Abstract
Background
Plasmodium infection among children is a serious public health problem. Asymptomatic malaria infection among humans serves as a significant reservoir for transmitting Plasmodium to uninfected Anopheles mosquitoes, fueling malaria endemicity and asymptomatic malaria may progress to clinical malaria. Therefore, prompt and accurate diagnosis of malaria infection is crucial for the management and control of malaria, especially in endemic areas. This study assessed the point prevalence of asymptomatic malaria infection and evaluated the performance of malaria Rapid Diagnostic Tests (RDT), light microscopy and nested PCR (nPCR) for the diagnosis of asymptomatic malaria infection in a paediatric population in the Atwima Nwabiagya North district, Ghana.
Methods
This cross-sectional study enrolled 500 asymptomatic children aged ≤ 5 years. After consent was obtained from a parent, blood samples were collected from each participant to assess for Plasmodium infection based on histidine rich protein-2 (pfHRP-2)-based malaria RDT, light microscopy and nPCR.
Results
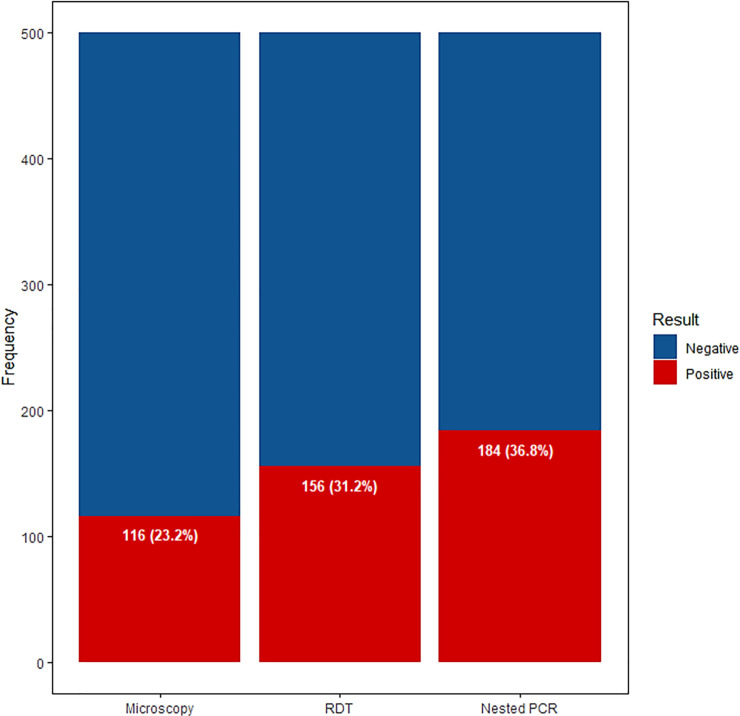
The point prevalence of asymptomatic malaria by microscopy, RDT, and nPCR were 116/500 (23.2%), 156/500 (31.2%), and 184/500 (36.8%), respectively. Using nPCR as the reference, RDT presented with a perfect sensitivity (100.0%), specificity (100.0%), accuracy (100.0%), and reliability (100.0%) in detecting asymptomatic P. falciparum infection. Likewise, microscopy presented with an excellent specificity and high accuracy in detecting both P. falciparum (100.0%; 85.6%) and P. malariae (100.0%; 100.0%). However, the sensitivity (56.4%) and reliability (56.4%) of microscopy was low for both P. falciparum.
Conclusion
The findings of this study indicate a high point prevalence of asymptomatic Plasmodium infection among children in Atwima Nwabiagya North district, Ghana. In the absence of the more sensitive PCR, pfHRP-2-based malaria RDT provides substantial diagnostic sensitivity, specificity, accuracy and reliability and is superior to microscopy.
Introduction
Malaria is a pervasive parasitic disease in the tropical and subtropical regions which is mostly prevalent in sub-Saharan Africa, Asia, and Latin America [1]. Currently, the World Health Organization (WHO) estimates 219 million cases and 435,000 malaria-related deaths globally [2]. In the WHO African Region, malaria causes significant morbidity and mortality with annual infection and mortality rates of 213 million and 380,000 individuals, respectively, and it claims the life of a child under five years every two minutes [3, 4]. Despite successes in global malaria control in previous years, recent data indicate insufficient progress. In Ghana, malaria remains a major cause of loss of days of healthy life, accounting for not less than 20% of child deaths, 40% of child hospital admissions, and more than 50% of outpatient attendances [5–8]. The enormous toll on life and both national and household economics [9] underscores the need for ongoing malaria diagnosis, treatment, and disease surveillance.
Clinically, the diagnosis of malaria is often based on signs and symptoms alone. However, due to overlapping symptoms between malaria and other infectious conditions, a malaria diagnosis based solely on signs and symptoms may be inaccurate leading to improper use of anti-malarial medication or the delay in proper diagnosis and treatment of an alternative condition [10]. As a result, the WHO recommends the use of microscopy or rapid diagnostic tests (RDT) as confirmatory diagnostic tools for malaria prior to initiation of treatment in suspected malaria cases, which also minimizes the likelihood of the development of drug resistant strains [11].
In many developing countries, microscopic examination of Giemsa-stained blood smears is considered the “gold standard” for malaria diagnosis and a mandatory test prior to antimalarial therapy. Though it is cost-effective, malaria microscopy is limited by several factors including quality control, limited availability of microscopes, time consuming for optimal film preparation, examination, and interpretation, diagnostic biases as a result of its dependence on operator’s experience and low diagnostic sensitivity [12–14]. Furthermore, bacteria, fungi, dirt, cell debris, and poor blood film preparation result in formation of artifacts and are associated with false positive results [15].
In an effort to improve diagnostic sensitivity and turnaround time and abate diagnostic errors related to microscopy, RDTs were developed. Currently, the most widely utilized RDTs exploit the presence of Plasmodium falciparum Histidine-Rich Protein-2 (pfHRP-2), parasite-specific Lactate Dehydrogenase (pLDH) or Plasmodium aldolase to detect parasitemia [16, 17]. The performance of RDT is influenced by manufacturing and environmental conditions in addition to its inability to quantify parasitemia and to accurately identify species other than P. falciparum [18–20]. Additionally, false negatives due to pfHRP-2 gene deletions and non-reliability for non-Plasmodium falciparum infections have been reported [21–23]. Moreover, persistence of pfHRP-2 antigens in circulation even after parasite clearance may result in false positive results; limiting RDT specificity [24].
Taken together, both RDT and microscopy are limited by low detection threshold, especially in low parasitaemic cases [20, 25]. As such, Polymerase Chain Reaction (PCR) based assays have been developed to remedy some of the limitations. PCR is very sensitive, particularly in cases of low density or mixed infection and it is valuable for accurate collection of malaria epidemiological data [26, 27]. However, the expensive, technical and time-consuming nature of PCR limits its utilization in routine practice, especially in remote or resource-limited settings.
Asymptomatic carriers of malaria parasites have recently received considerable attention as global strides towards malaria eradication are underway. Some reports indicate that asymptomatic malaria infection may serve as a significant reservoir, transmitting Plasmodium to uninfected Anopheles mosquitoes which fuels malaria endemicity [28, 29]. There is also the possibility that asymptomatic malaria will transition into clinical malaria. Thus, accurate diagnosis of asymptomatic malaria as a potential reservoir of infection, especially in children, is crucial. Although a number of studies on asymptomatic malaria in older children have been conducted across Ghana [30–32] and children under 5 in neighboring African countries [33–35], there remains a dearth of published data on asymptomatic malaria in children under 5 years in Ghana, particular in the northern sectors of the country where adequate health facilities are wanting. This study assessed the point prevalence of asymptomatic malaria infection and evaluated the performance of malaria RDT, light microscopy and nested PCR (nPCR) for the diagnosis of asymptomatic malaria infection in children under 5 years old in Atwima Nwabiagya North district, Ghana.
Materials and methods
Study design/area and participants
The study was conducted in July, 2015 in peri-urban and rural communities of the Atwima Nwabiagya North district in the Ashanti region of Ghana. The district lies approximately on latitude 6° 32’N and 6° 75’N and between longitude 1° 45’ W and 2° 00’ W. It is located in the western part of the region and shares common boundaries with Offinso Municipal (to the North), Ahafo Ano South and Atwima Mponua Districts (to the West), Amansie-West and Atwima Kwanwoma Districts (to the South), Kumasi Metropolis and Afigya Kwabre Districts (to the East). It covers an estimated area of 294.84 square kilometers and has an estimated population of 149,025 according to the 2010 Population and Housing Census [36].
The minimum sample size of 196 was calculated at 95% confidence level, 7% margin of error, and a response distribution of 50% using the Raosoft sample size calculator [37]. However, in an effort to enhance the statistical power of the study, a total of 500 asymptomatic children of age ≤ 5 years old were recruited to the study. Inclusion criteria were: lack of fever in the last 3 days, no history of anti-malarial treatment in the last 14 days, an axillary temperature less than 37.5°C, and no known acute/chronic or disease. Ethical approval for this study was obtained from the committee on Human Research, Publications and Ethics (CHRPE) of the School of Medical Sciences of the Kwame Nkrumah University of Science and Technology (CHRPE/AP/257/15). Written informed consent was obtained from parents/ guardians of all participating children after the aims and objectives of the study had been explained to them. Participation was voluntary, and respondents were assured that the information obtained was strictly for research and academic purposes only and were guaranteed the liberty to opt out from the study at their own convenience.
Data and sample collection
Parents/guardians of participating children were interviewed to obtain participants’ information on gender, age, history of malaria/fever and treatments, and presence of acute and/or chronic disease. Fingerprick blood samples were obtained from children who satisfied the inclusion criteria. About 8 μl of the blood was used for haemoglobin measurement using HemoPoint H2 Hemoglobin analyzer (Accuracy of 14.0 g/dl ± 0.3 g/dl; Linearity of 0–20 g/dl ± 0.3 g/dl; total precision CV <1.5%) (EKF Diagnostics, Stanbio Laboratory, USA). Anaemia was defined as haemoglobin level <11 g/dl, and graded as mild (10–10.9 g/dl), moderate (7–9.9 g/dl), and severe (<7 g/dl) [38]. Approximately 5 μl of blood was used for malaria diagnosis by RDT. Giemsa-stained thick and thin blood films were also prepared. Additionally, about 3–5 drops of the blood were spotted onto Whatman 903™ Filter Paper (Schleicher and Schuell BioScience, Inc., Keene, New Hampshire), air dried and individually kept in sealed plastic bag for subsequent nPCR analysis. All samples were tested for malaria by RDT, microscopy, and nested PCR. PCR was considered the gold standard.
Malaria diagnosis by RDT
Malaria RDT diagnosis was based on the detection of Histidine rich protein 2 (HRP-2) produced by P. falciparum only (paraHIT f, Span Diagnostics Limited, Surat, India). Testing and reporting was done according to the manufacturer’s instructions. Briefly, approximately 5 μl of the blood sample was transferred to the sample window using a micropipette, followed by 4 drops of the Reaction buffer into the buffer window. A sample was considered positive for P. falciparum malaria if the test line and control line appeared within the result window. The presence of the control line only, was considered a negative result. Results were declared invalid if the control line failed to appear within the result window, warranting re-testing. The test was done in duplicates. A third RDT was performed in the case of non-concordant result. Patients who tested positive were referred to the closest community health care facility for further diagnosis and treatment in accordance with the Ghana Health Service guidelines.
Malaria diagnosis by microscopy
Thick and thin blood smears were prepared (in duplicate) on clean, grease-free glass microscope slides immediately after sample collection. The films were allowed to air-dry and thin films were fixed with methanol. Both thick and fixed thin films were stained with 5% Giemsa solution for 30 minutes prior to microscopic examination. Examination and reporting of both thick and thin films were performed independently by two trained microscopists. The thin film was used to identify the specific species of Plasmodium. A film was considered positive by microscopy when both microscopists recorded a positive result for the same species. A film was considered negative only after observing at least 200 high-power fields (HPF) without finding parasites on a thick film. In the case of non-concordant result, a third examination was performed by a different microscopist. All microscopists were blinded to the results of RDT. Parasites were counted per 200 white blood cells (WBCs) per HPF from the thick film. The parasite density was calculated by assuming a WBC count of 8000/μl and 4.5 million RBC/μl in accordance with the WHO standard [39].
DNA extraction and molecular analysis
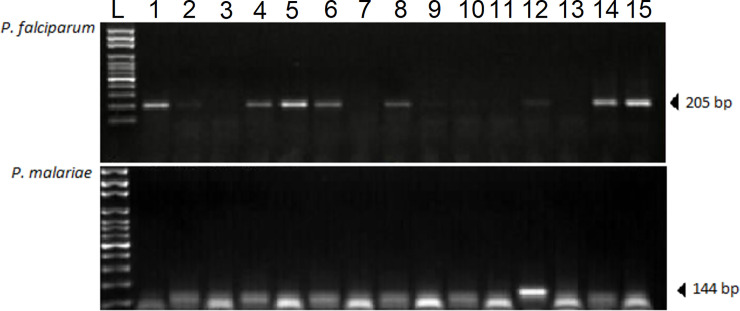
DNA isolation from Whatman filter papers was based on the Chelex-based technique as previously described [40]. Nested polymerase chain reaction was used for the determination of Plasmodium species, as previously described [41]. Briefly, Plasmodium genus was detected based on amplification of the outer genus-specific primers (rPLU1 and rPLU5). The reaction mixture for the initial outer reaction contained 4 mM of MgCl2, 200 μM DNTPs, 0.0625 μM of each primer and one unit of Taq DNA polymerase (Sigma-Aldrich, USA). For the primary reaction (Nested 1), the PCR cycling conditions consisted of an initial denaturation at 94°C for 4 min, denaturation at 94°C for 30 secs, annealing at 60°C for 1 min, extension at 72°C for 1 min (for 5 cycles), followed by denaturation at 94°C for 30 secs, annealing at 55°C for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 4 min (for 45 cycles). Subsequently, a secondary amplification reaction (Nested 2) using the genus-specific (rPLU3 and rPLU4) and species-specific primer pairs (rFAL1 and rFAL2, rMAL1 and rMAL2, rOVA1 and rOVA2) was performed with 1 μL of the product of the first amplification reaction as a template DNA as previously described [27, 42–44] (Table 1). All PCR reactions were performed using a GeneAmp PCR System 2700 (Applied Biosystems Incorporated, USA). The amplified products were separated by electrophoresis on 2% agarose gels, stained with 0.5 μg/mL ethidium bromide and visualized under UV light. (Fig 1).
Table 1. Nested PCR protocol and Plasmodium ssrRNA genes used in this study.
| Target species | Primer | Sequence (5'-3') | Reaction |
|---|---|---|---|
| Plasmodium genus-specific | rPLU1 | TCAAAGATTAAGCCATGCAAGTGA | Nested 1 |
| rPLU5 | CCTGTTGTTGCCTTAAACTTC | ||
| rPLU3 | TTTTTATAAGGATAACTACGGAAAAGCTGT | Nested 2 | |
| rPLU4 | TACCCGTCATAGCCATGTTAGGCCAATACC | ||
| Plasmodium species-specific | |||
| Plasmodium falciparum | rFAL1 | TTAAACTGGTTTGGGAAAACCAAATATATT | Nested 2 |
| rFAL2 | ACACAATGAACTCAATCATGACTACCCGTC | ||
| Plasmodium malariae | rMAL1 | ATAACATAGTTGTACGTTAAGAATAACCGC | Nested 2 |
| rMAL2 | AAAATTCCCATGCATAAAAAATTATACAAA | ||
| Plasmodium ovale | rOVA1 | ATCTCTTTTGCTATTTTTTAGTATTGGAGA | Nested 2 |
| rOVA2 | GGAAAAGGACACATTAATTGTATCCTAGTG |
Fig 1. Agarose gel electrophoresis showing nPCR products under UV light.
The top panel shows representative results of the P. falciparum-specific nested PCR. Lanes 1, 4, 5, 6, 8, 12, 14, and 15 contain the positive 205 bp PCR products expected. The bottom panel shows representative results of the P. malariae-specific nested PCR. Lane 12 contains the positive 144 bp PCR product expected. Smaller bands are likely due to low DNA concentration in those samples. L represents the molecular ladder.
Statistical analysis
Data processing was done using Microsoft Excel 2016. Statistical analysis and graphical presentation was performed using the R Language for Statistical Computing version 3.5.2 (R Core Team, Vienna, Austria) [45]. Categorical data were presented as frequency (percentages). Continuous data were presented as mean ± standard deviation (SD). Univariate logistic regression analysis was used to assess the association between sociodemographic characteristics and malaria infection for each test methods used in this study. The receiver operating characteristics (ROC) curve analysis was used to assess the diagnostic performance of malaria RDT and microscopy using PCR as the reference. Reliability was expressed as the J index [(TP×TN)—(FP×FN)]/ [(TP+FN) (TN+FP)]. All tests were two-sided and a 𝑝-value < 0.05 was considered statistically significant.
Results
A total of 500 children with mean age and haemoglobin level of 2.21 years and 10.26 g/dl, respectively, were included in this study. A higher proportion of the participants were male (51.2%), between 1–2 years of age (47.2%), and resided in the community of Barekuma (28.4%). A total of 189 (75.6%) were anaemic and the point prevalence of mild, moderate, and severe anaemia was 55.2%, 16.8%, and 3.6%, respectively. Only P. falciparum [parasite density = 1,1540 (2,000–34,000) parasites/μL] and P. malariae [parasite density = 2,100 (1,270–3,720) parasites/μL] were identified in this study. There were no mixed infections (Table 2).
Table 2. Baseline characteristics of the study population.
| Variables | Frequency (n = 500) | Percentage (%) |
|---|---|---|
| Age (years) | 2.21 ± 1.28* | |
| ≤1 | 62 | 12.4 |
| >1–2 | 236 | 47.2 |
| >2–3 | 54 | 14.8 |
| >3–4 | 76 | 15.2 |
| >4–5 | 52 | 10.4 |
| Sex | ||
| Male | 256 | 51.2 |
| Female | 244 | 48.8 |
| Residence | ||
| Abira | 88 | 17.6 |
| Adankwame | 94 | 18.8 |
| Barekese | 44 | 8.8 |
| Barekuma | 142 | 28.4 |
| Esaaso | 56 | 11.2 |
| Worapong | 76 | 15.2 |
| Anaemia | 368 | 75.6 |
| Mild | 276 | 55.2 |
| Moderate | 84 | 16.8 |
| Severe | 18 | 3.6 |
| Variable | Mean | Standard Deviation |
| Haemoglobin (g/dL) | 10.26 | ± 1.46 |
| Variable | Median | Interquartile Range |
| Parasite density (microscopy)‡ | ||
| P. falciparum (parasites/μL) | 11,540 | (2,000–34,000) |
| P. malariae (parasites/μL) | 2,100 | (1,270–3,720) |
*Anaemia was defined as haemoglobin level <11 g/dL and graded as mild (10–10.9 g/dL), moderate (7–9.9 g/dL), and severe (<7 g/dL).
The overall point prevalence of asymptomatic malaria by microscopy, RDT and nPCR were 116/500 (23.2%), 156/500 (31.2%), and 184/500 (36.8%), respectively (Fig 2).
Fig 2. Prevalence of asymptomatic malaria by microscopy, RDT, and nPCR.
The point prevalence of asymptomatic malaria was 98 (19.6%) for males and 86 (17.2%) for females based on nPCR. Using microscopy and RDT, the point prevalence among males was 66 (13.2%) vs 86 (17.2%), respectively, and 50 (10.0%) vs 70 (14.0%) among females, respectively. Upon stratification by age, the point prevalence of asymptomatic malaria was highest in children between 1–2 years of age based on nPCR (16.4%), microscopy (11.2%), and RDT (15.6%), respectively. A similar observation was made after age groups were stratified by gender. Children residing in the community of Worapong (13.2%) presented with the highest point prevalence of asymptomatic malaria, followed by Abira (8.0%), Barekuma and Adankwame (4.4%), and Barekese (4.0%), with the lowest being Esaaso (2.8%). Children in Abira [OR = 4.55, 95% CI (1.90–10.90), p<0.01], Barekese [OR = 4.55, 95% CI (1.58–13.08), p<0.01], and Worapong [OR = 36.0, 95% CI (11.52–112.48), p<0.0001] had significantly higher odds of asymptomatic malaria compared to Barekuma. A similar observation was made when using microscopy and RDT. In addition, children experiencing any anaemia had an increased odds of asymptomatic malaria (Table 3).
Table 3. Sociodemographic-stratified point prevalence and odds ratios for asymptomatic malaria infection by microscopy, RDT and nPCR.
| Variables | Microscopy | RDT | nPCR | |||
|---|---|---|---|---|---|---|
| Prevalence | OR (95% CI) | Prevalence | OR (95% CI) | Prevalence | OR (95% CI) | |
| Sex | ||||||
| Female | 50 (10.0) | 1 | 70 (14.0) | 1 | 86 (17.2) | 1 |
| Male | 66 (13.2) | 1.35 (0.75–2.44) | 86 (17.2) | 1.26 (0.74–2.15) | 98 (19.6) | 1.14 (0.68–1.14) |
| Age group (years) | ||||||
| ≤1 | 12 (2.4) | 1 | 14 (2.8) | 1 | 18 (3.6) | 1 |
| >1–2 | 56 (11.2) | 1.30 (0.48–3.48) | 78 (15.6) | 1.69 (0.67–4.27) | 82 (16.4) | 1.30 (0.55–3.09) |
| >2–3 | 18 (3.6) | 1.34 (0.42–4.30) | 22 (4.4) | 1.45 (0.48–4.35) | 30 (6.0) | 1.67 (0.60–4.46) |
| >3–4 | 22 (4.4) | 1.70 (0.55–5.28) | 26 (5.2) | 1.78 (0.61–5.23) | 32 (6.4) | 1.78 (0.65–4.87) |
| >4–5 | 8 (1.6) | 0.76 (0.19–3.04) | 16 (3.2) | 1.52 (0.47–4.98) | 22 (4.4) | 1.79 (0.60–5.38) |
| Female | ||||||
| ≤1 | 8 (3.3) | 1 | 12 (4.9) | 1 | 12 (4.9) | 1 |
| >1–2 | 22 (9.0) | 0.84 (0.23–3.04) | 30 (12.3) | 0.71 (0.23–2.24) | 34 (13.9) | 0.85 (0.27–2.64) |
| >2–3 | 10 (4.1) | 1.03 (0.23–4.58) | 14 (5.7) | 0.93 (0.25–3.52) | 20 (8.2) | 1.67 (0.46–6.06) |
| >3–4 | 4 (1.6) | 0.70 (0.11–4.59) | 6 (2.5) | 0.67 (0.13–3.41) | 10 (4.1) | 1.43 (0.32–6.46) |
| >4–5 | 6 (2.5) | 1.05 (0.19–5.76) | 8 (3.3) | 0.89 (0.19–4.11) | 10 (4.1) | 1.25 (0.28–5.53) |
| Male | ||||||
| ≤1 | 4 (1.6) | 1 | 2 (0.8) | 1 | 3 (2.3) | 1 |
| >1–2 | 34 (13.3) | 2.13 (0.43–10.60) | 48 (18.8) | 7.78 (0.95–63.80) | 48 (18.8) | 2.16 (0.54–8.67) |
| >2–3 | 8 (3.1) | 2.00 (0.30–13.27) | 8 (3.1) | 4.36 (0.42–45.26) | 10 (3.9) | 1.67 (0.31–8.93) |
| >3–4 | 18 (7.0) | 2.91 (0.53–16.09) | 20 (7.8) | 7.50 (0.84–66.86) | 22 (8.6) | 2.44 (0.54–11.03) |
| >4–5 | 2 (0.8) | 0.46 (0.04–5.79) | 8 (3.1) | 5.33 (0.51–56.24) | 12 (4.7) | 2.86 (0.53–15.47) |
| Anaemic status | ||||||
| Non-anaemic | 8 (1.6) | 1 | 16 (3.2) | 1 | 18 (3.6) | 1 |
| Anaemic | 108 (21.6) | 5.70 (1.97–16.48)** | 140 (28.0) | 3.90 (1.75–8.67)** | 166 (33.2) | 4.54 (2.11–9.71)*** |
| Residence | ||||||
| Barekuma | 16 (3.2) | 1 | 20 (4.0) | 1 | 22 (4.4) | 1 |
| Barekese | 8 (1.6) | 1.75 (0.47–6.48) | 10 (2.0) | 1.79 (0.54–5.96) | 20 (4.0) | 4.55 (1.58–13.08)** |
| Adankwame | 16 (3.2) | 1.62 (0.56–4.65) | 18 (3.6) | 1.45 (0.54–3.88) | 22 (4.4) | 1.67 (0.66–4.23) |
| Esaaso | 0 (0.0) | - | 10 (2.0) | 1.33 (0.41–4.30) | 14 (2.8) | 1.82 (0.62–5.30) |
| Abira | 28 (5.6) | 3.68 (1.39–9.71)** | 40 (8.0) | 5.08 (2.08–12.43)*** | 40 (8.0) | 4.55 (1.90–10.90)** |
| Worapong | 48 (9.6) | 13.50 (5.03–36.25)*** | 58 (11.9) | 19.66 (7.21–53.60)*** | 66 (13.2) | 36.00 (11.52–112.48)*** |
**; Significant at p<0.01
***; Significant at p<0.0001
Nested PCR detected 156 P. falciparum cases, of which microscopy identified 88 cases and did not identify 68 cases. RDT detected all 156 cases of P. falciparum. All 28 cases of P. malariae identified by nPCR were also detected by microscopy. RDT did not detect any P. malariae cases (Table 4).
Table 4. Comparison of microscopy and RDT with nPCR for the detection of asymptomatic malaria infection.
| Methods | P. falciparum | P. malariae | Negative |
|---|---|---|---|
| Microscopy | 88 | 28 | 384 |
| RDT | 156 | n/a | 344 |
| nPCR | 156 | 28 | 316 |
RDT presented with a perfect sensitivity (100.0%) specificity (100.0%), accuracy (100.0%), and reliability (100.0%) in detecting P. falciparum infection. Microscopy presented with a similar performance with respect to P. malariae infection. However, the sensitivity (56.4%), accuracy (85.6%) and reliability (56.4%) of microscopy was attenuated for detecting P. falciparum (Table 5).
Table 5. Diagnostic performance of microscopy and RDT in detecting asymptomatic malaria infection.
| Methods | Microscopy | RDT | |
|---|---|---|---|
| P. falciparum | P. malariae | P. falciparum | |
| Sensitivity (95% CI) | 56.4 (48.3–64.3) | 100.0 (87.7–100.0) | 100.0 (97.7–100.0) |
| Specificity (95% CI) | 100.0 (98.8–100.0) | 100.0 (98.8–100.0) | 100.0 (98.9–100.0) |
| PPV | 100.0 | 100.0 | 100.0 |
| NPV | 82.3 | 100.0 | 100.0 |
| TP | 88 | 28 | 156 |
| TN | 316 | 316 | 344 |
| FP | 0 | 0 | 0 |
| FN | 68 | 0 | 0 |
| Accuracy (%) | 85.6 | 100.0 | 100.0 |
| AUC (%) | 78.2 (74.2–81.8) | 100.0 (98.9–100.0) | 100.0 (99.3–100.0) |
| Reliability (%) | 56.4 | 100.0 | 100.0 |
nPCR was used as the reference
Discussion
Based on nested PCR, this study reports a high point prevalence (36.8%) of asymptomatic Plasmodium infection among a paediatric population in the Atwima Nwabiagya North district of Ghana. Asymptomatic malaria in children under 5 years has been reported in some African countries [33–35]. In Ghana, Crookston, et al. [46] reported an asymptomatic malaria prevalence of 31.8% based on PCR among children less than five years of age in Kumasi, Ghana which is similar to our study finding. Other studies in Ghana such as those by Dinko, et al. [31] in Kumasi and Danquah, et al. [47] in northern Ghana reported asymptomatic malaria among older children. The prevalence found in the studies by Dinko et al. (76.6%) and Danquah et al. (89.7%) are higher compared to this study and the discrepancy may be linked to the fact that older children tend to be the major asymptomatic carriers of Plasmodium compared to younger children [47, 48], possibly as a result of protective immunity acquired over the years.
We also found asymptomatic malaria to be more prevalent among male children compared to females, similar to the findings of Golassa et al. in Ethiopia [29]. The higher point prevalence of asymptomatic malaria among male children compared to females could be explained by the fact that, in Ghana, males are more exposed to both daytime and nighttime outdoor activities than females and thus, at higher risk of mosquito bites compared to females who are usually indoors. Our findings also indicate a strong association between presence of asymptomatic malaria and anaemia as consistent with studies by Crookston et al. [46], Verhoef et al. [49], and Stoltzfus et al. [50]. This study also presents information on the point prevalence of asymptomatic malaria in six different communities, providing data on potential hotspots for malaria screening and treatment. Children residing in Worapong presented with the highest point prevalence of asymptomatic malaria, followed by children in Abira. The higher point prevalence in Worapong compared to the other communities could be due to the relative accessibility of the communities or the agricultural practices of the specific communities. Barekese and Adankwame are economically diverse communities and on a main through-road, whereas Worapong is very difficult to get access due to poor roads and flooded rice field farming provides habitat for malaria transmitting mosquitoes. Nonetheless, owing to the fact that malaria-related morbidity and mortality is high among children and the propensity of asymptomatic malaria transitioning into clinical malaria, the children in these communities should be given substantial precedence during national and regional malaria surveillance exercises.
Due to the high point prevalence of asymptomatic malaria in Ghana [31, 32, 48], and the possibility that people with asymptomatic malaria infection may serve as a significant reservoir, transmitting Plasmodium to uninfected Anopheles mosquitoes [28, 29], prompt and accurate diagnosis of asymptomatic Plasmodium infection is crucial. In this study, despite the high specificity, microscopy presented with poor sensitivity and reliability for the detection of asymptomatic P. falciparum compared to nPCR as consistent with previous reports [14, 19, 27, 29, 44]. Taken together, these findings indicate that a negative result by microscopy does not exclude the presence of malaria infection since a substantial number of false negatives were associated with microscopy for P. falciparum. Microscopy thus seems to have an inherent limitation for asymptomatic P. falciparum detection, with proficiency of technicians and microscopists likely to be a significant contributor. This underscores the need for continued refinement through constant re-training to sharpen microscopists’ ability to detect malaria cases especially at low parasite densities, since refresher training has been reported to significantly improve the diagnostic accuracy of parasitological diagnosis of malaria by microscopy [51]. Meanwhile, the high specificity and positive predictive value of microscopy in detecting malaria parasites, regardless of the species, suggest that positive malaria microscopy is a good confirmation of malaria, regardless of the presence of symptoms. Thus, a positive result from microscopy could be trusted as the presence of the Plasmodium infection and anti-parasitic therapy should be guided by the species identified.
Strikingly, despite the possibility of false negatives due to pfHRP-2 gene deletions [21–23] and the persistence of pfHRP-2 antigens in circulation even after parasite clearance, which may increase the incidence of false positive results [24], malaria diagnosis by RDT provided a better estimate of asymptomatic P. falciparum infection, with a perfect sensitivity, specificity, accuracy and reliability compared to microscopy when nPCR is used as the reference. Indeed, RDT was able to correctly classify all P. falciparum cases detected by nPCR. This may be due to the fact that the RDT used in this study detects pfHRP-2 antigen and not malaria parasites, affording it an added advantage over microscopy through the detection of antigens produced in very low parasite densities below the detection threshold of microscopy. This finding strongly suggests that detection of pfHRP-2 by RDT accurately identifies P. falciparum infection in asymptomatic children and is an indication for anti-malarial therapy. However, it is worthy of note that there is the possibility that the pfHRP-2 antigen may be persistent in the blood, even in the absence of viable parasites. Our study excluded children who had received anti-malaria therapy in the prior 14 days. It is possible that if the study had included children who have had such therapy within the prior 14 days, we would have identified children who had persistent pfHRP-2 antigenemia but who were no longer parasitemic. Thus, interpretation should be done with caution. Additionally, it should be noted that the widely used RDT for malaria diagnosis in Ghana is pfHRP-2-based RDT which detects on P. falciparum but not P. malariae. The choice of this RDT is attributed to the relatively higher point prevalence of P. falciparum and its associated clinical significance in Ghana compared to the P. malariae which causes less severe clinical outcomes [52]. In other words, a negative pfHRP-2-based RDT does not exclude infection with non-falciparum malaria species.
Although the finding of this study and several other reports point to the fact that RDT should be used as a surrogate to microscopy due to the low sensitivity of microscopy, it is worthy of note that, microscopy allows for the quantification and calculation of malaria parasite densities, a function which RDT cannot be used to assess. Thus, microscopy should not be abandoned. We, however, recommend consistent re-training of malaria microscopists in the region to enhance their Plasmodium detection skills and abilities. Moreover, despite the high sensitivity and specificity, PCR is still expensive which limits its usefulness in routine malaria diagnosis.
Study strengths and limitations
The strength of the study is in the reporting of the point prevalence of asymptomatic malaria infection among children less than 5 years old in the northern sector of Ghana. We also highlight potential hotspots for malaria screening and treatment during national and regional malaria surveillance exercises. The study also corroborates previous reports on the usefulness of molecular detection methods for asymptomatic malaria diagnosis. We showed that, in the absence of PCR, RDT performs better in the diagnosis of asymptomatic malaria caused by P. falciparum among children compared to microscopy.
This study is however limited the fact that we used only a single brand of malaria RDT; the prevalence may not be the same when other commercially available test kits are used. Also, data on the use of long-lasting insecticidal nets was unavailable. Additionally, the study was conducted in a peri-urban setting and the findings may not be generalizable to other areas. Thus, we recommend that further studies be conducted in the larger population.
Conclusions
The findings of this study indicate a high point prevalence of Plasmodium infection and anemia among asymptomatic children in Atwima Nwabiagya North district of Ghana. Since Ghana remains in the control stage, there is the exigent need for effort intensification through detection of asymptomatic malaria, using highly sensitive diagnostic tools, in order to reach the pre-eradication stage of malaria. The use of microscopy for Plasmodium detection in children who are asymptomatic presents several challenges. However, in the absence of the more sensitive PCR, the use RDT provides substantial diagnostic sensitivity and reliability.
Supporting information
(DOCX)
Acknowledgments
The authors are grateful to all children and their parents/guardians who participated in the study. Authors are also grateful to the Student Learning Abroad Group of 2015 for their support as research assistants. A part of the work has been presented as a poster at the 2016 American Society for Microbiology General Meeting.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
All authors received support via salary from their respective institutions: University of Utah School of Medicine (TD, RB, SB), Kwame Nkrumah University of Science and Technology School of Medical Sciences (BO, AOO, DA, AOA, CA, EWO), and Komfo Anokye Teaching Hospital (EXA, JMB). The authors received no other specific funding for this study.
References
- 1.Addai-Mensah O, Gyamfi D, Amponsah FA, Annani-Akollor ME, Danquah KO, Boateng L, et al. Antierythropoietin Antibody Production Is Not Associated with Malaria and Malaria-Related Anaemia in Humans. The Scientific World Journal. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. World malaria report 2018 Geneva: World Health Organization; 2018. Available from: https://www.who.int/malaria/publications/world-malaria-report-2018/en/. [Google Scholar]
- 3.WHO. World Malaria Report 2019 Geneva: World Health Organization; 2019. Available from: https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019. [Google Scholar]
- 4.Addai-Mensah O, Annani-Akollor ME, Fondjo LA, Anto EO, Gyamfi D, Sallah L, et al. High-Sensitivity C-Reactive Protein: A Potential Ancillary Biomarker for Malaria Diagnosis and Morbidity. Disease Markers. 2019;2019:7 10.1155/2019/1408031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owusu-Agyei S, Asante K, Adjuik M, Adjei G, Awini E, Adams M. Epidemiology of malaria in the forest-savanna transitional zone of Ghana. Malaria Journal. 2009;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarpong N, Owusu-Dabo E, Kreuels B, Fobil J, Segbaya S, Amoyaw F, et al. Prevalence of malaria parasitaemia in school children from two districts of Ghana earmarked for indoor residual spraying: A cross-sectional study. Malaria Journal. 2015;14:260 10.1186/s12936-015-0772-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addai-Mensah O, Annani-Akollor M, Fondjo L, Sarbeng K, Anto E, Owiredu E, et al. Regular Antenatal Attendance and Education Influence the Uptake of Intermittent Preventive Treatment of Malaria in Pregnancy: A Cross-Sectional Study at the University Hospital, Kumasi, Ghana. J Journal of tropical medicine. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Initiative PM. Malaria Operational Plan Report Ghana. 2018. [Google Scholar]
- 9.Sicuri E, Vieta A, Lindner L, Constenla D, Sauboin C. The economic costs of malaria in children in three sub-Saharan countries: Ghana, Tanzania and Kenya. Malaria journal. 2013;12(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotepui M, Uthaisar K, Phunphuech B, Phiwklam N. A diagnostic tool for malaria based on computer software. Scientific reports. 2015;5:16656 10.1038/srep16656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Guidelines for the Treatment of Malaria. Third Edition: World Health Organization; 2015. Available from: https://www.who.int/malaria/publications/atoz/9789241549127/en/. [PubMed] [Google Scholar]
- 12.Alexander N, Schellenberg D, Ngasala B, Petzold M, Drakeley C, Sutherland C. Assessing agreement between malaria slide density readings. Malaria journal. 2010;9(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowers KM, Bell D, Chiodini PL, Barnwell J, Incardona S, Yen S, et al. Inter-rater reliability of malaria parasite counts and comparison of methods. Malaria journal. 2009;8(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrade BB, Reis-Filho A, Barros AM, Souza-Neto SM, Nogueira LL, Fukutani KF, et al. Towards a precise test for malaria diagnosis in the Brazilian Amazon: comparison among field microscopy, a rapid diagnostic test, nested PCR, and a computational expert system based on artificial neural networks. Malaria journal. 2010;9(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houwen B. Blood film preparation and staining procedures. Clinics in laboratory medicine. 2002;22(1):1–14. 10.1016/s0272-2712(03)00064-7 [DOI] [PubMed] [Google Scholar]
- 16.Abanyie FA, Arguin PM, Gutman J. State of malaria diagnostic testing at clinical laboratories in the United States, 2010: a nationwide survey. Malaria Journal. 2011;10(1):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ugah UI, Alo MN, Owolabi JO, Okata-Nwali OD, Ekejindu IM, Ibeh N, et al. Evaluation of the utility value of three diagnostic methods in the detection of malaria parasites in endemic area. Malaria journal. 2017;16(1):189 10.1186/s12936-017-1838-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moody A. Rapid diagnostic tests for malaria parasites. Clinical microbiology reviews. 2002;15(1):66–78. 10.1128/cmr.15.1.66-78.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahende C, Ngasala B, Lusingu J, Yong T-S, Lushino P, Lemnge M, et al. Performance of rapid diagnostic test, blood-film microscopy and PCR for the diagnosis of malaria infection among febrile children from Korogwe District, Tanzania. Malaria journal. 2016;15(1):391 10.1186/s12936-016-1450-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mtove G, Nadjm B, Amos B, Hendriksen IC, Muro F, Reyburn H. Use of an HRP2‐based rapid diagnostic test to guide treatment of children admitted to hospital in a malaria‐endemic area of north‐east Tanzania. Tropical medicine & international health. 2011;16(5):545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berhane A, Russom M, Bahta I, Hagos F, Ghirmai M, Uqubay S. Rapid diagnostic tests failing to detect Plasmodium falciparum infections in Eritrea: an investigation of reported false negative RDT results. Malaria journal. 2017;16(1):105 10.1186/s12936-017-1752-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathison BA, Pritt BS. Update on malaria diagnostics and test utilization. Journal of clinical microbiology. 2017;55(7):2009–17. 10.1128/JCM.02562-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berzosa P, de Lucio A, Romay-Barja M, Herrador Z, González V, García L, et al. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea. Malaria journal. 2018;17(1):333 10.1186/s12936-018-2481-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mfuh KO, Achonduh-Atijegbe OA, Bekindaka ON, Esemu LF, Mbakop CD, Gandhi K, et al. A comparison of thick-film microscopy, rapid diagnostic test, and polymerase chain reaction for accurate diagnosis of Plasmodium falciparum malaria. Malaria journal. 2019;18(1):73 10.1186/s12936-019-2711-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bejon P, Andrews L, Hunt-Cooke A, Sanderson F, Gilbert SC, Hill AV. Thick blood film examination for Plasmodium falciparum malaria has reduced sensitivity and underestimates parasite density. Malaria Journal. 2006;5(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosha JF, Sturrock HJ, Greenhouse B, Greenwood B, Sutherland CJ, Gadalla N, et al. Epidemiology of subpatent Plasmodium falciparum infection: implications for detection of hotspots with imperfect diagnostics. Malaria journal. 2013;12(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li P, Zhao Z, Wang Y, Xing H, Parker DM, Yang Z, et al. Nested PCR detection of malaria directly using blood filter paper samples from epidemiological surveys. Malaria journal. 2014;13(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alves FP, Gil LHS, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LHP. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. Journal of medical entomology. 2005;42(5):777–9. 10.1093/jmedent/42.5.777 [DOI] [PubMed] [Google Scholar]
- 29.Golassa L, Enweji N, Erko B, Aseffa A, Swedberg G. Detection of a substantial number of sub-microscopic Plasmodium falciparum infections by polymerase chain reaction: a potential threat to malaria control and diagnosis in Ethiopia. Malaria journal. 2013;12(1):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayanful-Torgby R, Quashie NB, Boampong JN, Williamson KC, Amoah LE. Seasonal variations in Plasmodium falciparum parasite prevalence assessed by varying diagnostic tests in asymptomatic children in southern Ghana. PloS one. 2018;13(6):e0199172 10.1371/journal.pone.0199172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dinko B, Oguike MC, Larbi JA, Bousema T, Sutherland CJ. Persistent detection of Plasmodium falciparum, P. malariae, P. ovale curtisi and P. ovale wallikeri after ACT treatment of asymptomatic Ghanaian school-children. International Journal for Parasitology: Drugs and Drug Resistance. 2013;3:45–50. 10.1016/j.ijpddr.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orish V, Aho KA, Ofori-Amoah J, Osei-Yobah J, Jamfaru I, Afeke I, et al. Asymptomatic Plasmodium falciparum infection and poor school performance in primary school children in the Volta Region of Ghana. Ethiopian journal of health sciences. 2018;28(6):749 10.4314/ejhs.v28i6.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akinbo F, Emekaili D, Mbarie A, Ogbogu M. Asymptomatic malaria in children under 5 years old in Benin City, Nigeria. Savannah Journal of Medical Research and Practice. 2015;4(2):66–71. [Google Scholar]
- 34.Ouédraogo M, Samadoulougou S, Rouamba T, Hien H, Sawadogo JE, Tinto H, et al. Spatial distribution and determinants of asymptomatic malaria risk among children under 5 years in 24 districts in Burkina Faso. Malaria journal. 2018;17(1):460 10.1186/s12936-018-2606-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wamae K, Wambua J, Nyangweso G, Mwambingu G, Osier F, Ndung’u F, et al. Transmission and age impact the risk of developing febrile malaria in children with asymptomatic Plasmodium falciparum parasitemia. The Journal of infectious diseases. 2019;219(6):936–44. 10.1093/infdis/jiy591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GSS. Population and housing census. Regional Analytical Report Ashanti Region. 2010:340. [Google Scholar]
- 37.Shanti R, Potluri M. Sample Size Calculator. Raosoft Inc; 2009. [Google Scholar]
- 38.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity: World Health Organization; 2011. Available from: https://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- 39.WHO. Basic malaria microscopy–Part I: Learner's guide. Second edition: World Health Organization; 2010. Available from: https://apps.who.int/iris/bitstream/handle/10665/44208/9789241547826_eng.pdf;jsessionid=F5D7D3AA5371D0B562103A5649F372C5?sequence=1. [Google Scholar]
- 40.Bereczky S, MÅrtensson A, Gil JP, FÄrnert A. Rapid DNA extraction from archive blood spots on filter paper for genotyping of Plasmodium falciparum. The American journal of tropical medicine and hygiene. 2005;72(3):249–51. [PubMed] [Google Scholar]
- 41.Snounou G, Singh B. Nested PCR analysis of Plasmodium parasites Malaria methods and protocols: Springer; 2002. p. 189–203. [DOI] [PubMed] [Google Scholar]
- 42.Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. A genus-and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. The American journal of tropical medicine and hygiene. 1999;60(4):687–92. 10.4269/ajtmh.1999.60.687 [DOI] [PubMed] [Google Scholar]
- 43.Fuehrer H-P, Fally MA, Habler VE, Starzengruber P, Swoboda P, Noedl H. Novel nested direct PCR technique for malaria diagnosis using filter paper samples. Journal of clinical microbiology. 2011;49(4):1628–30. 10.1128/JCM.01792-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doni NY, Zeyrek FY, Seyrek A. Detection of Plasmodium using filter paper and nested PCR for patients with malaria in Sanliurfa, in Turkey. Malaria journal. 2016;15(1):299 10.1186/s12936-016-1334-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Team RC. R: A language and environment for statistical computing. 2013. [Google Scholar]
- 46.Crookston BT, Alder SC, Boakye I, Merrill RM, Amuasi JH, Porucznik CA, et al. Exploring the relationship between chronic undernutrition and asymptomatic malaria in Ghanaian children. Malaria journal. 2010;9(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danquah I, Ziniel P, Eggelte TA, Ehrhardt S, Mockenhaupt FP. Influence of haemoglobins S and C on predominantly asymptomatic Plasmodium infections in northern Ghana. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2010;104(11):713–9. 10.1016/j.trstmh.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 48.Owusu ED, Buabeng V, Dadzie S, Brown CA, Grobusch MP, Mens P. Characteristics of asymptomatic Plasmodium spp. parasitaemia in Kwahu-Mpraeso, a malaria endemic mountainous district in Ghana, West Africa. Malaria journal. 2016;15(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verhoef H, West CE, Ndeto P, Burema J, Beguin Y, Kok FJ. Serum transferrin receptor concentration indicates increased erythropoiesis in Kenyan children with asymptomatic malaria. The American journal of clinical nutrition. 2001;74(6):767–75. 10.1093/ajcn/74.6.767 [DOI] [PubMed] [Google Scholar]
- 50.Stoltzfus RJ, Chwaya HM, Montresor A, Albonico M, Savioli L, Tielsch JM. Malaria, hookworms and recent fever are related to anemia and iron status indicators in 0-to 5-y old Zanzibari children and these relationships change with age. The Journal of nutrition. 2000;130(7):1724–33. 10.1093/jn/130.7.1724 [DOI] [PubMed] [Google Scholar]
- 51.Odhiambo F, Buff AM, Moranga C, Moseti CM, Wesongah JO, Lowther SA, et al. Factors associated with malaria microscopy diagnostic performance following a pilot quality-assurance programme in health facilities in malaria low-transmission areas of Kenya, 2014. Malaria journal. 2017;16(1):371 10.1186/s12936-017-2018-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owusu ED, Brown CA, Grobusch MP, Mens P. Prevalence of Plasmodium falciparum and non-P. falciparum infections in a highland district in Ghana, and the influence of HIV and sickle cell disease. Malaria journal. 2017;16(1):167 10.1186/s12936-017-1823-y [DOI] [PMC free article] [PubMed] [Google Scholar]