Abstract
Objectives
The purpose of this study was to assess the timing of changes in serial echocardiographic parameters in pediatric cancer survivors and to evaluate their associations with cardiomyopathy development.
Background
Pediatric cancer survivors undergo serial echocardiograms to screen for cardiotoxicity. It is not clear whether small longitudinal changes in functional or structural parameters over time have clinical significance.
Methods
This is a multicenter, retrospective, case-control study of ≥1-year survivors following the end of cancer therapy. Cardiomyopathy cases (fractional shortening [FS] ≤28% or ejection fraction [EF] ≤50% on ≥2 occasions) were matched to control subjects (FS ≥30%, EF ≥55%, not on cardiac medications) by cumulative anthracycline and chest radiation dose, follow-up duration, and age at diagnosis. Digitally archived clinical surveillance echocardiograms were quantified in a central core laboratory, blinded to patient characteristics. Using mixed models with interaction terms between time and case status, we estimated the least square mean differences of 2-dimensional, M-mode, pulsed wave Doppler, and tissue Doppler imaging–derived parameters over time between cases and control subjects.
Results
We identified 50 matched case-control pairs from 5 centers. Analysis of 412 echocardiograms (cases, n = 181; control subjects, n = 231) determined that indices of left ventricular systolic function (FS, biplane EF), diastolic function (mitral E/A ratio), and left ventricular size (end-diastolic dimension z-scores) were significantly different between cases and control subjects, even 4 years prior to the development of cardiomyopathy.
Conclusions
Longitudinal changes in cardiac functional parameters can occur relatively early in pediatric cancer survivors and are associated with the development of cardiomyopathy.
Key Words: cancer survivorship, cardiomyopathy, echocardiography, longitudinal
Abbreviations and Acronyms: EF, ejection fraction; FS, fractional shortening; PW, pulsed wave; TDI, tissue Doppler imaging
Central Illustration
Survival rates in pediatric cancer continue to improve with current 5-year overall survival rates at 80%, and over 430,000 pediatric cancer survivors living in the United States (1). However, cardiovascular disease is one of the leading contributors to late morbidity and mortality in this population (2). More specifically, those treated with anthracycline chemotherapy and/or chest radiation are at an increased risk of developing heart failure and cardiomyopathy (3,4). Many of these patients will have detectable cardiac dysfunction prior to the development of overt clinical heart failure (5). As a result, national and international groups have developed recommendations regarding echocardiographic surveillance to detect cardiac dysfunction early (6,7).
The adult cardio-oncology community has published echocardiographic guidelines for the detailed evaluation of cardiac function in adults during and after cancer therapy, including non-ejection fraction (EF)–based parameters such as global longitudinal strain (8). However, specific guidelines do not exist for pediatric patients, and there is a paucity of longitudinal data to facilitate comparable recommendations. With rare exceptions (9), most research studies examining echocardiographic changes in pediatric patients have been cross-sectional single-institution studies not enriched for cardiomyopathy cases, which makes it difficult to evaluate trajectories over time (10,11). The relative rarity at single centers of pediatric-age patients developing cardiac dysfunction has hampered the ability to rigorously study echocardiographic parameters longitudinally. Greater insight into these trajectories could help identify the subpopulation of patients who will develop subsequent, true cardiac dysfunction, and could potentially allow for earlier medical intervention.
We therefore undertook a retrospective multi-institutional study involving five pediatric cancer centers aimed at assessing the longitudinal trajectories of cardiac function and structure in pediatric cancer survivors prior to the onset of cardiomyopathy and contrasting these with closely matched survivor control subjects. Specifically, we collected and centrally analyzed serial echocardiograms previously obtained as part of routine care prior to the onset of cardiomyopathy and over a similar time period for matched control subjects. Cases who developed cardiac dysfunction were matched to control subjects, and detailed evaluation of echocardiographic indices of left ventricular (LV) systolic function, diastolic function, size, and geometry were undertaken. By taking advantage of serial echocardiograms within a multi-institutional case-control study design, we sought to address the question of which longitudinal changes in echocardiographic parameters are associated with the subsequent development of cardiac dysfunction in pediatric cancer survivors.
Methods
Patients
Eligibility for this retrospective case-control study was limited to individuals previously diagnosed with cancer at age <21 years and followed at 1 of 5 participating centers (City of Hope, Children’s Healthcare of Atlanta/Emory University, Hospital for Sick Children, Seattle Children’s Hospital, and Masonic Children’s Hospital/University of Minnesota). All participants had to survive at least 1 year beyond the end of initial cancer therapy. Cardiomyopathy cases were defined by echocardiography as having either a fractional shortening (FS) ≤28% or ejection fraction (EF) ≤50%, on at least 2 occasions, with at least 1 of those measurements occurring after completion of cancer therapy. Cases where these FS or EF criteria were met only once were also accepted if the qualifying measurement occurred after the completion of cancer therapy and led to the initiation of medical treatment for cardiomyopathy. Control subjects were individually matched to cases based on the following criteria (in order of descending priority): 1) cumulative anthracycline dose and type (50 mg/m2 increments for doxorubicin and daunorubicin; 25 mg/m2 for idarubicin and mitoxantrone); 2) chest radiotherapy (none, <15 Gy, 15 to 34 Gy, ≥35 Gy); 3) follow-up duration (±1, ±2, and ±3 years to within 5, 5 to 9, and ≥10 years of cancer diagnosis, respectively); 4) age at cancer diagnosis (±5 years); and 5) sex. Furthermore, control subjects had to have, by their institutional echocardiographic reports, FS ≥30% and EF ≥55%, without known qualitative changes concerning for cardiomyopathy during the same follow-up time interval as their matched case. To avoid issues with misclassification, control subjects also could not be treated with antihypertensive medications during the follow-up time interval. We also excluded patients with known congenital heart anomalies (except patent foramen ovale) or underlying genetic syndrome associated with abnormal cardiovascular development. The institutional review boards at all participating institutions approved the study procedures with waiver of consent.
Exposures
The medical records of cases and controls were reviewed. Information on patient demographics, the original cancer diagnosis, and any relapse, if applicable, was abstracted. Lifetime anthracycline and anthraquinone doses, by individual agent, were summed based on doxorubicin equivalency, as was any radiotherapy field potentially affecting the heart, using the Children’s Oncology Group version 5 guideline definitions (12), with the exception that mitoxantrone was considered 10 times more cardiotoxic than doxorubicin. Age and vital status at last follow-up were also recorded.
Outcomes
All available archived echocardiographic images for selected cases and matched control subjects were deidentified and submitted in DICOM format to a central core laboratory at Emory University for offline analysis, using a vendor-neutral software analysis package (TOMTEC Corporation, Chicago, Illinois). The submitted studies were obtained for routine clinical purposes from multiple institutions over a 13-year period (2004 to 2017). A single blinded reviewer (D.E.C.) analyzed all studies. Study parameters included those related to LV size and geometry (posterior wall thickness, end-diastolic dimension [EDD], and thickness-to-dimension ratio), LV systolic function (FS, EF derived by Biplane Simpson’s method, mitral Sʹ, and septal Sʹ), LV diastolic function (mitral E/A ratio, mitral Eʹ, mitral E/Eʹ, septal Eʹ, and septal E/Eʹ), and combined LV systolic/diastolic function (myocardial performance index [MPI], derived from both pulsed wave Doppler and tissue Doppler). Both 2-dimensional (2D) and M-mode measurements were made, but unless otherwise specified, all reported values are based on 2D views. We attempted to derive indices of deformation such as global longitudinal strain (GLS). However, only 10% of the submitted echocardiograms had adequate apical 2-, 3-, and 4- chamber views for accurate quantitation. This was felt to be an inadequate sample, and thus GLS was not included in the analysis.
Statistical analyses
Categorical patient characteristics and treatments are presented as frequency (percent) and were compared between cases and control subjects using chi-square tests. For continuous measures, normality was evaluated based on the Kolmogorov-Smirnov statistic, and measures are presented as mean ± SD if normally distributed or median (interquartile 25th and 75th percentiles) if not, with p values based on either Student’s t-tests or Wilcoxon tests, respectively. Because distributions of the echocardiographic parameters were normally distributed, mixed models were used to compare cases and control subjects over time prior to cardiomyopathy diagnosis or the same time for control subjects (case index time). Models included a random effect to account for matched pairs, and an autoregressive correlation structure was assumed for repeated measurements within individuals. Categorical time variables were created for echocardiogram timing prior to the index diagnosis, mapping continuous times to integer year categories as follows: index, <1, 1 to <2, 2 to <3, 3 to <4, 4 to <5, 5 to <6, 6 to <7, and ≥7 years prior to index time. Mixed models included indicator variables for all time categories, indicator of case status, and the interaction terms between time and case variables. Least square means (LSMs) with 95% confidence intervals (CIs) were estimated for each time category and displayed for cases and control subjects. Additional comparisons within specific time intervals (e.g., more than 2 years before the index time) were calculated using differences between LSMs, constructed from the relevant parameter and variance covariance estimates from the model.
During the time period more than 2 years prior to the index time, all endpoints were fit as a linear model as a function of time. To illustrate the trajectory of echocardiogram endpoints over this time for cases and control subjects, linear mixed models with continuous time, case status, and an interaction between time and case status were also fit for each endpoint; predicted means with 95% confidence bands were displayed.
Most echocardiograms were assessed post-therapy. However, an additional set of mixed models compared cases and control subjects with time categorized into 3 exclusive categories: 1) at the time of cancer diagnosis; 2) on cancer therapy; and 3) post-cancer therapy. These models all included an interaction term between case status and time, with results presented as LSMs (95% CIs) and p values. Data were analyzed using SAS (version 9.4, SAS Institute Inc., Cary, North Carolina), and a 2-sided p value <0.05 was considered statistically significant.
Results
By design, demographic and treatment characteristics among the 50 cases and 50 matched control subjects were similar (Table 1). Notably, 64% of cases were men (56% of control subjects; p = 0.414) and approximately 40% of the sample was of a minority racial/ethnic background. Among cases, the mean time interval from cancer diagnosis to the cardiomyopathy index time point was 6.4 ± 5.3 years. Most cases and control subjects received only 1 anthracycline or anthraquinone (76% or 78%, respectively); only 4% received 3 agents.
Table 1.
Demographic and Treatment Characteristics of Matched Cardiomyopathy Cases and Control Subjects
| Cases (n = 50) | Control Subjects (n = 50) | p Value∗ | |
|---|---|---|---|
| Female | 18 (36) | 22 (44) | 0.414 |
| Race/ethnicity | |||
| White, non-Hispanic | 26 (56) | 27 (63) | 0.547† |
| Black | 7 (15) | 4 (9) | |
| Asian | 7 (15) | 7 (16) | |
| Hispanic | 4 (9) | 5 (12) | |
| Multiracial | 2 (4) | 0 | |
| Unknown | 4 (8) | 7 914) | |
| Mean age at cancer diagnosis, yrs | 8.0 ± 5.5 | 7.2 ± 4.6 | 0.431 |
| Year of cancer diagnosis | 0.773 | ||
| 1991–1999 | 8 (16) | 7 (14) | |
| 2000–2005 | 17 (34) | 15 (30) | |
| 2006–2009 | 17 (34) | 22 (44) | |
| 2010–2015 | 8 (16) | 6 (12) | |
| Cancer diagnosis | 0.127 | ||
| Leukemia | 14 (28) | 14 (28) | |
| Lymphoma | 11 (22) | 15 (30) | |
| Sarcoma | 14 (28) | 18 (36) | |
| Other solid tumor | 11 (22) | 3 (6) | |
| Doxorubicin exposure | 41 (82) | 42 (84) | 0.790 |
| Median dose, mg/m2 | 260 (225–375) | 300 (200–375) | 0.934 |
| Daunorubicin exposure | 17 (34) | 14 (28) | 0.516 |
| Median dose, mg/m2 | 120 (95–300) | 100 (100–300) | 0.732 |
| Mitoxantrone exposure | 5 (10) | 5 (10) | 1.000 |
| Median dose, mg/m2 | 48 (48–50) | 48 (48–48) | n-e |
| Idarubicin exposure | 1 (2) | 2 (4) | 0.557 |
| Cumulative anthracycline dose, mg/m2‡ | 280 (200–450) | 300 (200–450) | 0.994 |
| Radiotherapy affecting the heart | 22 (44) | 21 (42) | 0.839 |
| Years of follow-up (cancer diagnosis to last follow-up) | 0.588 | ||
| <10 | 23 (46) | 29 (58) | |
| 10+ | 27 (54) | 21 (42) | |
| Years of follow-up from cancer diagnosis to first abnormal echo | N/A | ||
| <2 | 17 (34) | N/A | |
| 2–9 | 17 (34) | N/A | |
| 10+ | 16 (32) | N/A | |
| Age at first abnormal echo, yrs | |||
| 0–9 | 7 (14) | N/A | |
| 10–14 | 17 (34) | N/A | |
| 15–19 | 20 (40) | N/A | |
| 20–24 | 4 (8) | N/A | |
| 25–35 | 2 (4) | N/A | |
| Treated for cardiomyopathy | 27 (54) | N/A | |
| History of heart transplant | 1 (2) | N/A | |
| Mean age at last follow-up, yrs | 18.3 ± 5.2 | 17.2 ± 4.4 | 0.224 |
Values are n (%), mean ± SD, or median (interquartile 25th and 75th percentiles).
N/A = not applicable; n-e = not estimable.
Comparison of cases and control subjects based on chi-square test for categorical values; Student’s t-test, or Wilcoxon for continuous values.
White non-Hispanic vs. other.
Based on the following conversion for doxorubicin equivalence: daunorubicin 0.5, epirubicin 0.67, idarubicin 3.0, and mitoxantrone 10.0.
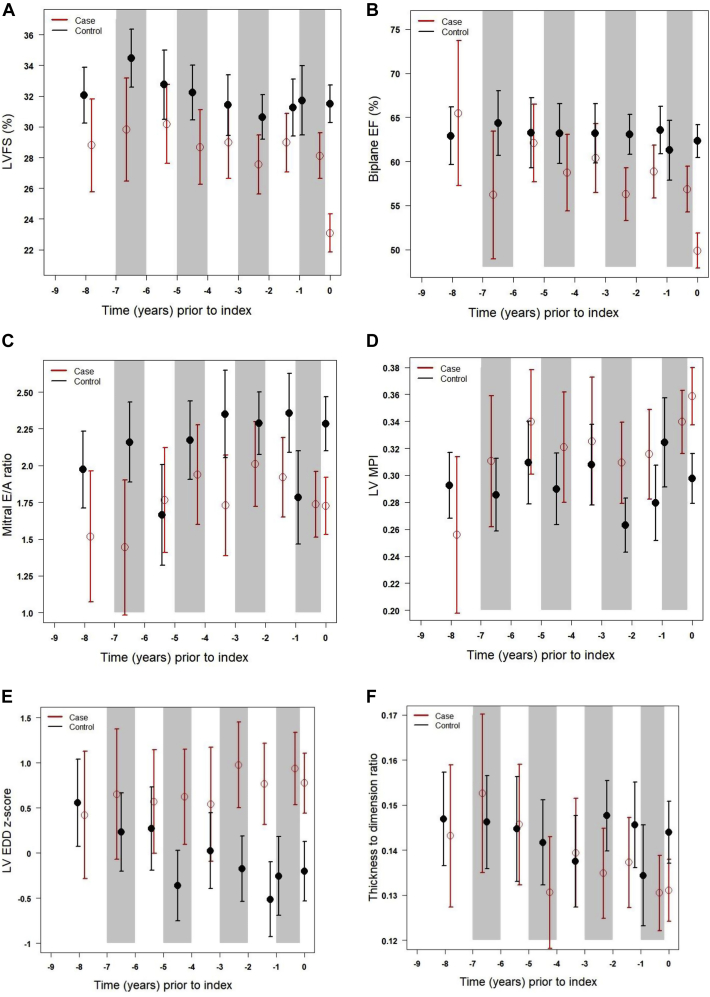
Detailed, core laboratory quantified measurements were performed on 412 echocardiograms (cases, 181 studies; control subjects, 231 studies), with a median number of 5 echocardiograms for cases and 4 echocardiograms for control subjects, and a mean follow-up time since cancer diagnosis of 5.4 ± 5.0 years for cases and 6.2 ± 4.4 years for control subjects. We then examined differences in LV systolic function, diastolic function, MPI, size, and geometry across categorical time periods. We observed significant differences between cases versus control subjects for FS, EF, mitral E/A ratio, and LVEDD across multiple time periods, whereas significant differences in MPI and LV thickness-dimension ratio were only seen at the index time point (Table 2, Figure 1). For example, even 2 to 3.9 years prior to the index point, significant differences in FS, EF, mitral E/A ratio, and LVEDD were observed, whereas the difference in MPI, derived by pulsed wave Doppler, was of borderline significance (p = 0.055). Combining estimates from all time periods other than the 2 years within the index time point, differences in FS, EF, mitral E/A ratio, and LVEDD remained significant. Results for LV FS and EDD by M-mode were similar to 2D results (data not shown).
Table 2.
Differences, Derived by LSM (95% Confidence Intervals), of Centrally Quantified Echocardiographic Parameters Between Cases and Control Subjects as a Function of Time Prior to the Index Time Point (i.e., Cardiomyopathy Diagnosis)∗
| ≥6 Yrs Prior to Index | 4 to <6 Yrs Prior to Index | 2 to <4 Yrs Prior to Index | <2 Yrs Prior to Index | Index Timepoint | All Times Prior to Index | >2 Yrs Prior to Index | |
|---|---|---|---|---|---|---|---|
| LV systolic function | |||||||
| 2D-derived FS, % | 4.0 (1.2 to 6.7) p = 0.005 |
3.1 (0.7 to 5.5) p = 0.013 |
2.8 (0.7 to 4.8) p = 0.008 |
3.0 (1.0 to 4.8) p = 0.003 |
8.4 (6.7 to 10.1) p < 0.001 |
3.2 (1.9 to 4.5) p < 0.001 |
3.3 (1.7 to 4.8) p < 0.001 |
| Biplane EF, % | 2.8 (−3.2 to 8.8) p = 0.364 |
2.8 (−1.4 to 7.0) p = 0.194 |
4.8 (1.5 to 8.1) p = 0.006 |
4.6 (1.6 to 7.6) p = 0.004 |
12.4 (9.8 to 15.0) p < 0.001 |
3.7 (1.3 to 6.1) p = 0.003 |
3.5 (0.5 to 6.4) p = 0.023 |
| Mitral Sʹ TDI, m/s | −0.005 (−0.025 to 0.015) p = 0.602 |
0.003 (−0.013 to 0.019) p = 0.700 |
0.007 (−0.007 to 0.021) p = 0.316 |
0.007 (−0.004 to 0.018) p = 0.193 |
0.027 (0.017 to 0.038) p < 0.001 |
0.003 (−0.005 to 0.011) p = 0.469 |
0.002 (−0.009 to 0.012) p = 0.745 |
| Septal Sʹ TDI, m/s | −0.005 (−0.014 to 0.003) p = 0.234 |
0.003 (−0.004 to 0.010) p = 0.406 |
0.003 (−0.004 to 0.009) p = 0.421 |
−0.003 (−0.008 to 0.003) p = 0.366 |
0.012 (0.007 to 0.017) p < 0.001 |
−0.001 (−0.004 to 0.003) p = 0.778 |
0.000 (−0.004 to 0.005) p = 0.952 |
| LV diastolic function | |||||||
| Mitral PW E/A ratio | 0.59 (0.21 to 0.96) p = 0.002 |
0.07 (−0.27 to 0.40) p = 0.699 |
0.45 (0.16 to 0.74) p = 0.002 |
0.24 (−0.02 to 0.50) p = 0.069 |
0.56 (0.32 to 0.80) p < 0.001 |
0.34 (0.16 to 0.51) p < 0.001 |
0.37 (0.16 to 0.58) p < 0.001 |
| Mitral Eʹ, m/s | 0.014 (−0.018 to 0.046); p = 0.405 |
0.004 (−0.022 to 0.029) p = 0.784 |
0.013 (−0.010 to 0.036) p = 0.268 |
−0.001 (−0.017 to 0.016) p = 0.930 |
0.045 (0.029 to 0.061) p < 0.001 |
0.007 (−0.007 to 0.021) p = 0.307 |
0.010 (−0.007 to 0.027) p = 0.257 |
| Mitral E/Eʹ ratio | 0.10 (−1.62 to 1.81) p = 0.913 |
−0.38 (−1.69 to 0.93) p = 0.567 |
0.06 (−1.23 to 1.35) p = 0.927 |
−0.26 (−1.15 to 0.62) p = 0.560 |
−0.77 (−1.63 to 0.08) p = 0.078 |
−0.12 (−0.92 to 0.67) p = 0.762 |
−0.08 (−1.04 to 0.89) p = 0.878 |
| Septal Eʹ TDI, m/s | −0.018 (−0.035 to −0.002) p = 0.032 |
0.010 (−0.004 to 0.023) p = 0.150 |
0.008 (−0.004 to 0.021) p = 0.192 |
−0.002 (−0.013−0.009) p = 0.751 |
0.018 (0.009 to 0.028) p < 0.001 |
0.000 (−0.008 to 0.007) p = 0.918 |
0.000 (−0.009 to 0.009) p = 0.991 |
| Septal E/Eʹ ratio | 1.74 (0.33 to 3.16) p = 0.016 |
−0.46 (−1.61 to 0.69) p = 0.431 |
−0.26 (−1.35 to 0.83) p = 0.639 |
−0.16 (−1.09 to 0.76) p = 0.729 |
−0.07 (−0.90 to 0.76) p = 0.867 |
0.21 (−0.46 to 0.89) p = 0.533 |
0.34 (−0.47 to 1.15) p = 0.413 |
| LV combined systolic/diastolic function | |||||||
| PW Doppler-derived MPI | 0.006 (−0.036 to 0.048) p = 0.788 |
−0.030 (−0.065 to 0.004) p = 0.088 |
−0.032 (−0.064 to 0.001) p = 0.055 |
−0.026 (−0.055 to 0.003) p = 0.084 |
−0.061 (−0.088 to −0.034) p < 0.001 |
−0.021 (−0.039 to −0.003) p = 0.026 |
−0.019 (−0.041 to 0.003) p = 0.093 |
| TDI-derived MPI | 0.020 (−0.043 to 0.084) p = 0.536 |
−0.042 (−0.091 to 0.008) p = 0.100 |
−0.032 (−0.075 to 0.011) p = 0.144 |
0.001 (−0.041 to 0.043) p = 0.962 |
−0.063 (−0.101 to −0.026) p = 0.001 |
−0.013 (−0.042 to 0.016) p = 0.374 |
−0.018 (−0.053 to 0.017) p = 0.314 |
| LV size and geometry | |||||||
| Posterior wall thickness, z-score | −0.26 (−0.81 to 0.30) p = 0.364 |
−0.11 (−0.58 to 0.35) p = 0.628 |
−0.16 (−0.57 to 0.25) p = 0.437 |
−0.03 (−0.39 to 0.33) p = 0.867 |
0.10 (−0.21 to 0.41) p = 0.532 |
−0.14 (−0.42 to 0.14) p = 0.330 |
−0.18 (−0.52 to 0.16) p = 0.305 |
| End-diastolic dimension, z-score | −0.28 (−1.04 to 0.47) p = 0.462 |
−0.87 (−1.49 to −0.25) p = 0.006 |
−1.09 (−1.64 to −0.55) p < 0.001 |
−0.81 (−1.31 to −0.32) p = 0.001 |
−1.18 (−1.62 to −0.74) p < 0.001 |
−0.77 (−1.19 to −0.34) p < 0.001 |
−0.75 (−1.24 to −0.26) p = 0.003 |
| Thickness to dimension ratio | −0.001 (−0.016 to 0.014) p = 0.864 |
0.005 (−0.008 to 0.018) p = 0.439 |
0.005 (−0.005 to 0.016) p = 0.321 |
0.006 (−0.004 to 0.016) p = 0.231 |
0.013 (0.004 to 0.022) p = 0.004 |
0.004 (−0.004 to 0.011) p = 0.323 |
0.003 (−0.006 to 0.012) p = 0.507 |
2D = 2-dimensional; EF = ejection fraction; FS = fractional shortening; LSM = least square means; LV = left ventricular; MPI = myocardial performance index; PW = pulsed wave Doppler derived; TDI = tissue Doppler imaging.
Differences were based on comparisons of LSMs from longitudinal mixed models for each measure, including an interaction between case status and time period, using an autoregressive correlation structure between echocardiograms for the same patient. For example, at the index time point, the estimated FS is 8.4% greater in control subjects compared with cases; at the <2-year time point, the estimated FS is 3.0% greater in control subjects compared with cases.
Figure 1.
Least Square Means for Echocardiographic Functional Parameters Across Time Periods Prior to Cardiomyopathy
Mean estimates (red open circles = cases; black filled circles = control subjects) with 95% confidence intervals (red bars = cases; black bars = control subjects) for categorical time periods prior to cardiomyopathy diagnosis (or index time for control subjects; denoted as time “0”) of (A) left ventricular fractional shortening (LVFS), (B) biplane Simpson’s derived ejection fraction (EF), (C) pulsed wave Doppler mitral E/A ratio, (D) pulsed wave Doppler-derived myocardial performance index (MPI), (E) left ventricular end-diastolic dimension (LVEDD), and (F) left ventricular wall thickness to dimension ratio. Shaded areas represent the same time period included in each category, with estimates and bars placed on the x-axis at the median time within the given period.
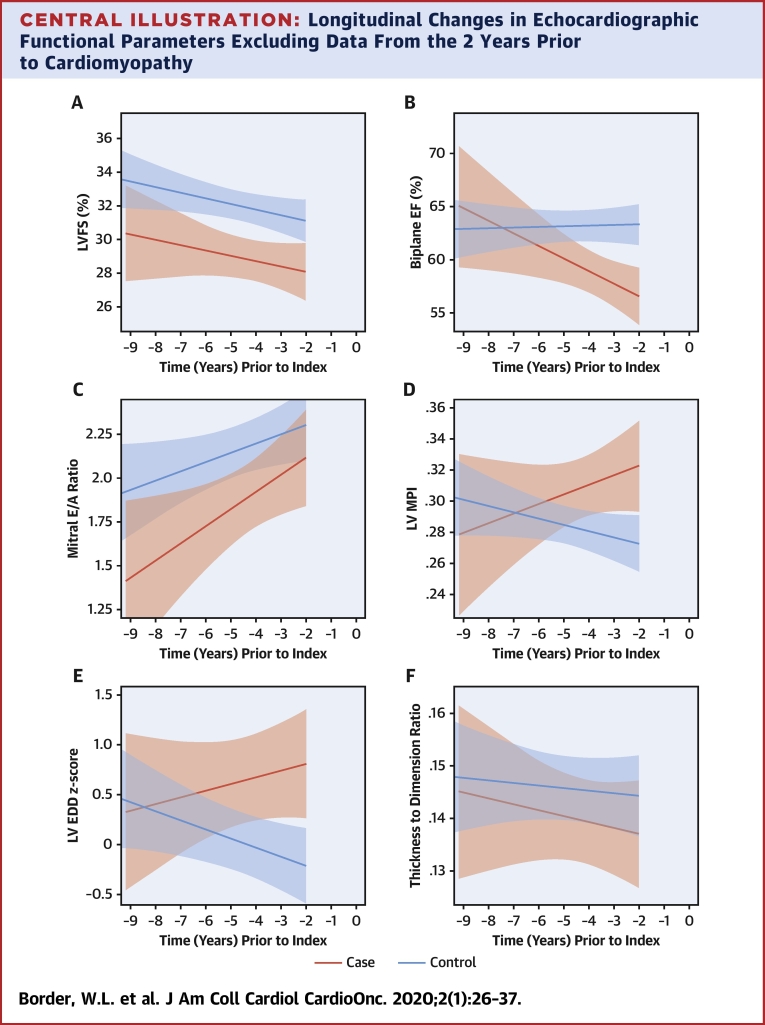
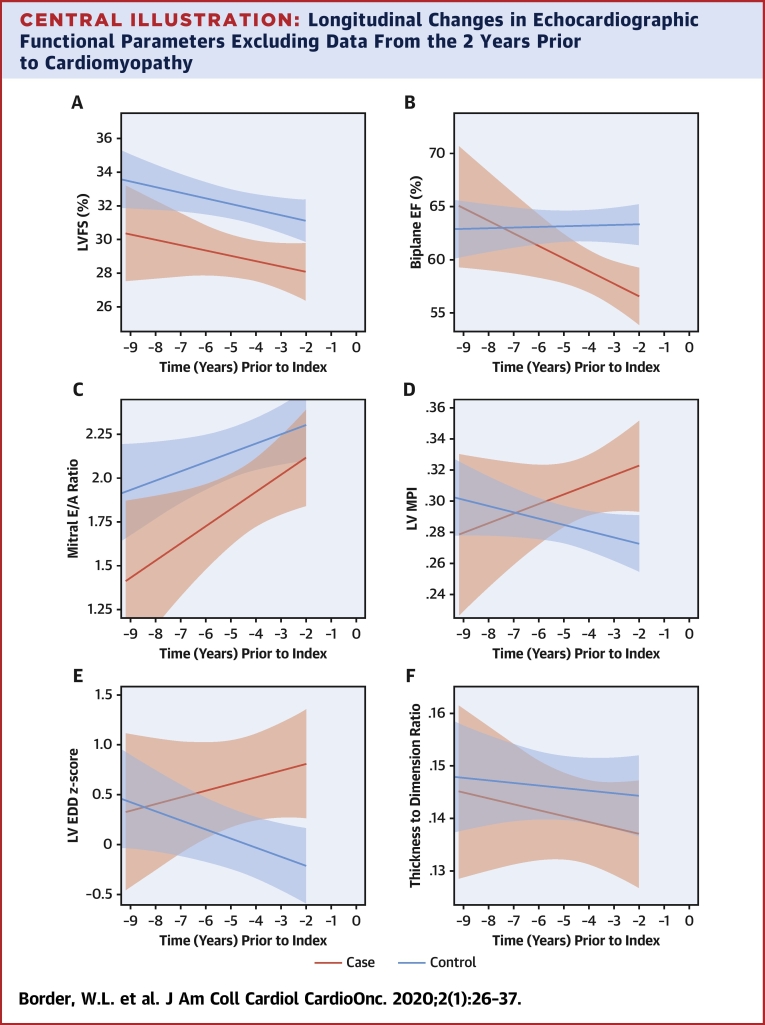
Given our interest in the trajectory of change prior to cardiomyopathy diagnosis, we also assessed differential model shapes and slopes over time, excluding data within 2 years of the index time point. In these models, which included an interaction of case status with time, significant nonlinearity was not detected, and therefore, linear models were used (Central Illustration). The slope of biplane EF (as well as septal Eʹ and septal E/Eʹ; data not shown) was significantly different between cases versus control subjects over this time period, but FS, mitral E/A ratio, pulsed wave Doppler MPI, LVEDD, and thickness-dimension ratio did not have significantly differing trajectories.
Central Illustration.
Longitudinal Changes in Echocardiographic Functional Parameters Excluding Data From the 2 Years Prior to Cardiomyopathy
Fitted line plots up to 2 years prior to cardiomyopathy, with 95% confidence intervals of (A) left ventricular (LV) fractional shortening, (B) biplane Simpson’s derived ejection fraction, (C) pulsed wave Doppler mitral E/A ratio, (D) pulsed wave Doppler-derived myocardial performance index, (E) LV end-diastolic dimension, and (F) left ventricular wall thickness to dimension ratio. Cardiomyopathy cases denoted by red line and controls denoted by the blue line, both based on a linear model. Time “0” denotes time point when cardiomyopathy was diagnosed.
Finally, we evaluated differences in central, core laboratory quantified echocardiographic parameters between cases and control subjects during 3 clinically defined time periods: at time of cancer diagnosis, while on cancer therapy, and after therapy up to and including the time of the index case echocardiogram (Table 3). Notably, significant differences between cases and control subjects were seen during the “on therapy” period.
Table 3.
Estimates, Derived by LSM of Centrally Quantified Echocardiographic Parameters for Cases and Control Subjects During 3 Time Periods: at the Time of Cancer Diagnosis; While on Cancer Therapy, and After Therapy up to and Including the Time of the Index Case Echocardiogram∗
| Case |
Control |
p Value | |||||
|---|---|---|---|---|---|---|---|
| n | Echo Time Points, n | LSM Estimate (95% CI) | n | Echo Time Points, n | LSM Estimate (95% CI) | ||
| 2D-derived FS, % | |||||||
| At cancer diagnosis | 15 | 15 | 31 (29 to 33) | 11 | 11 | 35 (33 to 38) | 0.010 |
| On therapy | 22 | 44 | 27 (26 to 29) | 14 | 3 | 34 (32 to 35) | <0.001 |
| Post-therapy | 37 | 121 | 26 (25 to 27) | 50 | 181 | 31 (30 to 32) | <0.001 |
| Biplane EF, % | |||||||
| At cancer diagnosis | 6 | 6 | 62 (57 to 66) | 3 | 3 | 64 (58 to 70) | 0.523 |
| On therapy | 10 | 13 | 55 (52 to 59) | 7 | 15 | 64 (61 to 68) | <0.001 |
| Post-therapy | 28 | 67 | 55 (53 to 56) | 41 | 99 | 63 (61 to 64) | <0.001 |
| Mitral PW E/A ratio | |||||||
| At cancer diagnosis | 13 | 13 | 1.61 (1.28 to 1.94) | 9 | 9 | 2.14 (1.75 to 2.53) | 0.035 |
| On therapy | 18 | 34 | 1.65 (1.42 to 1.88) | 13 | 33 | 1.78 (1.53 to 2.02) | 0.419 |
| Post-therapy | 37 | 114 | 1.84 (1.69 to 1.99) | 50 | 170 | 2.24 (2.12 to 2.37) | <0.001 |
| PW Doppler-derived MPI | |||||||
| At cancer diagnosis | 9 | 9 | 0.32 (0.28 to 0.36) | 9 | 9 | 0.29 (0.25 to 0.33) | 0.247 |
| On therapy | 14 | 26 | 0.33 (0.31 to 0.36) | 13 | 32 | 0.29 (0.26 to 0.31) | 0.006 |
| Post-therapy | 32 | 82 | 0.34 (0.32 to 0.35) | 50 | 159 | 0.29 (0.28 to 0.30) | <0.001 |
| LV end-diastolic dimension, z-score | |||||||
| At cancer diagnosis | 10 | 10 | 1.10 (0.54 to 1.67) | 6 | 6 | 0.34 (−0.32 to 1.00) | 0.088 |
| On therapy | 17 | 33 | 0.95 (0.53 to 1.38) | 14 | 31 | 0.10 (−0.33 to 0.53) | 0.006 |
| Post-therapy | 36 | 100 | 0.66 (0.34 to 0.97) | 47 | 157 | −0.11 (−0.39 to 0.17) | <0.001 |
| LV thickness-dimension ratio | |||||||
| At cancer diagnosis | 15 | 15 | 0.14 (0.13 to 0.15) | 11 | 11 | 0.15 (0.14 to 0.16) | 0.088 |
| On therapy | 22 | 44 | 0.13 (0.12 to 0.14) | 14 | 35 | 0.15 (0.14 to 0.16) | 0.003 |
| Post-therapy | 37 | 121 | 0.13 (0.13 to 0.14) | 50 | 182 | 0.14 (0.14 to 0.15) | 0.027 |
Abbreviations as in Table 2.
LSM, 95% confidence intervals (CIs), and p values for case-control comparisons were from longitudinal mixed models for each measure, including an interaction between case status and time period, and using an autoregressive correlation structure between echocardiograms for the same patient. As an example, the estimated FS is 31% in cases at cancer diagnosis as compared to 35% in controls at cancer diagnosis.
Discussion
This retrospective multi-institutional case-control study of pediatric cancer survivors demonstrated that there was a measurable decline in echocardiographic parameters of cardiac function and remodeling prior to developing overt cardiac dysfunction. This held true for indices of systolic function, diastolic function, and cardiac size. Even as far back as 4 years prior to the development of overt cardiac dysfunction, cases exhibited a decline in LV systolic function (both FS and EF) and diastolic function (mitral E/A) compared with matched control subjects. This trajectory has been difficult to demonstrate in the past, given the relative paucity of pediatric cancer survivors with cardiac dysfunction in individual centers.
Echocardiographic screening for cardiac dysfunction
Increased survival in pediatric cancer survivors has led to an associated increase in long-term cardiovascular complications such as cardiac dysfunction and heart failure (13,14). Select chemotherapy agents (e.g., anthracyclines) and chest radiotherapy are associated with this increased risk. Early detection of cardiac dysfunction would potentially provide the opportunity to intervene to affect pathological remodeling of the heart prior to the development of sustained abnormalities. This has led to the development of consensus-based guidelines that attempt to standardize the frequency and modality to best surveil for the development of cardiac dysfunction (7,12). Given the safety, convenience, and ubiquity of echocardiography, this modality has formed the backbone of this screening effort. However, many of the echocardiographic indices of cardiac function (such as EF and FS) have been considered binary variables (normal/not normal) in the past, making it difficult to be able to intervene before the abnormal finding is reported. This has started to change in more recent guidelines in adult cancer patients with an effort to account for trajectory of change in these indices (8).
Traditional echocardiographic measures of LV systolic function and remodeling
Early studies showed progressive cardiac dysfunction in pediatric cancer survivors primarily by documenting the development of abnormalities in systolic function as measured by fractional shortening and geometric changes, such as reduced LV mass and wall thinning (9). Similar changes have been demonstrated even in pediatric patients exposed to low doses of anthracycline (≤100 mg/m2), manifesting as decreased posterior wall thickness and decreased FS compared with control subjects (15). Interestingly, our study did not show significant differences in posterior wall thickness z-score between cases and control subjects, and only the LVEDD z-score was significantly greater in cases across multiple time intervals.
LVEF has surpassed FS as a more reliable measure of LV systolic function (16). However, the detection of abnormal FS or EF is felt to be a relatively late finding, occurring after injury and pathological remodeling have already occurred (17). This has led investigators to search for other more sensitive early markers of cardiac dysfunction. However, this has perhaps ignored the value of studying the rate of change in these more traditional indices over time, prior to crossing the threshold into dysfunction. This has also been difficult to document, given the relative lack of longitudinal data available in pediatric age cancer survivors. Lipshultz et al. (9) have previously published their experience among high-risk leukemia survivors, but among these participants, only 18 developed clinical cardiomyopathy. By evaluating serial echocardiograms in a larger group of cardiomyopathy cases prior to the onset of LV dysfunction, we were able to examine this trajectory of change and identify significant changes in FS and EF relative to control subjects up to 4 years prior. This trajectory of change raises the potential possibility of deriving a predictive equation to calculate risk of developing cardiac dysfunction in individual patients. However, our study was not sufficiently powered to be able to do this.
Echocardiographic measures of diastolic function
Diastolic dysfunction can occur in the setting of preserved systolic function, and hence, echocardiographic guidelines have recommended incorporating measures of diastolic function into standard practice (8,18). Adult studies have shown a high prevalence of diastolic abnormalities in anthracycline-exposed cancer survivors (19). However, the picture has been far more mixed in pediatric age cancer survivors, with some studies showing normal diastolic function (20) and others showing impaired relaxation and/or filling (21, 22, 23). Standard methods have included evaluating LV inflow by pulsed wave (PW) Doppler and reporting the E/A ratio as a measure of relaxation. In addition, tissue Doppler imaging (TDI) has been used to measure tissue velocities of the mitral annulus (free wall and septum), and report Eʹ velocities as a measure of relaxation. When combined with the mitral PW Doppler as the E/Eʹ ratio, this has been felt to reflect LV compliance and be a marker of restrictive physiology. In our study, when evaluated across multiple time points, only the mitral E/A ratio showed significant differences between cases and control subjects, even years before cardiomyopathy diagnosis.
Echocardiographic measures of combined systolic-diastolic function
MPI is a measure that incorporates both isovolumic contraction and isovolumic relaxation, and hence, has been used as a measure of combined systolic and diastolic function. It can be measured using both PW Doppler and TDI techniques. Studies in adult cancer survivors have shown the usefulness of MPI in detecting early cardiotoxicity (24). These findings have been replicated in pediatric age cancer survivors (25, 26, 27). In our series, differences in PW Doppler MPI and TDI MPI between cases and control subjects were noted at the time of cardiomyopathy diagnosis, but we only saw borderline differences in Doppler MPI in the years prior to cardiomyopathy diagnosis.
Advanced echocardiographic measures of deformation
Given the potential for regional myocardial abnormalities in pediatric cancer survivors and the limitations around the geometric and uniform contractility assumptions made by FS and EF, GLS has been recognized as a new method for early cardiotoxicity detection (28,29). In adult breast cancer patients, screening that includes EF and GLS has nearly twice the predictive power for future symptomatic disease compared with EF alone (30). The St. Jude Lifetime Cohort Study has provided some of the strongest data supporting a comprehensive approach utilizing EF, indices of diastolic function, and GLS. In a cohort of 1,820 adult cancer survivors, 5.8% had abnormal 3-dimensional LVEF, but in those with normal EF, 28% had abnormal GLS and 8.7% had diastolic dysfunction (31). In the present study, we attempted to perform post hoc measurements of GLS at the core echocardiography laboratory. However, this requires excellent quality apical 2-, 3-, and 4-chamber views to allow for accurate calculation of GLS. Due to the retrospective nature of our study, many of the images our study had access to (90%), especially in the earlier eras, did not have the required views or were not of an acceptable quality, and thus we had to abandon this component of the study.
Study limitations
The study required that echocardiograms were stored in DICOM format and could be uploaded to the central core laboratory for post hoc review and analysis. This meant that certain cases or control subjects could not be included in the study and may have biased us toward more of a contemporary cohort. In addition, this included less than complete echocardiographic data at earlier time points, such as time of cancer diagnosis, which limited our ability to determine the significance of any differences during those time periods. We also monitored for the issue of left truncated data (i.e., patients only having echocardiographic data starting after the time point at which their institution began archiving digitally). We attempted to minimize any bias that this could introduce among cases and control subjects by matching on echocardiogram follow-up duration and by institution.
Due to the retrospective nature of this study, we relied on the post hoc analysis of clinically obtained echocardiograms for routine surveillance from multiple institutions. We found significant variability in quality and completeness of the echocardiograms because they were not obtained with a specific research protocol in mind. Thus, not every echocardiographic measure could be obtained in each patient.
Conclusions
This multi-institutional study of 50 case-control pairs of pediatric cancer survivors demonstrated that there is a measurable longitudinal decline in many standard echocardiographic parameters of cardiac function prior to crossing the traditionally defined threshold of LV dysfunction (FS ≤28% or EF ≤50%). Cases and control subjects exhibited differences in indices of systolic function, diastolic function, and cardiac geometry. Although power limitations of the present sample size preclude development of cardiomyopathy risk prediction algorithms, these data indicate the potential role for an expanded analysis, which involves a larger cohort of cardiomyopathy cases to facilitate development of predictive models incorporating more sensitive echocardiographic indices, to identify those at risk of cardiomyopathy.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: This study demonstrates that there is a measurable decline in echocardiographic parameters of cardiac systolic function, diastolic function, and size in pediatric cancer survivors prior to the development of overt cardiac dysfunction. Even 4 years prior to overt dysfunction, cases exhibited a decline in LV systolic function (both FS and EF), diastolic function (mitral E/A), and size (LVEDD) compared with matched control subjects.
TRANSLATIONAL OUTLOOK: Future studies should consider an expanded analysis involving a larger cohort of pediatric cancer survivors with cardiomyopathy to facilitate the development of predictive models, incorporating echocardiographic indices, to allow for accurate identification of those at risk prior to the development of overt cardiac dysfunction.
Acknowledgments
The authors would like to thank the following research coordinators for their considerable work on this study: Cardiovascular Imaging Research Core at Emory/Children’s Healthcare of Atlanta (Heather Friedman, Nicole Krupa, Kelsey Zinck, and Cortlin Yancey); Children’s Healthcare of Atlanta Aflac Cancer Center (Rebecca Lewis); City of Hope Medical Center (Lanie Lindenfeld); Fred Hutchinson Cancer Research Center (Nancy Blythe); The Hospital for Sick Children (Emily Lam); and University of Minnesota Masonic Children’s Hospital (Susan C. Anderson).
Footnotes
This work was supported by a generous grant from The Rally Foundation, Atlanta, Georgia, and the National Institutes of Health (R01 CA211996). Dr. Leger has received research funding from Abbott (and upcoming Jazz funding); and has served on the advisory board of Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Amil Shah, MD, served as Guest Associate Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Robison L.L., Hudson M.M. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipshultz S.E., Adams M.J., Colan S.D. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 3.Mulrooney D.A., Yeazel M.W., Kawashima T. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger K.C., Mertens A.C., Sklar C.A. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Adams M.J., Lipshultz S.E. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer. 2005;44:600–606. doi: 10.1002/pbc.20352. [DOI] [PubMed] [Google Scholar]
- 6.Shankar S.M., Marina N., Hudson M.M. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121:e387–e396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 7.Armenian S.H., Hudson M.M., Mulder R.L. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–e136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz S.E., Lipsitz S.R., Sallan S.E. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 10.Hudson M.M., Rai S.N., Nunez C. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol. 2007;25:3635–3643. doi: 10.1200/JCO.2006.09.7451. [DOI] [PubMed] [Google Scholar]
- 11.van der Pal H.J., van Dalen E.C., Hauptmann M. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170:1247–1255. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 12.Children’s Oncology Group Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers, version 5.0. October 2018. http://www.survivorshipguidelines.org Available at:
- 13.Bloom M.W., Hamo C.E., Cardinale D. Cancer therapy-related cardiac dysfunction and heart failure: part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamo C.E., Bloom M.W., Cardinale D. Cancer therapy-related cardiac dysfunction and heart failure: part 2: prevention, treatment, guidelines, and future directions. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leger K., Slone T., Lemler M. Subclinical cardiotoxicity in childhood cancer survivors exposed to very low dose anthracycline therapy. Pediatr Blood Cancer. 2015;62:123–127. doi: 10.1002/pbc.25206. [DOI] [PubMed] [Google Scholar]
- 16.Yahalom J., Portlock C.S. Long-term cardiac and pulmonary complications of cancer therapy. Hematol Oncol Clin North Am. 2008;22:305–318. doi: 10.1016/j.hoc.2008.01.010. vii. [DOI] [PubMed] [Google Scholar]
- 17.Ewer M.S., Ewer S.M. Long-term cardiac safety of dose-dense anthracycline therapy cannot be predicted from early ejection fraction data. J Clin Oncol. 2009;27:6073–6075. doi: 10.1200/JCO.2009.25.5091. [DOI] [PubMed] [Google Scholar]
- 18.Zamorano J.L., Lancellotti P., Rodriguez Munoz D. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 19.Nagy A.C., Cserep Z., Tolnay E., Nagykalnai T., Forster T. Early diagnosis of chemotherapy-induced cardiomyopathy: a prospective tissue Doppler imaging study. Pathol Oncol Res. 2008;14:69–77. doi: 10.1007/s12253-008-9013-4. [DOI] [PubMed] [Google Scholar]
- 20.Harahsheh A., Aggarwal S., Pettersen M.D., L’Ecuyer T. Diastolic function in anthracycline-treated children. Cardiol Young. 2015;25:1130–1135. doi: 10.1017/S1047951114001760. [DOI] [PubMed] [Google Scholar]
- 21.Karakurt C., Kocak G., Ozgen U. Evaluation of the left ventricular function with tissue tracking and tissue Doppler echocardiography in pediatric malignancy survivors after anthracycline therapy. Echocardiography. 2008;25:880–887. doi: 10.1111/j.1540-8175.2008.00695.x. [DOI] [PubMed] [Google Scholar]
- 22.Santin J.C., Deheinzelin D., Junior S.P., Lopes L.F., de Camargo B. Late echocardiography assessment of systolic and diastolic function of the left ventricle in pediatric cancer survivors after anthracycline therapy. J Pediatr Hematol Oncol. 2007;29:761–765. doi: 10.1097/MPH.0b013e3181580ea2. [DOI] [PubMed] [Google Scholar]
- 23.Stapleton G.E., Stapleton S.L., Martinez A. Evaluation of longitudinal ventricular function with tissue Doppler echocardiography in children treated with anthracyclines. J Am Soc Echocardiogr. 2007;20:492–497. doi: 10.1016/j.echo.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Dodos F., Halbsguth T., Erdmann E., Hoppe U.C. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol. 2008;97:318–326. doi: 10.1007/s00392-007-0633-6. [DOI] [PubMed] [Google Scholar]
- 25.Eidem B.W., Sapp B.G., Suarez C.R., Cetta F. Usefulness of the myocardial performance index for early detection of anthracycline-induced cardiotoxicity in children. Am J Cardiol. 2001;87:1120–1122. doi: 10.1016/s0002-9149(01)01476-x. A9. [DOI] [PubMed] [Google Scholar]
- 26.Ishii M., Tsutsumi T., Himeno W. Sequential evaluation of left ventricular myocardial performance in children after anthracycline therapy. Am J Cardiol. 2000;86:1279–1281. doi: 10.1016/s0002-9149(00)01222-4. A9. [DOI] [PubMed] [Google Scholar]
- 27.Ruggiero A., De Rosa G., Rizzo D. Myocardial performance index and biochemical markers for early detection of doxorubicin-induced cardiotoxicity in children with acute lymphoblastic leukaemia. Int J Clin Oncol. 2013;18:927–933. doi: 10.1007/s10147-012-0458-9. [DOI] [PubMed] [Google Scholar]
- 28.Al-Biltagi M., Abd Rab Elrasoul Tolba O., El-Shanshory M.R., Abd El-Aziz El-Shitany N., El-Sayed El-Hawary E. Strain echocardiography in early detection of Doxorubicin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. ISRN Pediatr. 2012;2012:870549. doi: 10.5402/2012/870549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thavendiranathan P., Poulin F., Lim K.D., Plana J.C., Woo A., Marwick T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 30.Negishi K., Negishi T., Hare J.L., Haluska B.A., Plana J.C., Marwick T.H. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–498. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong G.T., Joshi V.M., Ness K.K. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–2522. doi: 10.1016/j.jacc.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]