Abstract
Background
NOD2 variants are the strongest genetic predictors for susceptibility to Crohn’s disease (CD). However, the clinical value of NOD2 on an individual patient level remains controversial. We aimed to define the predictive power of the major NOD2 mutations regarding complicated CD in a large single center cohort.
Methods
1076 CD patients were prospectively genotyped for the three common CD-associated NOD2 mutations rs2066844, rs2066845, and rs2066847, followed by detailed genotype-phenotype analyses.
Results
Overall, 434 CD patients (40.3%) carried at least one of the three main NOD2 mutations. A significantly higher minor allele frequency (15.6%) of the NOD2 frameshift mutation p.Leu1007fsX1008 (rs2066847) was seen in patients with aggressive disease compared to 8.2% in patients with mild disease (p = 2.6 x 10−5). Moreover, a total of 54 CD patients (5.0%) were homozygous for this NOD2 frameshift mutation. 100% of these patients had ileal disease compared to 82% of NOD2 wild-type carriers (p<0.0001). In homozygous carriers of the NOD2 frameshift mutation, 87% presented with ileal stenosis, 68.5% had fistulas, and 72.2% required CD-related surgery despite immunosuppressive therapy in 87% of these patients. All homozygous carriers of the 1007fs mutation who were active smokers had ileal stenosis and required CD-related surgery.
Conclusion
Homozygosity for Leu1007fsX1008 is an excellent biomarker for predicting complicated CD on an individual patient level. Active smoking and homozygosity for this mutation is associated with a 100% risk for developing ileal stenosis requiring CD-related surgery. In these patients, smoking cessation and early initiation of immunosuppressive strategies may be beneficial.
Introduction
The identification of the first genetic locus in the centromeric region of chromosome 16 associated with susceptibility to inflammatory bowel diseases (IBD) more than two decades ago provided first insights into the role of a genetic predisposition in the pathogenesis of IBD [1]. In this chromosomal region, NOD2 was identified five years later as the first gene to be associated with susceptibility to Crohn’s disease (CD) [2,3]. Three main NOD2 mutations, p.Arg702Trp (rs2066844), p.Gly908Arg (rs2066845), and p.Leu1007fsX1008 (rs2066847), are associated with a complicated disease course, particularly with a stricturing and penetrating disease behaviour [2–4]. Since 2001, several genetic studies including genome-wide association scans (GWAS) unrevealed the complex genetic background of the two IBD subtypes CD and ulcerative colitis (UC) [5–22]. Currently, more than 250 susceptibility regions for IBD have been confirmed, but only NOD2 has evolved from an initial candidate gene to a clinically useful genetic marker for disease prediction in CD [22,23].
In particular, a frameshift mutation in exon 11 of NOD2, p.Leu1007fsX1008 (rs2066847), has been successfully used as a predictive factor for therapeutic decisions in patients with CD [7,8]. Several clinical trials reported on CD patients with homozygosity for p.Leu1007fsX1008. Due to the limited number of patients in these subcohorts, the statistical power to reliably predict the disease course of CD was too low [12–15]. In 2006, we reported a subgroup of 19 patients with homozygosity for p.Leu1007fsX1008 in our IBD cohort [8]. At that time, this study represented the largest subgroup analysis of patients with p.Leu1007fsX1008 homozygosity and the respective phenotypes. Importantly, this patient population was characterized by an early onset of CD with a median age at diagnosis of 23.9 years and 100% ileal disease. Ninety-five percent of the patients had stricturing disease behaviour with long-segment stenosis in the terminal ileum, entero-enteral fistulas (52.6%), and the frequent need for CD-related surgery (73.7%) with a high prevalence of re-stenosis at the anastomosis (78.6%).
However, the value of NOD2 on an individual patient level remains controversial. In this study, we aimed to increase the CD patient cohort of homozygous carriers of the NOD2 mutation p.Leu1007fsX1008 in order to evaluate its predictive power for the CD disease course. We genotyped 1076 CD patients for the three main NOD2 mutations p.Arg702Trp (rs2066844), p.Gly908Arg (rs2066845), and p.Leu1007fsX1008 (rs2066847) and identified a total of 54 homozygous rs2066847 minor C allele carriers. The predictive power of the three common NOD2 mutations was subsequently analyzed by detailed genotype-phenotype correlations.
Patients and methods
Ethics statement
This study was approved by the ethics committee of the medical faculty of the Ludwig-Maximilians-University Munich. Written, informed consent was obtained from all patients prior to study inclusion. Study protocols were based on the ethical principles for medical research involving human subjects of the Helsinki Declaration.
Study population
In total, 1076 patients with CD were enrolled in this study. All patients were recruited at the University Hospital Munich-Grosshadern. Our study cohort included a predominantly Caucasian population (99.5% Caucasians, only 0.5% (n = 5 patients) were from India or of African descent).
Phenotypic parameters were collected independently of the results of the genotype analyses. Phenotypic data included demographic and clinical data (behaviour and anatomic location of IBD, disease-related complications, surgical or immunosuppressive therapy). Two senior gastroenterologists analyzed the data which were recorded by patient chart analysis and a detailed questionnaire based on an interview at the time of enrolment. For the analysis of demographic and phenotypic data, the diagnosis of CD was related to established international guidelines based on endoscopic, radiological, and histopathological parameters [24]. CD patients were classified according to the Montreal classification [25] including age at diagnosis (A), location (L), and behaviour (B) of disease.
The clinical course of CD was defined as “aggressive” in CD patients with a stricturing (B2) and/or penetrating disease behaviour (B3) and/or CD-related surgery. CD patients with a “mild” disease course did not have a stricturing or penetrating phenotype or CD-related surgery (B1 according to the Montreal Classification) [25].
DNA extraction and NOD2 genotyping
Amplification of NOD2 exons 4, 8, and 11 was performed according to standard procedures. PCR product size and quantity were analyzed by agarose gel electrophoresis. Fragments were purified with the QIAquick PCR purification kit (QIAGEN, Hilden, Germany) and sequenced with the ABI PRISM BigDye Terminator v3.1 Ready Reaction Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA). Sequences were analyzed on an ABI PRISM 377 DNA Sequencer (Applied Biosystems) using the Sequence Analysis program version 3.4.5 (Applied Biosystems). Control subject and patient sequence data were compared to the published NOD2 sequence, and all differences were documented. Different analyses of this IBD patient cohort have been previously published elsewhere [7–9,26–28].
Statistical analyses
All statistical tests were two-tailed and p-values < 0.05 were considered significant. Each genetic marker was tested for Hardy-Weinberg equilibrium. Single-marker allelic tests were performed with Pearson’s χ2 test. Odds ratios were calculated for the minor allele at each SNP. For evaluation of phenotypic consequences, we conducted logistic regression analyses. Data and haplotype analyses were done by using the PLINK v1.07 software (http://pngu.mgh.harvard.edu/purcell/plink/) [29] and R-2.4.1. (http://cran.r-project.org).
The combined effects of five important predictors (age at diagnosis, disease duration, smoking status, ileal involvement and homozygosity for p.Leu1007fsX1008 NOD2 mutation (rs2066847)) on the presence of stenoses and on the need for surgery were analyzed with multiple logistic regression. The models were fitted through a Bayesian approach with a weakly informative prior using the function “bayesglm” in the R package arm [30]. This approach was needed because of fitting problems due to quasi-complete separation for the effect of the p.Leu1007fsX1008 NOD2 mutation (rs2066847) on stenoses. For all other predictors in the model and for the effect of the p.Leu1007fsX1008 NOD2 mutation on surgery, p-values and odds ratios were closely similar with this approach as with conventional logistic regression (function “glm” in R base package stats). Odds ratios were calculated from the regression coefficients, and approximate 95% CI were calculated from 95% Wald confidence intervals as obtained with the function “coefci” in R package lmtest. To obtain more interpretable odds ratios, coefficients for age and disease duration were calculated for an increase by 10 years. Smoking (with three levels) was represented either by a single contrast (any smoking vs. non-smoking) or by two separate contrasts (active smoking vs. non-smoking and former smoking vs. non-smoking).
Results
Phenotypic characteristics of the study cohort
S1 Table shows the phenotypic characteristics of the study population. A total of 1076 CD patients were included in the analysis. There was an equal gender distribution (48.0% males, 52.0% females), and the mean age was 42.3 years with a mean disease duration of almost 12 years. The majority of CD patients (71.6%) was between 17 and 40 years of age at first diagnosis of CD, while 16.1% of the CD patients were younger than 17 years at the time of CD onset. 72.6% of the patients studied had either a stricturing or a penetrating disease behaviour (26.8% stricturing disease behaviour (B2); 45.8% penetrating disease behaviour (B3)), thus representing a CD cohort with a severe disease manifestation. 57.0% of the patients required CD-related surgery (e.g., ileocecal resection, fistulectomy, colectomy, or ileostomy). Over 80% of the CD patients were treated with immunosuppressive or biological therapies (S1 Table). Interestingly, almost one fifth of the patients (17.7%) had a positive family history of IBD, illustrating a strong genetic predisposition in this patient population. Importantly, more than one third of the CD patients (35.5%) were active smokers and more than one fifth were ex-smokers (21.8%) at the time of enrolment.
Allele frequencies of the three main NOD2 mutations rs2066844 (p.Arg702Trp), rs2066845 (p.Gly908Arg) and rs2066847 (p.Leu1007fsX1008)
Table 1 gives an overview of the observed minor allele frequencies and the genotype of the three main NOD2 mutations rs2066844 (p.Arg702Trp), rs2066845 (p.Gly908Arg), and rs2066847 (p.Leu1007fsX1008) in our CD patient cohort.
Table 1. Minor allele frequencies (MAF) of the three main NOD2 SNPs rs2066844 (p.Arg702Trp), rs2066845 (p.Gly908Arg), and rs2066847 (p.Leu1007fsX1008) for each genotype (homozygous, heterozygous and compound heterozygous) in patients with Crohn’s disease.
| Crohn’s disease | ||
|---|---|---|
| Gene marker | Genotype | MAF |
| (%) | ||
| rs2066844 (n = 1076) | ||
| p.Arg702Trp, minor allele | T | 8.0 (n = 172/2152) |
| Homozygous | TT | 1.1 (n = 24/2152) |
| Heterozygous | CT | 5.3 (n = 114/2152) |
| Compound heterozygous | CT/other NOD2 mutation | 1.6 (n = 34/2152) |
| rs2066845 (n = 1076) | ||
| p.Gly908Arg, minor allele | C | 3.9 (n = 84/2152) |
| Homozygous | CC | 0.2 (n = 4/2152) |
| Heterozygous | GC | 0.5 (n = 68/2152) |
| Compound heterozygous | GC/other NOD2 mutation | 3.2 (n = 12/2152) |
| rs2066847 (n = 1066) | ||
| p.Leu1007fsX1008, minor allele | insC | 13.4 (n = 286/2132)* |
| Homozygous | CC | 5.0 (n = 108/2132)* |
| Heterozygous | XC | 7.2 (n = 153/2132)* |
| Compound heterozygous | XC/other NOD2 mutation | 1.2 (n = 25/2132)* |
*For rs2066847 (p.Leu1007fsX1008), genotype status was only known in 1066 patients, resulting in 2132 alleles.
The observed minor allele frequencies of 8.0% (p.Arg702Trp), 3.9% (p.Gly908Arg), and 13.4% (p.Leu1007fsX1008) were comparable to previously reported allele frequencies, e.g., in a French IBD population [17]. 59.7% of the CD patients analyzed (n = 642) carried none of the three main NOD2 mutations, whereas 40.3% (n = 306) were carriers of one (or more) NOD2 variants. Of the 306 patients carrying NOD2 mutations, 28.4% were heterozygous carriers of one of the three main NOD2 mutants, 4.6% were compound heterozygous carriers, and 6.3% (n = 68) were homozygous (n = 12 with homozygosity for p.Arg702Trp, n = 2 with homozygosity for p.Gly908Arg, and n = 54 with homozygosity for p.Leu1007fsX1008, Table 1). Genotype frequencies for the NOD2 mutations were as follows: 59.7% were wildtype for NOD2 mutations, 16.1% were genotyped for rs2066844, 7.8% for rs2066845 and 26.8% for rs2066847 (Please note: Due to compound heterozygous carriers (see for details Table 1), the total percentage is > 100%; the genotype status of rs20066847 was known in n = 1066 CD patients).
NOD2 genotype-phenotype correlation in patients with Crohn’s disease demonstrates a gene-dosage effect of NOD2 mutations and reveals p.Leu1007fsX1008 as a highly predictive genetic marker for severe Crohn’s disease
Detailed genotype-phenotype analyses demonstrated a strong association between the number of affected alleles and the severity of Crohn’s disease in our CD cohort. Carriers with two NOD2 mutations, e. g., compound heterozygous and homozygous carriers, were significantly younger at the first diagnosis of IBD (p = 0.010 and p = 0.036, respectively) than NOD2 wild-type carriers (“wild-type” defined as the absence of the three main NOD2 mutations. There was no significant difference seen in patients with one affected allele with respect to age at diagnosis.
More patients with one or two altered NOD2 alleles had ileal involvement compared to NOD2 wild-type (95.9% of compound heterozygotes, 94.2% of homozygotes and 89.8% of NOD2 heterozygous mutation carriers compared to 82.0% of patients with the NOD2 wild-type, p = 0.318, p = 0.048 and p = 0.002 compared to 89.2% of patients with the NOD2 wild-type and ileal involvement).
Significant more compound heterozygous and homozygous NOD2 mutation carriers had a penetrating disease behavior (B3) compared to NOD2 wild-type patients (59.2% and 64.7% vs. 44.3%, respectively, p = 0.047 and p = 0.0003 compared to NOD2 wild-type carriers).
Contrarily, more NOD2 wild-type (29.6%) and heterozygous carriers (29.0%) had a non-stricturing and non-penetrating disease behavior (B1) compared to 14.3% and 10.3% of patients with compound heterozygosity and homozygosity for NOD2 mutations (p = 0.014 and p = 0.016, respectively; and p = 0.001 compared to 10.3% with B1 in homozygous carriers). In addition, for stenoses, fistulas, and need for CD-related surgery, there was a gene dosage with a more severe disease phenotype found amongst the 1007fs homozygous carriers.
No significant differences were seen for gender distribution, disease duration, body mass index (BMI), perianal fistulas, and the number of patients with a positive smoking history between the groups. No significant differences were also observed for p.Arg702Trp (rs2066844) and for p.Gly908Arg (rs2066845) regarding allele frequencies in CD patients with aggressive disease compared to mild CD (Table 2). In contrast, a higher minor allele frequency (15.6%) of the p.Leu1007fsX1008 (rs2066847) frameshift mutation was seen in patients with aggressive disease, compared to 8.2% in patients with mild disease which was highly significant (p = 2.63x10-5, Table 2).
Table 2. CD phenotype stratified by disease course (n = 1011).
| NOD2 | Aggressive disease behaviour | Mild disease behaviour | ||||
|---|---|---|---|---|---|---|
| SNP | (stenosis and/or fistula and/or CD-related surgery; n = 747) | (no stenosis, no fistula, no CD-related surgery = inflammatory disease type; n = 264) | p-value | OR | 95% CI | |
| rs2066844 | T-allele (%) | 119/1494 (8.0%) | 49/528 (9.3%) | 0.347 | 0.850 | [0.60–1.20] |
| C-allele (%) | 1375/1494 (92.0%) | 479/528 (90.7) | ||||
| rs2066845 | C-allele (%) | 64/1494 (4.3%) | 15/528 (2.8%) | 0.141 | 1.531 | [0.86–2.71] |
| G-allele (%) | 1430/1494 (95.7%) | 513/528 (97.2%) | ||||
| rs2066847 | C-allele (%) | 231/1482 (15.6%)* | 43/522 (8.2%)* | 2.633x10-5 | 2.057 | [1.46–2.90] |
| X-allele (%) | 1251/1482 (84.4%)* | 479/522 (91.8%)* |
Aggressive disease behaviour was defined as a stricturing and/or penetrating disease course and/or CD-related surgery. Given are the allele frequencies (upper line: minor allele frequency in bold, bottom line: major allele frequency) for CD patients with an aggressive and a mild disease behaviour, respectively, for the three main NOD2 mutants rs2068844, rs2066845, and rs2066847. Only the frameshift mutation p.Leu1007fsX1008 in exon 11 of the NOD2 gene (rs2066847) was significantly associated with aggressive disease behaviour in patients with CD (p<0.0001, Chi-squared test). For the other two NOD2 mutants, no significant differences were seen for the allele frequencies in patients with aggressive vs. mild disease behaviour. Parts of these data have been published elsewhere [8,26,28,31].
Overall, the number of affected alleles was significantly associated with the severity of CD. Compound heterozygous and homozygous carriers of NOD2 mutations had a more aggressive disease course compared to the NOD2 wild-type and heterozygous NOD2 mutation carriers (92.6% of the homozygous carriers, 87.8% of the compound heterozygous carriers vs. 72.0% of patients with NOD2 wild-type and 79.2% of the heterozygous carriers, p = 0.003, p = 0.023 and p = 0.065 compared to 72%, Table 3).
Table 3. CD phenotype stratified by the rs2066847 (p.Leu1007fsX1008) genotype in CD patients.
| NOD2 SNP rs2066847 | (1) | (2) | (3) | (1) vs. (3) | (2) vs. (3) |
|---|---|---|---|---|---|
| genotype status | Homozygous | Heterozygous | Wild-type | p-value | p-value |
| (n = 54) | (n = 178) | (n = 642) | OR (CI) | OR (CI) | |
| Location | |||||
| (n = 54) | (n = 171) | (n = 610) | |||
| Terminal ileum (L1) | 18 (33.3%) | 50 (29.2%) | 120 (19.7%) | 0.020 | 0.008 |
| 2.04 | 1.69 | ||||
| [1.12–3.72] | [1.15–2.48] | ||||
| Colon (L2) | 0 | 10 (5.9%) | 98 (16.0%) | 0.0002* | 0.001* |
| 0 | 0.32 | ||||
| [0–0.38] | [0.17–0.64] | ||||
| Ileocolon (L3) | 36 (66.7%) | 110 (64.3%) | 380 (62.3%) | 0.525 | 0.626 |
| 1.21 | 1.09 | ||||
| [0.67–2.18] | [0.77–1.55] | ||||
| Upper GI (L4) | 7 (13.0%) | 26 (15.2%) | 90 (14.8%) | 0.721 | 0.884 |
| 0.86 | 1.04 | ||||
| [0.38–1.96] | [0.65–1.66] | ||||
| Any ileal | 54 (100%) | 160 (93.6%) | 500 (82.0%) | <0.0001* | 0.0004* |
| involvement | NA | 3.2 | |||
| (L1+L3) | [3.05—infinite] | [1.68–6.10] | |||
| Behaviour 1 | |||||
| (n = 54) | (n = 168) | (n = 589) | |||
| Non-stricturing, Non-penetrat. (B1) | 4 (7.4%) | 37 (22.0%) | 177 (29.6%) | 0.001* | 0.043 |
| 0.19 | 0.66 | ||||
| [0.07–0.52] | [0.44–0.99] | ||||
| Stricturing (B2) | 13 (24.1%) | 54 (32.2%) | 156 (26.1%) | 0.700 | 0.149 |
| 0.88 | 1.31 | ||||
| [0.46–1.69] | [0.91–1.91] | ||||
| Penetrating (B3) | 37 (68.5%) | 77 (45.8%) | 265 (44.3%) | 0.001* | 0.847 |
| 2.66 | 1.03 | ||||
| [1.47–4.83] | [0.73–1.46] | ||||
| Surgery because of CD 3 | |||||
| (n = 54) | (n = 167) | (n = 575) | |||
| 39 (72.2%) | 109 (65.3%) | 306 (53.2%) | 0.009 | 0.006 | |
| 2.29 | 1.65 | ||||
| [1.23–4.24] | [1.15–2.36] | ||||
| Fistulas | |||||
| (n = 54) | (n = 167) | (n = 590) | |||
| 37 (68.5%) | 77 (46.1%) | 261 (44.2%) | 0.0009* | 0.668 | |
| 2.74 | 1.08 | ||||
| [1.51–4.98] | [0.76–1.52] | ||||
| Stenosis | |||||
| (n = 54) | (n = 164) | (n = 585) | |||
| 47 (87.0%) | 109 (66.5%) | 336 (57.4%) | 0.0001* | 0.038 | |
| 4.98 | 1.47 | ||||
| [2.21–11.19] | [1.02–2.11] | ||||
| Smoking | |||||
| Active smoker, ex-smoker | (n = 42) | (n = 120) | (n = 372) | ||
| 17 (40.5%) | 64 (53.3%) | 223 (60.0%) | 0.017 | 0.202 | |
| 0.45 | 0.76 | ||||
| [0.24–0.87] | [0.50–1.16] | ||||
| Disease course | |||||
| (n = 54) | (n = 168) | (n = 597) | |||
| Aggressive | 50 (92.6%) | 133 (79.2%) | 430 (72.0%) | 0.003 | 0.065 |
| 4.85 | 1.48 | ||||
| [1.73–13.65] | [0.98–2.23] | ||||
| Mild | 4 (7.4%) | 35 (20.8%) | 167 (28.0%) | 0.003 | 0.065 |
| 0.21 | 0.68 | ||||
| [0.07–0.58] | [0.45–1.02] | ||||
Group (1): Subcohort of 54 patients with homozygosity for p.Leu1007fsX1008. CD disease characteristics are based on the Montreal classification [25]. For each variable, the number of patients included is given. 1Disease behaviour was defined according to the Montreal classification. A stricturing disease phenotype was defined as the presence of stenoses without penetrating disease. The diagnosis of stenoses was made surgically, endoscopically, or radiologically (using MR enteroclysis). 2Immunosuppressive agents included azathioprine, 6-mercaptopurine, methotrexate, infliximab and/or adalimumab. 3 Only surgery related to CD-specific problems (e.g. fistulectomy, colectomy, ileostomy) was included. Group (2): CD patients heterozygous for the p.Leu1007fsX1008 (rs2066847) mutant (n = 178) including a total of 38 compound heterozygotes (n = 16 for p.Leu1007fsX1008/p.Gly908Arg (rs2066844) and n = 22 for p.Leu1007fsX1008/p.Arg702Trp (rs2066845)). Group (3): CD patients carrying the NOD2 wild-type (642 patients with none of the three main NOD2 mutations); group
* Given the large number of comparisons, we performed correction for multiple testing (for a total of n = 28 comparisons). Therefore, the threshold for a significant p-value was < 0.05 x 28 = 0.001786 and only p-values with an asterisk (*) remained significant after multiple testing.
Sub-analysis of NOD2 p.Leu1007fsX1008 homozygosity in patients with Crohn’s disease reveals associations with an aggressive disease phenotype characterized by ileal stenosis, fistulas, and need for CD-related surgery
The high prevalence of the NOD2 p.Leu1007fsX1008 mutation seen in CD patients with an aggressive disease course (e.g., CD patients with a penetrating and/or stricturing disease behavior and/or CD-related surgery) suggests that this mutation is highly associated with a severe CD phenotype. Our sub-analysis emphasizes the strong association of this NOD2 mutation with severe CD and with ileal involvement (Table 3).
The median age at diagnosis was 25.0 years in NOD2 wild-type patients (p.Leu1007fsX1008 XX genotype) compared to 22.0 years in heterozygous carriers (p.Leu1007fsX1008 XC genotype) and to 20.0 years in homozygous carriers of the minor C allele (p.Leu1007fsX1008 CC genotype; p = 0.203 for XX vs. XC genotype and p = 0.251 for XC vs. CC genotype, and p = 0.069 for CC vs XX genotype, respectively). Accordingly, the proportion of patients with age at first diagnosis of CD ≤ 16 years (A1 according to the Montreal classification) was lower in the NOD2 wild-type group than in the p.Leu1007fsX1008/XC or in the p.Leu1007fsX1008/CC group, although these differences did not reach statistical significance (14.3% of patients with A1 in the wild-type group (XX genotype), compared to 16.2% in the XC group and 22.2% in the CC group, p = 0.207 and p = 0.123, respectively).
Furthermore, our sub-analysis showed that homozygous carriers of the p.Leu1007fsX1008 mutation have a more severe CD phenotype with main localization in the terminal ileum (p = 0.020, respectively, Table 3).
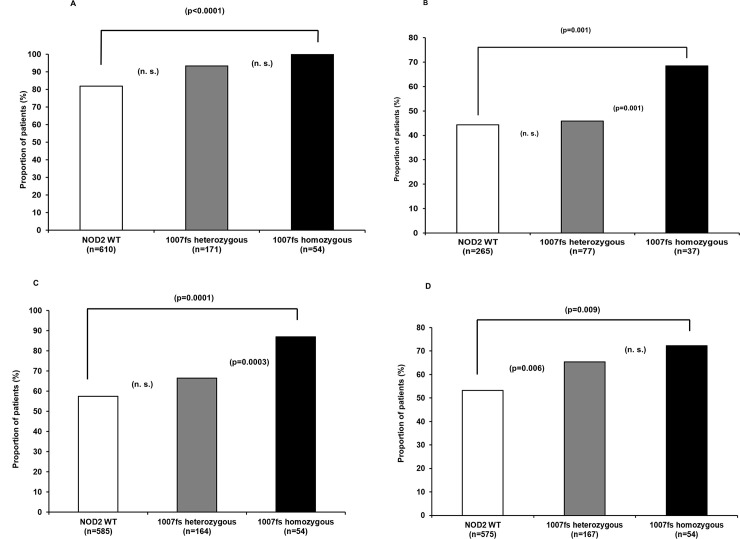
Most strikingly, homozygous carriers of p.Leu1007fsX1008 had significantly more severe disease compared to NOD2 wild-type carriers (p = 0.003, Table 3). In all homozygous carriers of the CC genotype, the main disease localization was in the terminal ileum compared to 93.6% in heterozygous carriers (XC genotype) and to 82.0% in patients of the NOD2 wild-type group (Table 3, Fig 1A, p<0.0001 for CC vs. wild-type). 68.5% of the patients with the CC genotype suffered from a penetrating disease (p = 0.001 for CC vs. wild-type), whereas there was no significant difference seen between the wild-type and the XC group (Table 3, Fig 1B, 44.3% vs. 45.8%, p = 0.847). 87% of the patients with homozygosity for p.Leu1007fsX1008 developed intestinal stenoses mainly in the terminal ileum compared to 66.5% of the patients in the XC group and 57.4% in the NOD2 wild-type group (p = 0.0001 for CC vs. wild-type and p = 0.0.038 for XC vs. wild-type and p = 0.0003 for CC vs. XC genotype, Table 3, Fig 1C). With 72.2%, the highest surgery rate was seen in CC-homozygous patients, compared with 65.3% in heterozygous carriers of the minor C allele (XC genotype) and with 53.2% in the NOD2 wild-type group (p = 0.118 for CC vs. XC genotype, p = 0.009 CC vs. wild-type and p = 0.006 for XC vs. wild-type, Table 3, Fig 1D).
Fig 1.
Gene dosage effect of the p.Leu1007fsX1008 mutation on (A) ileal involvement of CD (%), (B) development of fistulas (%), (C) development of stenoses (%), and (D) CD-related surgery (%).
Overall, 92.6% of the patients with homozygosity for p.Leu1007fsX1008 had either penetrating and/or stricturing disease, while 72.2% needed CD-related surgery (Table 3). In comparison, fewer patients in the NOD2 wild-type group (72%) and a lower proportion of heterozygous carriers of the minor C allele (XC genotype, 79.2%) had an aggressive disease phenotype (Table 3, p = 0.003 for CC vs. wild-type, p = 0.065 for XC vs. wild-type and p = 0.003 for CC vs. XC genotype).
Homozygous carriers of p.Leu1007fsX1008 who are active smokers are at 100% risk of developing intestinal stenosis requiring CD-related surgery
An important finding of this study was the strong influence of the NOD2 genotype and smoking status on the disease course of CD patients. We found a particular strong effect of the p.Leu1007fsX1008 mutation in CD patients with active smoking, resulting in a frequent need for CD related surgery.
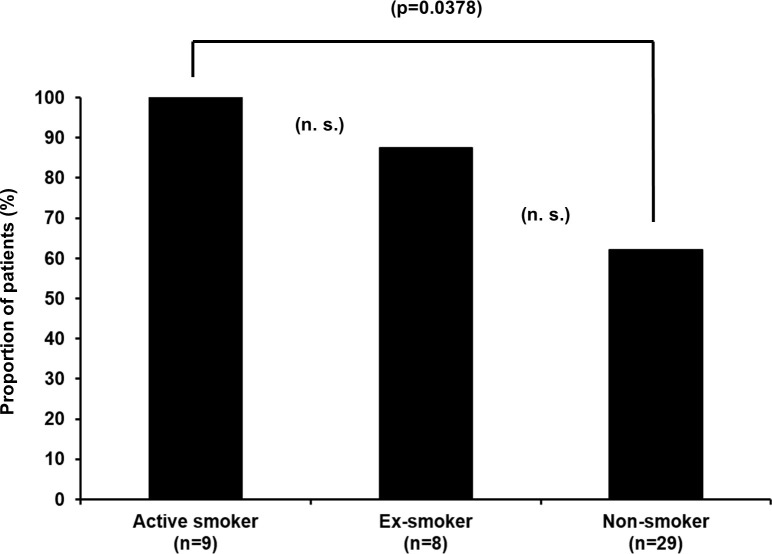
All patients in the subcohort of CD patients, who were homozygous carriers of p.Leu1007fsX1008 and were active smokers or had a recent smoking history, developed intestinal stenoses, and all these patients needed CD-related surgery (100%, Table 4 and Fig 2). In eight of them (88.9%), an ileocecal resection was performed, and one patient had fistula surgery (Table 4). All patients with a positive smoking history, who stopped smoking during follow-up, also developed intestinal stenosis (100%). The rate of surgery in this group was with 87.5% (n = 7/8) slightly lower compared to active smokers (surgery rate of 100% in active smokers) but higher than compared to non-smokers although this difference was statistically not significant (surgery rate of 62.1% in non-smokers, p = 0.232). In the subcohort of ex-smokers, seven patients with surgical interventions underwent ileocecal resection surgery (Table 4 and Fig 2).
Table 4. Risk of development of intestinal stenoses and subsequent need for CD-related surgery with respect to smoking history in homozygous carriers of the minor C allele of p.Leu1007fsX1008.
| CD patients with homozygosity for p.Leu1007fsX1008 and CD-related complications based on smoking status (n = 46) | ||||
|---|---|---|---|---|
| Stenoses | Surgery because of CD | |||
| (n =) | (%) | (n =) | (%) | |
| Smokers (n = 9) | 9 | 100.0 | 9 | 100.0 |
| Ex-smokers (n = 8) | 8 | 100.0 | 7 | 87.5 |
| Non-smokers (n = 29) | 23 | 79.3 | 18 | 62.1 |
Fig 2. Need for CD-related surgery in homozygous carriers of the p.Leu1007fsX1008 variant based on the smoking history (n = 46).
Overall, active smoking in patients with homozygosity for p.Leu1007fsX1008 was associated with the development of intestinal stenoses and the need for CD-related surgery in all cases. However, seven patients (all of them being non-smokers) with homozygosity for p.Leu1007fsX1008 (13.0%) did not develop intestinal stenoses. All of them received immunosuppressive therapy, and they were younger than 40 years at first diagnosis of CD. This suggests that early immunosuppressive therapy may alter the disease course in this patient subcohort.
Multiple logistic regression analysis confirms homozygosity for the NOD2 p.Leu1007fsX1008 mutation as major predictor for ileal stenosis and CD-related surgery
To analyze the specific contribution of homozygosity for the p.Leu1007fsX1008 NOD2 mutation on the risk for intestinal stenosis and CD-related surgery, we performed a multiple logistic regression analysis including predictors for which an effect on the development of CD-related intestinal stenoses and CD-related surgery has been demonstrated in previous studies. The combined effects of five important predictors (age at diagnosis, disease duration, smoking status, ileal involvement and homozygosity for the p.Leu1007fsX1008 NOD2 mutation (rs2066847)) on the presence of stenoses and on the need for CD-related surgery were analyzed by using multiple logistic regression as detailed in the Methods section. The results from multiple logistic regression for the association of the outcomes "stenoses" or "CD-related surgery" with the five predictors mentioned above are shown in Tables 5 and 6 and S2 and S3 Tables. In these analyses, smoking was represented by one (Tables 5 and 6) or by two contrasts (S2 and S3 Tables).
Table 5. Multiple logistic regression analysis including five important predictors (age at diagnosis, disease duration, smoking status, ileal involvement and homozygosity for the p.Leu1007fsX1008 NOD2 mutation (rs2066847)) on the presence of stenoses.
| Variable | p-value | OR (95% CI) |
|---|---|---|
| Age at diagnosis (per 10 years) | 0.447 | 1.084 [0.881–1.334] |
| Disease duration (per 10 years) | <0.001 | 1.934 [1.456–2.570] |
| Smoking status (any smoking versus non-smoking) | 0.007 | 1.968 [1.207–3.208] |
| Disease localization (any ileal involvement vs. none) | 0.027 | 2.122 [1.090–4.130] |
| Homozygosity for the NOD2 p.Leu1007fsX1008 mutation (yes vs. no) | 0.009 | 69.533 [2.945–1641.48] |
Smoking is represented by a single contrast; the outcome variable is "stenosis" (n = 639). Significant p-values are shown in bold numbers.
Table 6. Multiple logistic regression analysis including five important predictors (age at diagnosis, disease duration, smoking status, ileal involvement and homozygosity for the p.Leu1007fsX1008 NOD2 mutation (rs2066847)) on the need for CD-related surgery.
| Variable | p-value | OR (95% CI) |
|---|---|---|
| Age at diagnosis (per 10 years) | 0.013 | 1.309 [1.060–1.617] |
| Disease duration (per 10 years) | <0.001 | 2.793 [2.059–3.789] |
| Smoking status (any smoking versus non-smoking) | 0.117 | 1.487 [0.906–2.440] |
| Disease localization (any ileal involvement vs. none) | 0.213 | 1.571 [0.772–3.196] |
| Homozygosity for the NOD2 p.Leu1007fsX1008 mutation (yes vs. no) | 0.005 | 4.297 [1.557–11.863] |
Smoking is represented by a single contrast; the outcome variable is "CD-related surgery" (n = 629). Significant p-values are shown in bold numbers.
Our analyses confirmed the majority of previously known predictors of intestinal stenoses and CD-related surgery in our study cohort (Tables 5 and 6 and S2 and S3 Tables) but showed particular strong associations between homozygosity for the p.Leu1007fsX1008 NOD2 mutation and stenosis and CD-related surgery, respectively. We demonstrated that the odds ratios for the association of homozygosity for the p.Leu1007fsX1008 NOD2 mutation with stenoses or with CD-related surgery calculated from the models were larger than in univariable analyses and even larger than for all other previously known predictors of stenosis and CD-related surgery shown in Tables 5 and 6 and S2 and S3 Tables. A very strong association was found between NOD2 1007fs homozygosity and intestinal stenoses with an OR > 60 in both models applied (Table 5 and S2 Table). The higher odds ratios in the multivariate analysis (for NOD2 1007f homozygosity) are likely due to the fact that NOD2 1007fs homozygotes were more often non-smokers than NOD2 wildtype carriers (Table 4). The negative association between the two risk factors (homozygosity and smoking) would reduce the apparent strength of their association with stenoses or surgery when either predictor is considered separately.
To rule out an interaction between homozygosity for the NOD2 p.Leu1007fsX1008 mutation and smoking on the presence of stenoses or on the need for CD-related surgery, we performed three-way contingency tables by smoking status, homozygosity for rs2066847 and ileal stenosis (S4A Table) and CD-related surgery (S4B Table). There was no significant interaction for presence of ileal stenoses (p = 1.00) and for the need for CD-related surgery (p = 0.46). Thus, the effects of homozygosity for rs2066847 and smoking on the outcomes (ileal stenoses, CD-related surgery) did not depend on each other and the effect of smoking (increasing the risk of development of CD-related stenoses) was similar in patients with homozygosity for rs2066847 and in other CD patients (S4 Table).
In conclusion, these multivariate analyses strongly support our findings of NOD2 1007fs homozygosity as a major predictor of ileal stenosis and CD-related surgery.
Discussion
The main aim of this study was to evaluate the predictive power of the three major NOD2 mutations in combination with the smoking status regarding a complicated CD phenotype in a large patient cohort. Our study demonstrates that the NOD2 mutation p.Leu1007fsX1008, in particular homozygosity for the minor C allele, is an excellent genetic predictor for a complicated disease course in CD patients. Concomitant homozygosity and active smoking carry a 100% risk for developing ileal stenosis and a 100% risk for CD-related surgery. The very high predictive value of p.Leu1007fsX1008 homozygosity is important for guiding therapeutic decisions, such as the early initiation of an immunosuppressive therapy. This is supported by the fact that all homozygous p.Leu1007fsX1008 carriers, who did not develop ileal stenosis, received immunosuppressive therapy. Since 2001, numerous genetic studies on the IBD susceptibility were performed. However, no other genetic marker was identified as having the same risk effect on CD susceptibility and disease severity in adult Caucasian CD patients like NOD2 [5–23].
To date, our study represents the largest detailed genotype-phenotype analysis of p.Leu1007fsX1008 homozygous CD patients. Our results are in line with a meta-analysis of genome-wide association scans, followed by an extensive validation of significant findings, with a combined total of more than 75,000 cases and controls, which identified a total of 163 IBD loci that met genome-wide significance thresholds [22]. This study confirmed NOD2 as the main genetic marker for CD but did not provide a detailed phenotype analysis of p.Leu1007fsX1008 homozygous CD patients. Similarly, the IBDchip European Project, a retrospective multicenter cohort study, investigated the influence of CD-related SNPs on the clinical course of CD [18]. Among several SNPs included in the analysis, NOD2 was the most important genetic factor, being an independent predictive factor for ileal disease location, stenosing and penetrating disease, and the need for surgery and was as such also the strongest factor associated with a complicated disease course [18]. In our study, all 54 1007fs homozygous patients had ileal involvement, with penetrating disease behaviour in more than two thirds of the patients. The surgery rate was 72.2% with ileocecal resection as the main CD-related surgery in almost 85% of patients. More than one fifth of the patients were 16 years or younger at first diagnosis, with the youngest patients being six years old at the time of CD diagnosis. This demonstrates that homozygosity for p.Leu1007fsX1008 is a strong predictor for a severe CD disease course with an early disease onset. Only seven homozygous patients did not develop intestinal stenoses. They all received immunosuppressive therapy. However, their number is too small to draw final conclusions on the effect of immunosuppressive therapy in this patient subcohort but this observation suggests that the early start of an immunosuppressive therapy in these individuals (median of 14 months after first CD diagnosis) may have prevented the development of stricturing disease.
Our results are supported by the largest genotype-phenotype analysis performed in IBD patients which included a total of almost 30 000 IBD patients. In total, 156154 genetic variants were tested for genotype-phenotype associations while only three loci (NOD2, MHC, and 3p21) were associated with subphenotypes of IBD and NOD2 being strongly associated with a severe CD subphenotype [32].
Importantly, in our subcohort of patients with homozygosity for p.Leu1007fsX1008 and a history of active smoking, we demonstrated a uniform, very severe CD phenotype and an association with the development of intestinal stenoses and the need of CD-related surgery in all patients of this subcohort (100%). Similarly, when the subjects had stopped smoking during follow-up, this still was associated with the development of intestinal stenoses in all cases. To our knowledge, this represents the first detailed report on a strong association of NOD2 p.Leu1007fsX1008 homozygosity in combination with smoking and the subsequent CD disease course. Our findings are strongly supported by the results of a detailed multivariate analysis in which the effect size of 1007fs homozygosity on the development of stenosis and need for CD-related surgery was higher than that of other known predictors of intestinal stenosis and CD-related surgery such as disease duration and smoking status.
Van der Heide et al. suggested an association of p.Arg702Trp (rs2066844) with active smoking in CD and of p.Leu1007fsX1008 and p.Gly908Arg (rs2066845) with non-passive smoking [33]. Further clinical trials showed that smoking is an independent risk factor associated with an unfavorable outcome in CD patients [34–39]. The mechanism by which smoking alters the disease course has not been elucidated yet, but the existing evidence suggests an influence of smoking on the innate and acquired immune system and the intestinal microbiome which is supported by our findings pointing to NOD2 as a major co-variant by which smoking results in intestinal stenosis [34,36,39–43].
Interestingly, despite adequate knowledge on the association of smoking and an unfavorable CD outcome in the majority of CD patients, more than one third of patients (35.5%) are still active smokers [37]. Furthermore, smoking seems to be associated with higher societal costs and lower quality of life in IBD patients [37]. Thereby, smoking cessation may result in a reduced relapse incidence in CD patients compared to the relapse risk in non-smokers and may result in considerably lower societal costs [37,38].
However, the current study has some intrinsic limitations. The high proportion of patients in our CD cohort with “aggressive” CD (defined as stricturing or penetrating disease behaviour), which often requires CD related-surgery, may be also related to a typical referral center population in our specialized IBD center. Furthermore, the high prevalence of NOD2 mutations may be explained by the predominantly Caucasian population in our IBD cohort (99.5% Caucasians). In contrast, the association between NOD2 mutations and CD could not been demonstrated for Asian populations [44,45]. Furthermore, genetic testing focused on the three main NOD2 mutations, while rare NOD2 variants were not addressed in this study which would be possible using other techniques such as direct DNA sequencing techniques or genome-wide association scans (GWAS). Therefore, compound heterozygosity of NOD2 with other risk alleles was not addressed in this current study.
In conclusion, despite growing insights into the genetic background of IBD, NOD2 is still the most important genetic risk factor for CD. Our results demonstrate that in particular the NOD2 frameshift mutation p.Leu1007fsX1008 is a strong predictor for a complicated CD course, which requires an early medical intervention in order to prevent CD-related complications. The risk for complicated CD was particularly pronounced in homozygous carriers of the p.Leu1007fsX1008 mutation with a history of active smoking who all developed ileal stenoses and all required CD-related surgery. Given the strong predictive power of active smoking and 1007fs homozygosity for ileal stenosis and CD-related surgery, this NOD2 variant is a very useful biomarker for clinical and therapeutic decision making on an individual patient level. Our findings are an important step for initiating a more personalized therapy in CD and delivering precision medicine to these patients [19].
Supporting information
For each variable, the number of patients included is given. 1 Disease behaviour was defined according to the Montreal classification [25]. A stricturing disease phenotype was defined as the presence of stenoses without penetrating disease. The diagnosis of stenoses was made surgically, endoscopically, or radiologically (using MR enteroclysis). 2 Clinical course of CD was furthermore defined as “aggressive” in CD patients with a stricturing and/or penetrating disease behaviour and/or when CD-related surgery became necessary. Accordingly, a “mild” CD phenotype was defined as non-stricturing, non-fistulizing CD without CD-related surgery. 3 Immunosuppressive agents included azathioprine, 6-mercaptopurine, methotrexate, infliximab and/or adalimumab. 4 Only surgery related to CD-specific problems (e.g. fistulectomy, colectomy, ileostomy) was included.
(DOCX)
Smoking is represented by two contrasts; the outcome variable is "stenosis".
(DOCX)
Smoking is represented by two contrasts; the outcome variable is "CD-related surgery".
(DOCX)
Three-way contingency tables by smoking status, homozygosity for rs2066847 and (A) ileal stenosis and (B) need for CD-related surgery.
(DOCX)
Acknowledgments
We thank Dr. Jürgen Glas, Department of Preventive Dentistry and Periodontology, Ludwig-Maximilians-University (LMU) Munich, Munich, Germany, for technical support regarding the NOD2 genotyping and Dr. Sabine Güsewell, Clinical Trial Unit, Kantonsspital St. Gallen, Switzerland, for technical support with the statistical analyses.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
S. Brand was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (BR 1912/6-1) and the Else-Kröner-Fresenius-Stiftung (Else Kröner-Exzellenzstipendium 2010_EKES.32). F. Beigel was supported by the DFG (BE 4490/2-1). C. Tillack was supported by a grant of the Ludwig-Maximilians-University Munich (FöFoLe program). T. Olszak was supported by a grant of the German Research Council (Deutsche Forschungsgemeinschaft / DFG, OL/324-1).
References
- 1.Hugot JP, Laurent-Puig P, Gower-Rousseau C, Olson JM, Lee JC, Beaugerie L, et al. Mapping of a susceptibility locus for Crohn's disease on chromosome 16. Nature. 1996;379(6568):821–3. 10.1038/379821a0 [DOI] [PubMed] [Google Scholar]
- 2.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411(6837):599–603. 10.1038/35079107 [DOI] [PubMed] [Google Scholar]
- 3.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411(6837):603–6. 10.1038/35079114 [DOI] [PubMed] [Google Scholar]
- 4.Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, et al. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet. 2001;357(9272):1925–8. 10.1016/S0140-6736(00)05063-7 [DOI] [PubMed] [Google Scholar]
- 5.Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nature genetics. 2010;42(12):1118–25. 10.1038/ng.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International HapMap C, Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449(7164):851–61. 10.1038/nature06258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seiderer J, Schnitzler F, Brand S, Staudinger T, Pfennig S, Herrmann K, et al. Homozygosity for the CARD15 frameshift mutation 1007fs is predictive of early onset of Crohn's disease with ileal stenosis, entero-enteral fistulas, and frequent need for surgical intervention with high risk of re-stenosis. Scandinavian journal of gastroenterology. 2006;41(12):1421–32. 10.1080/00365520600703900 [DOI] [PubMed] [Google Scholar]
- 8.Seiderer J, Brand S, Herrmann KA, Schnitzler F, Hatz R, Crispin A, et al. Predictive value of the CARD15 variant 1007fs for the diagnosis of intestinal stenoses and the need for surgery in Crohn's disease in clinical practice: results of a prospective study. Inflammatory bowel diseases. 2006;12(12):1114–21. 10.1097/01.mib.0000235836.32176.5e [DOI] [PubMed] [Google Scholar]
- 9.Jurgens M, Brand S, Laubender RP, Seiderer J, Glas J, Wetzke M, et al. The presence of fistulas and NOD2 homozygosity strongly predict intestinal stenosis in Crohn's disease independent of the IL23R genotype. Journal of gastroenterology. 2010;45(7):721–31. 10.1007/s00535-010-0231-7 [DOI] [PubMed] [Google Scholar]
- 10.Pearson TA, Manolio TA. How to interpret a genome-wide association study. JAMA: the journal of the American Medical Association. 2008;299(11):1335–44. 10.1001/jama.299.11.1335 [DOI] [PubMed] [Google Scholar]
- 11.Manolio TA. Genomewide association studies and assessment of the risk of disease. The New England journal of medicine. 2010;363(2):166–76. 10.1056/NEJMra0905980 [DOI] [PubMed] [Google Scholar]
- 12.Radlmayr M, Torok HP, Martin K, Folwaczny C. The c-insertion mutation of the NOD2 gene is associated with fistulizing and fibrostenotic phenotypes in Crohn's disease. Gastroenterology. 2002;122(7):2091–2. [DOI] [PubMed] [Google Scholar]
- 13.Annese V, Lombardi G, Perri F, D'Inca R, Ardizzone S, Riegler G, et al. Variants of CARD15 are associated with an aggressive clinical course of Crohn's disease—an IG-IBD study. The American journal of gastroenterology. 2005;100(1):84–92. 10.1111/j.1572-0241.2005.40705.x [DOI] [PubMed] [Google Scholar]
- 14.Vavassori P, Borgiani P, Biancone L, D'Apice MR, Blanco Gdel V, Vallo L, et al. CARD15 mutation analysis in an Italian population: Leu1007fsinsC but neither Arg702Trp nor Gly908Arg mutations are associated with Crohn's disease. Inflammatory bowel diseases. 2004;10(2):116–21. 10.1097/00054725-200403000-00009 [DOI] [PubMed] [Google Scholar]
- 15.Lakatos PL, Lakatos L, Szalay F, Willheim-Polli C, Osterreicher C, Tulassay Z, et al. Toll-like receptor 4 and NOD2/CARD15 mutations in Hungarian patients with Crohn's disease: phenotype-genotype correlations. World journal of gastroenterology: WJG. 2005;11(10):1489–95. 10.3748/wjg.v11.i10.1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuthbert AP, Fisher SA, Mirza MM, King K, Hampe J, Croucher PJ, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology. 2002;122(4):867–74. 10.1053/gast.2002.32415 [DOI] [PubMed] [Google Scholar]
- 17.Lesage S, Zouali H, Cezard JP, Colombel JF, Belaiche J, Almer S, et al. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. American journal of human genetics. 2002;70(4):845–57. 10.1086/339432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleynen I, Gonzalez JR, Figueroa C, Franke A, McGovern D, Bortlik M, et al. Genetic factors conferring an increased susceptibility to develop Crohn's disease also influence disease phenotype: results from the IBDchip European Project. Gut. 2013;62(11):1556–65. 10.1136/gutjnl-2011-300777 [DOI] [PubMed] [Google Scholar]
- 19.Brand S. Moving the genetics of inflammatory bowel diseases from bench to bedside: first steps towards personalised medicine. Gut. 2013;62(11):1531–3. 10.1136/gutjnl-2012-304151 [DOI] [PubMed] [Google Scholar]
- 20.McGovern DP, Gardet A, Torkvist L, Goyette P, Essers J, Taylor KD, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nature genetics. 2010;42(4):332–7. 10.1038/ng.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nature genetics. 2008;40(8):955–62. 10.1038/ng.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momozawa Y, Dmitrieva J, Theatre E, Deffontaine V, Rahmouni S, Charloteaux B, et al. IBD risk loci are enriched in multigenic regulatory modules encompassing putative causative genes. Nat Commun. 2018;9(1):2427 10.1038/s41467-018-04365-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lennard-Jones JE. Classification of inflammatory bowel disease. Scandinavian journal of gastroenterology Supplement. 1989;170:2–6; discussion 16–9. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2005;19 Suppl A:5A–36A. [DOI] [PubMed] [Google Scholar]
- 26.Schnitzler F, Brand S, Staudinger T, Pfennig S, Hofbauer K, Seiderer J, et al. Eight novel CARD15 variants detected by DNA sequence analysis of the CARD15 gene in 111 patients with inflammatory bowel disease. Immunogenetics. 2006;58(2–3):99–106. 10.1007/s00251-005-0073-2 [DOI] [PubMed] [Google Scholar]
- 27.Schnitzler F, Friedrich M, Wolf C, Angelberger M, Diegelmann J, Olszak T, et al. The NOD2 p.Leu1007fsX1008 mutation (rs2066847) is a stronger predictor of the clinical course of Crohn's disease than the FOXO3A intron variant rs12212067. PloS one. 2014;9(11):e108503 10.1371/journal.pone.0108503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glas J, Seiderer J, Tillack C, Pfennig S, Beigel F, Jurgens M, et al. The NOD2 single nucleotide polymorphisms rs2066843 and rs2076756 are novel and common Crohn's disease susceptibility gene variants. PloS one. 2010;5(12):e14466 10.1371/journal.pone.0014466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81(3):559–75. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelman AJ, Grazia Pittau M and Sungsu Y. A weakly informative default prior distribution for logistic and other regression Models. The Annals of Applied Statistics 2. 2008;2:1360–83. [Google Scholar]
- 31.Schnitzler F, Seiderer J, Stallhofer J, Brand S. Dominant disease-causing effect of NOD2 mutations in a family with all family members affected by Crohn's disease. Inflammatory bowel diseases. 2012;18(2):395–6. 10.1002/ibd.21882 [DOI] [PubMed] [Google Scholar]
- 32.Cleynen I, Boucher G, Jostins L, Schumm LP, Zeissig S, Ahmad T, et al. Inherited determinants of Crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016. January 9;387(100014):156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Heide F, Nolte IM, Kleibeuker JH, Wijmenga C, Dijkstra G, Weersma RK. Differences in genetic background between active smokers, passive smokers, and non-smokers with Crohn's disease. The American journal of gastroenterology. 2010;105(5):1165–72. 10.1038/ajg.2009.659 [DOI] [PubMed] [Google Scholar]
- 34.Parkes GC, Whelan K, Lindsay JO. Smoking in inflammatory bowel disease: Impact on disease course and insights into the aetiology of its effect. Journal of Crohn's & colitis. 2014;8(8):717–25. [DOI] [PubMed] [Google Scholar]
- 35.To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: the adverse effects of tobacco smoking on the natural history of Crohn's disease. Aliment Pharmacol Ther. 2016;43(5):549–61. 10.1111/apt.13511 [DOI] [PubMed] [Google Scholar]
- 36.Chivese T, Esterhuizen TM, Basson AR, Watermeyer G. The influence of second-hand cigarette smoke exposure during childhood and active cigarette smoking on Crohn's disease phenotype defined by the Montreal classification scheme in a Western Cape population, South Africa. PLoS One. 2015;10(9):e0139597 10.1371/journal.pone.0139597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Severs M, van Erp SJ, van der Valk ME, Mangen MJ, Fidder HH, van der Have M, et al. Smoking is associated with extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2016;10(4):455–61. 10.1093/ecco-jcc/jjv238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nunes T, Etchevers MJ, Garcia-Sanchez V, Ginard D, Marti E, Barreiro-de Acosta M, et al. Impact of smoking cessation on the clinical course of Crohn's disease under current therapeutic algorithms: A multicenter prospective study. Am J Gastroenterol. 2016;111(3):411–9. 10.1038/ajg.2015.401 [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Lee A, Bowcock A, Zhu W, Li E, Ciorba M, et al. Influence of Crohn's disease risk alleles and smoking on disease location. Dis Colon Rectum. 2011;54(8):1020–5. 10.1007/DCR.0b013e31821b94b3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Wang Y, Shen J. Role of environmental factors in the pathogenesis of Crohn's disease: a critical review. Int J Colorectal Dis. 2019;34(12):2023–34. 10.1007/s00384-019-03441-9 [DOI] [PubMed] [Google Scholar]
- 41.Berkowitz L, Pardo-Roa C, Salazar GA, Salazar-Echegarai F, Miranda JP, Ramirez G, et al. Mucosal exposure to cigarette components induces intestinal inflammation and alters antimicrobial response in mice. Front Immunol. 2019;10:2289 10.3389/fimmu.2019.02289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindoso L, Mondal K, Venkateswaran S, Somineni HK, Ballengee C, Walters TD, et al. The effect of early-life environmental exposures on disease phenotype and clinical course of Crohn's disease in children. Am J Gastroenterol. 2018;113(10):1524–9. 10.1038/s41395-018-0239-9 [DOI] [PubMed] [Google Scholar]
- 43.Opstelten JL, Plassais J, van Mil SW, Achouri E, Pichaud M, Siersema PD, et al. Gut microbial diversity Is reduced in smokers with Crohn's disease. Inflamm Bowel Dis. 2016;22(9):2070–7. 10.1097/MIB.0000000000000875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirano A, Yamazaki K, Umeno J, Ashikawa K, Aoki M, Matsumoto T, et al. Association study of 71 European Crohn's disease susceptibility loci in a Japanese population. Inflamm Bowel Dis. 2013;19(3):526–33. 10.1097/MIB.0b013e31828075e7 [DOI] [PubMed] [Google Scholar]
- 45.Pugazhendhi S, Santhanam S, Venkataraman J, Creveaux I, Ramakrishna BS. NOD2 gene mutations associate weakly with ulcerative colitis but not with Crohn's disease in Indian patients with inflammatory bowel disease. Gene. 2013;512(2):309–13. 10.1016/j.gene.2012.10.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For each variable, the number of patients included is given. 1 Disease behaviour was defined according to the Montreal classification [25]. A stricturing disease phenotype was defined as the presence of stenoses without penetrating disease. The diagnosis of stenoses was made surgically, endoscopically, or radiologically (using MR enteroclysis). 2 Clinical course of CD was furthermore defined as “aggressive” in CD patients with a stricturing and/or penetrating disease behaviour and/or when CD-related surgery became necessary. Accordingly, a “mild” CD phenotype was defined as non-stricturing, non-fistulizing CD without CD-related surgery. 3 Immunosuppressive agents included azathioprine, 6-mercaptopurine, methotrexate, infliximab and/or adalimumab. 4 Only surgery related to CD-specific problems (e.g. fistulectomy, colectomy, ileostomy) was included.
(DOCX)
Smoking is represented by two contrasts; the outcome variable is "stenosis".
(DOCX)
Smoking is represented by two contrasts; the outcome variable is "CD-related surgery".
(DOCX)
Three-way contingency tables by smoking status, homozygosity for rs2066847 and (A) ileal stenosis and (B) need for CD-related surgery.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.