Dear Editor,
Coronavirus (CoV) is a positive-sense single-stranded RNA virus, comprising four subgroups: α, β, γ, and δ. Alpha-CoVs, such as human CoV 229E (HCoV-229E) and HCoV-NL63, cause upper respiratory tract inflammation and can trigger asthma exacerbation.1 , 2 In contrast, severe acute respiratory syndrome-CoV-2 (SARS-CoV-2), SARS-CoV, and Middle East respiratory syndrome-CoV (MERS-CoV) are β-CoVs that target epithelial cells in the upper and lower airways. SARS-CoV-2 is the etiological agent for CoV disease (COVID-19), which manifests as fever, olfactory dysfunction, pneumonia, and in some cases, acute respiratory distress syndrome (ARDS).3 The COVID-19 outbreak began in Wuhan, China, and has been rapidly spreading worldwide. COVID-19 tends to be severe in patients with comorbid conditions, such as diabetes and chronic obstructive pulmonary disease.4 The risk of severe COVID-19 may be elevated in patients with asthma, especially with non-allergic asthma,5 however, it remains unclear whether asthmatic patients are susceptible to SARS-CoV-2 infection. Information is also limited on whether COVID-19 can cause asthma exacerbation and on treatment of asthma exacerbation accompanied by COVID-19. Here, we report a case of exacerbation of previously well-controlled asthma associated with the onset of COVID-19.
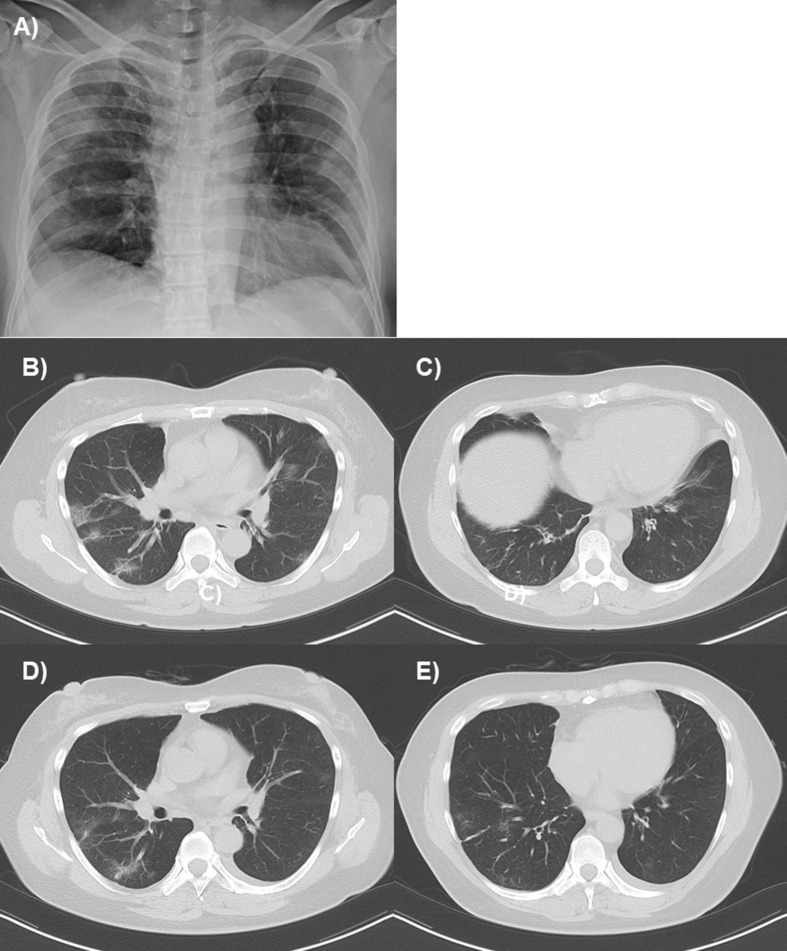
A 48-year-old woman with a history of asthma but no previous history of cigarette smoking was admitted to the hospital because of fever, wheezing, and dyspnea. She developed asthma at 34 years, which had been well-controlled under a combined treatment with inhaled corticosteroids, long-acting β-agonists, and a leukotriene receptor antagonist, accompanied by peripheral blood eosinophilia (618 cells/μL), but not by allergic sensitization or airflow limitation. Four days before admission, she had experienced fever and malaise, followed by sore throat and non-productive cough. On examination by a physician, she had a body temperature of 37.7 °C, percutaneous oxygen saturation (SpO2) of 98% on room air, and clear chest. Reverse transcriptase-polymerase chain reaction (RT-PCR) for SARS-CoV-2 was positive in a sample obtained from the nasopharynx, but influenza antigen tested negative. On admission, she had a body temperature of 37.7 °C, SpO2 of 86% on room air, and wheezing in the bilateral lung fields. No heart murmur or edema was observed. Blood tests revealed a peripheral blood leukocyte count of 8000/μL, with 72.6% neutrophils, 16.9% lymphocytes, and 0.6% eosinophils, and serum C-reactive protein level of 1.43 mg/dL. Chest radiography revealed mild opacities in the bilateral lungs (Fig. 1 A), and thoracic computed tomography showed multifocal ground glass opacities in the bilateral lungs, and bronchial wall thickening (Fig. 1B, C). She was diagnosed with asthma exacerbation and COVID-19 pneumonia.
Fig. 1.
Chest radiography and thoracic computed tomography on admission and discharge. Chest radiography on admission revealed mild opacities in the bilateral lungs (A). Thoracic computed tomography on admission shows multifocal ground-glass opacities in the bilateral lungs (B) and bronchial wall thickening (C). On discharge, these findings had mostly disappeared (D, E).
In addition to oxygen supplementation, inhalation of short-acting β agonists with a pressurized metered-dose inhaler to minimize the risk of aerosolization and infusion of methylprednisolone 160 mg/day were initiated. Inhalation of ciclesonide 1200 μg/day, oral favipiravir 1600 mg/day for 10 days, and infusion of nafamostat mesylate 250 mg/day for 8 days were administered for the treatment of COVID-19. Wheezes disappeared within 7 days; methylprednisolone administration and oxygen supplementation were terminated on days 5 and 10, respectively. RT-PCR result for SARS-CoV-2 was negative on day 21 after admission. Ground glass opacities and bronchial wall thickening had mostly disappeared (Fig. 1D, E).
To the best of our knowledge, there is only one case series of asthma exacerbation accompanied by SARS-CoV-2 infection.6 Treatment with systemic corticosteroids could successfully control asthmatic symptoms and hypoxemia, with minor impacts on the eradication of the virus.
Rhinovirus and respiratory syncytial virus (RSV) are the major pathogens associated with asthma exacerbation; however, there have been several reports of the presence of α-CoV in the upper airways during asthma exacerbation.1 Furthermore, an experimental infection with HCoV-229E caused wheezing, chest tightness, and shortness of breath in viral wheezers but not in healthy subjects.2 However, data are limited on whether β-CoV infection can cause asthma exacerbation. There have been no reports on whether COVID-19 is associated with exacerbations of chronic obstructive pulmonary disease, although it is a comorbidity associated with high mortality in COVID-19 cases.4 In the present case, sore throat, a relatively rare symptom observed in 5%–17% of the patients with COVID-19,3 , 4 preceded the appearance of wheezing and dyspnea, and therefore, coinfection with SARS-CoV-2 and other viruses, such as the rhinovirus and RSV could not be ruled out.
The administration of systemic corticosteroids in patients with COVID-19 is controversial.7, 8, 9 A study failed to demonstrate any beneficial effect of systemic corticosteroids on the prognosis of ARDS due to COVID-19.9 In addition, a report demonstrated that corticosteroid administration delayed viral clearance in MERS-CoV cases.10 In the present case, the virus was cleared 25 days after fever onset, which is slightly prolonged compared with the median period of SARS-CoV-2 clearance previously reported.7, 8, 9 For the patients with COVID-19 who develop asthma exacerbation, it might be important to reduce the dose and duration of systemic corticosteroids as much as possible and administer drugs with antiviral abilities, such as favipiravir, in combination.
Information is limited on the epidemiology and management of asthma exacerbation associated with COVID-19, and further accumulation of evidence is required.
Acknowledgment
Because this study was a retrospective case report that only utilizes the data on medical charts and thoracic CT, the requirement for obtaining informed consent from the studied subject was waived by the Institutional Review Board.
This study was partially funded by Research Grant on Allergic Disease and Immunology from the Japan Agency for Medical Research and Development under grant number JP20ek0410055.
Footnotes
Peer review under responsibility of Japanese Society of Allergology.
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Freymuth F., Vabret A., Brouard J., Toutain F., Verdon R., Petitjean J. Detection of viral, Chlamydia pneumoniae and Mycoplasma pneumoniae infections in exacerbations of asthma in children. J Clin Virol. 1999;13:131–139. doi: 10.1016/S1386-6532(99)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKean M.C., Leech M., Lambert P.C., Hewitt C., Myint S., Silverman M. A model of viral wheeze in nonasthmatic adults: symptoms and physiology. Eur Respir J. 2001;18:23–32. doi: 10.1183/09031936.01.00073101. [DOI] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Z., Hasegawa K., Ma B., Fujiogi M., Camargo C.A., Jr., Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. 2020;146:327–329. doi: 10.1016/j.jaci.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Codispoti C.D., Bandi S., Patel P., Mahdavinia M. Clinical course of asthma in 4 cases of COVID-19 infection. Ann Allergy Asthma Immunol. 2020;125:208–210. doi: 10.1016/j.anai.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang X., Mei Q., Yang T., Li L., Wang Y., Tong F. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu K., Chen Y., Yuan J., Yi P., Ding C., Wu W. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020;71:799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zha L., Li S., Pan L., Tefsen B., Li Y., French N. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020;212:416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]