Abstract
Background:
Prenatal exposures to phthalates and bisphenols are associated with impaired brain development in animals. However, epidemiological studies investigating the association between prenatal phthalate or bisphenol exposure and cognition have produced mixed findings and mostly had modest sample sizes and measured the exposure during the third trimester.
Objective:
We examined the association between pregnancy maternal urinary biomarkers of phthalate or bisphenol exposure and nonverbal intelligence quotient (IQ) in children 6 years of age.
Method:
The study sample consisted of 1,282 mother–child pairs participating in the Generation R Study, a population-based birth cohort in Rotterdam, Netherlands (enrollment 2002–2006). We measured maternal urinary concentrations of 18 phthalate metabolites and 8 bisphenols at , 18–25, and of gestation. Child nonverbal IQ was measured at 6 years of age using the Snijders-Oomen Nonverbal Intelligence Test–Revised. Linear regression models were fit for each of the three collection phases separately, the three collection phases jointly, and for the averaged prenatal exposure across pregnancy.
Results:
Higher urinary concentrations of phthalate metabolites during early pregnancy were associated with lower child nonverbal IQ score [e.g., B per 10-fold increase in summed low-molecular (95% CI: , )]. This association remained unchanged when adjusted for mid and late pregnancy exposures. We also observed an inverse association between late pregnancy di-n-octyl phthalate (DNOP) exposure and nonverbal IQ. Maternal urinary concentrations of bisphenols were not associated with child nonverbal IQ. There was no effect estimate modification by sex.
Conclusions:
We did not observe that maternal biomarkers of bisphenol exposure are associated with nonverbal IQ. We found that phthalate exposure in early pregnancy and DNOP exposure in late pregnancy are associated with lower nonverbal IQ scores in children. Our results might suggest that particularly early pregnancy is a sensitive window of phthalate exposure, but future studies are needed to replicate our findings. https://doi.org/10.1289/EHP6047
Introduction
Phthalates and bisphenols are synthetic compounds incorporated in many products. For example, phthalates such as di-(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), di-n-octyl phthalate (DNOP), and benzyl butyl phthalate (BBP) are primarily used as plasticizers for polyvinyl chloride and exist in food packaging materials, floor materials, clothing, toys, and medical devices. DBPs are also used as solvents and fixatives in paint and cosmetics (Cefic Chemdata International 2010; Sathyanarayana 2008; Schierow and Lee 2008). Bisphenol A (BPA) or common replacements such as bisphenol S (BPS) and bisphenol F (BPF) exist in products such as epoxy resin coatings of canned food containers, water bottles, storage containers, thermal paper, and baby bottles (Lehmler et al. 2018; Michałowicz 2014).
Phthalates and bisphenols are omnipresent in the environment, and these compounds have been detected in urine samples of mothers and their offspring (Casas et al. 2013). Prenatal exposure to these chemicals can occur because of their ability to cross the placental and blood–brain barriers (Schönfelder et al. 2002; Silva et al. 2004; Chou et al. 2011; Jensen et al. 2012). Studies suggest that prenatal phthalate and bisphenol exposure both interfere with the thyroid hormone system (Boas et al. 2009; Bonefeld-Jørgensen et al. 2007; Gao et al. 2017; Ghisari and Bonefeld-Jorgensen 2009; Lyche et al. 2009; Miodovnik et al. 2014), which is crucial for normal fetal brain development (Berbel et al. 2010). In addition, neurotoxic effects of phthalates and bisphenols may be mediated by anti-androgenic activity (Borch et al. 2006; Fang et al. 2017; Weiss 2012; Wolstenholme et al. 2011), disruption of brain dopaminergic activity (Bellinger 2013; Huang et al. 2017; Tanida et al. 2009), and interaction with peroxisome proliferator-activated receptors (Inadera 2015; Lyche et al. 2009; Miodovnik et al. 2014).
Studies in animals have shown that prenatal phthalate and bisphenol exposure impairs neurodevelopment in the offspring (Arcadi et al. 1998; Boberg et al. 2011; Kimura et al. 2016; X-J Li et al. 2013; Y Li et al. 2009; Poimenova et al. 2010; Sun et al. 2014; Tanida et al. 2009; Tian et al. 2010; Wolstenholme et al. 2011; Xu et al. 2012). With respect to phthalates, animal studies find inverse associations between prenatal exposure to DEHP and DBP and learning, memory, and brain development in the offspring (Arcadi et al. 1998; Boberg et al. 2011; X-J Li et al. 2013; Y Li et al. 2009; Tanida et al. 2009). In addition, prenatal exposure to DEHP can affect developmental plasticity of the hippocampus (Sun et al. 2014). Regarding bisphenols, animal studies have shown that gestational exposure to BPA was associated with alterations in brain morphology and brain function (Wolstenholme et al. 2011). Further, BPA-exposed rats and mice exhibit persistent learning and memory impairments (Poimenova et al. 2010; Tian et al. 2010; Xu et al. 2012) and low-dose BPA exposure disrupts hippocampal CA1 neuronal morphology, which is believed to persist into adulthood (Kimura et al. 2016; Tian et al. 2010; Xu et al. 2012).
However, epidemiological studies investigating the associations between prenatal phthalate or bisphenol exposure and cognitive functioning in children are limited and the findings are inconclusive. One study using mother–child pairs from inner-city New York reported that higher metabolite concentrations of DBP and di-isobutyl phthalate metabolites measured in the third trimester were associated with lower intelligent quotient (IQ) score in 328 children at 7 years of age (Factor-Litvak et al. 2014). Similarly, other studies () found that higher levels of DEHP metabolites during the third trimester were associated with a lower score on the mental development index (MDI) of the Bayley Scales of Infant Development at 0.5–2 years of age (Kim et al. 2011; Qian et al. 2019). Two studies found that third-trimester concentrations of DBP and mono(3-carboxypropyl) phthalate (mCPP) metabolites were inversely associated with the MDI at child age 2–3 y, but only in girls (Doherty et al. 2017; Whyatt et al. 2012). Another study found the averaged sum of high-molecular weight phthalates (HMWPs) and DEHP exposure across pregnancy to be inversely associated with IQ, but only in boys (Hyland et al. 2019). Yet, five other studies did not find any association between prenatal exposure to phthalates and cognition during the second (Kim et al. 2017; Li et al. 2019) and third trimester (Huang et al. 2015; Li et al. 2019; Nakiwala et al. 2018; Polanska et al. 2014) using data from 100–452 mother–child pairs. Research on bisphenol exposure mostly found little evidence of an association between prenatal exposure and child cognition. One study found an inverse association between prenatal BPA exposure measured in cord blood and offspring IQ at 7 years of age in 148 children (Lin et al. 2017). However, most other studies using maternal urine concentrations to determine exposure did not show an association between prenatal BPA exposure and cognitive functioning in 239–812 children at 1–8 years of age (Braun et al. 2011, 2017a, 2017b; Casas et al. 2015; Nakiwala et al. 2018; Stacy et al. 2017). Further, a recent study found first trimester exposure to a mixture of 26 endocrine-disrupting chemicals to be associated with lower IQ among boys (Tanner et al. 2020). Among a broad range of chemical biomarkers, the present study included phthalate metabolites and BPA, BPF, and BPS and identified BPF as the primary chemical of concern. In addition, concentrations of BPA, mono-ethyl phthalate (mEP), and monobenzyl phthalate (mBzP) had a considerable contribution to the overall mixture effect (Tanner et al. 2020).
The heterogeneity in epidemiological results may be explained by the fact that most of these studies had modest sample sizes, which may have limited the statistical power to consistently detect adverse associations. In addition, most studies measured prenatal exposure only during the third trimester, whereas other windows of susceptibility may exist (Selevan et al. 2000). Finally, only one previous study investigated the association between prenatal exposure to BPS and BPF with IQ. To address these limitations, we studied a large cohort from the Generation R Study, which is characterized by detailed follow-up information of the child and three repeated measurements (early, mid, and late pregnancy) of urinary phthalate and bisphenol biomarkers, including BPF and BPS. We investigated the extent to which maternal exposure to phthalates or bisphenols during pregnancy are associated with offspring’s nonverbal IQ at 6 years of age.
Methods
Study Participants and Follow-Up
Generation R is a prospective population-based birth cohort designed to identify early environmental and genetic determinants of growth and development (Kooijman et al. 2016). Briefly, all pregnant women who resided in the study area in Rotterdam, Netherlands, and had a delivery date between April 2002 and January 2006 were eligible. All eligible pregnant women who visited a midwife or obstetrician in Rotterdam were contacted by the Generation R Study staff for recruitment. The study staff were able to communicate with the pregnant women in Dutch, English, French, Portuguese, and Turkish. Among the 9,778 mothers who participated in the study, 8,879 (91%) were enrolled during pregnancy and the rest were enrolled during routine visits of the newborn to the child health centers. The enrollment procedure has been previously described in detail (Hofman et al. 2004; Jaddoe et al. 2006, 2008, 2010, 2012). Between February 2004 and January 2006, women provided spot urine specimens at the time of routine ultrasound examinations during early, mid, and late pregnancy. A total of 2,083 women provided a complete set of three urine specimens.
When the children turned 6 years of age, the families were invited to participate in an in-person follow-up visit to collect neurobehavioral data, biospecimens, and sociodemographic and health data. Of the 2,083 mother–child pairs with three urinary samples, 1,405 provided data at 6 years of age of the child. The availability of follow-up data was a requirement to allow studies of the associations between prenatal phthalates and bisphenol exposure and child health, including cognition. Of these 1,405 mother–child pairs, 1,282 had complete data on nonverbal IQ and comprised the study sample. Women in this subset had higher education and income levels, were slightly older, and were more likely to be of Dutch national origin than the broader Generation R cohort (Kooijman et al. 2016).
The study protocol underwent human subjects review at Erasmus Medical Center, Rotterdam, Netherlands (MEC 198.782.2001.31, MEC-2007-413). Mothers provided written informed consent for themselves and their children.
Bisphenol and Phthalate Measurements in Urine
Maternal spot urine specimens were collected during early ( of gestation, ), mid ( of gestation, ), and late pregnancy ( of gestation, ). Details on urine specimen collection have been described elsewhere (Kruithof et al. 2014). Briefly, all urine samples were collected (at 0800–1000 hours) in polypropylene urine collection containers that were kept for a maximum of 20 h in a cold room (4°C) before being frozen at in aliquots in polypropylene vials. The urine specimens were shipped on dry ice in polypropylene vials to the Wadsworth Center, New York State Department of Health, Albany, New York, for analysis of phthalate metabolite and bisphenol concentrations.
A detailed description of the analytical procedure is given elsewhere (Philips et al. 2018). Briefly, quantitative detection of phthalate metabolites was achieved by using a solid-phase extraction method followed by enzymatic deconjugation of the glucuronidated phthalate monoesters coupled with high performance liquid chromatography electrospray ionization–tandem mass spectrometry (HPLC-ESI-MS/MS) (Asimakopoulos et al. 2016), which allowed for the rapid detection of 18 metabolites of phthalates with limits of detection (LODs) in the range of . Quantitative detection of bisphenols was realized using a liquid–liquid extraction method followed by enzymatic deconjugation of the glucuronidated bisphenols coupled with HPLC-ESI-MS/MS, which permitted the detection of eight biomarkers of bisphenols (including BPA, BPF, and BPS) with an LOD range of . Similarly, samples were analyzed for creatinine using HPLC-ESI-MS/MS. Quantification of calibration check standards resulted in an LOD of .
Exposure biomarkers were excluded from further statistical analyses if more than 80% of the study population had concentrations below the LOD. Urinary biomarkers for exposure to phthalates were grouped according to their parent compound, biologic activity, and source of exposure in order to limit multiple comparisons. Phthalate groups included low-molecular weight phthalates (LMWPs), HMWPs, DEHP, DNOP, and phthalic acid (PA). For the LMWP group, we summed mono-methyl phthalate (mMP), mEP, mono-n-butyl phthalate (mBP), and mono-isobutyl phthalate (mIBP) metabolite concentrations. For the HMWP group, we summed mono-(2-ethyl-5-carboxypentyl) phthalate (mECPP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (mEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (mEOHP), mono-[(2-carboxymethyl)hexyl] phthalate (mCMHP), mCPP, mBzP, mono-hexyl phthalate (mHxP), and mono-2-heptyl phthalate (mHpP) metabolite concentrations. For the DEHP group, we summed mECPP, mEHHP, mEOHP, and mCMHP metabolite concentrations and used MCPP metabolite concentrations as a proxy for DNOP exposure. Finally, PA is an end metabolite of all phthalates. PA metabolite concentrations were therefore analyzed separately as a proxy for total phthalate exposure. Regarding bisphenol concentrations, we calculated the sum for total bisphenol concentrations. Prior to the statistical analyses, all concentrations below the LOD were replaced with the LOD divided by the square root of 2 (Hornung and Reed 1990).
Nonverbal IQ at Child Age 6 Years
Children’s nonverbal IQ was assessed by administering the Mosaics and Categories subtests from the Snijders-Oomen Nonverbal Intelligence Test–Revised (SON-R), a reliable and well-validated instrument (Jenkinson et al. 1996; Moore et al. 1998; Tellegen et al. 1998). The correlation between the total score of the SON-R 2.5-7 and the performance IQ score of the Wechsler Preschool and Primary Scale of Intelligence has been reported to be between 0.60 and 0.83, and the average reliability of the SON-R IQ score was 0.90 (Jenkinson et al. 1996; Moore et al. 1998; Tellegen et al. 2005). Further, the test is regarded as highly reliable and rated good (3 of 3) by the commission of Netherlands Institute for Psychologists. The two language-independent subtests that included items that probe visuospatial and abstract reasoning abilities were selected because of the multiethnic composition of the Generation R Study. Subtest raw test scores were converted into age-standardized nonverbal IQ scores. These standardized scores, based on the two subtests, correlated well () with those based on the complete instrument (Ghassabian et al. 2011).
Additional Data Collection
Maternal reproductive, sociodemographic, and cognitive data were assessed by questionnaires and/or observations. During the first prenatal visit, height, and weight were measured, but prepregnancy weight was self-reported by participants. These data were used to calculate body mass index (BMI). In addition, data was collected on maternal age (years), parity (0, 1, or ), smoking (no smoking during pregnancy, smoking until pregnancy recognized, and continued smoking during pregnancy), alcohol intake during pregnancy [no alcohol consumption during pregnancy, alcohol consumption until pregnancy recognized, continued occasionally (), and continued frequently ()], marital status (married/partner or single), household total net income [ (i.e., below the Dutch social security level), 1,200–2,000 euros/month, ], highest completed education level [low ( at general secondary school) intermediate ( of secondary education), and high (university degree or higher vocational training)], ethnicity (Dutch national origin, other-Western, and non-Western), and folic acid intake (none, started in first 10 wks of pregnancy, and started preconception). Maternal IQ was examined when mother–child pairs attended the 6-y examination and was assessed using a computerized Ravens Advanced Progressive Matrices Test, Set I (McKinzey et al. 2003). The test is a 12-item reliable and validated short version of the Raven’s Progressive Matrices to assess nonverbal cognitive ability (Chiesi et al. 2012). The consumption of fruit and vegetables was assessed in the first trimester using a modified version of a validated food frequency questionnaire and was adjusted for energy intake (Steenweg-de Graaff et al. 2012). Except for BMI (12%), smoking (10%), income (15%), folic acid intake (21%), and fruit and vegetable intake (25%), the percentage of missing values was below 10%.
Statistical Methods
Urinary phthalate metabolite and bisphenol concentrations were expressed on a creatinine basis and transformed (in micrograms per gram creatinine). Missing phthalate metabolite and bisphenol concentration values ( with missing data in one or two urinary collection periods during pregnancy) and all missing covariate data were imputed 10 times with the multivariate imputation by chained equations method in R (version 3.5.3; R Development Core Team) (van Buuren and Groothuis-Oudshoorn 2011). Urinary BPA and PA metabolite concentrations and the child nonverbal IQ score were included as predictors for the imputation of covariates. The outcome variable child nonverbal IQ was not imputed. We calculated intraclass correlation coefficients (ICCs) for individual measurements, for example, during early pregnancy (single-rater, relevant to the time-specific analyses), and for the mean of the three measurements across pregnancy (mean of k raters), using two-way mixed-effects models with absolute agreement (Koo and Li 2016). Further, we first performed regression analyses to estimate the associations of grouped phthalate and bisphenol urinary concentrations with nonverbal IQ for each collection phase (gestational age , 18–25, and ). Second, a mutually adjusted model was fitted in which the association of prenatal phthalate and bisphenol urinary concentrations from each time period on nonverbal IQ were jointly estimated. To test whether the association between prenatal urinary concentrations of phthalate metabolites and bisphenols and nonverbal IQ differed across time windows of exposure (), we used the multiple informant method, in which different exposure windows are treated as informants (Sánchez et al. 2011). We chose this strategy to identify possible windows of susceptibility and to be able to compare our results with other studies that used a single spot urine sample in pregnancy to determine phthalate and bisphenol concentrations. Third, we carried out regression analyses to estimate the association between the averaged prenatal urinary concentrations across pregnancy and nonverbal IQ. Urinary metabolite concentrations of phthalates and (especially) bisphenol concentrations vary over time. Therefore, the average is most likely a better approximation of each participant’s exposure during pregnancy than any exposure measurement on its own. Fourth, we presented restrictive cubic splines for untransformed grouped biomarker concentrations that were predictive of nonverbal IQ. Finally, several studies have suggested that sex may be a potential effect estimate modifier in the association of prenatal exposure to phthalates and bisphenols and neurodevelopmental outcomes (Braun et al. 2011; Casas et al. 2015; Doherty et al. 2017; Factor-Litvak et al. 2014; Hyland et al. 2019; Kim et al. 2011; Stacy et al. 2017; Whyatt et al. 2012). We therefore explored potential effect modification by sex via stratification, interaction terms, and augmented product terms (Buckley et al. 2017) ().
We present results from unadjusted and adjusted analyses. The adjustment variables were maternal age (continuous), ethnicity (categorical), education (categorical), income (categorical), marital status (categorical), alcohol consumption during pregnancy (categorical), maternal nonverbal IQ (continuous), prepregnancy BMI (continuous), parity (categorical), smoking during pregnancy (categorical), child sex (categorical), and child age at assessment (continuous). These potential confounders were selected a priori defined with a directed acyclic graph (DAG) (Textor et al. 2016) based on previous studies of prenatal phthalate and bisphenol exposure and child neurodevelopment and on biologically plausible covariate–exposure and covariate–outcome associations observed in our data (see Figure S1).
Sensitivity Analyses
Several sensitivity analyses were performed. First, we used another common method to adjust for creatinine by refitting visit-specific and averaged models, with concentrations expressed in nanograms per milliliter and creatinine concentration added as a separate covariate. We performed this sensitivity analyses to facilitate comparison with previous studies investigating prenatal exposure to nonpersistent chemicals and neurodevelopment that have used this adjustment method. Second, we performed post hoc analyses in which we explored the associations of individual phthalate metabolite and bisphenol concentrations with nonverbal IQ because individual phthalates and bisphenols within the summed groups may have different neurotoxic effects. Third, we used inverse probability weighting (stabilized weights) to correct for potential selection bias (Cole and Hernán 2008) and to provide results representative for the full Generation R Study cohort () given that children included in the analysis () were more likely to have parents who were of Dutch national origin, older, and from a higher socioeconomic (Kooijman et al. 2016). Baseline characteristics significantly different () between the initially recruited cohort and the current analysis sample are presented in Table S1. Fourth, because diet and the intake of healthy nutrients may confound the association between prenatal phthalate and bisphenol exposure (e.g., food packaging) and child cognition (e.g., healthy nutrients), we performed a sensitivity analyses in which we additionally adjusted for maternal fruit, vegetables, and folic acid intake. Fifth, to correct for multiple hypothesis testing, each -value was compared with a threshold, defined as 0.05 divided by the effective independent number of tests (Li et al. 2012). The corrected -value was calculated based on the correlation structure between the phthalate metabolite groups (LMWP, HMWP, DEHP, DNOP, and PA) for each time point separately (corrected , , ), and for the average exposures (corrected ).
Results
Sample Characteristics
At enrollment, most of the participating women were between 30 and 35 years of age (43%), nulliparous (61%), Dutch (54%), married (89%), and highly educated (50%) (Table 1). A large group had a prepregnancy BMI of between 18.5 and 25 (70%), a high income (68%), and did not consume alcoholic beverages (42%) or smoke (75%) during pregnancy.
Table 1.
Characteristics of study participants ().
| Maternal characteristicsa | Percentages | Phthalic acidb | Bisphenolsc | Nonverbal IQd | ||
|---|---|---|---|---|---|---|
| Median | P25, P75 | Median | P25, P75 | |||
| Age (y) | ||||||
| 2.0 | 109.2 | 65.4, 162.5 | 1.5 | 1.2, 2.6 | ||
| 11.5 | 85.5 | 61.9, 123.9 | 1.7 | 1.1, 2.8 | ||
| 26.8 | 82.6 | 52.1, 129.7 | 1.7 | 1.1, 2.8 | ||
| 42.9 | 84.7 | 52.5, 131.8 | 1.9 | 1.3, 2.9 | ||
| 16.8 | 85.8 | 54.5, 135.5 | 1.9 | 1.1, 2.9 | ||
| Prepregnancy BMI () | ||||||
| 2.5 | 88.6 | 45.4, 123.5 | 1.9 | 1.3, 3.1 | ||
| 69.8 | 79.9 | 51.1, 124.3 | 1.8 | 1.1, 2.8 | ||
| 19.1 | 95.6 | 58.2, 146.0 | 2.1 | 1.3, 3.3 | ||
| 8.6 | 95.0 | 67.7, 142.0 | 1.8 | 1.2, 3.0 | ||
| Parity | ||||||
| 0 | 60.5 | 86.0 | 54.0, 133.9 | 1.8 | 1.2, 2.8 | |
| 1 | 28.2 | 79.9 | 52.5, 119.3 | 1.9 | 1.2, 3.0 | |
| 11.3 | 88.2 | 54.2, 139.8 | 1.7 | 1.1, 2.8 | ||
| Ethnicity | ||||||
| Dutch | 53.9 | 81.1 | 50.4, 131.8 | 1.9 | 1.2, 2.9 | |
| Other-Western | 12.4 | 78.2 | 52.9, 128.6 | 1.7 | 1.2, 2.5 | |
| Non-Western | 33.7 | 88.3 | 58.7, 132.9 | 1.8 | 1.1, 2.9 | |
| Educatione | ||||||
| Low | 18.9 | 95.2 | 65.0, 151.7 | 1.8 | 1.2, 2.8 | |
| Intermediate | 30.7 | 85.9 | 55.3, 137.0 | 1.9 | 1.2, 3.1 | |
| High | 50.4 | 76.2 | 48.8, 122.1 | 1.8 | 1.1, 2.8 | |
| Household income (euros/month) | ||||||
| 15.4 | 91.1 | 62.4, 135.9 | 1.6 | 1.1, 2.8 | ||
| 1,200–2,000 | 17.0 | 86.9 | 56.2, 137.3 | 1.9 | 1.3, 3.0 | |
| 67.6 | 81.2 | 50.7, 127.4 | 1.8 | 1.2, 2.9 | ||
| Marital status | ||||||
| Married/living with partner | 88.5 | 82.8 | 52.0, 128.9 | 1.8 | 1.2, 2.9 | |
| No partner | 11.5 | 96.2 | 67.7, 151.0 | 1.8 | 1.1, 3.1 | |
| IQ score | ||||||
| 22.5 | 91.3 | 58.3, 145.5 | 1.9 | 1.1, 2.9 | ||
| 43.3 | 86.9 | 55.6, 134.8 | 1.8 | 1.2, 2.7 | ||
| 17.9 | 79.3 | 50.8, 125.2 | 1.8 | 1.2, 2.8 | ||
| 16.3 | 72.9 | 47.3, 118.6 | 1.9 | 1.3, 3.1 | ||
| Smoking | ||||||
| No smoking during pregnancy | 75.3 | 78.7 | 51.5, 122.7 | 1.8 | 1.1, 2.8 | |
| Until pregnancy recognized | 10.2 | 97.6 | 62.8, 140.1 | 2.0 | 1.4, 3.2 | |
| Continued during pregnancy | 14.5 | 105.6 | 71.2, 154.8 | 1.9 | 1.2, 3.1 | |
| Alcoholic beverage consumption | ||||||
| No alcohol consumption | 42.1 | 86.5 | 56.6, 137.6 | 1.8 | 1.1, 2.8 | |
| Until pregnancy recognized | 17.0 | 78.5 | 52.4, 132.7 | 1.7 | 1.1, 2.7 | |
| Continued occasionallyf | 35.2 | 85.5 | 51.3, 124.9 | 2.0 | 1.3, 3.0 | |
| Continued frequentlyg | 5.8 | 74.6 | 48.1, 129.7 | 2.0 | 1.4, 3.1 | |
| Infant characteristics | Percentages | Median | P25, P75 | Median | P25, P75 | |
| Sex of infant at birth | ||||||
| Male | 50.5 | 84.5 | 52.6, 130.7 | 1.9 | 1.2, 3.0 | |
| Female | 49.5 | 84.1 | 53.7, 132.3 | 1.7 | 1.2, 2.7 | |
Note: BMI, body mass index; IQ, intelligence quotient; P, percentile; SD, standard deviation.
There were missing observations for BMI (), parity (), ethnicity (), education (), household income (), marital status (), maternal IQ (), smoking (), and alcohol consumption ().
Average total phthalic acid in micrograms per gram creatinine by category of characteristics.
Average total bisphenols in micrograms per gram creatinine by category of characteristics.
Nonverbal IQ by category of characteristics.
Low: no education finished, primary education, lower vocational training, intermediate general school or at general secondary school. Intermediate: of secondary education, intermediate vocational training or first year of higher vocational training. High: University degree or higher vocational training.
Less than 1 glass/wk.
One or more glasses/wk for at least two trimesters.
Phthalate and Bisphenol Concentrations
The median LMWP metabolite concentrations for , 18–25, and of gestation were 240, 103, and creatinine, respectively (Table 2). The median HMWP measured at , 18–25, and of gestation were 69, 33, and creatinine. Total bisphenol concentrations comprised mostly BPA, and the median total bisphenol concentrations for , 18–25, and were 2, 1, and creatinine, respectively. Descriptive statistics of the individual biomarkers from our study sample can be found in Tables S2 and S3. The ICC for the grouped phthalate metabolite concentrations varied between 0.2 and 0.4 for a single measurement and varied between 0.4 and 0.6 for the mean of the three measurements (see Table S4). Regarding the bisphenol group, the ICCs for a single-measurement (0.05) and for the mean of the three measurements (0.14) were poor.
Table 2.
Descriptive statistics of creatinine-adjusted phthalate and bisphenol concentrations in urine samples measured in pregnancy.
| Variables | Min | P25 | P50 | P75 | Max | |
|---|---|---|---|---|---|---|
| Phthalate metabolite concentrations ()a | ||||||
| LMWP metabolites (weeks of gestation) | ||||||
| 1,274 | 0.4 | 109.5 | 239.5 | 603.3 | 102,789.2 | |
| 18–25 | 1,270 | 4.3 | 46.8 | 102.6 | 238.0 | 36,050.2 |
| 1,269 | 21.1 | 103.0 | 232.3 | 530.5 | 8,131.3 | |
| HMWP metabolites (weeks of gestation) | ||||||
| 1,274 | 0.6 | 39.6 | 68.5 | 126.6 | 2,837.4 | |
| 18–25b | 1,270 | 2.3 | 19.3 | 33.1 | 59.0 | 15,577.1 |
| b | 1,269 | 3.6 | 35.0 | 52.3 | 81.9 | 1,683.6 |
| DEHP metabolites (weeks of gestation) | ||||||
| 1,274 | 0.3 | 31.4 | 55.2 | 103.0 | 2,821.2 | |
| 18–25 | 1,270 | 1.8 | 14.4 | 25.0 | 46.3 | 15,574.4 |
| 1,269 | 3.2 | 29.6 | 45.0 | 71.0 | 1,673.5 | |
| DNOP metabolites (weeks of gestation) | ||||||
| 1,274 | 0 | 0.9 | 1.6 | 2.8 | 106.5 | |
| 18–25 | 1,270 | 0 | 0.4 | 0.8 | 1.4 | 65.8 |
| 1,269 | 0 | 1.2 | 1.9 | 3.0 | 72.0 | |
| PA (weeks of gestation) | ||||||
| 1,274 | 0.6 | 33.8 | 63.8 | 123.4 | 12,433.4 | |
| 18–25 | 1,270 | 4.3 | 56.5 | 111.6 | 250.1 | 2,885.2 |
| 1,269 | 2.6 | 44.2 | 73.9 | 127.1 | 1,562.4 | |
| Total bisphenol concentrations [weeks of gestation ()] | ||||||
| c | 1,274 | 0.1 | 1.0 | 2.1 | 5.2 | 982.8 |
| 18–25d | 1,270 | 0 | 0.6 | 1.2 | 2.5 | 277.6 |
| e | 1,269 | 0.1 | 1.1 | 2.0 | 4.2 | 145.4 |
Note: Concentrations below the LOD were imputed with LOD divided by the square root of 2. DEHP, di-2-ethylhexylphthalate metabolite; DNOP, di-n-octylphthalate metabolite; HMWP, high-molecular weight phthalate metabolite; LMWP, low-molecular weight phthalate metabolite; LOD, limit of detection; max, maximum; min, minimum; P, percentile; PA, phthalic acid.
Phthalate metabolites are grouped into the following categories: LMWP metabolites (sum of mono-methyl phthalate, mono-ethyl phthalate, mono-isobutyl phthalate, and mono--butyl phthalate); HMWP metabolites [sum of mono-(2-ethyl-5-carboxypentyl) phthalate, mono-(2-ethyl-5-hydroxyhexyl) phthalate, mono-(2-ethyl-5-oxohexyl) phthalate, mono-[(2-carboxymethyl)hexyl] phthalate, mono(3-carboxypropyl) phthalate, monobenzyl phthalate, mono-hexyl phthalate, and mono-2-heptyl phthalate; DEHP metabolites: [sum of mono (2-ethyl-5 carboxypentyl)phthalate, mono (2-ethyl-5 hydroxyhexyl)phthalate, mono-(2-ethyl-5-oxohexyl)phthalate, and mono-[(2-carboxymethyl)hexyl]phthalate]; DNOP metabolites [mono(3-carboxypropyl) phthalate]; and PA (a proxy for total phthalate exposure).
With the exclusion of mono-hexyl phthalate, and mono-2-heptyl phthalate metabolite concentrations.
Total bisphenol: sum of bisphenol A, bisphenol F, and bisphenol S.
Total bisphenol: sum of bisphenol A and bisphenol S.
Total bisphenol: sum of bisphenol A and bisphenol F.
Associations with Nonverbal IQ
Creatinine-adjusted LMWP, DNOP, and PA metabolite concentrations at were significantly associated with child nonverbal IQ score (Table 3). For example, a 10-fold higher LMWP, DNOP, or PA metabolite concentration was associated with lower nonverbal IQ scores of 1.7 points [95% confidence interval (CI): , ], 2.0 points (95% CI: , ), and 1.9 points (95% CI: , ), respectively. The associations for HMWP [ (95% CI: , 0.0)], and DEHP [ (95% CI: , 0.1)] metabolite concentrations with nonverbal IQ were comparable in magnitude. When adjusted for mid and late pregnancy exposures, the associations of child nonverbal IQ with grouped phthalate metabolite concentrations were similar. Mid and late pregnancy concentrations of grouped phthalate metabolites were generally not significantly associated with nonverbal IQ. However, a 10-fold increase in DNOP metabolite concentration at of gestation was associated with a 2.4-point lower nonverbal IQ score (95% CI: , 0.0). Further, the significant interaction terms between LMWP, HMWP, DNOP, and PA metabolite concentrations and timing of exposure (, , , and , respectively) suggested that the potential effects of prenatal LMWP, HMWP, DNOP, and PA metabolite concentrations on nonverbal IQ might differ depending on the timing of exposure. No significant interaction terms were observed for DEHP metabolite concentrations and timing of exposure. Further, compared with the associations observed at of gestation, we observed similar estimates in terms of magnitude for averaged LMWP [ (95% CI: , 0.4)] and PA [ (95% CI: , 1.0)] metabolite concentrations, and a greater estimate in terms of magnitude for averaged DNOP metabolite concentrations [ (95% CI: , 0.0)]. Finally, representative restrictive cubic splines for untransformed grouped phthalate metabolite concentrations at of gestation and DNOP metabolite concentrations at of gestation (see Figure S2) indicated a slightly steeper inverse association between exposure and outcome at lower levels of exposure.
Table 3.
Difference in child nonverbal IQ score at 6 years of age per increase in creatinine-adjusted maternal urine phthalate metabolite and bisphenol concentrations (measured in micrograms per gram creatinine), by timing of urine sampling.
| Phthalate metabolitea and bisphenol concentrations | Unadjusted | Adjustedb | Mutually adjustedc | |||
|---|---|---|---|---|---|---|
| B | 95% CI | B | 95% CI | B | 95% CI | |
| LMWP metabolites | ||||||
| of gestation | , | , | , | |||
| 18–25 wks of gestation | , | 0.27 | , 1.79 | 1.04 | , 2.67 | |
| of gestation | , | , 0.51 | , 0.90 | |||
| Averaged | , | , 0.36 | — | |||
| -Valued | 0.06 | |||||
| HMWP metabolites | ||||||
| of gestation | , | , 0.03 | , | |||
| 18–25 wks of gestatione | , 1.07 | 1.04 | , 2.87 | 1.19 | , 3.05 | |
| of gestatione | , 2.54 | 0.73 | , 3.26 | 0.79 | , 3.33 | |
| Averaged | , | , 2.78 | — | |||
| -Valued | 0.09 | |||||
| DEHP metabolites | ||||||
| of gestation | , | , 0.05 | , | |||
| 18–25 wks of gestation | , 1.49 | 0.92 | , 2.72 | 1.06 | , 2.88 | |
| of gestation | 0.20 | , 2.77 | 0.65 | , 3.09 | 0.71 | , 3.16 |
| Averaged | , 0.22 | 0.05 | , 2.36 | — | ||
| -Valued | 0.11 | |||||
| DNOP metabolites | ||||||
| of gestation | , | , | , | |||
| 18–25 wks of gestation | , 1.94 | 0.38 | , 2.32 | 0.87 | , 2.84 | |
| of gestation | , 1.43 | , | , 0.30 | |||
| Averaged | , | , 0.06 | — | |||
| -Valued | 0.03 | |||||
| PA metabolites | ||||||
| of gestation | , | , | , | |||
| 18–25 wks of gestation | , 0.91 | 0.85 | , 2.57 | 1.07 | , 2.80 | |
| of gestation | , | , 1.07 | , 1.42 | |||
| Averaged | , | , 1.04 | — | |||
| -Valued | 0.05 | |||||
| Bisphenol metabolites | ||||||
| of gestationf | 0.38 | , 1.86 | 0.49 | , 1.87 | 0.48 | , 1.87 |
| 18–25 wks of gestationg | 0.48 | , 2.15 | 0.13 | , 1.70 | 0.12 | , 1.69 |
| of gestationh | 1.05 | , 2.85 | 0.05 | , 1.72 | 0.06 | , 1.74 |
| Averaged | 1.81 | , 4.65 | 0.76 | , 3.42 | — | |
| -Valued | 0.92 | |||||
Note: —, not applicable; B, unstandardized beta; BMI, body mass index; CI, confidence interval; DEHP, di-2-ethylhexylphthalate; DNOP, di--octylphthalate; HMWP, high-molecular weight phthalate; IQ, intelligence quotient; LMWP, low-molecular weight phthalate; PA, phthalic acid.
Phthalate metabolites are grouped into the following categories: LMWP metabolites (sum of mono-methyl phthalate, mono-ethyl phthalate, mono-isobutyl phthalate, mono--butyl phthalate); HMWP metabolites [sum of mono-(2-ethyl-5-carboxypentyl) phthalate, mono-(2-ethyl-5-hydroxyhexyl) phthalate, mono-(2-ethyl-5-oxohexyl) phthalate, mono-[(2-carboxymethyl)hexyl] phthalate, mono(3-carboxypropyl) phthalate, monobenzyl phthalate, mono-hexyl phthalate, and mono-2-heptyl phthalate]; DEHP metabolites: [sum of mono (2-ethyl-5 carboxypentyl) phthalate, mono (2-ethyl-5 hydroxyhexyl) phthalate, mono-(2-ethyl-5-oxohexyl) phthalate, and mono-[(2-carboxymethyl)hexyl] phthalate]; DNOP metabolites [mono(3-carboxypropyl) phthalate]; and PA (a proxy for total phthalate exposure).
Adjusted for maternal age, maternal nonverbal IQ, sex of child, age of the child at assessment, ethnicity, education, income, marital status, maternal alcohol consumption, BMI, parity categories, and smoking.
Adjusted model with the inclusion of the three exposures in one model.
Tests whether exposure from different time points relates in the same manner to nonverbal IQ scores using the multiple informant method (Sánchez et al. 2011).
With the exclusion of mono-hexyl phthalate and mono-2-heptyl phthalate metabolite concentrations.
Total bisphenol: sum of bisphenol A, bisphenol F, and bisphenol S.
Total bisphenol: sum of bisphenol A and bisphenol S.
Total bisphenol: sum of bisphenol A and bisphenol F.
Effect Estimate Modification by Sex
No effect estimate modification by sex was observed for the association between maternal urinary concentrations of grouped phthalate metabolite concentrations and nonverbal IQ (see Table S5). Similarly, there was no effect estimate modification by sex for the association between maternal urinary bisphenol concentrations and nonverbal IQ.
Sensitivity Analyses
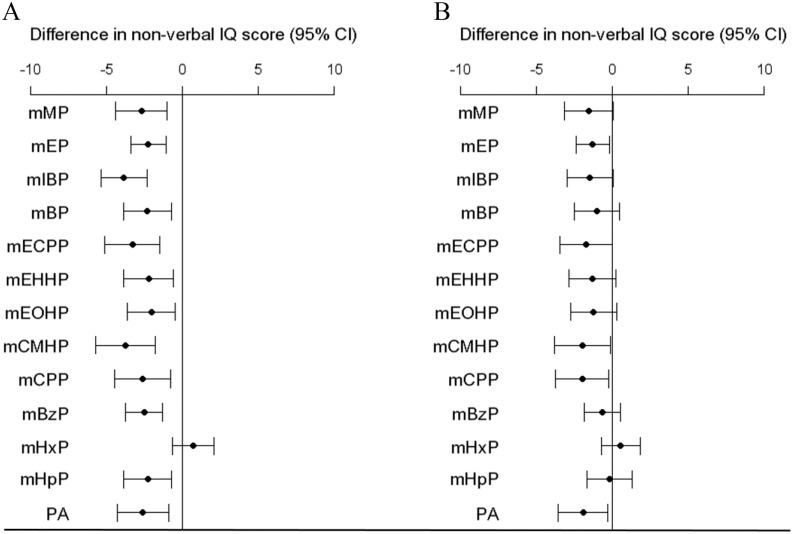
First, the results with concentrations expressed as nanograms per milliliter and creatinine concentration added as a separate covariate were similar to the results of the main analyses using creatinine-corrected concentrations (see Table S6). Second, associations between individual urine phthalate metabolite concentrations at of gestation and nonverbal IQ scores were inverse for all metabolites except for mHxP. Next, associations with mEP, mCMHP, and mCPP remained significant after confounder adjustment (Figure 1; see also Table S7). For example, the mean nonverbal IQ score was 1.3 points lower (95% CI: , ) in association with a 10-fold increase in urine mEP concentration at of gestation. Most mid and late pregnancy concentrations of phthalate metabolites were not significantly associated with nonverbal IQ. However, a 10-fold increase mCPP metabolite concentrations at of gestation was associated with a 2.4-point lower nonverbal IQ score (95% CI: , 0.0). As compared with the associations observed at of gestation, when we observed similar estimates in terms of magnitude for averaged mEP and mCMHP metabolite concentrations as well as a greater estimate in terms of magnitude for averaged mCPP metabolite concentrations. No associations were observed for the BPA, BPS, and BPF. Third, the results using inverse probability weighting to correct for potential selection bias (see Table S8) and the results in which we additionally adjusted for diet (see Table S9) were similar to the main results. Fourth, when the main results were corrected for the effective independent number of tests, the adjusted and mutually adjusted associations of maternal LMWP metabolite concentrations at with nonverbal IQ remained significant. Similarly, the adjusted association between PA metabolite concentrations at with nonverbal IQ survived the multiple testing correction.
Figure 1.
Difference in nonverbal IQ per increase in phthalate metabolite concentrations in micrograms per gram creatinine at of gestation. Corresponding numeric data are reported in Table S7. A) Unadjusted model. B) Adjusted for maternal age, maternal nonverbal IQ, sex of child, age of the child at assessment, ethnicity, education, income, marital status, maternal alcohol consumption, BMI, parity categories, and smoking categories. Note: BMI, body mass index; mBP, mono-n-butyl phthalate; mBzP, monobenzyl phthalate; mCMHP, mono-[(2-carboxymethyl)hexyl]phthalate; mCPP, mono(3-carboxypropyl) phthalate; mECPP, mono-(2-ethyl-5-carboxypentyl) phthalate; mEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; mEOHP, mono-(2-ethyl-5-oxyhexyl) phthalate; mEP, mono-ethyl phthalate; mHpP, mono-2-heptyl phthalate; mHxP, mono-hexyl phthalate; mIBP, mono-isobutyl phthalate; mMP, mono-methyl phthalate; PA, phthalic acid.
Discussion
In this large population-based study, we consistently observed that early pregnancy phthalate exposure was associated with lower child nonverbal IQ scores at 6 years of age. Urinary metabolite concentrations of early pregnancy LMWP, HMWP, DEHP, DNOP, and PA were associated with lower nonverbal IQ. We also found an inverse association between late pregnancy DNOP exposure and nonverbal IQ. Our findings did not support an association between other mid and late pregnancy grouped phthalate exposures, and we found little or no evidence of an association between prenatal bisphenol exposure and child nonverbal IQ. Finally, our results did not support effect modification by sex.
The results from our study may help clarify the so-far inconclusive results of epidemiological studies investigating the effect of prenatal exposure to phthalates on cognitive functioning. To our knowledge, most other previous studies have examined phthalate exposure during mid or late pregnancy. We did not find clear evidence for an association between mid and late pregnancy phthalate exposure and offspring IQ. Consistent with our results, previous studies have also found that prenatal phthalate metabolite concentrations measured at 14–27 wks of gestation (Kim et al. 2017), at 22–29 wks of gestation (Nakiwala et al. 2018), and at 26–36 wks of gestation (Huang et al. 2015) were not associated with IQ score measured in children. Similarly, Li et al. (2019) assessed the association between mid (range: 10–23 wks of gestation) and late pregnancy (19–35 wks of gestation) biomarkers of phthalate exposure and child IQ and did not find an association for most phthalate metabolite concentrations. However, they did report an inverse association for mid mBzP metabolite concentrations. Moreover, contrary to our results, several studies found third-trimester LMWP, DEHP, and HMWP exposure to be associated with cognition. A study demonstrated that a log-unit higher mBP and mIBP metabolite concentrations resulted in 2.7 lower IQ scores [ (, 95% CI: , ), (, 95% CI: , )] in children 7 years of age (Factor-Litvak et al. 2014). Other studies using the Bayles scales have estimated that higher DEHP and HMWP exposure was predictive of cognitive functioning in children 6 months to 3 years of age (Kim et al. 2011; Qian et al. 2019). In contrast to previous studies examining DNOP exposure in late pregnancy (Doherty et al. 2017; Li et al. 2019; Téllez-Rojo et al. 2013), we found an inverse association with late pregnancy mCPP metabolite concentrations and cognition. Differences in results may be due to ethnic differences and differences in cognitive measurement scales. For example, the study population of Factor-Litvak et al. (2014) consisted of Hispanic and African American women of inner-city New York with low socioeconomic status. Our study population comprised mainly Dutch participants and fewer socioeconomically deprived persons, limiting comparability of the results. Further, the Bayley Scales of Infant Development measures cognition through evaluation of sensory perception, knowledge, memory, problem solving, and early language at a young age. We used the SON-R test to measure cognition at 6 years of age, which is an age when IQ is arguably more stable than in infants or toddlers.
Regarding bisphenols, our results are in line with those of previous studies that also were unable to detect an association between maternal urinary BPA concentrations and cognitive functioning in children (Braun et al. 2017a, 2017b; Casas et al. 2015; Nakiwala et al. 2018; Stacy et al. 2017). BPA concentrations measured at 12 wks of gestation (Braun et al. 2017a), at 16–26 wks of gestation (Braun et al. 2017b; Stacy et al. 2017), and at 22–29 wks of gestation (Nakiwala et al. 2018) were not associated with IQ scores measured in children 1 and 8 years of age. Casas et al. (2015) used the Bayley Scales (at 1 year of age) and the McCarthy Scales of Children’s Abilities (at 4 years of age) to assess cognitive functioning but found no association for averaged first- and third-trimester BPA exposure. However, one study assessing BPA exposure using cord blood instead of maternal urine estimated an inverse association with IQ in children at 7 years of age (Lin et al. 2017).
To our knowledge, only one previous study investigated prenatal BPS and BPF exposure in relation to cognitive functioning in children (Tanner et al. 2020). The present study examined early pregnancy exposure to a mixture of endocrine-disrupting chemicals, including BPA, BPF, and BPS, and identified BPF as the primary chemical with a substantial contribution to the overall mixture effect on lower IQ among boys (Tanner et al. 2020). Manufacturers seeking BPA alternatives have turned to other bisphenols to produce BPA-free products (Grignard et al. 2012). However, experimental studies have shown that the BPA replacements have metabolism, potencies, and mechanisms of action that may be similar to that of BPA (Eladak et al. 2015). BPF and BPS also display endocrine-disruptive properties (Moreman et al. 2017; Viñas and Watson 2013). Further, an experimental study suggested that prenatal BPS exposure is able to induce hypothalamic neurogenesis (Kinch et al. 2015). However, we did not find that prenatal exposure to BPF and BPS was associated with nonverbal IQ in children.
Several studies have estimated sex differences in the association between prenatal phthalates or bisphenol exposure with neurobehavioral problems such as aggression, hyperactivity, inattention, emotional reactiveness, orientation, motor performance, anxiety, and depression and reduced masculine play in boys (Braun et al. 2009, 2011; Engel et al. 2009, 2010; Kobrosly et al. 2014; Perera et al. 2012; Roen et al. 2015; Swan et al. 2010; Yolton et al. 2011). In some studies of cognitive outcomes, effect modification by sex was estimated in the association of prenatal phthalate exposure and the Bayley Scales (Doherty et al. 2017; Kim et al. 2011; Téllez-Rojo et al. 2013; Whyatt et al. 2012) and IQ (Hyland et al. 2019), but the findings are inconsistent regarding the exact phthalate metabolites, timing of exposure, and the sex-specific effect of the associations.
The phthalate and bisphenol concentrations in the present study were generally of the same magnitude as those reported by several other studies of pregnant women. For example median mEHHP (), mEOHP (), mBP (), and BPA () concentrations in the present study were similar to those estimated in Canada (9, 6, 12, and , respectively) (Arbuckle et al. 2014) and Israel (6, 5, 10, and , respectively) (Machtinger et al. 2018). Concentrations measured in pregnant women from Korea, China, Taiwan, and the United States were, in general, somewhat higher (Factor-Litvak et al. 2014; Hyland et al. 2019; JI Kim et al. 2017; Y Kim et al. 2011; Qian et al. 2019; Whyatt et al. 2012; Woodruff et al. 2011). The difference in exposure levels between studies may be due to different dietary habits, methods and timing of urine sampling, product usage, and metabolic rate.
We found substantial confounding () of point estimates when adjusting for confounders and little change in the estimates when accounting for the exposure of other time windows. This suggests that the total effect of early pregnancy phthalate exposure on nonverbal IQ is mostly driven by the direct effect rather than by mid and late pregnancy phthalate exposure and that the effect of late pregnancy DNOP exposure is somewhat confounded by early and mid pregnancy exposure.
The critical windows of fetal neurodevelopment toxicity for phthalate exposure is uncertain. To the best of our knowledge, this is one of the first studies among humans that investigated early pregnancy phthalate exposure in relation to offspring IQ. Our results suggest that early pregnancy might be a critical period for potential effects of phthalate exposure on cognitive development. Although biological mechanisms that might contribute to associations are uncertain, animal studies have shown that phthalates may interfere with processes essential for the development of the fetal brain (Lyche et al. 2009; Miodovnik et al. 2014). A potential mechanism is via the disruption of the thyroid function (Lyche et al. 2009; Miodovnik et al. 2014). Thyroid hormones are important for fetal neurodevelopment from early pregnancy onward. Animal studies have showed that thyroid hormones are involved in neocorticogenesis and the development of the hippocampus and cytoarchitecture of the somatosensory cortex (Ausoó et al. 2004; Lavado-Autric et al. 2003). In addition, data from animal studies have shown that maternal hypothyroxinemia interferes with neuronal migration, differentiation, synaptogenesis, and cortical layer formation (Morreale de Escobar et al. 2004; Pathak et al. 2011). During early gestation, the fetus depends fully on maternal thyroid hormones that cross the placenta because the fetal thyroid function does not start before 12–14 wks of pregnancy (Morreale de Escobar et al. 2004, 2007). Moreover, after the onset of fetal thyroid hormone production, the fetus remains dependent on maternal thyroid hormones (Morreale de Escobar et al. 2004). Prenatal exposure to phthalates has been associated with changes in circulating thyroid hormone and low thyroid function in pregnant women, including during early pregnancy (Gao et al. 2017; Johns et al. 2015; Kuo et al. 2015; Yao et al. 2016), which is an important determinant of offspring neurodevelopment (Morreale de Escobar 2001; Haddow et al. 1999). For example, earlier studies in the same cohort as the present study (the Generation R Study cohort) found maternal thyroid function during early pregnancy to be associated with nonverbal IQ (Korevaar et al. 2016).
Another potential mechanism might be that prenatal phthalate exposure may affect neurodevelopment through interaction with peroxisome proliferator-activated receptors, a class of nuclear receptors involved in many physiologic processes central to neurodevelopment, including cellular reproduction and differentiation (Lyche et al. 2009; Miodovnik et al. 2014). Animal studies have shown that phthalates may induce peroxisome proliferator-activated receptor overexpression, resulting in apoptosis of undifferentiated neurons (Lin et al. 2011). Further, prenatal phthalate exposures may be associated with fetal growth (Marie et al. 2015), which is a strong predictor of neurodevelopment (Miller et al. 2016). It is conceivable that fetal exposure to phthalates may affect neurodevelopment via growth restriction. However, recent reviews do not provide a clear conclusion about the effects of phthalates on pregnancy outcomes such as gestational age, birth weight, and preterm birth (Marie et al. 2015; Radke et al. 2019; Zarean et al. 2016). Several phthalates are anti-androgenic, resulting in circulating testosterone and male reproductive tract abnormalities (Foster 2005; Hannas et al. 2011; Howdeshell et al. 2008; Johnson et al. 2012; Radke et al. 2018, 2020). Gonadal hormones are important for sex-specific brain development, and they also play a crucial role in adolescent brain remodeling (Cohen-Bendahan et al. 2005; Dahl et al. 2018). Other potential mechanisms include the disruption of calcium signaling and lipid metabolism, which are essential for normal neurodevelopmental processes in fetal life (Lyche et al. 2009; Miodovnik et al. 2014).
In the present study, we estimated that a 10-fold increase in early pregnancy phthalate metabolite concentrations and late pregnancy DNOP exposure was associated with 1.7–2.4 lower IQ points in children. Higher child IQ is associated with healthier behavior and lifetime achievements (including educational achievement, well-paid employment, enhanced social status, and the accompanying benefits to health) later in life (Batty and Deary 2004; Wraw et al. 2018). Several studies have estimated the socioeconomic impact of IQ loss. The burden and disease costs of exposure to chemicals has been estimated to be high (Trasande et al. 2015). For example, every IQ point lost from the U.S. average is estimated to have an annual cost of (Muir and Zegarac 2001).
A strength of the present study is the large sample size. The sample of our study was approximately two to three times larger than the abovementioned studies that investigated prenatal phthalates or bisphenol exposure and cognitive function in children. Another strength of the present study is the availability of more phthalate and bisphenol biomarkers as compared with previous studies investigating prenatal exposure to phthalates or bisphenols and neurodevelopment. Finally, the three repeated measures for exposure estimation in three time windows across pregnancy is yet another strength. This allowed us to investigate potential windows of susceptibility.
Our study has a few limitations that need to be considered. Although we adjusted for many potential confounders, we cannot rule out residual confounding by unknown unobserved background risk factors related to the likelihood of exposure and cognitive functioning. Second, we used three spot urine samples during early, mid, and late pregnancy for measurements of chemicals. Although this is more frequent than most other studies investigating prenatal exposure to phthalates and bisphenols and cognitive functioning, misclassification of exposure due to the limited number of samples may have occurred and could have resulted in less precise exposure–response estimates (Perrier et al. 2016; Vernet et al. 2019). This is particularly relevant for the time-specific analyses in which we relied on a single biomarker to estimate exposure. Phthalates and bisphenols have a short half-life and are quickly metabolized in the human body. Therefore, the use of multiple pooled urine specimens across trimesters is suggested to avoid exposure misclassification (Casas et al. 2018; Perrier et al. 2016; Vernet et al. 2019). Another limitation of the present study is the absence of information about the exact time of spot urine sampling. Because the urine spot samples were collected between 0800 and 1000 hours, there may have been a combination of first morning and random spot samples. Concentrations of chemicals, urine volume, and the rate of excretion vary with fluid intake, time of day, and other factors (Barr et al. 2005; Boeniger et al. 1993; Cornelis et al. 1996). Although, time of sample collection is unlikely to confound the association between phthalate and bisphenol exposure and nonverbal IQ, the difference in concentrations between morning and random spot urine could have increased the intra-individual variability. We used creatinine adjustment to account for urine dilution, which is advantageous because of its ease of measurement and the low cost and widespread availability of assays (Johnson et al. 2014). However, creatinine excretion rates may vary across pregnancy (Cheung and Lafayette 2013; Davison et al. 1980; Davison and Noble 1981), and studies have suggested that specific gravity rather than creatinine adjustment may be more appropriate in populations undergoing physiological changes in renal function, such as pregnant women (Abduljalil et al. 2012; MacPherson et al. 2018). For example, specific gravity has a slightly better within-person reproducibility and the least amount of systematic variation when compared with creatinine adjustment (MacPherson et al. 2018). However, high correlations () between creatinine and specific gravity in spot urines have been reported (Carrieri et al. 2000; Cone et al. 2009; Sauvé et al. 2015). Finally, the Generation R Study is representative of an urban population with varying ethnicities, socioeconomic statuses, and educational levels, and therefore less generalizable to populations where the phthalate and bisphenol exposure sources may differ.
In the present study, we did not observe that maternal biomarkers of bisphenols are associated with lower nonverbal IQ. We did observe that phthalate exposure in early pregnancy and DNOP exposure in late pregnancy is associated with lower nonverbal IQ scores in children. Our results might suggest that particularly early pregnancy is a sensitive window of phthalate exposure, but future studies are needed to replicate our findings.
Supplementary Material
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Center, Rotterdam, in close collaboration with the Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service, Rotterdam Homecare Foundation and Stichting Trombosedienst & Artsenlaboratorium Rijnmond. The authors extend thanks for the contribution of the participating parents and their children, general practitioners, hospitals, midwives, and pharmacies.
The general design of the Generation R Study is supported by the Erasmus Medical Center-Rotterdam, the Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development, Netherlands Organization for Scientific Research, and the Ministry of Health, Welfare and Sport, the Municipal Health Service Rotterdam area, and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond. This research received financial support from National Institutes of Health (NIH) grants R01ES022972 and R01ES029779. In addition, this work was supported in part by the Intramural Research Program of the NIH/National Institute of Environmental Health Sciences (ZIAES101575). H.T. was supported by grants from Netherlands Organization for Scientific Research (NWO; grant 024.001.003, Consortium on Individual Development, and NWO/ZonMW grant 016.VICI.170.200). M.G. is funded by a Miguel Servet fellowship (MS13/00054, CPII18/00018) awarded by the Spanish Institute of Health Carlos III. The research leading to these results received funding from the European Union Horizon 2020 Research and Innovation Programme under grant 733206 (LifeCycle Project). A.G. was supported by grant UH3OD023305 from the Environmental influences on Child Health Outcomes Program (NIH). V.W.V.J. received grant ERC-2014-CoG-648916 from the European Research Council.
References
- Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. 2012. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet 51(6):365–396, PMID: 22515555, 10.2165/11597440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, et al. 2014. Phthalate and bisphenol A exposure among pregnant women in Canada—results from the MIREC study. Environ Int 68:55–65, PMID: 24709781, 10.1016/j.envint.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Arcadi FA, Costa C, Imperatore C, Marchese A, Rapisarda A, Salemi M, et al. 1998. Oral toxicity of bis (2-ethylhexyl) phthalate during pregnancy and suckling in the Long–Evans rat. Food Chem Toxicol 36(11):963–970, PMID: 9771559, 10.1016/s0278-6915(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Asimakopoulos AG, Xue J, De Carvalho BP, Iyer A, Abualnaja KO, Yaghmoor SS, et al. 2016. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environ Res 150:573–581, PMID: 26654562, 10.1016/j.envres.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Ausoó E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. 2004. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology 145(9):4037–4047, PMID: 15087434, 10.1210/en.2004-0274. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113(2):192–200, PMID: 15687057, 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty GD, Deary IJ. 2004. Early life intelligence and adult health. BMJ 329(7466):585–586, PMID: 15361422, 10.1136/bmj.329.7466.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. 2013. Prenatal exposures to environmental chemicals and children’s neurodevelopment: an update. Saf Health Work 4(1):1–11, PMID: 23515885, 10.5491/SHAW.2013.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbel P, Navarro D, Ausó E, Varea E, Rodríguez AE, Ballesta JJ, et al. 2010. Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity. Cereb Cortex 20(6):1462–1475, PMID: 19812240, 10.1093/cercor/bhp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M, Main KM, Feldt-Rasmussen U. 2009. Environmental chemicals and thyroid function: an update. Curr Opin Endocrinol Diabetes Obes 16(5):385–391, PMID: 19625957, 10.1097/MED.0b013e3283305af7. [DOI] [PubMed] [Google Scholar]
- Boberg J, Christiansen S, Axelstad M, Kledal TS, Vinggaard AM, Dalgaard M, et al. 2011. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol 31(2):200–209, PMID: 21075200, 10.1016/j.reprotox.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J 54(10):615–627, PMID: 8237794, 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM. 2007. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect 115(suppl 1):69–76, PMID: 18174953, 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. 2006. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology 223(1–2):144–155, PMID: 16690193, 10.1016/j.tox.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. 2011. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 128(5):873–882, PMID: 22025598, 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Muckle G, Arbuckle T, Bouchard MF, Fraser WD, Ouellet E, et al. 2017a. Associations of prenatal urinary bisphenol A concentrations with child behaviors and cognitive abilities. Environ Health Perspect 125(6):067008, PMID: 28657891, 10.1289/EHP984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. 2009. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect 117(12):1945–1952, PMID: 20049216, 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Stacy SL, Erar B, Papandonatos GD, Bellinger DC, et al. 2017b. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology 62:192–199, PMID: 28736150, 10.1016/j.neuro.2017.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Doherty BT, Keil AP, Engel SM. 2017. Statistical approaches for estimating sex-specific effects in endocrine disruptors research. Environ Health Perspect 125(6):067013, PMID: 28665274, 10.1289/EHP334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri M, Trevisan A, Bartolucci GB. 2000. Adjustment to concentration-dilution of spot urine samples: correlation between specific gravity and creatinine. Int Arch Occup Environ Health 74(1):63–67, PMID: 11196084, 10.1007/s004200000190. [DOI] [PubMed] [Google Scholar]
- Casas M, Basagaña X, Sakhi AK, Haug LS, Philippat C, Granum B, et al. 2018. Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environ Int 121(pt 1):561–573, PMID: 30300814, 10.1016/j.envint.2018.09.046. [DOI] [PubMed] [Google Scholar]
- Casas M, Chevrier C, Hond ED, Fernandez MF, Pierik F, Philippat C, et al. 2013. Exposure to brominated flame retardants, perfluorinated compounds, phthalates and phenols in European birth cohorts: ENRIECO evaluation, first human biomonitoring results, and recommendations. Int J Hyg Environ Health 216(3):230–242, PMID: 22795704, 10.1016/j.ijheh.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Casas M, Forns J, Martínez D, Avella-García C, Valvi D, Ballesteros-Gómez A, et al. 2015. Exposure to bisphenol A during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Environ Res 142:671–679, PMID: 26343751, 10.1016/j.envres.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Cefic Chemdata International. 2010. Facts and Figures. The European Chemical Industry in a Worldwide Perspective. International Comparison of Production Growth. Brussels, Belgium: European Chemical Industry Council, Cefic Chemdata International. [Google Scholar]
- Cheung KL, Lafayette RA. 2013. Renal physiology of pregnancy. Adv Chronic Kidney Dis 20(3):209–214, PMID: 23928384, 10.1053/j.ackd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesi F, Ciancaleoni M, Galli S, Primi C. 2012. Using the Advanced Progressive Matrices (Set I) to assess fluid ability in a short time frame: an item response theory-based analysis. Psychol Assess 24(4):892–900, PMID: 22449036, 10.1037/a0027830. [DOI] [PubMed] [Google Scholar]
- Chou W-C, Chen J-L, Lin C-F, Chen Y-C, Shih F-C, Chuang C-Y. 2011. Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: a birth cohort study in Taiwan. Environ Health 10:94, PMID: 22050967, 10.1186/1476-069X-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bendahan CCC, van de Beek C, Berenbaum SA. 2005. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev 29(2):353–384, PMID: 15811504, 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cole SR, Hernán MA. 2008. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168(6):656–664, PMID: 18682488, 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone EJ, Caplan YH, Moser F, Robert T, Shelby MK, Black DL. 2009. Normalization of urinary drug concentrations with specific gravity and creatinine. J Anal Toxicol 33(1):1–7, PMID: 19161663, 10.1093/jat/33.1.1. [DOI] [PubMed] [Google Scholar]
- Cornelis R, Heinzow B, Herber RF, Christensen JM, Poulsen OM, Sabbioni E, et al. 1996. Sample collection guidelines for trace elements in blood and urine. IUPAC Commission of Toxicology. J Trace Elem Med Biol 10(2):103–127, PMID: 8829133, 10.1016/s0946-672x(96)80018-6. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Allen NB, Wilbrecht L, Suleiman AB. 2018. Importance of investing in adolescence from a developmental science perspective. Nature 554(7693):441–450, PMID: 29469094, 10.1038/nature25770. [DOI] [PubMed] [Google Scholar]
- Davison JM, Dunlop W, Ezimokhai M. 1980. 24‐hour creatinine clearance during the third trimester of normal pregnancy. Br J Obstet Gynaecol 87(2):106–109, PMID: 7362796, 10.1111/j.1471-0528.1980.tb04501.x. [DOI] [PubMed] [Google Scholar]
- Davison JM, Noble MCB. 1981. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol 88(1):10–17, PMID: 7459286, 10.1111/j.1471-0528.1981.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Doherty BT, Engel SM, Buckley JP, Silva MJ, Calafat AM, Wolff MS. 2017. Prenatal phthalate biomarker concentrations and performance on the Bayley Scales of Infant Development-II in a population of young urban children. Environ Res 152:51–58, PMID: 27741448, 10.1016/j.envres.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eladak S, Grisin T, Moison D, Guerquin M-J, N’Tumba-Byn T, Pozzi-Gaudin S, et al. 2015. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril 103(1):11–21, PMID: 25475787, 10.1016/j.fertnstert.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. 2010. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect 118(4):565–571, PMID: 20106747, 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, et al. 2009. Prenatal phthalate exposure and performance on the neonatal behavioral assessment scale in a multiethnic birth cohort. Neurotoxicology 30(4):522–528, PMID: 19375452, 10.1016/j.neuro.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, et al. 2014. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PLoS One 9(12):e114003, PMID: 25493564, 10.1371/journal.pone.0114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Zhu Q, Gu T, Shen X, Yang Y, Liang Y, et al. 2017. Anti-androgenic effects of bisphenol-A on spatial memory and synaptic plasticity of the hippocampus in mice. Horm Behav 93:151–158, PMID: 28576649, 10.1016/j.yhbeh.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Foster PMD. 2005. Mode of action: impaired fetal Leydig cell function—effects on male reproductive development produced by certain phthalate esters. Crit Rev Toxicol 35(8–9):713–719, PMID: 16417038, 10.1080/10408440591007395. [DOI] [PubMed] [Google Scholar]
- Gao H, Wu W, Xu Y, Jin Z, Bao H, Zhu P, et al. 2017. Effects of prenatal phthalate exposure on thyroid hormone concentrations beginning at the embryonic stage. Sci Rep 7(1):13106, PMID: 29026179, 10.1038/s41598-017-13672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A, Bongers-Schokking JJ, Henrichs J, Jaddoe VWV, Visser TJ, Visser W, et al. 2011. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the Generation R Study. Pediatr Res 69(5 pt 1):454–459, PMID: 21471776, 10.1203/PDR.0b013e3182125b0c. [DOI] [PubMed] [Google Scholar]
- Ghisari M, Bonefeld-Jorgensen EC. 2009. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett 189(1):67–77, PMID: 19463926, 10.1016/j.toxlet.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Grignard E, Lapenna S, Bremer S. 2012. Weak estrogenic transcriptional activities of bisphenol A and bisphenol S. Toxicol In Vitro 26(5):727–731, PMID: 22507746, 10.1016/j.tiv.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341(8):549–555, PMID: 10451459, 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Howdeshell KL, Wilson VS, Gray LE Jr.. 2011. Dose–response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol Sci 123(1):206–216, PMID: 21633115, 10.1093/toxsci/kfr146. [DOI] [PubMed] [Google Scholar]
- Hofman A, Jaddoe VWV, Mackenbach JP, Moll HA, Snijders RFM, Steegers EAP, et al. 2004. Growth, development and health from early fetal life until young adulthood: the Generation R Study. Paediatr Perinat Epidemiol 18(1):61–72, PMID: 14738548, 10.1111/j.1365-3016.2003.00521.x. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, et al. 2008. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague-Dawley rat in a cumulative, dose-additive manner. Toxicol Sci 105(1):153–165, PMID: 18411233, 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Huang B, Ning S, Zhang Q, Chen A, Jiang C, Cui Y, et al. 2017. Bisphenol A represses dopaminergic neuron differentiation from human embryonic stem cells through downregulating the expression of insulin-like growth factor 1. Mol Neurobiol 54(5):3798–3812, PMID: 27271280, 10.1007/s12035-016-9898-y. [DOI] [PubMed] [Google Scholar]
- Huang H-B, Chen H-Y, Su P-H, Huang P-C, Sun C-W, Wang C-J, et al. 2015. Fetal and childhood exposure to phthalate diesters and cognitive function in children up to 12 years of age: Taiwanese Maternal and Infant Cohort Study. PLoS One 10(6):e0131910, PMID: 26121592, 10.1371/journal.pone.0131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland C, Mora AM, Kogut K, Calafat AM, Harley K, Deardorff J, et al. 2019. Prenatal exposure to phthalates and neurodevelopment in the CHAMACOS cohort. Environ Health Perspect 127(10):107010, PMID: 31652105, 10.1289/EHP5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inadera H. 2015. Neurological effects of bisphenol A and its analogues. Int J Med Sci 12(12):926–936, PMID: 26664253, 10.7150/ijms.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaddoe VWV, Mackenbach JP, Moll HA, Steegers EAP, Tiemeier H, Verhulst FC, et al. 2006. The Generation R Study: design and cohort profile. Eur J Epidemiol 21(6):475–484, PMID: 16826450, 10.1007/s10654-006-9022-0. [DOI] [PubMed] [Google Scholar]
- Jaddoe VWV, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, et al. 2012. The Generation R Study: design and cohort update 2012. Eur J Epidemiol 27(9):739–756, PMID: 23086283, 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- Jaddoe VWV, van Duijn CM, van der Heijden AJ, Mackenbach JP, Moll HA, Steegers EAP, et al. 2008. The Generation R Study: design and cohort update until the age of 4 years. Eur J Epidemiol 23(12):801–811, PMID: 19101808, 10.1007/s10654-008-9309-4. [DOI] [PubMed] [Google Scholar]
- Jaddoe VWV, van Duijn CM, van der Heijden AJ, Mackenbach JP, Moll HA, Steegers EAP, et al. 2010. The Generation R Study: design and cohort update 2010. Eur J Epidemiol 25(11):823–841, PMID: 20967563, 10.1007/s10654-010-9516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson J, Roberts S, Dennehy S, Tellegen P. 1996. Validation of the Snijders-Oomen Nonverbal Intelligence Test–Revised 2½-7 for Australian children with disabilities. J Psychoeduc Assess 14(3):276–286, 10.1177/073428299601400307. [DOI] [Google Scholar]
- Jensen MS, Nørgaard-Pedersen B, Toft G, Hougaard DM, Bonde JP, Cohen A, et al. 2012. Phthalates and perfluorooctanesulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy. Environ Health Perspect 120:(6)897–903, PMID: 22398305, 10.1289/ehp.1104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-González LO, Del Toro LVA, et al. 2015. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol 13:4, PMID: 25596636, 10.1186/1477-7827-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KJ, Heger NE, Boekelheide K. 2012. Of mice and men (and rats): phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicol Sci 129(2):235–248, PMID: 22700540, 10.1093/toxsci/kfs206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Feehally J, Floege J. 2014. Comprehensive Clinical Nephrology E-Book. 5th ed Philadelphia, PA: Elsevier Health Sciences. [Google Scholar]
- Kim JI, Hong Y-C, Shin CH, Lee YA, Lim Y-H, Kim B-N. 2017. The effects of maternal and children phthalate exposure on the neurocognitive function of 6-year-old children. Environ Res 156:519–525, PMID: 28431379, 10.1016/j.envres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ha E-H, Kim E-J, Park H, Ha M, Kim J-H, et al. 2011. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) Study. Environ Health Perspect 119(10):1495–1500, PMID: 21737372, 10.1289/ehp.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura E, Matsuyoshi C, Miyazaki W, Benner S, Hosokawa M, Yokoyama K, et al. 2016. Prenatal exposure to bisphenol A impacts neuronal morphology in the hippocampal CA1 region in developing and aged mice. Arch Toxicol 90(3):691–700, PMID: 25804199, 10.1007/s00204-015-1485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch CD, Ibhazehiebo K, Jeong J-H, Habibi HR, Kurrasch DM. 2015. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci USA 112(5):1475–1480, PMID: 25583509, 10.1073/pnas.1417731112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, et al. 2014. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environ Health Perspect 122(5):521–528, PMID: 24577876, 10.1289/ehp.1307063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo TK, Li MY. 2016. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163, PMID: 27330520, 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IJzendoorn MH, et al. 2016. The Generation R Study: design and cohort update 2017. Eur J Epidemiol 31(12):1243–1264, PMID: 28070760, 10.1007/s10654-016-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korevaar TIM, Muetzel R, Medici M, Chaker L, Jaddoe VWV, de Rijke YB, et al. 2016. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol 4(1):35–43, PMID: 26497402, 10.1016/S2213-8587(15)00327-7. [DOI] [PubMed] [Google Scholar]
- Kruithof CJ, Kooijman MN, van Duijn CM, Franco OH, de Jongste JC, Klaver CCW, et al. 2014. The Generation R Study: biobank update 2015. Eur J Epidemiol 29(12):911–927, PMID: 25527369, 10.1007/s10654-014-9980-6. [DOI] [PubMed] [Google Scholar]
- Kuo F-C, Su S-W, Wu C-F, Huang M-C, Shiea J, Chen B-H, et al. 2015. Relationship of urinary phthalate metabolites with serum thyroid hormones in pregnant women and their newborns: a prospective birth cohort in Taiwan. PLoS One 10(6):e0123884, PMID: 26042594, 10.1371/journal.pone.0123884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado-Autric R, Ausó E, García-Velasco JV, del Carmen Arufe M, Escobar del Rey F, Berbel P, et al. 2003. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest 111(7):1073–1082, PMID: 12671057, 10.1172/JCI16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler H-J, Liu B, Gadogbe M, Bao W. 2018. Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. adults and children: the National Health and Nutrition Examination Survey 2013–2014. ACS Omega 3(6):6523–6532, PMID: 29978145, 10.1021/acsomega.8b00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M-X, Yeung JMY, Cherny SS, Sham PC. 2012. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum Genet 131(5):747–756, PMID: 22143225, 10.1007/s00439-011-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Papandonatos GD, Calafat AM, Yolton K, Lanphear BP, Chen A, et al. 2019. Identifying periods of susceptibility to the impact of phthalates on children’s cognitive abilities. Environ Res 172:604–614, PMID: 30878731, 10.1016/j.envres.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-J, Jiang L, Chen L, Chen H-S, Li X. 2013. Neurotoxicity of dibutyl phthalate in brain development following perinatal exposure: a study in rats. Environ Toxicol Pharmacol 36(2):392–402, PMID: 23736097, 10.1016/j.etap.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhuang M, Li T, Shi N. 2009. Neurobehavioral toxicity study of dibutyl phthalate on rats following in utero and lactational exposure. J Appl Toxicol 29(7):603–611, PMID: 19533667, 10.1002/jat.1447. [DOI] [PubMed] [Google Scholar]
- Lin C-C, Chien C-J, Tsai M-S, Hsieh C-J, Hsieh W-S, Chen P-C. 2017. Prenatal phenolic compounds exposure and neurobehavioral development at 2 and 7 years of age. Sci Total Environ 605–606:801–810, PMID: 28683424, 10.1016/j.scitotenv.2017.06.160. [DOI] [PubMed] [Google Scholar]
- Lin C-H, Chen T-J, Chen S-S, Hsiao P-C, Yang R-C. 2011. Activation of Trim17 by PPARγ is involved in di(2-ethylhexyl) phthalate (DEHP)-induced apoptosis on Neuro-2a cells. Toxicol Lett 206(3):245–251, PMID: 21856391, 10.1016/j.toxlet.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Lyche JL, Gutleb AC, Bergman Å, Eriksen GS, Murk AJ, Ropstad E, et al. 2009. Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health B Crit Rev 12(4):225–249, PMID: 20183522, 10.1080/10937400903094091. [DOI] [PubMed] [Google Scholar]
- Machtinger R, Berman T, Adir M, Mansur A, Baccarelli AA, Racowsky C, et al. 2018. Urinary concentrations of phthalate metabolites, bisphenols and personal care product chemical biomarkers in pregnant women in Israel. Environ Int 116:319–325, PMID: 29754027, 10.1016/j.envint.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson S, Arbuckle TE, Fisher M. 2018. Adjusting urinary chemical biomarkers for hydration status during pregnancy. J Expo Sci Environ Epidemiol 28(5):481–493, PMID: 29880833, 10.1038/s41370-018-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Vendittelli F, Sauvant-Rochat M-P. 2015. Obstetrical outcomes and biomarkers to assess exposure to phthalates: a review. Environ Int 83:116–136, PMID: 26118330, 10.1016/j.envint.2015.06.003. [DOI] [PubMed] [Google Scholar]
- McKinzey RK, Prieler J, Raven J. 2003. Detection of children’s malingering on Raven’s Standard Progressive Matrices. Br J Clin Psychol 42(Pt 1):95–99, PMID: 12675982, 10.1348/014466503762842048. [DOI] [PubMed] [Google Scholar]
- Michałowicz J. 2014. Bisphenol A–sources, toxicity and biotransformation. Environ Toxicol Pharmacol 37(2):738–758, PMID: 24632011, 10.1016/j.etap.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Miller SL, Huppi PS, Mallard C. 2016. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol 594(4):807–823, PMID: 26607046, 10.1113/JP271402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A, Edwards A, Bellinger DC, Hauser R. 2014. Developmental neurotoxicity of ortho-phthalate diesters: review of human and experimental evidence. Neurotoxicology 41:112–122, PMID: 24486776, 10.1016/j.neuro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Moore C, O’Keefe SL, Lawhon D, Tellegen P. 1998. Concurrent validity of the Snijders-Oomen Nonverbal Intelligence Test 2½–7–Revised with the Wechsler Preschool and Primary Scale of Intelligence–Revised. Psychol Rep 82(2):619–625, 10.2466/pr0.1998.82.2.619. [DOI] [Google Scholar]
- Moreman J, Lee O, Trznadel M, David A, Kudoh T, Tyler CR. 2017. Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ Sci Technol 51(21):12796–12805, PMID: 29016128, 10.1021/acs.est.7b03283. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G. 2001. The role of thyroid hormone in fetal neurodevelopment. J Pediatr Endocrinol Metabol 14(suppl 6):1453–1462, PMID: 11837499. [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregón MJ, del Rey FE. 2004. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 18(2):225–248, PMID: 15157838, 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregón MJ, del Rey FE. 2007. Iodine deficiency and brain development in the first half of pregnancy. Public health Nutr 10(12A):1554–1570, PMID: 18053280, 10.1017/S1368980007360928. [DOI] [PubMed] [Google Scholar]
- Muir T, Zegarac M. 2001. Societal costs of exposure to toxic substances: economic and health costs of four case studies that are candidates for environmental causation. Environ Health Perspect 109(suppl 6):885–903, PMID: 11744507, 10.1289/ehp.01109s6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakiwala D, Peyre H, Heude B, Bernard JY, Béranger R, Slama R, et al. 2018. In-utero exposure to phenols and phthalates and the intelligence quotient of boys at 5 years. Environ Health 17(1):17, PMID: 29458359, 10.1186/s12940-018-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak A, Sinha RA, Mohan V, Mitra K, Godbole MM. 2011. Maternal thyroid hormone before the onset of fetal thyroid function regulates reelin and downstream signaling cascade affecting neocortical neuronal migration. Cereb Cortex 21(1):11–21, PMID: 20368265, 10.1093/cercor/bhq052. [DOI] [PubMed] [Google Scholar]
- Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, et al. 2012. Prenatal bisphenol A exposure and child behavior in an inner-city cohort. Environ Health Perspect 120(8):1190–1194, PMID: 22543054, 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C. 2016. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 27(3):378–388, PMID: 27035688, 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VWV, Asimakopoulos AG, Kannan K, Steegers EAP, Santos S, et al. 2018. Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004–5. Environ Res 161:562–572, PMID: 29245124, 10.1016/j.envres.2017.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poimenova A, Markaki E, Rahiotis C, Kitraki E. 2010. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience 167(3):741–749, PMID: 20219646, 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- Polanska K, Ligocka D, Sobala W, Hanke W. 2014. Phthalate exposure and child development: the Polish Mother and Child Cohort Study. Early Hum Dev 90(9):477–485, PMID: 25038557, 10.1016/j.earlhumdev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Qian X, Li J, Xu S, Wan Y, Li Y, Jiang Y, et al. 2019. Prenatal exposure to phthalates and neurocognitive development in children at two years of age. Environ Int 131:105023, PMID: 31351385, 10.1016/j.envint.2019.105023. [DOI] [PubMed] [Google Scholar]
- Radke EG, Braun JM, Meeker JD, Cooper GS. 2018. Phthalate exposure and male reproductive outcomes: a systematic review of the human epidemiological evidence. Environ Int 121(Pt 1):764–793, PMID: 30336412, 10.1016/j.envint.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke EG, Braun JM, Nachman RM, Cooper GS. 2020. Phthalate exposure and neurodevelopment: a systematic review and meta-analysis of human epidemiological evidence. Environ Int 137:105408, PMID: 32045779, 10.1016/j.envint.2019.105408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke EG, Glenn BS, Braun JM, Cooper GS. 2019. Phthalate exposure and female reproductive and developmental outcomes: a systematic review of the human epidemiological evidence. Environ Int 130:104580, PMID: 31351310, 10.1016/j.envint.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roen EL, Wang Y, Calafat AM, Wang S, Margolis A, Herbstman J, et al. 2015. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Environ Res 142:739–745, PMID: 25724466, 10.1016/j.envres.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect 119(3):409–415, PMID: 21362588, 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S. 2008. Phthalates and children’s health. Curr Probl Pediatr Adolesc Health Care 38(2):34–49, PMID: 18237855, 10.1016/j.cppeds.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Sauvé J-F, Lévesque M, Huard M, Drolet D, Lavoué J, Tardif R, et al. 2015. Creatinine and specific gravity normalization in biological monitoring of occupational exposures. J Occup Environ Hyg 12(2):123–129, PMID: 25192246, 10.1080/15459624.2014.955179. [DOI] [PubMed] [Google Scholar]
- Schierow L-J, Lee MM. 2008. Phthalates in Plastics and Possible Human Health Effects. Washington, DC: Congressional Research Service, Library of Congress. [Google Scholar]
- Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. 2002. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect 110(11):A703–A707, PMID: 12417499, 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. 2000. Identifying critical windows of exposure for children’s health. Environ Health Perspect 108(suppl 3):451–455, PMID: 10852844, 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Herbert AR, Preau JL, Needham LL, Calafat AM. 2004. Detection of phthalate metabolites in human amniotic fluid. Bull Environ Contam Toxicol 72(6):1226–1231, PMID: 15362453, 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- Stacy SL, Papandonatos GD, Calafat AM, Chen A, Yolton K, Lanphear BP, et al. 2017. Early life bisphenol A exposure and neurobehavior at 8 years of age: identifying windows of heightened vulnerability. Environ Int 107:258–265, PMID: 28764921, 10.1016/j.envint.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenweg-de Graaff J, Roza SJ, Steegers EAP, Hofman A, Verhulst FC, Jaddoe VWV, et al. 2012. Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. Am J Clin Nutr 95(6):1413–1421, PMID: 22572645, 10.3945/ajcn.111.030791. [DOI] [PubMed] [Google Scholar]
- Sun W, Ban J-B, Zhang N, Zu Y-K, Sun W-X. 2014. Perinatal exposure to di‐(2‐ethylhexyl)‐phthalate leads to cognitive dysfunction and phospho‐tau level increase in aged rats. Environ Toxicol 29(5):596–603, PMID: 22610992, 10.1002/tox.21785. [DOI] [PubMed] [Google Scholar]
- Swan SH, Liu F, Hines M, Kruse RL, Wang C, Redmon JB, et al. 2010. Prenatal phthalate exposure and reduced masculine play in boys. Int J Androl 33(2):259–269, PMID: 19919614, 10.1111/j.1365-2605.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida T, Warita K, Ishihara K, Fukui S, Mitsuhashi T, Sugawara T, et al. 2009. Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: mixed exposure to phenol, phthalate, and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei. Toxicol Lett 189(1):40–47, PMID: 19481886, 10.1016/j.toxlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Tanner EM, Hallerbäck MU, Wikström S, Lindh C, Kiviranta H, Gennings C, et al. 2020. Early prenatal exposure to suspected endocrine disruptor mixtures is associated with lower IQ at age seven. Environ Int 134:105185, PMID: 31668669, 10.1016/j.envint.2019.105185. [DOI] [PubMed] [Google Scholar]
- Tellegen PJ, Winkel M, Wijnberg-Williams BJ, Laros JA. 1998. Snijders-Oomen Nonverbal Intelligence Test. SON-R 2½-7 Manual and Research Report. Lisse, Netherlands: Swets & Zeitlinger BV. [Google Scholar]
- Tellegen PJ, Winkel M, Wijnberg-Williams B, Laros JA. 2005. SON-R 2,5-7: Snijders-Oomen niet-verbale intelligentietest: Verantwoording en handleiding. [In Dutch.] Amsterdam, Netherlands: Hogrefe Uitgevers. [Google Scholar]
- Téllez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, et al. 2013. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ 461–462:386–390, PMID: 23747553, 10.1016/j.scitotenv.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH. 2016. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol 45(6):1887–1894, PMID: 28089956, 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- Tian YH, Baek JH, Lee SY, Jang CG. 2010. Prenatal and postnatal exposure to bisphenol A induces anxiolytic behaviors and cognitive deficits in mice. Synapse 64(6):432–439, PMID: 20169576, 10.1002/syn.20746. [DOI] [PubMed] [Google Scholar]
- Trasande L, Zoeller RT, Hass U, Kortenkamp A, Grandjean P, Myers JP, et al. 2015. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab 100(4):1245–1255, PMID: 25742516, 10.1210/jc.2014-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K. 2011. mice: multivariate imputation by chained equations in R. J Stat Softw 45(3):1–67, 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- Vernet C, Philippat C, Agier L, Calafat AM, Ye X, Lyon-Caen S, et al. 2019. An empirical validation of the within-subject biospecimens pooling approach to minimize exposure misclassification in biomarker-based studies. Epidemiology 30(5):756–767, PMID: 31373935, 10.1097/EDE.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñas R, Watson CS. 2013. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect 121(3):352–358, PMID: 23458715, 10.1289/ehp.1205826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. 2012. The intersection of neurotoxicology and endocrine disruption. Neurotoxicology 33(6):1410–1419, PMID: 22659293, 10.1016/j.neuro.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. 2012. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect 120(2):290–295, PMID: 21893441, 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ. 2011. The role of bisphenol A in shaping the brain, epigenome and behavior. Horm Behav 59(3):296–305, PMID: 21029734, 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 119(6):878–885, PMID: 21233055, 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraw C, Der G, Gale CR, Deary IJ. 2018. Intelligence in youth and health behaviours in middle age. Intelligence 69:71–86, PMID: 30100645, 10.1016/j.intell.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Hong X, Xie L, Li T, Yang Y, Zhang Q, et al. 2012. Gestational and lactational exposure to bisphenol-A affects anxiety- and depression-like behaviors in mice. Horm Behav 62(4):480–490, PMID: 23240141, 10.1016/j.yhbeh.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Yao H-Y, Han Y, Gao H, Huang K, Ge X, Xu Y-Y, et al. 2016. Maternal phthalate exposure during the first trimester and serum thyroid hormones in pregnant women and their newborns. Chemosphere 157:42–48, PMID: 27208644, 10.1016/j.chemosphere.2016.05.023. [DOI] [PubMed] [Google Scholar]
- Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. 2011. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol 33(5):558–566, PMID: 21854843, 10.1016/j.ntt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarean M, Keikha M, Poursafa P, Khalighinejad P, Amin M, Kelishadi R. 2016. A systematic review on the adverse health effects of di-2-ethylhexyl phthalate. Environ Sci Pollut Res Int 23(24):24642–24693, PMID: 27714658, 10.1007/s11356-016-7648-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.