On March 11, Covid-19, a novel coronavirus was declared a pandemic by the WHO, and at the time of writing this manuscript, Covid-19 has infected 3,913,643 people globally with 270,426 deaths.[1,2] The first case of Covid-19 in India was reported on January 30, 2020, from Kerala, the cases started increasing toward the end of March, and total Covid-19 cases in India have crossed the 50000 mark with over 1800 deaths.[3,4] [Table 1].
Table 1.
Total COVID-19 positive cases reported from India
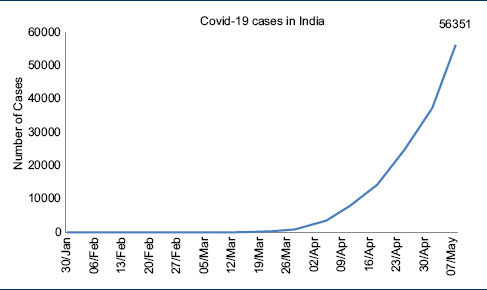
Some experts criticized India for testing too less numbers despite the call by the WHO to adopt “test, test, and test” approach.[5,6] India chose a different approach and went for total lockdown for 1.3 billion people on March 24.[6,7] This was complemented by the state directions for the compulsory use of face masks by the people when moving outdoors.[6]
Initial Indian strategy based on the advice of the Indian Council of Medical Research (ICMR) was that of limited testing of people with foreign travel, contacts of Covid-19 patients, and severe acute respiratory illness from all over the country.[8] This strategy backed by the national lockdown was successful, and India has apparently been able to flatten the curve of Covid-19 infections by extended the doubling rate of Covid-19 infections to 6 days from the earlier predicted time of 3 days in the initial phases.[7] Indian Health Ministry on 5th of May announced doubling time of 12 days which was later revised to 10.2 days on 7th of May, 2020, which is still much better than most developed countries.[9] This gave enough time for India to equip its hospitals by training the workforce, procurement, and manufacture of personal protective equipments, drugs, and ventilators for the Covid-19 cases. Meanwhile the developed countries such as the US, Italy, Spain, and the UK that went ahead with an approach of “test, test, and test” and delayed the lockdown resulted in a spike of infections [Table 2].[2] Till May 8th, India had tested only 984 people per million.[2] This testing vigor of India is in stark contrast to most other large nations who have tested over 20,000-30,000 people per million [Table 2].
Table 2.
Comparative Covid-19 burden and testing vigor of India and other nations
| Country | Cases/1 M Population | Deaths/1 M population | Tests/ 1M population | Test Positivity | Deaths/Cases |
|---|---|---|---|---|---|
| Spain | 5,494 | 558 | 41,332 | 13% | 10% |
| USA | 3,905 | 232 | 25,068 | 16% | 6% |
| Italy | 3,570 | 495 | 39,385 | 9% | 14% |
| UK | 3,045 | 451 | 22,605 | 13% | 15% |
| France | 2,678 | 398 | 21,213 | 13% | 15% |
| Germany | 2,022 | 88 | 32,891 | 6% | 4% |
| Israel | 1,893 | 28 | 49,963 | 4% | 1% |
| Iran | 1,228 | 77 | 6,485 | 19% | 6% |
| Russia | 1,214 | 11 | 32,913 | 4% | 1% |
| S. Korea | 211 | 5 | 12,666 | 2% | 2% |
| India | 41 | 1 | 984 | 4% | 3% |
Interestingly, Paul Romer, a Nobel-winning economist, has proposed to test 7% of the population every day and putting them on a rotating schedule every 2 weeks as the only long-term way of keeping the vast majority of people out in normal life.[10] For the United States, this would mean 150 million tests a week, and for India, this means 650 million tests. This could be one of the reasons that India did not choose the “test, test, and test” pathway proposed by the WHO. The order for 5 lakh rapid diagnostic kits was placed on March 28, by India, but the deadline was reported to have been postponed thrice by 5 days each as China supplied the available kits to the US.[11] The supply of testing kits to India and other developing countries, therefore, is highly likely to be affected due to the persistent demand and pressure on the suppliers from the US, Italy, France, Spain, Germany, the UK, and other developed nations. Despite all limitations, with 1,357,413 tests, India ranks 8th in the number of tests conducted till date by any country and is bound to surpass other nations in the times to come.[2]
Given the prevailing circumstances and available infrastructure at the onset of this pandemic, India has done a fairly decent job by keeping Covid-19 in check. Meanwhile, ICMR has steadily increased its testing capacity to match the rising number of cases in India [Table 3].[8]
Table 3.
Growth in the number of COVID-19 testing labs in India
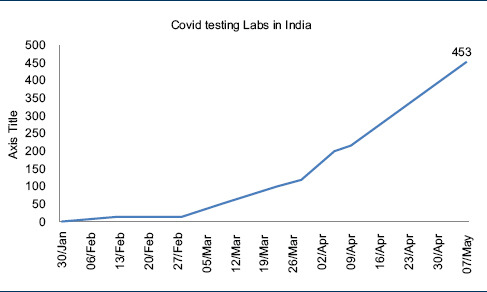
The WHO has approved the National Institute of Virology (NIV), Pune, as the reference center for confirmation of all Covid-19 cases diagnosed in peripheral laboratories in India.[12] As of now, all the suspected cases of Covid-19 in India are being tested using the real-time reverse transcriptase-polymerase chain reaction (rRT-PCR). A negative nucleic acid amplification test (NAAT) result, however, does not rule out Covid-19 infection because of poor overall sensitivity, and therefore, few important factors that must be kept in mind while reporting a negative result of NAAT include with following:[12]
Poor quality of specimen due to variance in the technique of obtaining nasopharyngeal and oropharyngeal swabs resulting in little patient material
Improper handling and shipping of the sample
Specimens collected late in the convalescence
Virus mutation or PCR inhibition.
Less stability of viral genome during the extraction process
Each laboratory in India has a capacity of testing roughly 100 samples per day. In the event of community transmission, this capacity is surely going to become overwhelmed. Foreseeing this eventuality, ICMR has adopted the following strategies to augment the testing capacity of the existing laboratories:
Validation of non-US-FDA/CE-IVD-approved RT PCR kits:[8] ICMR has validated 28 RT PCR kits, of which 10 were found satisfactory and are approved for use in India
-
Adoption of rapid antibody-based testing for Covid-19 on April 4, 2020:[8] The advisory is focused on testing in the containment zones and areas with clustering of cases. The rapid antibody tests approved by the US-FDA/CE-IVD or non-CE-IVD or validated by ICMR-NIV with marketing approval by DCGI may be used for the diagnosis of Covid-19
So far, 16 antibody-based kits have been evaluated by NIV, of which 8 are approved for use in India.[8] The latest update of ICMR advises rRT-PCR for influenza-like illness presenting within 7 days and rapid antibody-based tests after 7 days of illness[8]
Advisory on pooling of samples for molecular testing:[8] On April 13, 2020, ICMR released an advisory regarding the pooling of samples for molecular testing with an objective to increase the testing capacity of laboratories.[8] This protocol has been validated at the King George's Medical University, Lucknow, in India
Validation of Truenat™ beta CoV for test on Truenat™ workstation for Covid-19 testing by the ICMR on April 14, 2020:[8] Truenat™ machines are designed to work on a battery without the need for an air conditioner as is needed for GeneXpert® machines.[13] They were designed to detect tuberculosis at the primary health-care level. Already, 800 of these machines are installed in various parts of India, half of which are in the government sector.[14] The cost of Truenat™ workstation is roughly 6000 US$.[15] The kits for Turenat™ beta CoV will be supplied with a virus lysis buffer to render the sample non-infective at the bedside. All positive samples will need to be confirmed using rRT-PCR. Table 4 gives a comparison of available testing options in the near future with their advantages and limitations for Indian laboratories.
Table 4.
Advantages and limitations of the available testing options in India
| Type of test | Advantages | Limitations |
|---|---|---|
| rRT PCR | High specificity | Varied clinical sensitivity |
| Need for biosafety level-2 or 3 | ||
| Full coverall protection required | ||
| Limited availability of laboratories and skilled manpower | ||
| Costly (Rs. 5000/test) | ||
| Takes 5 h | ||
| Antibody based tests | Lower cost (Rs. 2000-3000/test) | Low sensitivity in 1st week |
| Rapid (3-15 min) | Limited supply of kits internationally Poor quality of kits | |
| Gloves, head cover and mask suffice as protective equipment | ||
| Stable at room temperature | ||
| Truenat™ beta CoV | Indian indigenous technology | Availability of kits |
| Low cost (Rs. 1000/test) | Need confirmatory testing by RT-PCR | |
| 800 machines installed in India | ||
| High specificity | ||
| The same machine can test TB, HIV | ||
| No need for Air conditioner | ||
| Easy to perform in primary care | ||
| Battery operated and portable | ||
| Pooled PCR | For low prevalence (<2%) areas | Pooling is not recommended at areas with >5% positivity rate |
| Cost effective for asymptomatic people and pre-operative screening | Not for contacts of Covid-19 patients |
Covid: Coronavirus disease, rRT-PCR: Real-time reverse transcriptase-polymerase chain reaction, CoV: Coronavirus, TB: Tuberculosis
Among all the above measures, pooled PCR- and Truenat™-based testing at 400–800 sites seems to be the most promising step at the moment. RDTs also are good in the 2nd week of infection. They should also be used for screening of health-care professionals who may have accidentally got infected resulting in mild symptoms and therefore did not get tested. In the US, a study of 9292 health-care professionals who were Covid-19 positive has shown that 90% did not require hospitalization and 8% did not develop any symptom at all.[16]
To conclude, India's strategic testing approach while ramping up its testing capabilities using innovative methods is helping keep the Covid-19 in check and has been complemented by the prolonged national lockdown and directions for universal use of masks in public places. India is all set to step-up the testing capabilities and to capitalize on the early advantage against Covid-19. This is a challenge that India cannot afford to loose.
REFERENCES
- 1.Novel Coronavirus (2019-nCoV) Situation Reports. [Last accessed on 2020 Apr 14]. Available from: https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/situation-reports .
- 2.Coronavirus Update (Live): 3,913,643 Cases and 270,426 Deaths from COVID-19 Virus Pandemic - Worldometer [Internet] [Last accessed on 2020 May 08]. Available from: https://www.worldometers.info/coronavirus/
- 3.2020 Coronavirus Pandemic in India. Wikipedia. 2020. [Last accessed on 2020 Apr 14]. Available from: https://en.wikipedia.org/w/index.php?title=2020_coronavirus_pandemic_in_India&oldid=950858032 .
- 4.India Coronavirus: 56,351 Cases and 1,889 Deaths - Worldometer [Internet] [Last accessed on 2020 May 08]. Available from: https://www.worldometers.info/coronavirus/country/india/
- 5.Biswas S. Coronavirus: Why is India Testing so Little. BBC News. 2020. Mar 20, [Last accessed on 2020 Apr 18]. Available from: https://wwwbbccom/news/world-asia-india-51922204 .
- 6.Ignore WHO, We Shall Listen to ICMR,” How Modi Govt Sidelined WHO and Averted a Major crisis. TFIPOST. 2020. [Last accessed on 2020 Apr 18]. Available from: https://tfipost.com/2020/04/ignore-who-we-shall-listen-toicmr-how-modi-govt-sidelined-who-and-averted-a-major-crisis/
- 7.2020 Coronavirus Lockdown in India. Wikipedia. 2020. [Last accessed on 2020 Apr 19]. Available from: https://en.wikipedia.org/w/index.php?title=2020_coronavirus_lockdown_in_India&oldid=951718458 .
- 8.Indian Council of Medical Research. New Delhi [Internet] [Last accessed on 2020 May 08]. Available from: https://www.icmr.gov.in/
- 9.May 8 SD| T| U, 2020, Ist 05:34. Covid-19: Doubling time worsens to 10.2 days in 1 week | India News - Times of India [Internet] The Times of India. [Last accessed on 2020 May 08]. Available from: https://timesofindia.indiatimes.com/india/covid-19-doubling-time-worsens-to-10-2-days-in-1-week/articleshow/75612580.cms .
- 10.Lesson From Singapore: Why We May Need to Think Bigger-The New York Times. [Last accessed on 2020 Apr 15]. Available from: https://wwwnytimescom/2020/04/14/upshot/coronavirus-singapore-thinking-bightml .
- 11.Delhi. Coronavirus: Are there Roadblocks in India's Fight Against the Deadly Covid-19? India Today. [Last accessed on 2020 Apr 14]. Available from: https://www.indiatoday.in/india/story/coronavirus-in-india-are-there-roadblocksin-indias-fight-against-the-deadly-covid19-1666001-2020-04-11 .
- 12.National Laboratories. [Last accessed 2020 Apr 14]. Available from: https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance .
- 13.Point-of-Care Molecular TB Test. FIND. [Last accessed on 2020 Apr 14]. Available from: https://www.finddx.org/tb/poc-truenat/
- 14.Coronavirus crisis: ICMR Allows TB-Testing Machine to Boost Screening Process. [Last accessed on 2020 Apr 14]. Available from: https://wwwbusinesstodayin/top-story/coronavirus-crisis-icmr-allows-tb- testing-machine-to-boost-screening-process/story/400695html .
- 15.TB Online-TB and MDR-TB Diagnosis Goes the “Make in India”Way. [Last accessed on 2020 Apr 14]. Available from: http://wwwtbonlineinfo/posts/2017/10/4/tb-and-mdr-tb-diagnosis-goes-make-india-way/
- 16.CDCMMWR. Characteristics of Health Care Personnel with COVID-19 — United States. [Last accessed on 2020 Apr 16];MMWR Morb Mortal Wkly Rep. 2020 69:477–81. doi: 10.15585/mmwr.mm6915e6. Available from: https://wwwcdcgov/mmwr/volumes/69/wr/mm6915e6htm . [DOI] [PMC free article] [PubMed] [Google Scholar]


