS using human ovarian cancer cell lines. TFS (1.5 μg/ml) produced significant antiproliferative effects against A2780/CP70 and OVCAR-3 cells by inducing p53-dependent apoptosis and S phase arrest. Further study showed that TFS decreased mitochondrial membrane potential, activated Caspase-3/7, Caspase-8 and Caspase-9 activities, and that the p53 inhibitor PFT-α reversed the TFS-induced cell growth inhibition and apoptosis. In addition, TFS inhibited the expression of Cdc25A, Cdk2, and CyclinD1 and upregulated Cyclin E and Cyclin A, suggesting that the Cdc25A-Cdk2-Cyclin E/A pathway was involved in TFS-induced S phase arrest. Furthermore, the S phase arrest was associated with a Chk2-Cdc25A DNA damage response. These results demonstrated that TFS has promising potential serving as functional food components for prevention of ovarian cancer.
Keywords: Tea (Camellia sinensis) flower, Saponins, Anti-cancer, Apoptosis, DNA damage, p53
Introduction
Ovarian cancer is the fifth leading cause of cancer-related deaths among women in the United States and continues to be a lethal malignancy among Chinese women (W. Chen et al., 2016; Siegel, Miller, & Jemal, 2015). Due to the lack of symptoms during the early development stages, ovarian cancer is usually diagnosed at an advanced stage, thus leading to a higher mortality rate compared with other cancers (Brasseur, Gevry, & Asselin, 2016). The standard protocol to treat ovarian cancer is surgery followed by chemotherapy. However, the high incidence of chemotherapy resistance indicates that commonly used drugs such as cisplatin and carboplatin may lose effectiveness over time, requiring the use of more toxic drugs. Discovery of effective alternative compounds is needed for the treatment of ovarian cancer (Moxley & McMeekin, 2010; X. Y. Zhang & Zhang, 2016).
Tea (Camellia sinensis) is a commonly consumed beverage worldwide. Among its numerous health benefits, the cancer chemoprevention effects of tea and tea constituents has been expensively investigated (Hajiaghaalipour, Kanthimathi, Sanusi, & Rajarajeswaran, 2015; Yang, Wang, Lu, & Picinich, 2009). A recent study found that tea consumption reduces the risk of ovarian cancer in women (Lee, Su, Pasalich, & Binns, 2013). Although the anti-cancer effects of tea leaves and tea constituents have been widely studied, less attention has been paid to the tea flowers. Even though tea flowers have similar chemical constituents compared with tea leaves (Lee et al., 2013), only a few studies have reported that tea flower extracts and polysaccharides extracted from tea flowers possess potential anti-cancer activities in vitro (Way et al., 2009; Xu, Ye, Sun, Tu, & Zeng, 2012). Our previous study found that a tea flower extract from tea (Camellia sinensis (L.) O. Kuntze, Theaceae) variety Longjing 43 has no mutagenic potential and very low acute and subchronic toxicity towards rats (Zurolo et al., 2011).
Triterpenoid saponins, which are present in various kinds of plants, demonstrate anti-cancer properties (Hanausek et al., 2001; Man, Gao, Zhang, Huang, & Liu, 2010). Triterpenoid saponins are characteristic ingredients of tea flowers, as compared with tea leaves which contain relatively low levels of saponins. We recently identified 21 triterpenoid saponins from tea flower extracts from tea flower extracts of selected cultivars (Shen et al., 2017). In recent years, much attention has been paid to the bioactive properties of saponins isolated from tea flowers. Several reports demonstrated that tea flower saponins have anti-hyperlipidemic, anti-hyperglycemic, anti-obesity, and gastroprotective effects in animal models (Matsuda et al., 2012; Matsuda, Nakamura, Morikawa, Muraoka, & Yoshikawa, 2016). However, little research has focused on the anti-cancer effects of saponins extracted from tea (Camellia sinensis) flower. It has been reported that TFS demonstrated antiproliferative effects on human digestive tract carcinoma cells by inducing apoptosis and cell cycle arrest, but the specific mechanisms have not been clearly elucidated (Kitagawa et al., 2016). We previously reported that black tea polyphenol theaflavins have shown anti-cancer properties using human ovarian cancer cell lines in vitro (Y. Gao, G. O. Rankin, Y. Tu, & Y. C. Chen, 2016; Y. Gao, G. O. Rankin, Y. Y. Tu, & Y. C. Chen, 2016; Tu et al., 2016). In the present study, we investigated the anti-cancer effects of tea flower saponins (TFS) and began to determine the specific mechanisms involved using human endothelial ovarian cancer cell lines, A2780/CP70 and OVCAR-3 as the cancer cell models, and a human normal ovarian surface epithelial cell line, IOSE-364 to determine TFS-induced effects on normal ovarian cells.
2. Materials and Methods
2.1. Preparation of tea flower saponins (TFS)
Dried flowers of tea (Camellia sinensis) were obtained from Zhejiang Yilongfang Ltd. The dried flowers were powdered and extracted with 70% methanol under refluxing. The methanol extract was subsequently fractionated into ethyl-acetate and n-butanol soluble fractions, with the n-butanol fraction separated by D101 macroporous adsorption resin and preparative HPLC to obtain TFS. After separation, TFS was identified and quantified by ultra-high performance chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS) as the methods described previously (Shen et al., 2017). TFS was dissolved in dimethyl sulfoxide (DMSO) to produce a 20 μg/ml stock solution and stored at −20°C.
2.2. Cell lines and reagents
Human ovarian cancer cell lines A2780/CP70 and OVCAR-3 were obtained from Dr. Jiang at West Virginia University. Normal ovarian surface epithelial cells IOSE-364 were provided by Dr. Auersperg at the University of British Columbia, Canada. All cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, and the cells were grown in a humidified incubator with 5% CO2 at 37°C. RPMI-1640 medium, Bovine Serum Albumin, DMSO, Hoechst 33342, DCFH-DA and pifithrin-α (PFT-α) and were purchased from Sigma-Aldrich (Sigma, St. Louis, MO, USA). CellTiter 96® AQueous One Solution Cell Proliferation Assay was obtained from Promega (Madison, WI, USA). Pierce LDH Cytotoxicity Assay Kit and Alaxa Fluor® 488 Annexin V/Dead Cell Apoptosis kit were purchased from Thermo Scientific (Waltham, MA, USA). Propidium iodide (PI), phosphate-buffered saline (PBS) and fetal bovine serum (FBS) were obtained Life Technologies (Invitrogen, Grand Island, NY, USA). Primary antibodies against PCNA, Cyclin A, CyclinD1, Cyclin E, Cdc25A, CDK4, γ-H2AX (Ser139), p21 Waf1/Cip1(12D1) were purchased from Cell Signaling Inc. (Danvers, MA, USA). Primary antibodies against pChk2 (Thr68), Chk2 (H-300), p53 (C11), p-p53 (Ser15), GAPDH (0411) and secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.3. Cell viability
Cell viability was measured using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS). Briefly, cells were plated in 96-well plate at a density of 1×104 cells per well. After incubation for 24 h, the cells were exposed to the indicated concentrations of TFS for 6, 12, and 24 h respectively. Cells were washed twice with phosphate-buffered saline (PBS), then 100 μl freshly prepared Aqueous One Solution (Promega, Madison, WI, USA) was added to each well and incubated at 37°C for 1 h. The absorbance at 490nm was measured with a microplate reader (SynergyTM Multi-Mode, BioTek, Vermont, USA). Cell viability was expressed as a percentage of control cells.
2.4. Cytotoxicity assay
The cytotoxicity effect of TFS on ovarian cancer cells and normal ovarian cancer cells was determined by a lactate dehydrogenase (LDH) assay using a LDH Cytotoxicity Assay Kit. Briefly, cells were seeded 96-well plate at 1×104 cells/ well in 100 μl culture medium. After a 24 h growth period, cells were treated with different concentrations of TFS for 6, 12 and 24 h. After incubation, 10 μl lysis buffer was added to the wells, and the plate was incubated for 45 min at 37°C with 5% CO2. Then 50 μl of each sample medium was transferred to a 96-well flat-bottom plate. 50 μl reaction mixture was added to each sample well and the plate was incubated at room temperature for 30 min in the dark. The absorbance was measured at 490 nm and 680 nm using the microplate reader (SynergyTM Multi-Mode, BioTek, Vermont, USA). The experiments were performed in triplicate and LDH cytotoxicity was calculated according to the manufacture’s instruction.
2.5. Hoechst 33342 staining
Hoechst 33342 staining was used to determine the degree of apoptosis present in each sample. In brief, A2780/CP70, OVCAR-3 and IOSE-364 cells with a density of 1×104 cells/well were seeded in 96-well plates and cultured for 24 h. After treatment with TFS at 0, 0.5, 1.0 or 1.5 μg/ml in the absence or presence of pifithrin-α (20 μM) for 24 h, cells were washed twice with PBS and stained with Hoechst 33342 (10μg/ml) in PBS for 15 min at 37°C. The cellular fluorescent changes were observed using fluorescence microscopy (ZEISS, Heidelberg, Germany).
2.6. Evaluation of mitochondrial membrane potential (ΔΨm)
A2780/CP70 and OVCAR-3 Cells were seeded in black 96-well plate at a density of 1×104 cells/well for 24 h. After incubation with different concentrations of TFS for 24 h, cells were washed twice with PBS followed by incubation with JC-1 dye solution at concentration of 10 μg/ml for 30 min. The fluorescent intensity was measured at excitation: emission of 485/590 and 485/535 for red aggregates and green monomers respectively using a fluorescence plate reader (SynergyTM Multi-Mode, BioTek, Vermont, USA).
2.7. Apoptosis analysis by flow cytometry
Cells were seeded in 60 mm dish palate at a density of 1×105 cells/well, after a 24 h growth for adhesion, cells were treated with different concentrations of TFS for 24 h and used for further analysis. For apoptosis analysis, an Alaxa Fluor® 488 Annexin V/Dead Cell Apoptosis kit (Thermo Scientific, MA, USA) was used in the experiment. Briefly, the treated cells were harvested and washed twice with cold PBS, followed by resuspending the cells in propidium iodide and annexin V buffer according to the manufacturer’s instruction. Samples were analyzed using a FACSCaliber flow cytometry system (BD Biosciences, USA).
2.8. Cellular caspase activity assay
A2780/CP70 and OVCAR-3 cells were seeded in 96-well plate at a density of 1×104cells/well. After a 24 h growth period, the cells were treated with various concentrations of TFS in the absence or presence of pifithrin-α (20 μM) for 24 h. Cells without any TFS treatment were used as controls. Caspase-Glo-3/7, −8 and −9 (Promega, USA) regents were added and incubated for 30 min. Luminescence was recorded using a microplate reader (BioTek, Vermont, USA). Experiments were conducted in triplicate and data were expressed as percentage of control group.
2.9. Cell cycle analysis by flow cytometry
Cells were seeded in 60 mm dish plate at a density of 1×105 cells/dish. After a 24 h growth period for adhesion, cells were treated with different concentrations of TFS for 24 h and used for further analysis. For cell cycle analysis, the cells were fixed with ice-cold 70% ethanol at 4°C overnight, cells were then resuspended in PBS containing RNaseA (180 μg/ml) and PI solution (50 μg/ml), and then analysed using FACSCaliber flow cytometry system (BD Biosciences, USA).
2.10. Western blot
A2780/CP70 and OVCAR-3 cells were treated with TFS in the absence or presence of pifithrin-α (20 μM) for 24 h as described above. Cells were then harvested and total protein was extracted using Mammalian Protein Extraction Reagent supplemented with 1% protease inhibitor cocktail. As we previous reported (Huang et al., 2015), total protein concentration was determined using a BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of total protein were subjected to SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were blocked at room temperature with 5% not-fat milk in Tris-buffer saline containing 0.1% Tween 20 (TBST) for 1 h and then incubated with specific primary antibodies overnight at 4°C overnight. After washing with TBST for 30 min, the membranes were incubated with secondary antibodies at room temperature for 2 h. Subsequently, the membranes were washed with TBST and bands visualization was quantified by NIH ImageJ software.
2.11. Statistical analysis
All data were expressed as mean±SEM and analysed by one-way analysis of variance with Newman-Keuls test using Graphpad Prism software. *P<0.05, **P<0.01, ***P<0.001 and #P<0.05.
3. Results
3.1. Characteristic, cytotoxicity and cellular proliferation inhibition
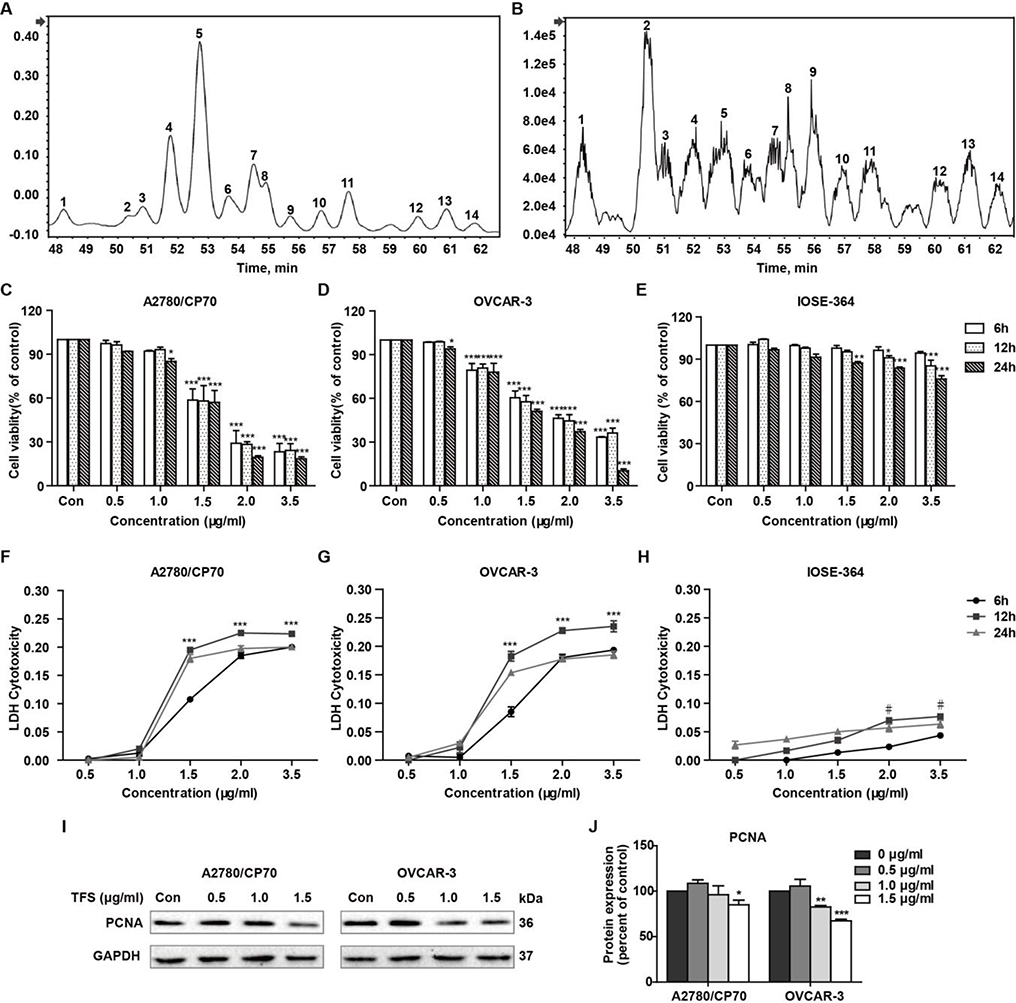
UPLC-Q-TOF/MS analysis was successfully characterized the saponins in the tea flower. We found that the bioactive compounds in this tea flower extract contained 14 triterpenoid saponins (Fig. 1A, B). The molecular formulas and molecular weights of these saponins were confirmed by high-resolution mass measurements, deprotonated molecular ions [M-H]-, isotope abundance for each pseudo-molecular ion and fragment ions (Table 1). The identification of the saponins were based on published fragmentation data and nominal massed calculated from known structures (Morikawa et al., 2007; Myose, Warashina, & Miyase, 2012; Sugimoto, Yoshikawa, Nakamura, & Matsuda, 2009; Yoshikawa et al., 2005; Yoshikawa, Nakamura, Kato, Matsuhira, & Matsuda, 2007; Yoshikawa et al., 2009; Yoshikawa, Sugimoto, Nakamura, & Matsuda, 2008). Meanwhile, as the commercial saponin standards from Camellia sinensis were not available, we quantified the content of individual saponin with individual ginsenoside Rd standard as the methods described previously (Shen et al., 2017) (Table 1).
Fig. 1 -. Cytotoxicity and proliferation inhibition effect of TFS on A2780/CP70, OVCAR-3 and IOSE-364 cells.
(A) UPLC/UV chromatograms of saponins extracted from tea (Camellia sinensis) flower. (B) UPLC-Q-TOF/MS total ion chromatograms of saponins extracted from tea (Camellia sinensis) flower. (C-E) Cell viability, 6, 12 and 24 h after treatment with different concentrations of TFS (0, 0.5, 1.0, 1.5, 2.0, and 3.5 μg/ml), was probed using the MTS assay. Values are mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control. (F-H) LDH release from cultured A2780/CP70, OVCAR-3 and IOSE-364 cells after receiving TFS. Cells were incubated with TFS (0, 0.5, 1.0, 1.5, 2.0, and 3.5 μg/ml) for 6, 12 and 24 h in fresh serum-free medium. LDH cytotoxicity was then detected using a LDH cytotoxicity assay kit. Data were expressed as mean ± SEM of triplicate experiments. Data donated ***(P < 0.001) are significant at all time points compared to control, data donated #(P<0.05) is significant at 12 h only compared to control. (I, J) Effects of TFS (0, 0.5, 1.0 and 1.5 μg/ml) on the expression of PCNA were assayed by Western blot. GAPDH was utilized for an endogenous reference to standardize protein levels. Protein intensity was expressed as mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control.
Table 1.
MS data in negative mode of saponins and the contents of saponins extracted from tea (Camellia sinensis) flower.
| Peak | Retention Time(min) | [M-H]- | MS2 | Formula | Peak identity | Content (mg/g) |
|---|---|---|---|---|---|---|
| 1 | 48.2553 | 1257.5890 | 1125,1077,993,975,813,653 | C61H94O27 | Foliatheasaponin I/III | 20.74 ± 0.13 |
| 2 | 50.4104 | 1229.6122 | 1097,1049,627 | C60H94O26 | Floratheasaponin G/I | 45.65 ± 0.46 |
| 3 | 50.8908 | 1301.6148 | 1139,1121,1007,989,667 | C63H98O28 | Teaseedsaponin D | |
| 4 | 51.8407 | 1285.6196 | 1123,1105,1007,989,845,667 | C63H98O27 | Chakasaponin V/ | 129.76 ± 1.10 |
| Floratheasaponin E | ||||||
| 5 | 52.8645 | 1271.6047 | 1139,1109,1091,1007,989,667 | C62H96O27 | Chakasaponin II | 315.25 ± 1.32 |
| 6 | 53.6629 | 1285.6203 | 1123,1105,1007,989,845,667 | C63H98O27 | Chakasaponin V/ | 61.22 ± 0.51 |
| Floratheasaponin E | ||||||
| 7 | 54.5142 | 1271.6048 | 1139,1091,989,667 | C62H96O27 | Floratheasaponin J | 158.90 ± 0.54 |
| 8 | 54.9511 | 1313.6145 | 1181,1049,989,807,654 | C64H98O28 | Unknown | |
| 9 | 55.7099 | 1273.6189 | 1141,1093,1009,669 | C62H98O27 | Floratheasaponin C | 15.11 ± 0.08 |
| 10 | 56.6404 | 1285.6204 | 1139,1123,1105,1007,989,667 | C63H98O27 | Chakasaponin V/ | 35.10 ± 0.17 |
| Floratheasaponin E | ||||||
| 11 | 57.4751 | 1271.6047 | 1139,1091,1007,989,667 | C62H96O27 | Floratheasaponin B | 75.31 ± 0.42 |
| 12 | 59.7542 | 1285.6204 | 1139,1007,989,667 | C63H98O27 | Unknown | 19.13 ± 0.05 |
| 13 | 60.6837 | 1271.6048 | 1139,1091,1007,989,959,667 | C62H96O27 | Floratheasaponin B | 44.38 ± 0.22 |
| 14 | 61.6496 | 1313.6159 | 1181,1049,667 | C64H98O28 | Unknown | 26.09 ± 0.16 |
To evaluate the anti-tumor potential of TFS for ovarian cancer, we treated ovarian cancer cells lines (A2780/CP70 and OVCAR-3) and human normal epithelial ovarian cell line (IOSE-364) with various concentrations (0, 0.5, 1.0, 1.5, 2.0, and 3.5 μg/ml) of TFS for 6, 12 and 24 h. The MTS colorimetric assay showed that concomitant loss of cell viability was detected in A2780/CP70 and OVCAR-3 cells in an obvious dose and time dependent manner (Fig. 1C, D), while TFS displayed relatively moderate cytotoxicity in normal ovarian cells (Fig. 1E). We also determined the LDH release to test the ability of TFS to perturb cell membrane integrity in culture medium. As shown in Fig. 1F and G, cultured A2780/CP70 and OVCAR-3 cells exhibited a remarkable increase in LDH levels at concentrations ranging from 1.5 to 3.5 μg/ml; but TFS only induce slight LDH release, even treating with 3.5 μg/ml in normal ovarian cells (Fig. 1H). To verify whether TFS caused cell proliferation inhibition, we also analyzed the endogenous expression of PCNA by Western blot and found it was significantly decreased in the TFS-treated group compared with control group (Fig. 1I, J). These results confirmed the occurrence of cell cytotoxicity and cellular proliferation inhibition induced by TFS. Results from the MTS and LDH assays indicated that 0 to 1.5 μg/ml TFS treatment for 24 h could induce significantly dose-dependent cell cytotoxicity in A2780/CP70 and OVCAR-3 cells but minimally affected IOSE-364 cells. Therefore, we used this condition for the further investigation.
3.2. TFS induces apoptosis in A2780/CP70 and OVCAR-3 cells
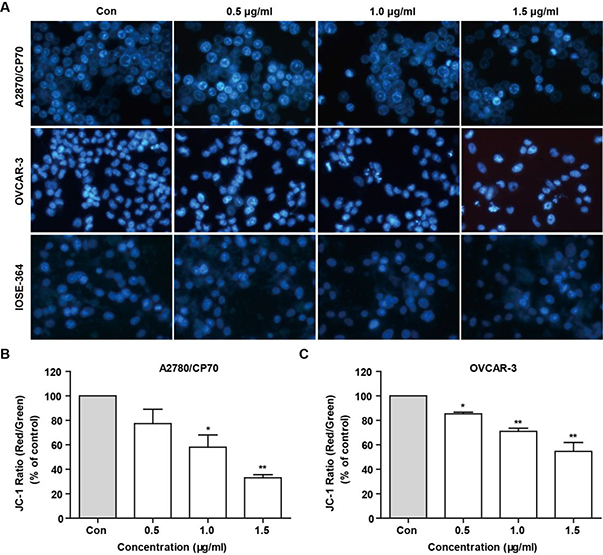
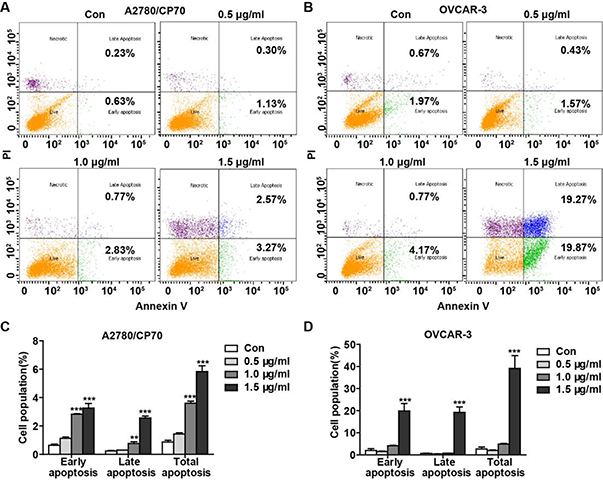
To assess whether TFS-induced cytotoxicity derived from its apoptotic effect, we used Hoechst 33342 staining and JC-1. As shown in Fig. 2A, treatment with TFS for 24 h could cause obvious nucleus shrinkage and chromatin condensation in A2780/CP70 and OVCAR-3 cells but not in IOSE-364 cells. Quantitation of the ratio of JC-1 aggregates to JC-1 monomers by fluorescence spectrophotometer showed that the mitochondrial membrane potential collapsed from 1.0 μg/ml TFS in A2780/CP70 and 0.5 μg/ml TFS in OVCAR-3 cells, respectively, which indicated the early appearance of apoptosis (Fig. 2B, C). To further confirm the pro-apoptotic effect of TFS, flow cytometry analysis via Annexin V/PI staining was performed. After treatment with TFS for 24 h, the percentage of apoptotic cells significantly increased dose-dependently in A2780/CP70 cells (Fig. 3A, C), as well as at the concentration of 1.5 μg/ml in OVCAR-3 cells (Fig. 3B, D).
Fig. 2 -. Detection of TFS induced cell apoptosis by Hoechst 33342 staining and JC-1 in A2780/CP70 and OVCAR-3 cells.
(A) After treated with TFS (0, 0.5, 1.0 and 1.5 μg/ml) for 24 h, cells were stained with Hoechst 33342 and visualized by fluorescence microscopy. (B, C) Bar charts represents the ratio of JC-1 aggregates to JC-1 monomers (ratio of 590:530 nm emission intensity), which reveals ΔΨm dissipation after 24 h treatment with TFS at various concentrations. Values are shown as mean ± SEM of three independent experiments. *P < 0.05 and **P < 0.01 versus control.
Fig. 3 -. Assay of TFS induced cell apoptosis using flow cytometry after staining with Annexin V-FITC/ PI.
(A, B) Flow cytometry analysis via Annexin V/PI staining was used to identify apoptosis after incubations of A2780/CP70 (A) and OVCAR-3 (B) cells with TFS (0, 0.5, 1.0 and 1.5 μg/ml) for 24 h. (C, D) Bar graphs manifested the percentage of cells at different stages and total stage of the apoptotic cells of A2780/CP70 (C) and OVCAR-3 (D) cells. Values are shown as mean ± SEM from three independent experiments. **P < 0.01 and ***P < 0.001 versus control.
3.3. TFS induces apoptosis via caspases activation in A2780/CP70 and OVCAR-3 cells
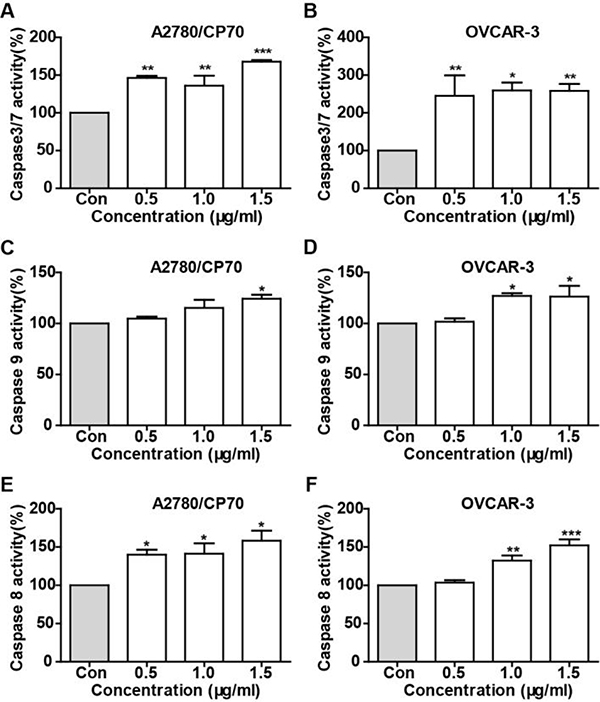
Caspases as a family of cysteine proteases, was believed to play a critical role in apoptotic responses. Initiator caspases (caspase-2, caspase-8 and caspase-9) as the early indicators of apoptosis, mainly mediate initiating apoptosis and programmed cell death. Activation of Caspase-9 and caspase-8 are makers for the intrinsic and extrinsic pathway, respectively. To demonstrate whether TFS induced apoptosis was related to activation of caspases, we determined the activities of caspase-3/7, −8 and −9 in A2780/CP70 and OVCAR-3 cells after treatment with TFS for 24 h. As shown in Fig. 4A and B, the activity of caspase-3/7 in TFS-treated cells was significantly increased versus control cells, which indicated that TFS induced apoptosis by activation of caspase-3/7. Meanwhile, caspase-9 (Fig. 4C and D) and caspase-8 (Fig. 4E and F) also showed significant high activity in TFS-treated cells, which suggested that both the intrinsic and extrinsic apoptotic pathway were activated in TFS induced apoptosis in both ovarian cancer cells.
Fig. 4 -. The effect of TFS on caspase activities in A2780/CP70 and OVCAR-3 cells.
Cells were treated with TFS (0, 0.5, 1.0 and 1.5 μg/ml) for 24 h and the caspase activities of caspase-3/7 (A, B), caspase-8 (C, D) and caspase-9 (E, F) were determined. Data shown were the mean of three independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001versus control.
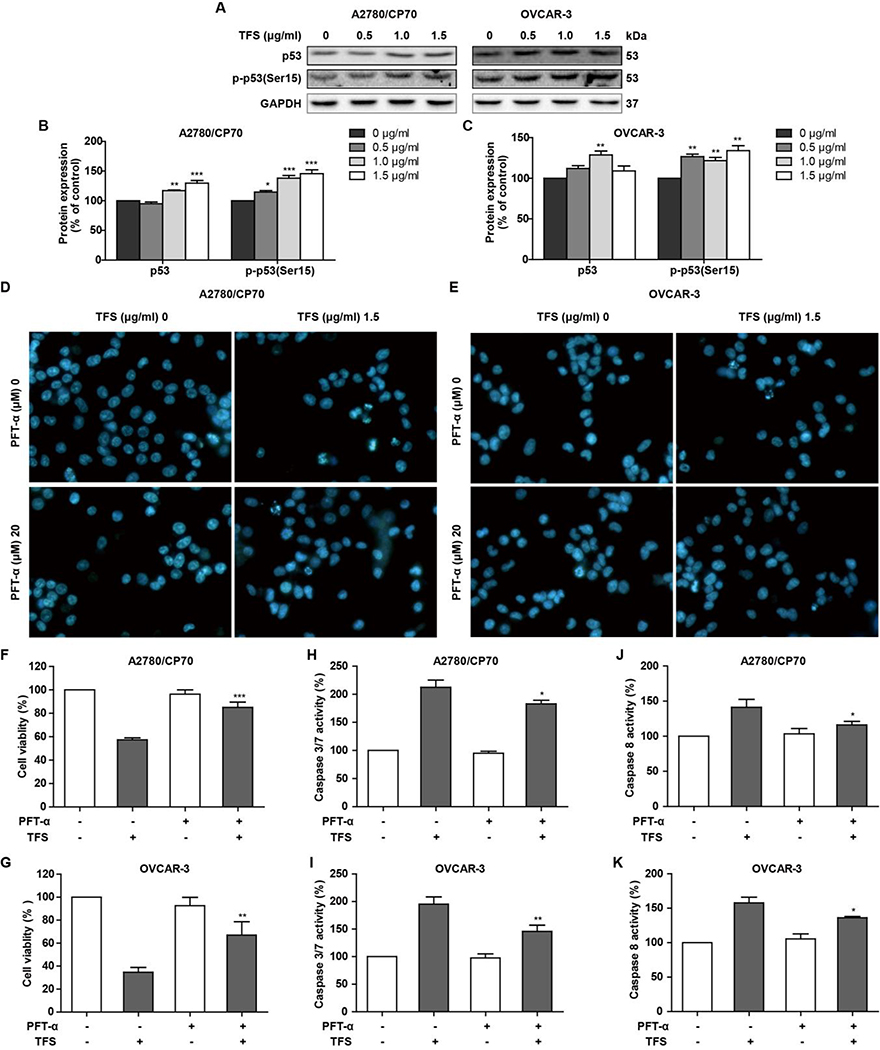
3.4. P53 contributes to TFS-induced apoptosis in A2780/CP70 and OVCAR-3 cells
TFS treatment also up-regulated protein expression level of p53 (Fig. 5A and B) in A2780/CP70 and OVCAR-3 cells. To explore the role of p53 in TFS-induced apoptosis, Hoechst 33342 staining, cell viability, and caspase activity levels were assessed in the presence and absence of the p53-specific inhibitor PFT-α (20 μM) in A2780/CP70 and OVCAR-3 cells. The results showed that PFT-α could decrease the apoptotic rate (Fig. 5D and E) and cytotoxicity (Fig. 5F and G) as well as lead to a decrease TFS-induced activities of caspase-3/7 (Fig5. H and I) and caspase-9 (Fig5. J and K). These results suggested that TFS-induced apoptosis is linked with p53 signaling.
Fig. 5 -. TFS-induced apoptosis in A2780/CP70 and OVCAR-3 cells is related with p53 upregulation.
(A-C) Expression levels of protein p53 and p-p53 in A2780/CP70 and OVCAR-3 cells were measured by western blot after treating with TFS (0, 0.5, 1.0 and 1.5 μg/ml) for 24 h. Each protein intensity was expressed as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control. (D, E) Cellular apoptosis was measured after cells were treated with TFS and p53 inhibitor PFT-α for 24 h and then stained with Hoechst 33342. (F-K) Cell viability, caspase 3/7 and caspase 8 activities were determined after treatment with TFS and p53 inhibitor PFT-α for 24 h. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control.
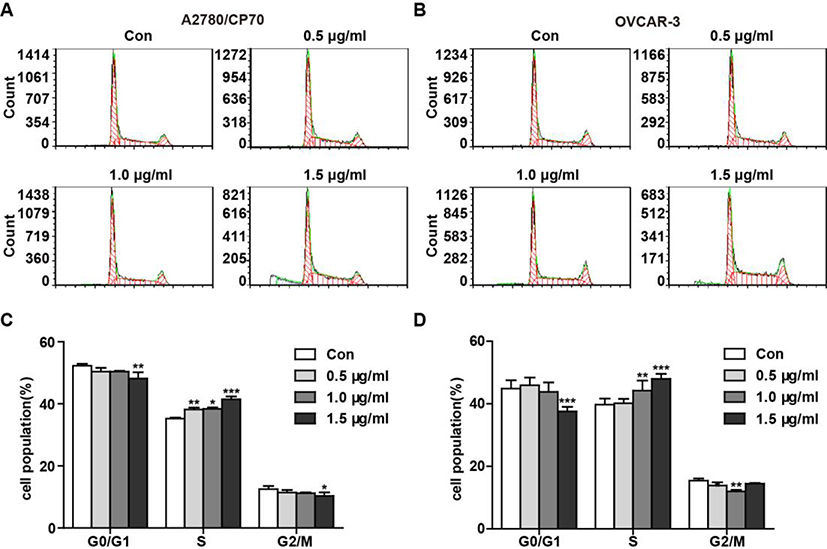
3.5. TFS triggers cell cycle arrest at the S phase by activating the CDC25A-Cdk2-Cyclin E/A signaling pathway
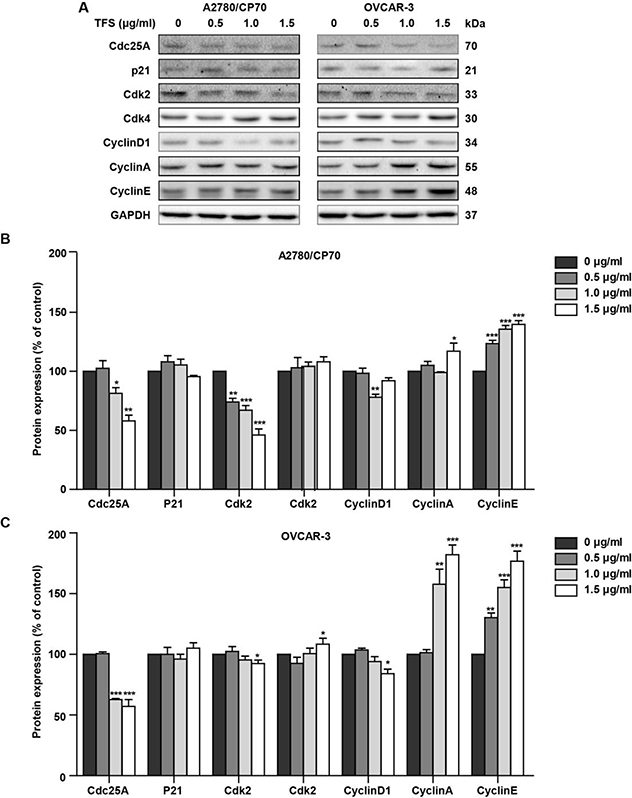
To further explore the mechanism by which TFS inhibited the cell proliferation, we also investigated the effects of TFS on cell cycle distribution. After incubation with TFS at concentrations of 0, 0.5, 1.0 or 1.5 μg/ml for 24 h, A2780/CP70 and OVCAR-3 cells were stained with propidium iodide (PI) and analyzed by flow cytometry (Fig. 6A and B). As expected, TFS induced cell cycle arrest at S phase starting from 0.5 μg/ml in A2780/CP70 cells and 1.0 μg/ml in OVCAR-3 cells (Fig. 6C and D). Meanwhile, we observed that 1.5 μg/ml dose of TFS resulted in a significant increase in the accumulation of cells in the sub-G1 phase in both cells (Fig. 6A and B), which clearly revealed apoptosis was occurring after treating with TFS. We further determined the expression of several cell-cycle related proteins by western blot. A2780/CP70 and OVCAR-3 cells incubated with TFS exhibited an increase in Cyclin E and Cyclin A, while the expression of Cdc25A, Cdk2 and CyclinD1 was clearly decreased (Fig. 7). As an important cell cycle regulator, Cdc25A was reported to decrease in S phase arrest and inhibit the activities of Cyclin E-Cdk2, Cyclin A-Cdk2 and Cyclin D1-Cdk4 complexes. Although Cyclin E and Cyclin A which are associated with S phase progression, were significantly upregulated in this experiment, Cdk2 was dose-dependently decreased in A2780/CP70 and OVCAR-3 cells, which lead to the reduction of kinase activities of Cyclin E-Cdk2 and Cyclin A-Cdk2 complexes. In addition, another index for S phase arrest, Cyclin D1, which is suppressed only in the S phase, was also inhibited. Moreover, the expression of p21 remained unchanged while Cdk4 slightly increased (Fig. 7). Taken together, these results indicated that the Cdc25A-Cdk2-Cyclin E/A pathway was involved in TFS-induced S phase arrest in ovarian cancer cells.
Fig. 6 -. TFS caused cell cycle arrest at S phase in A2780/CP70 and OVCAR-3 cells.
(A, B) Flow cytometry analysis of cell cycle phase distribution in A2780/CP70 (A) and OVCAR-3 (B) cells after treatment with 0, 0.5, 1.0 and 1.5 μg/ml TFS for 24 h. Cell number shown in the Y axis indicates peak value of the cell cycle phase. (C, D) Histograms displayed the percentage of cell cycle distribution in A2780/CP70 (C) and OVCAR-3 (D) cells. Data were expressed as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control cells which were not exposed to TFS.
Fig. 7 -. TFS induced S phase arrest in A2780/CP70 and OVCAR-3 cells via Cdc25A-Cdk2-Cyclin E/A pathway.
(A) Typical bands of protein Cdc25A, p21, Cdk2, Cdk4, Cyclin D1, Cyclin A, Cyclin E of A2780/CP70 and OVCAR-3 cells after treating with TFS (0, 0.5, 1.0 and 1.5 μg/ml) for 24 h. (B, C) Expression levels of Cdc25A, p21, Cdk2, Cdk4, Cyclin D1, Cyclin A and Cyclin E in A2780/CP70 and OVCAR-3 cells were measured and normalized to that of GAPDH protein. n=3, *P < 0.05, **P < 0.01 and ***P < 0.001 versus control.
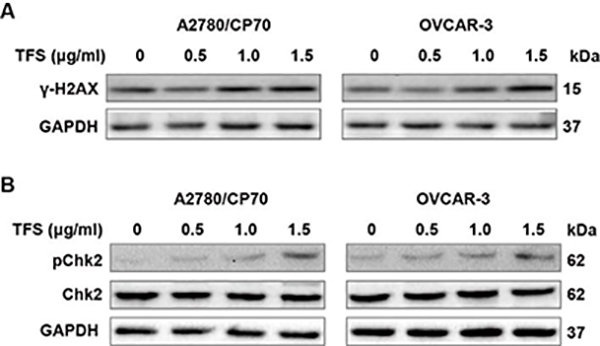
3.6. TFS induces DNA damage
Since DNA damage could lead to cell cycle arrest, we evaluated whether TFS induces DNA damage in A2780/CP70 and OVCAR-3 cells. Results showed a dramatic increase in γ-H2AX, which is a sensitive maker of DNA double strand breaks, in TFS-treated cells (Fig. 8A). Meanwhile, we analyzed the effects of TFS on expression levels of pChk2 and Chk2 and found that TFS induced the phosphorylation of Chk2 (Fig. 8B). In consideration that TFS also decreased the level of Cdc25A (Fig. 7), it is likely that TFS-induced S phase arrest derived from DNA damage and involved the activation of Chk2-Cdc25A pathway.
Fig. 8 -. TFS induces DNA damage in A2780/CP70 and OVCAR-3 cells.
(A) The protein expression levels of γ-H2AX in A2780/CP70 and OVCAR-3 cells were measured after treatment with TFS (0, 0.5, 1.0 and 1.5 μg/ml) for 24 h. (B) The protein expression levels of pChk2 and Chk2 in A2780/CP70 and OVCAR-3 cells were measured after treatment with TFS (0, 0.5, 1.0 and 1.5 μg/ml) for 24 h.
4. Discussion
Ovarian cancer remains a deadly disease among women due to its poor prognosis and drug resistance after chemotherapy. Saponins have emerged as promising anti-cancer agents against ovarian cancer, as evidenced by recent studies that several natural saponins could induce cell cycle arrest, trigger apoptosis in human ovarian cancer A2780 and HEY cell lines (X. Chen et al., 2016) and possess antitumor efficacy in human SKOV3 ovarian cancer xenografts in athymic mice (Xiao et al., 2012). A recent study reported that the total tea (Camellia sinensis) seed saponins exhibits cancer chemopreventive effects (Zhao et al., 2015). Saponins extracted from seeds of Camellia oleifera Abel. also show anti-proliferative activity against tumor cells and Oleiferasaponin C6 induces cell cycle arrest at G0/G1 phase and apoptosis in HL-60 and BEL-7402 cell lines (J. Zong et al., 2015; J. F. Zong et al., 2016). Here for the first time, we investigate the anti-cancer effect of tea (Camellia sinensis) flower saponins against ovarian cancer. Compared with seed saponins, we found that TFS contained 14 triterpenoid saponins and exhibited significant anti-proliferative effect against ovarian cancer cells in a fairly short time at low concentrations while only caused slight cytotoxicity in normal cells. We further revealed that both intrinsic and extrinsic apoptotic pathways were activated in TFS-induced p53-dependent apoptosis. We also showed that S-phase arrest induced by TFS in A2780/CP70 and OVCAR-3 was via the Cdc25A-Cdk2-Cyclin E/A pathway. Furthermore, S-phase arrest was associated with activation of the Chk2-Cdc25A DNA damage response.
It is known that a therapeutically useful cytotoxic compound must display selectivity between cancer cells and normal cells to have potential to become an anti-cancer agent (Gomes, Lefranc, Kijjoa, & Kiss, 2015). Our data demonstrated that TFS caused cytotoxicity and showed significant antiproliferative effects in both ovarian cancer cell lines at quite low concentrations. TFS induced decreased cell viability at higher concentrations, but the 1.5 μg/ml dose, at which A2780/CP70 and OVCAR-3 cells were significantly affected, only caused a slight decrease of cell viability in IOSE-364 cells. Thus, indicating that TFS could act selectively against ovarian cancer cells and demonstrating the safety of TFS, which is consistent with our previous report that tea flower extract had almost no cytotoxicity in rats (Zurolo et al., 2011).
Inducing apoptosis is an important mechanism for cancer treatments (Fesik, 2005). In the present study, we found that TFS induced apoptosis in both A2780/CP70 and OVCAR-3 cells, as evidenced by Hoechst 33342 staining, loss of mitochondrial membrane potential, Annexin V-FITC staining and the appearance of Sub-G1 peaks. p53 is a pivotal tumor suppressor that regulates cellular responses such as apoptosis, cell cycle arrest and tumor suppression (J. X. Zhang et al., 2015), and could be triggered when cells are under stress to induce apoptosis or cell cycle arrest (J. X. Zhang et al., 2015). In our study, we observed that TFS treatment leads to the upregulation of p53 expression in A2780/CP70 and OVCAR-3 cells. Knockdown of p53 by the p53 specific inhibitor PFT-α attenuates the inhibition of cell growth by TFS. PFT-α also decreases the apoptosis rate of A2780/CP70 and OVCAR-3 cells and reduces TFS-induced caspas-3/7 and caspase-8 activation. We, therefore, propose that TFS-induced apoptosis in A2780/CP70 and OVCAR-3 cells is in a p53-dependent signaling pathway.
We further hypothesized that the antiproliferative effect of TFS in A2780/CP70 and OVCAR-3 cells was involved with cell cycle arrest. Targeting the cell cycle is an important strategy for cancer therapies (Hartwell & Kastan, 1994). Moreover, cyclin-dependent kinases (CDK) and their regulatory partners the cyclins control the progression of the cell cycle, and inhibition of Cyclin D1-Cdk4/6 would be a benefit for cancer therapy (Malumbres & Barbacid, 2006; Williams & Stoeber, 2012). Furthermore, targeting the cell cycle, and CDK particularly, presents unique opportunities for drug discovery (Vermeulen, Van Bockstaele, & Berneman, 2003). We demonstrated that TFS induced cell cycle arrest at the S phase. Further study found that TFS downregulated the expression of Cdc25A and Cdk2. Since Cdc25A phosphatase plays a pivotal role in G1/S transition through inhibiting Cdk2, Cdc25A would be degraded once DNA damage occurred (Mailand et al., 2000). Though Cyclin E and Cyclin A, which are involved in the G1/S transition and S phase progression respectively (Eastman, 2004), were significantly upregulated. the downregulation of Cdk2 would reduce the formation of Cyclin E-Cdk2 and Cyclin A-Cdk2 complexes, and ultimately lead to S phase arrest. These results indicated that the Cdc25A-Cdk2-Cyclin E/A pathway is involved in TFS-induced S phase arrest in A2780/CP70 and OVCAR-3 cells and are consistent with a previous study in PNAS-4 induced S phase arrest in lung cancer cells (Yuan et al., 2015). The expression of Cyclin D1 was also significantly downregulated in both cells lines, and Cyclin D1 is an important index for S phase arrest. Taken together, our data clearly revealed that TFS induced cell cycle arrest at the S phase via the Cdc25A-Cdk2-Cyclin E/A pathway.
Treatment with TFS also induced the accumulation of γ-H2AX. γ-H2AX is regarded as a sensitive molecular marker of DNA damage which indicates DNA double-stranded breaks (Kinner, Wu, Staudt, & Iliakis, 2008). Significant Chk2 phosphorylation was observed after treatment of TFS in both ovarian cancer cell lines. Chk2 is activated by DNA damage and the activation of Chk2 could phosphorylate Cdc25A and lead to degradation of Cdc25A, thus ultimately failing to activate Cdk2 (Liao et al., 2015; Molinari, Mercurio, Dominguez, Goubin, & Draetta, 2000). We observed significant upregulation of pChk2 and downregulation of Cdc25A, which indicated that the Chk2-Cdc25A DNA damage response was involved during S phase arrest in ovarian cancer cells.
5. Conclusion
We have shown for the first time that saponins extracted from tea (Camellia sinensis) flower could efficiently induce p53-dependent apoptosis in A2780/CP70 and OVCAR-3 cells. Moreover, both the intrinsic and extrinsic apoptotic pathways were activated during apoptosis. TFS also triggered S phase arrest via the Cdc25A-Cdk2-Cyclin E/A pathway, as well as activating the Chk2-Cdc25A DNA damage response. Due to its lower cytotoxicity against a human normal ovarian cell line and a strong antiproliferative effect against ovarian cancer cells, we propose that TFS could be used as food additives for the prevention of ovarian cancer and may serve as a potential anti-cancer agent for ovarian cancer in the future.
Highlights.
Reported for the first time the anti-cancer effect of saponins extracted from Tea (Camellia sinensis) flowers (TFS) against ovarian cancer.
TFS showed significant growth inhibition effect against ovarian cancer cells at low concentrations.
TFS induced p53-dependent apoptosis and both intrinsic and extrinsic pathways were activated.
S phase arrest induced by TFS was via Cdc25A-Chk2-CyclinE/A pathway.
Chk2-Cdc25A DNA damage response pathway was activated in TFS induced S phase arrest.
Acknowledgements
This work was supported by the Changes of Health Function and Transformation Mechanisms of White Tea During Storage (KH20170151); the Collaborative Innovation Center of Chinese Oolong Tea Industry (Grant No. 2015-75); and the Natural Science Foundation of Zhejiang Province (LY16C200004). This work was also supported in part by NIH grants P20RR016477 from National Center for Research Resources and P20GM103434 from the National Institute for General Medical Sciences (NIGMS) awarded to the West Virginia IDeA Network of Biomedical Research Excellence. This work was also supported by Fortess S10 grant OD016165. We thank Dr. Kathy Brundage from the Flow Cytometry Core at West Virginia University for providing technical help on the cell cycle and apoptosis analyses.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Brasseur K, Gevry N, & Asselin E (2016). Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget. doi: 10.18632/oncotarget.14021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, … He J (2016). Cancer statistics in China, 2015. CA Cancer J Clin, 66(2), 115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- Chen X, Wu QS, Meng FC, Tang ZH, Chen XP, Lin LG, … Lu JJ (2016). Chikusetsusaponin IVa methyl ester induces G1 cell cycle arrest, triggers apoptosis and inhibits migration and invasion in ovarian cancer cells. [Article]. Phytomedicine, 23(13), 1555–1565. doi: 10.1016/j.phymed.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Eastman A (2004). Cell cycle checkpoints and their impact on anticancer therapeutic strategies. [Article]. Journal of Cellular Biochemistry, 91(2), 223–231. doi: 10.1002/jcb.10699 [DOI] [PubMed] [Google Scholar]
- Fesik SW (2005). Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer, 5(11), 876–885. doi: 10.1038/nrc1736 [DOI] [PubMed] [Google Scholar]
- Gao Y, Rankin GO, Tu Y, & Chen YC (2016). Inhibitory Effects of the Four Main Theaflavin Derivatives Found in Black Tea on Ovarian Cancer Cells. Anticancer Res, 36(2), 643–651. [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Rankin GO, Tu YY, & Chen YC (2016). Theaflavin-3, 3 ‘-digallate decreases human ovarian carcinoma OVCAR-3 cell-induced angiogenesis via Akt and Notch-1 pathways, not via MAPK pathways. [Article]. International Journal of Oncology, 48(1), 281–292. doi: 10.3892/ijo.2015.3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes NG, Lefranc F, Kijjoa A, & Kiss R (2015). Can Some Marine-Derived Fungal Metabolites Become Actual Anticancer Agents? Mar Drugs, 13(6), 3950–3991. doi: 10.3390/md13063950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajiaghaalipour F, Kanthimathi MS, Sanusi J, & Rajarajeswaran J (2015). White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chemistry, 169, 401–410. doi: 10.1016/j.foodchem.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Hanausek M, Ganesh P, Walaszek Z, Arntzen CJ, Slaga TJ, & Gutterman JU (2001). Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), suppress H-ras mutations and aneuploidy in a murine skin carcinogenesis model. Proc Natl Acad Sci U S A, 98(20), 11551–11556. doi: 10.1073/pnas.191363198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, & Kastan MB (1994). CELL-CYCLE CONTROL AND CANCER. [Review]. Science, 266(5192), 1821–1828. doi: 10.1126/science.7997877 [DOI] [PubMed] [Google Scholar]
- Huang H, Chen AY, Rojanasakul Y, Ye X, Rankin GO, & Chen YC (2015). Dietary compounds galangin and myricetin suppress ovarian cancer cell angiogenesis. J Funct Foods, 15, 464–475. doi: 10.1016/j.jff.2015.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinner A, Wu W, Staudt C, & Iliakis G (2008). Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res, 36(17), 5678–5694. doi: 10.1093/nar/gkn550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa N, Morikawa T, Motai C, Ninomiya K, Okugawa S, Nishida A, … Muraoka O (2016). The Antiproliferative Effect of Chakasaponins I and II, Floratheasaponin A, and Epigallocatechin 3-O-Gallate Isolated from Camellia sinensis on Human Digestive Tract Carcinoma Cell Lines. Int J Mol Sci, 17(12). doi: 10.3390/ijms17121979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Su D, Pasalich M, & Binns CW (2013). Tea consumption reduces ovarian cancer risk. [Article]. Cancer Epidemiology, 37(1), 54–59. doi: 10.1016/j.canep.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Liao YH, Ling JY, Zhang GY, Liu FJ, Tao SC, Han ZG, … Le HY (2015). Cordycepin induces cell cycle arrest and apoptosis by inducing DNA damage and up-regulation of p53 in Leukemia cells. [Article]. Cell Cycle, 14(5), 761–771. doi: 10.1080/15384101.2014.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand N, Falck J, Lukas C, Syljuasen RG, Welcker M, Bartek J, & Lukas L (2000). Rapid destruction of human Cdc25A in response to DNA damage. [Article]. Science, 288(5470), 1425–1429. doi: 10.1126/science.288.5470.1425 [DOI] [PubMed] [Google Scholar]
- Malumbres M, & Barbacid M (2006). Is Cyclin D1-CDK4 kinase a bona fide cancer target? Cancer Cell, 9(1), 2–4. doi: 10.1016/j.ccr.2005.12.026 [DOI] [PubMed] [Google Scholar]
- Man S, Gao W, Zhang Y, Huang L, & Liu C (2010). Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia, 81(7), 703–714. doi: 10.1016/j.fitote.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Matsuda H, Hamao M, Nakamura S, Kon’i H, Murata M, & Yoshikawa M (2012). Medicinal flowers. XXXIII. Anti-hyperlipidemic and anti-hyperglycemic effects of chakasaponins I-III and structure of chakasaponin IV from flower buds of Chinese tea plant (Camellia sinensis). Chem Pharm Bull (Tokyo), 60(5), 674–680. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Nakamura S, Morikawa T, Muraoka O, & Yoshikawa M (2016). New biofunctional effects of the flower buds of Camellia sinensis and its bioactive acylated oleanane-type triterpene oligoglycosides. J Nat Med, 70(4), 689–701. doi: 10.1007/s11418-016-1021-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Mercurio C, Dominguez J, Goubin F, & Draetta GF (2000). Human Cdc25 A inactivation in response to S phase inhibition and its role in preventing premature mitosis. [Article]. Embo Reports, 1(1), 71–79. doi: 10.1093/embo-reports/kvd018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa T, Nakamura S, Kato Y, Muraoka O, Matsuda H, & Yoshikawa M (2007). Bioactive saponins and glycosides. XXVIII. New triterpene saponins, foliatheasaponins I, II, III, IV, and V, from Tencha (the leaves of Camellia sinensis). Chemical & Pharmaceutical Bulletin, 55(2), 293–298. doi: 10.1248/cpb.55.293 [DOI] [PubMed] [Google Scholar]
- Moxley KM, & McMeekin DS (2010). Endometrial Carcinoma: A Review of Chemotherapy, Drug Resistance, and the Search for New Agents. [Review]. Oncologist, 15(10), 1026–1033. doi: 10.1634/theoncologist.2010-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myose M, Warashina T, & Miyase T (2012). Triterpene Saponins with Hyaluronidase Inhibitory Activity from the Seeds of Camellia sinensis. Chemical & Pharmaceutical Bulletin, 60(5), 612–623. [DOI] [PubMed] [Google Scholar]
- Shen X, Shi LZ, Pan HB, Li B, Wu YY, & Tu YY (2017). Identification of triterpenoid saponins in flowers of four Camellia Sinensis cultivars from Zhejiang province: Differences between cultivars, developmental stages, and tissues. [Article]. Industrial Crops and Products, 95, 140–147. doi: 10.1016/j.indcrop.2016.10.008 [DOI] [Google Scholar]
- Siegel RL, Miller KD, & Jemal A (2015). Cancer statistics, 2015. CA: A Cancer Journal for Clinicians, 65(1), 5–29. doi: 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- Sugimoto S, Yoshikawa M, Nakamura S, & Matsuda H (2009). MEDICINAL FLOWERS. XXV. STRUCTURES OF FLORATHEASAPONIN J AND CHAKANOSIDE II FROM JAPANESE TEA FLOWER, FLOWER BUDS OF CAMELLIA SINENSIS Medicinal flowers. XXV. Sturctures of floratheasaponin J and chakanoside II from Japanese tea flower, flower buds of Camellia sinensis. Heterocycles, 78(4), 1023–1029. doi: 10.3987/com-08-11568 [DOI] [Google Scholar]
- Tu YY, Kim E, Gao Y, Rankin GO, Li B, & Chen YC (2016). Theaflavin-3, 3 ‘-digallate induces apoptosis and G2 cell cycle arrest through the Akt/MDM2/p53 pathway in cisplatin-resistant ovarian cancer A2780/CP70 cells. [Article]. International Journal of Oncology, 48(6), 2657–2665. doi: 10.3892/ijo.2016.3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen K, Van Bockstaele DR, & Berneman ZN (2003). The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. [Review]. Cell Proliferation, 36(3), 131–149. doi: 10.1046/j.1365-2184.2003.00266.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way TD, Lin HY, Hua KT, Lee JC, Li WH, Lee MR, … Lin JK (2009). Beneficial effects of different tea flowers against human breast cancer MCF-7 cells. [Article]. Food Chemistry, 114(4), 1231–1236. doi: 10.1016/j.foodchem.2008.10.084 [DOI] [Google Scholar]
- Williams GH, & Stoeber K (2012). The cell cycle and cancer. [Review]. Journal of Pathology, 226(2), 352–364. doi: 10.1002/path.3022 [DOI] [PubMed] [Google Scholar]
- Xiao X, Zou J, Tri MBN, Bai P, Gao LB, Liu JS, … Wang H (2012). Paris saponin II of Rhizoma Paridis - A novel inducer of apoptosis in human ovarian cancer cells. [Article]. Bioscience Trends, 6(4), 201–211. doi: 10.5582/bst.2012.v6.4.201 [DOI] [PubMed] [Google Scholar]
- Xu RJ, Ye H, Sun Y, Tu YY, & Zeng XX (2012). Preparation, preliminary characterization, antioxidant, hepatoprotective and antitumor activities of polysaccharides from the flower of tea plant (Camellia sinensis). [Article]. Food and Chemical Toxicology, 50(7), 2473–2480. doi: 10.1016/j.fct.2011.10.047 [DOI] [PubMed] [Google Scholar]
- Yang CS, Wang X, Lu G, & Picinich SC (2009). Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer, 9(6), 429–439. doi: 10.1038/nrc2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M, Morikawa T, Yamamoto K, Kato Y, Nagatomo A, & Matsuda H (2005). Floratheasaponins A-C, acylated oleanane-type triterpene oligoglycosides with anti-hyperlipidemic activities from flowers of the tea plant (Camellia sinensis). Journal of Natural Products, 68(9), 1360–1365. doi: 10.1021/np0580614 [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Nakamura S, Kato Y, Matsuhira K, & Matsuda H (2007). Medicinal flowers. XIV. New acylated oleanane-type triterpene oligoglycosides with antiallergic activity from flower buds of Chinese tea plant (Camellia sinensis). Chemical & Pharmaceutical Bulletin, 55(4), 598–605. doi: 10.1248/cpb.55.598 [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Sugimoto S, Kato Y, Nakamura S, Wang T, Yamashita C, & Matsuda H (2009). Acylated Oleanane-Type Triterpene Saponins with Acceleration of Gastrointestinal Transit and Inhibitory Effect on Pancreatic Lipase from Flower Buds of Chinese Tea Plant (Camellia sinensis). Chemistry & Biodiversity, 6(6), 903–915. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Sugimoto S, Nakamura S, & Matsuda H (2008). Medicinal flowers. XXII - structures of chakasaponins V and VI, chakanoside I, and chakaflavonoside A from flower buds of Chinese tea plant (Camellia sinensis). Chemical & Pharmaceutical Bulletin, 56(9), 1297–1303. doi: 10.1248/cpb.56.1297 [DOI] [PubMed] [Google Scholar]
- Yuan Z, Guo WH, Yang J, Li L, Wang ML, Lei Y, … Wei YQ (2015). PNAS-4, an Early DNA Damage Response Gene, Induces S Phase Arrest and Apoptosis by Activating Checkpoint Kinases in Lung Cancer Cells. [Article]. Journal of Biological Chemistry, 290(24), 14927–14944. doi: 10.1074/jbc.M115.658419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JX, Wu D, Xing Z, Liang SJ, Han HB, Shi H, … Li QS (2015). N-Isopropylacrylamide-modified polyethylenimine-mediated p53 gene delivery to prevent the proliferation of cancer cells. [Article]. Colloids and Surfaces B-Biointerfaces, 129, 54–62. doi: 10.1016/j.colsurfb.2015.03.032 [DOI] [PubMed] [Google Scholar]
- Zhang XY, & Zhang PY (2016). Recent perspectives of epithelial ovarian carcinoma. Oncol Lett, 12(5), 3055–3058. doi: 10.3892/ol.2016.5107 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao WH, Li N, Zhang XR, Wang WL, Li JY, & Si YY (2015). Cancer chemopreventive theasaponin derivatives from the total tea seed saponin of Camellia sinensis. [Article]. Journal of Functional Foods, 12, 192–198. doi: 10.1016/j.jff.2014.11.017 [DOI] [Google Scholar]
- Zong J, Wang R, Bao G, Ling T, Zhang L, Zhang X, & Hou R (2015). Novel triterpenoid saponins from residual seed cake of Camellia oleifera Abel. show anti-proliferative activity against tumor cells. Fitoterapia, 104, 7–13. doi: 10.1016/j.fitote.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Zong JF, Wang DX, Jiao WT, Zhang L, Bao GH, Ho CT, … Wan XC (2016). Oleiferasaponin C-6 from the seeds of Camellia oleifera Abel.: a novel compound inhibits proliferation through inducing cell-cycle arrest and apoptosis on human cancer cell lines in vitro. Rsc Advances, 6(94), 91386–91393. doi: 10.1039/c6ra14467e [DOI] [Google Scholar]
- Zurolo E, Iyer A, Maroso M, Carbonell C, Anink JJ, Ravizza T, … Aronica, E. (2011). Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain, 134(Pt 4), 1015–1032. doi: 10.1093/brain/awr032 [DOI] [PubMed] [Google Scholar]