Abstract
Purpose
Under times of supply chain stress, the availability of some medical equipment and supplies may become limited. The current pandemic involving severe acute respiratory syndrome coronavirus 2 has highlighted limitations to the ordinary provision of personal protective equipment (PPE). For perioperative healthcare workers, N95 masks provide a stark example of PPE in short supply necessitating the creation of scientifically valid protocols for their decontamination and reuse.
Methods
We performed a systematic literature search of MEDLINE, Embase, Cochrane CENTRAL databases, and ClinicalTrials.gov to identify peer-reviewed articles related to N95 mask decontamination and subsequent testing for the integrity of mask filtration and facial seal. To expand this search, we additionally surveyed the official statements from key health agencies, organizations, and societies for relevant citations.
Results
Our initial database search resulted in five articles that met inclusion criteria, with 26 articles added from the expanded search. Our search did not reveal any relevant randomized clinical trials or cohort studies. We found that moist mask heating (65–80°C at 50–85% relative humidity for 20–30 min) and vaporous hydrogen peroxide treatment were supported by the literature to provide consistent viral decontamination without compromising mask seal and filtration efficiency. Other investigated decontamination methods lacked comprehensive scientific evidence for all three of these key criteria.
Conclusions
N95 mask reprocessing using either moist heat or vaporous hydrogen peroxide is recommended to ensure healthcare worker safety.
Electronic supplementary material
The online version of this article (10.1007/s12630-020-01770-w) contains supplementary material, which is available to authorized users.
Keywords: N95 cleaning, COVID-19, heat inactivation, hydrogen peroxide sterilization
Résumé
Objectif
Lorsque les chaînes d’approvisionnement sont mises sous pression, la disponibilité de certains équipements et fournitures médicaux pourrait devenir restreinte. La pandémie actuelle du syndrome respiratoire aigu sévère du coronavirus 2 a mis en lumière les limites de l’approvisionnement usuel des équipements de protection individuelle (EPI). Pour les travailleurs de la santé périopératoires, les masques N95 sont un exemple frappant d’EPI pouvant rapidement venir à manquer et nécessitant l’élaboration de protocoles scientifiquement rigoureux pour leur décontamination et leur réutilisation.
Méthode
Nous avons réalisé une recherche de littérature systématique dans les bases de données MEDLINE, Embase, Cochrane CENTRAL et sur ClinicalTrials.gov afin d’identifier les articles révisés par les pairs portant sur la décontamination des masques N95 et les tests subséquents pour vérifier l’intégrité de la filtration du masque et son étanchéité sur le visage. Afin d’étendre notre recherche, nous avons également passé en revue les énoncés officiels émanant des agences de santé, ainsi que des organismes et sociétés médicales majeurs pour en extraire les citations pertinentes.
Résultats
Notre recherche initiale des bases de données nous a permis d’extraire cinq articles respectant nos critères d’inclusion, et 26 articles ont été ajoutés à la suite de notre recherche étendue. Notre recherche n’a pas découvert d’études cliniques randomisées ou d’études de cohorte pertinentes. Nous avons observé que la décontamination du masque par chaleur humide (65–80°C à une humidité relative de 50–85 % pendant 20-30 min) et le traitement par vapeur de peroxyde d’hydrogène constituaient les deux mesures endossées par la littérature. En effet, ces modalités offrent une décontamination virale constante sans pour autant compromettre l’étanchéité du masque ou son efficacité de filtration. Les autres méthodes de décontamination étudiées ne possédaient pas de données probantes scientifiques exhaustives quant à ces trois critères clés.
Conclusion
Le retraitement des masques N95 à l’aide de chaleur humide ou de vapeur de peroxyde d’oxygène est recommandé pour assurer la sécurité des travailleurs de la santé.
The current coronavirus disease (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has strained the global availability of personal protective equipment (PPE).1 For anesthesiologists, this shortage has focused around the N95 mask, the gold standard PPE to protect against aerosol transmission within the healthcare setting. Current best-practices recommend their use during aerosol-generating medical procedures (AGMP) including intubation, airway suctioning, and extubation.2 While they are intended to be single-use and disposable, in the context of the COVID-19 pandemic, limited stores and production have prompted a widescale effort in evaluating both their extended use and reuse. For example, our institution has mobilized a PPE stewardship model to ensure the continued protection of perioperative healthcare workers (HCWs) that includes the development of a N95 decontamination pathway.3
The N95 mask is a filtering face mask respirator that combines particle filtering and a tight boundary between the mask and the face to effectively prevent the outward escape of user-generated aerosols and inward transport of infectious particles. These respirators are not resistant to oils and solvents (thus the “N” designation) and filter at least 95% of airborne particles greater than 0.3 µm. Filtration efficiency is size-based; 99.5% of particles greater than 0.75 µm are filtered but only 95% of particles 0.1–0.3 µm are filtered. This change in filtration efficiency leaves little available reduction in filtering capacity (from reprocessing) for the mask to still be effective for virus removal, with a SARS-CoV-2 virion size of 0.05 to 0.2 µm.4,5 Filtering is achieved mechanically by non-woven layers of synthetic material, such as polypropylene, and a permanent electrostatic charge to exclude charged particles, such as bacteria. Mechanical filtering occurs by inertial impaction, interception, and as a barrier to diffusion.6 The utility of the N95 mask contrasts with regular surgical masks, which do not have to achieve a predefined degree of filtration efficiency.6 As a result, surgical masks display a wide range of filtration capacities, reported to range from 10% to upwards of 90%.7 Importantly, the simultaneous use of multiple surgical masks will not produce the same filtering or protection as an N958 nor does it create an air-tight seal against the face.
In times of significantly increased N95 mask demand, a simple approach for conservation is to implement extended use, referring to the wearing of an N95 mask for prolonged periods and between multiple patient encounters. Extended use is generally well-tolerated, at least for short periods of time (most comprehensively tested from one to 12 hr, as recently reviewed).9 Healthy subjects wearing a properly fitting N95 mask have been shown to be able to perform one hour of moderate exercise without physiologic compromise, even in humid conditions.10,11 Further use, between 8 and 12 hours with interposed break periods, did not introduce physiologic disturbances but did lead to symptoms of significant discomfort for the wearers.12,13
Beyond extended use, the reuse of an N95 mask, with repeated doffing and re-donning for multiple patient encounters over extended time-periods (days), requires interval decontamination to optimize HCW safety. Reuse can lead to an increased risk of infection spread through the loss of filtration effectiveness or alterations to mask fit. The latter is a critical consideration as data suggests that as few as five consecutive donnings of an N95 mask can compromise the mask’s facial seal.14,15 While several decontamination methods are reported, the scientific literature often does not comprehensively validate or test biological decontamination, maintenance of filtration, and mask seal, for any single cleaning technique. Herein, we review the literature to synthesize the available evidence for N95 mask reuse, including decontamination approaches and methods to validate ongoing mask efficacy, providing a scientific basis for the implementation of mask reprocessing protocols. Where appropriate, we focus on decontamination methods that are more commonly available in medical facilities for the routine cleaning of medical equipment.
Methods
We conducted a rapid systematic literature search, following the interim recommendations by Cochrane Rapid Review Methods.16 This review was not registered on PROSPERO given the rapidly changing COVID-19 situation and related time sensitive nature.
In cases of N95 mask shortage during a pandemic, decontamination strategies have different abilities to provide biological sterility while maintaining mask filtration capacity and fit. To investigate which decontamination strategy is most effective, we selected the following studies: 1) original investigations assessing the efficacy or safety of decontamination methods for N95 respirators in the setting of SARS-CoV-2 and COVID-19; 2) studies using N95 respirators of any brand; and 3) peer-reviewed published articles and accepted articles available online prior to appearance in print. Archived pre-prints (not yet peer-reviewed) were excluded from our synthesis.
The electronic search was completed using the MEDLINE, Embase, and Cochrane CENTRAL databases and ClinicalTrials.gov from their inception to 13 May 2020. We developed this search strategy (provided in the eAppendix in the Electronic Supplementary Material) with the aid of an information specialist at University of Toronto. The literature search was limited to the English language. To expand this search and given the emergent nature of the current pandemic, we also surveyed the official statements from key health agencies, organizations, and societies, including the World Health Organization, Health Canada, Canadian Anesthesiologists’ Society, United States Center for Disease Control, and American Society of Anesthesiologists.
The results of the database searches and the official statements were screened and analyzed by three authors (B.E.S., K.A., and J.T.M.). Any discrepancies in eligibility assessment and data collection were solved by consensus within the whole group. Risk of bias assessment and a meta-analysis were not conducted because of the mixed nature (original investigations and health organization statements) included.
Results
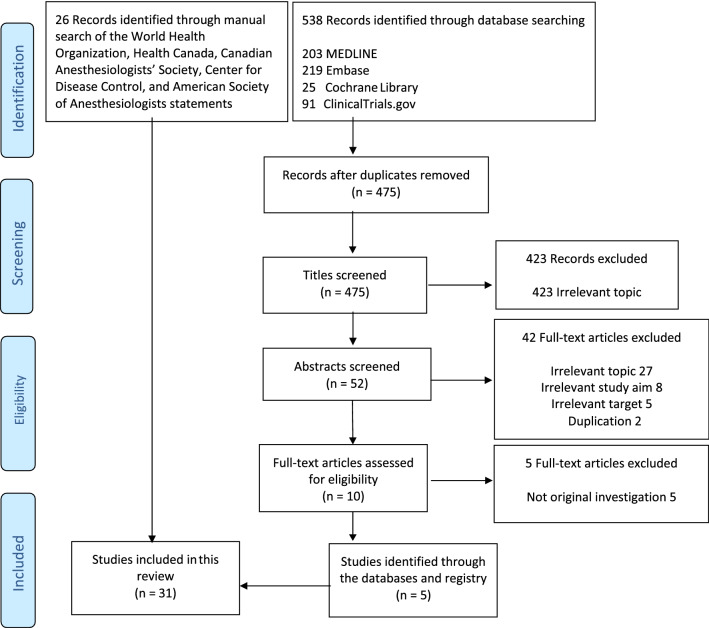
Using the included databases and registries yielded a total of 538 abstracts (Figure). Of these, 528 were excluded because they did not meet the eligibility criteria. Ten articles were further investigated with review of their full texts. We captured five papers specifically on the use of N95 masks against SARS-CoV-2.17–21 In addition, our manual search of recommendations from key health agencies, organizations, and Societies identified 16 papers evaluating decontamination methods22–37 and ten papers investigating barrier integrity testing38–47 that was not specifically related to SARS-CoV or SARS-CoV-2.
FIGURE.
Literature search flow diagram
For implementation, N95 decontamination methods should achieve virus inactivation at scale, without compromising filtration performance or mask fit, and present no irritation or health concern to the user. These criteria are difficult to achieve in practice and no single method for N95 decontamination and reuse has been uniformly accepted. Characteristics of common decontamination methods are summarized in Table 1 and discussed in detail below.
TABLE 1.
N95 decontamination methods
| Method | Equipment | Tested against SARS-CoV-2 | Tested against other biologicals | Pros | Cons | Approved by (HC/FDA) | Key refs |
|---|---|---|---|---|---|---|---|
| Heat | Oven +/- humidity control | Yes (not directly on N95) |
SARS-CoV-1, H1N1/H1N5 Influenza |
Simple and available technology; no chemicals | Risk of mask deformation for fit | No | [18, 23– 25] |
| Autoclave | Standard autoclave | Yes | Bacillus subtilis spores | Available technology; effective in B. subtilis spores |
No direct evidence SARS- CoV-2; risk of mask deformation for fit |
No | [26, 27] |
| HPV |
STERIS, Battelle (Bioquell) |
Yes | Geobacillus stearothermophilus spores, numerous bacteriophages and surrogate respiratory viruses | Low temperature; Breaks down into non- toxic by products | Limited availability, only mask strap breakdown up to 20–30 cycles | Yes | [21, 31, 48, 49] |
| HPGP | ASP STERRAD | Surrogates, no published data on SARS-CoV-2 | Geobacillus stearothermophilus spores | Low temperature; breaks down into non- toxic by products | Limited availability, limited to 3 decontamination cycles for mask integrity | Yes | [29, 36, 50] |
| iHP | SteraMist binary ionization | Yes | No published data | Low temperature | Limited availability, less testing overall on PPE integrity | No | [17] |
| EtO | Specialized | No | No | No impact on the filter function and mask appearance | A Known human carcinogen | No | [24, 29] |
| UV | Specialized lights | No | MS2 bacteriophage; H1N1 influenza | Simple and available technology; no chemicals | May not penetrate inner layers of masks; possibly due to shadowing | No | [51, 52] |
EtO = ethylene oxide; HPGP = hydrogen peroxide gas vapour; HPV = hydrogen peroxide vapour; iHP = ionized hydrogen peroxide; SARS-CoV = severe acute respiratory syndrome-associated coronavirus; SARS-CoV-2 = severe acute respiratory syndrome-associated coronavirus 2; UV = ultraviolet light. A STERRAD system uses low-temperature gas plasma combined with HPV
Heat, humidity, and autoclaving
The SARS-CoV-2 virus displays variable stability across tested temperature ranges. It remains stable at 4°C for prolonged periods, with only a 0.7 log-unit reduction of the 50% tissue culture infective dose (TCID50, measure of infectious titre) after 14 days.37 At 22°C and 65% relative humidity, virus particles can still be detected on the outside of a surgical mask after seven days (0.1% TCID50 of the original inoculum), indicating potential problems with ambient mask storage.37 Compared with results obtained by reverse transcriptase-polymerase chain reaction (RT-PCR) detection (measuring viral RNA), findings using TCID50 are more applicable to mask reuse as they quantify virus survival. Nevertheless, the complexity of measuring TCID50 limits its implementation.
In contrast to cold or ambient temperatures, SARS-CoV-2 is susceptible to heat inactivation. After five minutes at 70°C on a solid surface, there is a greater than six-fold reduction in TCID50.37 The need for combined humidity and heat in mask decontamination has only been studied for influenza, but indicates that 50–85% relative humidity is beneficial for viral inactivation.22 Similarly, a recent study comparing SARS-CoV (the virus responsible for the 2003 SARS pandemic) to SARS-CoV-2, showed that SARS-CoV is inactivated by a five to 30 min exposure to 70–75°C in liquid media, a susceptibility that likely extends to SARS-CoV-2.23
Few studies have simultaneously evaluated the effect of heat and humidity on viral inactivation, mask filtration, and fit under comparable conditions. Many mask models can undergo at least one cycle of elevated temperature (65–80°C) for 20–30 min without a decrease in their filtration efficacy and fit.24 There is only limited data for multiple cycles. Recent experiments suggest N95 masks can be exposed to up to 50 cycles of 85°C without changes in filtration efficacy and fit, but did not include the elevated humidity that is usually required to more broadly inactivate biological agents.18 In general, data suggest that N95 masks may be able to successfully undergo three 30-min decontamination cycles at 65–80°C with a high relative humidity without losing filtration or fit performance.24,25 The exposure of N95 masks to such a protocol does not alter the filtering capacity after three to five cycles. Recently, it was observed that heat (≤ 85°C) and variable humidity (up to 100% relative humidity) preserved filtration properties in melt-blown fabrics and N95-grade respirators.18 At 85°C and 30% relative humidity, the authors were able to perform 50 cycles of heat treatment without deterioration of filtration efficiency.
Autoclaving is a standard technique used by hospitals and academic labs to sterilize equipment, offering an attractive option because of the widespread availability of the instrumentation. Unlike oven heating (with or without added humidity), autoclaving utilizes an elevated temperature (greater than 120°C) and pressure (greater than 103 kPa, or 15 psi). Nevertheless, this method may significantly impact mask fit and function. Only a few studies have evaluated decontamination efficacy and mask quality after N95 autoclaving. The use of steam sterilization at 125°C for three minutes does not affect the electrostatic charge of the electret in the mask, but there is no evidence that this is sufficient for biological decontamination.26 In a study of heating a contaminated mask for 15 min at 121°C at 103 kPa, there was near 100% sterilization of bacteria (using B. subtilis spores), but virus survival was not evaluated.27 Steam alone is successful at decontaminating from avian coronavirus.20
There is conflicting evidence of the effectiveness of autoclave decontamination on N95 respirator filtration efficiency, with one study showing no increased penetration of 0.075 µm and 0.3 µm particles, but with another study stating significant mask degradation.28,29 Nevertheless, both studies showed that autoclave decontamination induced significant mask deformation. There is no direct evidence for SARS-CoV-2 sterilization using this technique.
Hydrogen peroxide or other chemical decontamination
Hydrogen peroxide, an oxidizer commonly found in cleaning agents, can eradicate a wide range of microorganisms, including nosocomial bacterial spores and viruses. It has been used in high concentrations for medical equipment sterilization for more than 30 years. Specifically, hydrogen peroxide vapour (HPV), hydrogen peroxide gas plasma (HPGP), and ionized hydrogen peroxide (iHP) are the three industry-standard techniques.17,30 Sterilization using hydrogen peroxide is a low-temperature technique preferable for cleaning medical equipment (i.e., endoscopes) that cannot withstand damage from the high temperature and humidity of autoclaving.
Reports on the use of HPV, HPGP, and iHP for decontamination of N95 respirators have shown varying levels of success. All three techniques successfully decontaminated N95 masks with pathogens more resistant than SARS-CoV-2, such as Geobacillus stearothermophilus (6-log spore reduction following treatment), and influenza A virus subtype H1N1 (5-log reduction following treatment).17,21,24,25,29,30 With HPV use, no change in N95 mask filter quality (filter efficiency > 98% and no change in airflow resistance) or fit was shown with three cycles of treatment (further claims of up to 20 cycles exist but are not validated: https://www.fda.gov/media/136386/download).24 With HPGP, no change in N95 respirator filtering quality is observed with one cycle of decontamination, but three or more cycles can impair filtration by greater than 5%.36 With iHP, no reports have evaluated respirator filter quality or fit, although far less investigation has been performed for this method. Of note, N95 respirators containing cellulose materials cannot be decontaminated using HPV and HPGP systems. The cellulose-containing materials absorb the hydrogen peroxide leading to decreased vapour concentrations and a potentially compromised or prematurely terminated decontamination cycle. A list of N95 respirators with and without cellulose is included in Table 2.
TABLE 2.
Examples of commercially available N95 respirators with and without cellulose
| Cellulose-containing N95 | Cellulose-free N95 |
|---|---|
| 3M 9105 (+S) | 3M 1860 (+S) |
| 3M 1804 (+S) | 3M 1870+ |
| 3M 1805 (+S) | 3M 8110S |
| 3M 8000 | 3M 8210 |
| 3M 8200 | 3M 8210+ |
| 3M 8212 | 3M 8210V |
| 3M 8214 | 3M 8211 |
| 3M 8233 | 3M 8211+ |
| 3M 8293 | 3M 8271 |
| 3M 8512 | 3M 8511 |
| 3M 8514 | 3M 8515 |
| 3M 9010 | 3M 8516 |
| 3M 9105 (+S) |
3M 8576 3M 8577 |
| 3M 9010 | |
| 3M 9210+ | |
| 3M 9211+ | |
| Gerson 1730 | |
| Moldex 1500 Series | |
| Moldex 2200 | |
| Kimberly Clark 46727 |
Detailed product information for the included masks can be found at the respective manufacturer’s website: 3M (https://www.3mcanada.ca/3M/en_CA/company-ca/); Gerson (https://www.gersonco.com/product/1730-n95-particulate-respirator/); Moldex (https://www.moldex.com/product-category/respiratory-protection/disposable-respirators/n95-respirators/); Kimberly Clark (https://www.kcprofessional.ca/products/scientific-ppe/respirators/lab/46827-kimberly-clark-n95-particulate-filter-respirator-and-surgical-mask-fluid-protection-pouch-style). Additional lists of National Institute of Occupational Safety and Health-approved N95 respirators are provided by the Centers for Disease Control at https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/n95list1.html. Notably, cellulose-containing N95 respirators are often incompatible with hydrogen peroxide-based decontamination methods, such as the Battelle Critical Care Decontamination System (https://www.battelle.org/docs/default-source/commercial-offerings/industry-solutions/battelle-ccds-n95-guidance.pdf)
The HPV/HPGP decontamination process consists of five steps: conditioning, pre-gassing, gassing, gassing dwell, and aeration.24,29,31 The latter phase allows for off-gassing and breakdown of HPV into oxygen and water vapour to minimize the risk of chemical contamination to the subsequent user. This process contrasts with other cleaning methods, such as formaldehyde and ethylene oxide, where significant chemical contamination can remain. The mechanism of action by which HPV eradicates microorganisms is primarily via oxidation, with the generation of hydroxy free radicals that can breakdown the cell wall and intracellular structures of microorganisms.31
The main drawback of HPV/HPGP is the lack of availability of the necessary machinery for the procedure. The four most common systems available are the Battelle Critical Care Decontamination System (CCDS, Battelle, Columbus, OH, USA) (HPV), Advanced Sterilized Products STERRAD system (HPV and HPGP, Advanced Sterilization Products, Irvine, California), the STERIS V-Pro sterilizers (HPV, Steris, Mentor, Ohio), and the ClarusR Bioquell system (HPV and HPGP, Bioquell, Andover, UK). Despite limited evidence on the effectiveness of hydrogen peroxide for the decontamination of SARS-CoV-2, the United States Food and Drug Administration (FDA) issued an emergency use authorization on 29 March 2020 for the use of the Battelle CCDS system for the decontamination of single-user N95 masks (up to 20 cleanings) (https://www.fda.gov/media/136386/download). Other hydrogen peroxide sterilization systems, such as models by STERIS and Advanced Sterilization Products, have since received similar emergency use authorizations by the FDA for the decontamination of non-cellulose-containing N95 masks for single-user reuse (https://www.fda.gov/media/136843/download and https://www.fda.gov/media/136882/download), but only for two decontamination cycles. Single-user reuse refers to the return of a specific N95 respirator to the original HCW who used the mask. Both standard and express cycles of the devices have been granted emergency use authorization.
Similar to HPV, ethylene oxide (EtO) gas has a long history of being used as a sterilant for healthcare materials, including heat- or moisture-sensitive medical devices, without deteriorating device elements (including a Centers for Disease Control and Prevention protocol for medical equipment sterilization, https://www.cdc.gov/infectioncontrol/guidelines/disinfection/sterilization/ethylene-oxide.html). Nevertheless, inhaled EtO is a known human carcinogen, and short-term exposure to EtO irritates the eyes and mucous membranes, and can lead to seizures, coma, and potentially death.32
There are a limited number of studies investigating the sterilizing effects of EtO on N95 masks. It has been shown that EtO decontamination does not impact the filter aerosol penetration, filter airflow resistance, or physical appearance of N95 masks.24,29 Nevertheless, these studies did not evaluate the efficiency of the decontamination methods to inactivate microorganisms. While residual EtO was below permissible exposure limits, two potential toxins (diacetone alcohol and ethylene glycol monoacetate) were detected after treatment of N95 rubber straps. To date, no clinical study of EtO decontamination of N95 masks has been conducted.
Ultraviolet light
The use of ultraviolet (UV) light, termed UV germicidal irradiation (UVGI), has also been suggested for N95 decontamination.19,33 No specific studies of UVGI were identified that matched our search criteria though there were at least two studies that might yet appear once they undergo peer review. For example, a method specifically aimed at sterilizing N95 respirators for SARS-CoV-2 that employs UV light between 100 and 280 nm was recently described (but is not yet peer-reviewed, https://www.nebraskamed.com/sites/default/files/documents/covid-19/n-95-decon-process.pdf) and simulated sunlight was shown to be SARS-CoV-2 viricidal.34 Biosafety cabinets with a manufacturer-reported fluence of 100 μWcm−2 were reported to effectively sanitize masks for reuse after approximately 15–20 min per side (pre-print, 10.1101/2020.03.25.20043489). As a proof of concept, light sensors were used to confirm that the entire surface area of the mask received an appropriate dose of radiation without shadow. Nevertheless, none of these studies measured live virus after treatment, and other UV tests illustrated a lack of biological decontamination.19 The use of UVGI may not inactivate viruses that have penetrated into the innermost layers of the N95 where UV transmission is reduced. Furthermore, there is a UV-mediated degradation of polymers and the maximum number of cycles has not been determined. Reassuringly, the elastic straps retain their structural integrity even at high UV doses.35
Post-decontamination barrier integrity testing
Before reuse, cleaned masks should undergo testing to ensure they can still provide an effective barrier for nano-sized particles such as SARS-CoV-2. Testing generally assesses “worst-case scenario” use of N95 masks by simulating prolonged wear in high exposure environments.
Aerosolized particles of differing sizes, characteristics (inert, biological, lipophilicity), and concentrations are flowed (cyclical or constant flows > 85 L·min−1 ± heat ± humidity) across N95 masks.38,39 Humidity is relevant as it reduces the electrostatic barrier and filtration capacity of N95 masks, particularly around the most penetrating particle size (MPPS; 0.05 µm).38,40 Detectors for measuring N95 mask filtration vary in their sensitivity to detect nanoparticles, important given the reported size of SARS-CoV-2 and the known MPPS for N95 masks.40,41
Testing N95 masks specifically for barrier capabilities against small viruses (viral filtration efficiency [VFE]), such as the SARS-CoV-2, is complicated by factors related to the virus, mask, and airflow. As such, VFE is rarely undertaken but is rather implied by successful proof of filtration efficiencies > 95% to inert compounds. In keeping with this concept, published accounts of filtration efficiencies of N95 masks show > 99% FE for the test bacteria and viruses.42 While the use of non-pathogenic viruses as surrogates for SARS-CoV-2 makes intuitive sense for testing N95 barrier function, they may not represent the most rigorous means of testing N95 barrier effectiveness to live particles. Biological agents as test particles are oftentimes inconsistent.43 There are considerable differences within or between bacterial and viral strains, yielding significant variability in size, electrostatic, and hydrophilic properties. Additionally, using biological substrates seldom represents the MPPS for N95 filters. The N95 filtration mechanisms combine to prevent penetration of a wide range of particles. The MPPS depends on filter properties, filtration mechanisms, airflow rates (L·min−1), types of flows (cyclical vs constant), filter fibre charge density, and aerosol particle charge distribution.38,44–46 Therefore, most N95 mask tests are not predicated on emulating all the physical properties of the pathogen in question. Rather, they are designed to test particles at the MPPS, where the N95 mask is most vulnerable, and assume that all other particle types will experience superior relative filtration efficiencies. As such, the reported filtration of biological particles that differ from the MPPS should be reported as better than 95%.22,39,47 Evaluated testing methods are summarized in Table 3. The sodium chloride aerosol test involving light photometry detection remains the National Institute for Occupational Safety and Health standard but is not readily available outside of commercial labs. Particle counters are more commonly found in occupational health and safety departments and are readily available for sale or rental from third party vendors.
TABLE 3.
N95 retesting strategies
| Test | Methodology | Considerations | References |
|---|---|---|---|
| Aerosolized sodium chloride | Detection by photometry or particle count (e.g., PortaCount) |
• NIOSH standard test (photometry) • Useful for N class masks (e.g., N95) • Capacity to test particles that are approximate the size of SARS-CoV-2 |
[42, 53, 54] |
| Aerosolized corn oil | Detection by photometry |
• Tests particles larger than the N95 MPPS • Less electrostatic charge than sodium chloride testing • Useful for assessing lipophilic particle filtration (non-N class masks) |
[55] |
| Beads (e.g., silica, latex, polystyrene) | Detection by spectrophotometry |
• Can be fluorescently tagged • Can be neutral or charged • Capacity to match size to MPPS |
[47, 56] |
| Bacteria (e.g., Staphylococcus aureus, Bacillus atrohaeus) | Detection by particle counting and viable growth | • Test biological particles with shapes, sizes, and charge similar to pathogen of interest | [55, 57] |
| Viruses (e.g., bacteriophage MS2 virus, T4, Bacillus subtilis phage, phiX, H1N1 influenza virus) | Detected by measuring viable virus |
• Test biological particles with shapes, sizes, and charge similar to pathogen of interest • Similar sizes to SARS-CoV-2 |
[39, 47, 56, 57] |
MPPS = most penetrating particle size; N = not resistant to oil; NIOSH = National Institute for Occupational Health and Safety; SARS-CoV-2 = severe acute respiratory syndrome-associated coronavirus 2
Discussion
During the current COVID-19 pandemic, HCWs have been forced to utilize PPE beyond the manufacturer recommended conditions. This has led to a proliferation of unpublished, non-peer-reviewed protocols for the cleaning and reuse of protective equipment. In the case of N95 masks, these protocols often fail to rigorously test physical form, function, and biological sterility post-decontamination. Most decontamination testing relies on a final evaluation of filtration capacity only, likely owing to the available, standardized equipment for this metric. Changes to the shape and facial seal of a mask after processing are rarely tested. Additionally, the evaluation of true biological decontamination is sparse, usually performed using surrogate viruses or non-viral spore-forming organisms. All three evaluations are important to ensuring the safety of the HCW, creating a deficiency of knowledge in comparing decontamination techniques. In our systematic literature search, we did not find any randomized-controlled trials or cohort studies, so evidence quality was not appraised.
Regardless of the decontamination methods, institutions will be required to evaluate N95 barrier integrity as the reprocessing may alter the N95 filter electrostatic barrier properties, pore sizes, fibre composition, and mask fit. Given the availability of suitable (e.g., MPPS-sized test particles) and accessible (e.g., Porta-Count, TSI, Shoreview, Minnesota) tests capable of assessing N95 barrier integrity, these tests are recommended prior to mask reuse. Of note, combinations of PPE equipment have been recommended for use during AGMP,2 including the use of face shields. This may further minimize the number of viral particles on the N95 mask and facilitate reprocessing. Moreover, the reprocessing of only “low-risk masks”, where the presumed burden of particles on the mask would be low (i.e., those worn for short periods of time or during care for patients with a low likelihood of infection), may further improve the reusability of N95 masks. The combination of N95 mask reuse and other adjunctive protection, like face shields, have not been specifically studied in the context of SARS-CoV-2.
Recommendations
While the scientific and peer-reviewed evidence available are sparse, only two methodologies are supported to provide proper mask cleaning while maintaining physical integrity: HPV and moist heat (65–80°C for 20–30 min, relative humidity of 50–85%). Although some evidence suggests that extended (5–20) cleaning cycles could be performed, the data only supports three cleanings for either technique. Given the wide availability of ovens with controllable humidity in hospital core facilities, this technique provides a viable option for HCWs when N95 masks are in short supply.
Ongoing research into PPE stewardship, allocation, and reuse will inform our best and safest practices as we continue to care for COVID-19-positive patients within emergency departments, critical care units, and operating rooms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the members of the Department of Anesthesia and Pain Medicine at the Hospital for Sick Children for their support and protected academic time. J.T.M. would like to thank the Wasser Family, the SickKids Foundation, and his colleagues for research chair funds.
Author contributions
Benjamin E. Steinberg, Kazuyoshi Aoyama, and Jason T. Maynes contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Mark McVey, David Levin, Asad Siddiqui, Farrukh Munshey, and Neil M. Goldenberg contributed to the acquisition, analysis, and interpretation of data. David Faraoni contributed to the acquisition and analysis of data, and conception and design of the study.
Disclosures
None.
Funding statement
Funding was provided through the Wasser Chair in Anesthesia and Pain Medicine, Hospital for Sick Children.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance - 2020. Available from URL: https://www.who.int/publications/i/item/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak (accessed July 2020).
- 2.Lockhart SL, Duggan LV, Wax RS, Saad S, Grocott HG. Personal protective equipment (PPE) for both anesthesiologists and other airway managers: principles and practice during the COVID-19 pandemic. Can J Anesth. 2020 doi: 10.1007/s12630-020-01673-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Mandhari M, Caldeira M, Dickinson M, Mc Donnell C. Perioperative personal protective equipment stewardship (POPPiES) Can J Anesth. 2020 doi: 10.1007/s12630-020-01676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian Y, Willeke K, Grinshpun SA, Donnelly J, Coffey CC. Performance of N95 respirators: filtration efficiency for airborne microbial and inert particles. Am Ind Hyg Assoc J. 1998;59:128–132. doi: 10.1080/15428119891010389. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. N95 Respirators and Surgical Masks. Posted on October 14, 2009 by Lisa Brosseau, ScD, and Roland Berry Ann. NIOSH Science Blog 2009; Available from URL: https://blogs.cdc.gov/niosh-science-blog/2009/10/14/n95/ (accessed July 2020).
- 7.Abd-Elsayed A, Karri J. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 doi: 10.1213/ANE.0000000000004841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derrick JL, Gomersall CD. Protecting healthcare staff from severe acute respiratory syndrome: filtration capacity of multiple surgical masks. J Hosp Infect. 2005;59:365–368. doi: 10.1016/j.jhin.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher EM, Shaffer RE. Considerations for recommending extended use and limited reuse of filtering facepiece respirators in health care settings. J Occup Environ Hyg. 2014;11:D115–D128. doi: 10.1080/15459624.2014.902954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Wu T, Powell JB, Roberge RJ. Physiologic and fit factor profiles of N95 and P100 filtering facepiece respirators for use in hot, humid environments. Am J Infect Control. 2016;44:194–198. doi: 10.1016/j.ajic.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Benson SM, Roberge RJ. Pulmonary and heart rate responses to wearing N95 filtering facepiece respirators. Am J Infect Control. 2013;41:24–27. doi: 10.1016/j.ajic.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 12.Radonovich LJ, Jr, Cheng J, Shenal BV, Hodgson M, Bender BS. Respirator tolerance in health care workers. JAMA. 2009;301:36–38. doi: 10.1001/jama.2008.894. [DOI] [PubMed] [Google Scholar]
- 13.Rebmann T, Carrico R, Wang J. Physiologic and other effects and compliance with long-term respirator use among medical intensive care unit nurses. Am J Infect Control. 2013;41:1218–1223. doi: 10.1016/j.ajic.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vuma CD, Manganyi J, Wilson K, Rees D. The effect on fit of multiple consecutive donning and doffing of N95 filtering facepiece respirators. Ann Work Expo Health. 2019;63:930–936. doi: 10.1093/annweh/wxz060. [DOI] [PubMed] [Google Scholar]
- 15.Bergman MS, Viscusi DJ, Zhuang Z, et al. Impact of multiple consecutive donnings on filtering facepiece respirator fit. Am J Infect Control. 2012;40:375–380. doi: 10.1016/j.ajic.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Garritty C, Gartlehner G, Kamel C, et al. Interim Guidance from the Cochrane Rapid Reviews Methods Group – 2020. Available from URL: https://methods.cochrane.org/rapidreviews/sites/methods.cochrane.org.rapidreviews/files/public/uploads/cochrane_rr_-_guidance-23mar2020-final.pdf (accessed July 2020).
- 17.Cheng VC, Wong SC, Kwan GS, Hui WT, Yuen KY. Disinfection of N95 respirators by ionized hydrogen peroxide during pandemic coronavirus disease 2019 (COVID-19) due to SARS-CoV-2. J Hosp Infect 2020; DOI: 10.1016/j.jhin.2020.04.003. [DOI] [PMC free article] [PubMed]
- 18.Liao L, Xiao W, Zhao M, et al. Can N95 Respirators be reused after disinfection? How many times? ACS Nano. 2020 doi: 10.1021/acsnano.0c03597. [DOI] [PubMed] [Google Scholar]
- 19.Cadnum JL, Li DF, Redmond SN, John AR, Pearlmutter B, Donskey CJ. Effectiveness of ultraviolet-C light and a high-level disinfection cabinet for decontamination of N95 respirators. Pathog Immun. 2020 doi: 10.20411/pai.v5i1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma QX, Shan H, Zhang CM, et al. Decontamination of face masks with steam for mask reuse in fighting the pandemic COVID-19: experimental supports. J Med Virol. 2020 doi: 10.1002/jmv.25921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman J, Pierce A, Mody J, et al. Institution of a novel process for N95 respirator disinfection with vaporized hydrogen peroxide in the setting of the COVID-19 pandemic at a large academic medical center. J Am Coll Surg. 2020 doi: 10.1016/j.jamcollsurg.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lore MB, Heimbuch BK, Brown TL, Wander JD, Hinrichs SH. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg. 2012;56:92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- 23.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV- 2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergman MS, Viscusi DJ, Heimbuch BK, Wander DJ, Sambol AR, Shaffer RE. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. J ENG FIBER FAB. 2020 doi: 10.1177/155892501000500405. [DOI] [Google Scholar]
- 25.Viscusi DJ, Bergman MS, Novak DA, et al. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J Occup Environ Hyg. 2011;8:426–436. doi: 10.1080/15459624.2011.585927. [DOI] [PubMed] [Google Scholar]
- 26.Lin TH, Chen CC, Huang SH, Kuo CW, Lai CY, Lin WY. Filter quality of electret masks in filtering 14.6–594 nm aerosol particles: effects of five decontamination methods. PLoS One 2017; 10.1371/journal.pone.0186217. [DOI] [PMC free article] [PubMed]
- 27.Lin TH, Tang FC, Hung PC, Hua ZC, Lai YC. Relative survival of Bacillus subtilis spores loaded on filtering facepiece respirators after five decontamination methods. Indoor Air. 2018 doi: 10.1111/ina.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viscusi DJ, King WP, Shaffer RE. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J Int Soc Respir Prot 2007; 24(III-IV): 93-107.
- 29.Viscusi DJ, Bergman MS, Eimer BC, Shaffer RE. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner F. Hydrogen peroxide and other oxidant disinfectants. In: Block SS, editor. Disinfection, Sterilization, and Preservation. Philadelphia: Lea & Febiger; 1983. pp. 240–250. [Google Scholar]
- 31.Schwartz A, Stiegel M, Greeson N, et al. Decontamination and reuse of N95 respirators with hydrogen peroxide vapor to address worldwide personal protective equipment shortages during the SARS-CoV-2 (COVID-19) pandemic. Appl Biosaf. 2020 doi: 10.1177/1535676020919932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rebsdat S, Mayer D. Ethylene oxide. In: Wiley-VC (Ed). Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley VCH Verlag GmbH & Co. KGaA; 2001: a10_117.
- 33.Tseng CC, Li CS. Inactivation of viruses on surfaces by ultraviolet germicidal irradiation. J Occup Environ Hyg. 2007;4:400–405. doi: 10.1080/15459620701329012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratnesar-Shumate S, Williams G, Green B, et al. Simulated sunlight rapidly inactivates SARS- CoV-2 on surfaces. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsley WG, Martin SB, Jr, Thewlis RE, et al. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergman MS, Viscusi DJ, Palmiero AJ, et al. Impact of three cycles of decontamination treatments on filtering facepiece respirator fit. J Int Soc Respir Prot. 2011;28:48–49. [Google Scholar]
- 37.Chin AW, Chu JTS, Perera MR, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahdavi A, Haghighat F, Bahloul A, Brochot C, Ostiguy C. Particle loading time and humidity effects on the efficiency of an N95 filtering facepiece respirator model under constant and inhalation cyclic flows. Ann Occup Hyg. 2015;59:629–640. doi: 10.1093/annhyg/mev005. [DOI] [PubMed] [Google Scholar]
- 39.Eninger RM, Honda T, Adhikari A, Heinonen-Tanski H, Reponen T, Grinshpun SA. Filter performance of N99 and N95 facepiece respirators against viruses and ultrafine particles. Ann Occup Hyg. 2008;52:385–396. doi: 10.1093/annhyg/men019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mostofi R, Wang B, Haghighat F, Bahloul A, Jaime L. Performance of mechanical filters and respirators for capturing nanoparticles–limitations and future direction. Ind Health. 2010;48:296–304. doi: 10.2486/indhealth.48.296. [DOI] [PubMed] [Google Scholar]
- 41.Gui M, Liu X, Guo D, et al. Electron microscopy studies of the coronavirus ribonucleoprotein complex. Protein Cell. 2017;8:219–224. doi: 10.1007/s13238-016-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rengasamy S, Miller A, Eimer BC. Evaluation of the filtration performance of NIOSH- approved N95 filtering facepiece respirators by photometric and number-based test methods. J Occup Environ Hyg. 2011;8:23–30. doi: 10.1080/15459624.2010.515556. [DOI] [PubMed] [Google Scholar]
- 43.Thiessen RJ. Filtration of respired gases: theoretical aspects. Respir Care Clin N Am. 2006;12:183–201. doi: 10.1016/j.rcc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Mahdavi A, Bahloul A, Haghighat F, Ostiguy C. Contribution of breathing frequency and inhalation flow rate on performance of N95 filtering facepiece respirators. Ann Occup Hyg. 2014;58:195–205. doi: 10.1093/annhyg/met051. [DOI] [PubMed] [Google Scholar]
- 45.Eshbaugh JP, Gardner PD, Richardson AW, Hofacre KC. N95 and p100 respirator filter efficiency under high constant and cyclic flow. J Occup Environ Hyg. 2008;6:52–61. doi: 10.1080/15459620802558196. [DOI] [PubMed] [Google Scholar]
- 46.He X, Reponen T, McKay RT, Grinshpun SA. Effect of particle size on the performance of an N95 filtering facepiece respirator and a surgical mask at various breathing conditions. Aerosol Science Technol. 2013;47:1180–1187. doi: 10.1080/02786826.2013.829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harnish DA, Heimbuch BK, Balzli C, et al. Capture of 0.1-μm aerosol particles containing viable H1N1 influenza virus by N95 filtering facepiece respirators. J Occup Environ Hyg 2016; 13: D46-9. [DOI] [PubMed]
- 48.Goyal SM, Chander Y, Yezli S, Otter JA. Evaluating the virucidal efficacy of hydrogen peroxide vapour. J Hosp Infect. 2014;86:255–259. doi: 10.1016/j.jhin.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer RJ, Morris DH, van Doremalen N, et al. Effectiveness of N95 respirator decontamination and reuse against SARS-CoV-2 virus. Emerging Infect Dis. 2010 doi: 10.3201/eid2609.201524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutala WA, Gergen MF, Weber DJ. Sporicidal activity of a new low-temperature sterilization technology: the Sterrad 50 sterilizer. Infect Control Hosp Epidemiol. 1999;20:514–516. doi: 10.1086/501662. [DOI] [PubMed] [Google Scholar]
- 51.Vo E, Rengasamy S, Shaffer R. Development of a test system to evaluate procedures for decontamination of respirators containing viral droplets. Appl Environ Microbiol. 2009;75:7303–7309. doi: 10.1128/AEM.00799-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mills D, Harnish DA, Lawrence C, Sandoval-Powers NM, Heimbuch BK. Ultraviolet germicidal irradiation of influenza- contaminated N95 filtering facepiece respirators. Am J Infect Control. 2018;46:e49–e55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilkes AR. Comparison of two techniques for measuring penetration of sodium chloride particles through breathing system filters. Br J Anaesth. 2002;89:541–545. doi: 10.1093/bja/aef231. [DOI] [PubMed] [Google Scholar]
- 54.Rengasamy S, Walbert G, Newcomb W, et al. Protection factor for N95 filtering facepiece respirators exposed to laboratory aerosols containing different concentrations of nanoparticles. Ann Occup Hyg. 2015;59:373–381. doi: 10.1093/annhyg/meu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rengasamy S, Zhuang Z, Niezgoda G, et al. A comparison of total inward leakage measured using sodium chloride (NaCl) and corn oil aerosol methods for air-purifying respirators. J Occup Environ Hyg. 2018;15:616–627. doi: 10.1080/15459624.2018.1479064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rengasamy S, Shaffer R, Williams B, Smit S. A comparison of facemask and respirator filtration test methods. J Occup Environ Hyg. 2017;14:92–103. doi: 10.1080/15459624.2016.1225157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lore MB, Sebastian JM, Brown TL, Viner AS, McCullough NV, Hinrichs SH. Performance of conventional and antimicrobial- treated filtering facepiece respirators challenged with biological aerosols. J Occup Environ Hyg. 2012;9:69–80. doi: 10.1080/15459624.2011.640273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.