Abstract
Objective:
Psychiatric illnesses, like medical illnesses, can sometimes be considered as progressing through stages. Understanding these stages can lead to a better understanding of pathophysiology, and clarification of prognosis and treatment needs. Opinions from experts in the field of anorexia nervosa (AN) were sought to create a model of stages of illness.
Method:
The Delphi approach was used to achieve consensus from a panel of 31 individuals from a range of disciplines with expertise in AN. Over three iterative rounds, participants rated agreement with statements about an overall staging framework and definitions of specific stages.
Results:
Agreement was reached about a longitudinal progression including Subsyndromal, Full Syndrome, Persistent Illness, and Partial and Full Remission. The panel achieved consensus in defining Subsyndromal AN as characterized by body image disturbance and mild to moderate restrictive eating. Overall, there was consensus that restrictive eating is central to the behavioral features of all stages of AN, and agreement that its absence is essential to any stage of health. There was little consensus about biological markers, other than body mass index, and no consensus about quality of life indices associated with different stages.
Discussion:
This panel discussion yielded an expert-informed staging model for AN. This model now needs to be tested for its validity. The lack of consensus in several areas highlighted other research questions to address in order to develop an empirically valid and scientifically useful model of the progression of AN.
Keywords: anorexia nervosa, biomarkers, Delphi, eating disorders, pathophysiology, remission, stages of illness, subsyndromal
1 |. INTRODUCTION
What kind of disorder is anorexia nervosa (AN)? Is it episodic, as mood disorders can be? Is it progressive, like some forms of schizophrenia? Or is it persistent, more similar to some forms of substance use disorders? Clinical presentations of AN vary in numerous factors, including age, developmental stage, and illness duration. Illness course also varies, with some individuals recovering quickly and fully and others manifesting mild, moderate, or severe levels of symptoms over an extended period (Eddy et al., 2017; Steinhausen, 2002). Classifying presentations into discrete stages of illness has had great clinical utility for medical illnesses (Association TCCotNYH, 1994; Liam et al., 2015; Odicino, Pecorelli, Zigliani, & Creasman, 2008). The prototypical framework comes from cancer care, where a range of biological markers are used to classify tumor staging, and each distinct stage has prognostic and treatment implications (Odicino et al., 2008). Illness-staging has drawn interest in psychiatry (though not necessarily universal support; Alda & Kapczinski, 2016; Dodd, Berk, Kelin, Mancini, & Schacht, 2013), and the need for a similar illness-staging framework for eating disorders has been well articulated (Treasure, Stein, & Maguire, 2015). Across psychiatry, it has been challenging to develop staging models that penetrate the work of researchers and clinicians (Frank, 2005). One barrier is that new definitions may require investigators to compromise in order to agree, and new definitions may interfere with comparisons with prior research where old definitions may have been used (Frank, 2005; Frank et al., 1991). A framework needs to have agreement from the field to increase the likelihood of its acceptance, and therefore to be of value. Expert input in the development of a staging model does not indicate model validity; rather, agreement within the field will facilitate the necessary science to determine the most useful staging model. Here, we present the results from a panel of experts surveyed about potential descriptions of stages and progression of illness in AN.
Broadly speaking, clinical staging frameworks aim to distinguish between phases of illness and are most useful when there is a predictable, longitudinal progression from one phase to the next. As the disease progresses, dissociable phases are distinguished by differences in treatment recommendations or prognosis. In AN, early descriptions alluded to the concept of stages, including a prodromal stage characterized by discomfort with fullness and mild food restriction that progresses to more severe food restriction, increased activity levels, and the onset of weight loss (Gull, 1873; Treasure et al., 2015). More recently, one self-report instrument was developed to assess stages of illness in AN (Maguire et al., 2012): while it did discriminate between “mild” and “severe,” it did not distinguish along other categories and its predictive validity for long-term illness course has not yet been examined (Maguire et al., 2017). There is also field-wide interest in identifying chronic AN (Tierney & Fox, 2009), as well as recovery (Bardone-Cone, Hunt, & Watson, 2018; Khalsa, Portnoff, McCurdy-McKinnon, & Feusner, 2017; Kordy et al., 2002). In developing a clinical staging framework, the goal for this panel was to identify specific clinical parameters that suggest an individual is in one stage versus another (Frank, 2005; Frank et al., 1991).
An illness-staging framework has potential to improve personalized treatment and advance scientific understanding of biobehavioral mechanisms of illness. Identifying discrete stages would allow for more homogeneous groups in research studies, which may improve understanding of illness mechanisms and/or development of more effective treatments. These groupings would also enhance the rigor of pooling across studies as science continues to demand larger samples sizes that are difficult to achieve in less common illnesses such as AN. One possible limitation in current neurobiological research in AN is that, without clear definitions of stages, heterogeneity may be clouding the detection of biological markers. As noted by Treasure et al. (2015), malnutrition affects the body, including the brain, and may lead to neuroprogression (a term used interchangeably with neuroadaptation to describe biological characteristics that may mark and/or shape pathophysiology) (Berk et al., 2011; Moylan, Maes, Wray, & Berk, 2013). Biomarkers of early illness may differ from persistent illness. Ideally, if there are stages of illness, this would be determined empirically and/or through advances in understanding pathophysiology. However, because the pathophysiology of AN remains uncertain, a staging framework may be a helpful step toward clarifying pathophysiology and advancing neuroscience research.
This study aimed to achieve expert consensus in definitions of stages of illness progression in AN using the Delphi approach (Linstone & Turoff, 1975). This method is an established technique for developing consensus by experts in the field and has been applied in the medical context when data-based guidelines or an accepted set of standards are lacking (Bader, McDonald, & Selby, 2009; Cabral et al., 2005; Eubank et al., 2016; Fish & Busby, 1996; Hasson, Keeney, & McKenna, 2000; Jones & Hunter, 1995). The Delphi method is a multistage process, in which panelists respond over several iterative rounds with the goal of reaching consensus. Each round builds off the last: panelists are asked to clarify and reassess their responses as they learn the views of the group. The putative strengths of this method are that opinions come from individuals who have a relevant expertise (Dalkey, Rourke, Lewis, & Snyder, 1972), that the responses are anonymous, and therefore cannot be unduly influenced by interpersonal factors (Fish & Busby, 1996), and that the forces of the group can be mobilized to move the experts toward consensus. The current study elicited an expert panel’s ideas on behavioral, cognitive, and biological features of stages of AN.
2 |. METHOD
In the Delphi method, participant selection (sampling) is purposive (not random) in order to recruit panelists who are experts in the topic under investigation. Inclusion criteria were used to identify and recruit participants with highly specialized knowledge of AN. “Expertise” was defined as greater than 10 years of experience in the field of AN, as well as membership in either the Eating Disorders Research Society or the Academy for Eating Disorders. To identify a range of participants meeting these criteria with geographic and disciplinary diversity, study investigators contacted 63 eating disorders professionals in relevant disciplines in both clinical and academic settings. Individuals were asked to nominate other professionals who met the established criteria outlined above. Forty-two individuals across specialties, including psychiatry, psychology, internal medicine, adolescent medicine, nutrition, and social work, were invited to participate.
In the initial contact, potential participants were informed that there would be three rounds of questions and were asked to commit to participating in all rounds. Respondents remained anonymous to one another throughout the study. At the conclusion of the study, panelists were offered the opportunity to be acknowledged in the publication.
Round 1 consisted of 16 open-ended questions developed by the authors (questions available upon request), in three parts, with opportunities for comments after each question. Part 1 provided a potential clinical staging framework that was drawn from existing proposals in the literature (Bardone-Cone et al., 2018; Treasure et al., 2015), and sought ideas about behavioral, cognitive, and biological components that might distinguish these stages from each other. The proposed stages included: Subsyndromal, Full Syndrome, Persistent, Partial Remission, Remission, and Full Syndrome, and Repeat Episode. Potential additional modifiers of Full Syndrome included “Early” and “First Episode.” Part 2 asked panelists to consider biological factors (bone density, gonadal hormones, thyroid function, white blood cell count, and cortisol levels) that might be useful in a staging model. Part 3 asked panelists to consider aspects of functional impairment (achievement different from expected at work or school and impairment in social relationships) that might distinguish between stages of illness. Responses to Round 1 were collated and systematically analyzed for themes to create items (statements) for Round 2.
Round 2 consisted of 47 statements, integrating responses from Round 1 and taken as close to verbatim from panelists as possible. These statements also aimed to refine terms and definitions of each proposed stage, including specification of time frames. Instructions to participants emphasized identifying specific clinical parameters (termed, “hinge points”) for each stage. Panelists were asked to rate their level of agreement with each item on a 5-point Likert scale, ranging from “Strongly Agree” to “Strongly Disagree.” Room for comment was included.
Round 3 consisted of 34 statements and a reference figure depicting the proposed stages. Items were omitted if there was consensus (two items, see Section 2.1) or less than 50% agreement (13 items) in Round 2. Each statement was accompanied by a histogram showing the distribution of responses for that item, the individual participant’s rating in Round 2, and a Likert scale for rating agreement.
2.1 |. Data analysis
Responses to Round 1 were analyzed using content analysis (Hsieh & Shannon, 2005), coded by the investigators separately (J.E.S. and D.R.G.) and then reviewed for consensus. Items were deemed idiosyncratic if they were attributable to a single respondent and these items were not integrated into future rounds. For Rounds 2 and 3, consensus was defined as ≥85% agreement (or disagreement) and “near consensus” was defined as ≥75% agreement (or disagreement) (Buchman, Attia, Dawson, & Steinglass, 2019; Mittnacht & Bulik, 2015; Noetel, Dawson, Hay, & Touyz, 2017). Frequencies were calculated to determine the percentage of panelists who responded “Agree” or “Strongly Agree” (or the opposite). Mean responses and SDs for each item in Round 3 were calculated.
3 |. RESULTS
Thirty-one individuals completed Round 1 and therefore constituted the expert panel. Participants included 15 MDs in psychiatry or medicine, 14 PhDs in psychology or social work, and 2 MD/PhDs. Panelists came from the United States (n = 23), the United Kingdom (n = 3), Canada (n = 2), Australia (n = 1), Austria (n = 1), and Israel (n = 1). One panelist declined to participate in Round 3 (98% panel retention).
In Round 2, 94% of the panel agreed that “restriction of food intake” is an element of Subsyndromal AN; 97% agreed that a Repeat Episode of Full Syndrome AN would occur after a clear period of Remission from AN. Responses and comments about the staging sequence in Round 2 were incorporated into the creation of Figure 1, which was disseminated in Round 3.
FIGURE 1.
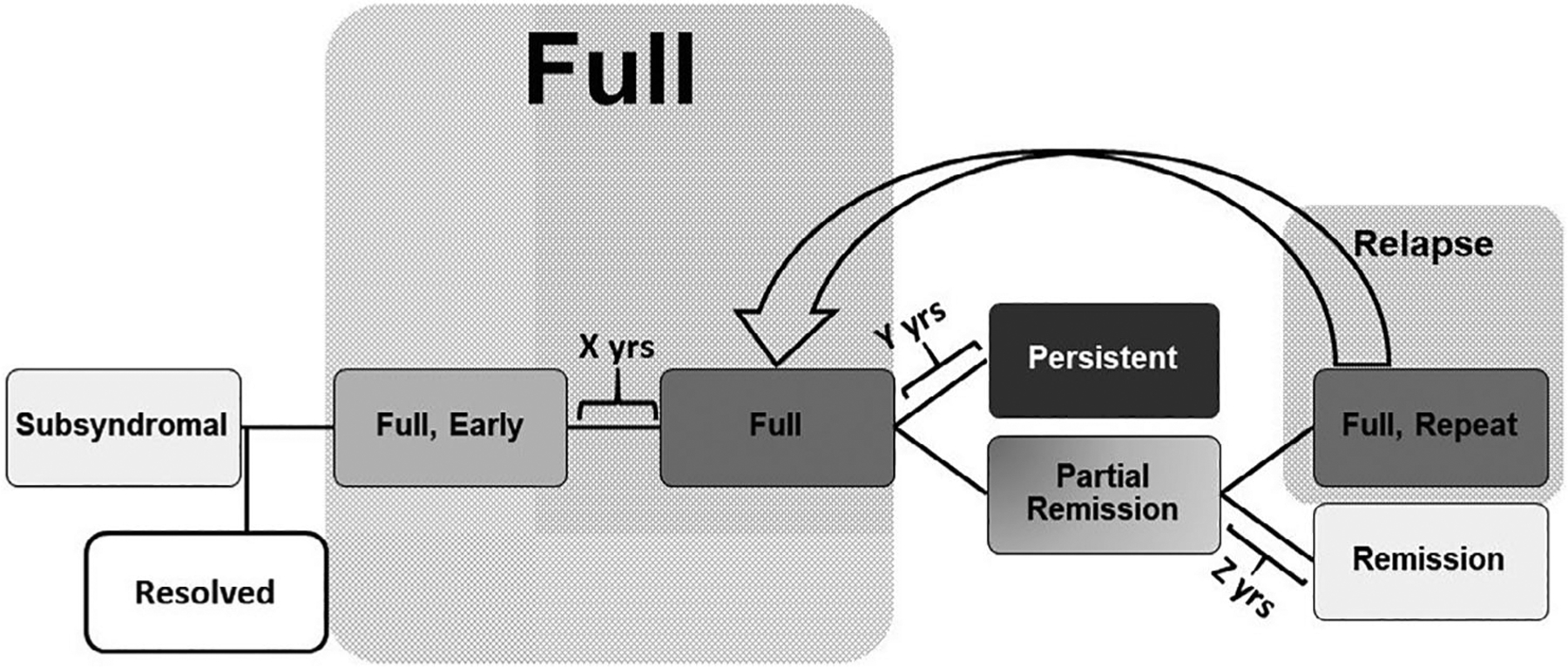
Anorexia Nervosa (AN) stages of illness sequencing, developed from Round 2 responses. The panel agreed that stages of illness in AN can be considered as progressing from Subsyndromal to Full Syndrome AN, followed by either Partial Remission or Persistent AN, and then by Full Remission or a new episode of Full Syndrome AN. The timeframe for Early AN (X) was the first 12 months of illness. There was near consensus that the timeframe for Persistent AN (Y) was more than 3 years. The timeframe for Remission (Z) was more than 1 year. AN, anorexia nervosa
In Round 2, there was less than 50% agreement for the following items, which were therefore omitted from Round 3. Regarding a Subsyndromal phase, there was <50% agreement about: excessive exercise as a required component, a specific time frame for weight loss, the omission of weight status, or body image disturbance as criteria. There was less than 50% agreement about several time frame qualifiers: Early AN as <3 years, Persistent AN as >1 year, and Remission as at least 3 or 6 months. Other items with less than 50% agreement were the use of a body mass index (BMI) threshold of 19.0 kg/m2 for Partial Remission or Remission, or the use of a threshold of at least two biological disturbances to distinguish Subsyndromal from Full Syndrome AN.
Results of Round 3 are presented in Tables 1 and 2. Overall, there was consensus about the use of a staging framework that includes Subsyndromal, Full Syndrome, Persistent Illness, Partial Remission, and Remission (see Figure 1). In identifying time frames, there was consensus that in a Full Syndrome, Early AN stage, symptoms would be present for less than 1 year (though only near consensus that a staging model would include this phase), Remission involves being asymptomatic for more than 1 year, and near-consensus that Full Syndrome AN must be present for more than 3 years to be considered Persistent. In defining behavioral characteristics, there was consensus about the specification that restrictive eating (or its reduction/absence) defines Subsyndromal, Partial Remission, and Remission, and that Remission also includes the absence of binge eating and purging. In considering cognitive components, there was consensus that all stages included disturbance in body image, and that EDE-Q scores are not useful for defining hinge points. For the biological components, there was consensus about BMI as a biological marker, with agreement that the threshold of 18.5 kg/m2 (and for adolescents, “10% below what is expected based on the growth curve”) defined illness. No consensus was reached for quality of life items (Table 2).
TABLE 1.
Panel responses to statements identifying and defining potential stages of illness in anorexia nervosa
| Item | Mean | SD | % Agreement |
|---|---|---|---|
| Overall framework | |||
| Stages of illness include partial remission and remission.a | 4.8 | 0.4 | 100 |
| A stages of illness framework of AN can include episodes.a | 4.4 | 0.6 | 97 |
| The Full Syndrome of AN includes a modifier describing persistent illness.a | 4.4 | 0.9 | 90 |
| The Full Syndrome of AN includes a modifier for early. | 4.2 | 0.8 | 83 |
| Subsyndromal | |||
| The cognitive component of Subsyndromal AN consists of body image disturbance, as defined in DSM-5: disturbance in the way in which one’s body weight or shape is experienced, or undue influence of body weight or shape on self-evaluation.a | 4.7 | 0.7 | 97 |
| The behavioral component of Subsyndromal AN is mild to moderate restrictive eating.a | 4.5 | 0.7 | 93 |
| Definition | |||
| Mild to moderate restrictive eating behavior and/or excessive exercise; body habitus not significantly underweight (e.g., weight loss not more than 10% of body weight; BMI not less than 18.5 kg/m2; appropriate BMI percentile for children and adolescents); and disturbance in body image. (EDE-Q not included)a | 4.4 | 0.9 | 87 |
| Mild to moderate restrictive eating behavior and/or excessive exercise; body habitus not significantly underweight (e.g., weight loss not more than 10% of body weight; BMI not less than 18.5 kg/m2; appropriate BMI percentile for children and adolescents); and disturbance in body image and EDE-Q global score of less than or equal to 3. | 3.8 | 0.8 | 80 |
| Mild, moderate, or severe restrictive eating behavior and/or excessive exercise; body habitus not significantly underweight (e.g., weight loss not more than 10% of body weight; BMI not less than 18.5 kg/m2; appropriate BMI percentile for children and adolescents); and disturbance in body image and EDE-Q global score of less than or equal to 3. | 3.7 | 0.8 | 80 |
| Full syndrome modifiers | |||
| In Full Syndrome, EARLY, symptoms are present for less than 1 year.a | 4.6 | 0.9 | 90 |
| In Persistent AN, Full Syndrome AN is present for more than 3 years. | 3.7 | 1.3 | 77 |
| Partial remission | |||
| In Partial Remission, the behavioral component of not more than minimal restriction is required.a | 4.4 | 0.8 | 93 |
| Definition | |||
| Minimal restrictive eating; partial weight restoration (e.g., BMI greater than 18.5 kg/m2, or within 10% of what is expected for the individual); improvement in fear of gaining weight OR disturbance in body image that interferes with achievement or maintenance of healthy weight. | 3.97 | 0.9 | 83 |
| Minimal restrictive eating; partial weight restoration (e.g., BMI greater than 18.5 kg/m2, or within 10% of what is expected for the individual); fear of gaining weight OR disturbance in body image that interferes with achievement or maintenance of healthy weight. (no EDE-Q) | 4.0 | 0.9 | 80 |
| Minimal restrictive eating; partial weight restoration (e.g., BMI greater than 18.5 kg/m2, or within 10% of what is expected for the individual); fear of gaining weight OR disturbance in body image that interferes with achievement or maintenance of healthy weight; EDE-Q less than or equal to 3. | 3.7 | 1.0 | 80 |
| Remission | |||
| Remission includes abstinence from binge eating and/or purging behaviors, as well.a | 4.8 | 0.6 | 97 |
| In Remission from AN, symptoms are absent for more than 12 months.a | 4.4 | 0.7 | 97 |
| In Remission from AN, BMI is greater than or equal to 18.5 kg/m2 or weight is restored to within 10% of expected (including growth curve or BMI percentile).a | 4.2 | 0.7 | 90 |
| Definition | |||
| Absence of restrictive eating; weight restoration; fear of gaining weight/disturbance in body image that does not interfere with maintenance of optimal weight. (no EDE-Q)a | 4.3 | 0.7 | 97 |
| Absence of restrictive eating AND absence of excessive exercise; weight restoration; fear of gaining weight/ disturbance in body image that does not interfere with maintenance of optimal weight; EDE-Q global score less than or equal to 2. | 3.9 | 1.2 | 80 |
| Absence of restrictive eating; weight restoration; fear of gaining weight/disturbance in body image that does not interfere with maintenance of optimal weight; EDE-Q global score less than or equal to 2. | 3.6 | 0.9 | 67 |
Abbreviations: AN, anorexia nervosa; BMI, body mass index; EDE-Q, Eating Disorder Examination-Questionnaire.
Consensus agreement.
Table 2.
Panel responses to biomarkers and quality of life in stages of anorexia nervosa
| Item | Mean | SD | % Agree | % Disagree |
|---|---|---|---|---|
| Biomarkers: Subsyndromal vs. Full Syndrome | ||||
| Bone density (osteoporosis or osteopenia) is a marker that distinguishes Subsyndromal AN from Full Syndrome AN (including any of the stage modifiers).a | 1.7 | 0.9 | 7 | 87 |
| High cholesterol is a marker that distinguishes Subsyndromal AN from Full Syndrome AN (including any of the stage modifiers). | 1.7 | 1.0 | 10 | 80 |
| Neutropenia is a marker that distinguishes Subsyndromal AN from Full Syndrome AN (including any of the stage modifiers). | 1.8 | 1.0 | 13 | 83 |
| Elevated cortisol is a marker that distinguishes Subsyndromal AN from Full Syndrome AN (including any of the stage modifiers). | 1.9 | 0.9 | 10 | 83 |
| Abnormal thyroid function (TSH, T3 and/or reverse T3) is a marker that distinguishes Subsyndromal AN from Full Syndrome AN (including any of the stage modifiers). | 1.9 | 0.1 | 13 | 73 |
| Bradycardia and/or prolonged QTc is a marker that distinguishes Subsyndromal AN from Full Syndrome AN (including any of the stage modifiers). | 2.4 | 1.1 | 23 | 67 |
| Biomarkers: Full Syndrome vs. Partial/Full Remission | ||||
| For females who had amenorrhea, the return of regular menses is a marker that distinguishes Remission from Full Syndrome AN (including any of the stage modifiers). | 4.1 | 1.1 | 80 | 10 |
| Resolution of transient medical complications of malnutrition is a marker that Remission from Full Syndrome AN (including any of the stage modifiers). | 3.8 | 1.1 | 77 | 13 |
| Normalization of gonadal hormones (estradiol, LH, FSH) is a marker that distinguishes Remission from Full Syndrome AN (including any of the stage modifiers). | 3.7 | 1.0 | 67 | 13 |
| For females who had amenorrhea, the return of regular menses is a marker that distinguishes Full Syndrome AN (including any of the stage modifiers) from Partial Remission. | 3.6 | 1.0 | 70 | 13 |
| Resolution of transient medical complications of malnutrition is a marker that distinguishes Full Syndrome AN (including any of the stage modifiers) from Partial Remission. | 3.5 | 1.0 | 70 | 13 |
| Quality of life | ||||
| Absence of functional impairment or return to baseline or appropriate developmental stage (e.g., in work, school, or relationships) is a marker that distinguishes Remission from Full Syndrome AN. | 3.7 | 1.1 | 77 | 17 |
| Achievement different from expected at work or school is a marker that distinguishes Subsyndromal AN from Full Syndrome AN (including any of the stage modifiers). | 2.0 | 0.8 | 0 | 73 |
Abbreviation: AN, anorexia nervosa.
Consensus DISAGREEMENT.
4 |. DISCUSSION
In this study, we successfully engaged a panel of 31 individuals with expertise in AN from different disciplines to provide opinions about stages of progression of illness. Developing consensus is challenging, presumably because of limited understanding of pathophysiology and limited data about determinants of illness course. And yet, staging models in psychiatry have been useful for psychosis, major depressive disorder, and bipolar disorder (Cosci & Fava, 2013; McGorry et al., 2014), suggesting this endeavor has merit. The ability to assemble a panel underscores that there is interest in the eating disorders field in developing a stages of illness model for AN, and that experts agree about some key aspects. First, there is agreement that the behavioral characteristic of restrictive eating is central to any stage of illness, and that its absence is central to any stage of health. In contrast to behavioral features of illness, experts are in less agreement about cognitive components of AN. Second, the panel was in agreement that AN progresses through stages, and can be episodic. There was consensus that there is a Subsyndromal phase that precedes the Full Syndrome (Figure 1).
The areas of disagreement, or failure to achieve consensus, were also informative. Not surprisingly, given the lack of existing data, the panel was hesitant to declare set durations for some stages. There was only low level of agreement on the existence of a modifier for “Early” AN, but agreement that if there were one, it would be within the first 12 months of illness. For Persistent Illness, there was agreement that there should be such a descriptor, but uncertainty about what might be a meaningful duration of illness to delineate as “Persistent.” These areas of doubt and the additional uncertainty around biological markers and quality of life indicators of different stages highlight the need for data to clarify if and when the proposed terms are meaningful.
Several questions for research emerge from the panel including: (a) whether the field can establish a Subsyndromal phase of AN, (b) whether there are biological or clinical features that reliably delineate the first 12 months of AN from later illness or signify a Persistent stage, (c) whether any biological markers other than BMI might signify Full Syndrome AN, and (d) whether biological markers can be useful for identifying adequate weight restoration.
The panel overwhelmingly agreed with the addition of a Subsyndromal stage of AN to the current lexicon, with over 90% agreeing that this stage could be characterized by body image disturbance and mild to moderate restrictive eating (without significant weight loss). Other areas of psychiatry have also concluded that staging models most advance the field by considering prodromal features (Alda & Kapczinski, 2016). The notion that subclinical eating disorder traits may be quite meaningful is bolstered by recent biological research showing that increases in self-reported subclinical eating disorder psychopathology were correlated with cortical thickness in regions essential to food perception, reward, and interoception (Wallace, Richard, Peng, Knodt, & Hariri, 2019). However, more research is necessary, including studies that use new or existing (Maguire et al., 2012) stage of illness measures to describe cognitive and behavioral features of individuals in a Subsyndromal phase, and to reliably distinguish Subsyndromal AN from nonpathological dieting.
Opinions were mixed about the existence of an Early stage of illness, though 12 months was agreed upon as constituting “Early.” This is an area where existing research is minimal, in part because of challenges in reliably defining onset of illness (Davis, Ranzenhofer, Posner, & Steinglass, 2019). Existing studies have explored questions around the age of onset and duration of illness (Raykos, Erceg-Hurn, McEvoy, Fursland, & Waller, 2018; Tan, Kwok, Zainal, & Lee, 2018), but provide less information about the clinical progression within the first 12 months of illness. Biological variables are even less studied in early illness, even though brain volume has been shown to be affected even among adolescents with AN (Frank, Shott, Hagman, & Yang, 2013; Fujisawa et al., 2015; Mainz, Schulte-Ruther, Fink, Herpertz-Dahlmann, & Konrad, 2012). Extrapolating from other areas of psychiatry, it seems likely that examining neuroprogression could be useful. In schizophrenia, for example, it has been shown that the lateral ventricles are commonly affected in chronic illness, but less so in first-episode (Steen, Mull, McClure, Hamer, & Lieberman, 2006). This raises the question: are there markers other than BMI that emerge early in illness, and perhaps mark the onset of pathology? Recent exploration of the genetics of AN (Watson et al., 2019) provides a hint that there may be more biological and metabolic markers that warrant study early in illness; clarifying aspects of progression may yield opportunities for intervention.
The panel clearly agreed that BMI is an important biological indicator of illness in Full Syndrome AN, and did not find other biological markers with greater value in determining acute illness, severity of AN, treatment needs, or prognosis. All other potential markers of starvation were either rejected or controversial. Glucose and insulin sensitivity, which have been shown to be low among patients during acute illness (Ilyas et al., 2019), were not raised by the panel as potential biological markers. General standards for weight restoration are useful clinical guidelines but may be less helpful in research on biology of illness. Among adolescents, it has been described that the majority of patients resumed menses within 6 months to a year after reaching a target weight of 90% of standard body weight, with the average weight for resumption of menses being 2 kg higher than the average weight for loss of menses (Golden et al., 1997). Numerous psychological, medical, and biological factors improve—but do not necessarily normalize—with weight restoration (Bargiacchi, 2014; Channon & deSilva, 1985; Frank et al., 2013; Friederich et al., 2012; Hubel et al., 2019; Meehan, Loeb, Roberto, & Attia, 2006; Nickel et al., 2018; Van den Eynde et al., 2012). These data all demonstrate the clinical importance of weight restoration, but do not yield biological markers to identify when weight restoration has been achieved. Gonadal hormones, which are sometimes used clinically to identify weight restoration, were not identified as a biomarker by this panel. This is perhaps understandable as existing data do not clarify whether normalization of gonadal hormones has prognostic value for maintaining psychiatric health, underscoring that there is no set of biological factors guaranteed to indicate physiological health (Schorr & Miller, 2017). As discussed by the panel, because individuals differ in medical presentations, it is a challenge to the field to identify broadly applicable biological markers of starvation and health. In particular, data are needed to identify biological markers that indicate when an individual is at sufficient weight to be at lower risk for relapse and higher likelihood of continued improvement in eating disorder symptoms.
Some individuals remain in a state of Full Syndrome AN and this panel agreed that there may be a meaningful distinction of a stage of illness that is Persistent AN, yet struggled with what the appropriate time frame should be. Existing studies have used definitions of persistence that range from 3 to 10 years (Broomfield, Stedal, Touyz, & Rhodes, 2017). Panelists in this study agreed that unremitting behavioral and psychological symptoms were defining features of a Persistent stage of AN (Hay & Touyz, 2018). The concept of “treatment resistance” was considered, but ultimately posed a challenge for our panel, perhaps because there are differences in how, when, and what treatments are accessible and whether patients (and their families) wish to partake in recommended approaches (Hay & Touyz, 2018). Whereas treatment resistance has been defined in major depressive disorder (Scott et al., 2013), there is no existing literature determining how many unsuccessful attempts at renourishing would constitute treatment resistance, especially in the United States given the limitations of the current managed care system.
5 |. SUMMARY AND CONCLUSIONS
Staging and classification models in psychiatry have garnered attention, with some indications that they have contributed to the understanding and treatment of illness. This is perhaps best exemplified in schizophrenia where the identification of a prodromal stage has had a major impact (McGorry et al., 2002; van der Gaag et al., 2013). Here, we successfully engaged experts to come together and outline a model of stages of illness in AN that would be useful to test for its empiric validity.
As with any Delphi approach, this study was limited by two major factors. First, it is critical to remember that consensus does not establish the validity of the stages or the model and should be considered a starting point for a research agenda—not an endpoint. In some psychiatric research, existing staging models have been shown to be unhelpful (e.g., depression; Dodd et al., 2013). Second, the findings are limited by the panelists perspectives and what the panel chooses to discuss. There may be biological markers, for example, that are highly relevant but were overlooked by this panel and therefore no discussion occurred. And, the panel in this study unfortunately did not include the full range of clinical disciplines: despite inviting nominations from organizations with members representing a broad range of clinical disciplines, the panel did not include dieticians or nurses. Though we achieved an international panel, the majority of participants were from the United States. Third, areas that did not achieve any consensus raise some additional questions about the transition from illness to health. For example, is quality of life improvement a key consideration? Individuals with AN report lower quality of life than those without eating disorders (Sy, Ponton, De Marco, Pi, & Ishak, 2013), and comparable to those with severe depression (Arkell & Robinson, 2008). One study has shown that improvements in quality of life are associated with, and may be dependent on, behavioral change and weight gain (Bamford et al., 2015). Fourth, this panel did not address distinctions between Remission and Recovery. Empiric validation of definitions of Recovery is actively under study within the field (Bardone-Cone et al., 2018). While meeting the definition of Recovery has been shown to have prognostic meaning— meeting criteria for Recovery is associated with staying well (Bardone-Cone et al., 2019)—efforts to validate remission and recovery in other areas of psychiatry have been disappointing. Research in major depressive disorder has suggested that there are no useful timeframes to predict maintenance of health (de Zwart, Jeronimus, & de Jonge, 2019). This research counters the notion of stages of illness in psychiatry, and is an alternative hypothesis that warrants further discussion and research.
The major strengths of this study include the size and breadth of the panel (spanning disciplines as well as clinical and research perspectives), and the ability to keep the panelists anonymous to each other as they pursued these definitions. The survey instructions also explicitly pushed the panelists to consider stages based on hinge points and to move away from criteria that might be simply descriptive. The specific research questions that emerged from this study would further the understanding of a staging model, and lead to more homogeneity in research groupings. Clarity about the neurobiology of stages of illness in AN could address questions about predisposing, precipitating, and perpetuating illness factors.
ACKNOWLEDGEMENTS
This Delphi study would not have been possible without the time and expertise of the panel members who participated. The authors would like to formally acknowledge the panelists, who gave their consent to be recognized, including: Wayne Bowers PhD, Cynthia Bulik PhD, Scott Crow MD, Gina Dimitropoulos MSW, PhD, Kamryn Eddy PhD, Martin Fisher MD, Victor Fornari MD, Guido Frank MD, Moria Golan PhD, Neville Golden MD, Allan Kaplan MD, Andreas Karwautz MD, PhD, Walter Kaye MD, Diane Klein MD, Richard Kreipe MD, Daniel Le Grange PhD, Sloane Madden MD, Laurel Mayer MD, Carrie McAdams MD, PhD, Diane Mickley MD, Carol Peterson PhD, Graham Redgrave MD, Lucy Serpell PhD, Janet Treasure PhD, Glenn Waller DPhil, B. Timothy Walsh MD, Ruth Weissman PhD, Jennifer Wildes PhD, Lucene Wisniewski PhD, Stephen Wonderlich PhD, and Joel Yager MD.
Funding information
Klarman Family Foundation, Grant/Award Number: Eating Disorders; National Institute of Mental Health, Grant/Award Number: K24 MH113737
Footnotes
CONFLICT OF INTEREST
Drs. Steinglass and Attia receive royalties from UpToDate. Dr. Glasofer and Ms. Dalack report no conflicts.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Alda M, & Kapczinski F (2016). Staging model raises fundamental questions about the nature of bipolar disorder. Journal of Psychiatry & Neuroscience, 41(5), 291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkell J, & Robinson P (2008). A pilot case series using qualitative and quantitative methods: Biological, psychological and social outcome in severe and enduring eating disorder (anorexia nervosa). The International Journal of Eating Disorders, 41(7), 650–656. [DOI] [PubMed] [Google Scholar]
- Association TCCotNYH. (1994). Nomenclature and criteria for diagnosis of diseases of the heart and great vessels (9th ed.). Boston, MA: Little, Brown and Company. [Google Scholar]
- Bader P, McDonald P, & Selby P (2009). An algorithm for tailoring pharmacotherapy for smoking cessation: Results from a Delphi panel of international experts. Tobacco Control, 18(1), 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford B, Barras C, Sly R, Stiles-Shields C, Touyz S, Le Grange D, … Lacey H (2015). Eating disorder symptoms and quality of life: Where should clinicians place their focus in severe and enduring anorexia nervosa? The International Journal of Eating Disorders, 48(1), 133–138. [DOI] [PubMed] [Google Scholar]
- Bardone-Cone AM, Alvarez A, Gorlick J, Koller KA, Thompson KA, & Miller AJ (2019). Longitudinal follow-up of a comprehensive operationalization of eating disorder recovery: Concurrent and predictive validity. The International Journal of Eating Disorders, 52(9), 1052–1057. [DOI] [PubMed] [Google Scholar]
- Bardone-Cone AM, Hunt RA, & Watson HJ (2018). An overview of conceptualizations of eating disorder recovery, recent findings, and future directions. Current Psychiatry Reports, 20(9), 79. [DOI] [PubMed] [Google Scholar]
- Bargiacchi A (2014). Brain imaging in early onset anorexia. Archives de Pédiatrie, 21(5), 548–551. [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, … Malhi GS (2011). Pathways underlying neuroprogression in bipolar disorder: Focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience and Biobehavioral Reviews, 35(3), 804–817. [DOI] [PubMed] [Google Scholar]
- Broomfield C, Stedal K, Touyz S, & Rhodes P (2017). Labeling and defining severe and enduring anorexia nervosa: A systematic review and critical analysis. The International Journal of Eating Disorders, 50(6), 611–623. [DOI] [PubMed] [Google Scholar]
- Buchman S, Attia E, Dawson L, & Steinglass JE (2019). Steps of care for adolescents with anorexia nervosa—A Delphi study. The International Journal of Eating Disorders, 52(7), 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral D, Katz JN, Weinblatt ME, Ting G, Avorn J, & Solomon DH (2005). Development and assessment of indicators of rheumatoid arthritis severity: Results of a Delphi panel. Arthritis and Rheumatism, 53(1), 61–66. [DOI] [PubMed] [Google Scholar]
- Channon S, & deSilva WP (1985). Psychological correlates of weight gain in patients with anorexia nervosa. Journal of Psychiatric Research, 19(2–3), 267–271. [DOI] [PubMed] [Google Scholar]
- Cosci F, & Fava GA (2013). Staging of mental disorders: Systematic review. Psychotherapy and Psychosomatics, 82(1), 20–34. [DOI] [PubMed] [Google Scholar]
- Dalkey NC, Rourke DL, Lewis R, & Snyder D (1972). Studies in the quality of life: Delphi and decision-making. Lexington, MA: Lexington Books. [Google Scholar]
- Davis L, Ranzenhofer LM, Posner J, & Steinglass JE (2019). When does anorexia nervosa start? Using an age of onset interview to measure duration of eating behaviors. Chicago, IL: Eating Disorders Research Society. [Google Scholar]
- de Zwart PL, Jeronimus BF, & de Jonge P (2019). Empirical evidence for definitions of episode, remission, recovery, relapse and recurrence in depression: A systematic review. Epidemiology and Psychiatric Sciences, 28(5), 544–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd S, Berk M, Kelin K, Mancini M, & Schacht A (2013). Treatment response for acute depression is not associated with number of previous episodes: Lack of evidence for a clinical staging model for major depressive disorder. Journal of Affective Disorders, 150(2), 344–349. [DOI] [PubMed] [Google Scholar]
- Eddy KT, Tabri N, Thomas JJ, Murray HB, Keshaviah A, Hastings E, … Franko DL (2017). Recovery from anorexia nervosa and bulimia nervosa at 22-year follow-up. The Journal of Clinical Psychiatry, 78(2), 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubank BH, Mohtadi NG, Lafave MR, Wiley JP, Bois AJ, Boorman RS, & Sheps DM (2016). Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Medical Research Methodology, 16, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish LS, & Busby DM (1996). The Delphi method In Moon SM (Ed.), Research methods in family therapy (pp. 469–482). New York, NY: Guilford Press. [Google Scholar]
- Frank E (2005). Describing course of illness: Does our language matter? The International Journal of Eating Disorders, 38(1), 7–8. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, … Weissman MM (1991). Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Archives of General Psychiatry, 48(9), 851–855. [DOI] [PubMed] [Google Scholar]
- Frank GK, Shott ME, Hagman JO, & Yang TT (2013). Localized brain volume and white matter integrity alterations in adolescent anorexia nervosa. Journal of the American Academy of Child and Adolescent Psychiatry, 52(10), 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich HC, Walther S, Bendszus M, Biller A, Thomann P, Zeigermann S, … Herzog W (2012). Grey matter abnormalities within cortico-limbic-striatal circuits in acute and weight-restored anorexia nervosa patients. NeuroImage, 59(2), 1106–1113. [DOI] [PubMed] [Google Scholar]
- Fujisawa TX, Yatsuga C, Mabe H, Yamada E, Masuda M, & Tomoda A (2015). Anorexia nervosa during adolescence is associated with decreased gray matter volume in the inferior frontal gyrus. PLoS One, 10(6), e0128548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, & Shenker IR (1997). Resumption of menses in anorexia nervosa. Archives of Pediatrics & Adolescent Medicine, 151(1), 16–21. [DOI] [PubMed] [Google Scholar]
- Gull WW (1873). Anorexia nervosa (apepsia hysterica). The British Medical Journal, 2, 527–528. [Google Scholar]
- Hasson F, Keeney S, & McKenna H (2000). Research guidelines for the Delphi survey technique. Journal of Advanced Nursing, 32(4), 1008–1015. [PubMed] [Google Scholar]
- Hay P, & Touyz S (2018). Classification challenges in the field of eating disorders: Can severe and enduring anorexia nervosa be better defined? Journal of Eating Disorders, 6, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HF, & Shannon SE (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. [DOI] [PubMed] [Google Scholar]
- Hubel C, Yilmaz Z, Schaumberg KE, Breithaupt L, Hunjan A, Horne E … Breen G (2019). Body composition in anorexia nervosa: Meta-analysis and meta-regression of cross-sectional and longitudinal studies. The International Journal of Eating Disorders, 52, 1205–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas A, Hubel C, Stahl D, Stadler M, Ismail K, Breen G, … Kan C (2019). The metabolic underpinning of eating disorders: A systematic review and meta-analysis of insulin sensitivity. Molecular and Cellular Endocrinology, 497, 110307. [DOI] [PubMed] [Google Scholar]
- Jones J, & Hunter D (1995). Consensus methods for medical and health services research. BMJ, 311(7001), 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Portnoff LC, McCurdy-McKinnon D, & Feusner JD (2017). What happens after treatment? A systematic review of relapse, remission, and recovery in anorexia nervosa. Journal of Eating Disorders, 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordy H, Kramer B, Palmer RL, Papezova H, Pellet J, Richard M, & Treasure J (2002). Remission, recovery, relapse, and recurrence in eating disorders: Conceptualization and illustration of a validation strategy. Journal of Clinical Psychology, 58(7), 833–846. [DOI] [PubMed] [Google Scholar]
- Liam CK, Andarini S, Lee P, Ho JC, Chau NQ, & Tscheikuna J (2015). Lung cancer staging now and in the future. Respirology, 20(4), 526–534. [DOI] [PubMed] [Google Scholar]
- Linstone HA, & Turoff M (1975). The Delphi method: Techniques and applications. Boston, MA: Addison-Wesley Pub. Co. [Google Scholar]
- Maguire S, Surgenor LJ, Le Grange D, Lacey H, Crosby RD, Engel SG, … Touyz S (2017). Examining a staging model for anorexia nervosa: Empirical exploration of a four stage model of severity. Journal of Eating Disorders, 5, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire S, Touyz S, Surgenor L, Crosby RD, Engel SG, Lacey H, … le Grange D (2012). The clinician administered staging instrument for anorexia nervosa: Development and psychometric properties. The International Journal of Eating Disorders, 45(3), 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainz V, Schulte-Ruther M, Fink GR, Herpertz-Dahlmann B, & Konrad K (2012). Structural brain abnormalities in adolescent anorexia nervosa before and after weight recovery and associated hormonal changes. Psychosomatic Medicine, 74(6), 574–582. [DOI] [PubMed] [Google Scholar]
- McGorry P, Keshavan M, Goldstone S, Amminger P, Allott K, Berk M, … Hickie I (2014). Biomarkers and clinical staging in psychiatry. World Psychiatry, 13(3), 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, … Jackson H (2002). Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Archives of General Psychiatry, 59(10), 921–928. [DOI] [PubMed] [Google Scholar]
- Meehan KG, Loeb KL, Roberto CA, & Attia E (2006). Mood change during weight restoration in patients with anorexia nervosa. The International Journal of Eating Disorders, 39(7), 587–589. [DOI] [PubMed] [Google Scholar]
- Mittnacht AM, & Bulik CM (2015). Best nutrition counseling practices for the treatment of anorexia nervosa: A Delphi study. The International Journal of Eating Disorders, 48(1), 111–122. [DOI] [PubMed] [Google Scholar]
- Moylan S, Maes M, Wray NR, & Berk M (2013). The neuroprogressive nature of major depressive disorder: Pathways to disease evolution and resistance, and therapeutic implications. Molecular Psychiatry, 18(5), 595–606. [DOI] [PubMed] [Google Scholar]
- Nickel K, Joos A, Tebartz van Elst L, Matthis J, Holovics L, Endres D, … Maier S (2018). Recovery of cortical volume and thickness after remission from acute anorexia nervosa. The International Journal of Eating Disorders, 51(9), 1056–1069. [DOI] [PubMed] [Google Scholar]
- Noetel M, Dawson L, Hay P, & Touyz S (2017). The assessment and treatment of unhealthy exercise in adolescents with anorexia nervosa: A Delphi study to synthesize clinical knowledge. The International Journal of Eating Disorders, 50(4), 378–388. [DOI] [PubMed] [Google Scholar]
- Odicino F, Pecorelli S, Zigliani L, & Creasman WT (2008). History of the FIGO cancer staging system. International Journal of Gynaecology and Obstetrics, 101(2), 205–210. [DOI] [PubMed] [Google Scholar]
- Raykos BC, Erceg-Hurn DM, McEvoy PM, Fursland A, & Waller G (2018). Severe and enduring anorexia nervosa? Illness severity and duration are unrelated to outcomes from cognitive behaviour therapy. Journal of Consulting and Clinical Psychology, 86(8), 702–709. [DOI] [PubMed] [Google Scholar]
- Schorr M, & Miller KK (2017). The endocrine manifestations of anorexia nervosa: Mechanisms and management. Nature Reviews. Endocrinology, 13(3), 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J, Leboyer M, Hickie I, Berk M, Kapczinski F, Frank E, … McGorry P (2013). Clinical staging in psychiatry: A cross-cutting model of diagnosis with heuristic and practical value. The British Journal of Psychiatry, 202(4), 243–245. [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, & Lieberman JA (2006). Brain volume in first-episode schizophrenia: Systematic review and meta-analysis of magnetic resonance imaging studies. The British Journal of Psychiatry, 188, 510–518. [DOI] [PubMed] [Google Scholar]
- Steinhausen HC (2002). The outcome of anorexia nervosa in the 20th century. The American Journal of Psychiatry, 159(8), 1284–1293. [DOI] [PubMed] [Google Scholar]
- Sy R, Ponton K, De Marco P, Pi S, & Ishak WW (2013). Quality of life in anorexia nervosa: A review of the literature. Eating Disorders, 21 (3), 206–222. [DOI] [PubMed] [Google Scholar]
- Tan SM, Kwok KFV, Zainal KA, & Lee HY (2018). Does late-onset anorexia nervosa exist? Findings from a comparative study in Singapore. Journal of Psychiatric Practice, 24(2), 97–103. [DOI] [PubMed] [Google Scholar]
- Tierney S, & Fox JR (2009). Chronic anorexia nervosa: A Delphi study to explore practitioners’ views. The International Journal of Eating Disorders, 42(1), 62–67. [DOI] [PubMed] [Google Scholar]
- Treasure J, Stein D, & Maguire S (2015). Has the time come for a staging model to map the course of eating disorders from high risk to severe enduring illness? An examination of the evidence. Early Intervention in Psychiatry, 9(3), 173–184. [DOI] [PubMed] [Google Scholar]
- Van den Eynde F, Suda M, Broadbent H, Guillaume S, Van den Eynde M, Steiger H, … Schmidt U (2012). Structural magnetic resonance imaging in eating disorders: A systematic review of voxel-based morphometry studies. European Eating Disorders Review, 20(2), 94–105. [DOI] [PubMed] [Google Scholar]
- van der Gaag M, Smit F, Bechdolf A, French P, Linszen DH, Yung AR, … Cuijpers P (2013). Preventing a first episode of psychosis: Meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophrenia Research, 149 (1–3), 56–62. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Richard E, Peng CS, Knodt AR, & Hariri AR (2019). Subclinical eating disorder traits are correlated with cortical thickness in regions associated with food reward and perception. Brain Imaging and Behavior, 10.1007/s11682-018-0007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson HJ, Yilmaz Z, Thornton LM, Hubel C, Coleman JRI, Gaspar HA … Bulik CM (2019). Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nature Genetics, 51(8), 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]


