Abstract
Background:
Despite proven benefits of isoniazid preventive therapy (IPT) for people living with HIV (PLHIV), its implementation remains limited in low-resource settings. There are also programmatic concerns of the completion rate of IPT particularly when full integration with other HIV services has not been achieved.
Aim:
The aim of this study was to determine the completion rate of IPT and predictive factors among PLHIV attending six government hospitals in Kebbi state, Northern Nigeria.
Methods:
This was a retrospective cohort study of program data spanning a 5-year period (December 2010–June 2016). Data were collected between January 2017 and June 2017.
Results:
A total of 1,134 IPT patients were enrolled of whom 740 (65.3%) were female. The mean age was 40.3 ± 3.7 years. Four hundred and fifty-four (40%) of those who initiated IPT completed the 6-month course. Of the 680 (60%) IPT noncompleters, 117 (17.2%) were lost to follow-up by month 1, 305 (44.9%) by month 2, 156 (22.9%) by month 3, 48 (7.1%) by month 4, and 54 (7.9%) by month 5. Being initiated on IPT by a pharmacist (adjusted odds ratio [aOR]: 23.7, 95% confidence interval [CI]: 16.5–33.9) and receiving ≤2 tuberculosis screening evaluation during IPT period (aOR: 0.58, 95% CI: 0.43–0.78) were associated with a higher and lower risk of completing IPT, respectively, whereas age, sex, and anti-retroviral therapy (ART) status were not significantly associated.
Conclusion:
IPT completion rate among PLHIV is relatively low, highlighting the need to strengthen IPT rollout in public health facilities in Nigeria. Pharmacy-led IPT adherence education and regular clinical evaluation may improve IPT completion rates, along with synchronizing and prepackaging IPT and ART resupplies for PLHIV.
Keywords: Human immunodeficiency virus, isoniazid preventive therapy, people living with HIV
BACKGROUND/RATIONALE
Tuberculosis (TB) is the leading cause of morbidity and mortality among people living with HIV (PLHIV) in developing countries. In 2017, TB was responsible for 37% of deaths among PLHIV.[1] The World Health Organization recommended 300 mg daily isoniazid (INH) preventive therapy (IPT) for 6 months as a proven strategy for the prevention and treatment of latent TB among PLHIV. Adherence and completion of the full course of IPT are important determinants of clinical benefits of IPT to patients and a critical indicator to measure the performance of the National TB Program and End TB Strategy. Several studies have documented the effectiveness and benefits of taking the full course of IPT in reducing the risk of TB and re-activation of latent TB in both developed and developing countries.[2,3,4] In Ethiopia, the use of IPT led to a 50% reduction in TB cases when compared with non-IPT group, whereas in Indonesia, IPT administration led to a 79% reduction in the risk of active TB among PLHIV.[5,6] Despite the proven effectiveness of IPT, its utilization has been suboptimal among PLHIV, especially in low-income countries. Studies from Botswana and Uganda also expressed concerns about adherence and treatment completion rate of IPT among PLHIVs.[7,8,9]
There are several barriers to IPT implementation, limiting completion of the full course of IPT. IPT completion rate appears to vary across settings, subpopulations, country policy guidelines on duration of treatment, availability of incentives, and whether IPT is provided alone or prepackaged with ART. For instance, studies from Zambia, Kenya, and Ethiopia concluded that under programmatic implementation, combination of IPT with additional package of interventions such as incentives for patient travel reimbursement, training of health-care workers, provision of Information, Education and Communication (IEC) materials and Standard Operating Procedure (SOPs), quality adherence counseling by physicians, patient follow-up, and continuous quality improvement have higher completion rate,[3,10,11,12,13] compared with implementation not combined with the above package of interventions.[14]
A systematic review of interventions to improve the adherence and completion of IPT concluded that completion rate was not only higher (82%; 66%–95%, confidence interval [CI]: 95%) among PLHIV and contacts compared with prisoners and immigrants (22%; 6%–43%, CI: 95%) but also varied inversely with the duration on IPT.[15] Higher completion rate was reported with a 3-month treatment regimen compared with a 9-month regimen.[15,16] The National TB Program in Nigeria recommends systematic TB screening of PLHIV using a 4-symptom clinical screening algorithm at every visit followed by the Genexpert test for symptomatic PLHIV.[17] The number of PLHIV placed on IPT has increased from 27,000 in 2006 to more 1.3 million PLHIV started in 2016.[1] Despite the progress, 18 of the 30 high TB/HIV burden countries did not report IPT among people newly enrolled in HIV care in 2016.[1] Data on the feasibility and completion rate of IPT, as well as predictive factors, are limited in Nigeria. One study in Nigeria reported a completion rate of 63%, but this was only by the 2nd month of treatment.[18] Another study from Northern Nigeria reported a higher completion rate of 93% among PLHIV on ART compared with 80% among Pre-ART.[19] None of these studies evaluated predictive factors influencing the completion of IPT among HIV-infected population when implemented under programmatic settings.
Objective
To inform the National TB Program in Nigeria about the feasibility of continued scale-up of IPT to other public ART clinics countrywide, we attempt to determine (1) the completion rates for a full course of IPT among PLHIV initiated on a 6-month IPT regimen and (2) predictive factors for completion of IPT.
METHODS
Study design
This retrospective cohort study aimed to assess IPT completion rate and predictive factors among PLHIV commenced on IPT across six government hospitals (5 secondary and 1 tertiary) in Kebbi state in Northern Nigeria between December 2010 and December 2016.
Setting
A cross-sectional study was conducted in six hospitals in Kebbi state, Nigeria, from January 1 to June 30, 2017. The hospitals include five secondary and one tertiary hospital in Kebbi state. The tertiary hospital provides specialized medical services, whereas the remaining five provide secondary services. They all provide services to a combined population of more than one million people in Kebbi state. The hospitals have also been designated to provide comprehensive HIV services, including Prevention of Mother to Child Transmission of HIV (PMTCT), TB/HIV, adult, and pediatric HIV care, support, and treatment services with the support of the United States Agency for International Development since 2007. The hospitals have also enrolled more than 10,000 patients in HIV care among which more than 7000 have been enrolled on ART and the remaining on pre-ART during the study period.
The hospitals operate a mix of models for IPT prescription, adherence education, and follow-up. In four of the hospitals, IPT is dispensed by the pharmacist after prior evaluation by physicians and ART. However, eligible patients are sometimes not placed on IPT by physicians due to workload, and in this case, the pharmacist may prescribe, and dispense IPT to such patients. IPT was given on a monthly basis, and participants who did not have documented evidence of making all six clinic visits to refill IPT due to loss to follow-up (LTFU), active TB, or having stopped treatment for any other reason were classified as not having completed IPT. Home tracing for participants lost to follow-up was not part of the routine IPT implementation. In these hospitals, the prescription of IPT was done separately from and not integrated with ART, such that patients receiving ART have to come for a second clinic within the same hospital for their repeat IPT prescriptions and final follow-up on months 1, 2, 3, 4, 5, and 6. IPT follow-up visits were rarely harmonized with other HIV care clinic appointments, but in many cases, different follow-up visits were necessary. Adherence to IPT was monitored through monthly prescription refill records in ART care card, IPT register, and pharmacy order form. During IPT clinic visits, patients were asked for symptoms of TB, investigated if symptomatic, and assessed for adverse events. Patients who miss 2 months or more of IPT refill visits are regarded as lost to follow-up and restarted when back in care. However, for those who miss <2 months of refill visits, prescription continues from the last refill without any need to restart according to the national guideline. Data for the study were collected by trained research assistants using predesigned data collection templates.
Participants
Our source population was HIV-infected individuals enrolled in HIV care and treatment between December 2010 and June 2016. The study participants are a subset of the source population and include adult PLHIV above 15 years old enrolled in HIV care and initiated on IPT between the periods. Exclusion criteria include incomplete documentation of IPT commencement and end date in ART care card, pharmacy order form, or the IPT register. Other exclusion criteria include the development of active TB during treatment (<10 patients), past history of TB, on treatment for TB, or having initiated IPT treatment <6 months before the study period. Individuals whose IPT commencement date was not within 6 months before the commencement of the study or younger than 15 years were excluded from the study. Patients agreeable to monthly IPT collection, with no signs and symptoms of active TB, and no clinical contraindications to INH were eligible and offered a 6-month IPT course according to the national guideline. Patients were ineligible for IPT if they had a previous history of alcohol abuse and kidney and/or liver abnormalities.[17] Routine TST was not required to screen for IPT eligibility in Nigeria.[17] All patients on IPT register between December 2010 and June 2016 were included in the analysis. Data collection took place between January and June 2017.
Variables
The dependent variable is the duration on IPT which is calculated by the difference between IPT start date and completion date (calculated from the number of IPT tablets prescribed as at last pickup date). The independent variables include sociodemographics and clinical characteristics such as age and sex of the patient, IPT start and completion date, adverse reaction to IPT, number of TB screening during IPT period, ART status during IPT initiation as well as LTFU status while on ART during IPT period.
Data sources/measurement
Relevant data (dependent and independent variables) were extracted into the data collection form. Adherence to ART was assessed from documents containing information on patient self-reported adherence and pill count data from returned, unused medication. Patient information such as age, sex, IPT start date, number of TB screening, adverse reaction to IPT, and ART status during and after IPT were extracted from ART care card, whereas the source of information for IPT completion and LTFU status was the IPT register. In this study, PLHIV who have taken IPT for 6 months or more are regarded as IPT completers, whereas a patient with documented evidence of <6 months of IPT intake is regarded as IPT non-completers.
Bias
The data collected from these aforementioned sources were also triangulated and compared with pharmacy order form to check for correctness and completeness of information captured with the data collection template. Where there are discrepancies with the ART care card and/or IPT register, the information in the pharmacy order form (IPT prescription form) is taken as a source and documented in the data collection template.
Study size
We utilized a record of PLHIV who were enrolled in care across six hospitals between December 2010 and June 2016 which were 10,321 in number.
Statistical methods
Data were entered into Microsoft Excel and imported into IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp. Data cleaning was done before analysis. The analysis contains a summary of patient sociodemographic and clinical characteristics. Bivariate and multivariate analyses were conducted to determine the relationship between independent and dependent variables (IPT completion). Only explanatory variables that were significantly associated with outcome variable (IPT completion), i.e., P < 0.05 on bivariate analysis, were included in logistic regression model to determine the independent predictors of IPT completion.
RESULTS
Participants
A total of 10,321 PLHIV were enrolled in care between December 2010 and June 2016, out of which 1,330 were excluded as being below the age of 15 years, whereas additional 380 were excluded due to missing demographic information. Of the remaining 8,991 patients, 7857 were excluded as a result of being diagnosed for TB (1,200) or having developed TB while on IPT (9) or having missing IPT data (6648). A total of 1134 patients were finally found to be eligible for the study as in the below flow chart [Figure 1].
Figure 1.
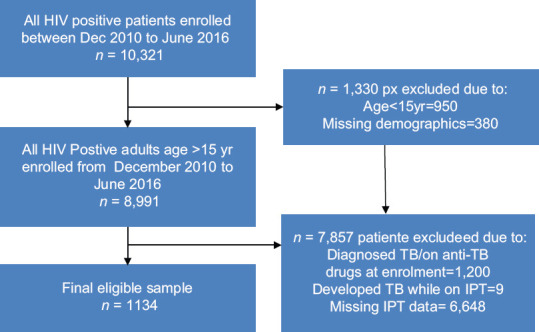
Flowchart showing the recruitment of the study population and arrival at sample size
Descriptive data
Table 1 shows the sociodemographic and clinical characteristics of patients placed on IPT between December 2010 and June 2016. Majority of the total respondents (740, 65.3%) were female. The mean age (±standard deviation) of the study population was 40.3 ± 3.7 years. Of the 1134 PLHIV on IPT, majority (73.6%, 835) were 25–49 years, mostly initiated by physicians (45.7%, 518/1134). Most of the patients (51.2%, 581/1134) were clinically screened for TB thrice during the IPT period. About 88.3% (1001/1134) were on ART, whereas 11.7% (133/1134) were pre-ART. Among 1001 patients on ART, 90.3% (903) were active on ART, whereas 9.7% (97) were LTFU on ART during the study period. Nearly 94.2% (1068/1134) of the patients initiated IPT within 72 h of initial clinical TB screening to rule out active TB, whereas 66 (5.8%) eligible patients were initiated >72 h later.
Table 1.
Basic sociodemographic and clinical characteristics of the retrospective survey of people living with human immunodeficiency virus placed on isoniazid preventive therapy at six government hospitals between December 2010 and December 2016, Kebbi state, Nigeria
| Variable | n (%) |
|---|---|
| Age (years) | |
| 15-19 | 56 (4.9) |
| 20-24 | 161 (14.2) |
| 25-49 | 835 (73.6) |
| Sex | |
| Male | 394 (34.7) |
| Female | 740 (65.3) |
| HCW initiating IPT | |
| Doctor | 518 (45.7) |
| ART nurse | 188 (16.6) |
| Number of TB screening while on IPT | |
| None | 62 (5.5) |
| One | 199 (17.5) |
| Two | 243 (21.4) |
| Three | 581 (51.2) |
| Four | 41 (3.6) |
| Five | 8 (0.7) |
| On ART | |
| No | 133 (11.7) |
| Yes | 1001 (88.3) |
| ART LTFU | |
| No | 903 (90.3) |
| Yes | 97 (9.7) |
| Duration on IPT (months) | |
| <6 (noncompleters) | 680 (60) |
| ≥6 (completers) | 454 (40) |
| Time to IPT initiation (h) | |
| <72 after… | 1068 (94.2) |
| ≥72 | 66 (5.8) |
IPT: Isoniazid preventive therapy, PLHIV: People living with human immunodeficiency virus, LTFU: Lost to follow-up, ART: Antiretroviral therapy, HCW: Health-care worker, TB: Tuberculosis
Outcome data
Figure 2 shows that the number of months IPT was received before stoppage among IPT noncompleters. Of the 1134 PLHIV who started IPT, the 6-month IPT completion rate was 40% (454/1134) and 680 (60%) did not complete 6 months of IPT. Of the 680 who did not complete 6 months of IPT, 117 (17.2%) stopped treatment after receiving a 1 month of supply of drugs in their first visit, majority (305, 44.9%) stopped after receiving 2 months of supply, 156 (22.9%) stopped after receiving 3 months of supply, 48 (7.1%) stopped after receiving 4 months, whereas only 54 (7.9%) made it to month 5 before stopping IPT treatment. Table 2 shows the IPT outcome by category of health-care workers who initiated IPT. Ninety-six percent (413/428) of the IPT completers were pharmacist initiated, 8% were physician initiated, and 0% nurse initiated.
Figure 2.
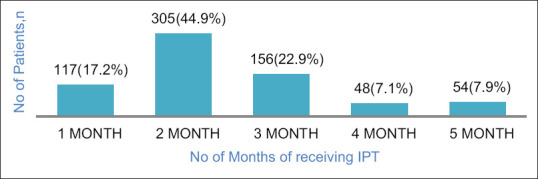
Number of months of isoniazid preventive therapy treatment collected by patients who failed to complete isoniazid preventive therapy
Table 2.
Isoniazid preventive therapy outcome by category of health-care worker initiating isoniazid preventive therapy
| HCW initiating IPT | Number of PLHIV initiated on IPT | IPT completers (%) | IPT noncompleters |
|---|---|---|---|
| Physicians | 518 | 41 (8) | 477 (92) |
| Nurse | 188 | 0 (0) | 188 (100) |
| Pharmacy | 428 | 413 (96) | 15 (4) |
IPT: Isoniazid preventive therapy, HCW: Health-care worker, PLHIV: People living with human immunodeficiency virus
Having adjusted for potential confounders (age, sex, ART status, etc.), the predictive factors associated with IPT completion are shown in Table 3. On both bivariate and multivariate analyses, factors associated with a higher IPT completion include having IPT initiated by a pharmacist (adjusted odds ratio [aOR]: 23.7, 95% CI: 16.5–33.9). In addition, having tw o or less TB screening during the course of IPT (aOR: 0.58, 95% CI: 0.43–0.78) was associated with a lower chance of IPT completion compared with those with more than two TB screening during the IPT period. Age, sex, ART status as well as ART LTFU status were not significantly associated with IPT completion.
Table 3.
Independent risk factors predicting completion of isoniazid preventive therapy among people living with human immunodeficiency virus in Kebbi State, Nigeria from December 2010 to December 2016
| Variable | Noncompleters <6 months | Completers >6 months | cOR | P | cOR |
|---|---|---|---|---|---|
| Age (years) | |||||
| <25 | 132 (60.8) | 85 (39.2) | 1.1 (0.77-1.42) | 0.772 | |
| ≥25 | 548 (59.8) | 369 (40.2) | Reference | ||
| Sex | |||||
| Male | 239 (60.7) | 155 (39.3) | 1.05 (0.81-1.34) | 0.727 | |
| Female | 441 (59.6) | 299 (40.4) | Reference | ||
| HCW cadre initiating IPT | |||||
| Doctors | 477 (92.1) | 41 (7.9) | Reference | <0.05 | Reference |
| Pharmacist | 203 (33.0) | 413 (67) | 23.7 (16.5-33.9) | 20.4 (14.2-29.5) | |
| Number of TB evaluation during follow-up visits | |||||
| Twice or less | 243 (48.2) | 261 (51.8) | 0.41 (0.32-0.52) | <0.05 | 0.58 (0.43-0.78) |
| More than twice | 437 (69.4) | 193 (30.6) | Reference | Reference | |
| ART LTFU status | |||||
| Yes | 584 (57.0) | 319 (43.0) | 1.16 (0.6-1.70) | 0.442 | |
| No | 84 (87.3) | 13 (12.7) | Reference | ||
| ART status | |||||
| Not on ART | 84 (63.2) | 49 (36.8) | 1.16 (0.8-1.7) | 0.424 | |
| On ART | 596 (59.5) | 405 (40.5) | Reference |
IPT: Isoniazid preventive therapy, LTFU: Lost to follow-up, ART: Antiretroviral therapy, HCW: Health-care worker, TB: Tuberculosis, OR: Odds ratio cOR: Crude Odds Ratio
DISCUSSION
The objective of the study was to determine IPT completion rate and predictive factors among adults living with HIV assessing IPT across six government hospitals in Kebbi state, Northern Nigeria. In this study, the IPT completion rate was 40%. Age, sex as well as ART status were not significant determinants of IPT completion, whereas pharmacist-initiated IPT and having more than 2 sessions of TB evaluation in follow-up clinic were significant predictors of IPT completion. Similar to our findings, a study in Tanzania reported that age was associated with IPT completion, but sex was not. This, however, contrasts with other studies where age <30 years and female sex were associated with a higher risk of IPT completion.[7,12] Our finding is also similar to Ethiopia study[20,21] but contrast with findings from Brazil and Zimbabwe where being on ART is associated with a higher IPT completion rate.[9,10]
The IPT completion rate of 40% in this study is higher than 33.6% completion rate reported in Uganda[7] but quite lower than completion rates of 81% and 86.1% reported in Zimbabwe and Malaysia, respectively.[9,21] The lower completion rate in our study could possibly be due to lack of synchronization of IPT with other HIV clinic appointments which could have increased the frequency of visits of patients. Studies in Malaysia, Zimbabwe, and Ethiopia found that co-administration of IPT with ART significantly reduced the incidence of IPT noncompletion.[9,20,21] Earlier, a qualitative study in Ethiopia concluded that integration of health system services was important factor to improve adherence and IPT completion rate[22] A prospective study in South Africa evaluating cohort of TB treatment-experienced HIV-infected patients in ART visits found a higher IPT completion rate of 86.8% when IPT visits were successfully integrated with existing ART visits.[23]
Pharmacist-initiated IPT delivery led to higher (twenty times) IPT completion rate compared with PLHIV initiated by physicians. A Tanzania study reported a 13% overall IPT noncompletion rate with 33% of noncompletion being physician initiated.[12] Our study shows that PLHIV exposed to fewer sessions of TB evaluation/screening (less than twice) during the IPT period were less likely to complete IPT compared with those exposed to multiple TB evaluation in follow-up clinics. This finding could mean that the low completion rate for physician-led IPT was a result of poor adherence counseling and patient education during the IPT period. Following a qualitative interview assessing barriers to IPT implementation among physicians managing ART clinics in Nairobi, Kenya, physicians reported that counseling patients on IPT would increase the waiting time and compromise health services for other patients.[24] Studies from Ethiopia, Kenya, and Zambia further emphasized the association of poor quality of physician interpersonal skills with IPT noncompletion adding that PLHIV receiving explanation about IPT adherence from health-care workers are more than seven times likely to adhere to and complete IPT.[20,24,25]
Furthermore, findings from studies in the United States and Turkey provided additional evidence regarding the impact of pharmacy-led patient education and monitoring on IPT completion. In these studies, patients receiving pharmacy-delivered IPT education and continuous monitoring were compared with those initiated by other health-care workers.[26,27,28,29] All the studies demonstrated higher medication adherence and IPT completion rate of 67%, 74%, and 93% from South California, Turkey, and Los Angelis, respectively. The completion rate among PLHIV exposed to pharmacy-initiated IPT in our study is 96% which is higher than studies from South Carolina and Turkey but comparable to that from Los Angelis. This higher completion for physician-initiated IPT may possibly be due to better documentation and patient-centered approaches of pharmacists. In addition, the study from Turkey utilized a more objective methodology that combined both qualitative and quantitative (urine IPT) assessments of IPT adherence, whereas many of the patients in Southern California study were foreign-born university students which may explain why the completion rates are lower than our study which relied on clinic attendance, self-reported adherence, and pill count.[25,26,27]
Our study has some limitations. First, the findings of the study must be interpreted and generalized with caution since the study took place in Northern Nigeria where the quality of health services is generally poorer and health indicators perform lower than what is available in the southern part of the country. Furthermore, additional variables such as ethnicity, religion, and marital status which have an influence on adherence were not measured in this study. The use of self-reported adherence, clinical attendance, and pill count are imperfect proxy measures of adherence and prone to errors. Providers could also forget to document pill intake when the patient has actually taken the medication resulting in possible underestimation of IPT completion rate. Despite the limitations, the study findings have implication for policy and practice. The result underlines the human resource challenges in many ART clinics and many other service delivery units in low-income countries where doctor–patient ratio is wide and a single doctor attends to hundreds of patients per day including those on ART. The study then provides evidence on need to monitor the implementation of the WHO's task shifting/sharing strategy adopted by Nigeria in 2006. The implementation will allow pharmacists to take a more involved role in the evaluation of patients for IPT eligibility, adherence counseling, patient education, prescription, and follow-up. As majority of all losses occurred within the first 2 months of treatment, this suggests the need to improve the initial adherence counseling and patient education on the importance of remaining adherent to IPT.
CONCLUSION
Our study indicates that pharmacist-initiated patient education and IPT treatment monitoring increase adherence to prescribed IPT treatment regimens and treatment completion rates. These results suggest that health systems and hospitals have an opportunity to expand the role of pharmacists in the care of PLHIV with latent TB infection. The National TB Program needs to adopt and scale up innovative approaches integrating and synchronizing IPT and ART visits through prepackaging of the two medications. This could minimize the frequency of clinic visit for PLHIVs. There is also a need for further research in the form of randomized, controlled trials in order to demonstrate the outcome-based value of pharmacist-initiated patient education and monitoring in terms of disease-specific goals of therapy such as reduction in TB incidence and mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. HIV Associated Tuberculosis Achievements in 2016. World Health Organization; 2017. Available from: Available from http://wwwwhoint/tb/publications/tbhiv_factsheetpdf . Retrieved December 23, 2019. [Google Scholar]
- 2.Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD., Jr Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–72. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 3.Churchyard GJ, Fielding KL, Lewis JJ, Coetzee L, Corbett EL, Godfrey-Faussett P, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370:301–10. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 4.Fielding KL, Grant AD, Hayes RJ, Chaisson RE, Corbett EL, Churchyard GJ, Thibela TB. Design and methods of a cluster randomised trial of the effect of community-wide isoniazid preventive therapy on tuberculosis amongst gold miners in South Africa. Contemp Clin Trials. 2011;32:382–92. doi: 10.1016/j.cct.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Assebe LF, Reda HL, Wubeneh AD, Lerebo WT, Lambert SM. The effect of isoniazid preventive therapy on incidence of tuberculosis among HIV-infected clients under pre-ART care, Jimma, Ethiopia: A retrospective cohort study. BMC Public Health. 2015;15:346. doi: 10.1186/s12889-015-1719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satiavan I, Hartantri Y, Werry B, Nababan Y, Wisaksana R, Alisjahban B. Effect of isoniazid preventive therapy on tuberculosis incidence in people living with HIV-AIDS at Hasan Sadikin hospital. IOP Conf Series Earth Environ Sci. 2018;125:12006. [Google Scholar]
- 7.Namuwenge PM, Mukonzo JK, Kiwanuka N, Wanyenze R, Byaruhanga R, Bissell K, et al. Loss to follow up from isoniazid preventive therapy among adults attending HIV voluntary counseling and testing sites in Uganda. Trans R Soc Trop Med Hyg. 2012;106:84–9. doi: 10.1016/j.trstmh.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 8.HIV and AIDS Treatment in Practice. IPT for TB in People with HIV: Barriers to National Implementation. London, UK: National AIDS Manual; 2008. [Last accessed on 2018 Aug 23]. Available from: http://wwwaidsmapcom/files/file1002936pdf . [Google Scholar]
- 9.Takarinda KC, Choto RC, Harries AD, Mutasa-Apollo T, Chakanyuka-Musanhu C. Routine implementation of isoniazid preventive therapy in HIV-infected patients in seven pilot sites in Zimbabwe. Public Health Action. 2017;7:55–60. doi: 10.5588/pha.16.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durovni B, Cavalcante SC, Saraceni V, Vellozo V, Israel G, King BS, et al. The implementation of isoniazid preventive therapy in HIV clinics: The experience from the TB/HIV in Rio (THRio) study. AIDS. 2010;24(Suppl 5):S49–56. doi: 10.1097/01.aids.0000391022.95412.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaeh S, Kempker R, Stenehjem E, Blumberg HM, Temesgen O, Ofotokun I, et al. Improving tuberculosis screening and isoniazid preventive therapy in an HIV clinic in Addis Ababa, Ethiopia. Int J Tuberc Lung Dis. 2013;17:1396–401. doi: 10.5588/ijtld.13.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munseri PJ, Talbot EA, Mtei L, Fordham von Reyn C. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int J Tuberc Lung Dis. 2008;12:1037–41. [PubMed] [Google Scholar]
- 13.Makoni A, Chemhuru M, Tshimanga M, Gombe NT, Mungati M, Bangure D. Evaluation of the isoniazid preventive therapy (IPT) program in Shurugwi District, Midlands Province, Zimbabwe, January 2013 to August 2014. BMC Res Notes. 2015;8:476. doi: 10.1186/s13104-015-1451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Guidelines on the Management of Latent Tuberculosis Infection. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 15.Tortajada C, Martínez-Lacasa J, Sánchez F, Jiménez-Fuentes A, De Souza ML, García JF, et al. Is the combination of pyrazinamide plus rifampicin safe for treating latent tuberculosis infection in persons not infected by the human immunodeficiency virus? Int J Tuberc Lung Dis. 2005;9:276–81. [PubMed] [Google Scholar]
- 16.Spyridis NP, Spyridis PG, Gelesme A, Sypsa V, Valianatou M, Metsou F, et al. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: Results of an 11-year randomized study. Clin Infect Dis. 2007;45:715–22. doi: 10.1086/520983. [DOI] [PubMed] [Google Scholar]
- 17.Federal Ministry of Health. National Tuberculosis, Leprosy and Buruli Ulcer Management and Control Guidelines. 6th ed. Abuja Federal Ministry of Health; 2015. [Google Scholar]
- 18.Hulda IN. Use of Isoniazid Preventive Therapy on HIV/AIDS Patient in a Tertiary Health Facility South Eastern Nigeria. 2015. Available from: https://wwwresearchgatenet/publication/276105049_Use_of_Isoniazid_Preventive_Therapy_on_HIVAIDS_Patient_in_a_Tertiary_Health_Facility_South_Eastern_Nigeria . Retrieved on November 26, 2019.
- 19.Kabiru US, Chiegil JE, Adeyemi SO, Martins OF. Isoniazid Preventive Therapy Implementation among People Living with HIV/AIDS Enrolled in Care at Specialist Hospital Yola, Nigeria', Texila. Int J Public Health. 2017;5:1–10. [Google Scholar]
- 20.Yirdaw KD, Jerene D, Gashu Z, Edginton ME, Kumar AM, Letamo Y, et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoS One. 2014;9:e104557. doi: 10.1371/journal.pone.0104557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim CL, Wong PS, Pereirasamy L, Ang PP, Leong KN, Chow TS. Outcome of Isoniazid Preventive Therapy in Adults Living with HIV in Penang, Malaysia. J Infect Dis Prev Med. 2016;4:133. [Google Scholar]
- 22.Mindachew M, Deribew A, Memiah P, Biadgilign S. Perceived barriers to the implementation of Isoniazid preventive therapy for people living with HIV in resource constrained settings: A qualitative study. Pan Afr Med J. 2014;17:26. doi: 10.11604/pamj.2014.17.26.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maharaj B, Gengiah TN, Yende-Zuma N, Gengiah S, Naidoo A, Naidoo K. Implementing isoniazid preventive therapy in a tuberculosis treatment-experienced cohort on ART. Int J Tuberc Lung Dis. 2017;21:537–43. doi: 10.5588/ijtld.16.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mwangi PM. Implementation of Isoniazid Preventive Therapy among HIV Infected Children in Three Health Facilities in Nairobi County. 2013. Avalable from: http://erepositoryuonbiacke/bitstream/handle/11295/99719/Peninah%20Final%20Thesispdf?sequence=1&isAllowed=y . Retrieved on November 26, 2019. [DOI] [PMC free article] [PubMed]
- 25.Bartlett EE, Grayson M, Barker R, Levine DM, Golden A, Libber S. The effects of physician communications skills on patient satisfaction; recall, and adherence. J Chronic Dis. 1984;37:755–64. doi: 10.1016/0021-9681(84)90044-4. [DOI] [PubMed] [Google Scholar]
- 26.Dunbar J. Predictors of patient adherence: Patient characteristics. In: Shumaker S, Schroon E, Ockene J, editors. The Handbook of Health Behaviour Change. New York: Springer; 2004. pp. 348–60. [Google Scholar]
- 27.Hess K, Goad J, Wu J, Johnson K. Isoniazid completion rates for latent tuberculosis infection among college students managed by a community pharmacist. J Am Coll Health. 2009;57:553–5. doi: 10.3200/JACH.57.5.553-556. [DOI] [PubMed] [Google Scholar]
- 28.Clark PM, Karagoz T, Apikoglu-Rabus S, Izzettin FV. Effect of pharmacist-led patient education on adherence to tuberculosis treatment. Am J Health Syst Pharm. 2007;64:497–505. doi: 10.2146/ajhp050543. [DOI] [PubMed] [Google Scholar]
- 29.Last JP, Kozakiewicz JM. Development of a pharmacist-managed latent tuberculosis clinic. Am J Health Syst Pharm. 2009;66:1522–3. doi: 10.2146/ajhp090034. [DOI] [PubMed] [Google Scholar]


