Abstract
Background
Kidneys transplanted from deceased-donors with serum creatinine-defined acute kidney injury (AKI) have similar allograft survival as non-AKI kidneys but are discarded at a higher rate. Urine injury biomarkers are sensitive markers of structural kidney damage and may more accurately predict graft outcomes.
Materials and Methods
In the 2010–2013 multicenter Deceased Donor Study of 2430 kidney transplant recipients from 1298 donors, we assessed the association of donor urine injury biomarkers microalbumin, NGAL, KIM-1, IL-18, and L-FABP with graft failure (GF) and death-censored GF (dcGF) using Cox proportional hazard models (median follow-up 4 years). We examined if serum creatinine-defined donor AKI modified this association to assess the relationship between subclinical donor AKI (elevated biomarkers without creatinine-defined AKI) and graft failure. Through chart review of a sub-cohort (1137 recipients), we determined associations between donor injury biomarkers and a 3-year composite outcome of GF, mortality, or eGFR <=20mL/min/1.73m2.
Results
Risk of GF, dcGF, and 3-year composite outcome did not vary with donor injury biomarker concentrations after adjusting for donor, transplant, and recipient characteristics (adjusted HR ranged from 0.96 to 1.01 per log-2 increase in biomarker). Subclinical injury in transplanted kidneys without AKI was not associated with GF.
Conclusions
AKI measured using injury biomarkers was not associated with posttransplant graft outcomes (at median 4 years posttransplant). When assessing posttransplant graft viability, clinicians can prioritize other donor and recipient factors over donor kidney injury, measured by either serum creatinine or urine injury biomarkers.
Keywords: kidney transplantation, graft survival, acute kidney injury (AKI), urine injury biomarkers, subclinical AKI
Introduction
From 2000 to 2015, 36700 kidneys (17% of all kidneys which were procured) were discarded in the United States.1,2 Many of these discarded organs may be viable for transplantation.1–5 With over 90000 people on the kidney transplant waiting list, expanding the pool of viable donor organs will save lives. Kidney transplants increase survival and quality of life and decrease cost compared to prolonged dialysis;6–8 unfortunately, approximately 5000 people on the waitlist die each year waiting for a transplant.9
While increasing transplant volume is a goal for some transplant centers, transplant center programs may also be reluctant to accept kidneys perceived as lower quality because of concerns about regulatory penalties or the financial costs of managing transplant complications.10–13 All transplant centers receive evaluations on 1-year post-transplant mortality and graft failure rates from the Scientific Registry for Transplant Recipients. These reports adjust for donor organ characteristics, but many transplant centers perceive the adjustment as insufficient. Organs are often viewed unfavorably and discarded when associated with poor biopsy findings, poor function, prolonged ischemia, or poor donor medical history.1 Donor kidneys may be injured over time from chronic kidney disease (CKD) or more acutely from disseminated intravascular coagulopathy, hemodynamic instability, or other causes of acute kidney injury (AKI). The Kidney Donor Risk Index (KDRI), which comprises 10 donor characteristics and intends to function as a summary measure of graft quality, is supplied to transplant centers with every organ offer.14
Transplant centers may be able to utilize more donor organs with AKI to expand the supply of deceased-donor kidneys if posttransplant outcomes are acceptable. Our group previously found that AKI donor kidneys (increasing admission-to-terminal serum creatinine by AKI Network Stages) are discarded at a higher rate than non-AKI donor kidneys (30% versus 18%), but AKI donor kidneys that are selected for transplant have similar long-term graft survival (median follow-up = 4 years) to non-AKI donor kidneys.15,16 Since serum creatinine is widely available, it remains the primary means by which donor AKI is identified. However, serum creatinine is a relatively insensitive marker of AKI or acute tubular injury (ATI). Urine injury biomarkers such as microalbumin, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), IL-18, and liver-type fatty acid binding protein (L-FABP) are evident within 1–4 hours of acute tubular injury.17,18 These biomarkers can help identify subclinical AKI – elevated kidney injury without serum creatinine-defined AKI.19–22 Among kidneys from hospitalized deceased donors without serum creatinine-defined AKI, our group found rates of mild and severe acute tubular injury measured by urine injury biomarkers to be 18% and 9%, respectively.19
Our group previously found that donor urine injury biomarkers NGAL and L-FABP were associated with lower 6-month eGFR only among recipients without posttransplant DGF but the relationship with medium to long-term graft survival was unclear.23 This study investigated the association between donor urine injury biomarkers microalbumin, NGAL, KIM-1, IL-18, and L-FABP and longer-term posttransplant allograft survival (median follow-up 4 years).
Materials and Methods
The Deceased Donor Study is a multicenter, observational analysis of deceased donors and corresponding kidney transplant recipients from five organ procurement organizations (OPOs) between May 2010 and December 2013. Donors that did not lead to transplantation, donors with age less than 16 years, and donors without urine samples were excluded. We also generated a sub-cohort by performing direct chart reviews of recipients from donors at 13 transplant centers, which allowed us to ascertain detailed posttransplant outcomes (e.g., poor graft function). The five participating OPOs were Gift of Life Donor Program, Philadelphia, PA; New Jersey Sharing Network, New Providence, NJ; Gift of Life Michigan, Ann Arbor, MI; New York Organ Donor Network, New York, NY; and New England Organ Bank, Waltham, MA. This study was approved by the scientific review boards from participating OPOs and the institutional review boards from the 13 transplant centers. A thorough description of data quality and accuracy efforts in this study is provided in Supplemental Methods S1.
This study used data from the Organ Procurement and Transplantation Network (OPTN), which was supplemented through our study’s database of clinical and biomarker data. The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the OPTN, and has been described elsewhere.24,25 The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. The analyses are based on OPTN data as of July 31, 2017 and may be subject to change due to future data submission or correction by transplant centers.
Donor urine injury biomarker data
Prior to organ procurements, 10mL of fresh donor urine was collected using an indwelling urinary catheter tube, transferred on ice, and then frozen (median time to freezing was 6.3 hours, interquartile range 4.9–8.0 hours). The urine was stored at −80 degrees Celsius until monthly shipments to the Yale University Biorepository. Samples were then thawed, centrifuged, and separated into 1-mL aliquots and then stored at −80 degrees Celsius. We measured the following urine injury biomarkers from stored samples: microalbumin (mg/dL), NGAL (ng/dL), KIM-1 (pg/mL), IL-18 (pg/mL), and L-FABP (pg/mL). Urine creatinine was measured using the RxDaytona immunoturbidimetry assay (Randox Laboratories, Boston, MA). Biomarker measurements are described more completely in the supplemental materials (Supplemental Methods S2) and in our group’s previous work.23
Primary outcomes
In the full study cohort of recipients, we assessed posttransplant all-cause graft failure (GF) and death-censored graft failure (dcGF). All-cause GF was defined as all-cause mortality, return to dialysis, or re-transplantation with median posttransplant follow-up of 4 (IQR = 3,5) years. In the chart review sub-cohort of recipients, we measured a 3-year composite outcome of graft failure, death, or estimated glomerular filtration rate (eGFR) <=20 mL/min/1.73m2, which was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.26 The threshold of 20 mL/min/1.73m2 was used because the OPTN uses this value in determining eligibility for re-transplantation (OPTN policy 8.4).27 Outcomes were ascertained using data from OPTN until July 31, 2017 and chart reviews until November 4, 2016.
Statistical analysis
For donor and recipient descriptive statistics, means with standard deviations and frequencies with percentages were reported. We used Cox proportional hazard models (clustered by donor28) to analyze the association between log-transformed (base 2) urine injury biomarker concentrations and primary outcomes. We report hazard ratios of both unadjusted and adjusted models accounting for KDRI, donor urine creatinine, cold ischemia time, number of human leukocyte antigen mismatches, and the following recipient variables: age, black race, sex, previous kidney transplant, diabetes as the cause of end-stage kidney disease, panel reactive antibody, body mass index (BMI), and pre-emptive transplant status. Historically, the expanded-criteria donor (ECD) designation is defined as donors >= 60 years old, or age 50–59 years with at least two of the following: cerebrovascular accident as the cause of death, preexisting hypertension, or terminal serum creatinine >1.5 mg/dL. The KDRI29,30 was computed for each donor. We used Kolmogorov-type supremum tests31 to evaluate proportional hazards assumptions.
In a secondary analysis, we assessed whether the association between donor urinary injury biomarkers and all-cause graft failure differed between kidneys with and without creatinine-defined AKI. To do so, in unadjusted Cox proportional hazard models we included an interaction term between log-transformed donor biomarkers and AKI, defined as Stage 1 or higher using the AKI Network criteria.32 A significant interaction term indicated effect modification by serum creatinine-defined AKI and unadjusted hazard ratios were estimated separately by donor AKI status. As our group previously used injury biomarkers to demonstrate the presence of mild and severe acute tubular injury among kidneys without creatinine-defined AKI,19 we assessed the relationship between subclinical AKI (elevated injury without creatinine-defined AKI) and graft outcomes through this analysis. Stage 1 donor AKI was defined as a terminal serum creatinine that increased from the admission value by greater than or equal to 0.3 mg/dL or greater than 1.5 times the admission serum creatinine.
All statistical tests and confidence intervals were two-sided with a significance level of 0.05. We used SAS 9.4 software for Windows (SAS Institute, Cary, NC).
Results
Cohort and sub-cohort characteristics
Of the 1679 donors who contributed urine samples to the study, 1298 donors were eligible for the study and main analyses (Figure 1). The full DDS cohort consisted of 2430 kidney transplant recipients from the 1298 donors. Our study sub-cohort included 1137 recipients (from 862 donors) who underwent transplantation at one of our study’s 13 transplant centers. The donor cohort had the following characteristics: mean age of 41 ± 15 years, 60% male, 16% black, mean KDRI of 1.29, and mean admission to procurement time of 5 days. The recipient cohort had the following characteristics: mean age of 53 ± 15 years, 61% male, 39% black, and mean of 46 ± 38 months on dialysis prior to transplantation. Complete donor and recipient characteristics are described in Table 1. Sub-cohort characteristics are presented in Supplemental Table S1. Supplemental Table S2 compares donor characteristics of kidneys that were transplanted versus discarded. Transplanted kidneys had lower mean KDRI (1.27 ± 0.40 versus 1.85 ± 0.52) and were more likely to not have AKI (76% versus 63%) compared to discarded kidneys.
Figure 1.
Flowchart for study enrollment. OPO, organ procurement organization.
Table 1:
Donor, recipient, and transplant characteristics
| Deceased Donor Characteristics | (N=1298) | Recipient and transplant characteristics | (N=2430) | ||
|---|---|---|---|---|---|
| Age, years | 41 ± 15 | Age, years | 53 ± 15 | ||
| Male | 784 (60%) | Male | 1492 (61%) | ||
| Black race | 205 (16%) | Black race | 956 (39%) | ||
| Hispanic ethnicity | 171 (13%) | Hispanic ethnicity | 279 (11%) | ||
| BMI, kg/m2 | 28 ± 7 | BMI, kg/m2 | 28.0 ± 5.8 | ||
| Hypertension | 399 (31%) | Cause ESRD | Diabetes | 726 (30%) | |
| Diabetes | 130 (10%) | Hypertension | 643 (26%) | ||
| Cause of death | Head trauma | 396 (31%) | Glomerulonephritis | 391 (16%) | |
| Anoxia | 425 (34%) | Graft failure | 174 (7%) | ||
| Stroke | 427 (34%) | Other or unknown | 496 (20%) | ||
| Other | 18 (1%) | Preemptive transplant | 274 (11%) | ||
| Hepatitis C seropositive | 48 (4%) | Dialysis duration, months | 46 ± 38 | ||
| ECD | 246 (19%) | Previous kidney transplant | 315 (13%) | ||
| DCD | 206 (16%) | Pre-transplant blood transfusion | 438 (18%) | ||
| KDRI | 1.29 ± 0.41 | PRA | Continuous, % | 21 ± 35 | |
| KDPI, % | 48 ± 27 | 0% | 1545 (64%) | ||
| Admission to procurement, days | 5 ± 7 | 1–20% | 178 (7%) | ||
| Admission SCr, mg/dL | 1.10 ± 0.61 | 21–80% | 326 (13%) | ||
| Terminal SCr, mg/dL | 1.17 ± 0.85 | >80% | 381 (16%) | ||
| Urine creatinine, mg/dL | 49.09 ± 48.41 | Cold ischemia time, hours | 15.3 ± 7.1 | ||
| Number of individual kidneys transplanted | 1 | 166 (13%) | HLA Mismatch | Continuous | 4.2 ± 1.5 |
| 2 | 1132 (87%) | 0 | 153 (6%) | ||
| Donor AKI stage | No AKI | 976 (75%) | 1 | 21 (1%) | |
| Stage 1 | 211 (16%) | 2 | 82 (3%) | ||
| Stage 2 | 62 (5%) | 3 | 292 (12%) | ||
| Stage 3 | 49 (4%) | 4 | 642 (26%) | ||
| 5 | 820 (34%) | ||||
| 6 | 414 (17%) | ||||
Values are mean (SD) or n (%). ECD, expanded-criteria donor; DCD, donation after cardiovascular determination of death; KDRI, kidney donor risk index; KDPI, kidney donor profile index; SCr serum creatinine; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; PRA; panel reactive antibody; HLA, human leukocyte antigen.
Urine injury biomarkers and graft failure among full and sub-cohort
The total combined follow-up of the cohort was 9479 patient-years with a median follow-up of 4 (IQR: 3.0–5.0) years. Of the 2430 recipients in the full cohort, 623 (26%) experienced all-cause graft failure during the entire follow-up period. There were 313 death-censored graft failures and 402 deaths, with 92 deaths occurring after graft failure. The overall event rate for all-cause and death-censored graft failure among the full cohort was 66.2 (95% CI=61.1, 71.7) and 33.3 (95% CI=29.7, 37.3) per 1000 person-years, respectively. Of the 1137 recipients in the study sub-cohort, 214 (19%) recipients experienced the 3-year composite outcome of graft failure, death, or eGFR <= 20 mL/min/1.73m2, with an event rate of 76.1 (95% CI=66.3, 87.4) per 1000 person-years (Table 2).
Table 2.
Association between urinary injury biomarkers and all-cause and death-censored graft failure (full cohort) and composite eGFR ≤ 20, graft failure, or death (subcohort)
| Full cohort | Sub-cohort | |||||
| All-Cause Graft Failure (n=2430) | Death Censored GF (n=2430) | Composite eGFR≤20, GF, or death (n=1137) | ||||
| Event rate per 1000 py 66.2 (61.1, 71.7) | Event rate per 1000 py 33.3 (29.7, 37.3) | Event rate per 1000 py 76 (66, 87) | ||||
| Log2 Transformed Urine Biomarker | Hazard Ratios (95% Confidence Interval) | Hazard Ratios (95% Confidence Interval) | ||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Microalbumin | 1.02 (0.97, 1.06) | 1.00 (0.96, 1.06) | 0.99 (0.93, 1.06) | 0.98 (0.91, 1.05) | 0.98 (0.91, 1.07) | 0.96 (0.88, 1.05) |
| NGAL | 1.02 (0.99, 1.05) | 1.00 (0.97, 1.03) | 1.01 (0.97, 1.05) | 0.99 (0.95, 1.04) | 0.99 (0.95, 1.04) | 0.99 (0.94, 1.04) |
| KIM-1 | 0.99 (0.94, 1.03) | 0.97 (0.92, 1.02) | 1.00 (0.94, 1.06) | 0.99 (0.93, 1.07) | 1.00 (0.93, 1.08) | 1.01 (0.92, 1.11) |
| IL-18 | 0.99 (0.95, 1.03) | 0.98 (0.93, 1.02) | 0.97 (0.92, 1.03) | 0.96 (0.9, 1.03) | 0.96 (0.89, 1.03) | 0.96 (0.89, 1.05) |
| L-FABP | 1.00 (0.96, 1.03) | 0.98 (0.95, 1.01) | 0.99 (0.95, 1.04) | 0.98 (0.93, 1.03) | 0.97 (0.92, 1.03) | 0.97 (0.91, 1.02) |
Adjusted for KDRI, Urine Creatinine, cold ischemia time, Recipient age, black race, sex, previous kidney transplant, diabetes as the cause of end-stage kidney disease, number of human leukocyte antigen mismatches, panel reactive antibody, BMI, pre-emptive transplant
The donor urine injury biomarkers microalbumin, NGAL, KIM-18, IL-18, and L-FABP were neither associated with graft failure in the full cohort nor the sub-cohort. Unadjusted and adjusted hazard ratios are presented in Table 2. In these models, KDRI was significantly associated with increased graft failure (not shown). In a supplementary analysis combining all biomarkers into one model, there was no significant association between biomarkers and graft failure outcomes (not shown).
Supplemental Table S3 shows urine injury biomarker concentrations by all-cause graft failure, death-censored graft failure, and the 3-year composite of graft failure, death, or eGFR <= 20 ml/min/1.73m2.
Urine injury biomarkers and graft failure by serum creatinine-defined donor AKI
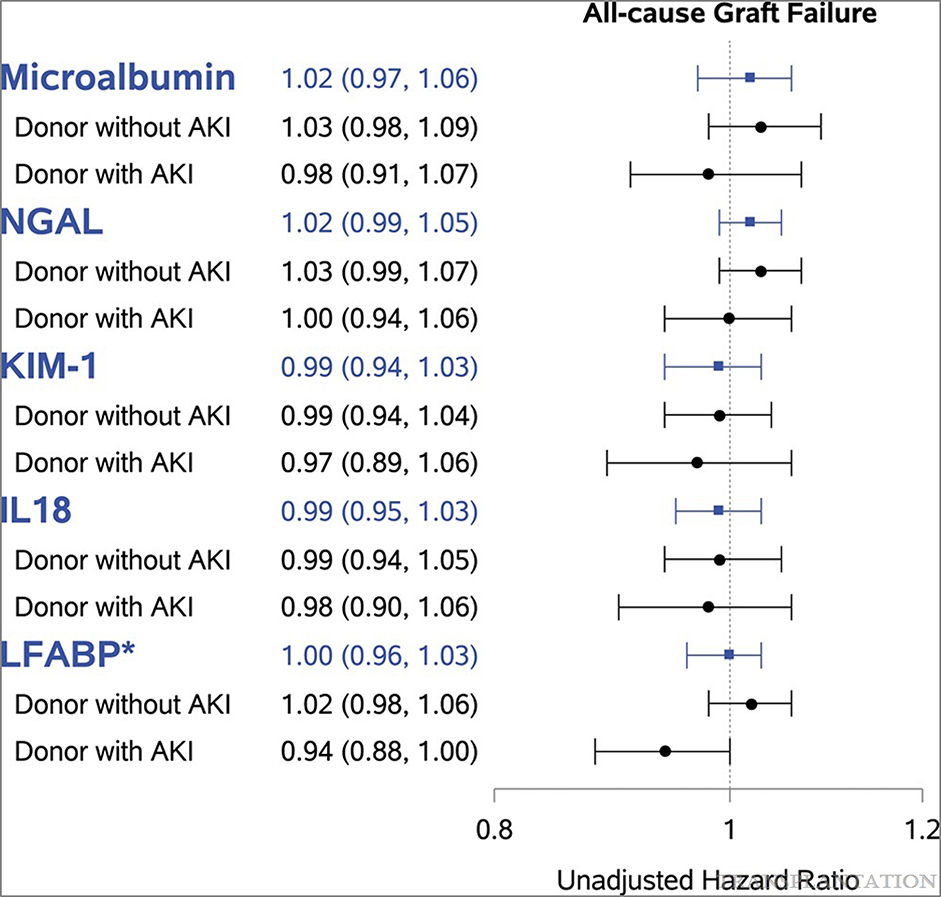
Of the 2430 kidney transplant recipients in our cohort, 1845 (76%) received kidneys from donors without serum creatinine-defined donor AKI. Among the recipient cohort without donor AKI, 475 (26%) recipients had all-cause graft failure. The event rate for all-cause graft failure was 65.4 (95% CI=55.7, 76.8) per 1000 person-years in the AKI group and 65.8 (60.2, 72.0) per 1000 person-years in the non-AKI group. In a secondary analysis, associations between urine biomarker and all-cause graft failure did not differ by serum creatinine-defined donor AKI except for LFABP (p-value for interaction <0.05; Figure 2). In donors with AKI, elevated LFABP was modestly protective of all-cause graft failure (unadjusted HR = 0.94, 95% CI = 0.88, 1.00). Overall, lack of significant effect modification of AKI on the association between biomarker concentration and graft failure suggests no relationship between subclinical AKI and graft failure. Figure 2 presents the full results and hazard ratios of this secondary analysis.
Figure 2.
Urine biomarker association with all-cause graft failure does not differ by donor AKI status (full cohort). Unadjusted hazard ratio (95% CI) per log-2-transformed urine biomarkers presented in figure for all recipients (full cohort, n = 2430) in blue, followed by HR by donor AKI strata in black. *P for interaction term between donor AKI and urine biomarkers was only significant for L-FABP (P < 0.05). AKI defined as stage 1 or higher using the AKI Network criteria. Event rate per 1000 person-y: for recipients from donors without AKI (n = 1845) was 65.8 (60.2, 72.0) and recipients from donors with AKI (n = 585) was 65.4 (55.7, 76.8). AKI, acute kidney injury; CI, confidence interval; HR, hazard ratio; KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid binding protein; NGAL, neutrophil gelatinase-associated lipocalin.
Discussion
This multicenter observational analysis of long-term graft survival among kidney transplant recipients from deceased donors revealed that elevated donor urine injury biomarkers microalbumin, NGAL, KIM-1, IL-18, and L-FABP prior to organ procurement were not associated with long-term graft survival. Risk of all-cause graft and death-censored graft failure did not change with elevated urine injury biomarkers. Additionally, in our sub-cohort analysis, donor injury biomarkers were not associated with the 3-year composite outcome. Lastly, subclinical donor AKI defined by elevated urine injury biomarkers and normal serum creatinine was not associated with graft failure.
Given that urine injury biomarkers are more sensitive measures of AKI than serum creatinine,17–21 these findings are generally consistent with and enhance existing literature showing that, among kidneys that are transplanted, clinical donor AKI by serum creatinine is not associated with worse recipient outcomes.16,33–50 A recent meta-analysis of fourteen studies with 15345 deceased donors concluded that there is no significant difference in recipient allograft survival from AKI versus non-AKI donor groups at 12 months through 120 months post-transplantation.51 A review by Heilman et al. concluded that kidneys with biopsy-confirmed cortical necrosis less than 10% and no more than mild chronic changes had comparable long-term outcomes to kidneys without injury.52 Recent studies and the meta-analysis by Zheng et al. also suggest higher rates of DGF despite similar long-term recipient graft survival among recipients with AKI versus non-AKI donors.51 Our previous work showed that biomarkers could identify injury in the absence of creatinine-defined AKI (subclinical AKI).19 Our current findings show that the lack of association between injury biomarkers and graft failure did not differ by creatinine-defined AKI status (none versus Stage 1 or greater). Thus, subclinical AKI among kidneys without creatinine-defined AKI was not associated with long-term graft survival.
One analysis by Boffa et al. of 11219 kidneys in the UK differed from other studies with evidence for 20% greater risk of all-cause graft failure within 1 year for kidneys from donors with AKI as well as a slight but significantly worse 5-year graft survival for AKI kidneys compared with non-AKI kidneys (78% vs. 76%, p=0.009).53 The authors of the UK study advised caution for using donor kidneys with the most severe AKI (Stage 3) because of an apparent 3-fold risk for primary non-function compared with non-AKI donor kidneys.53 Among our recipient cohort, only 4% received kidneys with Stage 3 donor AKI versus 9% in the UK cohort, which may contribute to differences in findings between our study and Boffa et al. Our cohort may not have had sufficient power to detect differences in long-term outcomes among those with highest levels of donor kidney injury.
We hypothesize several reasons for our findings of similar long-term graft outcomes despite higher donor kidney injury biomarkers. First, donor AKI in the setting of DGF may induce protective upregulation of anti-ischemic proteins and cell repair processes through a mechanism similar to ischemic preconditioning.54–56 Second, the most important factors determining graft function may take place after transplantation. Third, recipient mortality driven by factors like infection, cardiovascular disease, and frailty may generate noise in isolating the role of donor injury on long-term graft failure, which may affect our analysis of all-cause graft failure. However, our analysis of death-censored graft failure showed no association with injury biomarkers. With longer follow-up, this study supplements an earlier analysis of our group investigating donor urine injury biomarkers and early posttransplant graft survival.23 Our earlier study suggested that while donor urine injury biomarkers were associated with donor AKI, they were associated with lower eGFR at 6 months only among recipients without DGF. Our current analysis with several years of follow-up after transplantation suggests that graft survival did not significantly change with increased urine injury biomarker concentrations, despite the increased rates of DGF shown in our previous work.
Some important limitations should be considered when evaluating our study’s findings. While our observational analysis adjusted for a variety of donor and recipient characteristics, including KDRI, residual confounding may exist. Consequently, donor kidneys with injury that are transplanted may differ from those with injury that are discarded and be healthier. Also, we did not measure urine injury biomarkers over time posttransplantation, which may have provided more insight into the mechanism of similar graft survival across donor kidney injury levels. Our definition of AKI is limited as it relies on admission creatinine, which may not be reflective of the donors’ true baseline (e.g., pre-renal injury prior to hospitalization). Also, the use of a single measurement of terminal serum creatinine to define AKI, without accounting for the different creatinine trends throughout the donor’s hospital stay prior to demise, may not accurately capture the true course the kidney has undertaken. Our cohort may not have had sufficient power to assess long-term recipient outcomes from donor kidneys with highest levels of injury (i.e., 4% of our cohort received Stage 3 AKI kidneys). Lastly, given median national graft survival times of approximately 10 years,57 our median follow-up of four years may have been inadequate to detect potential effects of donor AKI on CKD at later stages in the post-transplant course.
Future investigations should evaluate mechanisms by which donor kidneys with AKI have similar post-transplant graft survival compared with non-AKI donor kidneys so that clinicians and transplant centers can better assess and predict long-term graft survival. These studies can measure urine injury biomarkers over time, both before and after transplant. We hypothesize that additional injury occurs during implantation and posttransplantation, and thus may make donor kidney injury a less important factor in long-term graft survival. Other donor and recipient factors may better predict posttransplant injury and be more important in predicting long-term allograft success.58–61 Furthermore, donor AKI may induce protective processes that support long-term recovery and survival (e.g., ischemic preconditioning). Future studies can also investigate the role of other emerging biomarkers of repair like YKL-40 and uromodulin in predicting long-term graft outcomes.
We found no association between injury biomarkers and posttransplant graft survival (at median four years posttransplant). Our results suggest that, when evaluating long-term graft survival, transplant centers and clinicians can prioritize other donor and recipient factors that correlate with long-term outcomes over donor kidney injury, measured by either serum creatinine or urine injury biomarkers.
Supplementary Material
Acknowledgements
The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. These organizations were not involved in study design, analysis, interpretation, or manuscript creation.
This work will be presented on a poster at the American Transplant Congress in June 2019.
Disclosures
This work was supported by the National Institute of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK-93770 and grant K24DK090203 and George M. O’Brien Kidney Center at Yale Grant P30DK079310 to Dr. Parikh; grant K23DK105207 awarded to Dr. Harhay from the NIH/NIDDK; a grant to Dr. Hall by the NIH/National Center for Advancing Translational Sciences (NCATS) under award numbers UL1TR002538 and KL2TR002539; and the Health Resources and Services Administration contract 234-2005-37011C.
The authors of this manuscript have conflicts of interest to disclose. N. Koyawala: None. I.E. Hall: None. Y. Jia: None. S.G. Mansour: None. P.P. Reese: Epidemiology consulting from COHRDATA related to dialysis outcomes. Grant/Research Support from Merck, CVS. Initiated grants from Merck to the University of Pennsylvania to support the THINKER (kidney), USHER (heart) and SHELTER (lung) trials of transplanting organs from donors with Hepatitis C into Hepatitis C negative recipients. Initiated grants from CVS to the University of Pennsylvania to support studies of medication adherence. Associate Editor, American journal of Kidney Disease. H.R. Thiessen-Philbrook: None. E. Akalin: None. J.S. Bromberg: Grants/Research support from National Institute of Allergy and Infectious Diseases, National Institute of Diabetes and Digestion and Kidney Diseases. Clinical trials with Astellas, Novartis, Qiagen, CareDx, Angion, Medeor. Executive clinical editor, Transplantation. M.D. Doshi: None. M.N. Harhay: None. S. Mohan: Grant/Research support from NIH(NIDDK/NIAID/NIHMD/NIBIB). Consulting fees from Jazz Pharma. Deputy Editor, Kidney International Reports. T. Muthukumar: None. B. Schroppel: Consulting Fees from Pfizer, Astellas, Novartis, Amgen, Alexion, Sanofi. P. Singh: None. F.L. Weng: Grant/Research Support from Angion Biomedica, Merck, Shire, CareDx, Astellas, CSL Behring, Novartis, Medeor Therapeutics. Ownership Interest Pfizer. C.R. Parikh: Consulting Fee from Renalytix. Grant/Research Support from George M. O’Brien Kidney Center at Yale Grant, National Institute of Diabetes and Digestion and Kidney Diseases, National Heart, Lung and Blood Institute. Data Safety and Monitoring Board of Genfit, Abbott.
Abbreviations
- AKI
acute kidney injury
- ATI
acute tubular injury
- BMI
body mass index
- CKD
chronic kidney disease
- DcGF
death censored graft failure
- ECD
expanded-criteria donor
- GFR
glomerular filtration rate
- GF
graft failure
- HRSA
health resources and services administration
- KDRI
kidney donor risk index
- KIM-1
kidney injury molecule-1
- L-FABP
liver-type fatty acid binding protein
- NGAL
neutrophil gelatinase-associated lipocalin
- OPO
organ procurement organizations
- OPTN
organ procurement and transplantation network
- UNOS
united network for organ sharing
References
- 1.Mohan S, Chiles MC, Patzer RE, et al. Factors leading to the discard of deceased donor kidneys in the United States. Kidney International. 2018;94(1):187–198. doi: 10.1016/j.kint.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrero WJ, Naik AS, Friedewald JJ, et al. Predictors of Deceased Donor Kidney Discard in the United States. Transplantation. 2017;101(7):1690–1697. doi: 10.1097/TP.0000000000001238 [DOI] [PubMed] [Google Scholar]
- 3.Stewart DE, Garcia VC, Rosendale JD, Klassen DK, Carrico BJ. Diagnosing the Decades-Long Rise in the Deceased Donor Kidney Discard Rate in the United States. Transplantation. 2017;101(3):575–587. doi: 10.1097/TP.0000000000001539 [DOI] [PubMed] [Google Scholar]
- 4.Mohan S, Foley K, Chiles MC, et al. The weekend effect alters the procurement and discard rates of deceased donor kidneys in the United States. Kidney Int. 2016;90(1):157–163. doi: 10.1016/j.kint.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husain SA, Chiles MC, Lee S, et al. Characteristics and Performance of Unilateral Kidney Transplants from Deceased Donors. Clin J Am Soc Nephrol. 2018;13(1):118–127. doi: 10.2215/CJN.06550617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. New England Journal of Medicine. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303 [DOI] [PubMed] [Google Scholar]
- 7.Tonelli M, Wiebe N, Knoll G, et al. Systematic Review: Kidney Transplantation Compared With Dialysis in Clinically Relevant Outcomes. American Journal of Transplantation. 2011;11(10):2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x [DOI] [PubMed] [Google Scholar]
- 8.Howard K, Salkeld G, White S, et al. The cost-effectiveness of increasing kidney transplantation and home-based dialysis. Nephrology. 2009;14(1):123–132. doi: 10.1111/j.1440-1797.2008.01073.x [DOI] [PubMed] [Google Scholar]
- 9.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 Annual Data Report: Kidney. American Journal of Transplantation. 2018;18(S1):18–113. doi: 10.1111/ajt.14557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schold JD, Buccini LD, Srinivas TR, et al. The association of center performance evaluations and kidney transplant volume in the United States. Am J Transplant. 2013;13(1):67–75. doi: 10.1111/j.1600-6143.2012.04345.x [DOI] [PubMed] [Google Scholar]
- 11.Reese PP, Harhay MN, Abt PL, Levine MH, Halpern SD. New Solutions to Reduce Discard of Kidneys Donated for Transplantation. J Am Soc Nephrol. 2016;27(4):973–980. doi: 10.1681/ASN.2015010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper M, Formica R, Friedewald J, et al. Report of National Kidney Foundation Consensus Conference to Decrease Kidney Discards. Clin Transplant. 2019;33(1):e13419. doi: 10.1111/ctr.13419 [DOI] [PubMed] [Google Scholar]
- 13.Saidi RF, Elias N, Kawai T, et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: realities and costs. Am J Transplant. 2007;7(12):2769–2774. doi: 10.1111/j.1600-6143.2007.01993.x [DOI] [PubMed] [Google Scholar]
- 14.Organ Procurement and Transplantation Network. A Guide to Calculating and Interpreting the Kidney Donor Profile Index (KDPI). Health Resources and Services Administration; 2018. https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf. Accessed February 4, 2019. [Google Scholar]
- 15.Hall IE, Schröppel B, Doshi MD, et al. Associations of deceased donor kidney injury with kidney discard and function after transplantation. Am J Transplant. 2015;15(6):1623–1631. doi: 10.1111/ajt.13144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall IE, Akalin E, Bromberg JS, et al. Deceased-donor acute kidney injury is not associated with kidney allograft failure. Kidney International. November 2018. doi: 10.1016/j.kint.2018.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slocum JL, Heung M, Pennathur S. Marking renal injury: can we move beyond serum creatinine? Transl Res. 2012;159(4):277–289. doi: 10.1016/j.trsl.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol. 2011;22(5):810–820. doi: 10.1681/ASN.2010080796 [DOI] [PubMed] [Google Scholar]
- 19.Moledina DG, Hall IE, Thiessen-Philbrook H, et al. Performance of Serum Creatinine and Kidney Injury Biomarkers for Diagnosing Histologic Acute Tubular Injury. American Journal of Kidney Diseases. 2017;70(6):807–816. doi: 10.1053/j.ajkd.2017.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parikh CR, Moledina DG, Coca SG, Thiessen-Philbrook HR, Garg AX. Application of new acute kidney injury biomarkers in human randomized controlled trials. Kidney Int. 2016;89(6):1372–1379. doi: 10.1016/j.kint.2016.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonventre JV. Diagnosis of acute kidney injury: from classic parameters to new biomarkers. Contrib Nephrol. 2007;156:213–219. doi: 10.1159/000102086 [DOI] [PubMed] [Google Scholar]
- 22.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57(17):1752–1761. doi: 10.1016/j.jacc.2010.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reese PP, Hall IE, Weng FL, et al. Associations between Deceased-Donor Urine Injury Biomarkers and Kidney Transplant Outcomes. J Am Soc Nephrol. 2016;27(5):1534–1543. doi: 10.1681/ASN.2015040345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickinson DM, Bryant PC, Williams MC, et al. Transplant data: sources, collection, and caveats. Am J Transplant. 2004;4 Suppl 9:13–26. doi: 10.1111/j.1600-6135.2004.00395.x [DOI] [PubMed] [Google Scholar]
- 25.Health Resources and Services Administration (HRSA), Department of Health and Human Services (HHS). Organ procurement and transplantation network. Final rule. Fed Regist. 2013;78(128):40033–40042. [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Organ Procurement and Transplantation Network. OPTN Policies, 2018. https://optn.transplant.hrsa.gov/governance/policies/. Accessed December 29, 2018.
- 28.Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations In: Klein JP, Goel PK, eds. Survival Analysis: State of the Art. Nato Science. Dordrecht: Springer Netherlands; 1992:237–247. doi: 10.1007/978-94-015-7983-4_14 [DOI] [Google Scholar]
- 29.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–236. doi: 10.1097/TP.0b013e3181ac620b [DOI] [PubMed] [Google Scholar]
- 30.Zhong Y, Schaubel DE, Kalbfleisch JD, Ashby VB, Rao PS, Sung RS. Reevaluation of the Kidney Donor Risk Index (KDRI). Transplantation. 2018;Online First. doi: 10.1097/TP.0000000000002498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin DY, Wei LJ, Ying Z. Checking the Cox Model with Cumulative Sums of Martingale-Based Residuals. Biometrika. 1993;80(3):557–572. doi: 10.2307/2337177 [DOI] [Google Scholar]
- 32.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu CC, Ho HC, Yu TM, et al. Kidneys from standard-criteria donors with different severities of terminal acute kidney injury. Transplant Proc. 2014;46(10):3335–3338. doi: 10.1016/j.transproceed.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 34.Heilman RL, Smith ML, Kurian SM, et al. Transplanting Kidneys from Deceased Donors With Severe Acute Kidney Injury. Am J Transplant. 2015;15(8):2143–2151. doi: 10.1111/ajt.13260 [DOI] [PubMed] [Google Scholar]
- 35.Molina M, Apaza J, González Monte E, et al. Results of kidney transplantation from deceased donors with acute kidney injury. Transplant Proc. 2015;47(1):42–44. doi: 10.1016/j.transproceed.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 36.Si Nga H, Takase HM, Bravin AM, et al. Good Outcomes in Kidney Transplantation With Deceased Donor With Acute Kidney Injury: Donor’s Age and Not Acute Kidney Injury Predicts Graft Function. Transplant Proc. 2016;48(7):2262–2266. doi: 10.1016/j.transproceed.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 37.Lee MH, Jeong E-G, Chang JY, et al. Clinical outcome of kidney transplantation from deceased donors with acute kidney injury by Acute Kidney Injury Network criteria. J Crit Care. 2014;29(3):432–437. doi: 10.1016/j.jcrc.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 38.Farney AC, Rogers J, Orlando G, et al. Evolving experience using kidneys from deceased donors with terminal acute kidney injury. J Am Coll Surg. 2013;216(4):645–655; discussion 655–656. doi: 10.1016/j.jamcollsurg.2012.12.020 [DOI] [PubMed] [Google Scholar]
- 39.Oppong YD, Farber JL, Chervoneva I, Martinez Cantarin MP. Correlation of acute tubular injury in reperfusion biopsy with renal transplant outcomes. Clin Transplant. 2016;30(7):836–844. doi: 10.1111/ctr.12757 [DOI] [PubMed] [Google Scholar]
- 40.Kim JH, Kim YS, Choi MS, et al. Prediction of clinical outcomes after kidney transplantation from deceased donors with acute kidney injury: a comparison of the KDIGO and AKIN criteria. BMC Nephrol. 2017;18(1):39. doi: 10.1186/s12882-017-0461-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali T, Dimassi W, Elgamal H, et al. Outcomes of kidneys utilized from deceased donors with severe acute kidney injury. QJM. 2015;108(10):803–811. doi: 10.1093/qjmed/hcv033 [DOI] [PubMed] [Google Scholar]
- 42.Klein R, Galante NZ, de Sandes-freitas TV, de Franco MF, Tedesco-silva H, Medina-pestana JO. Transplantation With Kidneys Retrieved From Deceased Donors With Acute Renal Failure. Transplantation Journal. 2013;95(4):611–616. doi: 10.1097/TP.0b013e318279153c [DOI] [PubMed] [Google Scholar]
- 43.Benck U, Schnuelle P, Krüger B, et al. Excellent graft and patient survival after renal transplantation from donors after brain death with acute kidney injury: a case–control study. Int Urol Nephrol. 2015;47(12):2039–2046. doi: 10.1007/s11255-015-1127-5 [DOI] [PubMed] [Google Scholar]
- 44.Kumar MSA, Khan SM, Jaglan S, et al. Successful Transplantation of Kidneys from Deceased Donors with Acute Renal Failure: Three-year Results. Transplantation. 2006;82(12):1640–1645. doi: 10.1097/01.tp.0000250908.62948.8f [DOI] [PubMed] [Google Scholar]
- 45.Kayler LK, Garzon P, Magliocca J, et al. Outcomes and Utilization of Kidneys from Deceased Donors with Acute Kidney Injury. American Journal of Transplantation. 2009;9(2):367–373. doi: 10.1111/j.1600-6143.2008.02505.x [DOI] [PubMed] [Google Scholar]
- 46.Deroure B, Kamar N, Depreneuf H, et al. Expanding the criteria of renal kidneys for transplantation: use of donors with acute renal failure. Nephrol Dial Transplant. 2010;25(6):1980–1986. doi: 10.1093/ndt/gfq009 [DOI] [PubMed] [Google Scholar]
- 47.Jung CW, Park KT, Kim SY, et al. Clinical Outcomes in Kidney Transplantation Patients From Deceased Donors With Acute Kidney Injury. Transplantation Proceedings. 2013;45(8):2941–2945. doi: 10.1016/j.transproceed.2013.08.048 [DOI] [PubMed] [Google Scholar]
- 48.Jacobi J, Rebhan D, Heller K, et al. Donor acute kidney injury and short-term graft outcome in renal transplantation. Clin Transplant. 2014;28(10):1131–1141. doi: 10.1111/ctr.12425 [DOI] [PubMed] [Google Scholar]
- 49.Yuan XP, Han M, Wang XP, et al. Kidney Transplantation From Cardiac Death Donors With Terminal Acute Renal Failure. Transplantation Proceedings. 2014;46(4):1057–1060. doi: 10.1016/j.transproceed.2013.11.055 [DOI] [PubMed] [Google Scholar]
- 50.Wiwattanathum P, Ingsathit A, Kantachuvesiri S, Arpornsujaritkun N, Tirapanich W, Sumethkul V. Stabilization of estimated glomerular filtration rate in kidney transplantation from deceased donors with acute kidney injuries. World Journal of Transplantation. 2016;6(4):712–718. doi: 10.5500/wjt.v6.i4.712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Y-T, Chen C-B, Yuan X-P, Wang C-X. Impact of acute kidney injury in donors on renal graft survival: a systematic review and Meta-Analysis. Ren Fail. 2018;40(1):649–656. doi: 10.1080/0886022X.2018.1535982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heilman RL, Smith ML, Smith BH, et al. Long-term outcomes following kidney transplantation from donors with acute kidney injury. Transplantation. May 2019. doi: 10.1097/TP.0000000000002792 [DOI] [PubMed] [Google Scholar]
- 53.Boffa C, van de Leemkolk F, Curnow E, et al. Transplantation of Kidneys From Donors With Acute Kidney Injury: Friend or Foe? Am J Transplant. 2017;17(2):411–419. doi: 10.1111/ajt.13966 [DOI] [PubMed] [Google Scholar]
- 54.Gassanov N, Nia AM, Caglayan E, Er F. Remote ischemic preconditioning and renoprotection: from myth to a novel therapeutic option? J Am Soc Nephrol. 2014;25(2):216–224. doi: 10.1681/ASN.2013070708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y, Lang X-B, Zhang P, Lv R, Wang Y-F, Chen J-H. Remote ischemic preconditioning for prevention of acute kidney injury: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2014;64(4):574–583. doi: 10.1053/j.ajkd.2014.04.029 [DOI] [PubMed] [Google Scholar]
- 56.Stokfisz K, Ledakowicz-Polak A, Zagorski M, Zielinska M. Ischaemic preconditioning – Current knowledge and potential future applications after 30 years of experience. Advances in Medical Sciences. 2017;62(2):307–316. doi: 10.1016/j.advms.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 57.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605–612. doi: 10.1056/NEJM200003023420901 [DOI] [PubMed] [Google Scholar]
- 58.Han M, Jeong JC, Koo TY, et al. Kidney donor risk index is a good prognostic tool for graft outcomes in deceased donor kidney transplantation with short, cold ischemic time. Clin Transplant. 2014;28(3):337–344. doi: 10.1111/ctr.12318 [DOI] [PubMed] [Google Scholar]
- 59.Jun H, Jung CW, Lim S, Kim MG. Kidney Donor Risk Index as the Predictor for the Short-term Clinical Outcomes After Kidney Transplant From Deceased Donor With Acute Kidney Injury. Transplant Proc. 2017;49(1):88–91. doi: 10.1016/j.transproceed.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 60.Molnar AO, van Walraven C, Fergusson D, Garg AX, Knoll G. Derivation of a Predictive Model for Graft Loss Following Acute Kidney Injury in Kidney Transplant Recipients. Can J Kidney Health Dis. 2017;4:2054358116688228. doi: 10.1177/2054358116688228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagarajan M, Ramanathan S, Dhanapriya J, Dineshkumar T, Subramaniyan TB, Gopalakrishnan N. Impact of acute kidney injury on renal allograft survival. Ren Fail. 2017;39(1):40–44. doi: 10.1080/0886022X.2016.1244076 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.