Abstract
Vitamin D3 (cholecalciferol) is endogenously produced in the skin of primates when exposed to the appropriate wavelengths of ultra violet light (UV-B). Common marmosets (Callithrix jacchus) maintained indoors require dietary provision of vitamin D3 due to lack of sunlight exposure. The minimum dietary vitamin D3 requirement and the maximum amount of vitamin D3 that can be metabolized by marmosets is unknown. Observations of metabolic bone disease and gastrointestinal malabsorption have led to wide variation in dietary vitamin D3 provision amongst research institutions, with resulting variation in circulating 25-hydroxyvitamin D3 (25(OH)D3), the accepted marker for vitamin D sufficiency/deficiency. Multiple studies have reported serum 25(OH)D3 in captive marmosets, but 25(OH)D3 is not the final product of vitamin D3 metabolism. In addition to serum 25(OH)D3, we measured the most physiologically active metabolite, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), and the less well understood metabolite, 24,25-dihydroxyvitamin D3 (24,25(OH)2D3) to characterize the marmoset’s ability to metabolize dietary vitamin D3. We present vitamin D3 metabolite and related serum chemistry value colony reference ranges in marmosets provided diets with 26,367 (Colony A, N = 113) or 8,888 (Colony B, N = 52) international units (IU) of dietary vitamin D3 per kilogram of dry matter. Colony A marmosets had higher serum 25(OH)D3 (426ng/ml (SD 200) versus 215ng/ml (SD 113)) and 24,25(OH)2D3 (53ng/ml (SD 35) versus 7ng/ml (SD 5)). There was no difference in serum 1,25(OH)2D3 between the colonies. Serum 1,25(OH)2D3 increased and 25(OH)D3 decreased with age, but the effect was weak. Marmosets tightly regulate metabolism of dietary vitamin D3 into the active metabolite 1,25(OH)2D3; excess 25(OH)D3 is metabolized into 24,25(OH)2D3. This ability explains the tolerance of high levels of dietary vitamin D3 by marmosets, however, our data suggest that these high dietary levels are not required.
Keywords: Vitamin D3, marmoset, Callithrix jacchus, diet
Introduction
Marmosets (Callithrix jacchus) are maintained in laboratory facilities to study topics such as neuroscience, reproduction, aging, and infectious disease (Fox et al., 2019; Miller, 2017). Recommended macro and micro nutrient requirements are available, but there is marked variety in both what types of food (primary diets and supplemental food items) are fed to marmosets and how those food items are presented (National Research Council (U.S.). Committee on Animal Nutrition. & National Research Council (U.S.). Panel on Nonhuman Primate Nutrition., 2003). The amount of dietary vitamin D3 has received interest due to its suggested role in metabolic bone disease secondary to chronic malabsorption, a common clinical syndrome affecting marmosets (Jarcho et al., 2013). There are two forms of vitamin D, ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3); New World monkeys are unable to use vitamin D2 (Marx et al., 1989; Ziegler et al., 2015). Vitamin D3 is photosynthesized in skin from 7-dehydroxycholesterol following absorption of ultraviolet B (UV-B) light (285–315 nanometers) (Hatt & Sainsbury, 1998). Most laboratory housing facilities do not provide access to natural sunlight or UV-B lamps and vitamin D3 is acquired from the diet. Captive marmosets can vary widely in their apparent digestibility of energy and serum 25-hydroxyvitamin D3 positively correlates with digestive efficiency (Jarcho et al., 2013). Observations of chronic diarrhea or weight loss (evidence of gastrointestinal malabsorption and decreased apparent digestibility) and metabolic bone disease (osteomalacia secondary to vitamin D3 and calcium insufficiency) in marmosets have led to inclusion of vitamin D3 in commercial diets and additional supplementation by institutions beyond what is recommended by the National Research Council (Jarcho et al., 2013). Higher vitamin D3 levels in feed has led to a decline in metabolic bone disease (Power et al., 1997). The recommended amount of dietary vitamin D3 for primates is 2,000–3,000 international units (IU) per kilogram (kg) of dry feed (National Research Council (U.S.). Committee on Animal Nutrition. & National Research Council (U.S.). Panel on Nonhuman Primate Nutrition., 2003). Commercially available diets designed for marmosets have 6,000–10,000 IU vitamin D3 per kg and some colonies supplement this with additional vitamin D3. The minimum vitamin D3 requirement to maintain mineral homeostasis and the threshold at which toxicity or significant phenotypic change is observed in marmosets is unknown (Power & Koutsos, 2019).
Ingested vitamin D3 must be metabolized to 1,25(OH)2D3 to elicit physiological effects. Initial hydroxylation by the liver enzyme 25-hydroxylase (CYP2R1) produces 25(OH)D3. Circulating 25(OH)D3 has a half-life of two to three weeks and serum levels are used to characterize an individual’s vitamin D status (Lips, 2007). A second hydroxylation is completed by 1-a-hydroxylase (CYP27B1), primarily in the kidneys, to produce 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the most physiologically active metabolite which binds to the vitamin D receptors. Production of 1,25(OH)2D3 is tightly regulated (Pasquali et al., 2015). 25(OH)D3 and 1,25(OH)2D3 can undergo another hydroxylation by 24-hydroxylase (CYP24A1) to generate 24,25-dihydroxyvitamin D3 (24,25(OH)2D3) and 1,24,25-trihydroxyvitamin D3 (1,24,25(OH)3D3), respectively (Dirks et al., 2018; Stubbs et al., 2014). Although vitamin D receptor affinity by 24,25(OH)2D3 and 1,24,25(OH)3D3 has been suggested (Bikle, 2014), as well as non-genomic effects of 24,25(OH)2D3 related to pro-inflammatory signaling (Wehmeier et al., 2016), these metabolites are generally regarded as pathways for the excretion of excess 25(OH)D3 (Jones et al., 2014).
Although 1,25(OH)2D3 is primarily considered a regulator of calcium metabolism, almost all nucleated cells have vitamin D receptors (Mellanby, 2016) and receptor activation regulates gene transcription and affects cell proliferation, cell differentiation, and formation of proteins that impact cell phenotype (Garg et al., 2013; Teixeira et al., 2012; Zendehdel & Arefi, 2019). Thus, 1,25(OH)2D3 affects a wide range of physiological processes including immunological, cardiovascular, and neoplastic diseases (Fox et al., 2019; Jones, 2008; Mellanby, 2016; Tang et al., 2017). Marmosets have higher serum levels of 1,25(OH)2D3 than rhesus and humans (Shinki et al., 1983; Takahashi et al., 1985; Ziegler et al., 2015). Marmosets require higher levels of 1,25(OH)2D3 due to increased vitamin D response element binding protein that competes with the intracellular vitamin D receptors for 1,25(OH)2D3 (Adams et al., 2003).
Serum levels of 25(OH)D3 in marmosets have been measured with radioimmunoassay (RIA) and liquid chromatography-tandem mass spectrometry (LC-MS) (Bosseler et al., 2018; Ziegler et al., 2015). LC-MS is able to differentiate vitamin D2 from D3 and to measure all vitamin D metabolites (Tang et al., 2017; Ziegler et al., 2015). There have been limited evaluations of 1,25(OH)2D3 in marmosets; most studies report 25(OH)D3 (Shinki et al., 1983; Takahashi et al., 1985). Evaluation of 24,25(OH)2D3 and 1,25(OH)2D3 in conjunction with 25(OH)D3, provides a more complete picture of vitamin D3 metabolism that can be used to guide dietary supplementation recommendations in marmosets without UVB exposure.
An opportunity to evaluate the impact of dietary vitamin D3 on serum vitamin D3 metabolites was identified at the Wisconsin National Primate Research Center (WNPRC). Two common marmoset colonies are maintained at the WNPRC that receive different levels of dietary vitamin D3; Colony A is the original colony maintained since 1990 and Colony B was acquired in 2015. Colony B has continued to receive the diet implemented by the institution of origin to facilitate projects evaluating the optimal diet for marmosets. This information will allow an evidence-based decision on what diet is optimal for future use. Colony A’s primary diet contains 26,367 IU vitamin D3 per kg of dry matter diet. Colony B’s primary diet contains 8,888 IU dietary vitamin D3 per kg dry matter diet. Both colonies receive daily supplemental food items for enrichment (same frequency and portion sizes). Our first aim was to establish colony reference ranges for 25(OH)D3, 24,25(OH)2D3, 1,25(OH)2D3, and related serum chemistry values (calcium, phosphorous, and albumin). Vitamin D3 facilitates calcium absorption which impacts phosphorous levels. Low albumin has been associated with gastrointestinal malabsorption and may be indicative of impaired ability to absorb vitamin D3 (Baxter et al., 2013). We also evaluated the impact of age and sex. Our second aim was to compare vitamin D3 metabolites between the colonies and assess the marmoset’s ability to metabolize different levels of dietary vitamin D3.
Methods
Marmosets resided at two different facilities at the WNPRC (Madison, WI). Facilities maintained temperature of 75 to 85 °F and humidity of 30 to 70%. A 12:12 hour light cycle with fluorescent bulbs (Colony A: GE42556 F32T8XLSPX50HLEC; Colony B: GE66474 F34/CX41/WM/ECO) was used and there was no exposure to natural light. Marmosets were socially housed as same sex pairs, opposite sex pairs, or family groups unless single housing was required for study. Colony A marmosets (42 females, 71 males) were from 0.8 to 13 years in age (median = 3 years). Colony B marmosets (20 females, 32 males) were from 0.8 to 11.2 years in age (median = 4.1 years).
Marmosets in both colonies are fed twice a day (between 0800–1000 AM and 1230–1430 PM). Routine enrichment food items consisted of a protein in the morning and produce in the afternoon. Diet and enrichment consumption were not monitored and the proportion of enrichment consumed relative to primary diet may vary between marmosets. Socially housed marmosets that are dominant may have access to a larger proportion of enrichment food items. Marmosets consume 3 – 7% of their body weight daily in dry matter (Power & Myers, 2009). Previous dietary intake studies in this colony found daily dry matter intake of approximately 15 grams (calculated from Power et al., 2019). Colony A receives Mazuri Callitrichid High Fiber Diet (Mazuri 5MI6, Land O’Lakes, Arden Hills, MN) which contains vitamin D3 as 5730 IU/kg with no more than 10% moisture content (6,367 IU/kg of dry matter). It is supplemented with vitamin D3 (Teklad TD.10936 Vitamin D Premix 4000 IU/g) prior to reconstitution with water to bring the total vitamin D3 to 26,367 IU/ kg of dry matter (13,300 IU/kg as fed). Approximately 400 IU vitamin D3 is consumed daily per marmoset in Colony A based on the expected daily consumption of 15 grams dry matter diet. Colony B receives Teklad New World Primate Diet 8794 (Envigo, New Jersey) containing vitamin D3 8,000 IU per kilogram with no more than 10% moisture content (8,888 IU/kg of dry matter). On average, approximately IU 135 IU vitamin D3 is expected to be consumed daily per marmoset in Colony B. Colony A receives water from a municipal source and Colony B receives water from a well that has been filtered and softened.
Blood (1.5 mL) was drawn from the femoral vessels between 800 AM and 100 PM; food was withheld the day of blood collection until post draw. Serum was aliquoted and stored at −80 C until metabolite analysis (Vitamin D Panel, LC-MS/MS, Assay Services WNPRC, Madison, WI) or serum chemistry evaluation (Merriter Laboratories, Madison, WI). If insufficient blood volume for both tests was available, vitamin D3 metabolite analysis was prioritized.
All procedures were approved by the Institutional Animal Care and Use Committee of the College of Letters & Sciences and the Office of the Vice Chancellor of Research and Graduate Education at University of Wisconsin, Madison, as a part of the Primate Center Colony Resource protocol. Animal care and use is consistent with the ASP Principles for Ethical Treatment of Non-Human Primates and compliant with the US National Research Council’s Guide for the Care and Use of Laboratory Animals, the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals, and USDA Animal Welfare Act and Regulations.
An LC-MS/MS panel was used to measure vitamin D3 metabolites (Ziegler et al., 2018). As the serum level of vitamin D3 metabolites are on average higher in the common marmoset than in the Old World monkeys, the volume used for the extraction and analyses was reduced to 50 μl. The separation was attained on the same column described using the same QTRAP 5500 Quadrupole – Linear Ion Trap Mass Spectrometer equipped with an ESI source in positive mode. Coefficient of variation ranged from 7.1–17.5% for all forms of vitamin D. Serum vitamin D3, 24,25(OH)2D3, and 25(OH)D3 were measured.
Marmoset serum samples were analyzed for 1,25(OH)2D3 by liquid chromatography-tandem mass spectrometry (LC-MS/MS). 43 samples from Colony A were of insufficient volume to facilitate this measurement. A 10 μL aliquot of the extracted eluate was separated using Shimadzu XR pumps on a 2.6 um C18 100 × 2.1 mm column (Phenomenex, Torrance, CA). Column temperature was 104°F and the flow rate was 0.15 mL/min. Solvent A consisted of 0.1% formic acid in water and solvent B was 0.1% formic acid acetonitrile. The mobile phase gradient started at 30% B and was held for 2 minutes then increased to 60% over 6 min. The gradient was then increased to 98% B and held for 3 minutes and then re-equilibrated for four minutes at 30% B. A QTRAP 5500 triple quadrapole MS (Sciex) was used for detection with electrospray ionization in positive mode. Data was analyzed using Analyst (Sciex) software and interassay variability was as follows: 3.94–5.87% (1,25(OH)2D3), 8.43–15.19% (24,25(OH)2D3), 4.58–9.88% (25(OH)D3), and 3.81–13.29% (D3).
R, JMP® and SPSS were used to complete statistical analysis (R Foundation for Statistical Computing, Vienna, Austria; JMP®, SAS Institute Inc., Cary, NC; IBM). Normality of data distribution was evaluated via Shapiro Wilk test. The means of normally distributed data were compared via a T-test. The means of non-normally distributed data were evaluated with the Wilcoxon rank sum test. Pearson’s correlation was used to evaluate relationships between age and serum parameters. Statistical tests were performed on the entire data set and on each colony’s data separately. Analysis of covariance was used to test for differences in slopes of regression lines between colonies’ relationships among vitamin D3 metabolites and serum chemistry values. The significance threshold was set at .05.
Results
Colony 90% reference ranges, median, mean, and standard deviation for 25(OH)D3, 24,25(OH)2D3, 1,25(OH)2D3, and related serum chemistry values are presented in Tables 1 and 2 and in Figure 1. There was no difference between groups in mean serum levels of vitamin D3 (Z=0.73, p=.464) and 1,25(OH)2D3 (Z=0.77, p=.442). 25(OH)D3 (Z=−6.34, p <.001) and 24,25(OH)2D3 serum levels were higher (Z=−8.01, p <.001) in Colony A compared to Colony B (Table 1, Figure 1). Serum 25(OH)D3 and 24,25(OH)2D exhibited a positive correlation in both colonies (Figure 2; all samples, rs=.88, p<.0001; Colony A, rs=.81, p<.0001; Colony B, rs=.78, p<.0001). There was a significant difference between the slopes of colonies A and B in the relationship of 24,25(OH)2D3 to 25(OH)D3 (ANCOVA; F=19.843, df=1, p<0.001; Figure 2).
Table 1.
References ranges of vitamin D3 metabolites for both colonies. 25(OH)D3 and 24,25(OH)2D3 means were greater in the Colony A compared to the Colony B. There was no difference in 1,25(OH)2D3 means. Means were compared with Wilcoxon rank sum test.
| Colony | Vitamin D3 Metabolite | n | 90% | Mean (SD) | Median | P value (A vs B) |
|---|---|---|---|---|---|---|
| A | Vitamin D3 ng/mL | 113 | 0.4–3.21 | 1.3 (1.4) | 0.94 | 0.464 |
| 25(OH)D3 ng/mL | 113 | 43.56–681.6 | 425.84 (200.48) | 467 | <.001 | |
| 24,25(OH)2D3 ng/mL | 113 | 2.33–112.8 | 52.6 (35.4) | 54.2 | <.001 | |
| 1,25(OH)2D3 pg/mL | 70 | 145.86–936.40 | 561.12 (330.36) | 523.93 | 0.442 | |
| B | Vitamin D3 ng/mL | 52 | 0.4–4.37 | 1.56 (1.54) | 1.23 | 0.464 |
| 25(OH)D3 ng/mL | 52 | 49–463 | 215.46 (112.85) | 209 | <.001 | |
| 24,25(OH)2D3 ng/mL | 52 | 1.36–15.31 | 7.37 (5.30) | 6.8 | <.001 | |
| 1,25(OH)2D3 pg/mL | 52 | 230.77–1138.65 | 598.15 (291.86) | 483.5 | 0.442 | |
Table 2.
Colony reference ranges for selected serum chemistry values.
| Colony | Serum chemistry | n | 90% | Mean (SD) | Median | P value (A vs B) |
|---|---|---|---|---|---|---|
| A | Albumin g/dL | 108 | 3.74–5.37 | 4.6 (0.51) | 4.6 | .347 |
| Calcium mg/dL | 108 | 9.20–11.63 | 10.4 (0.74) | 10.4 | .77 | |
| Phosphorus mg/dL | 108 | 2.14–5.10 | 3.39 (0.9) | 3.25 | <.001 | |
| B | Albumin g/dL | 47 | 3.84–5.46 | 4.68 (0.52) | 4.75 | .347 |
| Calcium mg/dL | 47 | 9.37–11.57 | 10.36 (0.73) | 10.4 | .77 | |
| Phosphorus mg/dL | 47 | 2.47–6.83 | 4.52 (1.34) | 4.3 | <.001 | |
Figure 1.
A: Box plot of colony 25(OH)D3 serum levels.
B: Box plot of colony 1,25(OH)2D3 serum levels.
C: Box plot of colony 24,25(OH)2D3 serum levels.
Figure 2:
The relationship between 24,25(OH)2D3 and 25(OH)D3 serum levels by colony.
We found no difference in mean serum calcium (t(154)=0.28, p = .77) or albumin (t (156)=−0.94, p=.347) between colonies. Mean phosphorus levels were higher (Z=3922.5, p<.001) in Colony B compared to Colony A, but means for both groups were within normally accepted values for marmosets and the difference may not be of clinical significance (Table 2)(Fox et al., 2019). However, serum calcium was positively associated with both 25(OH)D3 and 24,25(OH)2D3 (r=0.285 and 0.293, respectively, p<0.001) while phosphorus was negatively correlated with these vitamin D3 metabolites (r=−0.347 and −0.348, respectively, p<0.001), which resulted in the calcium: phosphorus ratio being highly correlated with serum 25(OH)D3 and 24,25(OH)2D3 (r=0.452 and 0.425, respectively, p<.001). Analysis of covariance found colony (F=5.77; df=1; p=.017), sex (F=8.68; df=1; p=.04), age (F=13.93; df=1; p<.001) and 25(OH)D3 (F=25.86; df=1; p<.001) as significant factors explaining calcium: phosphorus ratio, with a significant difference in calcium: phosphorus ratio between colonies (estimated marginal means at age = 4.07 years and 25(OH)D3 at 368.3ng/ml of 3.17 ± 0.07 compared to 2.81 ± 0.12, for colony A and B, respectively, p=0.017). Many individuals in colony A had high ratios (13% greater than 4:1 calcium-to-phosphorus).
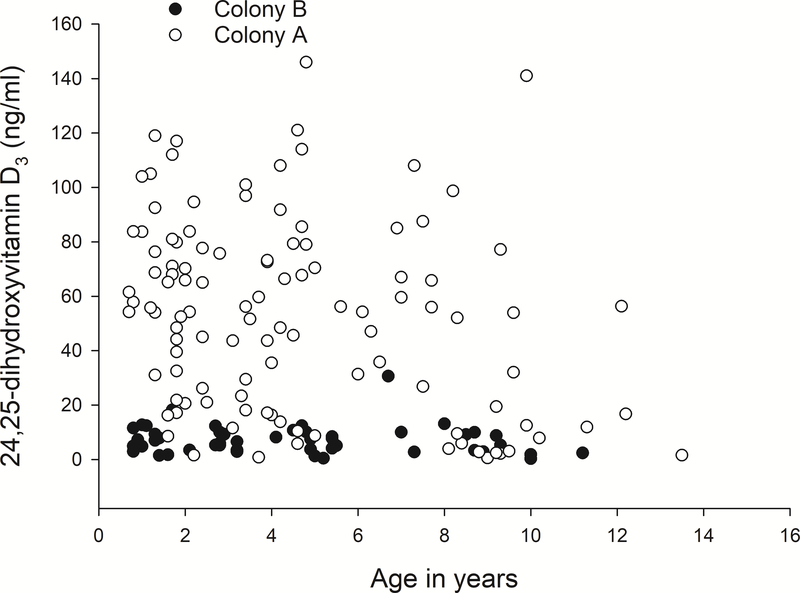
For the entire dataset, age had consistent effects on serum vitamin D3 metabolites and serum chemistry values, with serum 1,25(OH)2D3 increasing with age and all other serum parameters decreasing. However, when examined within each colony not all of these relationships were significant (Table 3). Both colonies displayed a decrease in 25(OH)D3 and increase in 1,25(OH)2D3 with age, but the association was not strong (Figure 3).
Table 3.
Relationship between age and serum vitamin D3 metabolites and serum chemistry values.
| Colony | n | r | P value | |
|---|---|---|---|---|
| All data | 25(OH)D3 ng/mL | 165 | −.260 | .001 |
| 24,25(OH)2D3 ng/mL | 164 | −.219 | .005 | |
| 1,25(OH)2D3 pg/mL | 122 | .258 | .004 | |
| A | 25(OH)D3 ng/mL | 113 | −.282 | .003 |
| 24,25(OH)2D3 ng/mL | 113 | −.281 | .003 | |
| 1,25(OH)2D3 pg/mL | 70 | .223 | .064 | |
| B | 25(OH)D3 ng/mL | 52 | −.278 | .046 |
| 24,25(OH)2D3 ng/mL | 52 | −.114 | .421 | |
| 1,25(OH)2D3 pg/mL | 52 | .324 | .019 | |
| All data | Albumin g/dL | 158 | −.286 | .001 |
| Calcium mg/dL | 156 | −.399 | .001 | |
| Phosphorous mg/dL | 156 | −.305 | .001 | |
| A | Albumin g/dL | 108 | −.375 | .000 |
| Calcium mg/dL | 108 | −.453 | .000 | |
| Phosphorous mg/dL | 108 | −.224 | .020 | |
| B | Albumin g/dL | 49 | −.089 | .54 |
| Calcium mg/dL | 47 | −.266 | .067 | |
| Phosphorous mg/dL | 47 | −.599 | .000 | |
Figure 3.
A: The relationship between age and 25(OH)D3 serum levels.
B: The relationship between age 1,25(OH)2D3 serum levels.
C: The relationship between age and 24,25(OH)2D3 serum levels.
The sex effect on vitamin D3 metabolites and serum chemistry values was evaluated. Colony A females (672.13 pg/mL (401.06); n=32) had higher values of 1,25(OH)2D3 compared to males (467.73 pg/ mL (221.64); n=38; Z=2.26, p=.024). There was no difference between females (620.08 pg/mL (352.49); n=20) and males (584.43 pg/mL (251.96); n=32) in Colony B (Z = −0.122, p=.902). We found no difference between the mean 25(OH)D3 (Colony A: females 400.45 ng/mL (173.11), males 440.86 ng/mL (214.80), Z=−1.16, p=.244; Colony B: females 189 ng/mL (165), males 231 ng/mL (230), Z=−1.326, p=.185 ) and 24,25(OH)2D3 (Colony A: females 56.12 ng/mL (34.37), males 50.52 ng/mL (36.01), Z=0.826, p=.409; Colony B: females 6.84 ng/mL (4.54), males 7.7 ng/mL (5.76), Z=−0.376, p=.707) values of males and females within either colony.
Males had higher albumin (females 4.41 g/dL (0.52), males 4.84 g/dL (0.45), t(48)= 3.15, p=.003), calcium (females 10.04 mg/dL (0.8), males 10.57 mg/dL (0.6), t(46)=−2.6, p=.012), and phosphorous (females 3.98 mg/dL (1.37), males 4.87 mg/dL (1.22), Z= 164, p .019) means than females in Colony B. We found no difference between these values in Colony A (albumin: females 4.61 g/dL (0.48), males 4.59 g/dL (0.53), t(106)=0.130, p=.897), calcium: females 10.34 mg/dL (0.73), males 10.43 mg/dL (0.74), t(106)= −0.599, p=.551, phosphorous: females 3.35 mg/dL (0.96), males 3.42 mg/dL (0.87), Z= 1311, p =.638).
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Discussion
25(OH)D3, 24,25(OH)2D3, 1,25(OH)2D3, calcium, albumin, and phosphorous ranges are presented for marmoset colonies consuming diets with 26,367 or 8,888 IU per kg of dry matter of vitamin D3. Colony A marmosets consume on average approximately 400 IU vitamin D3 per day and Colony B marmosets 135 IU per day as a component of their primary diet. Concurrent evaluation of vitamin D3 metabolites provided insight into the marmoset’s ability to regulate vitamin D3 metabolism. There was no difference between the serum vitamin D3 means of the colonies; this was expected as the half-life of vitamin D3 is four to twenty hours (Garg et al., 2013). Colony A had higher mean serum 25(OH)D3 and 24,25(OH)2D3. This is consistent with provision of greater amounts of dietary vitamin D3. Active metabolite 1,25(OH)2D3 serum levels were found to be the same between colonies. This indicates excess 25(OH)D3 is metabolized to 24,25(OH)2D3 and that 1,25(OH)2D3 production is tightly regulated. The range of 1,25(OH)2D3 serum levels are wider than measurements in macaques and humans (Ziegler et al., 2015). Previous studies have reported 1,25(OH)2D3 serum levels measured via HPLC/ RIA, but comparisons between RIA and LC/MS/MS found 1,25(OH)2D3 to be lower when measured by RIA (Ziegler et al., 2015).
The correlation between age and vitamin D3 metabolites differs from previous studies that found no impact of age on 25(OH)D3 in captive colonies of Callithrix jacchus and Callithrix pencillata with exposure to sunlight (Bosseler et al., 2018; Teixeira et al., 2010; Teixeira et al., 2012). There was a consistent decline in 25(OH)D3 with age in both colonies, though the effect was not strong. Indicators of lipid malabsorption have been shown to increase with age in common marmosets (Power et al., 2019) which would be consistent with the decline in 25(OH)D3 with age found in this study. We are unsure why 1,25(OH)2D3 serum levels would increase with age.
Sex did not impact serum 25(OH)D3 in other marmoset colonies (Bosseler et al., 2018; Jarcho et al., 2013; Teixeira et al., 2010; Teixeira et al., 2012). Colony A female marmosets had higher serum 1,25(OH)2D3 than males, but there was no difference in Colony B. Variables not measured include variation in feed intake or clinical disease (digestive efficiency mediated by gastrointestinal disease or renal disease impacting vitamin D3 metabolism) between colonies.
Vitamin D3 insufficiency for marmosets is suggested as less than 50 ng/mL serum 25(OH)D3 in callitrichids (Power et al., 1997). Marmosets diagnosed with bone disease have levels less than 20 ng/mL (Power et al., 1997; Shinki et al., 1983; Yamaguchi et al., 1986). We identified ten marmosets (4 females and 6 males) with 25(OH)D3 values less than 50 ng/mL. The percent of the affected population sampled was 6% in both populations (Colony A 7/113 and Colony B 3/52). 1,25(OH)2D3 values for these ten marmosets were 72–1095 pg/mL. Similar deficiency levels indicate that both levels of dietary vitamin D3 are sufficient to maintain > 50 ng/mL serum 25(OH)D3. Hypovitaminosis D elicits bone remodeling and secondary hyperparathyroidism to maintain mineral homeostasis (Dusso, 2011). Adequate vitamin D3 is defined as the 25(OH)D3 serum concentration associated with a plateau in parathyroid hormone (PTH) concentration (Saliba et al., 2011). We did not measure PTH in this study, but future comparison would characterize effect of elevated 25(OH)D3 and 24,25(OH)2D3 on PTH.
Table 4 provides reported 25(OH)D3 levels in marmoset colonies. When comparing reports of 25(OH)D3, important considerations include: concentration of vitamin D3 in the feed (ideally reported as a unit of dry matter to compare diets of varying moisture levels), proportion of primary diet consumed relative to supplemental food items, environmental exposure to UVB rays, gastrointestinal or renal disease incidence within colony, colony age distribution, and assay used to measure vitamin D3 metabolites. No difference was found between 25(OH)D3 detected via radioimmunoassay and liquid chromatography mass spectrometry; this provides evidence that comparison between these assays is feasible (Ziegler et al., 2015). Overall, increased dietary provision of vitamin D3 leads to increased serum 25(OH)D3. The range of 25(OH)D3 values within each marmoset colony was wide; suggesting pronounced individual variability likely associated with digestive efficiency or food intake variation (Bosseler et al., 2018; Jarcho et al., 2013; Ziegler et al., 2015).
Table 4.
Reported 25(OH)D3 means and standard deviations in marmoset colonies.
| Facility | Publication | N | Assay | Vitamin D3 IU/kg feed (as fed) | Mean 25(OH)D3 (ng/mL) | Standard deviation 25(OH)D3 (ng/mL) |
|---|---|---|---|---|---|---|
| Brazil, wild population | Teixeira, 2012 | 15 | RIA | 0† | 61.7 | 20.8 |
| Brazil, University of Brasilia Primatology Center | Teixeira, 2010 | 29 | RIA | 0† | 121 | 33 |
| Netherlands, Biomedical Primate Research Centre | Bosseler, 2018 | 67 | RIA | 3000† | 82.8 | 41.2 |
| Texas, Southwestern National Primate Research Center | Jarcho, 2013 | 35 | RIA | 8000 | 119 | 46.2 |
| Wisconsin, New England Primate Research Center, Colony B | Unpublished, 2015 | 28 | LCMS | 8000 | 179.76 | 56.41 |
| Wisconsin, New England Primate Research Center, Colony B | Current data | 52 | LCMS | 8000 | 215 | 112 |
| Texas, Southwestern National Primate Research Center | Jarcho, 2013 | 35 | RIA | 9000 | 89.4 | 32.3 |
| Wisconsin, Wisconsin National Primate Research Center, Colony A | Ziegler, 2015 | 25 | LCMS | 13,300 | 394.81 | 43.5 |
| Wisconsin, Wisconsin National Primate Research Center, Colony A | Current data | 133 | LCMS | 13,300 | 425.84 | 200.48 |
Populations had access to sunlight which facilitates endogenous vitamin D3 production
The minimum amount of dietary vitamin D3 necessary for marmosets and toxicity threshold remains unknown. Increased activation of vitamin D receptors may impact phenotype before evidence of toxicity is observed (Jones, 2008). A strategy to ensure adequate vitamin D3 is provided in the diet is providing the minimum dietary D3 needed to achieve 25(OH)D3 levels reported in wild Callithrix pencillata (61.7 ng/mL mean, standard deviation 20.8 ng/mL) (Teixeira et al., 2012). 110 IU vitamin D3 per 100 g of body weight has been suggested (Hatt & Sainsbury, 1998; Takahashi et al., 1985), but our data would suggest that this level is high, and that marmosets that ingest vitamin D3 at these levels will upregulate their ability to convert 25(OH)D3 to 24,25(OH)2D3. Provision of 8000 IU D3 per kg of diet (8,888 IU per kg dry matter) in Colony B provides approximately 135 IU per marmoset (assumes consumption of 15 grams dry matter feed in an average 350–400 g marmoset), which would be approximately 30 IU vitamin D3 per 100g body weight. This dietary level results in 25(OH)D3 serum levels greater than found in wild marmosets and a lower vitamin D3 dose may be sufficient. Individual marmosets with clinical conditions predisposing to gastrointestinal malabsorption (Jarcho et al., 2013) or marmosets that don’t regularly consume an adequate amount of the primary diet may require vitamin D3 supplementation beyond what is provided in the primary diet. Human conditions also noted in marmosets that might warrant screening include: bone fractures, osteoporosis, chronic kidney disease, pregnancy, and lipid malabsorption (Dirks et al., 2018).
In summary, we found that marmosets consuming diets with 26,367 IU/kg dry matter vitamin D3 had higher serum levels of both 25(OH)D3 and 24,25(OH)2D3 than marmosets consuming diets with 8,888 IU/kg of dry matter vitamin D3. 1,25(OH)2D3 serum levels were the same. The ability of marmosets to greatly upregulate their conversion of 25(OH)D3 to 24,25(OH)2D3 appears to explain their tolerance of high levels of dietary vitamin D3 that would be toxic to other species. Whether elevated 24,25(OH)2D3 has any significant biological effects is uncertain but should be explored if marmosets continue to be fed diets high in vitamin D3. The association of a high serum calcium: phosphorus ratio with high 25(OH)D3 and 24,25(OH)2D3 does indicate that feeding marmosets high levels of vitamin D3 may have potential metabolic consequences. The elevated 25(OH)D3 serum levels compared to wild populations, similar 1,25(OH)2D3 despite difference in dietary vitamin D3, and elevated 24,25(OH)2D3 serum levels in Colony A suggests lower dietary vitamin D3 levels may be sufficient, but care must be taken to ensure marmosets with lower digestive efficiency or reduced primary diet intake receive adequate supplementation. Further studies to evaluate the impact of dietary vitamin D3 on active metabolites and PTH are necessary to refine supplementation recommendations and reduce nutritional induced variability amongst research colonies.
Acknowledgements
We acknowledge the veterinary technical support provided by Megan Sosa and Matthew Zblewski, statistical consultations provided by Nicholas Keuler, and generation of technique for sample analysis by Amita Kapoor and Robinson Goy. We’d also like to thank our animal research technologist teams for ensuring the marmoset’s environmental, nutritional, and behavioral needs are met every day. Funding for this research was provided by Public Health Services grant R24OD020347 and was made possible in part by NCRR/ORIP grant P51OD011106 to the Wisconsin National Primate Research Center, University of Wisconsin, Madison.
References
- Adams JS, Chen H, Chun RF, Nguyen L, Wu S, Ren SY, Barsony J, & Gacad MA (2003, February). Novel regulators of vitamin D action and metabolism: Lessons learned at the Los Angeles zoo. J Cell Biochem, 88(2), 308–314. 10.1002/jcb.10333 [DOI] [PubMed] [Google Scholar]
- Baxter VK, Shaw GC, Sotuyo NP, Carlson CS, Olson EJ, Zink MC, Mankowski JL, Adams RJ, Hutchinson EK, & Metcalf Pate KA (2013). Serum albumin and body weight as biomarkers for the antemortem identification of bone and gastrointestinal disease in the common marmoset. PLoS One, 8(12), e82747 10.1371/journal.pone.0082747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD (2014, Mar). Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol, 21(3), 319–329. 10.1016/j.chembiol.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosseler L, Bakker J, Duchateau L, Remarque E, Langermans JAM, Cornillie P, & Chiers K (2018, February). 25-OH-vitamin D, parathyroid hormone, and calcium serum levels in captive common marmosets (Callithrix jacchus): Reference values and effect of age, sex, season, and closure of long bone epiphyses. J Med Primatol. 10.1111/jmp.12334 [DOI] [PubMed] [Google Scholar]
- Dirks NF, Ackermans MT, Lips P, de Jongh RT, Vervloet MG, de Jonge R, & Heijboer AC (2018, April). The When, What & How of Measuring Vitamin D Metabolism in Clinical Medicine. Nutrients, 10(4). 10.3390/nu10040482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusso AS (2011, September). Kidney disease and vitamin D levels: 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and VDR activation. Kidney Int Suppl (2011), 1(4), 136–141. 10.1038/kisup.2011.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JG, Marini RP, Wachtman LM, Tardif SD, & Mansfield K (2019). The common marmoset in captivity and biomedical research. Academic Press, an imprint of Elsevier,. [Google Scholar]
- Garg M, Rosella O, Lubel JS, & Gibson PR (2013, November). Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm Bowel Dis, 19(12), 2634–2643. 10.1097/01.MIB.0000436957.77533.b2 [DOI] [PubMed] [Google Scholar]
- Hatt JM, & Sainsbury AW (1998, July). Unusual case of metabolic bone disease in a common marmoset (Callithrix jacchus). Vet Rec, 143(3), 78–80. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Power ML, Layne-Colon DG, & Tardif SD (2013, February). Digestive efficiency mediated by serum calcium predicts bone mineral density in the common marmoset (Callithrix jacchus). Am J Primatol, 75(2), 153–160. 10.1002/ajp.22093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G (2008, August). Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr, 88(2), 582S–586S. 10.1093/ajcn/88.2.582S [DOI] [PubMed] [Google Scholar]
- Lips P (2007, November). Relative value of 25(OH)D and 1,25(OH)2D measurements. J Bone Miner Res, 22(11), 1668–1671. 10.1359/jbmr.070716 [DOI] [PubMed] [Google Scholar]
- Marx SJ, Jones G, Weinstein RS, Chrousos GP, & Renquist DM (1989, December). Differences in mineral metabolism among nonhuman primates receiving diets with only vitamin D3 or only vitamin D2. J Clin Endocrinol Metab, 69(6), 1282–1290. 10.1210/jcem-69-6-1282 [DOI] [PubMed] [Google Scholar]
- Mellanby RJ (2016, April). Beyond the skeleton: the role of vitamin D in companion animal health. J Small Anim Pract, 57(4), 175–180. 10.1111/jsap.12458 [DOI] [PubMed] [Google Scholar]
- Miller CT (2017, March). Why marmosets? Dev Neurobiol, 77(3), 237–243. 10.1002/dneu.22483 [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.). Committee on Animal Nutrition., & National Research Council (U.S.). Panel on Nonhuman Primate Nutrition. (2003). Nutrient requirements of nonhuman primates (2nd rev. ed.). National Academies Press.
- Pasquali M, Tartaglione L, Rotondi S, Muci ML, Mandanici G, Farcomeni A, Marangella M, & Mazzaferro S (2015, June). Calcitriol/calcifediol ratio: An indicator of vitamin D hydroxylation efficiency? BBA Clin, 3, 251–256. 10.1016/j.bbacli.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power ML, Koutsos L (2019). Marmoset Nutrition and Dietary Husbandry In Fox JG, Marini RP, Wachtman LM, Tardif SD, & Mansfield K (Eds.), The Common Marmoset in Captivity and Biomedical Research (pp. 63–73). Academic Press, an imprint of Elsevier,. [Google Scholar]
- Power ML, Adams J, Solonika K, Colman RJ, Ross C, & Tardif SD (2019, August). Diet, digestion and energy intake in captive common marmosets (Callithrix jacchus): research and management implications. Sci Rep, 9(1), 12134 10.1038/s41598-019-48643-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power ML, & Myers EW (2009, December). Digestion in the common marmoset (Callithrix jacchus), a gummivore-frugivore. Am J Primatol, 71(12), 957–963. 10.1002/ajp.20737 [DOI] [PubMed] [Google Scholar]
- Power ML, Oftedal OT, Savage A, Blumer ES, Soto LH, Chen TC, & Holick MF (1997). Assessing Vitamin D Status of Callitrichids: Baseline Data from Wild Cotton-Top Tamarins (Saguinus oedipus) in Colombia. In (Vol. 16, pp. 39–46). Washington, DC: Zoo Biology. [Google Scholar]
- Saliba W, Barnett O, Rennert HS, Lavi I, & Rennert G (2011, December). The relationship between serum 25(OH)D and parathyroid hormone levels. Am J Med, 124(12), 1165–1170. 10.1016/j.amjmed.2011.07.009 [DOI] [PubMed] [Google Scholar]
- Shinki T, Shiina Y, Takahashi N, Tanioka Y, Koizumi H, & Suda T (1983, July). Extremely high circulating levels of 1 alpha,25-dihydroxyvitamin D3 in the marmoset, a new world monkey. Biochem Biophys Res Commun, 114(2), 452–457. [DOI] [PubMed] [Google Scholar]
- Stubbs JR, Zhang S, Friedman PA, & Nolin TD (2014, November). Decreased conversion of 25-hydroxyvitamin D3 to 24,25-dihydroxyvitamin D3 following cholecalciferol therapy in patients with CKD. Clin J Am Soc Nephrol, 9(11), 1965–1973. 10.2215/cjn.03130314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Suda S, Shinki T, Horiuchi N, Shiina Y, Tanioka Y, Koizumi H, & Suda T (1985, April). The mechanism of end-organ resistance to 1 alpha,25-dihydroxycholecalciferol in the common marmoset. Biochem J, 227(2), 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JCY, Nicholls H, Piec I, Washbourne CJ, Dutton JJ, Jackson S, Greeves J, & Fraser WD (2017, August). Reference intervals for serum 24,25-dihydroxyvitamin D and the ratio with 25-hydroxyvitamin D established using a newly developed LC-MS/MS method. J Nutr Biochem, 46, 21–29. 10.1016/j.jnutbio.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Teixeira DS, Castro LC, Nóbrega YK, Almeida RC, Gandolfi L, & Pratesi R (2010, April). 25-hydroxy-vitamin D levels among Callithrix penicillata primate species raised in captivity. J Med Primatol, 39(2), 77–82. 10.1111/j.1600-0684.2009.00399.x [DOI] [PubMed] [Google Scholar]
- Teixeira DS, Nobrega YK, Valencia CE, Gandolfi L, Pratesi R, & Castro LC (2012, December). Evaluation of 25-hydroxy-vitamin D and parathyroid hormone in Callithrix penicillata primates living in their natural habitat in Brazil. J Med Primatol, 41(6), 364–371. 10.1111/jmp.12021 [DOI] [PubMed] [Google Scholar]
- Wehmeier K, Onstead-Haas LM, Wong NC, Mooradian AD, & Haas MJ (2016, August). Pro-inflammatory signaling by 24,25-dihydroxyvitamin D3 in HepG2 cells. J Mol Endocrinol, 57(2), 87–96. 10.1530/JME-16-0009 [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Kohno Y, Yamazaki T, Takahashi N, Shinki T, Horiuchi N, Suda T, Koizumi H, Tanioka Y, & Yoshiki S (1986, July). Bone in the marmoset: a resemblance to vitamin D-dependent rickets, type II. Calcif Tissue Int, 39(1), 22–27. [DOI] [PubMed] [Google Scholar]
- Zendehdel A, & Arefi M (2019, January). Molecular evidence of role of vitamin D deficiency in various extraskeletal diseases. J Cell Biochem. 10.1002/jcb.28185 [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Kapoor A, Binkley NC, Rice KS, Rogers J, Jolly CJ, & Phillips-Conroy JE (2018, December). Comparison of vitamin D metabolites in wild and captive baboons. Am J Primatol, 80(12), e22935 10.1002/ajp.22935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Kapoor A, Hedman CJ, Binkley N, & Kemnitz JW (2015, July). Measurement of 25-hydroxyvitamin D(2&3) and 1,25-dihydroxyvitamin D(2&3) by tandem mass spectrometry: A primate multispecies comparison. Am J Primatol, 77(7), 801–810. 10.1002/ajp.22403 [DOI] [PMC free article] [PubMed] [Google Scholar]