Abstract
Objectives:
To evaluate neutrophil-enhanced Pseudomonas aeruginosa (PA) biofilm formation on silicone hydrogel contact lenses and to determine the effect of epithelial biodebris on PA adherence in contact lens storage cases.
Methods:
A fully invasive PA corneal isolate stably conjugated to green fluorescent protein was used. Unworn lotrafilcon A contact lenses were incubated at various ratios of PA to polymorphonuclear neutrophil (PMN) for 24 hours at 37°C. Lens-associated PA was evaluated using laser scanning confocal microscopy and nonviable PA were visualized using propidium iodide. Viable bacteria were enumerated by colony-forming unit (CFU) analysis. For acute epithelial cell studies, PA viability was determined after coincubation with freeze–thaw epithelial cell lysates in 96-well polystyrene plates. Levels of residual cellular debris and bacterial viability were further assessed in used contact lens storage cases.
Results:
Laser scanning confocal microscopy demonstrated that cotreatment with PMA-stimulated neutrophils increased PA adherence over 24 hours to lens surfaces with a striking alteration of PA architecture. Propidium iodide staining showed that the adherent bacteria consisted of a mixture of viable and nonviable PA; a PMN-associated increase in viable PA was confirmed by CFU (PA:PMN 0.1:1, P = 0.025; PA:PMN 1:1, P = 0.005). Acute epithelial cell debris studies revealed a significant increase in viable PA in 96-well plates in the presence of epithelial freeze–thaw lysates (PA:debris 1:1, P = 0.002; PA:debris 100:1, P = 0.002). Crystal violet staining of used lens storage cases revealed residual cellular debris at all time points, which was independent of microbial contamination; all lens cases used for periods of 9 months or more were uniformly associated with high levels of viable microorganisms.
Conclusion:
These results demonstrate that prolonged corneal inflammation with the presence of PMNs when confronted with simultaneous PA challenge in extended contact lens wear has the potential to stimulate biofilm formation on silicone hydrogel contact lenses. These findings further suggest that a persistent buildup of extracellular debris in lens storage cases may contribute to the heavy biofilms reported on these surfaces.
Keywords: Contact lens, Inflammation, Infection, Contact lens storage cases, Pseudomonas aeruginosa
Microbial keratitis (MK) is a severe potentially blinding complication of contact lens wear and for more than three decades, Pseudomonas aeruginosa (PA), an opportunistic gramnegative bacteria, has been repeatedly identified as the most common pathogen associated with contact lens–related MK.1–4 The clinical picture of an established contact lens–related MK includes the presence of an overlying epithelial defect accompanied by intense polymorphonuclear neutrophil (PMN)–mediated inflammation. Prolonged PMN recruitment during the course of the disease contributes to tissue destruction, and in severe cases, leads to scarring and vision loss.5 Early clinical and bench studies implicated hypoxic damage to the corneal epithelial surface arising from low oxygen transmissible contact lenses worn on an extended wear basis as a significant risk factor for inflammation and infection6–12; however, despite the widespread acceptance of silicone hydrogels in the lens-wearing population and a corresponding substantial improvement in corneal physiology, the risk for MK remains unchanged and the incidence of corneal inflammatory events (CIE) have doubled.13,14 Although factors including longer wearing times for silicone hydrogel lenses during extended wear and patient characteristics such as new adopters or problem solver prescribing may be likely contributors, a recent study evaluating the risk factors for CIE during silicone hydrogel lens wear identified substantial lens bioburden as a significant causative factor (8.66 hazard ratio) with PA identified as a frequent contributor to these adverse lens-related events.15
Biofilms are bacterial aggregates enclosed in an extracellular matrix that confer protection, allowing organisms to thrive under adverse conditions.16 In animal models of infection, extended contact lens wear with concurrent bacterial challenge has been shown to result in significant neutrophil accumulation and biofilm formation on posterior lens surfaces.17,18 Clinically, although biofilms are infrequently found on contact lenses, the presence of lens-associated biofilms have been reported with culture-proven MK during contact lens wear,19 including lenses worn on an extended wear basis.20 We recently have reported that PA can exploit the robust neutrophil response that occurs during bacterial challenge in extended wear to enhance biofilm formation on hydrogel contact lenses surfaces.21 Specifically, negatively charged DNA and F-actin released by necrotic neutrophils accumulate during prolonged inflammation complexed with positively charged histones, forming a scaffold or matrix to which PA adhere.22 Importantly, the dissolution of these complexes using a combination of DNAase and polyaspartic acid was effective in eliminating biofilm formation in vitro and was consistent with an observed reduction in PA internalization in corneal epithelial cells in vivo, in an established rabbit contact lens model.21,23
Recently, there has been a significant focus on microbial contamination of lens storage cases and their potential as a contributing factor in contact lens–related MK and other adverse events.13,24–27 Up to 80% of lens cases recovered in clinical trials have been reported to demonstrate some level of microbial colonization, including bacteria, fungi, and Acanthamoeba.28–32 Studies have investigated the ability of various species of bacteria to form biofilms in lens storage cases33,34; however, these studies have employed the use of sterile cases that had not previously been used. The purpose of this study is to extend our earlier hydrogel findings and further evaluate the ability of dying neutrophils to enhance PA biofilm formation on silicone hydrogel contact lenses. As the effects of DNA and F-actin are not cell type specific, we investigated a potential new role for accumulated epithelial cell biodebris to contribute to increased PA adherence in used contact lens storage cases and assessed the role of duration of lens case use in contributing to this phenomenon.
MATERIALS AND METHODS
Bacterial Strains, Media, and Culture Conditions
Bacteria were grown on Mueller Hinton II (MH) agar slants at 37°C overnight. Pseudomonas aeruginosa (PA) concentrations were adjusted using a SmartSpec 3000 spectrophotometer (Bio-Rad, Hercules, CA) at 650 nm to obtain an optical density (OD) of 0.30 (5 × 108 colony-forming unit [CFU]/mL). Pseudomonas aeruginosa strain PA6487 was an ocular isolate that was stably conjugated with green fluorescent protein (GFP) and was a gift of Dr Suzanne M. Fleiszig (University of California, Berkeley).
Neutrophil Isolation
Neutrophils for all assays were isolated from healthy volunteers by the plasma-Percoll method, as previously described.35 The blood draw procedure was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center.
Formation of Pseudomonas aeruginosa Biofilms on Contact Lens Surfaces
Contact lenses were lotrafilcon A silicone hydrogel lenses (CIBA, Duluth, GA), which were rinsed in sterile phosphate-buffered saline (PBS) before the assay. The plasma-Percoll isolated human neutrophils were resuspended in Roswell Park Memorial Institute (RPMI) and activated by the addition of 25 nM (or 60 ng/mL) phorbol 12-myristate 13-acetate (PMA; Sigma, St Louis, MO). After 1 minute of stimulation, neutrophils were pelleted by centrifugation; the supernatant was removed and diluted to a concentration of 2.5 × 106 cells per milliliter in RPMI and 2% heat inactivated platelet-poor plasma, this concentration was held constant for all experiments. Using a Costar 3526 sterile polystyrene plate (Corning, Lowell, MA), 500 μL of neutrophil suspension was added to the wells. Activation was microscopically verified after 4 hours of incubation at 37°C. Then, 500 μL of PA was added to each well to make PA:PMN ratios of 1:1 or 0.1:1 in a final well volume of 1 mL. Unworn whole lenses were then placed into all wells. Control lenses were combined with PA in the absence of neutrophils. For assays using lysed neutrophils, cells underwent a single freeze–thaw cycle. Lenses and bacteria were incubated at 37°C for 24 to 48 hours to allow for biofilm growth.
Laser Scanning Confocal Microscopy of Biofilm Formation on Contact Lens Surfaces
Laser scanning confocal microscopy was used to directly visualize adherent PA on contact lens surfaces. Before PA exposure, lenses were cut into four equal segments to facilitate imaging. Pseudomonas aeruginosa was allowed to adhere to contact lenses in the presence and absence of neutrophils in an identical manner, as described above. Nonviable bacteria with neutrophils were assessed by propidium iodide (PI) exclusion assay. Propidium iodide staining was performed by adding 1 mL of a 1:500 dilution of PI in PBS to the lens pieces for 15 minutes. All lenses were washed twice before imaging. Lenses were imaged using a Leica SP2 laser scanning confocal microscope (Leica Microsystems, Heidelberg, Germany) with a ×63 water objective. A 488 nm Ar/ArKr laser was used for excitation of GFP, and a 535 nm GreNe laser was used to excite PI.
Quantization of Viable Bacteria Adherent to Contact Lens Surfaces
Colony-forming unit analysis was used to quantify the amount of viable PA adherent to lens surfaces. After overnight incubation in PA with or without neutrophils, lenses were washed twice in PBS and then individually placed in 500 μL of RPMI for homogenization with a Polytron benchtop homogenizer (Brinkman Instruments, Westbury, NY). An RPMI of 500 μL was run through the homogenizer to remove any excess lens/bacteria. After homogenization, the samples were vortexed, serially diluted in RPMI, and plated in triplicate on MH agar plates for overnight growth and CFU analysis.
Live/Dead Assay of Corneal Epithelial Cells
A live/dead assay was used to confirm the loss of viability of corneal epithelial cells after a single freeze–thaw cycle. Telomerase-immortalized human corneal epithelial cells (hTCEpi) were used as the cell source for epithelial debris experiments. The hTCEpi cells were cultured, as previously described.36 Corneal epithelial cells were harvested at a concentration of 2.5 × 106 cells per milliliter and held constant through all experiments. For epithelial freeze–thaw lysates, 1 mL of corneal epithelial cells were exposed to a single overnight freeze–thaw cycle at −20°C. For live/dead assays, hTCEpi cells were seeded onto Lab-Tek II chambered 1.5 cover glass and allowed to grow overnight in at 37°C. Cells were stained using a calcein-ethidium homodimer-1 (live-dead assay; Invtirogen, Carlsbad, CA) according to manufacturer’s instructions and allowed to incubate at room temperature for 45 minutes. For freeze–thaw lysates, identical concentrations of cells were centrifuged as needed, and excess fluid was removed from all wells. Cells were stained in suspension, as described above. Stained cellular debris was plated onto Lab-Tek II chambered 1.5 cover glass for subsequent analysis. Imaging was performed using a Leica SP2 laser scanning confocal microscope (Leica Microsystems, Heidelberg, Germany) with a ×20 objective.
Crystal Violet Assay for Biodebris in Used Contact Lens Case Wells
Crystal violet (CV) is a dye that is commonly used as a histologic stain but also binds DNA and has a high affinity for proteins. Crystal violet was used in this study to compare the density of residual cellular debris in used contact lens storage cases. Sterile unused lens cases from Alcon Laboratories (Fort Worth, TX) and Abbott Medical Optics (Santa Ana, CA) were used as positive and negative controls in all experiments. As a positive control, viable corneal epithelial cells were resuspended at 2.5 × 106 cells per milliliter in PBS with 1% Luria broth (LB) and subjected to a freeze–thaw cycle for use as necrotic cellular debris. Next, 1 mL of cellular debris was added to a sterile contact lens case and allowed to sit at room temperature for 3 days. No debris was added to the negative control. After removing the liquid from the respective wells of all control and used lens cases, 2 mL of CV was added and incubated at room temperature for 15 minutes. The CV was removed, and the lens cases were washed twice with distilled water, then air dried at room temperature for 2 hours. Crystal violet was subsequently dissolved using 2 mL of ethanol for 15 minutes at room temperature, and 400 μL of each solution was transferred in triplicate into a Cellstar sterile polystyrene flat-bottom 96-well cell culture plate (Greiner Bio-one, Monroe, NC) and read on an iMark Microplate Absorbance Reader (Bio-Rad) at an OD of 595 nm. Values in the negative control samples were averaged for adjustment of each experimental well.
MTT Assay for Microbial Viability in Used Contact Lens Case Wells and 96-Well Culture Plates
96-Well Cell Culture Plates
A (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) MTT assay was used to determine bacterial viability. The MTT detects cell viability by the reduction of a tetrazolium salt in metabolically active cells. In this assay, MTT was used to determine the number of viable adherent bacteria in a 96-well plate both in the presence and absence of epithelial debris. Viable corneal epithelial cells were resuspended at 2.5 × 106 cells per milliliter in PBS with 1% LB and subjected to a freeze–thaw cycle for use as cellular debris. Then, 200 μL of freeze-thawed corneal epithelial cell lysates were added to each experimental well of a Cellstar sterile polystyrene flatbottom 96-well cell culture plate (Greiner Bio-one). Next, 200 μL of 2.5 × 108 CFU per milliliter and 2.5 × 106 CFU per milliliter was added to their respective wells in duplicate and incubated at 37°C for 24 hours. A well with 1× PBS containing 1% LB (PBS:LB) was used as a negative control. Liquid was removed from each well and washed once with PBS:LB. Then, 180 μL of LB was mixed with 18 μL of MTT and added to each well. The mixture was allowed to incubate at 37°C for 2 hours. Then, 200 μL of sodium dodecyl sulfate hydrochloric acid (SDS-HCl) was added to each well and incubated at 37°C for 4 hours. The OD of the 96-well plates was measured using an iMark Microplate Absorbance Reader (Bio-Rad) at an OD of 595 nm with a reference OD of 655 nm. Values in the negative control samples were averaged for adjustment of each experimental well.
Contact Lens Storage Cases
New and used flat-well contact lens storage cases were used to evaluate bacterial contamination by MTT assay. Used contact lens cases were collected from campus participants at the University of Texas Southwestern Medical Center. Participation was voluntary, and all volunteers signed an informed consent before submitting their lens case for analysis. Lens cases were subsequently grouped into approximate age categories based on information recorded from the participant at the time of collection. Lens case collection and analysis was approved by the Institutional Review Board the University of Texas Southwestern Medical Center. Sterile unused lens cases from Alcon Laboratories and Abbott Medical Optics were used as positive and negative controls in all experiments. After removing all liquid and lenses from the respective wells, 1 mL of LB was added to each well with 100 μL of MTT and allowed to incubate at 37°C for 2 hours. Then, 1 mL of SDS-HCl was added to dissolve the MTT and allowed to incubate at 37°C for 4 hours. Each sample was mixed, and 400 μL of each was placed into a 96-well plate in triplicate. Solutions were analyzed using an iMark Microplate Absorbance Reader (Bio-Rad) at an OD of 595 nm with a reference OD of 655 nm. Values in the negative control samples were averaged for adjustment of each experimental well.
Statistical Analysis
Data were analyzed using Sigma Plot (Systat Software, San Jose, CA). For comparisons between 2 groups, a t test or Wilcox rank sum test was used when appropriate. For comparisons between multiple groups, a two-way analysis of variance was performed. A Holm–Sidak post hoc multiple comparison test was used to identify differences between the groups. Statistical significance was set at P < 0.05.
RESULTS
Neutrophil-Enhanced Pseudomonas aeruginosa Adherence on Silicone Hydrogel Contact Lenses
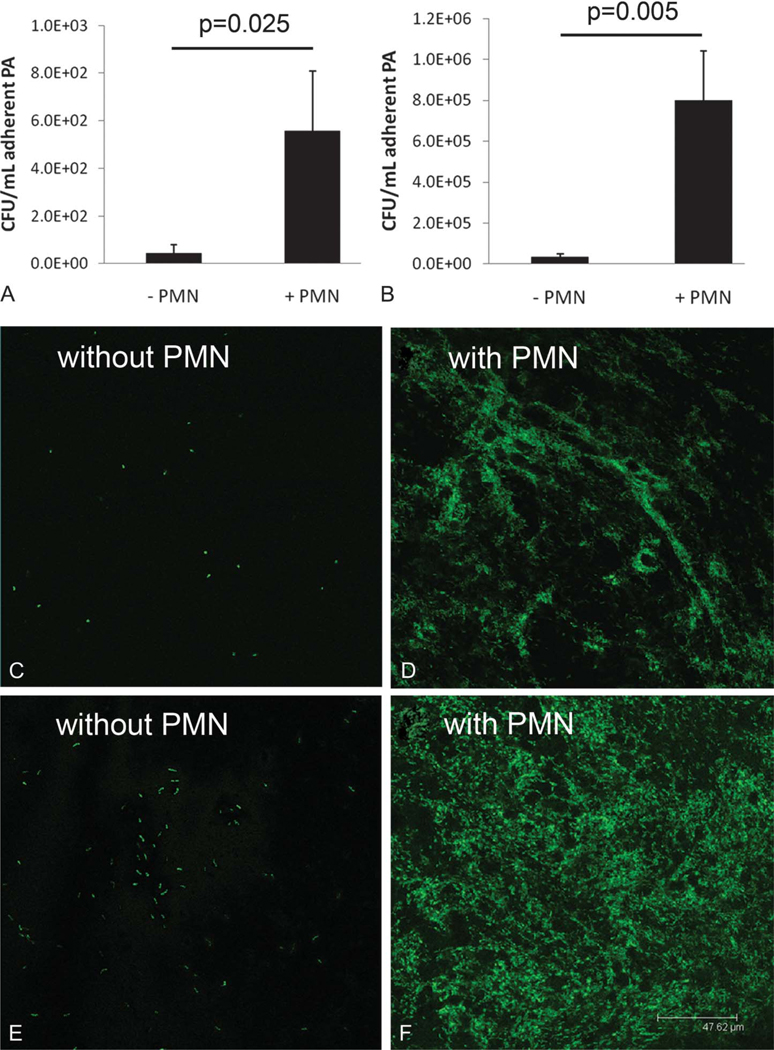
Assessment of viable PA to lotafilcon A contact lenses showed a significant increase in lens-associated PA when incubated in the presence of PMA-stimulated neutrophils over 24 hours at a PA:PMN ratio of 0.1:1 (Fig. 1A, P = 0.025). This was a 12.5-fold increase compared with lenses incubated in PA alone. Keeping the neutrophil concentration constant, the effect was more robust, with a 22.5-fold increase in lens-associated PA when incubated at a PA:PMN ratio of 1:1 (Fig. 1B, P = 0.025). Similar to CFU findings, laser scanning confocal microscopy demonstrated an increase in GFP-labeled PA to the lens surface when incubated in the presence of PMA-stimulated neutrophils compared with PA alone at both PA:PMN ratios tested. Unlike lenses incubated in the presence of PA alone, neutrophil-enhanced PA adherence to the lotrafilcon A lens showed a denser, more ordered arrangement of PA on the lens surface (Figs. 1C–F). This effect was further enhanced at 48 hours (data not shown). Counterstaining GFP-labeled PA with PI revealed a mixture of viable and nonviable bacteria adherent to the lens surface for both lenses incubated in PA alone and those incubated in the presence of PMA-stimulated neutrophils at a PA:PMN ratio of 1:1 (Figs. 2A, B). All PMA-stimulated neutrophils stained positive for PI, confirming the loss of viability after 24 hours of coincubation in the presence of PA. Interestingly, PA appeared to coalesce around dying neutrophils (Fig. 2B) and was occasionally detected adherent to dead cells (Fig. 2D). When incubated in the presence of freeze–thaw neutrophil lysates, PA displayed increased adherence to the lens surface similar to that seen with stimulated cells (Fig. 2C).
FIG. 1.
Neutrophil-enhanced PA adherence to silicone hydrogel lenses. (A) PA:PMN ratio of 0.1:1 showed a 12.5× increase in CFU per milliliter compared with PA alone (P = 0.025, t test). (B) PA:PMN ratio of 1:1 showed a 22.5× increase in CFU per milliliter compared with PA alone (P = 0.005, t test). Data are representative of 3 independent experiments (n = 3). (C–F), Laser scanning confocal microscopy of GFP-labeled PA on contact lens surfaces. Lenses were incubated for 24 hours at PA:PMN ratios of 0.1:1 (C, D) and 1:1 (E, F). Neutrophil concentration remained constant at 2.5 × 106 cells per milliliter. There was a dramatic increase in lens-associated PA in the presence of PMA-stimulated neutrophils at both inoculum levels. Scale: 47.62 μm.
FIG. 2.
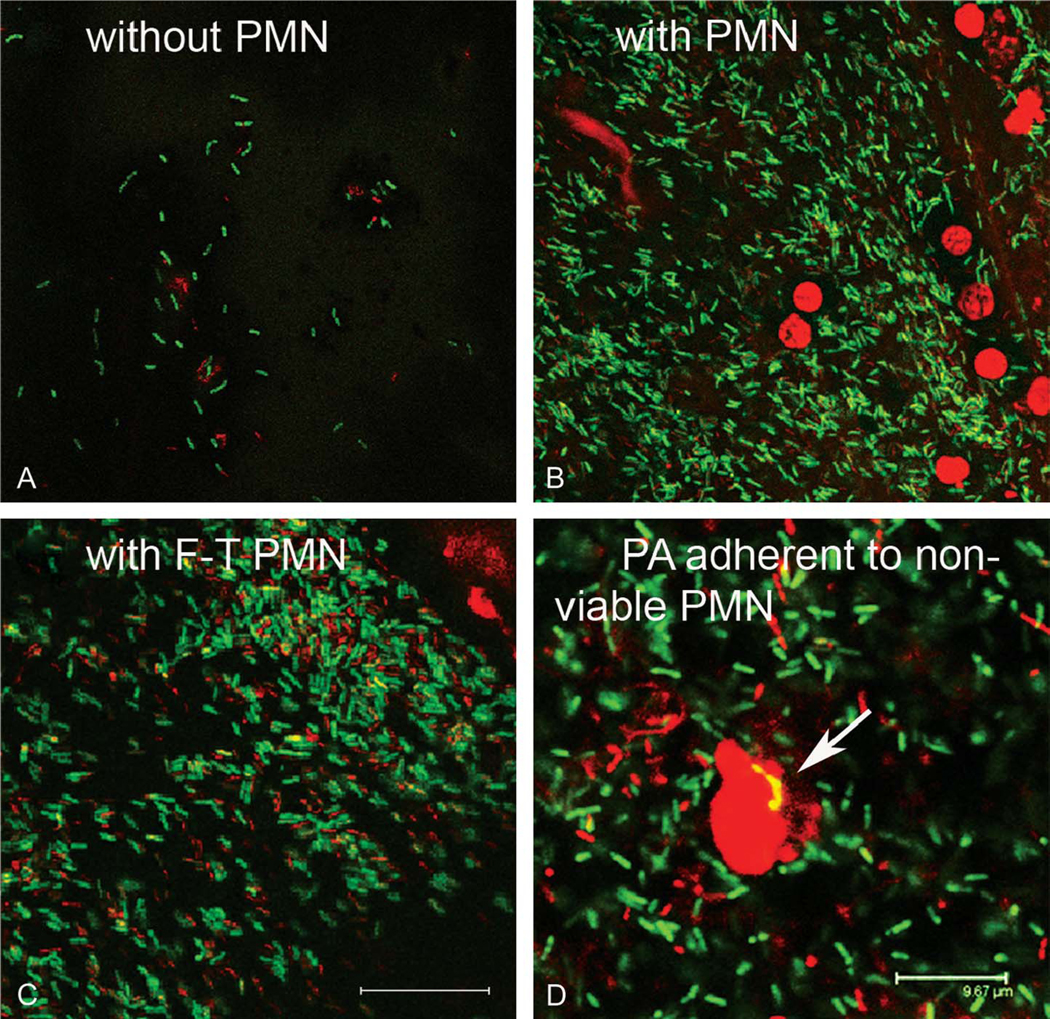
Assessment of viable lens-associated PA. Contact lenses were incubated for 24 hours at a PA:PMN ratio of 1:1. There was significant increase in viable and nonviable PA after exposure to either PMA-stimulated neutrophils or freeze–thaw neutrophils cell lysates compared with PA alone. (A) PA alone; (B) PA with PMA-stimulated neutrophils; (C) PA with freeze–thaw (F-T) neutrophil cell lysates. Scale: 14.58 μm. (D) Zoomed image of PA incubated in the presence of PMA-stimulated neutrophils showing PA adherent to dead cells (arrow). Scale: 9.7 μm.
Epithelial Cellular Debris-Enhanced Pseudomonas aeruginosa Adherence in 96-Well Plates
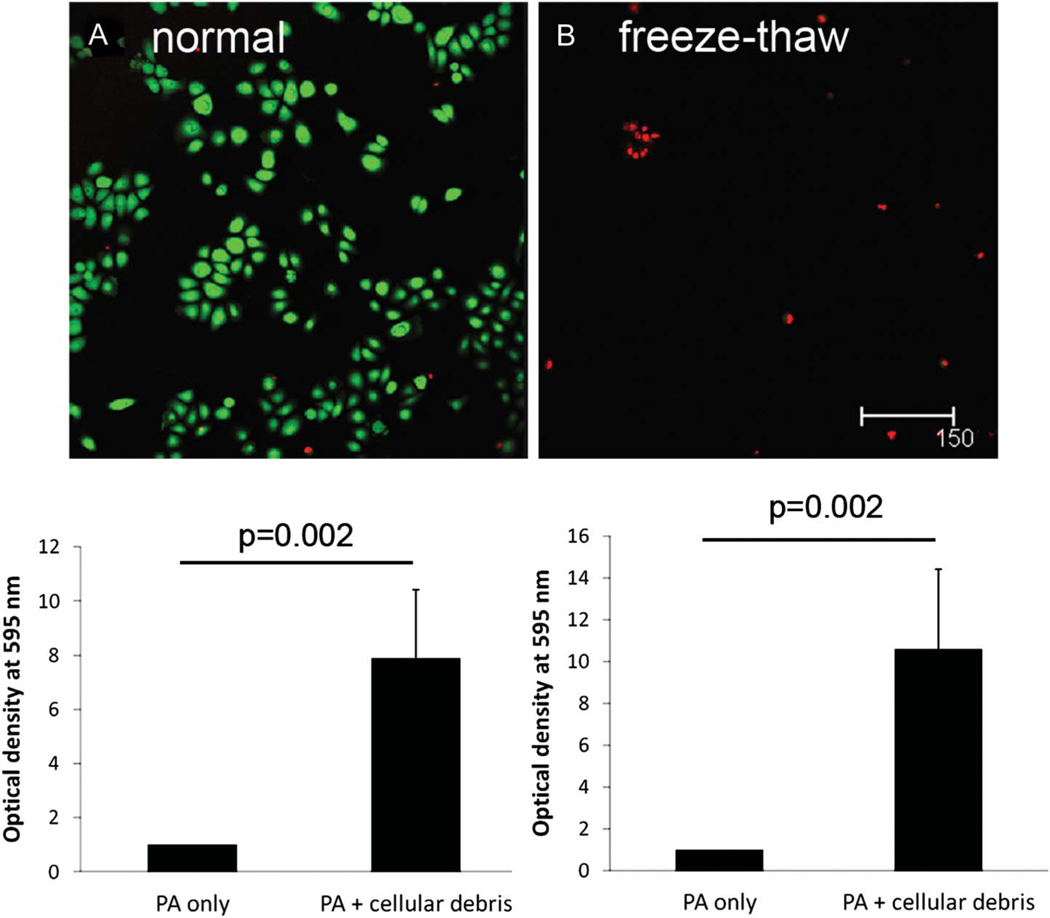
Laser scanning confocal microscopy confirmed the loss of viability of corneal epithelial cells after a single freeze–thaw cycle (Figs. 3A, B). When incubated with epithelial cell freeze–thaw lysates at a PA-tocellular debris ratio of 1:1 in a 96-polystyrene-well plate over 24 hours, there was a 7.9-fold increase in viable PA adherent to the lens well surface compared with PA alone (Fig. 3C; P = 0.002). This effect was increased in the presence of a higher inoculum, with a 10.6-fold increase in adhered PA at a PA-to-cellular debris ratio of 100:1 (Fig. 3D; P = 0.002).
FIG. 3.
Epithelial debris-enhanced PA adherence. Live/dead cytotoxicity viability assay was used to confirm loss of corneal epithelial cell viability after a single freeze–thaw cycle. (A) Viable hTCEpi cell control (green). (B) Nonviable freeze–thaw corneal epithelial cell debris (red); Scale: 150 μm. Viable adherent PA in the presence of epithelial was examined using an MTT assay. (C) PA-to-cellular debris ratio of 1:1 showed a 7.9× increase in viable PA adherent to a 96-well plate compared with PA alone (P = 0.002, t test). (D) PA-to-cellular debris ratio of 100:1 showed a 10.6× increase in CFU per milliliter compared with PA alone (P = 0.002, t test). Data are representative of three independent experiments. Freeze–thaw epithelial cell lysates held constant at a prefreeze concentration of 1 × 106 cells per milliliter.
Effect of Lens Case Age on Debris Buildup and Adherence of Pseudomonas aeruginosa
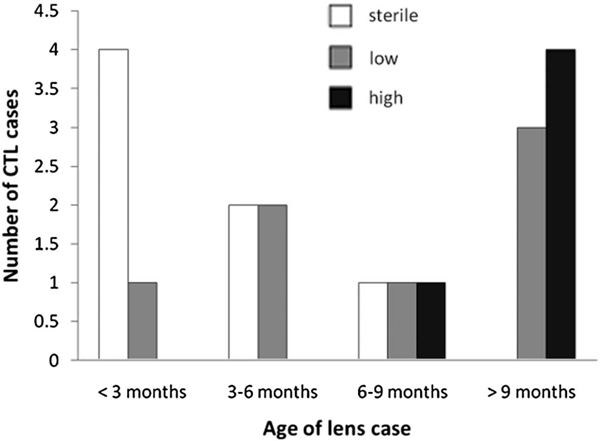
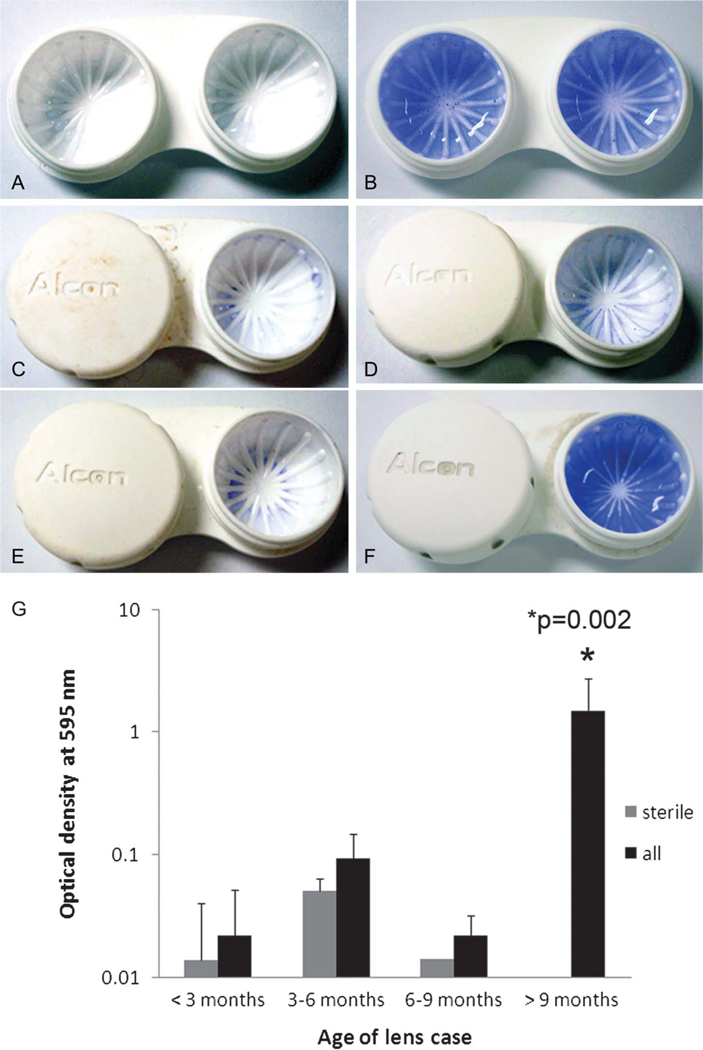
A total of 19 used lens storage cases were collected for analysis. Twelve lens cases were from Alcon Laboratories, four were from Abbott Medical Optics, two were from Bausch & Lomb, and one case was generic. Lens cases were grouped according to approximate time in use provided by the wearers. The distribution of lens cases by time of use is shown in Figure 4. An MTT assay for the presence of viable microorganisms indicated an overall contamination rate of 66%. There was an increase in the number of contaminated lens cases and the level of microbial contamination as a function of increasing time of use (Fig. 4). All cases used for 9 months or longer demonstrated high levels of microbial activity. Crystal violet staining of sterile lens storage cases showed dark blue staining evenly across the well surface after a 3-day incubation with freeze–thaw epithelial cell lysates using approximately 1×106 cells per milliliter (Fig. 5B); by contrast, no staining was evident in the sterile unused lens case in the absence of exogenous cellular debris (Fig. 5A). In used lens cases, there were low-to-moderate levels of CV staining in all cases used for periods of <9 months. In these cases, CV staining was present regardless of the presence of viable microbes (Fig. 5G). Levels of CV staining were not significantly different between these groups (P = 0.953). For cases used for durations >9 months, all cases had significantly higher levels of CV staining compared with all other time points (Fig. 5G; P = 0.002).
FIG. 4.
Distribution of used contact lens cases as a function of age and level of microbial contamination. Level of microbial contamination was classified as sterile (no metabolic activity demonstrated by MTT); low (positive metabolic activity but failed to show visual color change; and high (positive metabolic activity accompanied by visual color change). Select “high” cases required additional dilutions before readings could be obtained because of extremely elevated levels of viable microorganisms. All cases used for durations >9 months were contaminated.
FIG. 5.
Crystal violet staining of used lens storage cases. (A) Sterile case, negative control. (B) 3-day incubation in freeze–thaw epithelial cell lysate containing 1 × 106 cells per milliliter, positive control. (C–F) Representative contact lens storage cases at respective time points; (C) <3 months of use; (D) 3 to 6 months of use; (E) 6 to 9 months of use; (F) >9 months of use. (G) A significantly elevated amount of CV staining was detected in storage cases used for periods of >9 months compared with shorter durations (P = 0.002, 2-way analysis of variance, Holm–Sidak multiple comparison test). There was no difference between levels of CV staining between sterile and contaminated cases at earlier time points (P = 0.953).
DISCUSSION
The results of this study suggest that prolonged neutrophil recruitment during extended lens wear contributes to an increase in lens-associated PA adherence to silicone hydrogel contact lenses. This finding was evident even at low inoculum levels (PA:PMN ratio of 0.001:1, data not shown). In the current study, we detected a significant increase in viable PA adherent to lens surfaces in the presence of PMA-stimulated neutrophils when tested at various PA:PMN ratios. These findings are consistent with an earlier study that demonstrated a robust increase in viable PA adherent to hydrogel contact lenses over 24 hours when incubated in the presence of PMA-stimulated neutrophils.21 Although biofilm formation, as defined by the presence of a true extracellular matrix, was not evaluated in this study, there was a significant dramatic alteration in the density and organization of lens-associated PA in the presence of dying neutrophils that corresponded with the significant increase in CFU values reported. In addition to viable PA recovered from the lens surface, there was a similar visually apparent increase in nonviable adherent PA detected. The presence of extracellular bacterial DNA arising from bacteriolysis has previously been identified as an essential component to early biofilm formation and in vivo37,38 can further exacerbate an inflammatory response through continued neutrophil activation.39
Although true biofilms are infrequently found on contact lens surfaces, they have been confirmed in cases of culture proven MK after extended lens wear.19,20 In the presence of bacterial challenge, prolonged neutrophil recruitment accelerates the ability of PA to adhere to lens surfaces and when unable to effectively clear the offending organism, an ensuing feedback loop heightens the potential risk for biofilm formation on the posterior lens surface. The infrequent occurrence of biofilms detected on lens surfaces after wear is likely because of the robust antimicrobial capabilities of the ocular surface, which defend against biofilm formation in daily and short-term extended wear. The ability of neutrophils to enhance PA adherence to contact lens surfaces with resultant increased bacterial uptake in the corneal epithelium after 24 hours of lens wear in vivo has been previously demonstrated in a rabbit contact lens model through the dissolution of the DNA and F-actin scaffolds.21 Thus, the ability of neutrophils to facilitate PA adherence to lens surfaces with a subsequent increase in microbial invasion of the epithelium may explain, in part, the increase in lens-associated bacterial bioburden that has been identified as a risk factor for CIE reported during extended silicone hydrogel lens wear.15
In this study, we used PMA-stimulated neutrophils that were harvested by venipuncture from healthy human volunteers. It has previously been established that during the process of extravasation into the cornea and tears, neutrophils undergo surface modifications, including an upregulation of cell surface adhesion molecules, which may potentially alter the cellular phenotype of these immune cells.40 However, an important consideration for this study is the mechanism by which dying neutrophils facilitate PA biofilm formation. Once activated, the mature terminally differentiated neutrophil has an intrinsically short life span on the order of hours in inflamed tissues, which is vital to limiting neutrophil-mediated tissue destruction.41 In the presence of lipopolysaccharide, there is a preferential increase in neutrophil necrosis, which corresponds with a reduction in neutrophil-mediated apoptotic cell death.42 In both this study and our previous study, we have shown that cellular debris released by necrotic neutrophils that accumulate in response to persistent inflammation provide the scaffolding material that facilitate enhanced PA adhesion to both hydrogel and silicone hydrogel lens surfaces. We confirmed this finding through the use of freeze–thaw PMN lysates, which showed a similar aggregation of PA to the lens surface. This finding is also in agreement with reports by a previous group that demonstrated preferential association of PA strain PAO1 with necrotic neutrophils, whereas PA failed to associated with neutrophils undergoing UV-induced apoptotic cell death.23
The second novel finding in this study is the potential for epithelial cell debris accumulated in the lens case to independently enhance PA adherence and potential biofilm formation. Recently, a significant emphasis has been placed on lens case hygiene and the ability of various strains of bacteria to form biofilms in unused lens cases.33,34 Using a static assay, we demonstrated for the first time, the ability of epithelial cell debris to enhance PA adhesion in sterile, flat-bottom, 96-well plates. The effect, which was similar to that seen in our neutrophil studies, showed a significant increase in viable PA in the presence of epithelial debris compared with PA alone. This would suggest that in the presence of substantial buildup, accumulated residual debris has the potential to enhance microbial load in lens storage cases and accelerate bacterial biofilm formation irrespective of the lens case surface. This new finding has important clinical implications, particularly in the use of silver impregnated antibacterial lens cases, because once a cellular debris carpet has been formed, the antimicrobial properties could be significantly attenuated.
To investigate the presence of biodebris accumulation in used lens cases, we used a CV assay. Crystal violet, although commonly used for the detection of bacterial biofilms, has a high affinity for protein and DNA. As a protein and DNA marker, CV and related stains have been used in forensic analysis to detect latent fingerprints on a variety of surfaces.43,44 In this study, we used CV as a useful marker of residual epithelial biodebris in the wells of lens storage cases. Importantly, we confirmed that all used lens cases, regardless of duration of use, possessed some chronic level of CV staining. This effect was seen in cases used for short durations of 3 months or less and extended throughout the initial 6 to 9 months of use. The presence of CV alone at these time points was not indicative of microbial contamination because there was no significant difference in CV staining levels in sterile compared with contaminated cases. For older lens cases used for periods of 9 months or longer, there was heavy staining identical to that that detected in sterile unused lens cases that underwent prolonged incubation with high amounts of cellular debris.
The primary limitation to this study lies in the absence of any clinical information pertaining to lens case hygiene habits, lens-wearing schedules, and care products used. A large-scale clinical study to evaluate the effects of cleaning regimens and compliance on the formation of the debris-laden carpet is clearly needed. Moreover, although we did not perform microbial testing to identify case-associated microorganisms in this study, which has already been previously reported by others, we used an MTT assay to confirm the presence of viable microorganisms in used lens cases. The MTT, a marker of metabolic activity, is routinely used as an indirect measure of cell number in proliferation and viability assays. The use of MTT as a measure of adherent bacteria in flat-well lens storage cases has already been validated.33 Using a similar protocol, MTT was used in this study to compare contaminated lens cases with levels of CV staining. Importantly, the MTT assay confirmed that all cases aged 9 months or older with high levels of CV staining were positive for microbial activity. The increased frequency of microbial contamination in lens cases used for durations of 9 months or longer is in agreement with a previous report correlating lens case age with high levels of microbial contamination.31
In summary, these findings confirm our earlier results and further support the view that significant neutrophil accumulation in the postlens environment during prolonged contact lens wear may contribute to lens-related infection through an enhancement in PA adherence and biofilm formation on lens surfaces. Similarly, the presence of substantial epithelial debris in the lens case that accumulates over time also independently has the potential to greatly accelerate biofilm formation on these surfaces. As chemically preserved multipurpose solutions have been shown to have variable efficacy against the heavier biofilms formed in lens storage cases,45,46 the elimination and removal of nonviable biodebris is an essential step in lens case cleaning regimens. Thus, the new findings reported here clearly support earlier practitioner recommendations that a mechanical cleaning step, such as rubbing and rinsing, is required as part of a lens case hygiene regimen and that lens cases should be replaced on average at no longer than 6-month intervals.31,33
Acknowledgments
Supported by the National Institutes of Health (R01 EY018219) (D.M.R.), the National Eye Institute Core grant (EY020799), the OneSight Research Foundation, Dallas, TX (D.M.R.), a Career Development Award (D.M.R.), and an unrestricted grant from the Research to Prevent Blindness, Inc, New York, NY.
Contact lenses were donated by CIBA Vision.
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Schein OD, Glynn RJ, Poggio EC, et al. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. Microbial Keratitis Study Group. N Engl J Med 1989; 321:773–778. [DOI] [PubMed] [Google Scholar]
- 2.Pachigolla G, Blomquist P, Cavanagh HD. Microbial keratitis pathogens and antibiotic susceptibilities: a 5-year review of cases at an urban county hospital in North Texas. Eye Contact Lens 2007;33:45–49. [DOI] [PubMed] [Google Scholar]
- 3.Ormerod LD, Smith RE. Contact lens-associated microbial keratitis. Arch Ophthalmol 1986;104:79–83. [DOI] [PubMed] [Google Scholar]
- 4.Mondino BJ, Weissman BA, Farb MD, et al. Corneal ulcers associated with daily-wear and extended-wear contact lenses. Am J Ophthalmol 1986;102:58–65. [DOI] [PubMed] [Google Scholar]
- 5.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci 2007;84:273–278. [DOI] [PubMed] [Google Scholar]
- 6.Imayasu M, Petroll WM, Jester JV, et al. The relation between contact lens oxygen transmissibility and binding of Pseudomonas aeruginosa to the cornea after overnight wear. Ophthalmology 1994;101:371–388. [DOI] [PubMed] [Google Scholar]
- 7.Cavanagh HD, Ladage PM, Li SL, et al. Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding and corneal epithelium. Ophthalmology 2002;109:1957–1969. [DOI] [PubMed] [Google Scholar]
- 8.Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology 2001;108: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 9.Ren DH, Petroll WM, Jester JV, et al. Adherence of Pseudomonas aeruginosa to shed rabbit corneal epithelial cells after overnight wear of contact lenses. CLAO J 1997;23:63–68. [PubMed] [Google Scholar]
- 10.Ren DH, Petroll WM, Jester JV, et al. The Relationship between contact lens oxygen permeability and binding of Pseudomonas aeruginosa to human corneal epithelial cells after overnight and extended Wear. CLAO J 1999;25: 80–100. [PubMed] [Google Scholar]
- 11.Ren DH, Yamamoto K, Ladage PM, et al. Adaptive effects of 30-night wear of hyper-O2 transmissible contact lenses on bacterial binding and corneal epithelium. Ophthalmology 2002;109:27–39. [DOI] [PubMed] [Google Scholar]
- 12.Ladage PM, Yamamoto K, Li L, et al. Corneal epithelial homeostasis following daily and overnight lens wear. Contact Lens Anterior Eye 2002;25: 11–21. [DOI] [PubMed] [Google Scholar]
- 13.Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens related microbial keratitis in Australia. Ophthalmology 2008;115:1655–1662. [DOI] [PubMed] [Google Scholar]
- 14.Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low dk hydrogel extended contact lens wear: a meta-analysis. Optom Vis Sci 2007;84:247–256. [DOI] [PubMed] [Google Scholar]
- 15.Szczotka-Flynn L, Lass JH, Sethi A, et al. Risk factors for corneal infiltrative events during continuous wear of silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci 2010;51:5421–5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zegans ME, Becker HI, Budzik J, et al. The role of bacterial biofilms in ocular infections. DNA Cell Biol 2002;21:415–420. [DOI] [PubMed] [Google Scholar]
- 17.Szliter EA, Barrett RP, Gabriel MM, et al. Pseudomonas aeruginosa-induced inflammation in the rat extended-wear contact lens model. Eye Contact Lens 2006;32:12–18. [DOI] [PubMed] [Google Scholar]
- 18.Tam C, Mun JJ, Evans DJ, et al. The impact of inoculation parameters on the pathogenesis of contact lens-related infectious keratitis. Invest Ophthalmol Vis Sci 2010;51:3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin-Borlace L, Stapleton F, Matheson M, et al. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J Appl Microbiol 1998;84:827–838. [DOI] [PubMed] [Google Scholar]
- 20.Stapleton F, Dart J. Pseudomonas keratitis associated with biofilm formation on a disposable soft contact lens. Br J Ophthalmol 1995;79:864–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson DM, Parks QM, Young RL, et al. Disruption of contact lens-associated Pseudomonas aeruginosa biofilms formed in the presence of neutrophils. Invest Ophthalmol Vis Sci 2011;52:2844–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker TS, Tomlin KL, Worthen GS, et al. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun 2005;73:3693–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parks QM, Young RL, Poch KR, et al. Neutrophil enhancement of Pseudomonas aeruginosa biofilm development: human F-actin and DNA as targets for therapy. J Med Microbiol 2009;58:492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willcox M, Power KN, Stapleton F, et al. Potential sources of bacteria that are isolated from contact lenses during wear. Optom Vis Sci 1997;74:1030–1038. [DOI] [PubMed] [Google Scholar]
- 25.Hall BJ, Jones L. Contact lens cases: the missing link in contact lens safety? Eye Contact Lens 2010;36:101–105. [DOI] [PubMed] [Google Scholar]
- 26.Vermeltfoort PB, Hooymans JM, Busscher HJ, et al. Bacterial transmission from lens storage cases to contact lenses-effects of lens care solutions and silver impregnation of cases. J Biomed Mater Res B Appl Biomater 2008;87: 237–243. [DOI] [PubMed] [Google Scholar]
- 27.Szczotka-Flynn LB, Pearlman E, Ghannoum M. Microbial contamination of contact lenses, lens care solutions, and their accessories: a literature review. Eye Contact Lens 2010;36:116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devonshire P, Munro FA, Abernety C, et al. Microbial contamination of contact lens cases in the west of Scotland. Br J Ophthalmol 1993;77: 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larkin DF, Kilvington S, Easty DL. Contamination of contact lens storage cases by Acanthamoeba and bacteria. Br J Ophthalmol 1990;74: 133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willcox MDP, Carnt NA, Diec J, et al. Contact lens case contamination during daily wear of silicone hydrogels. Optom Vis Sci 2010;87:456–464. [DOI] [PubMed] [Google Scholar]
- 31.Wu YT, Zhu H, Harmis NY, et al. Profile and frequency of microbial contamination of contact lens cases. Optom Vis Sci [published online ahead of print January 22, 2010] doi: 10.1097/OPX.0b013e3181cf86ee [DOI] [PubMed] [Google Scholar]
- 32.Gray TB, Cursons RT, Sherwan JF, et al. Acanthamoeba, bacterial, and fungal contamination of contact lens storage cases. Br J Ophthalmol 1995;79:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu YT, Zhu H, Willcox M, et al. Removal of biofilm from contact lens storage cases. Invest Ophthalmol Vis Sci 2010;51:6329–6333. [DOI] [PubMed] [Google Scholar]
- 34.Wu YT, Zhu H, Willcox M, et al. The effectiveness of various cleaning regimens and current guidelines in contact lens case biofilm removal. Invest Ophthalmol Vis Sci 2011;52:5287–5292. [DOI] [PubMed] [Google Scholar]
- 35.Haslett C, Guthrie LA, Kopaniak MM, et al. Modulation of neutrophil function by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson DM, Li L, Fisher S, et al. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci 2005;46:470–478. [DOI] [PubMed] [Google Scholar]
- 37.Whitchurch CB, Tolker-Nielsen T, Ragas PC, et al. Extracellular DNA required for bacterial biofilm formation. Science 2002;295:1487. [DOI] [PubMed] [Google Scholar]
- 38.Allesen-Holm M, Barken KB, Yang L, et al. A characterization of DNA-release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 2006;59:1114–1128. [DOI] [PubMed] [Google Scholar]
- 39.Fuxman Bass JI, Russo DM, Gabelloni ML, et al. Extracellular DNA: a major proinflammatory component of Pseudomonas aeruginosa biofilms. J Immunol 2010;184:6386–6395. [DOI] [PubMed] [Google Scholar]
- 40.Lee S, Bowrin K, Hamad AR, et al. Extracellular matrix lumican deposited on the surface of neutrophils promotes migration by binding β2 integrin. J Biol Chem 2009;284:23662–23669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofman P Molecular regulation of neutrophil apoptosis and potential targets for therapeutic strategy against the inflammatory process. Inflamm Allergy 2004;3:1–9. [DOI] [PubMed] [Google Scholar]
- 42.Geering B, Simon H-U. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ 2011;18:1457–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldman MA, Meloan CE, Lamber JL. A new method for recovering latent fingerprints from skin. J Forensic Sci 1982;27:806–811. [PubMed] [Google Scholar]
- 44.Zamir A, Springer E, Glattstein B. Fingerprints and Dna: STR typing of DNA extracted from adhesive tape after processing for fingerprints. J Forensic Sci 2000;45:687–688. [PubMed] [Google Scholar]
- 45.Szczotka-Flynn LB, Imamura Y, Chandra J, et al. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea 2009;28:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson LA, Sawant AD, Ahearn DG. Comparative efficacies of soft contact lens disinfectant solutions against microbial films in lens cases. Arch Ophthalmol 1991;109:1155–1157. [DOI] [PubMed] [Google Scholar]